- 1College of Stomatology, Xi’an Jiaotong University, Xi’an, China
- 2Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi’an Jiaotong University, Xi’an, China
- 3Department of Oral and Maxillofacial Surgery, College of Stomatology, Xi’an Jiaotong University, Xi’an, China
- 4Department of Surgery, Institute of Integrated Traditional Chinese and Western Medicine, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 5Department of Neurology, School of medicine, Sichuan provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
Acute pancreatitis (AP) is a severe inflammatory condition that frequently leads to systemic inflammatory response syndrome and multiple organ failure. Despite the increasing occurrence, targeted therapies remain unavailable. CircRNAs have recently been implicated in AP pathogenesis, regulating biological processes such as apoptosis, pyroptosis, and inflammation through mechanisms including miRNA sponging, protein scaffolding, and translation. Their disease-specific roles make them promising candidates for clinical translation. This review highlights the potential of circRNAs as novel diagnostic tools and therapeutic targets in AP, aiming to guide future advancement of innovative biomarkers and therapeutic strategies.
1 Introduction
With a 1%–5% case fatality rate in extreme cases, acute pancreatitis (AP)-a condition characterized by inflammation of the pancreas-manifests as severe abdominal pain and can progress to multiple organ failure and pancreatic necrosis (Petrov and Yadav, 2019). The primary pathological mechanism involves premature activation of pancreatic digestive enzymes, causing inflammation and autodigestion (Boxhoorn et al., 2020), Additional contributors include aberrant calcium ion levels (Huang et al., 2017; Sutton, 2020), oxidative stress, and inflammatory cell infiltration (Sendler et al., 2013; Sendler et al., 2020). Patients may experience persistent pain, recurrent episodes, and long-term functional impairment, which considerably reduce quality of life and increase socioeconomic burden. AP exhibits a broad spectrum of severity, ranging from mild manifestations to life-threatening complications.
Circular RNAs (circRNAs) are a class of single-stranded, covalently closed RNAs made up of exons, introns, or a combination of both (Zhang et al., 2016). They are produced through back-splicing (Zhang et al., 2016) or via lariat-derived RNA intermediates (Jeck and Sharpless, 2014). While the vast majority of circRNAs are considered non-protein-coding and function as miRNA sponges, a subset of circRNAs has been found to possess coding potential and can be translated into functional peptides or proteins (Du et al., 2017a). These molecules hold significant clinical potential and play essential roles in cell growth and proliferation.
While numerous reviews have summarized the roles of circRNAs across different diseases, this article focuses specifically on their implications in AP, offering a systematic integration of circRNA mechanisms—such as miRNA sponging and translational regulation—within the AP context. Furthermore, we emphasize the translational potential, discussing recent advances and preclinical evidence that support circRNAs as both biomarkers and therapeutic targets for AP. For instance, circRNAs contribute significantly to AP pathogenesis, by c modulating crucial signaling pathways including inflammation, apoptosis, and pyroptosis. Moreover, circRNAs have emerged as promising diagnostic biomarkers and therapeutic targets for the early detection of AP (Liu C. et al., 2020), owing to their exceptional stability conferred by the covalently closed circular structure (which confers resistance to RNase R degradation) and their highly tissue-specific expression patterns. As a case in point, a study by Liu et al. (2020) demonstrated the utility of differentially expressed circRNAs in the plasma of AP patients for early diagnosis.
Despite the accumulating literature in this area, a dedicated review that synthesizes the current knowledge of circRNAs specifically in AP—particularly regarding their mechanisms and clinical applicability—is lacking. Therefore, this review aims to particularly regarding their mechanisms summarizing the functional roles and regulatory mechanisms of circRNAs in AP and evaluating their potential for diagnosis and targeted therapy, ultimately providing a foundational resource for future research and clinical translation.
1.1 Literature search and selection
This narrative review synthesizes current evidence on the roles and mechanistic insights of circular RNAs (circRNAs) in acute pancreatitis (AP). Literature searches were performed in electronic databases, including PubMed and Web of Science, using key terms such as “circular RNAs,” “circRNAs,” “acute pancreatitis,” and related synonyms. Study selection adhered to the following criteria:
Inclusion criteria: (1) original research focusing on the function, mechanism, or clinical relevance of circRNAs in AP; (2) studies conducted in human subjects, animal models, or cell lines; and (3) articles published in peer-reviewed journals.
Exclusion criteria: (1) conference abstracts, reviews, editorials, or dissertations; (2) articles whose full text was inaccessible or contained non-extractable data; and (3) studies irrelevant to AP.
Owing to the emerging nature of circRNAs research in AP, the search yielded a circumscribed body of core literature that met these criteria, all of which has been incorporated into this synthesis.
2 Circular RNA
2.1 Circular RNA biosynthesis
Two processes are involved in the synthesis of circRNAs: the lariat-driven pathway (Jeck and Sharpless, 2014), and direct back-splicing (Zhang et al., 2016; Chen, 2020). Direct back-splicing is facilitated by complementary sequences within lengthy flanking introns (Liang and Wilusz, 2014) or through the dimerization of RNA-binding proteins (RBPs) (Kristensen et al., 2019). These complementary sequences, known as reverse complementary matches (RCMs), which can be either repeated or non-repeated elements (Ivanov et al., 2015), are essential for circular RNAs formation. Inverted repeating Alu elements are particularly common RCMs. Furthermore, RBPs such as Quaking (QKI) (Conn et al., 2015), Muscleblind (MBL) (Liang et al., 2017), FUS-binding protein (Errichelli et al., 2017), and NF90/NF110 proteins (Li et al., 2017) are known to attach to the introns on both sides of the exons to generate circRNAs, hence increasing circRNAs creation in RBP dimerization. In contrast to canonical back-splicing, circRNAs can be generated from lariat structure, especially when introns lack inverted repeat sequences like Alu elements (Jeck and Sharpless, 2014). In such cases, the lariat may contain exons. The lariat can produce an exonic circular RNA if it is not broken down but instead processed further (Barrett et al., 2015).
Based on genomic origin, circRNAs are primarily classified into four types: exonic (EcircRNAs) (Jeck et al., 2013), intronic (IcircRNAs) (Zhang et al., 2013), exon-intron (EIciRNAs) (Salzman et al., 2013), and intergenic circRNAs (PMC, 2025). While the majority of circRNAs are generated through pre-mRNA back-splicing, a minor fraction originates from lariat introns via linear splicing. (Figure 1). In addition to this classification, circRNAs can be categorized by the genomes of subcellular organelles. For instance, mitochondrial genome-encoded circRNAs (mecciRNAs) have been identified and implicated in regulating mitochondrial function and cardiac pathology (Liu et al., 2025). Furthermore, the mitochondrial circRNA SCAR (steatohepatitis-associated circRNA ATP5B regulator) plays crucial cellular roles in various tissues (Wu et al., 2020; Zhao et al., 2020; Costa et al., 2021; Wu et al., 2023).
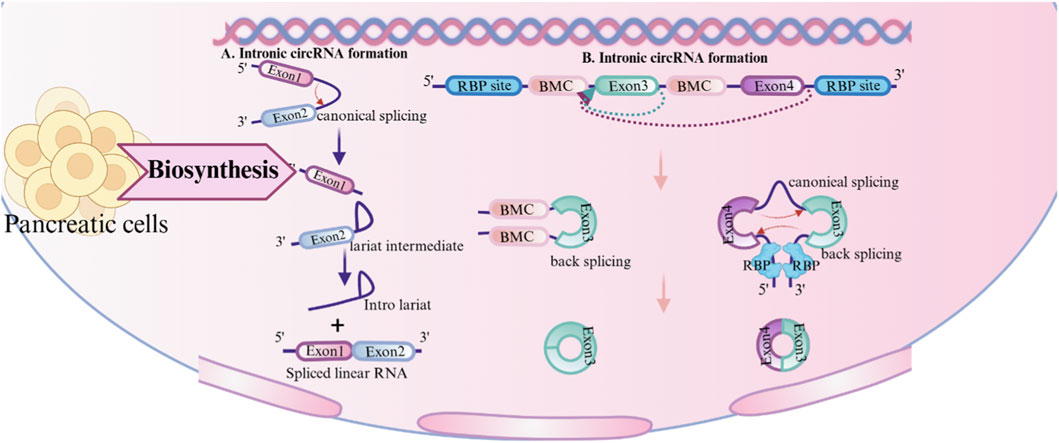
Figure 1. Synthesis of circular RNA (circRNA). circRNA: circular RNA; RBP: RNA-binding protein; RCM: Reverse complementary matches.
2.2 Regulation of circRNAs synthesis
Beyond the core mechanisms of back-splicing and RBP modulation, circRNAs biogenesis is further shaped by structural and sequence features. Longer exons exhibit increased susceptibility to circularization due to the higher availability of splice sites, thereby enhancing circRNAs formation efficiency (Liang and Wilusz, 2014). Suboptimal base pairing in intronic repeats—where GU mismatches replace canonical Watson-Crick pairs—can still promote the biogenesis of circRNAs by enabling partial RNA duplex formation, thus facilitating splice site recognition (Liang and Wilusz, 2014). The presence of polyadenylation signals competes with back-splicing machinery, as these elements may redirect pre-mRNA processing toward linear splicing pathways, thereby suppressing circRNAs generation (Liang and Wilusz, 2014). Notably, adenosine-to-inosine (A-to-I) RNA editing mediated by Adenosine Deaminase Acting on RNA 1 (ADAR1) disrupts double-stranded RNA (dsRNA) structures (e.g., reverse complementary matches between intronic sequences), thereby preventing RNA-binding protein (RBP) recruitment or proper alignment of splice sites, which ultimately inhibits circRNAs formation (Ivanov et al., 2015) (Figure 1).
2.3 CircRNAs degradation
Circular RNA degradation can be divided into four categories according to the auxiliary factors needed: structure-mediated, m6A-mediated, RNase L-mediated, and miRNA-mediated (Liu et al., 2019). Due to their covalently closed circular structure, circRNAs are highly stable, and their degradation primarily relies on endonucleases that catalyze internal cleavage. For example, the circRNA CDR1as/ciRS-7 can recruit miR-671-loaded Ago2 to CDR1as/ciRS-7, causing Ago2 to cleave the molecule endonucleolytically, which in turn causes exonucleolytic RNA degradation (Hansen et al., 2011). Additionally, m6A-modified circRNAs can recruit the adaptor protein HRSP12 through the m6A mark;, HRSP12 then bridges endoribonuclease RNase P/MRP and the m6A reader protein YTHDF2, thereby initiating circRNAs degradation (Park et al., 2019). In contrast, the mechanism underlying RNase L-mediated circRNAs degradation remains poorly understood, though Liu et al. have proposed that it may be related to circRNAs tending to form a 16–26-base pair (bp) imperfect RNA duplex (Liu et al., 2019). Finally, structure-dependent decay involves the UPF1(Up-frameshift 1) RNA helicase and G3BP1(GTPase-activating protein SH3 domain-binding protein 1) stress granule assembly factor, which facilitate the degradation of circRNAs (Fischer et al., 2020).
3 Functions of CircRNAs
3.1 miRNA sponge
CircRNAs mostly operate through interactions with other molecules, with the most widely studied mechanism being miRNA sponges (Panda, 2018; Thomson and Dinger, 2016). For instance, ciRS-7, a prominent circRNAs, possesses over 70 conserved binding sites of miR-7, enabling it to regulate the expression of its target genes by interaction with miR-7 (Hansen et al., 2013; Memczak et al., 2013). The effect of ciRS-7 on miR-7 (either inhibition or protection) may be contingent upon the specific cellular milieu (Kleaveland et al., 2018). Moreover, additional circRNAs, including circHIPK3 (Zheng et al., 2016), circPVT1 (Verduci et al., 2017), and circBIRC6 (Yu et al., 2017), have been validated to possess analogous miRNA sponge capabilities, therefore elucidating the diverse role of circRNAs in gene regulation (Figure 2).
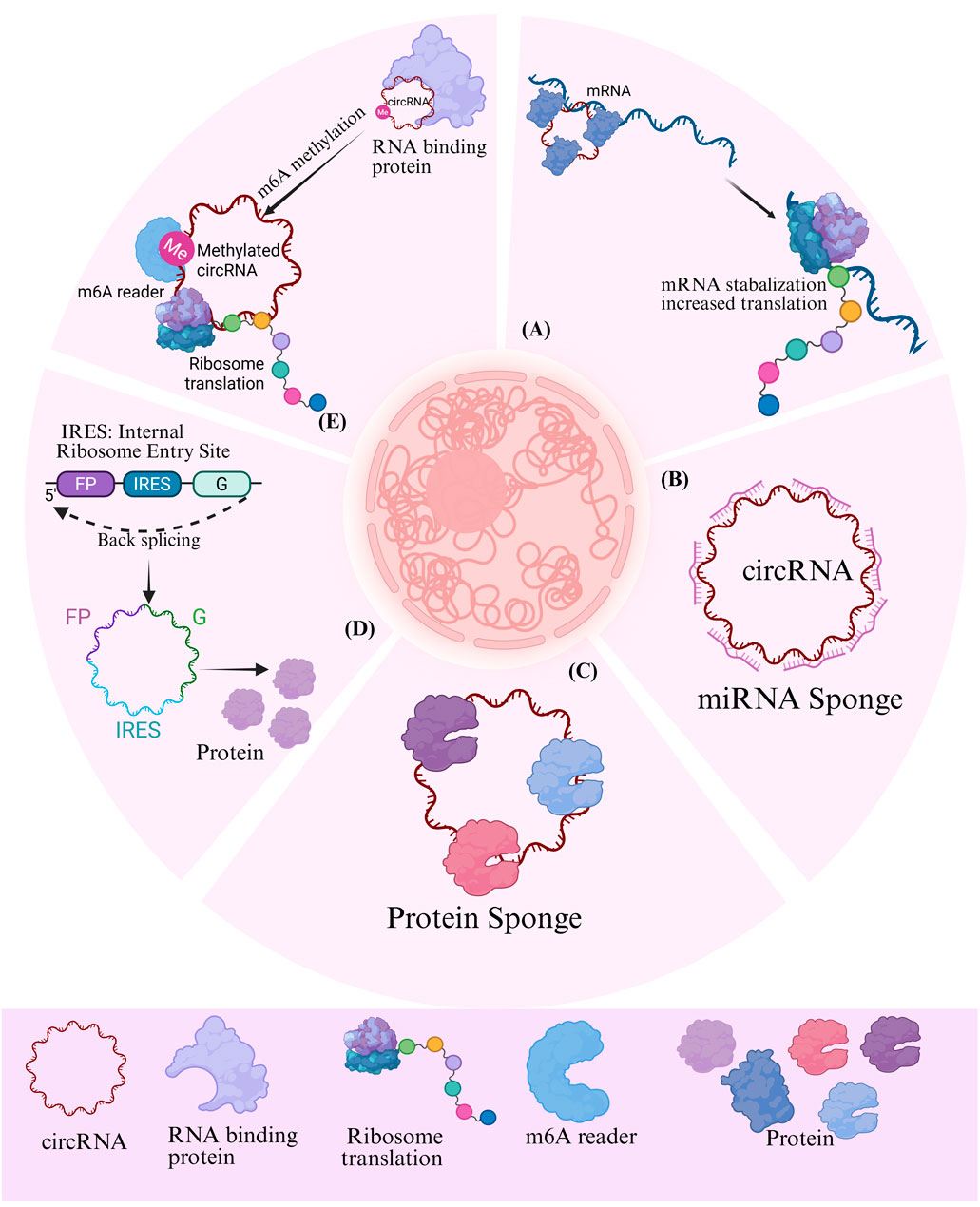
Figure 2. Potential role of circRNAs in the development and progression of pancreatitis. m6A: methylated N6 adenosine; IRES: internal ribosomal entry site; IL F2: Interleukin Enhancer-Binding Factor 2/Nuclear Factor 45; IL F3: Interleukin Enhancer-Binding Factor 3/Nuclear Factor 90; MBL: Muscleblind-like protein.
3.2 Protein modulator
The function interactions between circRNAs and proteins can be categorized into several key mechanistic themes. (Zhou et al., 2020). First, circRNAs can serve as scaffolds to facilitate protein-protein interactions, thereby promoting post-translational modification or trans-activation of the bound proteins (Du et al., 2017b; H et al., 2019). Second, they can act as decoys by binding to proteins and and inhibiting their interactions with other biomolecules, such as DNA (Xia et al., 2018; Chen et al., 2016),RNA (Barbagallo et al., 2018; Barbagallo et al., 2019), and other proteins (Xueting et al., 2019). Third, circRNAs participate in epigenetic regulation by recruiting transcription factors, nucleosome remodeling complexes (Chen et al., 2018; Liu B. et al., 2020), or modifying enzymes to specific genomic loci (Jie et al., 2020). Additionally, circRNAs can form complexes with proteins and mRNA to either increase translation by stabilizing mRNA (Guarnerio et al., 2019) or repressing it (Chen et al., 2020). Finally, the subcellular localization of circRNAs regulating proteins, such as nuclear circRNAs associated with proteins (Yang Q. et al., 2017), cytoplasm circRNAs facilitating nuclear protein input (Yang Z-G. et al., 2017) or altering protein distribution through nuclear export mechanism (Wang S. et al., 2019).
3.3 Transcriptional regulation
CircRNAs influence gene transcription through several distinct mechanisms. First, they can bind to transcription factors and act as co-activators to directly modulate transcriptional activity (Li Z. et al., 2015; Guarnerio et al., 2020). Second, by competing with linear mRNAs for splice sites, circRNAs can promote exon skipping, thereby altering splicing patterns and gene expression outcomes (Liang et al., 2017; Ashwal-Fluss et al., 2014; Kelly et al., 2015; Conn et al., 2017). Third, interactions with specific RNA-binding proteins can allow circRNAs to alter the regulatory functions of these proteins (Ashwal-Fluss et al., 2014). Finally, circRNAs can indirectly influence gene expression by sequestering miRNAs (Xia et al., 2018).
3.4 Template for translation
The absence of a 5′cap and 3′polyadenylate tail necessitates unique processes for the translation of circRNAs. At present, two recognized forms of circRNAs translation exist: m6A-dependent translation and IRES-mediated translation. Research indicates that m6A-modified circRNAs can initiate translation through the collaborative function of m6A reading proteins (e.g., YTHDF3) and translation initiation factors (e.g., eIF4G2) (Yang Y. et al., 2017; Di Timoteo et al., 2020). IRES translations are either endogenous or synthetically engineered circRNAs that incorporate IRES. Ribosomes can be directly recruited for translation (Macejak and Sarnow, 1991; Chen and Sarnow, 1995; Zhang et al., 2020). It is noteworthy that these two mechanisms can be synergistic: m6A alteration can improve IRES activity, hence dramatically enhancing the translation efficiency of circRNAs (Di Timoteo et al., 2020; Legnini et al., 2017) (Figure 2).
4 The role of circRNAs methylation modification in AP
N6-methyladenosine (m6A) modification is prevalent in eukaryotic mRNA, but its mechanism of action in circRNAs remains ambiguous. Research indicates that m6A modification in circRNAs is governed by the same “writer” (e.g., METTL3), “eraser” (e.g., FTO), and “reader” (e.g., YTHDF2) as mRNA (Zhang et al., 2020; Shulman and Stern-Ginossar, 2020), and exhibits specificity for cell type (Zhou et al., 2017). This modification critically influences the fate of circRNAs by governing two opposing outcomes: cap-independent translation (Yang Y. et al., 2017) and RNA decay (Park et al., 2019) (Ries et al., 2019). On one hand, m6A modification can promote the translation of circRNAs. By recruiting initiation factors and the ribosomal complex through YTHDF family readers, m6A drives cap-independent translation, enabling a subset of circRNAs to produce functional peptides that contribute to disease pathogenesis (Yang Y. et al., 2017). On the other hand, m6A-modified circRNAs can also undergo accelerate the degradation. For instance, RNase P/MRP complexes or reader proteins such as YTHDF2 can recognize m6A-modified circRNAs and facilitate their decay. Additionally, certain m6A-modified circRNAs are susceptible to cleavage by RNase P/MRP complexes (Park et al., 2019), indicating a dynamic and context-dependent regulatory layer (Zhang et al., 2020; Shulman and Stern-Ginossar, 2020).
A study in a severe acute pancreatitis (SAP) mouse model identified 57 circRNAs with aberrant m6A modification, which were implicated in pathological processes such as autophagy (Wu et al., 2021). For example, the constructed m6A-circRNA-miRNA network indicated that miR-24-3p and miR-26a may modulate disease progression through their interaction with m6A-circRNAs (Shrinivas et al., 2019) (Wu et al., 2021). Furthermore, the demethylase ALKBH5 (alkylation repair homolog protein 5) was found to be upregulated in this model, suggesting that active m6A demethylation contributes to SAP progression (Wu et al., 2021).
These findings highlight the m6A-circRNA axis as a promising therapeutic frontier. Testable hypotheses can now be formulated: for example, does ALKBH5 gain-of-function in pancreatic acinar cells exacerbate AP by reshaping the m6A landscape of key circRNAs? Conversely, could inhibiting ALKBH5 or therapeutically targeting m6A-modified circRNAs present a viable strategy to ameliorate SAP? Future investigations to validate these mechanisms in human samples and through in vivo functional studies will be crucial for translating these insights into novel diagnostics and therapies approaches.
5 Circular RNA as biomarkers
Exosomes, which are frequently found in blood and other body fluids (Ma et al., 2019), serve as reliable extracellular transporters for proteins, nucleic acids, and other bioactive molecules (van Niel et al., 2018). CircRNAs are abundantly present in extracellular vesicles (Li Y. et al., 2015; Wang Y. et al., 2019), and their expression profiles can differentiate between healthy and diseased states, highlighting their potential as diagnostic biomarkers (Li Y. et al., 2015).
CircRNAs can be used as predictive biomarkers as well as diagnostic biomarkers. For instance, postoperative atrial fibrillation can be predicted based on the presence of particular circRNAs in the patient’s plasma. Furthermore, the presence of specific circRNAs in the plasma following the initiation of the disease can also predict organ dysfunction (Vausort et al., 2016; Plasma Circular RNAs, 2016). In AP, recent clinical evidence confirms circRNAs as promising diagnostic markers. A study profiling blood circRNAs in AP patients identified six differentially expressed circRNAs (e.g., hsa_circRNA_101015, hsa_circRNA_101211, hsa_circRNA_103470), three of which significantly increased with disease severity and showed high diagnostic accuracy via receiver operating characteristic (ROC) analysis (Liu C. et al., 2020). This establishes circRNAs as AP-specific biomarkers.
However, while circRNAs hold promise as diagnostic biomarkers for AP, they should be evaluated against established diagnostic markers such as amylase, lipase, C-reactive protein (CRP), and clinical scoring systems like BISAP (Bedside Index for Severity in Acute Pancreatitis) and Ranson scores. Amylase and lipase have long been the gold standard for diagnosing AP, though their diagnostic accuracy can be limited by sensitivity and specificity, especially in mild cases or delayed presentations (Guda et al., 2018). CRP is widely used to assess the inflammation level and severity, but it is a non-specific acute-phase reactant that can be elevated in a wide array of other inflammatory and infectious conditions, limiting its specificity for AP (Rm et al., 2016). The BISAP and Ranson scores are valuable for predicting the severity and mortality of AP, but they rely on a combination of clinical and laboratory parameters that require 24–48 h to complete and can be complex for rapid bedside application (Mounzer et al., 2012).
Comparatively, circRNAs may offer more specificity and sensitivity due to their unique expression patterns in AP. Recent studies have demonstrated that circRNAs are not only upregulated in AP but also correlate strongly with disease severity, offering an early and non-invasive diagnostic tool (Yang et al., 2020). Furthermore, circRNAs can be detected in exosomes, which are stable and easy to isolate from blood or plasma samples, presenting a significant advantage over traditional markers that may require serial measurements and lack organ specificity (Li Y. et al., 2015; Wang Y. et al., 2019). This suggests that integrating circRNAs into diagnostic panels could complement or even surpass the current biomarkers in terms of both accuracy and ease of use, particularly when combined with other markers in multi-biomarker approaches (Liu C. et al., 2020).
To benchmark circRNAs against existing biomarkers, further large-scale clinical studies are required. These studies should compare the diagnostic accuracy, sensitivity, specificity, and prognostic value of circRNAs with conventional biomarkers like amylase, lipase, CRP, and the BISAP/Ranson scores. Additionally, evaluating the potential of circRNAs in combination with these existing markers could improve the overall diagnostic and prognostic capabilities for AP.
6 The role of circRNAs in AP
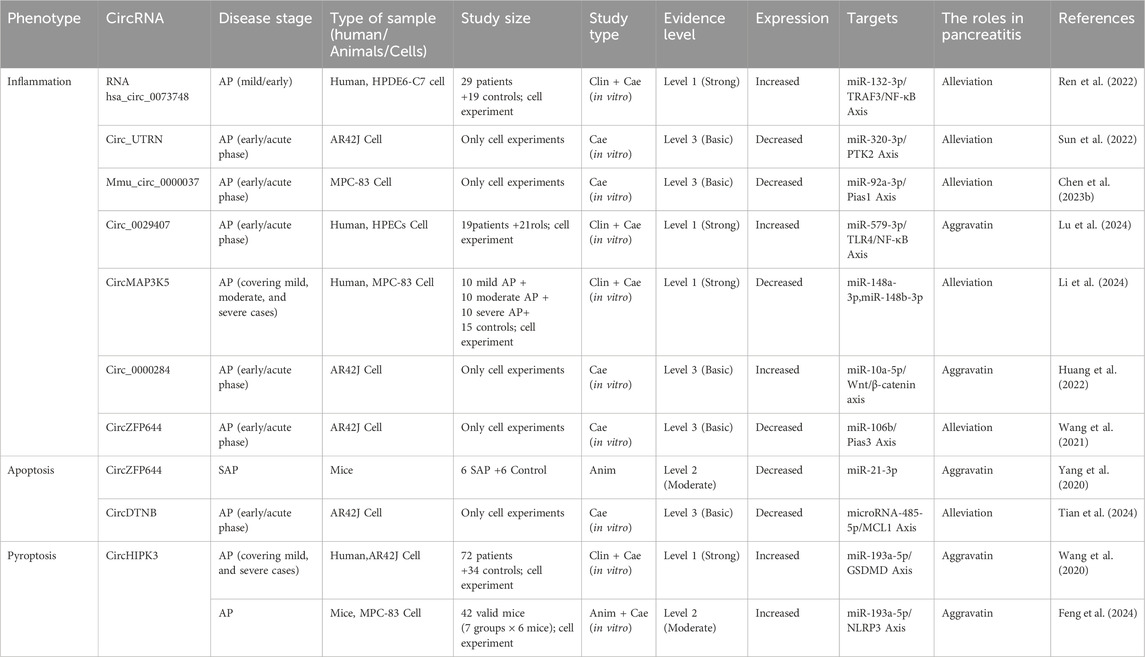
CircRNAs participate in the occurrence and progression of AP by regulating inflammatory responses, cell death, epigenetic modifications, and other critical mechanisms, with their aberrant expression intimately linked to disease progression. This article provides a comprehensive analysis of the association between circRNAs and pancreatitis, examining inflammation, apoptosis, and pyroptosis in detail (Figure 3; Table 1).
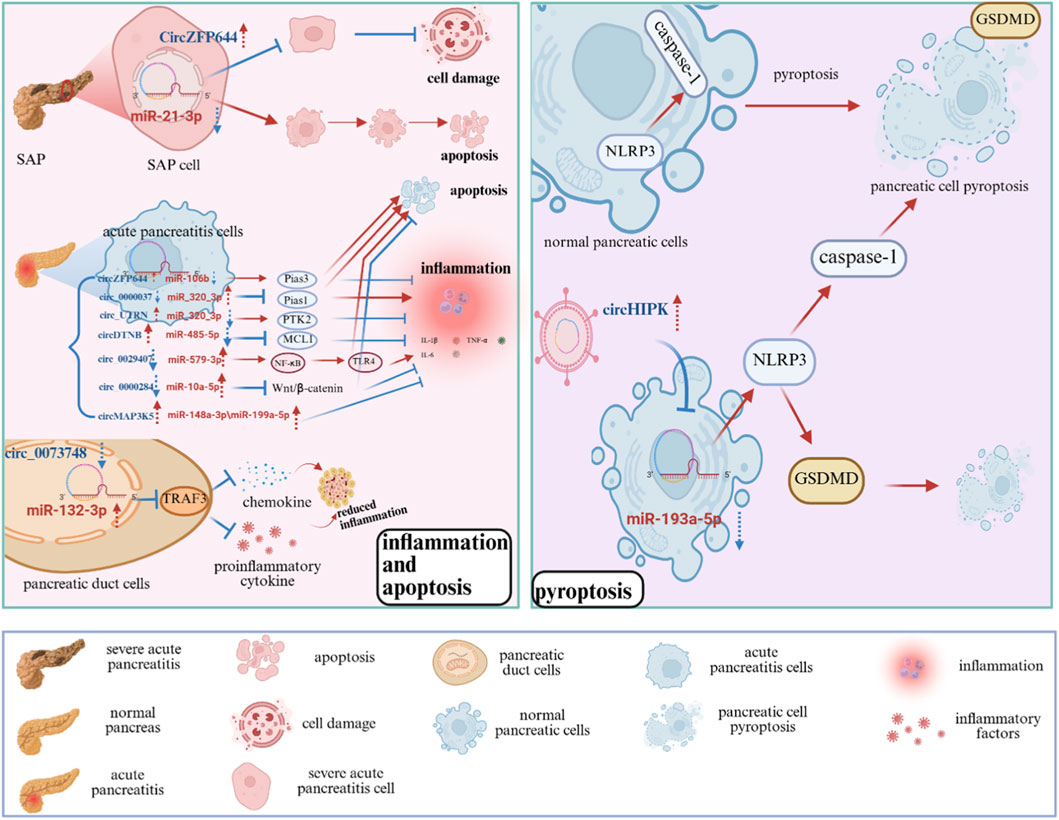
Figure 3. Potential role of circRNAs in the development and progression of pancreatitis. The role of circRNAs in AP, involving key processes such as inflammation, apoptosis, and pyroptosis, and highlighting their potential as diagnostic biomarkers and therapeutic targets. SAP: Severe Acute Pancreatitis; Pias3: Protein Inhibitor of Activated STAT 3; MCL1: Myeloid Cell Leukemia 1; NLRP3: NOD-, LRR- and pyrin domain-containing protein 3; GSDMD: Gasdermin D; TRAF3: TNF Receptor-Associated Factor 3; PTK2: Protein Tyrosine Kinase 2; TNF-α: Tumor Necrosis Factor-alpha; IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; TLR4: Toll-Like Receptor four.
6.1 Inflammation
The pathogenesis of pancreatitis mostly involves the abnormal activation of trypsinogen, which induces pancreatic cell damage, releases damage-associated molecular patterns (DAMPs), triggers the innate immune response, activates the inflammasome, and promotes the production of IL-1β (Yang et al., 2020; Kono and Rock, 2008). This additionally upregulates leukocyte adhesion molecules and facilitates neutrophil infiltration (Kubes and Mehal, 2012; Frossard et al., 1999). Additionally, pancreatitis can facilitate the interaction of microbiome-associated molecular patterns (MAMPs) with pathogen recognition receptors (PRRs) by modifying intestinal permeability, leading to the activation of NF-κB (Rakonczay et al., 2008) and fostering pancreatic inflammation (Gianotti et al., 1993; Rychter et al., 2009). These processes intensify the inflammatory response through pro-inflammatory cytokines (e.g., IL-6, IL-1β, TNF-α) (Mayer et al., 2000; Malmstrøm et al., 2012Malmstrøm et al., 2012) and chemokines (e.g., IL-8, MCP-1) (Malmstrøm et al., 2012; Regnér et al., 2008; Sakai et al., 2003; Watanabe et al., 2017).
6.1.1 Circ_0073748
Significant upregulation of circ_0073748 was detected in the plasma and caerulein-stimulated pancreatic duct cells of AP patients. Circ_0073748 acts as a molecular sponge for miR-132-3p, and regulates TRAF3 expression. TRAF3 is a member of the TNF Receptor-Associated Factor (TRAF) family (Bishop, 2016) and plays a crucial role in immune signaling. Inhibiting of circ_0073748 expression diminishes its interaction with miR-132-3p, thereby enhancing the miRNA-mediated suppression of TRAF3 and ultimately reducing the activation of the NF-κB signaling pathway, which significantly mitigates pancreatic duct cell injury, inflammation, and oxidative stress (Ren et al., 2022). While these findings are mechanistically insightful, the evidence is primarily derived from in vitro Caerulein-induced models, and clinical validation in larger patient cohorts is warranted.
6.1.2 Circ_UTRN
In pancreatic acinar cells, the overexpression of circ_UTRN acts as a molecular sponge for miR-320-3p, and alleviated the inhibition of PTK2 (Protein Tyrosine Kinase 2) by miR-320-3p, thereby enhancing the expression of PTK2 (Sun et al., 2022). Consequently, it reduces the secretion of inflammatory mediators (such as TNF-α, IL-1β, and IL-6) and suppresses the inflammatory response in pancreatic acinar cells. Thus, circ_UTRN also counteracts the apoptosis inhibition caused by caerulein and facilitates the removal of injured cells. This mechanismawaits validation in human tissues or in vivo models to confirm its translational potential.
6.1.3 Circ_0000037
In the AP model, circ_0000037 functions as a sponge for miR-92a-3p, thereby relieving miR-92a-3p-mediated suppression of its target gene PIAS1, which ultimately upregulates PIAS1 expression. PIAS1 is a crucial anti-inflammatory protein whose overexpression mitigates inflammation and cell death. In pancreatitis, the expression of circ_0000037 is downregulated, whereas miR-92a-3p is upregulated, and PIAS1 is downregulated, thereby promoting apoptosis and inflammation in pancreatic cells. Thus, circ_0000037 alleviates AP progression by regulating the miR-92a-3p/PIAS1 axis (Sun et al., 2022). While, this axis is supported by functional assays in cellular models; however, clinical correlation and direct interaction evidence are lacking.
6.1.4 circ_0029407
Li et al. discovered that circ_0029407 was markedly elevated in the serum of AP patients and in human pancreatic epithelial cells stimulated by hyaluronan. This finding provides stronger evidence by combining patient-derived data with in vitro validation. Inhibiting the expression of circ_0029407 alleviates cell damage induced by hyaluronan, thereby promoting cell proliferation, inhibiting apoptosis, and reducing the secretion of pro-inflammatory cytokines. Subsequent research has demonstrated that circ_0029407 acts as a competitive endogenous RNA (ceRNA) for miR-579-3p to modulate the TLR4/NF-κB signaling pathway,. thereby influencing the inflammatory response and the damage of pancreatic cells (Lu et al., 2024; Ma et al., 2023).
6.1.5 circMAP3K5
Research indicates that the expression level of circMAP3K5 in patients with AP is significantly downregulated and inversely correlated with disease severity. This observed correlation enhances the clinical relevance of the findings. Mechanistic studies revealed that circMAP3K5 functions as a ceRNA, primarily by sponging miRNAs such as miR-148a-3p and miR-199a-5p. Through this mechanism, circMAP3K5 overexpression alleviates the repression of target genes, leading to a marked reduction in the production of inflammatory factors such as IL-1β, IL-6, and TNF-α (Li et al., 2024). Further in vivo functional studies are warranted to establish a causal relationship.
6.1.6 circ_0000284
Circ_0000284 promotes the pathogenesis of AP by sponging miR-10a-5p, which leads to activation of the Wnt/β-catenin signaling pathway, thereby enhancing inflammation and inhibiting apoptosis. The Wnt/β-catenin pathway is a signaling cascade consisting of a collection of proteins that is crucial for embryonic development and the maintenance of adult tissue homeostasis (Liu et al., 2022). The canonical Wnt/β-catenin pathway regulates cell proliferation, survival, differentiation, and migration through the nuclear translocation of β-catenin and the activation of TCF/LEF transcription factors (Niehrs, 2012). Experiments demonstrated that circ_0000284 sponges miR-10a-5p, thereby activating the Wnt/β-catenin pathway, and exacerbating inflammation and apoptosis (Huang et al., 2022). Current evidence is based on experimental models; clinical association and pathway specificity require further investigation.
In conclusion, circRNAs are of great significance in the occurrence and development of pancreatitis. They regulate AP pathogenesis by acting as competing endogenous RNAs (ceRNAs) to modulate inflammatory responses. Protective circRNAs (e.g., circ_0073748, circ_0000037, circ_0029407, circMAP3K5) suppress inflammation via miRNA sponging: circ_0073748 upregulates TRAF3 to inhibit NF-κB, circ_0000037 elevates PIAS1 to reduce apoptosis, circ_0029407 blocks TLR4/NF-κB via miR-579-3p, and circMAP3K5 sequesters miR-148a-3p/miR-199a-5p to curb cytokine release. Conversely, pro-inflammatory circRNAs (circ_UTRN, circ_0000284) exacerbate damage: circ_UTRN promotes PTK2 expression via miR-320-3p sponging, while circ_0000284 activates Wnt/β-catenin signaling by sponging miR-10a-5p. Notably, the level of supporting evidence varies considerably across these proposed axes. Findings from patient-derived samples (e.g., circ_0029407, circMAP3K5) carry greater translational weight, whereas mechanisms solely established in Caerulein-induced models (e.g., circ_0073748, circ_UTRN, circ_0000037) require further clinical correlation and validation in more physiologically relevant systems.
Despite these insights, the field faces several limitations: the predominant reliance on caerulein-induced cell/animal models limits clinical translatability; small patient cohorts and the use of unvalidated functional assays (e.g., lack of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) validation for circ_0000037/PIAS1 interactions) compromise the robustness of the findings; and static analyses overlook dynamic ceRNA networks across disease stages. Future work should utilize patient-derived organoids, integrate multi-omics data, and develop targeted therapeutics (e.g., circRNA-specific ASOs) to bridge these gaps, thereby accelerating the translation of circRNA research into clinical applications for AP.
6.2 Apoptosis
Apoptosis regulates programmed cell death through intrinsic and extrinsic pathways involving key regulators such as Bcl-2 family proteins and caspases (Gon et al., 1996; Liu et al., 1996). Apoptosis is characterized by DNA fragmentation, vesiculation, and the release of phagocytic signals (Katsumori et al., 2011; Ravichandran, 2010), facilitating the clearance of the cell in a non-inflammatory way. Recent findings indicate that circRNAs modulate apoptotic pathways by interacting with apoptosis-related molecules, suggesting their significant involvement in pancreatitis pathogenesis. The core mechanisms of AP encompass aberrant trypsinogen activation (Aghdassi et al., 2018), dysregulated calcium signaling (Wen et al., 2015), and mitochondrial dysfunction (Biczo et al., 2018). Recent findings indicate that circRNAs may be involved in regulating the apoptosis of pancreatic acinar cells through regulating calcium homeostasis and mitochondrial activity.
6.2.1 circZFP644
MiR-21-3p is elevated in severe acute pancreatitis (SAP) and correlates with the suppression of apoptosis and the enhancement of necrosis in pancreatic cells. CircZFP644 functions as a competitive endogenous RNA (ceRNA) by binding to miR-21-3p, thereby diminishing its expressionand mitigating its inhibitory effect on apoptosis, which ultimately reduces pancreatic damage and necrosis (Yang et al., 2020). In addition, Wang et al. discovered that the expression of circZFP644 was significantly downregulated in AP models, and the overexpression of circZFP644 suppressed the secretion of inflammatory factors (TNF-α, IL-1β, IL-6), while promoting apoptosis. Mechanism investigations indicate that circZFP644 modulates Pias3 expression via targeting miR-106b, and the inhibition of miR-106b can further diminish the inflammatory response and augment apoptosis. These findings indicate that circZFP644 in AP possesses anti-inflammatory properties and facilitates apoptosis, demonstrating a dual role (Wang et al., 2021). These conclusions are drawn primarily from cell line models (e.g., AR42J); human tissue validation and in vivo functional studies are necessary to confirm pathophysiological relevance.
6.2.2 circDTNB
In AP models of the pancreatic acinar cell line AR42J, the downregulation of circDTNB was observed to exacerbate inflammation and apoptosis. Subsequent research indicated that circDTNB alleviated the suppressive effect of miR-485-5p on MCL1 (myeloid cell leukemia 1) by competitively interacting with miR-485-5p, thereby diminishing cytotoxicity and inflammatory response (Tian et al., 2024). MCL1 is a crucial anti-apoptotic protein of Bcl-2family, capable of inhibiting the pro-apoptosis proteins Bax and Bak to prevent the formation of the mitochondrial apoptosis-induced channel, hence ensuring cell survival. It is significant in B cells, T cells, and neurons, and plays a vital role in the development of hematopoietic stem cells (Thomas et al., 2010). These results indicate that circDTNB serves a protective role in AP through the miR-485-5p/MCL1 axis. This mechanism is supported by in vitro evidence but lacks correlation with human AP samples or in vivo validation.
CircRNAs intricately modulate pancreatic acinar cell apoptosis in AP through ceRNA networks targeting miRNAs linked to mitochondrial dysfunction and calcium signaling. CircZFP644 acts as a pro-apoptotic factor by sponging miR-21-3p to upregulate Pias3, enhancing caspase-dependent cell death and reducing necrosis (Yang et al., 2020; Wang et al., 2021), while circDTNB protects cells by sequestering miR-485-5p to stabilize MCL1, inhibiting Bax/Bak-mediated apoptosis (Tian et al., 2024; Thomas et al., 2010). The evidence supporting these apoptotic regulatory networks remains predominantly experimental and preclinical. The current findings, largely derived from caerulein-induced AR42J cell models and not validated in primary human acinar cells or tissues, limit their clinical generalizability.
Despite their therapeutic potential, current evidence is limited by reliance on caerulein-induced AR42J cell models and lacks direct human tissue validation, raising concerns about translational relevance. Mechanistic gaps persist, including incomplete downstream apoptosis pathway characterization (e.g., caspase activity) and unverified specificity of circRNA-miRNA interactions. Inconsistent expression patterns further challenge reproducibility. Future studies should employ human-derived organoids, multi-omics integration, and standardized apoptosis assays to enhance rigor, while therapeutically targeting circRNAs with chemically modified mimics/inhibitors may bridge bench-to-bedside translation.
6.3 Pyroptosis
Pyroptosis is a form of programmed cell death initiated by the activation of inflammasomes, such as NLRP3 (NACHT, LRR and PYD domains-containing protein 3), which activates caspase-1 and cleaves gasdermin D (GSDMD). This process intensifies tissue damage by producing inflammatory mediators such as IL-1β (Boucher et al., 2018). In AP, the NLRP3/caspase-1/GSDMD axis induces pyroptotic death and exacerbates systemic inflammation (Gao et al., 2021), whereas the inhibition of caspase-1 significantly alleviates inflammation in the AP model (Zhang et al., 2014). Recent studies have demonstrated that circRNAs not only regulate thepancreatic cell death but also influence pyroptosis by modulating inflammasome activity and key pyroptotic factors, such as caspase-1 and GSDMD.
6.3.1 circHIPK3
Research indicates that circHIPK3 facilitates pyroptosis and inflammation in pancreatic cells by disrupting the function of miR-193a-5p. Specifically, circHIPK3 functions as a molecular sponge for miR-193a-5p, resulting in reduced miRNA levels and subsequent disinhibition of GSDMD. GSDMD is a key executor of protein-mediated pyroptosis (Balasubramanian et al., 2024). Upon GSDMD activation, cells undergo pyroptosis, releasing numerous inflammatory factors (such as IL-1β, IL-6, IL-8, and TNF-α) and further amplifying the inflammatory response (Wang et al., 2020). Moreover, the molecular sponging activity of circHIPK3 alleviates the inhibitory effect of miR-193a-5p on the NLRP3 inflammasome, leading to its activation, which then promotes the activation of caspase-1 and GSDMD, thereby enhancing pyroptosis and inflammation. In conclusion, targeted inhibition of circHIPK3 can significantly diminish the inflammatory response in AP (Feng et al., 2024). The evidence for this axis is derived from mechanistic studies in cellular models; its operation in human disease contexts and potential as a biomarker or therapeutic target requires further investigation in clinical cohorts.
7 CircRNAs across the spectrum of pancreatic diseases
While the roles of circRNAs in AP are increasingly being elucidated, their functions in chronic pancreatitis (CP) and pancreatic cancer (PC) present a more complex and evolving picture. This comparative analysis aims to situate the findings in AP within the broader context of pancreatic pathologies, highlighting both conserved and divergent mechanisms.
7.1 AP vs. CP: Bridging the gap via fibrosis
A striking gap exists in the literature regarding circRNAs’ expression and function specifically in CP. Unlike AP, which is characterized by sudden inflammation and injury, CP involves persistent, fibrotic destruction of the pancreas. We propose that fibrosis pathways involving TGF-β and pancreatic stellate cell (PSC) activation are highly pertinent to CP pathogenesis. Given the established roles of circRNAs in regulating TGF-β signaling and fibrosis in other organ systems (Dai et al., 2021; Jiang and Ning, 2019), it is highly plausible that they contribute to CP pathogenesis by modulating PSC activation and extracellular matrix deposition. The absence of studies directly linking circRNAs to CP underscores a significant opportunity for future research, potentially identifying circRNAs that differentiate acute flare-ups from chronic background inflammation or that predict progression to fibrosis.
7.2 AP vs. PC: The inflammatory-oncogenic nexus
The circRNAs’ landscape in PC is extensively studied, often revealing roles in proliferation, invasion, and metastasis—processes less relevant to AP. However, intriguing parallels exist, particularly through shared inflammatory-oncogenic pathways. A key link involves the NLRP3 inflammasome and the glycolysis/PKM2 axis. For example, circHIPK3 is upregulated in both AP and PC. In AP, it promotes NLRP3 inflammasome-mediated pyroptosis and inflammation (Gao et al., 2021), whereas in PC, circERC1 inhibits pyroptosis—a pro-inflammatory cell death process—by disrupting the hnRNPA1-PKM2-NLRP3 axis, thereby promoting tumor cell survival and drug resistance (Gu et al., 2022). This suggests that circRNAs can exert context-dependent functions, promoting inflammation in AP and supporting survival in PC. This nexus between inflammation, metabolic reprogramming (glycolysis/PKM2), and cancer risk provides a fertile ground for discovering circRNAs that bridge acute injury and malignant transformation.
7.3 Conclusion and future perspectives
In summary, circRNAs in AP primarily act as mediators of rapid inflammatory response and cell death, while in PC, they are often co-opted to drive sustained growth and survival. The role in CP remains largely hypothetical but likely involves regulating fibrotic and chronic inflammatory processes. Future studies should prioritize profiling circRNAs’ expression in CP patient samples. Verifiable predictions include: (1) identifying a CP-specific circRNA signature that differentiates it from AP and PC; (2) determining whether circRNAs associated with AP resolution (e.g., those with anti-inflammatory effects) are dysregulated in CP could reveal mechanisms underlying disease chronicity. Promising candidate circRNAs for CP research include those known to regulate TGF-β signaling (e.g., circTGFBR2, circSMAD2) or macrophage polarization (e.g., circRNA_0057344) in other fibrotic diseases. Similarly, exploring if certain “pro-inflammatory” circRNAs in AP share identity with “oncogenic” circRNAs in PC could provide insights into the molecular link between inflammation and cancer, potentially identifying shared therapeutic targets for the entire pancreatic disease spectrum.
8 Summary and outlook
As an important regulator of AP, circRNAs have shown great potential as a biomarker and therapeutic target in multiple studies. For example, their inherent biophysical properties—including high abundance in body fluids, exceptional stability due to the covalently closed circular structure, and often tissue-specific expression patterns—make them particularly suitable as non-invasive diagnostic tools (Liu C. et al., 2020; Wang et al., 2020). In addition, the expression changes of these circRNAs can reflect disease progression or treatment response, offering a promising approach for monitoring therapeutic efficacy. Beyond diagnostic applications, circRNAs have also emerged as potential therapeutic targets by modulating signaling pathways associated with inflammatory responses, pyroptosis, and apoptosis (Lu et al., 2024; Wang et al., 2021). Although many findings of circRNAs’ involvement in AP regulation have been reported, most of them are preliminary observations. For example, many studies in the literature have focused on the circRNA/miRNA/mRNA axis, but the regulatory mechanisms upstream of circRNAs are lacking. Only one article reported the relationship between m6A modification of circRNAs in a mouse SAP model (Wu et al., 2021); however, the role of m6A modification in other forms of AP and its consistency between human patients and mouse models remain unexplored. Additionally, the same circRNAs can exert opposing functions when binding to different downstream factors to affect AP, but there is a lack of research on the cooperation and competition between downstream factors (Yang et al., 2020; Wang et al., 2021). Compared to fields like cancer (Hang et al., 2018; Li P. et al., 2015) or cardiovascular diseases (Ruberto and Foo, 2025)where circRNAs biogenesis regulation, specific functional mechanisms (Chen, 2020), and extensive clinical validation studies are more advanced, research on circRNAs in AP is still in its relatively early stages. Future AP research should prioritize elucidating these upstream regulatory mechanisms and resolving the context-dependent functions of individual circRNAs.
To bridge these gaps, future studies should prioritize the following: (1) Utilizing more physiologically relevant human model systems, such as patient-derived organoids or primary acinar/stellate cell cultures, to reduce reliance on caerulein-based rodent models and enhance clinical translatability. (2) Applying multi-omics approaches (epitranscriptomic, proteomic, and single-cell transcriptomic) to map upstream regulators (e.g., RNA-binding proteins like QKI or METTL3/14) that control circRNA expression and modification in AP. (3) Conducting large-scale, multi-center clinical validation studies to correlate specific circRNAs (e.g., circHIPK3, circ_0073748) with standardized clinical outcomes across diverse AP cohorts. (4) Developing circRNA-targeting therapeutics, such as CRISPR-based knockdown or nanoparticle-delivered circRNA mimics/inhibitors, and testing their efficacy in well-characterized preclinical models of AP.
At present, although there are many studies on circRNAs and AP, there are few studies on circRNAs and chronic pancreatitis (CP). CP is characterized by gland fibrosis, which is markedly different from the glandular inflammatory tissue of AP. There is still a lack of in-depth research on circRNAs and CP, and more studies are urgently needed to clarify the mechanism of action of circRNAs in chronic lesions and open up new horizons for the treatment of pancreatic disease. Future studies on circRNAs in CP could focus on their roles in pancreatic stellate cell activation (Liu et al., 2024), extracellular matrix remodeling, and persistent inflammation, drawing parallels and distinctions from circRNAs functions established in fibrosis of other organs like liver or kidney.
Further clinical validation of these findings is essential, particularly through confirmatory studies in large cohorts, in-depth mechanistic dissection of circRNA functions, and the development of circRNA-targeted therapeutics, which will be key focuses of future research (Chen R. et al., 2023). Moreover, integrating circRNAs with other molecular markers or therapeutic targets could enhance diagnostic accuracy and treatment efficacy. Leveraging advanced technologies like single-cell sequencing to map cell type-specific circRNA expression patterns during disease progression, and developing novel delivery systems for pancreatic-targeted circRNA therapeutics, represent critical future directions (Shrinivas et al., 2019). The application of single-cell sequencing and spatial transcriptomics could further resolve cell type-specific circRNA networks and interaction maps during AP progression and the transition to CP. Furthermore, systematically investigating the dynamic changes in circRNA regulatory networks across the AP-CP continuum could reveal key drivers of disease progression.
Overall, circRNAs provide a new perspective for the diagnosis and treatment of AP. With the advancement of molecular biology technology, circRNAs is expected to become an important tool in precision medicine, promoting personalized diagnosis and treatment of patients with AP, enabling earlier disease detection and more effective interventions.
Author contributions
JC: Writing – original draft. XC: Writing – review and editing. FH: Writing – review and editing, Conceptualization.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82172909).
Acknowledgements
We are thanks BioRender for Figure 1; BioRender for Figure 2, Figure 3 and graphical abstract.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aghdassi, A. A., John, D. S., Sendler, M., Weiss, F. U., Reinheckel, T., Mayerle, J., et al. (2018). Cathepsin D regulates cathepsin B activation and disease severity predominantly in inflammatory cells during experimental pancreatitis. J. Biol. Chem. 293, 1018–1029. doi:10.1074/jbc.M117.814772
Ashwal-Fluss, R., Meyer, M., Pamudurti, N. R., Ivanov, A., Bartok, O., Hanan, M., et al. (2014). circRNA biogenesis competes with Pre-mRNA splicing. Mol. Cell 56, 55–66. doi:10.1016/j.molcel.2014.08.019
Balasubramanian, A., Hsu, A. Y., Ghimire, L., Tahir, M., Devant, P., Fontana, P., et al. (2024). The palmitoylation of gasdermin D directs its membrane translocation and pore formation during pyroptosis. Sci. Immunol. 9, eadn1452. doi:10.1126/sciimmunol.adn1452
Barbagallo, D., Caponnetto, A., Cirnigliaro, M., Brex, D., Barbagallo, C., D’Angeli, F., et al. (2018). CircSMARCA5 inhibits migration of glioblastoma multiforme cells by regulating a molecular axis involving splicing factors SRSF1/SRSF3/PTB. Int. J. Mol. Sci. 19, 480. doi:10.3390/ijms19020480
Barbagallo, D., Caponnetto, A., Brex, D., Mirabella, F., Barbagallo, C., Lauretta, G., et al. (2019). CircSMARCA5 regulates VEGFA mRNA splicing and angiogenesis in glioblastoma multiforme through the binding of SRSF1. Cancers (Basel) 11, 194. doi:10.3390/cancers11020194
Barrett, S. P., Wang, P. L., and Salzman, J. (2015). Circular RNA biogenesis can proceed through an exon-containing lariat precursor. eLife 4, e07540. doi:10.7554/eLife.07540
Biczo, G., Vegh, E. T., Shalbueva, N., Mareninova, O. A., Elperin, J., Lotshaw, E., et al. (2018). Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology 154, 689–703. doi:10.1053/j.gastro.2017.10.012
Bishop, G. A. (2016). TRAF3 as a powerful and multitalented regulator of lymphocyte functions. J. Leukoc. Biol. 100, 919–926. doi:10.1189/jlb.2MR0216-063R
Boucher, D., Monteleone, M., Coll, R. C., Chen, K. W., Ross, C. M., Teo, J. L., et al. (2018). Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 215, 827–840. doi:10.1084/jem.20172222
Boxhoorn, L., Voermans, R. P., Bouwense, S. A., Bruno, M. J., Verdonk, R. C., Boermeester, M. A., et al. (2020). Acute pancreatitis. Lancet 396, 726–734. doi:10.1016/S0140-6736(20)31310-6
Chen, L.-L. (2020). The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 21, 475–490. doi:10.1038/s41580-020-0243-y
Chen, C. Y., and Sarnow, P. (1995). Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 268, 415–417. doi:10.1126/science.7536344
Chen, Q., Sun, L., and Chen, Z. J. (2016). Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 17, 1142–1149. doi:10.1038/ni.3558
Chen, N., Zhao, G., Yan, X., Lv, Z., Yin, H., Zhang, S., et al. (2018). A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 19, 218. doi:10.1186/s13059-018-1594-y
Chen, L., Kong, R., Wu, C., Wang, S., Liu, Z., Liu, S., et al. (2020). Circ-MALAT1 functions as both an mRNA translation brake and a microRNA sponge to promote self-renewal of hepatocellular cancer stem cells. Adv. Sci. (Weinh) 7, 1900949. doi:10.1002/advs.201900949
Chen, R., Wang, S. K., Belk, J. A., Amaya, L., Li, Z., Cardenas, A., et al. (2023a). Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 41, 262–272. doi:10.1038/s41587-022-01393-0
Chen, H., Tu, J., He, L., Gao, N., and Yang, W. (2023b). Mmu_circ_0000037 inhibits the progression of acute pancreatitis by miR-92a-3p/Pias1 axis. Immun. Inflamm. Dis. 11, e819. doi:10.1002/iid3.819
Conn, S. J., Pillman, K. A., Toubia, J., Conn, V. M., Salmanidis, M., Phillips, C. A., et al. (2015). The RNA binding protein quaking regulates formation of circRNAs. Cell 160, 1125–1134. doi:10.1016/j.cell.2015.02.014
Conn, V. M., Hugouvieux, V., Nayak, A., Conos, S. A., Capovilla, G., Cildir, G., et al. (2017). A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants 3, 17053. doi:10.1038/nplants.2017.53
Costa, M. C., Calderon-Dominguez, M., Mangas, A., Campuzano, O., Sarquella-Brugada, G., Ramos, M., et al. (2021). Circulating circRNA as biomarkers for dilated cardiomyopathy etiology. J. Mol. Med. Berl. 99, 1711–1725. doi:10.1007/s00109-021-02119-6
Dai, X., Cheng, Y., Wang, C., Huang, J., and Chao, J. (2021). Role of circular RNAs in visceral organ fibrosis. FOOD Chem. Toxicol. 150, 112074. doi:10.1016/j.fct.2021.112074
Di Timoteo, G., Dattilo, D., Centrón-Broco, A., Colantoni, A., Guarnacci, M., Rossi, F., et al. (2020). Modulation of circRNA metabolism by m6A modification. Cell Rep. 31, 107641. doi:10.1016/j.celrep.2020.107641
Du, W. W., Yang, W., Chen, Y., Wu, Z.-K., Foster, F. S., Yang, Z., et al. (2017a). Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 38, 1402–1412. doi:10.1093/eurheartj/ehw001
Du, W. W., Fang, L., Yang, W., Wu, N., Awan, F. M., Yang, Z., et al. (2017b). Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 24, 357–370. doi:10.1038/cdd.2016.133
Errichelli, L., Dini Modigliani, S., Laneve, P., Colantoni, A., Legnini, I., Capauto, D., et al. (2017). FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 8, 14741. doi:10.1038/ncomms14741
Feng, M., Qin, B., Luo, F., Zhu, X., Liu, K., Li, K., et al. (2024). Qingjie huagong decoction inhibits pancreatic acinar cell pyroptosis by regulating circHipk3/miR-193a-5p/NLRP3 pathway. Phytomedicine 126, 155265. doi:10.1016/j.phymed.2023.155265
Fischer, J. W., Busa, V. F., Shao, Y., and Leung, A. K. L. (2020). Structure-Mediated RNA decay by UPF1 and G3BP1. Mol. Cell 78, 70–84.e6. doi:10.1016/j.molcel.2020.01.021
Frossard, J. L., Saluja, A., Bhagat, L., Lee, H. S., Bhatia, M., Hofbauer, B., et al. (1999). The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology 116, 694–701. doi:10.1016/s0016-5085(99)70192-7
Gao, L., Dong, X., Gong, W., Huang, W., Xue, J., Zhu, Q., et al. (2021). Acinar cell NLRP3 inflammasome and gasdermin D (GSDMD) activation mediates pyroptosis and systemic inflammation in acute pancreatitis. Br. J. Pharmacol. 178, 3533–3552. doi:10.1111/bph.15499
Gianotti, L., Munda, R., Alexander, J. W., Tchervenkov, J. I., and Babcock, G. F. (1993). Bacterial translocation: a potential source for infection in acute pancreatitis. Pancreas 8, 551–558. doi:10.1097/00006676-199309000-00004
Gon, S., Gatanaga, T., and Sendo, F. (1996). Involvement of two types of TNF receptor in TNF-alpha induced neutrophil apoptosis. Microbiol. Immunol. 40, 463–465. doi:10.1111/j.1348-0421.1996.tb01095.x
Gu, H., Deng, W., Zhang, Y., Chang, Y., Shelat, V. G., Tsuchida, K., et al. (2022). NLRP3 activation in tumor-associated macrophages enhances lung metastasis of pancreatic ductal adenocarcinoma. Transl. Lung Cancer Res. 11, 858–868. doi:10.21037/tlcr-22-311
Guarnerio, J., Zhang, Y., Cheloni, G., Panella, R., Mae Katon, J., Simpson, M., et al. (2019). Intragenic antagonistic roles of protein and circRNA in tumorigenesis. Cell Res. 29, 628–640. doi:10.1038/s41422-019-0192-1
Guarnerio, J., Zhang, Y., Cheloni, G., Panella, R., Katon, J. M., Simpson, M., et al. (2020). Author correction: intragenic antagonistic roles of protein and circRNA in tumorigenesis. Cell Res. 30, 188. doi:10.1038/s41422-019-0262-4
Guda, N. M., Muddana, V., Whitcomb, D. C., Levy, P., Garg, P., Cote, G., et al. (2018). Recurrent acute pancreatitis: international state-of-the-science conference with recommendations. Pancreas 47, 653–666. doi:10.1097/MPA.0000000000001053
H, L., F, Y., A, H., X, W., E, F., Y, C., et al. (2019). Therapeutic targeting of circ-CUX1/EWSR1/MAZ axis inhibits glycolysis and neuroblastoma progression. EMBO Mol. Med. 11, e10835. doi:10.15252/emmm.201910835
Hang, D., Zhou, J., Qin, N., Zhou, W., Ma, H., Jin, G., et al. (2018). A novel plasma circular RNA circFARSA is a potential biomarker for non-small cell lung cancer. Cancer Med. 7, 2783–2791. doi:10.1002/cam4.1514
Hansen, T. B., Wiklund, E. D., Bramsen, J. B., Villadsen, S. B., Statham, A. L., Clark, S. J., et al. (2011). miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 30, 4414–4422. doi:10.1038/emboj.2011.359
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. doi:10.1038/nature11993
Huang, W., Cane, M. C., Mukherjee, R., Szatmary, P., Zhang, X., Elliott, V., et al. (2017). Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release. Gut 66, 301–313. doi:10.1136/gutjnl-2015-309363
Huang, H., Chen, W., Lu, J., Zhang, S., Xiang, X., Wang, X., et al. (2022). Circ_0000284 promoted acute pancreatitis progression through the regulation of miR-10a-5p/Wnt/β-Catenin pathway. Chem. and Biodivers. 19, e202101006. doi:10.1002/cbdv.202101006
Ivanov, A., Memczak, S., Wyler, E., Torti, F., Porath, H. T., Orejuela, M. R., et al. (2015). Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 10, 170–177. doi:10.1016/j.celrep.2014.12.019
Jeck, W. R., and Sharpless, N. E. (2014). Detecting and characterizing circular RNAs. Nat. Biotechnol. 32, 453–461. doi:10.1038/nbt.2890
Jeck, W. R., Sorrentino, J. A., Wang, K., Slevin, M. K., Burd, C. E., Liu, J., et al. (2013). Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157. doi:10.1261/rna.035667.112
Jiang, X., and Ning, Q. (2019). Circular RNAs as novel regulators, biomarkers and potential therapies in fibrosis. EPIGENOMICS 11, 1107–1116. doi:10.2217/epi-2019-0001
Jie, M., Wu, Y., Gao, M., Li, X., Liu, C., Ouyang, Q., et al. (2020). CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol. Cancer 19, 56. doi:10.1186/s12943-020-01160-2
Katsumori, S., Jun, S., and Shigekazu, N. (2011). Constitutive exposure of phosphatidylserine on viable cells. Proc. Natl. Acad. Sci. U. S. A. 108, 19246–19251. doi:10.1073/pnas.1114799108
Kelly, S., Greenman, C., Cook, P. R., and Papantonis, A. (2015). Exon skipping is correlated with exon circularization. J. Mol. Biol. 427, 2414–2417. doi:10.1016/j.jmb.2015.02.018
Kleaveland, B., Shi, C. Y., Stefano, J., and Bartel, D. P. (2018). A network of noncoding regulatory RNAs acts in the mammalian brain. Cell 174, 350–362.e17. doi:10.1016/j.cell.2018.05.022
Kono, H., and Rock, K. L. (2008). How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289. doi:10.1038/nri2215
Kristensen, L. S., Andersen, M. S., Stagsted, L. V. W., Ebbesen, K. K., Hansen, T. B., and Kjems, J. (2019). The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691. doi:10.1038/s41576-019-0158-7
Kubes, P., and Mehal, W. Z. (2012). Sterile inflammation in the liver. Gastroenterology 143, 1158–1172. doi:10.1053/j.gastro.2012.09.008
Legnini, I., Di Timoteo, G., Rossi, F., Morlando, M., Briganti, F., Sthandier, O., et al. (2017). Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37.e9. doi:10.1016/j.molcel.2017.02.017
Li, Z., Huang, C., Bao, C., Chen, L., Lin, M., Wang, X., et al. (2015a). Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 22, 256–264. doi:10.1038/nsmb.2959
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015b). Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25, 981–984. doi:10.1038/cr.2015.82
Li, P., Chen, S., Chen, H., Mo, X., Li, T., Shao, Y., et al. (2015c). Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin. Chim. Acta 444, 132–136. doi:10.1016/j.cca.2015.02.018
Li, X., Liu, C.-X., Xue, W., Zhang, Y., Jiang, S., Yin, Q.-F., et al. (2017). Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol. Cell 67, 214–227.e7. doi:10.1016/j.molcel.2017.05.023
Li, J., Sun, Z., Ning, C., Lin, C., Shen, D., Huang, G., et al. (2024). Identification and validation of disease severity-related circular RNA in acute pancreatitis. ABBS 56, 1406–1409. doi:10.3724/abbs.2024115
Liang, D., and Wilusz, J. E. (2014). Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 28, 2233–2247. doi:10.1101/gad.251926.114
Liang, D., Tatomer, D. C., Luo, Z., Wu, H., Yang, L., Chen, L.-L., et al. (2017). The output of protein-coding genes shifts to circular RNAs when the pre-mRNA processing machinery is limiting. Mol. Cell 68, 940–954.e3. doi:10.1016/j.molcel.2017.10.034
Liu, C.-X., Li, X., Nan, F., Jiang, S., Gao, X., Guo, S.-K., et al. (2019). Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell 177, 865–880.e21. doi:10.1016/j.cell.2019.03.046
Liu, C., Zhu, X., Niu, X., Chen, L., and Ge, C. (2020a). Elevated hsa_circRNA_101015, hsa_circRNA_101211, and hsa_circRNA_103470 in the human blood: novel biomarkers to early diagnose acute pancreatitis. BIOMED Res. Int. 2020, 2419163. doi:10.1155/2020/2419163
Liu, B., Ye, B., Zhu, X., Yang, L., Li, H., Liu, N., et al. (2020b). An inducible circular RNA circKcnt2 inhibits ILC3 activation to facilitate colitis resolution. Nat. Commun. 11, 4076. doi:10.1038/s41467-020-17944-5
Liu, J., Xiao, Q., Xiao, J., Niu, C., Li, Y., Zhang, X., et al. (2022). Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Sig Transduct. Target Ther. 7, 3–23. doi:10.1038/s41392-021-00762-6
Liu, B., Li, C., Bo, Y., Tian, G., Yang, L., Si, J., et al. (2024). Let-7f-5p regulated by Hsa_circ_0000437 ameliorates bleomycin-induced skin fibrosis. J. Cell Biochem. 125, e30629. doi:10.1002/jcb.30629
Liu, X., Kim, C. N., and Yang, L. (1996). Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157. doi:10.1016/s0092-8674(00)80085-9
Liu, X., Wang, Q., Li, X., Yang, Y., Deng, Y., Wang, X., et al. (2025). Fast degradation of MecciRNAs by SUPV3L1/ELAC2 provides a novel opportunity to tackle heart failure with exogenous MecciRNA. Circulation 151, 1272–1290. doi:10.1161/CIRCULATIONAHA.124.070840
Lu, X., Shi, C., and Fan, C. (2024). Involvement of circ_0029407 in caerulein-evoked cytotoxicity in human pancreatic cells via the miR-579-3p/TLR4/NF-κB pathway. Mol. Biotechnol. 67, 1978–1990. doi:10.1007/s12033-024-01175-w
Ma, H., Xu, Y., Zhang, R., Guo, B., Zhang, S., and Zhang, X. (2019). Differential expression study of circular RNAs in exosomes from serum and urine in patients with idiopathic membranous nephropathy. Arch. Med. Sci. 15, 738–753. doi:10.5114/aoms.2019.84690
Ma, W., Wang, C., and Xiao, J. (2023). A testlet diagnostic classification model with attribute hierarchies. Appl. Psychol. Meas. 47, 183–199. doi:10.1177/01466216231165315
Macejak, D. G., and Sarnow, P. (1991). Internal initiation of translation mediated by the 5’ leader of a cellular mRNA. Nature 353, 90–94. doi:10.1038/353090a0
Malmstrøm, M. L., Hansen, M. B., Andersen, A. M., Ersbøll, A. K., Nielsen, O. H., Jørgensen, L. N., et al. (2012). Cytokines and organ failure in acute pancreatitis: inflammatory response in acute pancreatitis. Pancreas 41, 271–277. doi:10.1097/MPA.0b013e3182240552
Mayer, J., Rau, B., Gansauge, F., and Beger, H. G. (2000). Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut 47, 546–552. doi:10.1136/gut.47.4.546
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. doi:10.1038/nature11928
Mounzer, R., Langmead, C. J., Wu, B. U., Evans, A. C., Bishehsari, F., Muddana, V., et al. (2012). Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 142, 1476–e16. doi:10.1053/j.gastro.2012.03.005
Niehrs, C. (2012). The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779. doi:10.1038/nrm3470
Panda, A. C. (2018). Circular RNAs Act as miRNA sponges. Adv. Exp. Med. Biol. 1087, 67–79. doi:10.1007/978-981-13-1426-1_6
Park, O. H., Ha, H., Lee, Y., Boo, S. H., Kwon, D. H., Song, H. K., et al. (2019). Endoribonucleolytic cleavage of m6A-Containing RNAs by RNase P/MRP complex. Mol. Cell 74, 494–507.e8. doi:10.1016/j.molcel.2019.02.034
Petrov, M. S., and Yadav, D. (2019). Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 175–184. doi:10.1038/s41575-018-0087-5
Plasma Circular RNAs (2016). Hsa_circRNA_025016, predict postoperative atrial fibrillation after isolated off-pump coronary artery bypass grafting - PMC n.d. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5850143/(Accessed June 2, 2025).
PMC (2025). Circular RNAs as potential biomarkers for cancer diagnosis and therapy - PMC n.d. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4937728/(Accessed March 3, 2025).
Rakonczay, Z., Hegyi, P., Takács, T., McCarroll, J., and Saluja, A. K. (2008). The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut 57, 259–267. doi:10.1136/gut.2007.124115
Ravichandran, K. S. (2010). Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J. Exp. Med. 207, 1807–1817. doi:10.1084/jem.20101157
Regnér, S., Appelros, S., Hjalmarsson, C., Manjer, J., Sadic, J., and Borgstrom, A. (2008). Monocyte chemoattractant protein 1, active carboxypeptidase B and CAPAP at hospital admission are predictive markers for severe acute pancreatitis. Pancreatology 8, 42–49. doi:10.1159/000114866
Ren, S., Pan, L., Yang, L., Niu, Z., Wang, L., Gao, Y., et al. (2022). Interfering hsa_circ_0073748 alleviates caerulein-induced ductal cell injury in acute pancreatitis by inhibiting miR-132-3p/TRAF3/NF-κB pathway. Cell Cycle 21, 172–186. doi:10.1080/15384101.2021.2014653
Ries, R. J., Zaccara, S., Klein, P., Olarerin-George, A., Namkoong, S., Pickering, B. F., et al. (2019). m6A enhances the phase separation potential of mRNA. Nature 571, 424–428. doi:10.1038/s41586-019-1374-1
Rm, B., Md, S., Je, J., Cg, E., J, S.-V., M, L.-R., et al. (2016). “International symposium on intensive care and emergency medicine: brussels, Belgium,”, 20. London, England: Critical Care. doi:10.1186/s13054-016-1208-6
Ruberto, F., and Foo, R. (2025). Atrial fibrillation: a measure from one circular RNA. Cardiovasc Res. 121, 974–976. doi:10.1093/cvr/cvaf083
Rychter, J. W., van Minnen, L. P., Verheem, A., Timmerman, H. M., Rijkers, G. T., Schipper, M. E. I., et al. (2009). Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery 145, 157–167. doi:10.1016/j.surg.2008.09.011
Sakai, Y., Masamune, A., Satoh, A., Nishihira, J., Yamagiwa, T., and Shimosegawa, T. (2003). Macrophage migration inhibitory factor is a critical mediator of severe acute pancreatitis. Gastroenterology 124, 725–736. doi:10.1053/gast.2003.50099
Salzman, J., Chen, R. E., Olsen, M. N., Wang, P. L., and Brown, P. O. (2013). Cell-Type specific features of circular RNA expression. PLOS Genet. 9, e1003777. doi:10.1371/journal.pgen.1003777
Sendler, M., Dummer, A., Weiss, F. U., Krüger, B., Wartmann, T., Scharffetter-Kochanek, K., et al. (2013). Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut 62, 430–439. doi:10.1136/gutjnl-2011-300771
Sendler, M., van den Brandt, C., Glaubitz, J., Wilden, A., Golchert, J., Weiss, F. U., et al. (2020). NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology 158, 253–269.e14. doi:10.1053/j.gastro.2019.09.040
Shrinivas, K., Sabari, B. R., Coffey, E. L., Klein, I. A., Boija, A., Zamudio, A. V., et al. (2019). Enhancer features that drive Formation of transcriptional condensates. Mol. Cell 75, 549–561.e7. doi:10.1016/j.molcel.2019.07.009
Shulman, Z., and Stern-Ginossar, N. (2020). The RNA modification N6-methyladenosine as a novel regulator of the immune system. Nat. Immunol. 21, 501–512. doi:10.1038/s41590-020-0650-4
Sun, Q., Liang, R., Li, M., and Zhou, H. (2022). Circ_UTRN ameliorates caerulein-induced acute pancreatitis in vitro via reducing inflammation and promoting apoptosis through miR-320-3p/PTK2 axis. J. Pharm. Pharmacol. 74, 861–868. doi:10.1093/jpp/rgab161
Sutton, R. (2020). Parenchymal pressure injury Ca2+ entry mechanism in pancreatitis. Cell Calcium 88, 102208. doi:10.1016/j.ceca.2020.102208
Thomas, L. W., Lam, C., and Edwards, S. W. (2010). Mcl-1; the molecular regulation of protein function. FEBS Lett. 584, 2981–2989. doi:10.1016/j.febslet.2010.05.061
Thomson, D. W., and Dinger, M. E. (2016). Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 17, 272–283. doi:10.1038/nrg.2016.20
Tian, X., Zhang, Y., Peng, M., and Hou, Y. (2024). Regulatory axis of circular RNA DTNB, microRNA-485-5p, and myeloid cell leukemia 1 attenuates inflammation and apoptosis in caerulein-treated AR42J cells. Funct. Integr. Genomics 24, 140. doi:10.1007/s10142-024-01411-1
van Niel, G., D’Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. doi:10.1038/nrm.2017.125
Vausort, M., Salgado-Somoza, A., Zhang, L., Leszek, P., Scholz, M., Teren, A., et al. (2016). Myocardial infarction-associated circular RNA predicting left ventricular dysfunction. J. Am. Coll. Cardiol. 68, 1247–1248. doi:10.1016/j.jacc.2016.06.040
Verduci, L., Ferraiuolo, M., Sacconi, A., Ganci, F., Vitale, J., Colombo, T., et al. (2017). The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol. 18, 237. doi:10.1186/s13059-017-1368-y
Wang, S., Zhang, Y., Cai, Q., Ma, M., Jin, L. Y., Weng, M., et al. (2019a). Circular RNA FOXP1 promotes tumor progression and Warburg effect in gallbladder cancer by regulating PKLR expression. Mol. Cancer 18, 145. doi:10.1186/s12943-019-1078-z
Wang, Y., Liu, J., Ma, J., Sun, T., Zhou, Q., Wang, W., et al. (2019b). Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol. Cancer 18, 116. doi:10.1186/s12943-019-1041-z
Wang, J., Li, X., Liu, Y., Peng, C., Zhu, H., Tu, G., et al. (2020). CircHIPK3 promotes pyroptosis in Acinar cells through regulation of the miR-193a-5p/GSDMD axis. Front. Med. 7, 88. doi:10.3389/fmed.2020.00088
Wang, J., Fu, J., Xu, C., Jia, R., Zhang, X., and Zhao, S. (2021). Circ_ZFP644 attenuates caerulein-induced inflammatory injury in rat pancreatic acinar cells by modulating miR-106b/Pias3 axis. Exp. Mol. PATHOLOGY 121, 104644. doi:10.1016/j.yexmp.2021.104644
Watanabe, T., Kudo, M., and Strober, W. (2017). Immunopathogenesis of pancreatitis. Mucosal Immunol. 10, 283–298. doi:10.1038/mi.2016.101
Wen, L., Voronina, S., Javed, M. A., Awais, M., Szatmary, P., Latawiec, D., et al. (2015). Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology 149, 481–92.e7. doi:10.1053/j.gastro.2015.04.015
Wu, Z., Sun, H., Wang, C., Liu, W., Liu, M., Zhu, Y., et al. (2020). Mitochondrial genome-derived circRNA mc-COX2 functions as an oncogene in chronic lymphocytic leukemia. Mol. Ther. Nucleic Acids 20, 801–811. doi:10.1016/j.omtn.2020.04.017
Wu, J., Yuan, X.-H., Jiang, W., Lu, Y.-C., Huang, Q.-L., Yang, Y., et al. (2021). Genome-wide map of N6-methyladenosine circular RNAs identified in mice model of severe acute pancreatitis. World J. Gastroenterology 27, 7530–7545. doi:10.3748/wjg.v27.i43.7530
Wu, R., Huang, S., Xie, J.-F., Wen, N.-L., Wen, M., and Zhong, S.-E. (2023). CircRNA SCAR improves high-glucose-induced mitochondrial dysfunction and permeability damage in retinal microvascular endothelial cells. Horm. Metab. Res. 55, 555–562. doi:10.1055/a-2108-9820
Xueting, H., Duoguang, W., and Xiaotian, H. (2019). circGSK3β promotes metastasis in esophageal squamous cell carcinoma by augmenting β-catenin signaling. Mol. Cancer 18, 160. doi:10.1186/s12943-019-1095-y
Xia, P., Wang, S., Ye, B., Du, Y., Li, C., Xiong, Z., et al. (2018). A Circular RNA protects dormant hematopoietic stem cells from DNA sensor cGAS-Mediated exhaustion. Immunity 48, 688–701.e7. doi:10.1016/j.immuni.2018.03.016
Yang, Q., Du, W. W., Wu, N., Yang, W., Awan, F. M., Fang, L., et al. (2017a). A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell Death Differ. 24, 1609–1620. doi:10.1038/cdd.2017.86
Yang, Z.-G., Awan, F. M., Du, W. W., Zeng, Y., Lyu, J., Wu, D., et al. (2017b). The Circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol. Ther. 25, 2062–2074. doi:10.1016/j.ymthe.2017.05.022
Yang, Y., Fan, X., Mao, M., Song, X., Wu, P., Zhang, Y., et al. (2017c). Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27, 626–641. doi:10.1038/cr.2017.31
Yang, Y., Ren, J., Huang, Q., Wu, J., Yuan, X., Jiang, W., et al. (2020). CircRNA expression profiles and the potential role of CircZFP644 in mice with severe acute pancreatitis via sponging miR-21-3p. Front. Genet. 11, 206. doi:10.3389/fgene.2020.00206
Yu, C.-Y., Li, T.-C., Wu, Y.-Y., Yeh, C.-H., Chiang, W., Chuang, C.-Y., et al. (2017). The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency. Nat. Commun. 8, 1149. doi:10.1038/s41467-017-01216-w
Zhang, Y., Zhang, X.-O., Chen, T., Xiang, J.-F., Yin, Q.-F., Xing, Y.-H., et al. (2013). Circular intronic long noncoding RNAs. Mol. Cell 51, 792–806. doi:10.1016/j.molcel.2013.08.017
Zhang, X.-H., Li, M.-L., Wang, B., Guo, M.-X., and Zhu, R.-M. (2014). Caspase-1 inhibition alleviates acute renal injury in rats with severe acute pancreatitis. World J. Gastroenterol. 20, 10457–10463. doi:10.3748/wjg.v20.i30.10457
Zhang, Y., Xue, W., Li, X., Zhang, J., Chen, S., Zhang, J.-L., et al. (2016). The biogenesis of nascent circular RNAs. Cell Rep. 15, 611–624. doi:10.1016/j.celrep.2016.03.058
Zhang, L., Hou, C., Chen, C., Guo, Y., Yuan, W., Yin, D., et al. (2020). The role of N6-methyladenosine (m6A) modification in the regulation of circRNAs. Mol. Cancer 19, 105–111. doi:10.1186/s12943-020-01224-3
Zhao, Q., Liu, J., Deng, H., Ma, R., Liao, J.-Y., Liang, H., et al. (2020). Targeting mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell 183, 76–93.e22. doi:10.1016/j.cell.2020.08.009
Zheng, Q., Bao, C., Guo, W., Li, S., Chen, J., Chen, B., et al. (2016). Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 7, 11215. doi:10.1038/ncomms11215
Zhou, C., Molinie, B., Daneshvar, K., Pondick, J. V., Wang, J., Wittenberghe, N. V., et al. (2017). Genome-Wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 20, 2262–2276. doi:10.1016/j.celrep.2017.08.027
Keywords: CircRNAs, acute pancreatitis, biomarker, therapeutic tool, chronic pancreaititis
Citation: Chen J, Chen X and He F (2025) Circular RNAs at the crossroads of acute pancreatitis: bridging molecular mechanisms to diagnostic and therapeutic innovations. Front. Genet. 16:1650363. doi: 10.3389/fgene.2025.1650363
Received: 20 June 2025; Accepted: 10 November 2025;
Published: 24 November 2025.
Edited by:
Nicoletta Potenza, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Liang Chen, University of Science and Technology of China, ChinaZeyu Wu, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2025 Chen, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: XiaoLing Chen, Y2hlbnhsMjAyMzA0QDE2My5jb20=; Fei He, aGVmZWloaHNoQHhqdHUuZWR1LmNu
 Jing Chen
Jing Chen XiaoLing Chen
XiaoLing Chen Fei He1,2,3*
Fei He1,2,3*