- 1Department of Psychiatric-Neuroimaging-Genetics and Comorbidity Laboratory (PNGC-Lab), Tianjin Mental Health Centre, Mental Health Teaching Hospital of Tianjin Medical University, Tianjin Anding Hospital, Tianjin, China
- 2Department of Psychiatry, College of Basic Medical Science, Tianjin Medical University, Tianjin, China
- 3Department of Psychiatry, First Hospital/First Clinical Medical College of Shanxi Medical University, Taiyuan, China
- 4MDT Center for Cognitive Impairment and Sleep Disorders, First Hospital of Shanxi Medical University, Taiyuan, China
- 5Department of Psychiatry, Institute of Mental Health, Jining Medical University, Jining, China
- 6GHM Institute of CNS Regeneration, Jinan University, Guangzhou, China
- 7Department of Pattern Recognition, China National Key Laboratory, Institute of Automation, Chinese Academy of Sciences, Beijing, China
- 8Department of Pattern Recognition, University of Chinese Academy of Sciences, Beijing, China
- 9Department of Pharmacy, The First Hospital of Hebei Medical University, Shijiazhuang, China
Few studies have been conducted to explore the influence of the catechol-o-methyltransferase (COMT) genotype on the severity of and treatment efficacy on auditory verbal hallucination (AVH) symptoms in healthy individuals with AVHs (Hi-AVHs). We hypothesized that the efficacy of dopamine antagonist treatment on AVHs in Hi-AVHs may be influenced by their COMT genotype and may be accompanied by corresponding brain alterations. To preliminarily investigate and test our hypothesis in an artificially controlled pilot study, we enrolled 42 Hi-AVHs as subjects and used magnetic resonance imaging and genetic methods to explore the basis brain features to investigate whether the efficacy of dopamine antagonist treatment on AVHs in Hi-AVH subjects was influenced by their COMT genotype or not. We found that COMT-met genotype subjects’ treatment response was better than that of COMT-val subjects. Although COMT-met genotype subjects demonstrated an increase in global functional connectivity density (gFCD) but no difference on gray matter volume (GMV) compared to COMT-val genotype subjects at baseline, notably, we found that both groups demonstrated gFCD and GMV reduction after treatment, but the reduction was more widespread in COMT-met genotype subjects than in COMT-val genotype subjects. This is the first study to report that Hi-AVH subjects’ baseline brain functional features are influenced by their COMT genotypes and that the COMT-met genotype subjects exhibit better responses to dopamine antagonists but have more widespread GMV and gFCD reduction than subjects with the COMT-val genotype. Despite several limitations, these findings may provide auxiliary information to further explain the mechanisms of AVHs and provide a clue for scholars to further explore specific treatment targets for AVHs in Hi-AVH subjects or in schizophrenia patients.
Introduction
According to the previous findings, even according to the strictest criteria, 0.7% of the general population has experienced auditory verbal hallucinations (AVHs); these subjects are usually called healthy individuals with AVHs (Hi-AVHs) (Johns et al., 2004; Sommer et al., 2010; Upthegrove et al., 2016). Many hypotheses of AVHs have been established in recent decades; each hypothesis explains AVHs from a different perspective (Jones, 2010; Liemburg et al., 2012; Cho and Wu, 2014; McCarthy-Jones et al., 2014; Northoff, 2014; Wilkinson, 2014; Alderson-Day et al., 2015, 2017; Hugdahl, 2015; Conde et al., 2016; Baumeister et al., 2017; Curcic-Blake et al., 2017; Wilkinson and Fernyhough, 2017). To date, however, no hypothesis has achieved general acceptance (Wilkinson and Fernyhough, 2017). Many studies have proposed that investigating AVHs in Hi-AVHs subjects can provide important information to help clarify the mechanisms of AVHs (Jones, 2010; Hugdahl, 2015; Wilkinson and Fernyhough, 2017).
The catechol-o-methyltransferase (COMT) genotype influences brain functional (including auditory processing, which is highly related to AVHs) and dopaminergic alterations both in healthy people and in patients with schizophrenia (Lu et al., 2007; Kang et al., 2010; Gothelf et al., 2011; Edgar et al., 2012; Tian et al., 2013a,b; Li et al., 2015; Steiner et al., 2018) and the efficacy of dopamine antagonists in patients with schizophrenia (Olgiati et al., 2009; Sagud et al., 2010; Huang et al., 2016). These previous findings converge to indicate that the reciprocal interactions between COMT genotype, dopamine levels, and structural and/or functional alterations in the human brain are related to mental disorder symptoms, such as AVHs (Lu et al., 2007; Kang et al., 2010; Sagud et al., 2010; Gothelf et al., 2011; Edgar et al., 2012; Tian et al., 2013a,b; Li et al., 2015; Huang et al., 2016; Steiner et al., 2018).
COMT-met genotype patients with schizophrenia respond more strongly than COMT-val genotype patients to dopamine antagonists. In particular, patients with positive symptoms respond more strongly to dopamine antagonists in the former group than in the latter, and this response is associated with corresponding brain structural and functional alterations (Edgar et al., 2012; Lei et al., 2015; Gong et al., 2016). AVHs are the classic positive symptoms (Tandon, 2013; Reed et al., 2018). However, no study has reported the efficacy of antipsychotics on AVHs in schizophrenic patients. Most studies refer to AVHs as part of the positive symptom cluster and do not report them as a distinct category (Olgiati et al., 2009; Sagud et al., 2010; Huang et al., 2016). To the best of our knowledge, only one study has reported that the efficacy of transcranial direct current stimulation (tCDS) as a supplement to antipsychotic treatment of AVHs is weaker in schizophrenia patients with the COMT-met genotype than in those with the COMT-val genotype (Chhabra et al., 2018). A recent systematic review reported that antipsychotics can improve AVHs in patients with borderline personality disorder (Slotema et al., 2018). This study indicates the feasibility of exploring the efficacy of antipsychotics on AVHs. Exploring the pathological features of Hi-AVH subjects and the efficacy of antipsychotic treatment on them can provide new fundamental information for exploring the mechanisms of AVHs in patients with schizophrenia. The recruitment of Hi-AVHs subjects can prevent many confounding factors, such as other positive symptoms.
To the best of our knowledge, few studies have been conducted to explore the influence of the COMT genotype on the severity of AVH symptoms in healthy individuals with AVHs, to explore the relationship between brain structural and functional alterations and the COMT genotype, or to explore the efficacy of dopamine antagonists on AVHs in Hi-AVH subjects in order to explore the corresponding brain structural or functional alterations that accompany the efficacy of treatment. We proposed a hypothesis that the efficacy of dopamine antagonist treatment on AVHs symptoms in Hi-AVHs subjects is influenced by the COMT genotype and is accompanied by structural and functional brain alterations.
In the present pilot study, we adopt genotyping, functional connectivity density mapping, and statistical parametric mapping (SPM) techniques to explore the influence of the COMT genotype on AVH symptoms in Hi-AVH subjects and explore the influence of the COMT genotype on the efficacy of dopamine antagonist treatment on AVHs in Hi-AVH subjects and the accompanying brain structural and functional alterations.
Samples and Methods
Samples
For the present pilot study, we recruited by advertisement in 1,000 communities (total resident population greater than 200,000) to enroll Hi-AVH volunteers from 1,1,2016 to 6,31,2018. In accordance with previous studies (Haddock et al., 1999; Xu et al., 2012; Dollfus et al., 2018), we adopted the traditional Auditory-Verbal Hallucinations Rating Scale (AHRS) extracted from the Psychotic Symptom Rating Scales (PSYRATS) to assess the severity of the AVH symptoms. We enrolled 300 healthy people with diagnosed AVHs. Among them, only 115 subjects reported that they had suffered mental distress caused by the AVHs and volunteered to accept treatment. None of the subjects who participated in the treatment processing reported psychiatric positive family history, childhood trauma, abuse, or any other negative life events. Simultaneously, none of the subjects achieved the diagnostic criteria of any clearly mental disorders according to DSM-IV, depending on the assessment by two senior psychiatrists according to the SCID-NP semistructured interview (Phillips et al., 2009). The Tianjin Anding Hospital ethics review board approved this study. All patients provided written consent. The assessments were carried out in compliance with the Declaration of Helsinki guidelines and approved by the institutional ethics committee.
Methods
Genotyping
The genotypes were grouped by allele dominance according to the available literature (Chhabra et al., 2018). Blood collection and genotyping were performed as previously reported (Chhabra et al., 2018). In brief, 5 ml of peripheral blood was collected in K2EDTA-treated vacutainers (Becton & Dickinson, NJ, USA), and genomic DNA was extracted using commercial spin columns (Qiagen, Inc., Limburg, the Netherlands). The quality of extracted DNA was determined by UV spectrophotometry (Thermo Scientific, Waltham, MA, USA). Then, the genomic DNA was subjected to COMT genotyping at rs4680 using the TaqMan 5′ nuclease allelic discrimination assay. The genotyping was performed by real-time polymerase chain reaction (PCR) in a 96-well plate (StepOne Plus™ Real-Time PCR Systems, Applied Biosystems) with predesigned, commercially made primers and allele-specific minor groove binding probes (FAM and VIC; Applied Biosystems, Foster City, CA, USA) in a reaction volume of 10 μl (10 ng of genomic sample DNA, assay mix, and PCR Universal Master Mix with AmpErase® Uracil-DNA Glycosylase) as follows: 60°C for 30 s, and 95°C for 10 min, followed by 50 cycles of 92°C for 15 s and 60°C for 90 s. PCR was performed in duplicate with both positive and negative controls. In accordance with previous studies, COMT-met is the subjects with COMT-met/met, while COMT-val is the subjects with COMT-met/val and COMT-val/val (Kang et al., 2010).
Magnetic Resonance Imaging (MRI) Data Acquisition
All MRI data were obtained on a 3.0-tesla MR system (Discovery MR750, General Electric, Milwaukee, WI, USA). Tight but comfortable foam padding was used to stabilize head position, and earplugs were used to reduce scanner noise during image acquisition. A sagittal 3D T1-weighted brain volume sequence with 188 sagittal slices was performed with the following scan parameters: repetition time (TR) = 8.2 ms; echo time (TE) = 3.2 ms; inversion time (TI) = 450 ms; flip angle (FA) = 12°; field of view (FOV) = 256 mm × 256 mm; matrix = 256 × 256; slice thickness = 1 mm, no gap. Resting-state functional MRI (fMRI) scans were performed using a gradient-echo single-shot echo-planar imaging sequence with scan parameters of TR/TE = 2000/45 ms, FOV = 220 mm × 220 mm, matrix = 64 × 64, FA = 90°, slice thickness = 4 mm, gap = 0.5 mm, 32 interleaved transverse slices, and 180 volumes. During fMRI scans, all subjects were instructed to keep their eyes closed, to relax, to move as little as possible, to think of nothing in particular, and not to fall asleep.
fMRI Data Preprocessing
Resting-state fMRI data were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The first 10 volumes for each subject were discarded to allow for scanner stabilization and the participants’ adaption to the scanning environment. The remaining volumes were preprocessed after a slice-timing correction. All subjects’ fMRI data were within defined motion thresholds (i.e., translational and rotational motion parameters less than 2 mm or 2°). Several nuisance covariates (six motion parameters and average BOLD signals of the ventricles and white matter) were regressed out of the data. Framewise displacement (FD), which indexes volume-to-volume changes in head position, was also calculated. If the FD of the specific volume was over 0.5, spike volumes were also regressed out. The datasets were bandpass filtered with frequencies ranging from 0.01 to 0.08 Hz. Individual structural images were linearly coregistered to the mean functional image, and the transformed structural images were linearly coregistered to the Montreal Neurological Institute (MNI) space. Finally, the motion-corrected functional volumes were spatially normalized to the MNI space using parameters that were estimated during linear coregistration. The functional images were resampled into 3-mm cubic voxels.
gFCD Calculation
We calculated the gFCD of each voxel using an in-house script on the Linux platform as previously reported (Tomasi and Volkow, 2010, 2011). The strength of the functional connectivity between voxels was evaluated using Pearson’s linear correlation with a correlation coefficient threshold of R > 0.6. The gFCD calculation was restricted to voxels in the cerebral gray matter regions using a cerebral gray matter mask. The gFCD at a given voxel x0 was computed as the total number of functional connections, k(x0), between x0 and all other voxels using a “growing” algorithm that was developed on the Linux platform. This processing calculation was repeated for all voxels x0 in the whole brain. To increase the normality of the distribution, we applied grand mean scaling to gFCD by dividing by the mean value of the qualified voxels in the whole brain. Finally, to minimize differences in the functional brain anatomy across subjects, we spatially smoothed the FCD maps with a 6 × 6 × 6 mm3 Gaussian kernel.
Gray Matter Volume (GMV) Calculation
The GMV of each voxel was calculated using Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). With the standard unified segmentation model, we segmented structural MR images into gray matter (GM), white matter, and cerebrospinal fluid. After an initial affine registration of the GM concentration map into the MNI space using the technique of diffeomorphic anatomical registration through exponentiated Lie algebra, GM concentration images were nonlinearly warped and then converted to a voxel size of 1.5 × 1.5 × 1.5 mm3. The nonlinear determinants were first derived from the spatial normalization step and then multiplied by the GM concentration map to obtain the GMV of each voxel. Finally, the GMV images were smoothed with a 6 × 6 × 6 mm3 full width at half-maximum Gaussian kernel. The normalized, modulated, and smoothed GMV maps were used for statistical analyses after spatial preprocessing.
Statistical Analysis
A two-sample t-test was used to compare gFCD between groups in a voxelwise manner with adjustment for age and sex. A familywise error (FWE) method was used to correct for multiple comparisons (p < 0.05). If a significant difference between groups was found in the mean gFCD of each cluster, it was extracted for each subject and then used for region of interest (ROI)-based comparison between groups. The possible effect of GMV on global FCD changes was excluded by comparing the GMV of each ROI between groups as an added covariate of no interest. For these ROI-based analyses, the effect size of each comparison was described using Cohen’s d. To further investigate whether the gFCDs were correlated with clinical variables, we used a partial correlation analysis to analyze the relationship of ROI-based analyses with antipsychotic doses of chlorpromazine equivalents, illness duration, and adjusted for age and sex. Partial correlation analyses were also performed to investigate the relationship between AVH scores and gFCD values, adjusted for age, gender, educational level, and antipsychotic dose. The Bonferroni method was used to correct for multiple comparisons (p < 0.05).
Results
Sample Information
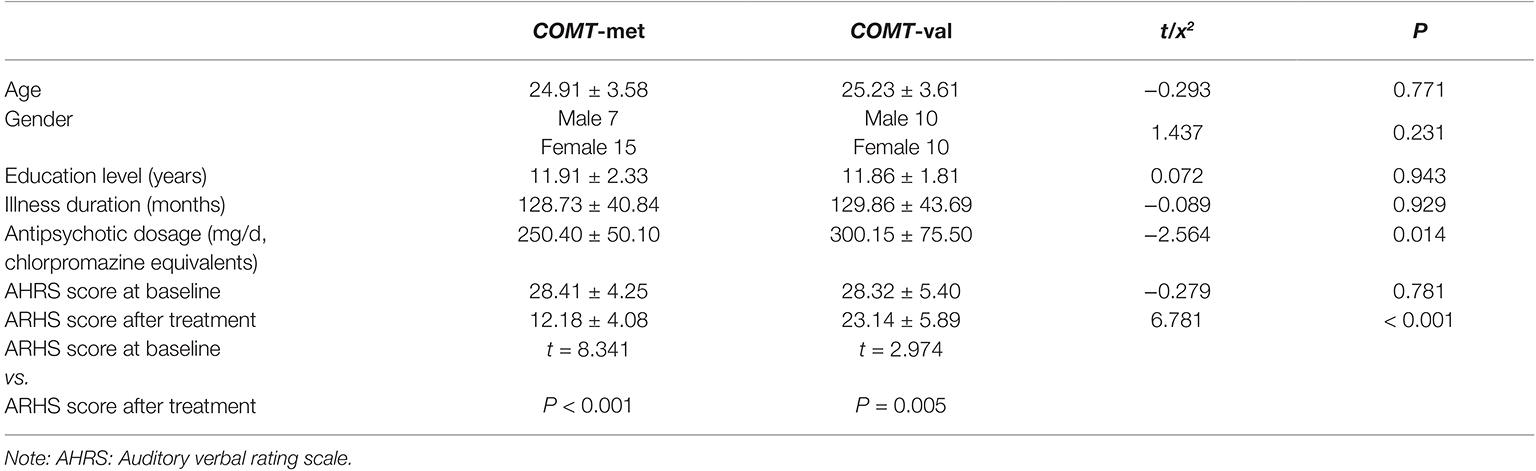
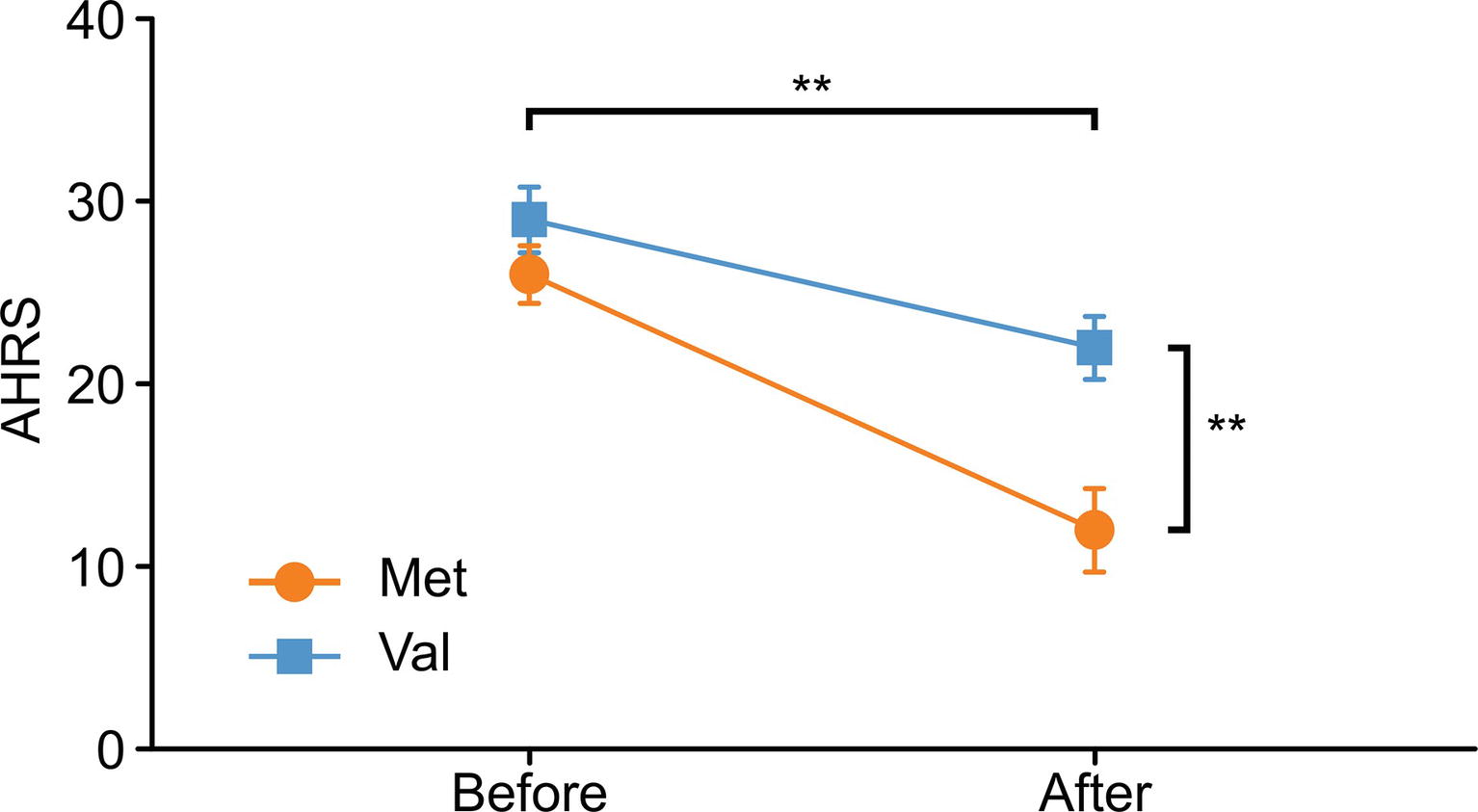
Ultimately, 34 COMT-met subjects and 45 COMT-val subjects underwent dopamine antagonist treatment for 6 months. However, the MRI data from only 25 COMT-met subjects and 21 COMT-val subjects could be used for analysis. We sought to assess accurately how the COMT genotype influenced the antipsychotic efficacy and accompanying corresponding brain alterations. We factitiously discarded information from three COMT-met subjects and one COMT-val subject (a flaw of the present study; please see the section on limitations), preserving only 22 COMT-met subjects and 20 COMT-val subjects for further analysis to guarantee comparability. All the sociodemographic, genotype, and treatment response information are shown in Table 1. There were no significant group differences in gender, age, educational level, illness duration, or baseline AVH symptom severity. During the treatment, all subjects took risperidone (Johns and Johns, Xi’an Yang-Sen Pharmaceutical Co., Ltd.) as treatment, and their antipsychotic dosages (in chlorpromazine equivalents) ranged from 500 to 100 mg/d. Antipsychotic dosage showed a significant difference between genotypes, with COMT-val subjects receiving higher dosages than COMT-met subjects. Surprisingly, however, despite largely comparable medication regimens, the efficacy of antipsychotics on AVHs was remarkably different between two groups (Table 1 and Figure 1, Note: ** < 0.001).
gFCD and GMV Differences Between Two Groups at Baseline
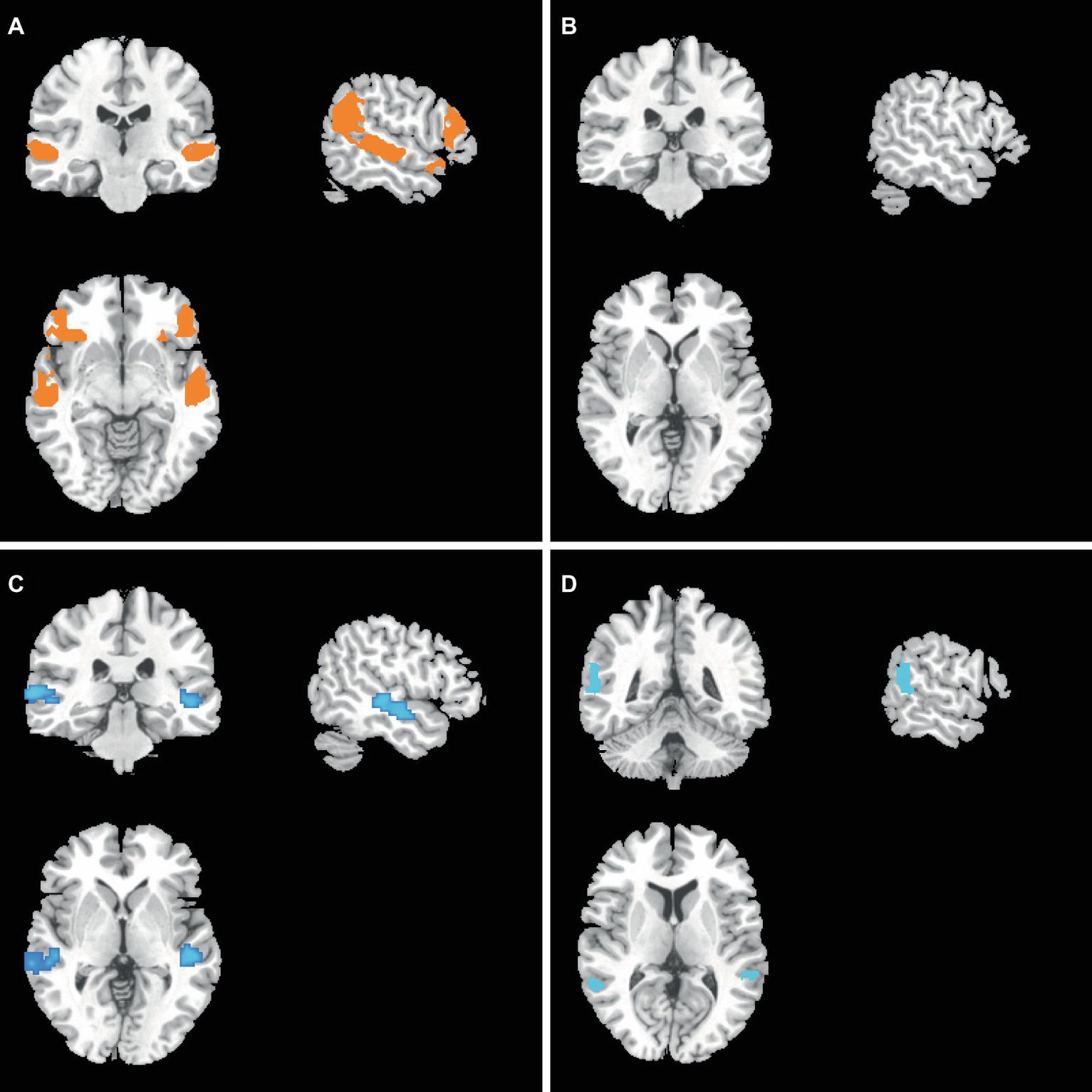
At baseline, compared to the COMT-val genotype subjects, the current study found that COMT-met genotype subjects demonstrated higher gFCDs located in the auditory cortex (superior temporal gyrus or Wernicke brain region, p < 0.05, corrected with FWE) (Figure 2A). Simultaneously, the ventral lateral prefrontal lobe, which was related to mood regulation, also demonstrated higher gFCD (Figure 2A). However, GMV showed no significant differences between two groups (Figure 2B).
gFCD and GMV Differences Between Two Groups After Treatment
After treatment, compared to the COMT-val genotype subjects, the present study found that COMT-met genotype subjects demonstrated lower gFCD in the middle temporal gyrus (Figure 2C). More notably, COMT-met genotype subjects’ GMV was also significantly lower in the lateral parietal lobe (Figure 2D).
gFCD and GMV Differences Before and After Treatment in Each Group
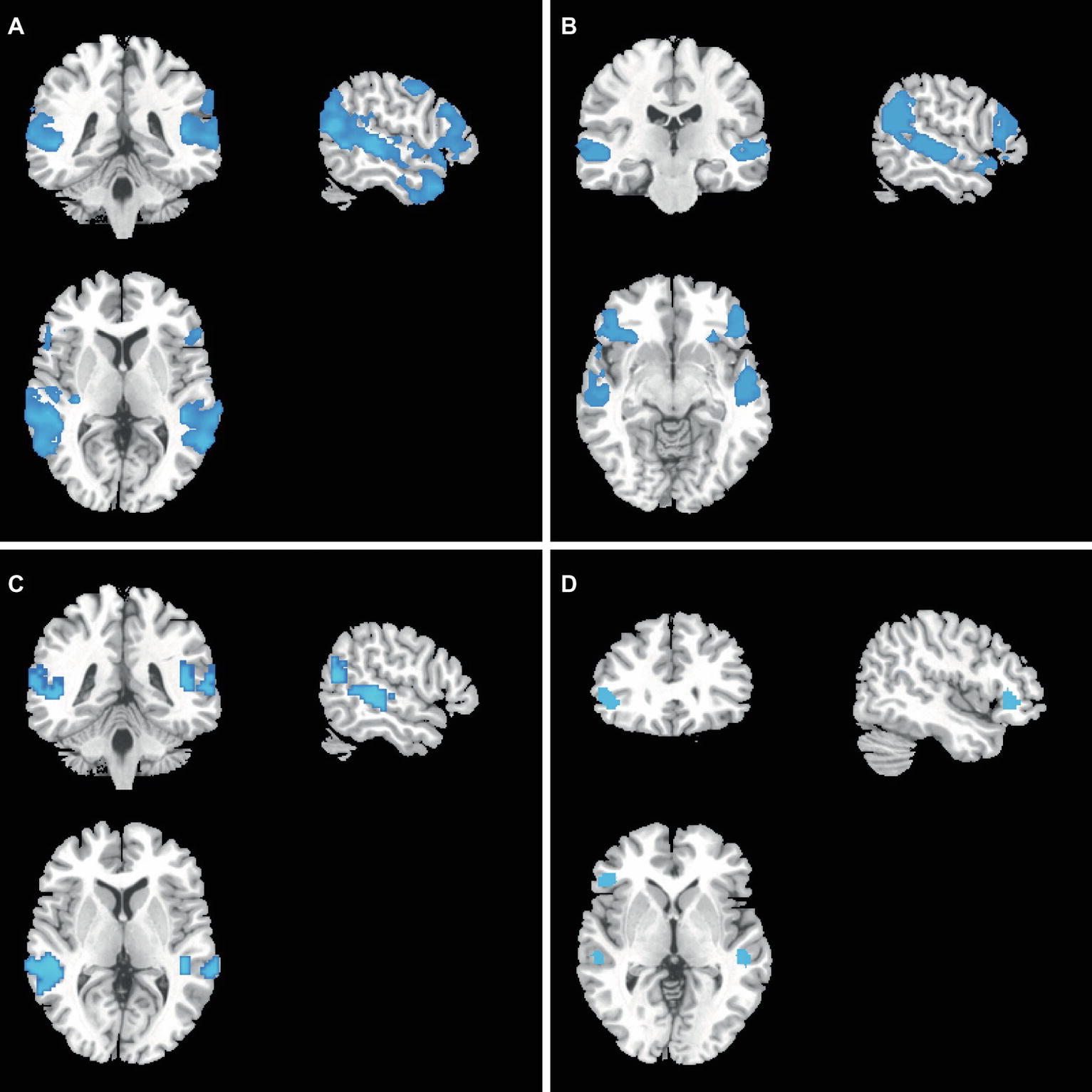
In the COMT-met subjects, gFCD was lower in the lateral parietal lobe, dorsolateral prefrontal cortex, posterior superior temporal gyrus, temporal pole, and motor cortex after treatment (Figure 3A), while GMV was lower in the lateral frontal lobe, lateral temporal lobe, and lateral parietal lobe (Figure 3B). However, in the COMT-val subjects, gFCD was lower in the posterior superior temporal gyrus and posterior parietal lobe (Figure 3C), and GMV was lower in the superior temporal gyrus and ventral lateral prefrontal lobe (Figure 3D).
The Association Among gFCD, GMV, and AVHs
In each group, we found no statistical correlations between gFCD and antipsychotic dosage in chlorpromazine equivalents, illness duration, or AVH scores before or after treatment. Similarly, we also did not find any statistical correlations between GMV and antipsychotic dosages in chlorpromazine equivalents, illness duration, or AVH scores before or after treatment.
Discussion
The present artificially controlled pilot study is the first one to demonstrate that the efficacy of dopamine antagonists on Hi-AVH subjects was influenced by the COMT genotype and was accompanied by corresponding brain structural and functional alterations. More importantly, we not only compared treatment efficacy and brain alterations between two groups but also assessed the difference before and after treatment in each group, providing supplementary information to further clarify the pathological features of Hi-AVHs with specific COMT genotypes. Although this pilot study has several limitations, it can at least provide a clue to guide further study.
Mounting studies have confirmed that GMV, usually referred to as structural alterations, affects many functional activities and subsequently causes mental and behavioral alterations (Asami et al., 2013; Rogers and De Brito, 2016). gFCD is an index evaluating functional connectivity, and many previous studies have also reported that gFCD indexes informational communication capacity to some extent. gFCD increase indicates that information communication throughout the brain is enhanced and vice versa (Goni et al., 2014; Teodoro et al., 2018). The GMV and gFCD alterations in Hi-AVH subjects after treatment indicate that antipsychotics may normalize structural and functional aberrations of the brain, subsequently alleviating AVH symptoms, although the treatment efficacy is influenced by the COMT genotype.
In this pilot study, we found that COMT-met Hi-AVH subjects achieved markedly better treatment efficacy than those with the COMT-val genotype; correspondingly, brain structural and functional alterations are also more widespread after treatment in the former group than in the latter. However, we did not find any correlation between the gFCD or GMV alterations accompanying AVH alleviation and the dosage of antipsychotics or the duration of illness. More interestingly, we also found that COMT-met subjects had a broader scope of brain structural and functional aberrations before and after treatment than COMT-val subjects. These aberrant brain regions are involved in multiple types of information processing; for example, the superior temporal gyrus participates in the processing of auditory information, the ventral lateral prefrontal lobe and lateral parietal lobe participate in cognitive information processing, the posterior superior temporal gyrus and posterior parietal lobe are related to language processing, and the temporal pole participates in language processing and multisensory integration (Fan et al., 2014; Xu et al., 2015). These findings indicated that Hi-AVH subjects also had functional aberrations in many brain regions that modulate multiple types of functional activity in the brain, not limited to auditory information processing. More importantly, after treatment, all subjects also demonstrated normalization of hyperfunctional activity, which indicated that GMV was impaired in all subjects. More notably, the GMV reduction before and after treatment was more widespread among COMT-met subjects than among COMT-val subjects, which indicated that antipsychotics may have caused GMV reduction or the normalization of enlarged GMV before treatment. This complex phenomenon requires further study of genotypically similar normal controls and Hi-AVH subjects to clarify. However, some studies have reported that antipsychotics may cause GMV reduction (Asami et al., 2013; Rogers and De Brito, 2016). As for the normalization of aberrant functional activity by antipsychotic treatment, this finding has been confirmed by many studies (Gong et al., 2016). Therefore, we do not expand on the explanation in this paper. Our data may support the postulation that antipsychotics normalize enlarged GMV before treatment, which has very little support from current available literature. Of course, additional attention and studies are required to confirm this conclusion, since the subjects in our study took antipsychotics for only 6 months.
AVHs in schizophrenia have been reported by many studies from multiple perspectives, from a clinical to a combination of neuroimaging, electrophysiological, and neurotransmitter perspectives (Lu et al., 2007; Kang et al., 2010; Gothelf et al., 2011; Edgar et al., 2012; Steiner et al., 2018). Multiple specific figures are also corroborated by many studies and some are generally accepted to some extent. However, few studies have reported on AVHs in Hi-AVH subjects. Our pilot study also found that AVH symptoms in Hi-AVHs are nearly as severe as in schizophrenia patients, and many subjects desired treatment. Further study is needed to explore specific strategies by which to treat these subjects, especially considering the GMV impairment caused by antipsychotics. In only 6 months of treatment, the GMV decreased at a remarkable speed. One possible explanation is that the GMV in Hi-AVH subjects was enlarged compared with that of healthy controls, while antipsychotics normalized the enlarged GMV, thus causing GMV reduction. However, this postulation is highly speculative and cannot be adequately tested with the current data. Therefore, further study is urgently needed to address this hypothesis.
Limitations
By listing the flaws of this pilot study, we hope to help other scholars avoid similar weaknesses in their research. This study has at least 12 limitations, which we list in the following paragraphs; we sincerely hope that international scholars will provide constructive comments to guide our subsequent studies.
First, to evaluate the practicability of this study, we adopted many methods to ensure that the subjects could complete the full study. However, despite our best efforts, only 42 subjects with adequate data can be used for the whole analysis. In order to improve the accuracy of investigating the influences of COMT genotype on the treatment efficacy of dopamine antagonist on the AVH symptoms in the Hi-AVH subjects, a long-term cohort study with large sample will be necessary. Given this pilot study, we should rethink our method to assure that large samples are sufficiently enrolled in the study. Second, to explore potentially objective evaluation indices, we discarded four samples that deviated substantially from other samples. The present study was only a pilot study, and we need to strengthen our study methods so that heterogeneous samples can be analyzed. Third, here we adopted the relatively simple indices of gFCD and GMV to explore brain alterations. More precise MRI data analysis methods are currently available, and we should adopt the most advanced method to analyze brain alterations in future studies. Fourth, in this pilot study, we considered only the COMT genotype. Other genes, such as FOX2 gene, NRG1, and other newly identified genes, were not examined. We should adopt genomic, transcriptomic, and even proteomic methods for further studies. Fifth, here we used a 3.0-T scanner to acquire MRI data. Currently, a 7.0-T scanner is in use in China. A higher resolution scanner should be applied in future studies to explore the subtle alterations in the brain. Sixth, we did not consider treatment time or reciprocal gene interaction effects, which should be taken into account in further studies. Seventh, an important flaw in this pilot study is that, limited by the condition, at baseline, we did not enroll healthy controls with COMT-met and COM-val to compare their brain alterations with those of similarly genotyped Hi-AVH subjects. Therefore, with current data, we cannot clarify whether the brain structural and functional alterations exist or not in the Hi-AVH subjects at baseline. However, comparison was performed in one patient before and after treatment in the present study. This self-control comparison may somewhat remedy the study flaw. In future studies, we must solve this problem to achieve a more precise understanding of AVHs-related brain structural alterations in the Hi-AVHs. Eighth, we did not enroll schizophrenia patients with AVHs for comparison, which limits the information that can be gained from the present study. The ninth limitation of this pilot study is that we did not compare cognitive alterations before antipsychotic treatments. To the best of our knowledge, there are no studies reporting any influence of antipsychotics on the cognitive ability of psychiatrically healthy subjects. Conversely, many studies have reported that antipsychotics (including risperidone) have positive effects on cognitive impairments in patients with schizophrenia (Suzuki and Gen, 2012; Desamericq et al., 2014), which indicates that antipsychotics can improve cognitive impairments or, at least, does not impair cognitive ability. Hence, we need to clarify the effect of antipsychotics on the cognitive ability of Hi-AVHs in the future. Tenth, when we found the GMV were lower after six-month treatment, we worried about the influences of the treatment on the subjects’ social and cognitive function. Thus, we adopted Global Assessment of Functioning (GAF) scale (Vaskinn and Abu-Akel, 2018) and Wisconsin Card Sorting Test (WCST) (Westwood et al., 2016) to evaluate each subject, which was used as a remedy method to define whether the dopamine antagonist caused the functional impairment or not in the subject. Fortunately, all subjects scored within the normal range. This problem should be avoided in the future study. More importantly, as mentioned above, the GMV in the subjects receiving treatment decreased so quickly in the Hi-AVH subjects that we must be highly vigilant. According to our pilot study, we suggest that the dopamine antagonist is not the appropriate treatment for Hi-AVH subjects. Eleventh, in this pilot study, we calculated the gFCD both with and without the global signal and found little difference between the two values. As there is no consensus as whether to include the global signal or not in calculating gFCD, we reported the gFCD with the global signal here. Twelfth, as this was a pilot study, we did not compare differences in the demographic and clinical data between the subjects who completed and those who did not complete the full study. In this study, we aimed to observe the influence of the COMT genotype on the efficacy of atypical antipsychotics on AVHs in the Hi-AVH subjects. However, we did not genotype COMT in the subjects who failed to complete the study, which was a flaw of this study.
Conclusion
In this artificially controlled pilot study, despite many limitations existed, we report for the first time that COMT genotypes influence the functional features of brains and the efficacy of dopamine antagonists on the treatment of AVHs in Hi-AVH subjects. COMT-met genotype subjects responded more strongly to dopamine antagonists but also had more serious GMV and FCD reductions than COMT-val subjects. Although the design of this pilot study is less than optimal, these findings can at least provide primary information to further explain the mechanisms of AVHs and to help reveal specific targets for the treatment of AVHs in Hi-AVH subjects or in schizophrenia patients.
Author Contributions
CZhuo and CZhou had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. CZhuo and CZhou contributed to the study concept and design. YX, LZ, and RJ participated in acquisition, analysis, or interpretation of data. CZhuo, YX, LZ, and RJ performed statistical analysis. CZhuo and CZhou contributed to administrative, technical, or material support. CZhuo involved in drafting of the manuscript.
Funding
This work was supported by grants from the Tianjin Health Bureau Foundation (2014KR02 to CZhuo), National Natural Science Foundation of China (81871052 to CZhuo), the Key Projects of the Natural Science Foundation of Tianjin, China (17JCZDJC35700 to CZhuo), National Key Research and Development Program of China (2016YFC1307004 to YX), and Multidisciplinary Team for Cognitive Impairment of Shanxi Science and Technology Innovation Training Team (201705D131027 to YX).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alderson-Day, B., Lima, C. F., Evans, S., Krishnan, S., Shanmugalingam, P., Fernyhough, C., et al. (2017). Distinct processing of ambiguous speech in people with non-clinical auditory verbal hallucinations. Brain 140, 2475–2489. doi: 10.1093/brain/awx206
Alderson-Day, B., McCarthy-Jones, S., and Fernyhough, C. (2015). Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci. Biobehav. Rev. 55, 78–87. doi: 10.1016/j.neubiorev.2015.04.016
Asami, T., Whitford, T. J., Bouix, S., Dickey, C. C., Niznikiewicz, M., Shenton, M. E., et al. (2013). Globally and locally reduced MRI gray matter volumes in neuroleptic-naive men with schizotypal personality disorder: association with negative symptoms. JAMA Psychiatry 70, 361–372. doi: 10.1001/jamapsychiatry.2013.665
Baumeister, D., Sedgwick, O., Howes, O., and Peters, E. (2017). Auditory verbal hallucinations and continuum models of psychosis: a systematic review of the healthy voice-hearer literature. Clin. Psychol. Rev. 51, 125–141. doi: 10.1016/j.cpr.2016.10.010
Chhabra, H., Shivakumar, V., Subbanna, M., Kalmady, S. V., Bose, A., Agarwal, S. M., et al. (2018). Gene polymorphisms and response to transcranial direct current stimulation for auditory verbal hallucinations in schizophrenia. Acta Neuropsychiatr. 30, 218–225. doi: 10.1017/neu.2018.4
Cho, R., and Wu, W. (2014). Is inner speech the basis of auditory verbal hallucination in schizophrenia? Front. Psychiatry 5:75. doi: 10.3389/fpsyt.2014.00075
Conde, T., Goncalves, O. F., and Pinheiro, A. P. (2016). A cognitive neuroscience view of voice-processing abnormalities in schizophrenia: a window into auditory verbal hallucinations? Harv. Rev. Psychiatry 24, 148–163. doi: 10.1097/HRP.0000000000000082
Curcic-Blake, B., Ford, J. M., Hubl, D., Orlov, N. D., Sommer, I. E., Waters, F., et al. (2017). Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog. Neurobiol. 148, 1–20. doi: 10.1016/j.pneurobio.2016.11.002
Desamericq, G., Schurhoff, F., Meary, A., Szoke, A., Macquin-Mavier, I., Bachoud-Levi, A. C., et al. (2014). Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur. J. Clin. Pharmacol. 70, 127–134. doi: 10.1007/s00228-013-1600-y
Dollfus, S., Jaafari, N., Guillin, O., Trojak, B., Plaze, M., Saba, G., et al. (2018). High-frequency neuronavigated rTMS in auditory verbal hallucinations: a pilot double-blind controlled study in patients with schizophrenia. Schizophr. Bull. 44, 505–514. doi: 10.1093/schbul/sbx127
Edgar, J. C., Hunter, M. A., Huang, M., Smith, A. K., Chen, Y., Sadek, J., et al. (2012). Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophr. Res. 140, 250–257. doi: 10.1016/j.schres.2012.06.009
Fan, L., Wang, J., Zhang, Y., Han, W., Yu, C., and Jiang, T. (2014). Connectivity-based parcellation of the human temporal pole using diffusion tensor imaging. Cereb. Cortex 24, 3365–3378. doi: 10.1093/cercor/bht196
Gong, Q., Lui, S., and Sweeney, J. A. (2016). A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am. J. Psychiatry 173, 232–243. doi: 10.1176/appi.ajp.2015.15050641
Goni, J., van den Heuvel, M. P., Avena-Koenigsberger, A., Velez de Mendizabal, N., Betzel, R. F., Griffa, A., et al. (2014). Resting-brain functional connectivity predicted by analytic measures of network communication. Proc. Natl. Acad. Sci. USA 111, 833–838. doi: 10.1073/pnas.1315529111
Gothelf, D., Hoeft, F., Ueno, T., Sugiura, L., Lee, A. D., Thompson, P., et al. (2011). Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J. Psychiatr. Res. 45, 322–331. doi: 10.1016/j.jpsychires.2010.07.008
Haddock, G., McCarron, J., Tarrier, N., and Faragher, E. B. (1999). Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol. Med. 29, 879–889. doi: 10.1017/S0033291799008661
Huang, E., Zai, C. C., Lisoway, A., Maciukiewicz, M., Felsky, D., Tiwari, A. K., et al. (2016). Catechol-O-methyltransferase Val158Met polymorphism and clinical response to antipsychotic treatment in schizophrenia and schizo-affective disorder patients: a meta-analysis. Int. J. Neuropsychopharmacol. 19. pii: pyv132. doi: 10.1093/ijnp/pyv132
Hugdahl, K. (2015). Auditory hallucinations: a review of the ERC “VOICE” project. World J. Psychiatry 5, 193–209. doi: 10.5498/wjp.v5.i2.193
Johns, L. C., Cannon, M., Singleton, N., Murray, R. M., Farrell, M., Brugha, T., et al. (2004). Prevalence and correlates of self-reported psychotic symptoms in the British population. Br. J. Psychiatry 185, 298–305. doi: 10.1192/bjp.185.4.298
Jones, S. R. (2010). Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr. Bull. 36, 566–575. doi: 10.1093/schbul/sbn129
Kang, C., Xu, X., Liu, H., and Yang, J. (2010). Association study of catechol-O-methyltransferase (COMT) gene Val158Met polymorphism with auditory P300 in Chinese Han patients with schizophrenia. Psychiatry Res. 180, 153–155. doi: 10.1016/j.psychres.2008.07.008
Lei, W., Li, N., Deng, W., Li, M., Huang, C., Ma, X., et al. (2015). White matter alterations in first episode treatment-naive patients with deficit schizophrenia: a combined VBM and DTI study. Sci. Rep. 5:12994. doi: 10.1038/srep12994
Li, M. L., Xiang, B., Li, Y. F., Hu, X., Wang, Q., Guo, W. J., et al. (2015). Morphological changes in gray matter volume correlate with catechol-O-methyl transferase gene Val158Met polymorphism in first-episode treatment-naive patients with schizophrenia. Neurosci. Bull. 31, 31–42. doi: 10.1007/s12264-014-1491-7
Liemburg, E. J., Vercammen, A., Ter Horst, G. J., Curcic-Blake, B., Knegtering, H., and Aleman, A. (2012). Abnormal connectivity between attentional, language and auditory networks in schizophrenia. Schizophr. Res. 135, 15–22. doi: 10.1016/j.schres.2011.12.003
Lu, B. Y., Martin, K. E., Edgar, J. C., Smith, A. K., Lewis, S. F., Escamilla, M. A., et al. (2007). Effect of catechol O-methyltransferase val(158)met polymorphism on the p50 gating endophenotype in schizophrenia. Biol. Psychiatry 62, 822–825. doi: 10.1016/j.biopsych.2006.11.030
McCarthy-Jones, S., Green, M. J., Scott, R. J., Tooney, P. A., Cairns, M. J., Wu, J. Q., et al. (2014). Preliminary evidence of an interaction between the FOXP2 gene and childhood emotional abuse predicting likelihood of auditory verbal hallucinations in schizophrenia. J. Psychiatr. Res. 50, 66–72. doi: 10.1016/j.jpsychires.2013.11.012
Northoff, G. (2014). Are auditory hallucinations related to the brain’s resting state activity? A ‘neurophenomenal resting state hypothesis’. Clin. Psychopharmacol. Neurosci. 12, 189–195. doi: 10.9758/cpn.2014.12.3.189
Olgiati, P., Mandelli, L., Lorenzi, C., Marino, E., Adele, P., Ferrari, B., et al. (2009). Schizophrenia: genetics, prevention and rehabilitation. Acta Neuropsychiatr. 21, 109–120. doi: 10.1111/j.1601-5215.2009.00360.x
Phillips, M. R., Zhang, J., Shi, Q., Song, Z., Ding, Z., Pang, S., et al. (2009). Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet 373, 2041–2053. doi: 10.1016/S0140-6736(09)60660-7
Reed, G. M., Keeley, J. W., Rebello, T. J., First, M. B., Gureje, O., Ayuso-Mateos, J. L., et al. (2018). Clinical utility of ICD-11 diagnostic guidelines for high-burden mental disorders: results from mental health settings in 13 countries. World Psychiatry 17, 306–315. doi: 10.1002/wps.20581
Rogers, J. C., and De Brito, S. A. (2016). Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiatry 73, 64–72. doi: 10.1001/jamapsychiatry.2015.2423
Sagud, M., Muck-Seler, D., Mihaljevic-Peles, A., Vuksan-Cusa, B., Zivkovic, M., Jakovljevic, M., et al. (2010). Catechol-O-methyl transferase and schizophrenia. Psychiatr. Danub. 22, 270–274.
Slotema, C. W., Blom, J. D., Niemantsverdriet, M. B. A., and Sommer, I. E. C. (2018). Auditory verbal hallucinations in borderline personality disorder and the efficacy of antipsychotics: a systematic review. Front. Psychiatry 9:347. doi: 10.3389/fpsyt.2018.00347
Sommer, I. E., Daalman, K., Rietkerk, T., Diederen, K. M., Bakker, S., Wijkstra, J., et al. (2010). Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr. Bull. 36, 633–641. doi: 10.1093/schbul/sbn130
Steiner, G. Z., Fernandez, F. M., Coles, M., Karamacoska, D., Barkus, E., Broyd, S. J., et al. (2018). Interrogating the relationship between schizotypy, the catechol-o-methyltransferase (COMT) Val158Met polymorphism, and neuronal oscillatory activity. Cereb. Cortex. doi: 10.1093/cercor/bhy171
Suzuki, H., and Gen, K. (2012). The influence of switching from haloperidol decanoate depot to risperidone long-acting injection on the clinical symptoms and cognitive function in schizophrenia. Hum. Psychopharmacol. 27, 470–475. doi: 10.1002/hup.2249
Tandon, R. (2013). Schizophrenia and other psychotic disorders in DSM-5. Clin. Schizophr. Relat. Psychoses 7, 16–19. doi: 10.3371/CSRP.TA.032513
Teodoro, T., Edwards, M. J., and Isaacs, J. D. (2018). A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: systematic review. J. Neurol. Neurosurg. Psychiatry. 89, 1308–1319. doi: 10.1136/jnnp-2017-317823
Tian, T., Qin, W., Liu, B., Jiang, T., and Yu, C. (2013a). Functional connectivity in healthy subjects is nonlinearly modulated by the COMT and DRD2 polymorphisms in a functional system-dependent manner. J. Neurosci. 33, 17519–17526. doi: 10.1523/JNEUROSCI.2163-13.2013
Tian, T., Qin, W., Liu, B., Wang, D., Wang, J., Jiang, T., et al. (2013b). Catechol-O-methyltransferase Val158Met polymorphism modulates gray matter volume and functional connectivity of the default mode network. PLoS One 8:e78697. doi: 10.1371/journal.pone.0078697
Tomasi, D., and Volkow, N. D. (2010). Functional connectivity density mapping. Proc. Natl. Acad. Sci. USA 107, 9885–9890. doi: 10.1073/pnas.1001414107
Tomasi, D., and Volkow, N. D. (2011). Functional connectivity hubs in the human brain. NeuroImage 57, 908–917. doi: 10.1016/j.neuroimage.2011.05.024
Upthegrove, R., Broome, M. R., Caldwell, K., Ives, J., Oyebode, F., and Wood, S. J. (2016). Understanding auditory verbal hallucinations: a systematic review of current evidence. Acta Psychiatr. Scand. 133, 352–367. doi: 10.1111/acps.12531
Vaskinn, A., and Abu-Akel, A. (2018). The interactive effect of autism and psychosis severity on theory of mind and functioning in schizophrenia. Neuropsychology. 33, 195–202. doi: 10.1037/neu0000499
Westwood, H., Stahl, D., Mandy, W., and Tchanturia, K. (2016). The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: a systematic review and meta-analysis. Psychol. Med. 46, 1809–1827. doi: 10.1017/S0033291716000581
Wilkinson, S. (2014). Accounting for the phenomenology and varieties of auditory verbal hallucination within a predictive processing framework. Conscious. Cogn. 30, 142–155. doi: 10.1016/j.concog.2014.09.002
Wilkinson, S., and Fernyhough, C. (2017). “Auditory verbal hallucinations and inner speech: A predictive processing perspective” in Before Consciousness: In Search of the Fundamentals of Mind. ed. Z. Radman. Imprint Academic [M] 207–335. Exeter (UK).
Xu, L., Qin, W., Zhuo, C., Zhu, J., Liu, H., Liu, X., et al. (2015). Selective functional disconnection of the dorsal subregion of the temporal pole in schizophrenia. Sci. Rep. 5:11258. doi: 10.1038/srep11258
Keywords: COMT genotypes, auditory verbal hallucinations, dopamine antagonists, brain alterations, health individuals
Citation: Zhuo C, Xu Y, Zhang L, Jing R and Zhou C (2019) The Effect of Dopamine Antagonist Treatment on Auditory Verbal Hallucinations in Healthy Individuals Is Clearly Influenced by COMT Genotype and Accompanied by Corresponding Brain Structural and Functional Alterations: An Artificially Controlled Pilot Study. Front. Genet. 10:92. doi: 10.3389/fgene.2019.00092
Edited by:
Weihua Yue, Peking University Sixth Hospital, ChinaCopyright © 2019 Zhuo, Xu, Zhang, Jing and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanjun Zhuo, Y2h1YW5qdW56aHVvdGptaEAxNjMuY29t
Chunhua Zhou, emhvdWNodW5odWE4MEAxMjYuY29t
 Chuanjun Zhuo
Chuanjun Zhuo Yong Xu
Yong Xu Li Zhang6
Li Zhang6