- 1Institute of Pharmacology of Natural Products and Clinical Pharmacology, University of Ulm, Ulm, Germany
- 2Department of Pharmacology, Qena Faculty of Medicine, South Valley University, Qena, Egypt
- 3Dr. Margarete Fischer-Bosch Institute of Clinical Pharmacology, Stuttgart, Germany
- 4University of Tübingen, Tübingen, Germany
- 5Transplant Center, Klinikum Stuttgart, Stuttgart, Germany
- 6Central Institute for Clinical Chemistry and Laboratory Medicine, Klinikum Stuttgart, Stuttgart, Germany
- 7Departments of Clinical Pharmacology, Pharmacy and Biochemistry, University of Tübingen, Tübingen, Germany
Background: Although there is evidence that the CYP3A4*22 variant should be considered in tacrolimus dosing in renal transplantation, its impact beyond tacrolimus dose requirements remains controversial.
Methods: In a cohort of 121 kidney transplant recipients, we analyzed the CYP3A4*1B, CYP3A4*22, and CYP3A5*3 alleles and the ABCB1 variants 1236C>T, 2677G>T/A, and 3435C>T for their impact on exposure and dose requirement. Relevant clinical outcome measures such as acute rejection within the first year after transplantation, delayed graft function, and renal function at discharge (estimated glomerular filtration rate) were evaluated.
Results: Extensive metabolizer (n = 17, CYP3A4*1/*1 carriers with at least one CYP3A5*1 allele) showed significantly higher tacrolimus dose requirement (P = 0.004) compared with both intermediate metabolizer (IM, n = 93, CYP3A5*3/*3 plus CYP3A4*1/*1 or CYP3A4*22 carriers plus one CYP3A5*1 allele), and poor metabolizer (n = 11, CYP3A4*22 allele in combination with CYP3A5*3/*3) after onset of therapy. Significantly higher dose requirement was observed in CYP3A5 expressers (P = 0.046) compared with non-expressers again at onset of therapy. Using the log additive genetic model, the area under the curve for the total observation period up to 16 days was significantly associated with the CYP3A5*3 genotype (P = 3.34 × 10-4) as well as with the IM or extensive metabolizer phenotype (P = 1.54 × 10-4), even after adjustment for multiple testing. Heterozygous carriers for CYP3A4*22 showed significantly higher areas under the curve than the CYP3A4*1/*1 genotype in the second week post-transplantation (adjusted P = 0.016). Regarding clinical outcomes, acute rejection was significantly associated with human leukocyte antigen mismatch (≥3 alleles; OR = 12.14, 95% CI 1.76, 525.21, P = 0.019 after correction for multiple testing). Graft recipients from deceased donors showed higher incidende of delayed graft function (OR 7.15, 95% CI 2.23, 30.46, adjusted P = 0.0008) and a lower estimated glomerular filtration rate at discharge (P = 0.0001). Tested CYP3A4 or CYP3A5 variants did not show any effects on clinical outcome parameters. ABCB1 variants did neither impact on pharmacokinetics nor on clinical endpoints.
Conclusion: At our transplantation center, both CYP3A5*3 and, to a lesser extent, CYP3A4*22 affect tacrolimus pharmacokinetics early after onset of therapy with consequences for steady-state treatment in routine clinical practice.
Introduction
Tacrolimus, a macrolide isolated from Streptomyces tsukubaensis, is an inhibitor of T cell proliferation widely used in solid organ transplantation. After kidney transplantation, it is part of the maintenance immunosuppressive regimen including mycophenolic acid and corticosteroids (Kasiske et al., 2010).
Tacrolimus dosing remains a clinical challenge, as its complex pharmacokinetics leads to large interindividual variability in blood levels, with drug toxicity or insufficient immunosuppression being the consequence. Although strategies to determine the optimal starting dose of tacrolimus have been explored, including algorithms for tacrolimus clearance (Passey et al., 2012), initial dosing is still guided by body weight in clinical practice.
The impact of pharmacogenetics on tacrolimus metabolism has been extensively studied. The influence of CYP3A5 expresser/non-expresser status on pharmacokinetics of tacrolimus is well established, and dosing recommendations have been published [e.g., Guideline of the Clinical Pharmacogenetics Implementation Consortium (Birdwell et al., 2015) and the Recommendations of the Dutch Pharmacogenetic Working Group (Dutch Pharmacogenetics Working Group, 2018)].
However, the effect of genotype-adjusted dosing on clinically important outcome measures [e.g., acute rejection (AR)] is still a matter of discussion. AR is regarded as one of the strongest predictors of allograft survival and thus a clinically critical outcome parameter (Matas et al., 1994). A meta-analysis published in 2015 concluded that CYP3A5 non-expressers have a significantly increased risk for transplant rejection (Rojas et al., 2015), but a prospective randomized trial failed to demonstrate a benefit in terms of AR, nephrotoxicity, or survival (Thervet et al., 2010). Also, CYP3A5-guided dosing did not lower the incidence of AR in another recent prospective investigation (Shuker et al., 2016). The absence of consistent effects on clinical outcomes found for variation in CYP3A5 mandates the search for additional genetic or nongenetic factors. Many variants of the tacrolimus-metabolizing enzyme CYP3A4 have been reported (https://www.pharmvar.org/htdocs/archive/cyp3a4.htm), but the frequency distribution of these variants is usually very low, and functional consequences are missing. However, the CYP3A4*22 allele, an intronic base change from G to A (rs35599367), has been recently correlated with reduced expression of the CYP3A4 enzyme (Wang et al., 2011) and associated with alteration of the pharmacokinetics of several CYP3A4 drugs like tacrolimus, cyclosporine, and statins (Elens et al., 2013).
Regarding pharmacokinetics, the high interindividual variability in tacrolimus trough levels (C0) even after including CYP3A5 genetics is not sufficiently explained as well. For several variants in drug-metabolizing enzymes or transporters, significant associations with tacrolimus C0 levels have been reported. However, in a validation study, a panel of 44 variants in selected candidate genes associated with tacrolimus pharmacokinetics was investigated, and variants in CYP3A5 (including others that are in strong linkage disequilibrium with CYP3A5*3) and the CYP3A4*22 allele hold true for validation (Oetting et al., 2018a). In line with these results, a genome-wide association study comprising 1,345 European Americans (a sub-cohort from the DeKAF genomics study) carrying CYP3A5 loss-of-function alleles (*3,*6, or *7) found an additional association of the CYP3A4*22 allele with tacrolimus C0 levels (Oetting et al., 2018b).
Moreover, heterozygous variant carriers for CYP3A4*22 have been shown to require lower mean tacrolimus doses and have an increased risk of supra-therapeutic tacrolimus levels (Elens et al., 2011a; Pallet et al., 2015). However, if additive information on CYP3A4*22 carrier status does improve the prediction of tacrolimus levels and dose requirements in a clinically significant way, it is still a matter of debate (Elens et al., 2011a; Moes et al., 2014; Elens and Haufroid, 2017; Lloberas et al., 2017).
Thus, the aim of this retrospective single-center study was to further elucidate the impact of the CYP3A4*22 variant on tacrolimus dose requirement and blood levels as well as its impact on AR in the first year after transplantation, delayed graft function (DGF), and renal function upon discharge. We report on 121 kidney transplant recipients whose tacrolimus levels have been closely monitored in the early period after transplantation. In addition to the CYP variants, we selected three polymorphisms of ABCB1 (encoding P-glycoprotein), since contradictory data regarding tacrolimus pharmacokinetics and ABCB1 genetics in renal transplant patients (reviewed by Tron et al., 2018) have been reported.
Patients and Methods
Patient Population
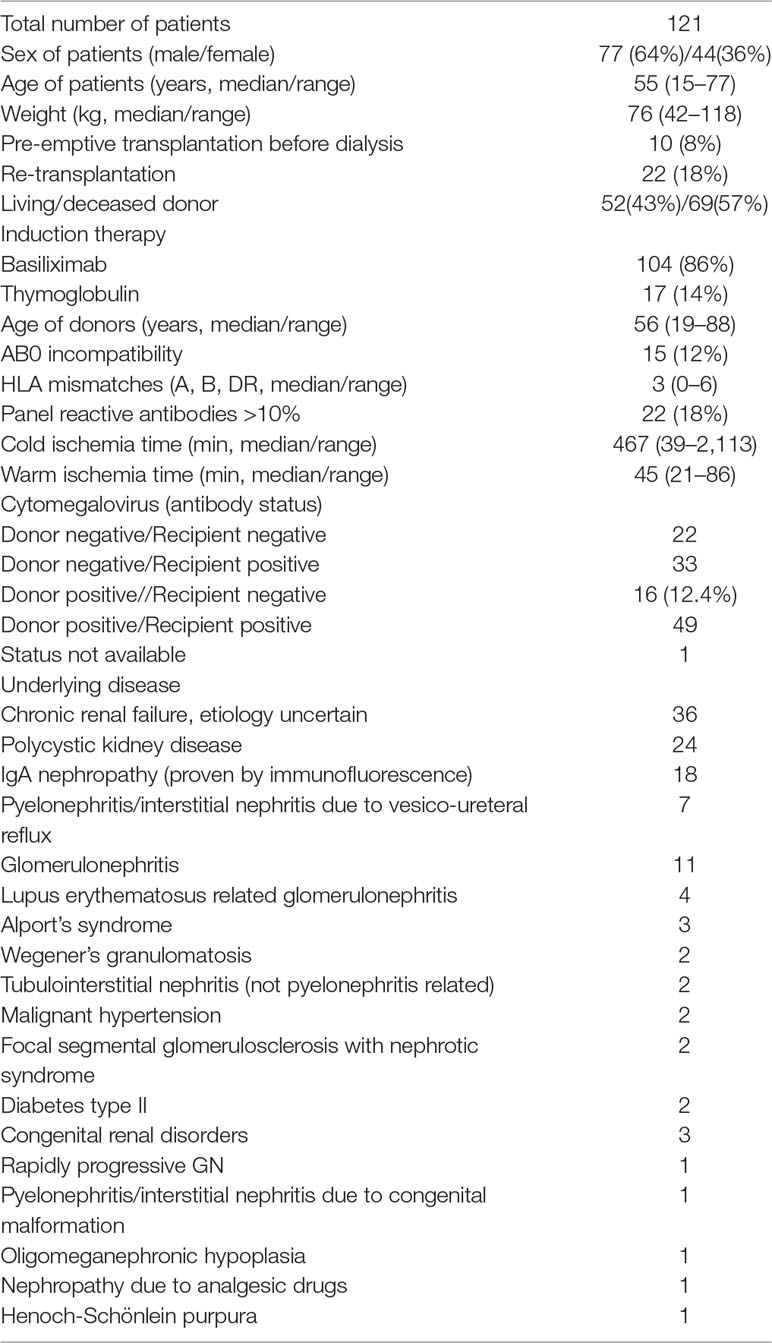
Data from 121 patients who underwent kidney transplantation between 2009 and 2015 at the Transplantation Center of Klinikum Stuttgart, Germany, were available for analysis. The study was approved by the ethics committee of University Hospital, Tuebingen, Germany (616/2013BO2). All patients signed a written informed consent. As induction therapy, 86% (104/121) of kidney transplant recipients received the interleukin-2 receptor antagonist basiliximab, while the remaining 14% received thymoglobulin due to high immunologic risk. The immunosuppressive maintenance therapy after renal transplantation at the transplantation center at Klinikum Stuttgart, Germany is a triple therapy of tacrolimus, mycophenolate mofetil, and prednisolone. Tacrolimus therapy is started at the day of renal transplantation with a dosage of 0.1 mg/kg/day orally and subsequently adjusted to achieve a target tacrolimus C0 concentration of 6 to 8 µg/l in the first 3 months after transplantation. Thereafter, the target C0 level is 4 to 6 µg/l. The daily dosage of tacrolimus until day 16 after transplantation and at discharge of the patient from the hospital was considered for pharmacogenetic analyses. Mycophenolate sodium 720 mg was given twice a day during the first 3 months starting from day 1. Prednisolone was uniformly administered to all patients according to the therapy protocol, with intravenous 250 mg peri-operatively, and then orally as 0.5 mg per kg body weight until day 14, then continued with 20 mg/day and tapering. Moreover, all recipients receive as part of the standard treatment protocol proton-pump-inhibitors, colecalciferol plus calcium carbonate, cotrimoxazole, and, for the first 4 days, piperacillin/tazobactam intravenously. Depending on the cytomegalovirus (CMV) status, antiviral prophylaxis with valgancyclovir is administered only to CMV-negative recipients receiving a CMV positive graft. Demographic characteristics of the study cohort are shown in Table 1.
Genotyping
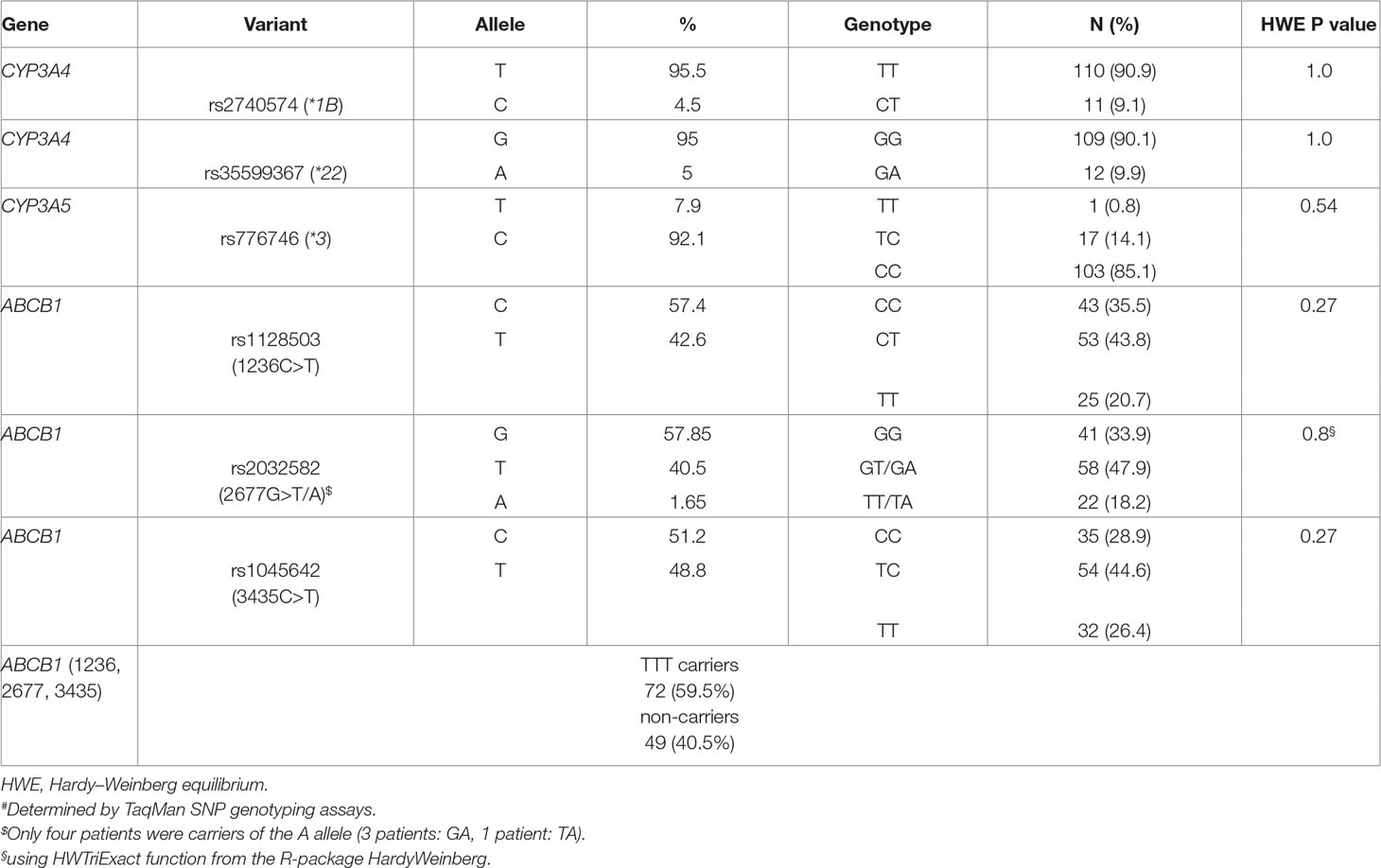
Genomic DNA was isolated from 200-µl ethylenediaminetetraacetic acid whole blood using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. For genotyping, the following variants were selected: CYP3A4*1B (rs2740574), CYP3A4*22 (rs35599367), and CYP3A5*3 (rs776746) and the three most relevant ABCB1 variants 1236C>T (rs1128503), 2677G>T,A (rs2032582), and 3435C>T (rs1045642). Genotyping was performed using TaqMan assays on 7900HT Fast Real-Time PCR System and the allelic discrimination method according to the manufacturer’s instructions (Applied Biosystems, Darmstadt, Germany). CYP3A4*1: assay C___1837671_50; CYP3A4*22: assay C__59013445_10; CYP3A5*3: assay C__26201809_30; ABCB11236C>T, assay C___7586662_10; triallelic ABCB12677: assays C_11711720D_40 (for T allele) and C_11711720C_30 (for A allele); ABCB13435C>T: assay C___7586657_20).
As described previously (Elens et al., 2011b), the combined CYP3A4/5 genotypes were defined as follows: poor metabolizers (PM, patients carrying the CYP3A4*22 allele in combination with homozygosity for CYP3A5*3), intermediate metabolizer (IM, CYP3A5*3/*3 plus CYP3A4*1/*1 or CYP3A4*22 carriers plus one CYP3A5*1 allele), and extensive metabolizer (EM, CYP3A4*1/*1 with at least one CYP3A5*1 allele).
Tacrolimus Blood Levels
Trough concentrations C0 (nanogram per milliliter) available from the first 2 weeks after transplantation or until the patient was discharged were retrospectively gathered from patient files. Tacrolimus concentrations are measured in whole blood by a validated liquid chromatography with tandem mass spectrometry as a routine laboratory procedure (Valbuena et al., 2016). Dose-adjusted C0 values were calculated by dividing the C0 by the total daily dose (nanogram per milliliter per milligram per day). Area under the time–concentration curve (AUC) was used as an estimate of the exposure to tacrolimus over time, since C0 was not available at every single postoperative day for every patient. Separate calculations were made for the first week (AUC1–7days), the second week (AUC8–14days), and for the whole follow-up period (AUC1–16days). AUC is reported as tacrolimus concentration divided by daily dose times days, i.e. (ng/mL)/ (mg/day) × days.
Clinical Outcome Parameters
Clinical outcome measures for this study were AR, DGF, and renal function at discharge from the hospital. AR was defined as an acute deterioration in allograft function associated with specific pathologic changes in graft biopsies occurring during the first year after transplantation. DGF was defined as the need for dialysis within the entire 16 days post-transplantation for this study. Estimated glomerular filtration rate based on serum creatinine (eGFR) was used as measure for renal function at discharge from the hospital.
Statistical Analysis
Statistical analyses were performed with R-3.5.0 (R Core Team, 2018), including additional packages coin_1.2-2, quantreg_5.36, and SNPassoc_1.9-2 (Koenker, 2004; Hothorn et al., 2006; González et al., 2007; Champely et al., 2018). Observed and expected allele and genotype frequencies within populations were compared using Hardy–Weinberg equilibrium calculations. Here, for the tri-allelic ABCB12677G>T/A variant, the HWTriExact function from the R-package HardyWeinberg_v.1.6.1 was used (Graffelman and Camarena, 2008; Graffelman, 2015). Linkage disequilibrium computation was performed using Haploview (Barrett et al., 2005).
AUC values were estimated from the available individual C0 levels using the trapezoid rule. The differences in quantitative variables (AUC, tacrolimus blood levels, and weight-adjusted daily tacrolimus doses) among individuals with different genotypes were investigated using Kruskal–Wallis tests, Wilcoxon–Mann–Whitney tests, or median regression as appropriate. The variants were considered in four different genetic models: codominant, dominant, recessive, and log additive. For the tri-allelic ABCB1 2677G>T,A variant, T and A allele were combined before applying these models.
These models were also applied for the multivariate logistic/linear regression analyses of genetic variants and their association with AR, DGF, or square root transformed eGFR. Here, human leukocyte antigen (HLA) mismatch (≥3 vs <3), panel reactive antibodies (>10% vs ≤10%), AB0 compatibility, (yes vs no), donor source (living vs. deceased), type of agent used for induction (basiliximab vs. thymoglobulin), previous transplantation (yes vs. no), and valgancyclovir therapy (yes vs. no) were considered as covariates. The association of AR, DGF, and eGFR with confounders was tested with Fisher tests; for the continuous variable eGFR, Wilcoxon rank sum tests were employed.
Post hoc power calculation was performed based on a two-sample t-test, the respective effect and samples sizes in our cohort, and a two-sided significance level of 5%. Weight-adjusted doses at day 10 were log-transformed in order to estimate coefficients of determination based on univariate linear models.
All statistical tests were two-sided, and significance level was set to 5%. Where indicated, P-values were adjusted for multiple testing according to Holm (1979).
Results
Genotyping Results and Genotype Frequencies
All genotype frequencies were in Hardy–Weinberg equilibrium. The two CYP3A4 variants *1B and *22 were not linked (D’ = 0.19, r2 = 0.0). There were no homozygotes for the CYP3A4*22 allele, and 9.9% were heterozygous carriers. The minor allele frequency for CYP3A4*22 was 5%. Seventeen patients were heterozygous carriers for CYP3A5*1, and one patient carried two functional alleles (*1/*1). In total, 11 patients (8.7%) were classified as PM (CYP3A4*22 allele in combination with CYP3A5*3/*3), 93 patients (77.0%) as IM (CYP3A5*3/*3 plus CYP3A4*1/*1 or CYP3A4*22 carriers plus one CYP3A5*1 allele), and 17 patients (14.3%) as EM (CYP3A4*1/*1 carriers with at least one CYP3A5*1 allele). For the ABCB1 variants, linkage was observed between 1236C>T and 2677G>T (D’ = 0.88, r2 = 0.72), 1236C>T and 3435C>T (D’ = 0.75, r2 = 0.45), as well as 2677G>T and 3435C>T (D’ = 0.95, r2 = 0.64). LD plots with D’ and r2 are shown in Supplementary Figure 1. Of our patients, 59.5% carried the T-T-T haplotype. Frequencies for all tested variants are given in Table 2.
Effect of Genotypes on Tacrolimus Dose Requirements
As illustrated in Figure 1, already at day 1 after onset of therapy, a significantly higher dose requirement in CYP3A5 expressers (with at least one functional CYP3A5 allele, n = 18) was observed compared with non-expressers (CYP3A5*3/*3; P = 0.046). This association remains significant for the total observation period of 16 days. These data are also supported by a significantly higher dose requirement (P = 0.004) for EM (n = 17) patients compared with both IM (n = 93) and PM (n = 11) subjects for the entire observation period. A trend of significance toward a decreased dose requirement in heterozygous CYP3A4*22 patients was evident after the first week of therapy, being statistically significant at day 10 (P = 0.03). A post hoc power calculation for CYP3A4*22 showed a power of 63.5%. Here, we used the data at day 10 after transplantation; a dose/weight showed a plateau after this time point. Moreover, based on univariate linear models with log-transformed weight-adjusted doses, estimated coefficients of determination were 2.7% for CYP3A4*1B, 4.8% for CYP3A4*22, 18.7% for CYP3A5*3, and 21.2% for the CYP3A4/5 combined genotypes. In addition, carriers of CYP3A4*1B showed a significant higher tacrolimus dose requirement after day 12 of treatment (p = 0.026), keeping in mind that CYP3A4*1B and CYP3A5*3 are in strong linkage disequilibrium (D’ = 0.90, r2 = 0.45). None of the selected ABCB1 variants showed significant effects on the tacrolimus dose requirement.
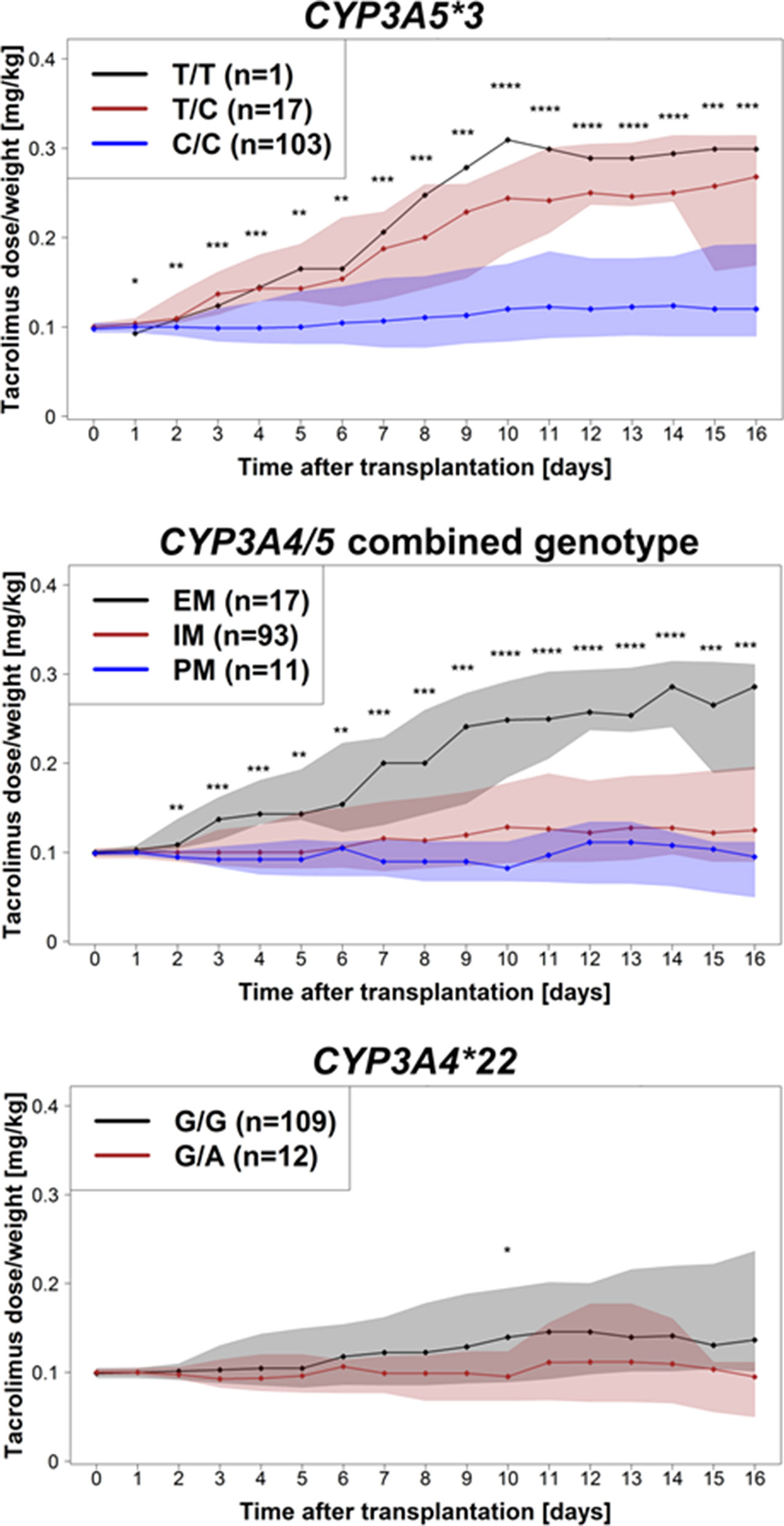
Figure 1 Tacrolimus dose requirement (mg/kg body weight/day) for the entire observation period of 16 days after transplantation for CYP3A5*3, CYP3A4/CYP3A5 combined genotypes, and CYP3A4*22. Dots represent medians of tacrolimus dose/body weight at the different days; shaded areas are defined by 25 and 75% quantiles. Significance levels are shown as asterisk: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Exposure to Tacrolimus
An overview of the distribution of median tacrolimus levels for the entire observation period is shown in Figure 2A. Individuals carrying two nonfunctional alleles of CYP3A5*3/*3 had significantly increased AUCs calculated from the C0 measurements compared with CYP3A5 expressers even after consideration of multiple testing (log additive model: first week P = 9.60 × 10-4, second week P = 8.54 × 10-4, days 1–16 P = 3.34 × 10-4). In the same timeframe, significant increased AUCs were found in PM and IM compared with EM (log additive model: first week P = 0.002, second week P = 3.86 × 10-5, days 1–16 P = 1.54 × 10-4). Heterozygous carriers of the CYP3A4*22 variant showed significantly higher median AUCs from days 8–14 (log additive model: adjusted P = 0.016), while for the entire study period of 16 days, only a trend of significance was found (adjusted P = 0.176). Results for the tested CYP3A4 and CYP3A5 variants are shown in Table 3. For CYP3A4*1B and variants of ABCB1 (individual variants as well as the T-T-T haplotype), no significant effects on the AUC of dose-adjusted tacrolimus concentrations were revealed. Table 2 of the Supplementary Material summarizes the results for all tested variants and genetic models.
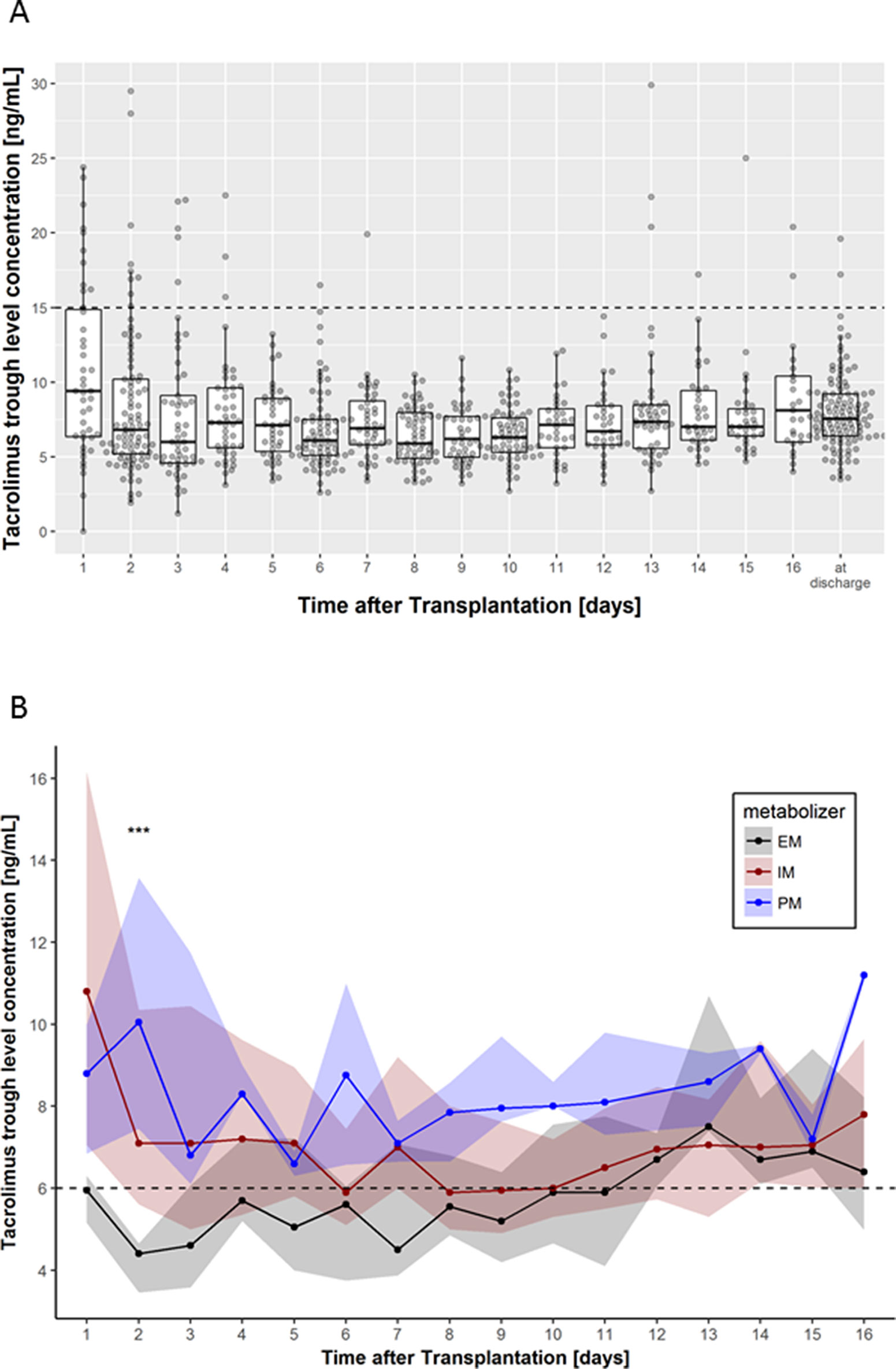
Figure 2 (A) Box-scatter plots per day of tacrolimus trough levels measured using liquid chromatography with tandem mass spectrometry. Thick lines represent median levels, boxes represent the interquartile range from 25 and 75% percentiles, and whiskers extend from the 25 or 75% percentile to the highest (or lowest, respectively) value not further than 1.5 times the interquartile range. The supra-therapeutic level of 15 ng/ml is shown using a horizontal dashed line. (B) Median tacrolimus trough levels according to CYP3A4/5 combined genotype (EM, extensive metabolizer; IM, intermediate metabolizer, PM, poor metabolizer). Dots represent the medians per day, while shaded areas are defined by 25 and 75% quantiles. Levels below 6 ng/ml are considered to be subtherapeutic. Significance levels are shown as asterisk: ***P < 0.001.
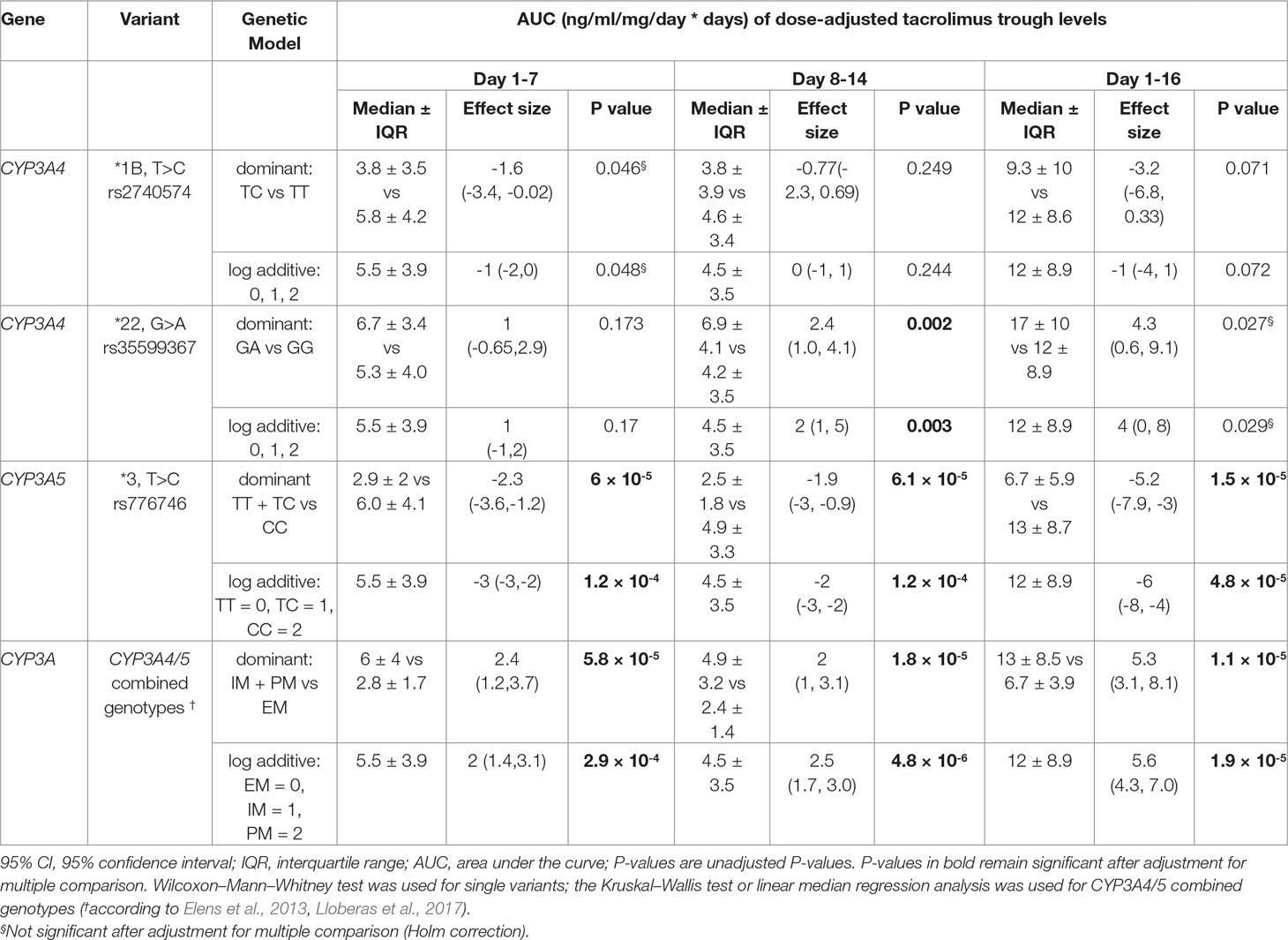
Table 3 Univariate analysis of AUC of dose-adjusted tacrolimus trough levels for CYP3A4 and CYP3A5 variants.
Supra-Therapeutic and Subtherapeutic Tacrolimus Levels
Thirty six of 754 measurements showed supra-therapeutic tacrolimus levels (defined as >15 ng/ml). Frequencies of supra-therapeutic measurements were not different (P = 0.36) between EM (3/111), IM (28/575), or PM (5/68). Of the 36 supra-therapeutic levels, 27 fell into the first 4 days of treatment, and 10 of them occurred on the first day (Figure 2A). In addition, five patients (1 EM and 3 IM) showed supra-therapeutic levels only after day 13.
Subtherapeutic tacrolimus levels (defined as <6 ng/ml) during the entire observation time were more common in EM patients (54%; P < 4.7 × 10-9) compared with IM (37%) or PM (9%). Moreover, the target C0 tacrolimus level of >6 ng/ml was achieved in EM patients not before day 12 of onset of therapy (Figure 2B).
Impact of CYP3A4/5 and ABCB1 Variants on Clinical Outcome
AR in the first year after transplantation and DGF within the observation period of 16 days were observed in 18 patients (14.9%) and 30 patients (25%), respectively. eGFR at discharge from the hospital was >60 ml/min in 27 patients. Most patients achieved an eGFR between >30 and ≤59 ml/min (n = 64 patients). Seventeen patients retained a severely decreased renal function (>15 and ≤30 ml/min), while 12 patients had an eGFR below <15 ml/min at discharge.
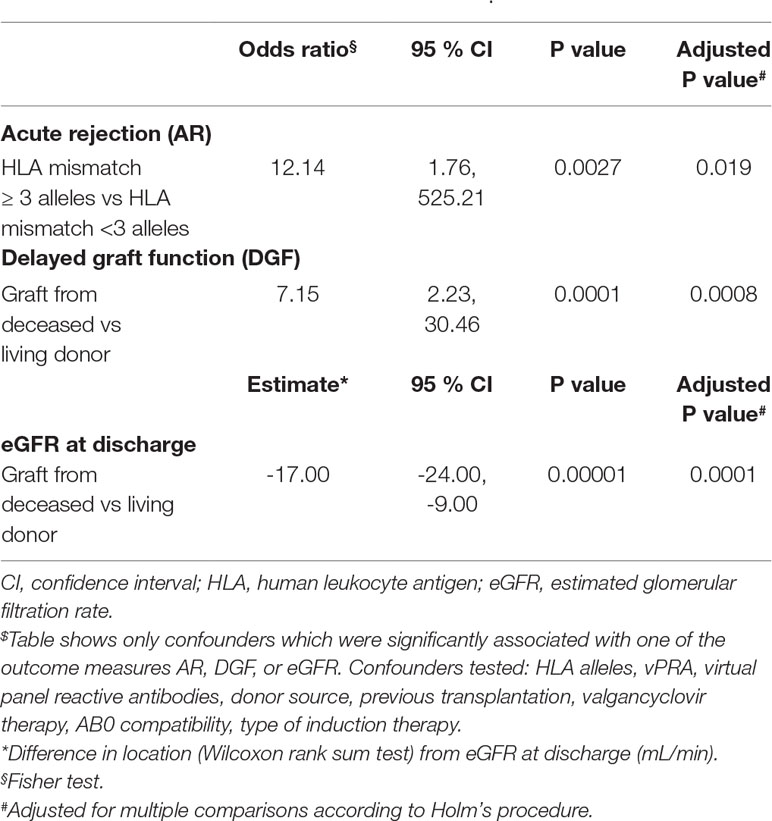
No statistical significant effects of all tested genetic variants and related phenotypes (EM, IM, and PM) on AR, DGF, or renal function were found after correction for multiple comparisons (Table 4). However, AR was significantly associated with ≥3 HLA allelic mismatches (OR = 12.14, 95% CI 1.76, 525.21, P = 0.019 even after multiple testing). Donor source (deceased vs. living) was associated with a higher frequency of DGF (OR 7.15, 95% CI 2.23, 30.46, adjusted P = 0.0008) and a lower eGFR at discharge (mean 37.41 ml/min, SD ± 18.58 ml/min versus mean 53.75 ml/min, SD ± 18.58 ml/min, P = 0.0001) (Table 5). With regard to concomitant medication, valgancyclovir treatment did not impact on AR, DGF, or eGFR.
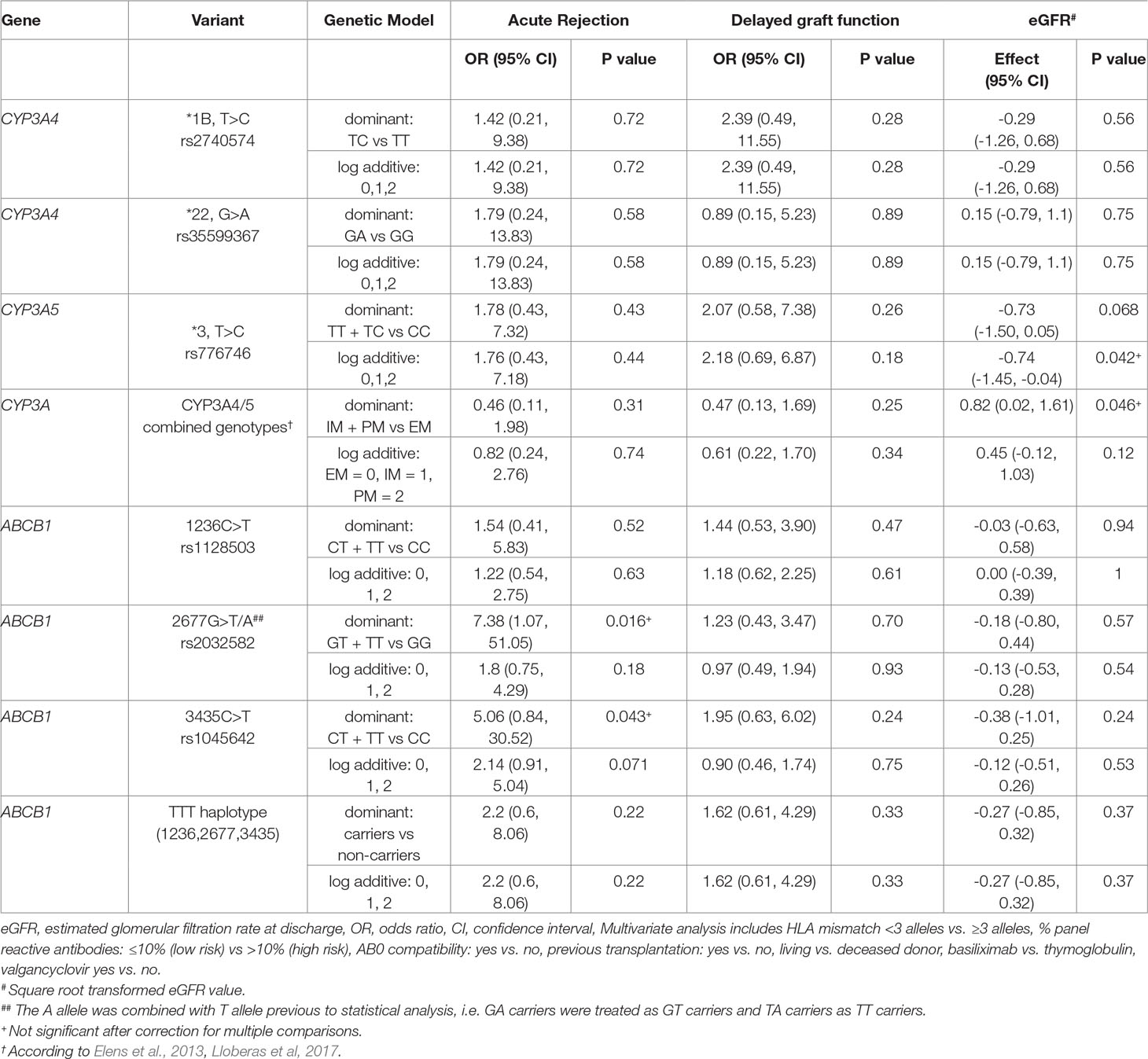
Table 4 Impact of selected CYP3A4/5 and ABCB1 variants on acute rejection, delayed graft function and estimated glomerular filtration rate at discharge (multivariate regression analysis).
Discussion
This study aimed to elucidate the impact of CYP3A*22 and CYP3A5 and selected functional relevant ABCB1 variants on pharmacokinetics and outcome parameters in clinical practice in a well-defined single center cohort of renal transplant patients treated with tacrolimus.
Tacrolimus Dose Requirements
An important aspect to this study was to investigate the effect of the variants on the tacrolimus dose. To minimize rejection and toxicity after transplantation, the target tacrolimus C0 level at our center is 6–8 ng/ml during the first 3 months, which is in agreement with international recommendations (Kasiske et al., 2009). Already at day 1, CYP3A5 expressers (n = 18) had significantly (P = 0.046) higher dose requirements than CYP3A5 non-expressers (CYP3A5*3/*3). For the CYP3A4/5 combined genotypes, EM (n = 17) showed also a significantly higher dose requirement than both IM (n = 93) or PM (n = 11) at onset of therapy. The IM and PM groups did not show a significant difference in the daily doses, in contrast to Gijsen et al. (2013). In line with other studies (Elens et al., 2011a; Elens et al., 2013; Gijsen et al., 2013; Pallet et al., 2015), tacrolimus dosing for the entire observation time was lower in patients who were heterozygous carriers of CYP3A4*22. However, statistically significant differences between CYP3A4*1/*22 and CYP3A4*1/*22 were only observed at day 10 keeping in mind that the number of variant carriers for CYP3A4*22 is low in our study cohort. The CYP3A4/5 combined genotypes explain a greater part of variability in dose requirement (21.2%) compared with the isolated contribution of CYP3A4*22 (4.8%) corroborating previous data about the relevance of CYP3A5 for tacrolimus dosing.
CYP3A4*22 and Tacrolimus Pharmacokinetics
The wide range of tacrolimus C0 levels observed in patients treated with the same dose of tacrolimus can be explained in part by the genetic variation in CYP3A5. Yet, although CYP3A4*22 contributes to a lower rate to tacrolimus metabolism, CYP3A4*22 related higher C0 tacrolimus levels have been reported in some cohorts (Elens et al., 2011a). For instance, the impact of CYP3A4*22 was described by Scheibner et al. (2018), who reported a median 342% increase of dose-adjusted tacrolimus C0 levels in transplant patients homozygous for CYP3A4*22 and CYP3A5*3 allele, compared with controls.
In our cohort, the minor allele frequency for the CYP3A4*22 allele was 5%, i.e., 12 of 121 patients carried the CYP3A4*22 allele heterozygously. This frequency is in line with the allele frequency of 4.4% in non-Finnish Europeans as reported by the Genome Aggregation Database (Lek et al., 2016) and other research groups (van Schaik et al., 2002; Elens et al., 2013). Classification into extensive (EM, 14%), intermediate (IM, 77%), and PM (9%) revealed a similar distribution as reported by Lloberas et al. (2017).
The exposure to tacrolimus (AUC of dose-adjusted levels) in our study was significantly higher (P = 0.016) in heterozygous CYP3A4*22 carriers compared with CYP3A4*1/*1 (days 8 to 14). This observation holds true for the entire observation period up to 16 days with a trend of significance after correction for multiple testing. Several studies reported a significantly reduced tacrolimus clearance (with a higher C0) in heterozygous patients for CYP3A4*1/*22 in the early period after renal transplantation (Elens et al., 2011a; Pallet et al., 2015; Lloberas et al., 2017). In contrast, other studies did not find clinically significant effects for this association (Tavira et al., 2011; Santoro et al., 2013; Moes et al., 2014). One explanation for this observation is that different time frames were selected to assess tacrolimus clearance, exemplarily demonstrated by the studies of Elens et al. (2011a) and Lloberas et al. (2017) with a time frame up to 1 year after transplantation.
CYP3A5 and CYP3A4/5 Combined Genotype and Tacrolimus Pharmacokinetics
Throughout the first 16 days post-transplantation, CYP3A5 non-expressers (CYP3A5*3/*3) showed significantly higher dose-adjusted tacrolimus AUC than expressers (CYP3A5*1/*3 or CYP3A5*1/*1). This resembles data from a meta-analysis by Rojas et al. (2015). Regarding the CYP3A4/5 combined genotypes, both the PM or IM cluster have significantly higher dose-adjusted tacrolimus AUCs in the first 16 days post-transplantation than EM. AUCs show a huge interindividual variability in IM and PM and do not differ significantly between these groups. Assessment of the CYP3A4/5 combined genotypes regarding AUC was not superior compared with the monogenic CYP3A5 analysis.
Supra- and Subtherapeutic Levels
Few studies report on an increased risk for supra-therapeutic tacrolimus concentrations (C0 > 15ng/ml) in heterozygous carriers of CYP3A4*22 (Pallet et al., 2015) as well as in homozygous-variant patients (Lloberas et al., 2017). In the former study, an initial dose of 0.2 mg/kg body weight has been used, which is twice the daily dose used in our protocol. The latter study aimed for a higher target C0 in the first week of transplantation. Most probably due to our lower dosing regimen (0.1 mg/kg body weight), supra-therapeutic tacrolimus levels were found only on 36 of 754 occasions, with a majority occurring in the first 4 days of tacrolimus therapy (Figure 2A). Supra-therapeutic levels in our cohort were not associated with the CYP3A4/5 combined genotype, which may also be explained by lower dosing of tacrolimus. Subtherapeutic levels (<6 ng/ml) may be a concern in CYP3A5 expressers or EM patients due to an enhanced metabolism toward inactive metabolites (Lloberas et al., 2017). In our cohort, measurements of <6 ng/ml were in fact most common in EM patients. In consequence, during the first 11 days of therapy, the median C0 was below the target value of 6 ng/ml in EM.
Clinical Outcome
Generally, highest incidence of AR is reported within the first year after transplantation (The Scientific Registry of Transplant Recipients/Organ Procurement and Transplantation Network, 2012). Although genetic variation in drug metabolism has been confirmed to lead to different tacrolimus blood levels, a meta-analysis concluded that tacrolimus C0 does not predict the risk of AR after renal transplantation (Bouamar et al., 2013).
These data are supported by our study indicating that variation in CYP3A and/or ABCB1 did not impact on AR, DGF, or renal function (eGFR) on discharge, when controlling for confounders like HLA mismatch, % panel reactive antibodies, AB0 compatibility, valgancyclovir use, donor source, number of transplant, and type of induction therapy. CYP3A4*22 has been associated with DGF, but only with cyclosporine (Elens et al., 2012) and not for tacrolimus use. HLA mismatches are generally recognized as a major barrier to long-term engraftment and supported by our data. AR occurring within 1 year of transplantation was significantly associated with ≥3 HLA allelic mismatches. Moreover, DGF and a reduced eGFR at discharge were significantly more frequent in recipients with a deceased donor graft. These observations corroborate previous data (Perico et al., 2004; Ghods et al., 2007; Siedlecki et al., 2011) and underline the major impact of transplantation relevant factors like the HLA mismatches and the donor source of the transplant for clinical outcome variables.
Limitations
Our study has some limitations. It is a retrospective analysis study. The number of patients in our single-center study was relatively small (n = 121), which may explain why we did not observe significant effects of the tested genotypes on the clinical endpoints. We only performed genetic analysis using DNA from recipients but not from donors, which may explain missing genetic associations observed by others, e.g., ABCB1 variants and DGF (Woillard et al., 2010; Hauser et al., 2012). Although all our transplant patients received the same standard therapeutic regimen and we explicitly considered concomitant valgancyclovir therapy as a confounding factor in our multivariate analysis, additional medications given to transplant recipients may impact tacrolimus pharmacokinetics with consequences on outcome variables.
Conclusion
Our results confirm previous data indicating that CYP3A variants explain interindividual variability of tacrolimus pharmacokinetics but do not impact on clinical outcome variables like AR, DLF, or eGFR. Notably, CYP3A4*22 isolated or in combination with CYP3A5 genotypes did not decisively improve the well-established value of CYP3A5 pharmacogenetic testing only to predict tacrolimus dosing. ABCB1 did neither significantly impact on tacrolimus pharmacokinetics nor on clinical outcome parameters.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
All subjects have given their written informed consent. The study was approved by the ethics committee of University Hospital, Tuebingen, Germany (616/2013BO2).
Author Contributions
EA-K was responsible for acquisition of all source data and contributed to the writing of the manuscript. SW performed or supervised statistical analyses and critically revised the manuscript. RT performed statistical analyses. ES was responsible for genotyping. CO recruited the patients and provided the clinical data. EW, MSc, and MSh were responsible for funding, conception of this study, interpretation of results, and critical revision of the manuscript. SJ performed statistical analysis and wrote the manuscript.
Funding
This study was supported in part by the Robert Bosch Stiftung Stuttgart, Germany and in part by the Horizon 2020-PHC-2015 grant U-PGx 668353.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the excellent technical assistance of Andrea Jarmuth in genotyping.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00871/full#supplementary-material
References
Barrett, J. C., Fry, B., Maller, J., Daly, M. J. (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics (Oxford, England) 21, 263–265. doi: 10.1093/bioinformatics/bth457
Birdwell, K. A., Decker, B., Barbarino, J. M., Peterson, J. F., Stein, C. M., Sadee, W., et al. (2015). Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 98, 19–24. doi: 10.1002/cpt.113
Bouamar, R., Shuker, N., Hesselink, D. A., Weimar, W., Ekberg, H., Kaplan, B., et al. (2013). Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials(†). Am. J. Transplant. 13, 1253–1261. doi: 10.1111/ajt.12191
Champely, S., Ekstrom, C., Dalgaard, P., Gill, J., Weibelzahl, S., Anandkumar, A., et al. (2018). Basic Functions for Power Analysis: Power analysis functions along the lines of Cohen.
Elens, L., Haufroid, V. (2017). Genotype-based tacrolimus dosing guidelines: with or without CYP3A4*22? Pharmacogenomics 18, 1473–1480. doi: 10.2217/pgs-2017-0131
Elens, L., Bouamar, R., Hesselink, D. A., Haufroid, V., van der Heiden, I. P., van Gelder, T., et al. (2011a). A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin. Chem. 57, 1574–1583. doi: 10.1373/clinchem.2011.165613
Elens, L., Bouamar, R., Hesselink, D. A., Haufroid, V., van Gelder, T., van Schaik, R. H. N. (2012). The new CYP3A4 intron 6 CT polymorphism (CYP3A4*22) is associated with an increased risk of delayed graft function and worse renal function in cyclosporine-treated kidney transplant patients. Pharmacogenet. Genomics 22, 373–380. doi: 10.1097/FPC.0b013e328351f3c1
Elens, L., van Gelder, T., Hesselink, D. A., Haufroid, V., van Schaik, R. H. N. (2013). CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics 14, 47–62. doi: 10.2217/pgs.12.187
Elens, L., van Schaik, R. H., Panin, N., Meyer, M., Wallemacq, P., Lison, D., et al. (2011b). Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics 12, 1383–1396. doi: 10.2217/pgs.11.90
Ghods, A. J., Savaj, S., Abbasi, M., Heidari, H., Rokhsatyazdi, H. (2007). The incidence and risk factors of delayed graft function in 689 consecutive living unrelated donor renal transplantation. Transplant. Proc. 39, 846–847. doi: 10.1016/j.transproceed.2007.04.011
Gijsen, V. M. G. J., van Schaik, R. H., Elens, L., Soldin, O. P., Soldin, S. J., Koren, G., et al. (2013). CYP3A4*22 and CYP3A combined genotypes both correlate with tacrolimus disposition in pediatric heart transplant recipients. Pharmacogenomics 14, 1027–1036. doi: 10.2217/pgs.13.80
González, J. R., Armengol, L., Solé, X., Guinó, E., Mercader, J. M., Estivill, X., et al. (2007). SNPassoc: an R package to perform whole genome association studies. Bioinformatics (Oxford, England) 23, 644–645. doi: 10.1093/bioinformatics/btm025
Graffelman, J. (2015). Exploring Diallelic Genetic Markers: the HardyWeinberg Package. J. Stat. Soft. 64, 1–23. doi: 10.18637/jss.v064.i03
Graffelman, J., Camarena, J. M. (2008). Graphical tests for Hardy–Weinberg equilibrium based on the ternary plot. Hum. Hered. 65, 77–84. doi: 10.1159/000108939
Hauser, I. A., Kruck, S., Gauer, S., Nies, A. T., Winter, S., Bedke, J., et al. (2012). Human pregnane X receptor genotype of the donor but not of the recipient is a risk factor for delayed graft function after renal transplantation. Clin. Pharmacol. Ther. 91, 905–916. doi: 10.1038/clpt.2011.346
Hothorn, T., Hornik, K., de Wiel, van, Zeileis, A. (2006). A Lego System for Conditional Inference. Am. Stat. 60, 257–263. doi: 10.1198/000313006X118430
Kasiske, B. L., Zeier, M. G., Chapman, J. R., Craig, J. C., Ekberg, H., Garvey, C. A., et al. (2009). KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 9, 10–13.
Kasiske, B. L., Zeier, M. G., Chapman, J. R., Craig, J. C., Ekberg, H., Garvey, C. A., et al. (2010). KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 77, 299–311. doi: 10.1038/ki.2009.377
Koenker, R. (2004). Quantreg: An R package for quantile regression and related methods. doi: 10.1017/CBO9780511754098
Lek, M., Karczewski, K. J., Minikel, E. V., Samocha, K. E., Banks, E., Fennell, T., et al. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291. doi: 10.1038/nature19057
Lloberas, N., Elens, L., Llaudó, I., Padullés, A., van Gelder, T., Hesselink, D. A., et al. (2017). The combination of CYP3A4*22 and CYP3A5*3 single-nucleotide polymorphisms determines tacrolimus dose requirement after kidney transplantation. Pharmacogenet. Genomics 27, 313–322. doi: 10.1097/FPC.0000000000000296
Matas, A. J., Gillingham, K. J., Payne, W. D., Najarian, J. S. (1994). The impact of an acute rejection episode on long-term renal allograft survival (t1/2). Transplantation 57, 857–859. doi: 10.1097/00007890-199403270-00015
Moes, D. J. A. R., Swen, J. J., Hartigh, J., van der Straaten, T., van der Heide, J. J. H., Sanders, J. S., et al. (2014). Effect of CYP3A4*22, CYP3A5*3, and CYP3A Combined Genotypes on Cyclosporine, Everolimus, and Tacrolimus Pharmacokinetics in Renal Transplantation. CPT Pharmacometrics Syst. Pharmacol. 3, e100. doi: 10.1038/psp.2013.78
Oetting, W. S., Wu, B., Schladt, D. P., Guan, W., Remmel, R. P., Dorr, C., et al. (2018a). Attempted validation of 44 reported SNPs associated with tacrolimus troughs in a cohort of kidney allograft recipients. Pharmacogenomics 19, 175–184. doi: 10.2217/pgs-2017-0187
Oetting, W. S., Wu, B., Schladt, D. P., Guan, W., Remmel, R. P., Mannon, R. B., et al. (2018b). Genome-wide association study identifies the common variants in CYP3A4 and CYP3A5 responsible for variation in tacrolimus trough concentration in Caucasian kidney transplant recipients. Pharmacogenomics J. 18, 501–505. doi: 10.1038/tpj.2017.49
Pallet, N., Jannot, A.-S., El Bahri, M., Etienne, I., Buchler, M., Ligny, B. H., et al. (2015). Kidney transplant recipients carrying the CYP3A4*22 allelic variant have reduced tacrolimus clearance and often reach supratherapeutic tacrolimus concentrations. Am. J. Transplant. 15, 800–805. doi: 10.1111/ajt.13059
Passey, C., Birnbaum, A. K., Brundage, R. C., Schladt, D. P., Oetting, W. S., Leduc, R. E., et al. (2012). Validation of tacrolimus equation to predict troughs using genetic and clinical factors. Pharmacogenomics 13, 1141–1147. doi: 10.2217/pgs.12.98
Perico, N., Cattaneo, D., Sayegh, M. H., Remuzzi, G. (2004). Delayed graft function in kidney transplantation. Lancet 364, 1814–1827. doi: 10.1016/S0140-6736(04)17406-0
R Core Team. (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rojas, L., Neumann, I., Herrero, M. J., Bosó, V., Reig, J., Poveda, J. L., et al. (2015). Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 15, 38–48. doi: 10.1038/tpj.2014.38
Santoro, A. B., Struchiner, C. J., Felipe, C. R., Tedesco-Silva, H., Medina-Pestana, J. O., Suarez-Kurtz, G. (2013). CYP3A5 genotype, but not CYP3A4*1b, CYP3A4*22, or hematocrit, predicts tacrolimus dose requirements in Brazilian renal transplant patients. Clin. Pharmacol. Ther. 94, 201–202. doi: 10.1038/clpt.2013.68
Scheibner, A., Remmel, R., Schladt, D., Oetting, W. S., Guan, W., Wu, B., et al. (2018). Tacrolimus Elimination in Four Patients With a CYP3A5*3/*3 CYP3A4*22/*22 Genotype Combination. Pharmacotherapy 38(7), e46–e52. doi: 10.1002/phar.2131
Shuker, N., Bouamar, R., van Schaik, R. H. N., Clahsen-van Groningen, M. C., Damman, J., Baan, C. C., et al. (2016). A randomized controlled trial comparing the efficacy of Cyp3a5 genotype-based with body-weight-based tacrolimus dosing after living donor kidney transplantation. Am. J. Transplant. 16, 2085–2096. doi: 10.1111/ajt.13691
Siedlecki, A., Irish, W., Brennan, D. C. (2011). Delayed graft function in the kidney transplant. Am. J. Transplant. 11, 2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x
Tavira, B., Coto, E., Garciá, E. C., Díaz-Corte, C., Ortega, F., Arias, M., et al. (2011). Pharmacogenetics of tacrolimus after renal transplantation: analysis of polymorphisms in genes encoding 16 drug metabolizing enzymes. Clin. Chem. Lab. Med. 49, 825–833. doi: 10.1515/CCLM.2011.143
The Scientific Registry of Transplant Recipients/Organ Procurement and Transplantation Network. (2012). SRTR & OPTN Annual Data Report: Chapter Kidney. Accessed March 25, 2019, https://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/01_kidney_13.pdf.
Thervet, E., Loriot, M. A., Barbier, S., Buchler, M., Ficheux, M., Choukroun, G., et al. (2010). Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin. Pharmacol. Ther. 87, 721–726. doi: 10.1038/clpt.2010.17
Tron, C., Lemaitre, F., Verstuyft, C., Petitcollin, A., Verdier, M.-C., Bellissant, E. (2018). Pharmacogenetics of Membrane Transporters of Tacrolimus in Solid Organ Transplantation. Clin. Pharmacokinet. 58(5), 593–613. doi: 10.1007/s40262-018-0717-7
Valbuena, H., Shipkova, M., Kliesch, S.-M., Müller, S., Wieland, E. (2016). Comparing the effect of isotopically labeled or structural analog internal standards on the performance of a LC-MS/MS method to determine ciclosporin A, everolimus, sirolimus and tacrolimus in whole blood. Clin. Chem. Lab. Med. 54, 437–446. doi: 10.1515/cclm-2015-0519
van Schaik, R. H. N., van der Heiden, I. P., van den Anker, J. N., Lindemans, J. (2002). CYP3A5 variant allele frequencies in Dutch Caucasians. Clin. Chem. 48, 1668–1671.
Wang, D., Guo, Y., Wrighton, S. A., Cooke, G. E., Sadee, W. (2011). Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J. 11, 274–286. doi: 10.1038/tpj.2010.28
Woillard, J.-B., Rerolle, J.-P., Picard, N., Rousseau, A., Guillaudeau, A., Munteanu, E., et al. (2010). Donor P-gp polymorphisms strongly influence renal function and graft loss in a cohort of renal transplant recipients on cyclosporine therapy in a long-term follow-up. Clin. Pharmacol. Ther. 88, 95–100. doi: 10.1038/clpt.2010.62
Keywords: tacrolimus, renal transplantation, pharmacogenetics, CYP3A5, CYP3A4*22, ABCB1, therapeutic drug monitoring
Citation: Abdel-Kahaar E, Winter S, Tremmel R, Schaeffeler E, Olbricht CJ, Wieland E, Schwab M, Shipkova M and Jaeger SU (2019) The Impact of CYP3A4*22 on Tacrolimus Pharmacokinetics and Outcome in Clinical Practice at a Single Kidney Transplant Center. Front. Genet. 10:871. doi: 10.3389/fgene.2019.00871
Received: 02 April 2019; Accepted: 20 August 2019;
Published: 26 September 2019.
Edited by:
José A. G. Agúndez, University of Extremadura, SpainReviewed by:
Julio Benitez, University of Extremadura, SpainVangelis G. Manolopoulos, Democritus University of Thrace, Greece
Copyright © 2019 Abdel-Kahaar, Winter, Tremmel, Schaeffeler, Olbricht, Wieland, Schwab, Shipkova and Jaeger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Schwab, bWF0dGhpYXMuc2Nod2FiQGlrcC1zdHV0dGdhcnQuZGU=
†These authors share first authorship
 Emaad Abdel-Kahaar1,2†
Emaad Abdel-Kahaar1,2† Stefan Winter
Stefan Winter Roman Tremmel
Roman Tremmel Elke Schaeffeler
Elke Schaeffeler Matthias Schwab
Matthias Schwab Maria Shipkova
Maria Shipkova Simon U. Jaeger
Simon U. Jaeger