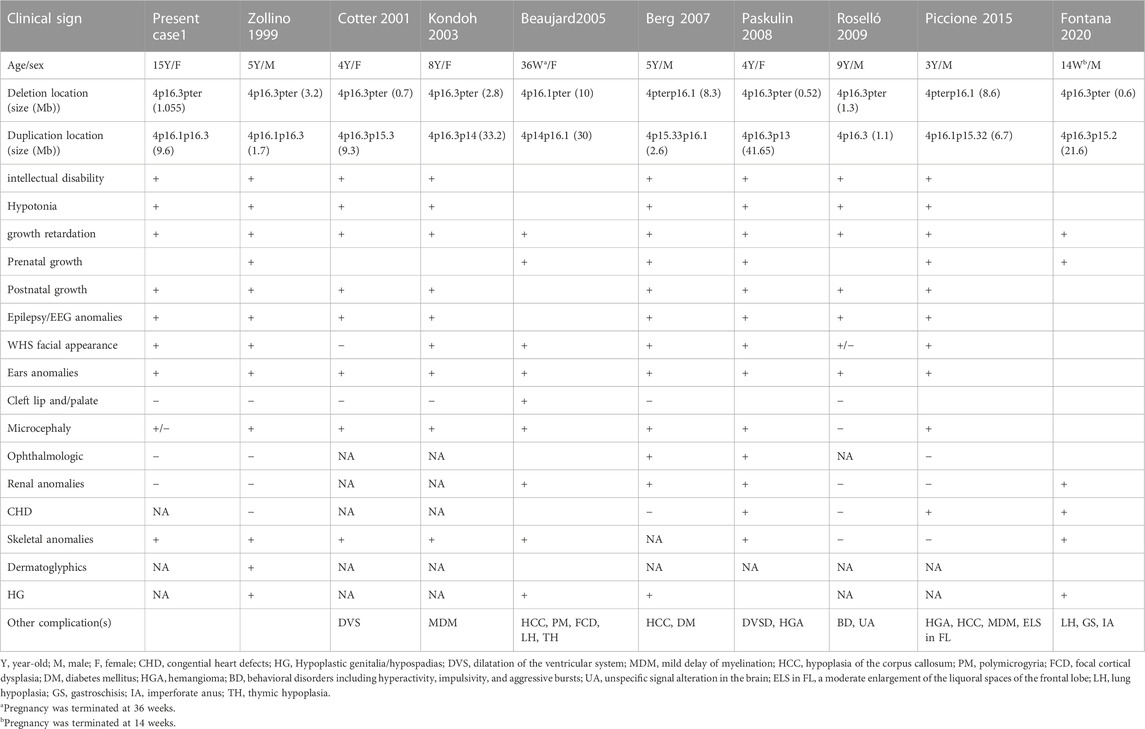
- 1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Department of Pediatrics, Peking University First Hospital, Beijing, China
Structural rearrangements of chromosome 4p gives rise to a group of rare genomic disorders that mainly result in two different clinical entities: Wolf-Hirschhorn syndrome (WHS) and partial 4p trisomy. The severity of the phenotype depends on the size of the deletion or locus duplication. Here, we present two unrelated individuals with a copy number variation of chromosome 4p. Inverted duplication deletions (inv dup-del) in 4p are particularly rare. Case 1 describes a 15-year-old girl with a 1.055 Mb deletion of terminal 4p, distal to the recognized critical region of WHS, and a large duplication of 9.6 Mb in size from 4p16.3 to p16.1. She had postnatal development delay, intellectual disability (especially pronounced in speech), seizure/electroencephalogram anomalies, and facial dysmorphic features. This unusual chromosomal imbalance resulted in the WHS phenotype rather than the 4p trisomy syndrome phenotype. Case 2 describes a 21-month-old boy with a 1.386 Mb terminal 4p deletion who presented with slight developmental delay, borderline intellectual disability, and seizures. Combined with previous reported cases of 4 pter del-dup or pure 4p terminal deletions, our observations suggest that terminal chromosome 4p deletion is more pathogenic than the concomitant partial 4p duplication, and some regions of the 4p terminal may have regulatory effects on the remaining region of 4p. About nine cases have been reported thus far to date, and our study delineates further genotype-phenotype correlations about terminal 4p duplication-deletions for predicting disease prognosis and patient counseling.
1 Introduction
The short arm of chromosome 4 (4p) is approximately 50.86 Mb in size and contains approximately 379 genes, approximately 45% of which are located 10 Mb distal to 4p (Hannes et al., 2008). Rearrangements of chromosome 4p mainly cause two different clinical entities: partial 4p trisomy and Wolf-Hirschhorn syndrome (WHS). Wilson et al. (1970) first described 4p trisomy, which is associated with large duplications containing 2/3 of chromosome 4p. 4p trisomy is characterized by a constellation of facial dysmorphic features, prenatal and postnatal growth retardation, intellectual disability, cardiac anomalies, and renal anomalies. The dysmorphic features include a prominent glabella, bulbous nose with a flat nasal bridge, retrognathia and malformed ears with abnormal helix and antihelix.
In the 1960s, Wolf et al. (1965) and Hirschhorn et al. (1965) published the first patients with WHS. WHS is a multiple congenital anomaly syndrome, with clinical manifestations including pre- and postnatal growth retardation, intellectual disability, seizures (or EEG anomalies), major malformations and a characteristic facial anomaly of a broad nasal bridge, microcephaly, high forehead with prominent glabella, ocular hypertelorism, epicanthus, highly arched eyebrows, short philtrum, downturned corners of the mouth, micrognathia, and hypoplastic ears with pits or tags. Prominent forehead, hypertelorism, and the wide bridge of the nose continuing to the forehead lead to the appearance of a typical ‘Greek warrior helmet’. Major malformations include congenital heart defects, ocular colobomas, renal abnormalities, skeletal abnormalities and midline defects such as cleft palate and hypospadias (Nevado et al., 2020). WHS is also classified as a contiguous gene syndrome and is usually caused by partial deletion of terminal 4p. Wieczorek et al. (2008) and Zollino et al. (2008) found that the severity of WHS clinical characteristics, such as craniofacial anomalies and intellectual disability, were positively correlated with the length of the 4p terminal deletion. Thus, different lengths of 4p deletions may contribute to the heterogeneity in clinical presentations of WHS patients.
Two critical regions have been identified as candidate regions underlying WHS pathogenesis. The first is WHSCR, which is limited to a 165 kb interval around 1.9–2.1 Mb from the telomere. The second is WHSCR-2, which lies within a 300–600 kb interval 1.6–1.3 Mb from the telomere and is contiguous distally to WHSCR. Most patients carry a terminal deletion, and haploinsufficiency of these genes located in the critical region contributes to the phenotypic features of WHS. Simple 4p deletions, whether they are terminal or interstitial deletions, account for about 70% of all WHS cases. Approximately 22% of cases have an unbalanced translocation, and the remaining cases have complex rearrangements, such as inverted duplications (Battaglia et al., 2015). Cases of 4p inverted duplication deletions (inv dup-del) are rare and heterogeneous. Thus far, only nine patients have been reported. The proposed mechanism of Inv del-dup includes terminal deletion with two chromatid breaks, followed by re-connection of the broken ends on the chromatids after DNA replication, like a U type, to form a dicentric intermediate (Figure 1) (Gordon and Halliday, 1995; L R Rowe et al., 2014).
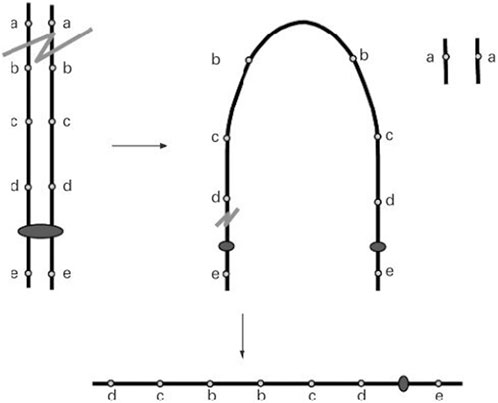
FIGURE 1. A double-strand break of one chromosome is repaired by fusion of the two sister chromatids (U-type exchange) which results in a dicentric chromosome. Pre-zygotic breakage of the dicentric outside the fusion region leads to a monocentric chromosome with a terminal deletion of the ‘‘a’’ locus and an inverted duplication of the ‘‘b’’, ‘‘c’’, and ‘‘d’’ loci with no single copy region between the duplication (L R Rowe et al., 2014).
Here, we report two cases of rearrangements of chromosome 4p. Cases with inverted duplication of distal 4p accompanied by a subtelomeric deletion are rare, and our case provides further information relevant to the clinical phenotype and genotype of the deletion and duplication of 4p.
2 Case description
2.1 Case 1
The patient was a 15-year-old girl who was the second child of apparently healthy, non-consanguineous parents, with no similar cases in the family. Her mother was pregnant and gave birth at 36 years of age. The pregnancy was uneventful, and there was no teratogenic exposure. Height, weight, and head circumference measurements were all around the 50th percentile since birth. She had no hypotonia. The facial characteristics (Figure 2) were unremarkable, and were limited to large and protruding eyes, high nasal bridge, short philtrum, downturned corners of the mouth, and low-set and large ears. The patient’s developmental milestones were delayed. Gross motor development was slightly delayed, and language was also delayed and limited to single words or short sentences. She began to have febrile seizures at the age of 8 years, myoclonic seizures which lasted for 9 years, and was seizure-free after valproic acid administration. EEG examination showed normal background activity and spike-wave or poly-spike-wave discharges in the central and posterior cortical regions during waking and sleep. She had learning difficulties and her intellectual performance appeared to be within the lower limits. Although she had attended a normal school, her academic difficulties resulted in her academic performance always being lower than that of her colleagues. She was diagnosed with scoliosis, bilateral clinodactylous of the index and fifth fingers, and flat feet with bilateral clinodactyly of toes 2 and 5. The teeth in this patient were sparse. The patient had normal hearing and vision. Magnetic resonance imaging revealed a left temporal arachnoid cyst with abnormal subcortical signals in the left frontal lobe and right parietal lobe. The patients’ cardiac and renal examinations were normal.
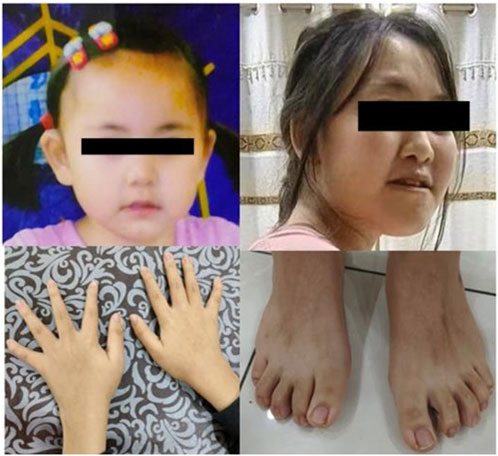
FIGURE 2. The picture of the patient (case 1). The upper picture is when the patient is at 2 years old and 15 years old, respectively. Below are photos of the patient’s hands and feet.
2.2 Case 2
A 21-month-old boy was born at full term via cesarean section after an uneventful pregnancy. He is the second child of an apparently healthy, non-consanguineous parent, with no similar cases in the family. His birth weight, length, and head circumference were not recorded. Motor and language development were slightly delayed. The patient’s facial characteristics were unremarkable. He had complex febrile seizures at 10 months of age and daily seizures characterized by eyelid myoclonus and drop attacks lasting for 2–3 s, occurring more than 10 times a day. EEG examination showed generalized spike-wave discharges. Cardiac and renal examinations were normal.
2.3 Clinical examinations of the patients
Peripheral blood samples were obtained from the two patients and their parents. DNA was extracted from the patients peripheral blood samples, then, DNA was fragmented, and DNA libraries were constructed by end filling, adapter ligation and polymerase chain reaction (PCR) amplification. DNA libraries were subjected to massively parallel sequencing on the NovaSeq6000 (Illumina, San Diego, CA, United States) to generate raw sequencing reads. The TruSeg Library Construction Kit was used to build the database and the paired-end sequencing strategy was performed according to the manufacturer’s instructions. For each sample, raw reads were filtered to remove low quality reads by using FastQC, high-quality sequences were obtained (Q30 > 80%) from the raw data. Clean reads were mapped to the reference genome GRCh37/hg19 to obtain bioinformatic results and determine the existence of chromosomal variation and CNVs. The Database of Genomic Variants, DECIPHER database, ClinVar, OMIM, and ClinGen were used for interpretation and classification of the clinical significance of candidate CNVs according to previously reported guidelines.
2.4 Results
For case 1, CNV-seq results showed a 1.055 Mb deletion of terminal 4p and a contiguous large duplication of 9.6 Mb in size extending from 4p16.3 to p16.1. The patient in case 2 was found to carry a 1.386 Mb terminal deletion of 4p using CNV-seq. The results of genomic maps can be seen in Figure 3.
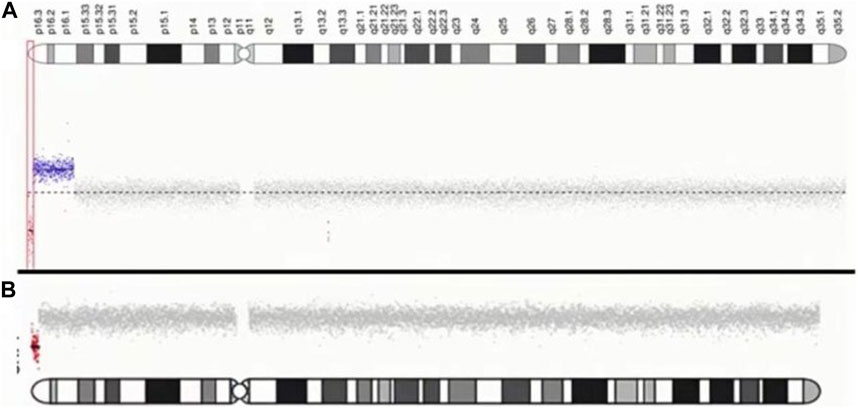
FIGURE 3. Characterization of chromosome 4p of two patients. (A) case1: The red dot indicates the location of the submicroscopic deletion. The blue dot indicates the location of the submicroscopic duplication. (B) case2: The red dot indicates the location of the submicroscopic deletion.
3 Discussion
We have reported two cases with a 1.055 Mb 4p terminal deletion, a contiguous large duplication of 9.6 Mb, and a pure 1.386 Mb 4p terminal deletion. Case 1 was a girl with a mild WHS phenotype, including mild facial anomalies, epilepsy, skeletal anomalies, mild postnatal developmental delay, and intellectual disability, especially in speech, while clinical manifestations of 4p trisomy syndrome were practically absent. This patient carried an inverted duplication for 4p16.3-p16.1, a deletion of 4p16.3-4-pter, a 1.055 Mb 4p terminal deletion, distal to the critical region of WHS, and a large duplication of 9.6 Mb in size from 4p16.3 to p16.1, comprising the WHS critical region.
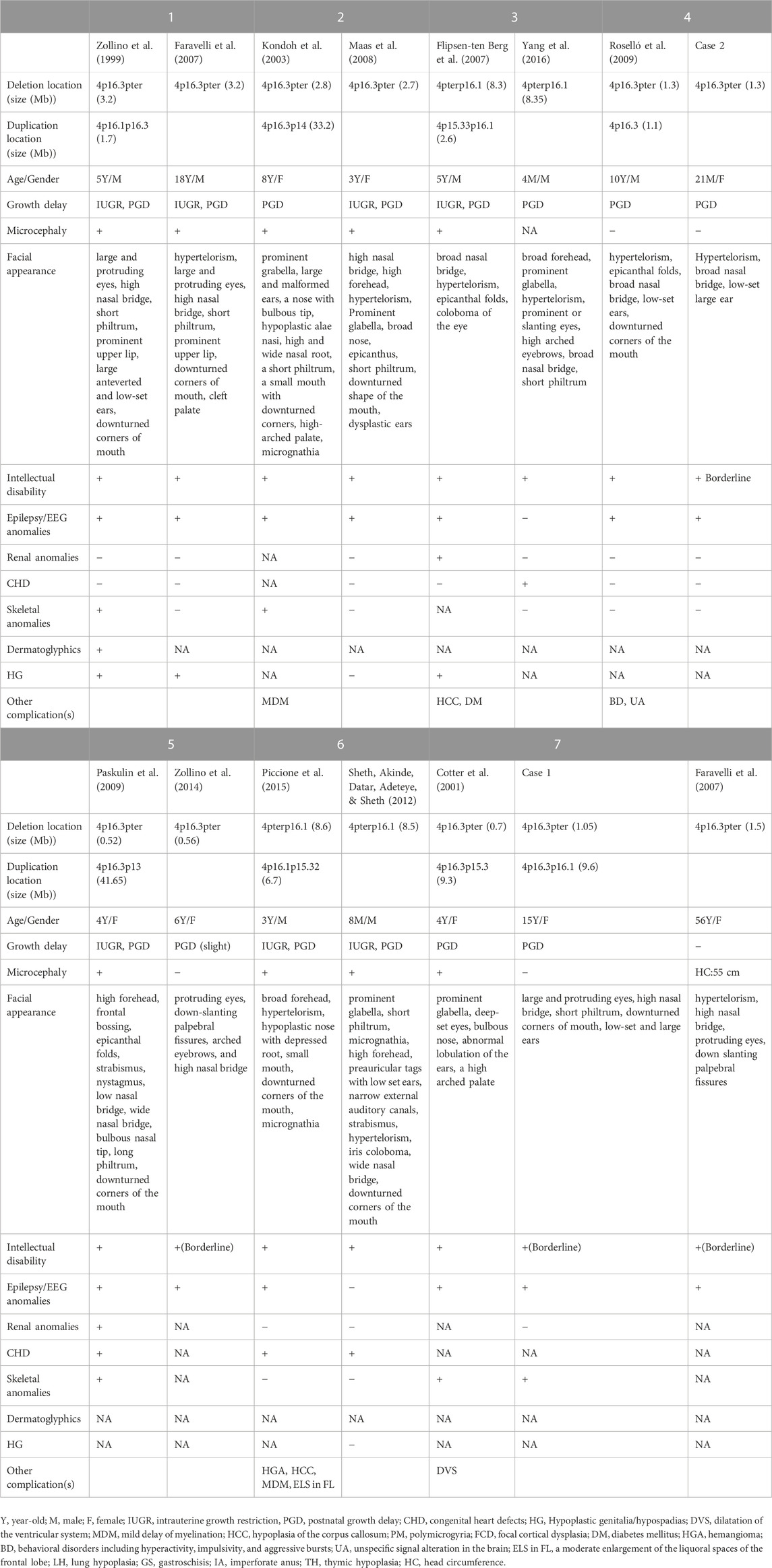
To date, patients with inverted terminal deletion duplication of 4p are relatively rare, and approximately nine cases have been reported (Table 1). Among them, two 4 pter del-dup cases were detected prenatally, and the pregnancies were terminated at 14 and 36 weeks, respectively. To demonstrate the roles of partial 4p deletion and 4p duplication in the phenotypic features of patients, we compared the clinical presentation of patients carrying a 4 pter del-dup with patients carrying similar pure 4p terminal deletions (Table 2). The phenotype of patients with a 4 pter del-dup was generally consistent with that of patients carrying a similarly sized pure 4p deletion. In groups 1, 2, 3, and 6, patients carried a relatively large deletion of terminal 4p, encompassing the accepted critical region of WHS, which appeared to be more consistent with WHS syndrome. Seizure onset in WHS generally occurs within the first 2 years of the patient’s life with a peak incidence at 6–12 months of age; therefore, patients with pure 4p deletions in groups 3 and 6 were too young to develop seizures (Battaglia & Carey, 2005). The size of the deletion seems to be more prognostic of phenotypic severity than the size of the duplication.
Two seizure-susceptibility regions have been identified in WHS. The distal candidate region is a 197 kb segment starting from 368 kb to 565 kb of the terminus of 4p, while the proximal seizure candidate region maps from 0.76 to 1.3 Mb from the end of 4p terminus (South et al., 2008). Almost all the patients suffered from seizures and had a deletion encompassing both distal regions. There is an overlap region of 0.5 Mb from terminal between these patients. It has been reported that 4p terminal deletions between 200 and 400 kb are likely benign (Hannes & Vermeesch, 2008). Therefore, we propose that the region spanning 0.4–0.52 Mb from the terminus of 4p is a seizure candidate region (Figure 4) and a total of 3 RefSeq genes reside within this region. Only PIGG (phosphatidylinositol glycan anchor biosynthesis, class G; PIGG) are in the OMIM database and have already been proposed as candidate genes for seizures. PIGG is a glycosylphosphatidylinositol that anchors proteins to the cell surface. Homozygous and compound heterozygous variants of PIGG have been reported in five patients with intellectual disability, hypotonia, and early onset seizures, whereas parents and siblings with heterozygous variants of PIGG were asymptomatic carriers (Makrythanasis et al., 2016; Corrêa et al., 2022). This means that haploinsufficiency for PIGG is not sufficient to develop seizures, and several genes may function synergistically to promote seizures. In addition, we believe that this region may be associated with mild or borderline intellectual disability, notably speech delay.
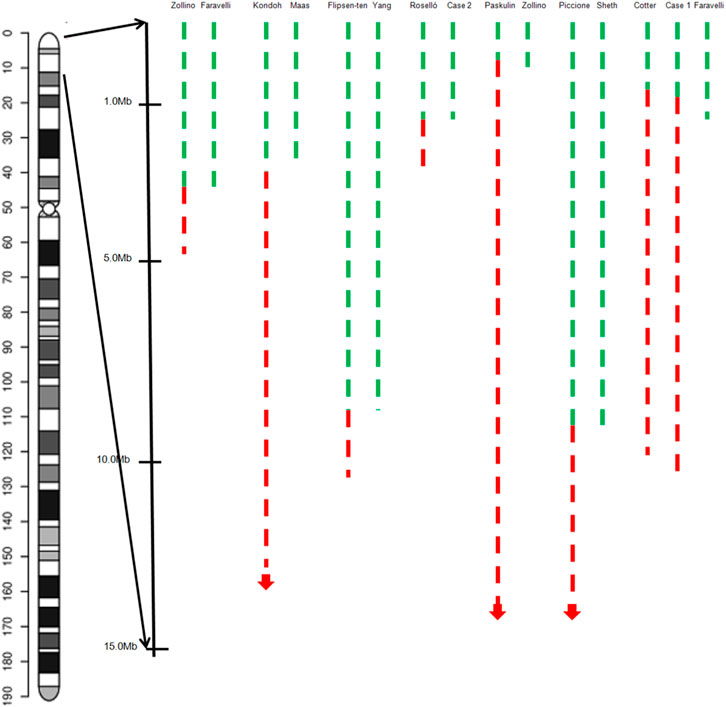
FIGURE 4. Graphical representation of the overview of the molecular cytogenetic region involved in a case with inv del-dup of 4p and pure terminal deletion of 4p in the literature. Green dashed lines indicate the deleted segments on chromosome 4p, red dashed lines the duplicated regions.
Several patients with 4 pter del-dup reported by Paskulin et al. (2009) and Cotter et al. (2001) carried a deletion of terminal 4p, distal to the critical region, and presented with characteristics corresponding to 4p trisomy syndrome. Cotter et al. (2001) reported a 4-year-old girl with a 4p terminal deletion about 0.7 Mb distal to the WHS critical region and a 9.3 Mb duplication from 4p16.3 to p15.3, giving rise to typical findings of 4p trisomy including prominent glabella, bulbous nose, multiple congenital anomalies, seizure disorder, and severe intellectual disability. A 3.2 Mb 4p terminal deletion and 1.7 Mb duplication was detected in another patient diagnosed with WHS. Zollino et al. (1999) speculated that the critical WHS region plays a role as a ‘‘master’’ regulatory sequence, with strong pleiotropic effects on the other genes in a dose-dependent manner. Our patient and the patient reported by Roselló et al. (2009) carried a 1.055 Mb and 1.3 Mb 4p terminal deletion, respectively, and presented with a more pronounced WHS syndrome phenotype. Hannes et al. (2010) reported a 7-year-old boy clinically diagnosed with mild WHS features, and molecular karyotyping revealed a deletion 600 kb proximal to WHSCR and WHSCR-2. They suggested that the WHSCR1-2 flanking sequence directly or indirectly contributes to the severity of WHS. South et al. (2008) also found that WHS patients with 4p deletions flanking the WHSCR display a specific facial gestalt. So, combined with our patients, we speculate that the region 0.7 Mb–1.3 Mb from the 4p terminus may harbor controlling genes that regulate the expression of genes in the rest of the 4p region, especially in the accepted WHS critical region (Figure 3).
Although partial 4p trisomy and WHS are two different clinical entities, there is overlap in the clinical phenotype between pure duplication starting from the 4p terminal (as in 4p trisomy), and pure deletion of the 4p terminal (as in WHS). Both are characterized by similar features, such as developmental and neuropsychomotor delays, especially predominant in speech delay, as well as microcephaly, low weight, wide nasal bridge/root, dysplastic ears, and other organ anomalies. Two interstitial microduplications encompassing the WHS critical region have been reported (between 1.3Mb and 1.9 Mb from terminal 4p), both have overgrowth features such as macrocephaly, overgrowth in height, and bone age (Cyr et al., 2011; Palumbo et al., 2015). None of these phenotypes were found in the nine patients mentioned above or in the two cases discussed in the present study. This study also demonstrated that particular genes or regions located in the 4p terminal region, like 0.7 Mb–1.3 Mb from the 4p terminus, have a regulatory influence on the critical region of WHS.
Genotype-phenotype correlations contribute to the identification of genes involved in the pathogenesis of genetic syndromes. Combined with previously reported cases, we found that the region 0.7 Mb–1.3 Mb from the 4p terminus may have a regulatory effect on the rest of the 4p region, especially in the accepted WHS critical region, and has a critical effect on patient phenotypes. Structural rearrangements of chromosome 4p are complicated, and in cases of 4p inv dup-del, the phenotypes are heterogeneous. More accurate clinical descriptions of patients need to be reported, and the roles and functions of genes located on 4p need to be elucidated.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minors' legal guardian for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ drafted the initial manuscript. XZ, HoL, HY, and YJ were responsible for genetic analysis. XZ, WL, JL, and ZY collected clinical information. WS revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Xuanwu Hospital Science Program for Fostering Young Scholars (grant number: QNPY2021006) and National Natural Science Foundation of China (grant number: 81571267).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Battaglia, A., and Carey, J. C. (2005). Seizure and EEG patterns in Wolf-Hirschhorn (4p-) syndrome. Brain Dev. 27 (5), 362–364. doi:10.1016/j.braindev.2004.02.017
Battaglia, A., Carey, J. C., and South, S. T. (2015). Wolf-hirschhorn syndrome: A review and update. Am. J. Med. Genet. C Semin. Med. Genet. 169 (3), 216–223. doi:10.1002/ajmg.c.31449
Corrêa, T., Mayndra, M., and Santos-Rebouças, C. B. (2022). Distinct epileptogenic mechanisms associated with seizures in wolf-hirschhorn syndrome. Mol. Neurobiol. 59 (5), 3159–3169. doi:10.1007/s12035-022-02792-9
Cotter, P. D., Kaffe, S., Li, L., Gershin, I. F., and Hirschhorn, K. (2001). Loss of subtelomeric sequence associated with a terminal inversion duplication of the short arm of chromosome 4. Am. J. Med. Genet. 102 (1), 76. doi:10.1002/1096-8628(20010722)102:1<76::aid-ajmg1389>3.0.co;2–480
Cyr, A. B., Nimmakayalu, M., Longmuir, S. Q., Patil, S. R., Keppler-Noreuil, K. M., and Shchelochkov, O. A. (2011). A novel 4p16.3 microduplication distal to WHSC1 and WHSC2 characterized by oligonucleotide array with new phenotypic features. Am. J. Med. Genet. A 155a (9), 2224–2228. doi:10.1002/ajmg.a.34120
Faravelli, F., Murdolo, M., Marangi, G., Bricarelli, F. D., Di Rocco, M., and Zollino, M. (2007). Mother to son amplification of a small subtelomeric deletion: A new mechanism of familial recurrence in microdeletion syndromes. Am. J. Med. Genet. A, 143a(11), 1169–1173. doi:10.1002/ajmg.a.31723
Flipsen-ten Berg, K., van Hasselt, , Eleveld, M. J., van der Wijst, S. E., Hol, F. A., and de Vroede, M. A. (2007). Unmasking of a hemizygous WFS1 gene mutation by a chromosome 4p deletion of 8.3 Mb in a patient with Wolf-Hirschhorn syndrome. Eur. J. Hum. Genet. 15 (11), 1132–1138. doi:10.1038/sj.ejhg.5201899
Gordon, A. J., and Halliday, J. A. (1995). Inversions with deletions and duplications. Genetics 140 (1), 411–414. doi:10.1093/genetics/140.1.411
Hannes, F., Drozniewska, M., Vermeesch, J. R., and Haus, O. (2010). Duplication of the Wolf-Hirschhorn syndrome critical region causes neurodevelopmental delay. Eur. J. Med. Genet. 53 (3), 136–140. doi:10.1016/j.ejmg.2010.02.004
Hannes, F., and Vermeesch, J. R. (2008). Benign and pathogenic copy number variation on the short arm of chromosome 4. Cytogenet Genome Res. 123 (1-4), 88–93. doi:10.1159/000184695
Kondoh, Y., Toma, T., Ohashi, H., Harada, N., Yoshiura, K., Ohta, T., et al. (2003). Inv dup del(4)(:p14--> p16.3::p16.3--> qter) with manifestations of partial duplication 4p and Wolf-Hirschhorn syndrome. Am. J. Med. Genet. A 120a (1), 123–126. doi:10.1002/ajmg.a.20208
Maas, N. M., Van Buggenhout, G., Hannes, F., Thienpont, B., Sanlaville, D., and Kok, K. (2008). Genotype-phenotype correlation in 21 patients with Wolf-Hirschhorn syndrome using high resolution array comparative genome hybridisation (CGH). J. Med. Genet. 45 (2), 71–80. doi:10.1136/jmg.2007.052910
Makrythanasis, P., Kato, M., Zaki, M. S., Saitsu, H., Nakamura, K., and Santoni, F. A. (2016). Pathogenic variants in PIGG cause intellectual disability with seizures and hypotonia. Am. J. Hum. Genet. 98 (4), 615–626. doi:10.1016/j.ajhg.2016.02.007
Nevado, J., Ho, K. S., Zollino, M., Blanco, R., Cobaleda, C., and Golzio, C. (2020). International meeting on Wolf-Hirschhorn syndrome: Update on the nosology and new insights on the pathogenic mechanisms for seizures and growth delay. Am. J. Med. Genet. A 182 (1), 257–267. doi:10.1002/ajmg.a.61406
Palumbo, O., Palumbo, P., Ferri, E., Riviello, F. N., Cloroformio, L., Carella, M., et al. (2015). Report of a patient and further clinical and molecular characterization of interstitial 4p16.3 microduplication. Mol. Cytogenet 8, 15. doi:10.1186/s13039-015-0119-6
Paskulin, G. A., Riegel, M., Cotter, P. D., Kiss, A., Rosa, R. F., and Zen, P. R. (2009). Inv dup del(4)(:p13-->p16.3::p16.3-->qter) in a girl without typical manifestations of Wolf-Hirschhorn syndrome. Am. J. Med. Genet. A 149a (6), 1302–1307. doi:10.1002/ajmg.a.32888
Piccione, M., Salzano, E., Vecchio, D., Ferrara, D., Malacarne, M., and Pierluigi,., M. (2015). 4p16.1-p15.31 duplication and 4p terminal deletion in a 3-years old Chinese girl: Array-CGH, genotype-phenotype and neurological characterization. Eur. J. Paediatr. Neurol. 19 (4), 477–483. doi:10.1016/j.ejpn.2015.02.002
Roselló, M., Monfort, S., Orellana, C., Ferrer-Bolufer, I., Quiroga, R., Oltra, S., et al. (2009). Submicroscopic duplication of the Wolf-Hirschhorn critical region with a 4p terminal deletion. Cytogenet Genome Res. 125 (2), 103–108. doi:10.1159/000227833
Rowe, L. R., J-Y Lee, , Rector, L., and B Kaminsky, E. (2014). U-type exchange is the most frequent mechanism for inverted duplication with terminal deletion rearrangements. J. Med. Genet. 2009 (46), 694–702. doi:10.1136/jmg.2008.065052
Sheth, F., Akinde, O. R., Datar, C., Adeteye, O. V., and Sheth, J. (2012). Genotype-phenotype characterization of wolf-hirschhorn syndrome confirmed by FISH: Case reports. Case Rep. Genet. 2012, 878796. doi:10.1155/2012/878796
South, S. T., Hannes, F., Fisch, G. S., Vermeesch, J. R., and Zollino, M. (2008). Pathogenic significance of deletions distal to the currently described Wolf-Hirschhorn syndrome critical regions on 4p16.3. Am. J. Med. Genet. C Semin. Med. Genet. 148c (4), 270–274. doi:10.1002/ajmg.c.30188
Wilson, M. G., Towner, J. W., and Negus, L. D. (1970). Wolf-Hirschhorn syndrome associated with an unusual abnormality of chromosome no. 4. J. Med. Genet. 7 (2), 164–170. doi:10.1136/jmg.7.2.164
Wolf, U., Reinwein, H., Porsch, R., Schröter, R., and Baitsch, H. (1965). Deficiency on the short arms of a chromosome No. 4. Humangenetik 1 (5), 397–413.
Yang, W. X., Pan, H., Li, L., Wu, H. R., Wang, S. T., and Bao, X. H. (2016). Analyses of genotypes and phenotypes of ten Chinese patients with wolf-hirschhorn syndrome by multiplex ligation-dependent probe amplification and array comparative genomic hybridization. Chin. Med. J. Engl. 129 (6), 672–678. doi:10.4103/0366-6999.177996
Zollino, M., Murdolo, M., Marangi, G., Pecile, V., Galasso, C., Mazzanti, L., et al. (2008). On the nosology and pathogenesis of wolf-hirschhorn syndrome: Genotype-phenotype correlation analysis of 80 patients and literature review. Am. J. Med. Genet. C Semin. Med. Genet. 148c (4), 257–269. doi:10.1002/ajmg.c.30190
Zollino, M., Orteschi, D., Ruiter, M., Pfundt, R., Steindl, K., and Cafiero, C. (2014). Unusual 4p16.3 deletions suggest an additional chromosome region for the Wolf-Hirschhorn syndrome-associated seizures disorder. Epilepsia 55 (6), 849–857. doi:10.1111/epi.12617
Keywords: chromosome 4p, terminal 4p deletion, wolf-Hirschhorn syndrome, partial duplication 4p, 4p trisomy
Citation: Zhang X, Lu H, Yang H, Ji Y, Liu H, Liu W, Li J, Yang Z and Sun W (2023) Genotype-phenotype correlation of deletions and duplications of 4p: case reports and literature review. Front. Genet. 14:1174314. doi: 10.3389/fgene.2023.1174314
Received: 26 February 2023; Accepted: 30 May 2023;
Published: 14 June 2023.
Edited by:
Zhiqiang Li, Qingdao University, ChinaReviewed by:
Priyanka Srivastava, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaCesar Cobaleda, Spanish National Research Council (CSIC), Spain
Copyright © 2023 Zhang, Lu, Yang, Ji, Liu, Liu, Li, Yang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Sun, Ym11c3Vubnl3QDE2My5jb20=
 Xuan Zhang
Xuan Zhang Hongjuan Lu
Hongjuan Lu Hanran Yang1
Hanran Yang1 Wenjian Liu
Wenjian Liu