- Texas A&M Health Science Center, Bryan, USA
Maternal ethanol consumption during pregnancy can lead to a stereotypic cluster of fetal craniofacial, cardiovascular, skeletal, and neurological deficits that are collectively termed the fetal alcohol spectrum disorder (FASD). Fetal ethanol exposure is a leading non-genetic cause of mental retardation. Mechanisms underlying the etiology of ethanol teratology are varied and complex. This review will focus on the developing brain as an important and vulnerable ethanol target. Near the end of the first trimester, and during the second trimester, fetal neural stem cells (NSCs) produce most of the neurons of the adult brain, and ethanol has been shown to influence NSC renewal and maturation. We will discuss the neural developmental and teratological implications of the biogenesis and function of microRNAs (miRNAs), a class of small non-protein-coding RNAs that control the expression of gene networks by translation repression. A small but growing body of research has identified ethanol-sensitive miRNAs at different stages of NSC and brain maturation. While many miRNAs appear to be vulnerable to ethanol at specific developmental stages, a few, like the miR-9 family, appear to exhibit broad vulnerability to ethanol across multiple stages of NSC differentiation. An assessment of the regulation and function of these miRNAs provides important clues about the mechanisms that underlie fetal vulnerability to alterations in the maternal-fetal environment and yields insights into the genesis of FASD.
Heavy ethanol exposure during pregnancy can lead to fetal growth retardation, and to a constellation of craniofacial, cardiovascular, skeletal, and brain growth defects (Clarren et al., 1978; Clarren, 1986) that are collectively termed “Fetal Alcohol Spectrum Disorder” (FASD; Lemoine et al., 1968; Jones et al., 1973). Brain defects can include, microencephaly, the loss of the corpus callosum, and the emergence of heterotopias. FASD is a leading, non-genetic cause of mental retardation. Epidemiological studies (SAMHSA, 2009) show that 6.8% of pregnant women report continued ethanol consumption into the third trimester of pregnancy. Moreover, 2–5% of school-aged children in the US are estimated to exhibit symptoms associated with fetal ethanol exposure (May et al., 2009). Importantly, nutrition (Thomas et al., 2009; Keen et al., 2010), and drugs of abuse like nicotine (Chen et al., 1998, 1999) also contextually enhance or mitigate effects of prenatal ethanol exposure. It is important to understand the genesis of fetal vulnerability to disrupters of the maternal-fetal environment.
Neural Stem Cells and Neurogenesis; Second Trimester Brain Vulnerability
The gestational period spanning the end of the first trimester through the second trimester of fetal development is a specific window of vulnerability, because during this period, neural stem cells (NSCs) produce the majority of adult neurons. The enormous rate of proliferation and maturation amplifies effects of early perturbations in the maternal-fetal environment, so that relatively minor disruptions in genetic and epigenetic instructions that guide stem cell maturation will significantly alter the structure and function of the mature brain.
Cerebral Cortical Neurogenesis, an Example of Ethanol Vulnerability
Most neurons of the adult human cerebral cortex are generated near the end of the first trimester through the second trimester of fetal development (Bystron et al., 2008). The human brain adds ∼2,500 new neurons every minute during this developmental window (Noback et al., 2005), indicating an enormous rate of NSC mitosis (mouse mitotic cycles can shorten to 8 h/cycle; Caviness et al., 1995). Newly generated neuroblasts of the ventricular zone (VZ), migrate directly to the cortical plate, or to the sub-ventricular zone (SVZ), where they may undergo additional mitosis before migrating to the cortical plate (Noctor et al., 2004).
Ethanol exposure during neurogenesis has been shown to decrease the thickness and cell proliferation rate within the VZ, while increasing the thickness and increasing proliferation within the SVZ (Miller, 1989; Miller and Nowakowski, 1991). Ethanol also disrupts the laminar organization of the emerging cortical plate (Kotkoskie and Norton, 1988), and consistent with autopsy observations in human FASD children, ethanol exposure during the period of neurogenesis also promotes the formation of subpial heterotopias (Mooney et al., 2004).
These studies showed that the neurogenic window constituted a critical period of vulnerability, but did not specifically identify NSCs as an ethanol target. We found that ethanol exposure did not kill NSCs (Prock and Miranda, 2007), showing that ethanol is not toxic to NSCs, compared to its toxicity to maturing neurons (Luo et al., 1997; Cheema et al., 2000; Bonthius et al., 2002). Ethanol promoted neuroepithelial cell proliferation, while simultaneously depleting the numbers of cells expressing NSC markers like ABCG2, CD133, and Sca-1 (Santillano et al., 2005). Ethanol also promoted asymmetrical cell division and the appearance of radial glial-like cells (Santillano et al., 2005). Radial glia are precursors of neuroblasts that exit the VZ (Tramontin et al., 2003; Noctor et al., 2004), and also serve as a conduit for neuronal migration out of the VZ into the intermediate SVZ, and from the SVZ into the emerging cortical plate (Anton et al., 1997; Hansen et al., 2010). These data suggest a mechanism (Figure 1) for the premature depletion or “thinning” of the VZ and its NSCs, and the corresponding increase in thickness and cell proliferation of the SVZ that was observed in in vivo experiments (Miller, 1989). Moreover, as predicted from the observed appearance of radial-glia-like precursors, ethanol-treated NSCs exhibited increased migration during their early differentiation (Camarillo and Miranda, 2008), providing a mechanism for the appearance of subpial heterotopias that have been observed in the brains of FASD children and in experimental models (Mooney et al., 2004). It is likely that NSCs are heterogeneous, and that NSCs from different brain regions may exhibit varied responses to ethanol. For example, while we observed increased maturation of cerebral cortical NSCs in response to ethanol, others showed that, in neural crest models, ethanol stalls cells in G1 (Luo et al., 1999), and retards NSC maturation (Zhou et al., 2011).
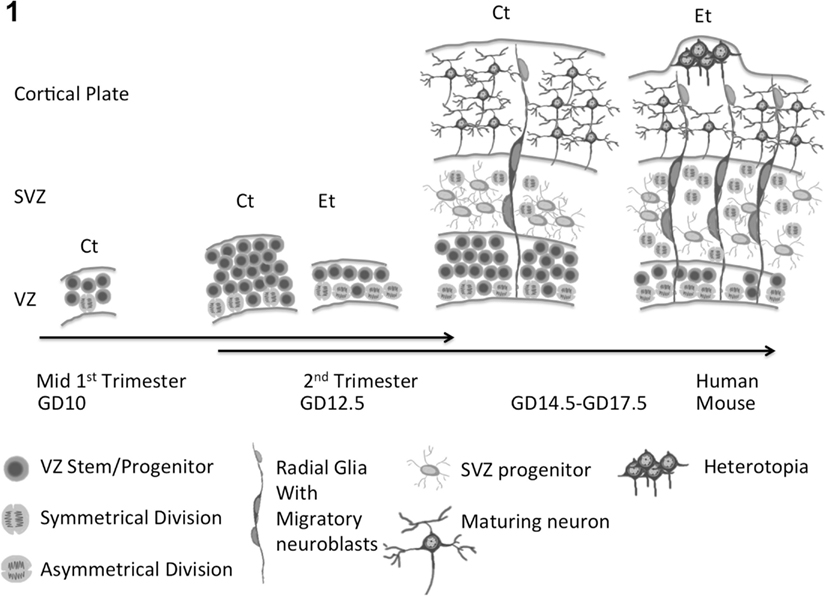
Figure 1. Composite model for ethanol effects on fetal neural development. Ethanol promotes asymmetric neural stem cell (NSC) proliferation, leading to premature depletion of NSCs in the ventricular zone (VZ) and ultimately loss of reserve neuron generation capacity. The appearance of radial glial-like neuronal progenitors, supports expansion, and increased proliferation of neuronal progenitors in the sub-ventricular zone (SVZ) and increased migration of neuronal progenitor cells out of the VZ and SVZ, leading to the formation of heterotopias or displaced neuronal aggregates.
These data collectively show that ethanol action on NSCs during the critical period of neurogenesis explains many neural defects associated with in utero ethanol exposure. A question that arises is, “what mechanisms mediate ethanol’s, effects on NSCs?” One answer may be, “microRNAs.”
MicroRNAs are Targets of Ethanol
MicroRNAs (miRNAs) are 17–25 nucleotide long, non-protein-coding RNAs that are coded within the genomes of all plants and animals and are important for the renewal and maturation of stem cells (Yi and Fuchs, 2011). MiRNAs are initially transcribed as long primary transcripts (pri-miRNAs) and processed within the nucleus by Drosha/DGCR8 to generate shorter “pre-miRNAs” (Lee et al., 2003; Han et al., 2006). Pre-miRNAs are translocated to the cytoplasm where the Dicer enzyme complex further processes them into mature double-strand RNA molecules (Zhang et al., 2002). One strand, the “guide strand” is preferentially loaded onto the RNA-induced silencing complex (RISC), to destabilize mRNA transcripts or repress translation (Lim et al., 2005).
In 2007, we obtained the first evidence showing that miRNAs mediate ethanol’s teratogenic effects (Sathyan et al., 2007). We screened 218 mouse miRNAs for ethanol sensitivity in fetal NSCs. While 38% of sampled miRNAs were expressed in fetal NSCs, only a few, miR-9, -21, -153, -335, were significantly decreased by ethanol exposure. Subsequent reports have expanded the list of ethanol-sensitive miRNAs. Wang et al. (2009) showed that ethanol exposure between the latter half of the first trimester to the middle of the second trimester-equivalent period in mice, altered ∼3% of assessed mature miRNAs in whole brain RNA samples obtained at the end of the second trimester-equivalent period. Recently, Guo et al. (2011) showed that differentiated neurons exposed to a “chronic intermittent ethanol” paradigm exhibited significant alterations in ∼11% (42 out of 385) of detected miRNAs.
Are Specific Types of miRNAs Susceptible to Ethanol Exposure?
In these early stages, it is difficult to identify unique principles that connect ethanol exposure to miRNA expression. The variety of experimental models and exposure conditions lend additional complexity to the assessment of miRNA changes. However, the accumulated data permit some conclusions.
a. Ethanol appears to influence a relatively small subset of expressed miRNAs. The apparent absence of a global effect on miRNAs suggests that ethanol does not generally target the miRNA processing machinery during development.
b. Ethanol appears to influence miRNAs that are a normal constituent of the cellular repertoire for a given stage of differentiation. None of the published screens has identified a miRNA that was uniquely expressed following ethanol exposure, but undetectable under control conditions. Therefore, ethanol is unlikely to promote lineage transformation of NSCs via a miRNA-dependent mechanism. For example, miR-124 which is sufficient to direct NSCs toward a neuronal lineage (Makeyev et al., 2007; Visvanathan et al., 2007) and is uniquely expressed in neurons (Smirnova et al., 2005), has not been identified as an ethanol-sensitive target in any of the published screens, whereas miR-9 which is ethanol-sensitive, is expressed by cells belonging to both neuronal and glial lineages (Smirnova et al., 2005). These data lend themselves to the prediction that ethanol regulation of miRNAs is unlikely to mediate transformation of a committed neuron to a glial cell, but may subtly bias miRNA expression to favor development along a specific lineage.
c. The numbers of ethanol-sensitive miRNAs appear to increase with developmental stage. It is possible that the sheer number of ethanol-sensitive miRNAs increases with differentiation stage (from ∼4% in our observations of fetal NSCs to ∼11% in the observations of Guo and colleagues in differentiated neurons). This increase may reflect increasing complexity and diversity of miRNAs that is recruited during neuronal maturation (Guo et al., 2011).
d. MicroRNAs exhibit developmental stage-specific sensitivity to ethanol. Because miRNAs play an important role in cell and tissue maturation, it is likely that each stage of NSC differentiation requires expression of new miRNAs. Therefore, new ethanol-sensitive miRNAs are likely to emerge as a function of tissue differentiation stage. A comparative analysis of our data on NSCs with data obtained with more differentiated cells and neural tissue (Wang et al., 2009; Guo et al., 2011) suggests that this hypothesis is generally true. MiRNAs like miR-335 and miR21 which are ethanol-sensitive in NSCs, and important for regulating NSC fate (Sathyan et al., 2007), are no longer observed to be ethanol-sensitive at the end of the second trimester-equivalent period, coincident with the depletion of NSC number, the disappearance of the VZ and the relative increase in the size of the SVZ. Instead other ethanol-sensitive miRNAs emerge, including miR-10a/10b (Wang et al., 2009), which serve as negative regulators of the Hox gene family (Woltering and Durston, 2008). The Hox family plays an important role in neuronal migration (Geisen et al., 2008), and elevated levels of miR-10 promote tumor cell invasion (another migratory behavior) by translation inhibition of HoxD10 (Liu et al., 2012). These data collectively suggest that ethanol-mediated elevations in miR-10a/b, during a critical window for neuronal migration, may dysregulate neuronal migration, whereas regulation of miR-21 and miR-335 at earlier time periods regulate NSC behavior.
e. Some miRNAs are ethanol-sensitive over multiple developmental windows. MiR-9 for example exhibits sensitivity to ethanol at multiple stages of development, from the embryo and fetus to the adult (Sathyan et al., 2007; Pietrzykowski et al., 2008; Wang et al., 2009; Balaraman et al., 2012; Tal et al., 2012). While ethanol decreased miR-9 expression in early development models in the mouse and fish (Sathyan et al., 2007; Balaraman et al., 2012; Tal et al., 2012), ethanol induces miR-9 expression at later developmental stages (Wang et al., 2009) and in the adult (Pietrzykowski et al., 2008). The functional implication of a presumptive developmental switch in ethanol regulation of miR-9 is unclear. Moreover, though miR-9 is targeted by ethanol throughout development, it is likely that downstream effects on translation will change simply because the transcriptome itself is significantly altered during NSC maturation.
Assessment of miRNAs for Clues about Mechanisms of Ethanol Teratology
Tal et al. (2012) reported that zebrafish exposed to between 4 and 24 h post-fertilization (hpf) exhibited decreased miR-9. Loss of miR-9 resulted in increased daylight-related hyperactivity in juvenile fish, recapitulating increased hyperactivity in FAS children (Mattson et al., 2001). These data suggest common mechanisms for ethanol teratology emerged early in vertebrates, and that some ethanol-sensitive miRNAs mediate behavioral deficits associated with fetal alcohol spectrum disorder (FASD).
MiR-9 plays a significant role in early neural tube patterning (Leucht et al., 2008), telencephalic neurogenesis, and early differentiation (Packer et al., 2008; Shibata et al., 2008, 2011). Importantly, a murine double knockout of miR-9-2 and miR-9-3 genes (miR-9-2/-3−/−) results in FAS-like phenotypes, fetal growth retardation and microencephaly (Shibata et al., 2011). Moreover, miR-9-2/-3−/− mice exhibited decreased cortical plate thickness at the end of the neurogenic period. Shibata and colleagues showed that miR-9 acts directly as a negative regulator of Foxg1, a gene that has been shown to prevent NSC differentiation and promote proliferation (Martynoga et al., 2005). Consistent with that finding, Shibata et al. additionally observed evidence for increased NSC proliferation in GD12.5, miR-9-2/-3−/− mouse brain (i.e., at the beginning of the second trimester-equivalent period). This group also discovered that, during the later half of the second trimester-equivalent period (i.e., after GD16.5, or the tail-end of the neurogenic window), Foxg1 expression was no longer suppressed in miR-9-2/-3−/− mutant mice. They suggested that this latter refractory period for Foxg1 regulation was due to a compensatory, miR-9-depletion-mediated, increase in the expression of the RNA-binding protein, ELAVL2 which in turn, stabilized Foxg1 mRNA transcripts. These data coincide well with our findings that ethanol depleted the expression of miR-9 while promoting NSC proliferation and induced expression of ELAVL2 mRNA in murine NSCs (Santillano et al., 2005; Sathyan et al., 2007), and further suggests that miR-9 plays a critical role in early second trimester ethanol teratology in coordination with other ethanol-sensitive miRNAs (Figures 2A,B). Added support for miR-9 as a mediator of ethanol neuro-teratology comes from the analysis of human familial mutations of the Foxg1 locus. Mutations in cis-regulatory elements of the Foxg1 locus are associated with fetal growth retardation, microcephaly, and mental retardation (Kortum et al., 2011), i.e., features associated with FAS.
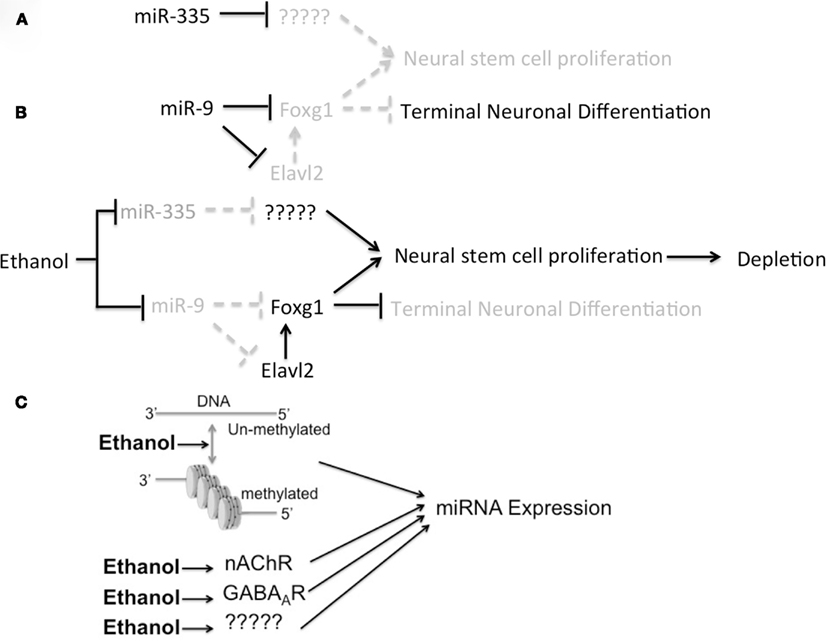
Figure 2. Model for miRNA-mediated effects of ethanol on NSC maturation and ethanol regulation of miRNAs. (A) MiRNAs like miR-9 and miR-335 may repress NSC proliferation and promote maturation by repressing specific mRNA networks. (B) Ethanol represses these miRNAs in fetal NSCs, and would be projected to attenuate terminal neuronal maturation and aberrant NSC proliferation, ultimately depleting the NSC pool. (C) The collective data supports two mechanisms for ethanol control of miRNAs, i.e., by regulating the epigenetic landscape of the genome and by influencing the activity of ligand-gated ion channels like nicotinic acetylcholine receptors (nAChRs) and GABAA receptors (GABAARs).
Mechanisms of Ethanol and Teratogen Regulation of miRNAs
Two early reports (Sathyan et al., 2007; Balaraman et al., 2012), suggest that GABAA receptors (GABAAR) and nicotinic Acetylcholine receptors (nAChRs) mediate some ethanol effects on miRNA expression. Moreover, several of the ethanol-sensitive miRNAs are localized with chromosomal regions that are susceptible to epigenetic modification. Therefore ligand-gated ion channel receptors and epigenetic modifications are potential mediators of ethanol’s effects on fetal miRNAs (Figure 2C).
GABAAR and nAChRs
We previously observed that one miRNA, miR21 was regulated by ethanol in a GABAAR dependent manner, i.e., the non-competitive GABAAR antagonist, picrotoxin, prevented the ethanol-mediated decrease in miR21 (Sathyan et al., 2007). However, another miRNA, miR-335 was not regulated by a GABAAR-dependent mechanism. These data suggest that GABAA-dependent mechanisms selectively target a sub-population of ethanol-sensitive miRNAs. All of the tested ethanol-sensitive NSC miRNAs are also regulated in a nAChR-dependent manner, since the non-selective nAChR antagonist, mecamylamine attenuated the general miRNA-inducing effects of nicotine (Balaraman et al., 2012). In fact nicotine and ethanol appear to behave as functional antagonists in regulating sensitive NSC miRNAs suggesting that these agents, which are often co-abused during pregnancy (SAMHSA, 2009), may also target common miRNA-regulated gene networks. One model for miRNA regulation that emerges from these admittedly limited studies is that nAChRs serve as general regulators, while GABAARs are more selective regulators of ethanol-sensitive NSC miRNAs. The nAChR-dependence of ethanol-sensitive miRNAs is particularly intriguing, since recent evidence shows that the dominant α4β2 nAChR isoform predominantly localizes to the endoplasmic reticulum (Richards et al., 2011), in proximity to sites for miRNA-mediated translation control. Therefore nAChRs may well share translation control with some miRNAs at the endoplasmic reticulum.
miRNA Regulation by Epigenetic Mechanisms
Ethanol has recently been shown to alter methylation patterns in differentiating NSCs (Zhou et al., 2011) and therefore, ethanol may regulate miRNAs by epigenetic mechanisms as well. A comparison of miR-21 and miR-335 provides important insights into the role of epigenetic mechanisms in miRNA expression. We found that these miRNAs served as functional antagonists to each other, and that miR21 was regulated by GABAAR-dependent mechanisms but miR-335 was not. A comparison of the chromosome structure associated with the miR-21 and miR-335 gene loci points to an alternate mechanism for miR-335 regulation. The mammalian-specific miR-335 is coded as an intronic miRNA, within the MEST/Peg1 imprinted gene locus, which contains GC-rich regions, termed CpG islands (Table 1). The miR-335/Peg1 locus is part of a network of imprinted genes that controls fetal growth. Loss of this locus is associated with fetal growth retardation in a mouse model (Lefebvre et al., 1998), while hyper-methylation of the paternal allele in humans is associated with the Russell–Silver syndrome (Kagami et al., 2007), characterized by growth retardation and mild mental retardation. Therefore expression of the miR-335/Peg1 locus is controlled epigenetically, whereas the miR21 locus, which is not associated with such methylation-sensitive chromatin, appears to be controlled by alternate mechanisms. Moreover, transcription at the Peg1/miR-335 locus is shut down as the growth of organs slows down with development (Lui et al., 2008; Finkielstain et al., 2009), further supporting a role in organogenesis. The Peg1/miR-335 locus has been implicated in early cortical development (Sansom et al., 2005). We showed that miR-335 is pro-apoptotic and decreases NSC proliferation, and conversely, miR-335 suppression (equivalent to the effect of ethanol exposure) resulted in increased NSC proliferation and resistance to apoptosis (Sathyan et al., 2007). Therefore, the brain effects of the miR-335 locus may also be susceptible to epigenetic programming.
Emerging evidence from the field of cancer biology shows that the three mammalian miR-9 genes are also susceptible to epigenetic programming. MiR-9-1, miR-9-2, and miR-9-3, can be independently targeted in a variety of cancers for hyper-methylation and consequently, inactivation (Trankenschuh et al., 2010; Tsai et al., 2011; Heller et al., 2012; Minor et al., 2012). Consequently, ethanol may also regulate the expression of miR-9 genes via epigenetic mechanisms (Figure 2C). Moreover, since ethanol appears to suppress miR-9 during early fetal development, but induce miR-9 at later developmental periods, it is possible that temporal alterations in methylation of miR-9 genes may account for the switch in ethanol’s effects. A human genome map1 of other known ethanol-sensitive miRNAs shows that several ethanol-sensitive miRNAs are either bracketed by, or proximal to CpG islands, and therefore presumptive targets for DNA methylation and epigenetic programming (Table 1).
Concluding Thoughts
Teratogens including ethanol have complex effects on developing tissues and cells, making the search for cohesive principles, elusive. The significant promise of research on miRNA involvement in fetal ethanol effects, is that we will finally be able to develop cohesive models for the susceptibility of developing biological systems to alterations in the maternal-fetal environment, including the effects of ethanol exposure. Moreover, since the maternal-fetal environment is often complex, and the developing fetus may be exposed to multiple prenatal stressors (teratogens, drugs of abuse, nutrition deficiencies etc.), it is likely that the miRNA profile of developing cells will be the product of such complexity. In this context, it is particularly interesting to note that drugs like ethanol and nicotine that are commonly co-abused, target an overlapping cohort of miRNAs in fetal NSCs (Balaraman et al., 2012). Clearly, research on miRNAs and teratology is in its infancy; but as outlined in this review, the possibilities for understanding and ultimately, for therapeutic intervention in teratology are enormous.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research described here was made possible in part, by a grant from NIAAA, R01AA013440 to Rajesh C. Miranda.
Footnote
- ^UCSC genome browser, http://genome.ucsc.edu
References
Anton, E. S., Marchionni, M. A., Lee, K. F., and Rakic, P. (1997). Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124, 3501–3510.
Balaraman, S., Winzer-Serhan, U., and Miranda, R. C. (2012). Opposing actions of ethanol and nicotine on microRNAs are mediated by nicotinic acetylcholine receptors in fetal cerebral cortical-derived neural progenitor cells. Alcohol. Clin. Exp. Res. doi:10.1111/j.1530-0277.2012.01793.x. [Epub ahead of print].
Bonthius, D. J., Tzouras, G., Karacay, B., Mahoney, J., Hutton, A., McKim, R., and Pantazis, N. J. (2002). Deficiency of neuronal nitric oxide synthase (nNOS) worsens alcohol-induced microencephaly and neuronal loss in developing mice. Brain Res. Dev. Brain Res. 138, 45–59.
Bystron, I., Blakemore, C., and Rakic, P. (2008). Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 9, 110–122.
Camarillo, C., and Miranda, R. C. (2008). Ethanol exposure during neurogenesis induces persistent effects on neural maturation: evidence from an ex vivo model of fetal cerebral cortical neuroepithelial progenitor maturation. Gene Expr. 14, 159–171.
Caviness, V. S. Jr., Takahashi, T., and Nowakowski, R. S. (1995). Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 18, 379–383.
Cheema, Z. F., West, J. R., and Miranda, R. C. (2000). Ethanol induces Fas/Apo [apoptosis]-1 mRNA and cell suicide in the developing cerebral cortex. Alcohol. Clin. Exp. Res. 24, 535–543.
Chen, W. J., Parnell, S. E., and West, J. R. (1998). Neonatal alcohol and nicotine exposure limits brain growth and depletes cerebellar Purkinje cells. Alcohol 15, 33–41.
Chen, W. J., Parnell, S. E., and West, J. R. (1999). Effects of alcohol and nicotine on developing olfactory bulb: loss of mitral cells and alterations in neurotransmitter levels. Alcohol. Clin. Exp. Res. 23, 18–25.
Clarren, S. K. (1986). Neuropathology in fetal alcohol syndrome, in Alcohol and Brain Development, ed. J. R. West (Oxford University Press, New York), 158–166.
Clarren, S. K., Alvord, E. C. Jr., Sumi, S. M., Streissguth, A. P., and Smith, D. W. (1978). Brain malformations related to prenatal exposure to ethanol. J. Pediatr. 92, 64–67.
Finkielstain, G. P., Forcinito, P., Lui, J. C., Barnes, K. M., Marino, R., Makaroun, S., Nguyen, V., Lazarus, J. E., Nilsson, O., and Baron, J. (2009). An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology 150, 1791–1800.
Geisen, M. J., Di Meglio, T., Pasqualetti, M., Ducret, S., Brunet, J. F., Chedotal, A., and Rijli, F. M. (2008). Hox paralog group 2 genes control the migration of mouse pontine neurons through slit-robo signaling. PLoS Biol. 6, e142. doi:10.1371/journal.pbio.0060142
Guo, Y., Chen, Y., Carreon, S., and Qiang, M. (2011). Chronic intermittent ethanol exposure and its removal induce a different miRNA expression pattern in primary cortical neuronal cultures. Alcohol. Clin. Exp. Res. doi: 10.1111/j.1530-0277.2011.01689.x. [Epub ahead of print]..
Han, J., Lee, Y., Yeom, K. H., Nam, J. W., Heo, I., Rhee, J. K., Sohn, S. Y., Cho, Y., Zhang, B. T., and Kim, V. N. (2006). Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125, 887–901.
Hansen, D. V., Lui, J. H., Parker, P. R., and Kriegstein, A. R. (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561.
Heller, G., Weinzierl, M., Noll, C., Babinsky, V., Ziegler, B., Altenberger, C., Minichsdorfer, C., Lang, G., Dome, B., End-Pfutzenreuter, A., Arns, B. M., Grin, Y., Klepetko, W., Zielinski, C. C., and Zochbauer-Muller, S. (2012). Genome-wide microRNA expression profiling identifies miR-9-3 and miR-193a as targets for DNA methylation in non-small cell lung cancers. Clin. Cancer Res. 18, 1619–1629.
Jones, K. L., Smith, D. W., Ulleland, C. N., and Streissguth, P. (1973). Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1, 1267–1271.
Kagami, M., Nagai, T., Fukami, M., Yamazawa, K., and Ogata, T. (2007). Silver-Russell syndrome in a girl born after in vitro fertilization: partial hypermethylation at the differentially methylated region of PEG1/MEST. J. Assist. Reprod. Genet. 24, 131–136.
Keen, C. L., Uriu-Adams, J. Y., Skalny, A., Grabeklis, A., Grabeklis, S., Green, K., Yevtushok, L., Wertelecki, W. W., and Chambers, C. D. (2010). The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. Biofactors 36, 125–135.
Kortum, F., Das, S., Flindt, M., Morris-Rosendahl, D. J., Stefanova, I., Goldstein, A., Horn, D., Klopocki, E., Kluger, G., Martin, P., Rauch, A., Roumer, A., Saitta, S., Walsh, L. E., Wieczorek, D., Uyanik, G., Kutsche, K., and Dobyns, W. B. (2011). The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J. Med. Genet. 48, 396–406.
Kotkoskie, L. A., and Norton, S. (1988). Prenatal brain malformations following acute ethanol exposure in the rat. Alcohol. Clin. Exp. Res. 12, 831–836.
Lee, Y., Ahn, C., Han, J., Choi, H., Kim, J., Yim, J., Lee, J., Provost, P., Radmark, O., Kim, S., and Kim, V. N. (2003). The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419.
Lefebvre, L., Viville, S., Barton, S. C., Ishino, F., Keverne, E. B., and Surani, M. A. (1998). Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat. Genet. 20, 163–169.
Lemoine, P., Harouseau, H., Borteryu, J. T., and Menuet, J. C. (1968). Les enfants des parents alcooliques: Anomalies observees apropos de 127 cas. Ouest Med. 21, 476–482.
Leucht, C., Stigloher, C., Wizenmann, A., Klafke, R., Folchert, A., and Bally-Cuif, L. (2008). MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat. Neurosci. 11, 641–648.
Lim, L. P., Lau, N. C., Garrett-Engele, P., Grimson, A., Schelter, J. M., Castle, J., Bartel, D. P., Linsley, P. S., and Johnson, J. M. (2005). Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773.
Liu, Z., Zhu, J., Cao, H., Ren, H., and Fang, X. (2012). miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int. J. Oncol. 40, 1553–1560.
Lui, J. C., Finkielstain, G. P., Barnes, K. M., and Baron, J. (2008). An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R189–R196.
Luo, J., West, J. R., Cook, R. T., and Pantazis, N. J. (1999). Ethanol induces cell death and cell cycle delay in cultures of pheochromocytoma PC12 cells. Alcohol. Clin. Exp. Res. 23, 644–656.
Luo, J., West, J. R., and Pantazis, N. J. (1997). Nerve growth factor and basic fibroblast growth factor protect rat cerebellar granule cells in culture against ethanol-induced cell death. Alcohol. Clin. Exp. Res. 21, 1108–1120.
Makeyev, E. V., Zhang, J., Carrasco, M. A., and Maniatis, T. (2007). The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell 27, 435–448.
Martynoga, B., Morrison, H., Price, D. J., and Mason, J. O. (2005). Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol. 283, 113–127.
Mattson, S. N., Schoenfeld, A. M., and Riley, E. P. (2001). Teratogenic effects of alcohol on brain and behavior. Alcohol Res. Health 25, 185–191.
May, P. A., Gossage, J. P., Kalberg, W. O., Robinson, L. K., Buckley, D., Manning, M., and Hoyme, H. E. (2009). Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 15, 176–192.
Miller, M. (1989). Effects of prenatal exposure to ethanol on neocortical development: II. Cell proliferation in the ventricular and subventricular zones of the rat. J. Comp. Neurol. 287, 326–338.
Miller, M. W., and Nowakowski, R. S. (1991). Effect of prenatal exposure to ethanol on the cell cycle kinetics and growth fraction in the proliferative zones of fetal rat cerebral cortex. Alcohol. Clin. Exp. Res. 15, 229–232.
Minor, J., Wang, X., Zhang, F., Song, J., Jimeno, A., Wang, X. J., Lu, X., Gross, N., Kulesz-Martin, M., Wang, D., and Lu, S. L. (2012). Methylation of microRNA-9 is a specific and sensitive biomarker for oral and oropharyngeal squamous cell carcinomas. Oral Oncol. 48, 73–78.
Mooney, S. M., Siegenthaler, J. A., and Miller, M. W. (2004). Ethanol Induces Heterotopias in Organotypic Cultures of Rat Cerebral Cortex. Cereb. Cortex 14, 1071–1080.
Noback, C. R., Strominger, N. L., Demarest, R. J., and Ruggiero, D. A. (eds) (2005). “Development and growth of the nervous system,” in The Human Nervous System (New York: Humana Press), 101–128.
Noctor, S. C., Martinez-Cerdeno, V., Ivic, L., and Kriegstein, A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144.
Packer, A. N., Xing, Y., Harper, S. Q., Jones, L., and Davidson, B. L. (2008). The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J. Neurosci. 28, 14341–14346.
Pietrzykowski, A. Z., Friesen, R. M., Martin, G. E., Puig, S. I., Nowak, C. L., Wynne, P. M., Siegelmann, H. T., and Treistman, S. N. (2008). Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron 59, 274–287.
Prock, T. L., and Miranda, R. C. (2007). Embryonic cerebral cortical progenitors are resistant to apoptosis, but increase expression of suicide receptor DISC-complex genes and suppress autophagy following ethanol exposure. Alcohol. Clin. Exp. Res. 31, 694–703.
Richards, C. I., Srinivasan, R., Xiao, C., Mackey, E. D., Miwa, J. M., and Lester, H. A. (2011). Trafficking of alpha4* nicotinic receptors revealed by superecliptic phluorin: effects of a beta4 amyotrophic lateral sclerosis-associated mutation and chronic exposure to nicotine. J. Biol. Chem. 286, 31241–31249.
SAMHSA. (2009). The NSDUH Report: Substance Use among Women during Pregnancy and Following Childbirth, Vol. NSDUH09-0521. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies.
Sansom, S. N., Hebert, J. M., Thammongkol, U., Smith, J., Nisbet, G., Surani, M. A., McConnell, S. K., and Livesey, F. J. (2005). Genomic characterisation of a Fgf-regulated gradient-based neocortical protomap. Development 132, 3947–3961.
Santillano, D. R., Kumar, L. S., Prock, T. L., Camarillo, C., Tingling, J. D., and Miranda, R. C. (2005). Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 6, 59. doi:10.1186/1471-2202-6-59
Sathyan, P., Golden, H. B., and Miranda, R. C. (2007). Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J. Neurosci. 27, 8546–8557.
Shibata, M., Kurokawa, D., Nakao, H., Ohmura, T., and Aizawa, S. (2008). MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J. Neurosci. 28, 10415–10421.
Shibata, M., Nakao, H., Kiyonari, H., Abe, T., and Aizawa, S. (2011). MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J. Neurosci. 31, 3407–3422.
Smirnova, L., Grafe, A., Seiler, A., Schumacher, S., Nitsch, R., and Wulczyn, F. G. (2005). Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 21, 1469–1477.
Tal, T. L., Franzosa, J. A., Tilton, S. C., Philbrick, K. A., Iwaniec, U. T., Turner, R. T., Waters, K. M., and Tanguay, R. L. (2012). MicroRNAs control neurobehavioral development and function in zebrafish. FASEB J. 26, 1452–1461.
Thomas, J. D., Abou, E. J., and Dominguez, H. D. (2009). Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol. Teratol. 31, 303–311.
Tramontin, A. D., Garcia-Verdugo, J. M., Lim, D. A., and Alvarez-Buylla, A. (2003). Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb. Cortex 13, 580–587.
Trankenschuh, W., Puls, F., Christgen, M., Albat, C., Heim, A., Poczkaj, J., Fleming, P., Kreipe, H., and Lehmann, U. (2010). Frequent and distinct aberrations of DNA methylation patterns in fibrolamellar carcinoma of the liver. PLoS ONE 5, e13688. doi:10.1371/journal.pone.0013688
Tsai, K. W., Liao, Y. L., Wu, C. W., Hu, L. Y., Li, S. C., Chan, W. C., Ho, M. R., Lai, C. H., Kao, H. W., Fang, W. L., Huang, K. H., and Lin, W. C. (2011). Aberrant hypermethylation of miR-9 genes in gastric cancer. Epigenetics 6, 1189–1197.
Visvanathan, J., Lee, S., Lee, B., Lee, J. W., and Lee, S. -. K. (2007). The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 21, 744–749.
Wang, L. L., Zhang, Z., Li, Q., Yang, R., Pei, X., Xu, Y., Wang, J., Zhou, S. F., and Li, Y. (2009). Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum. Reprod. 24, 562–579.
Woltering, J. M., and Durston, A. J. (2008). MiR-10 represses HoxB1a and HoxB3a in zebrafish. PLoS ONE 3, e1396. doi:10.1371/journal.pone.0001396
Yi, R., and Fuchs, E. (2011). MicroRNAs and their roles in mammalian stem cells. J. Cell. Sci. 124(Pt 11), 1775–1783.
Zhang, H., Kolb, F. A., Brondani, V., Billy, E., and Filipowicz, W. (2002). Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 21, 5875–5885.
Keywords: microRNA, fetal alcohol spectrum disorders, FASD, miR-9, neural stem cells, cerebral cortical development
Citation: Miranda RC (2012) MicroRNAs and fetal brain development: implications for ethanol teratology during the second trimester period of neurogenesis. Front. Gene. 3:77. doi: 10.3389/fgene.2012.00077
Received: 20 March 2012; Accepted: 23 April 2012;
Published online: 16 May 2012.
Edited by:
Matthew Reilly, National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism, USAReviewed by:
Peter G. Clote, Boston College, USARicardo Silva, Universidad Simón Bolívar, Venezuela
Copyright: © 2012 Miranda. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Rajesh C. Miranda, Department of Neuroscience and Experimental Therapeutics, College of Medicine, Texas A&M Health Science Center, Medical Research and Education Building, 8447 State Highway 47, Bryan, TX 77807-3260, USA e-mail:bWlyYW5kYUBtZWRpY2luZS50YW1oc2MuZWR1