- Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh, India
The World Health Organization (WHO) and American Psychiatric Association (APA) have recognised premenstrual dysphoric disorder (PMDD) as an independent diagnostic entity, legitimising the distress and socio-occupational impairment experienced by affected women. However, the biological validity of this diagnosis remains inexplicit. This illness has also been criticised for a feminist-led, sympathetic reaction to the modern cultural challenges of urban, literate, employed, high-functioning women. This article systematically reviews existing literature on PMDD using the criteria established by Robins and Guze for the validity of a psychiatric diagnosis (clinical description, laboratory study, exclusion of other disorders, follow-up study, and family study). Despite the early recognition of premenstrual syndrome (PMS) in the 1950s, the research has encountered challenges due to two groups of proponents viewing it with psychologising bias and medicalising bias. PMDD is currently understood as the most severe form of PMS, characterised by the presence of psychological features. Recent evidence suggests that PMDD perhaps has neurodevelopmental underpinnings (attention deficit hyperactive disorder, adverse childhood experiences) affecting the fronto-limbic circuit that regulates the emotions. In addition, the affected individuals exhibit an increased sensitivity to gonadal hormonal fluctuations as observed during premenstrual, pregnancy, and perimenopausal phases of life. The prevalence is comparable between high-income countries and low- and middle-income countries (LAMIC), refuting the notion that it mostly affects modern women. Instead, a greater prevalence is observed in LAMIC. Despite the fact that educated women possess knowledge regarding the importance of getting help, there is a prevalent issue of inadequate help-seeking behaviour. This can be attributed to the perception of seeking help as an isolating experience, which is influenced by profound internalised stigma and discrimination in the workplace. Future studies must aim to develop culturally validated assessment tools and more research to understand the life course of the illness, in addition to systematically examining for more biological validators (animal models, genetics, imaging, neurotransmitters).
1. Introduction
The somatic, affective, and cognitive symptoms during the luteal stage of the menstrual cycle are commonly known as premenstrual symptoms. These symptoms are on a spectrum of mild-to-moderate severity that is often culturally normalised. The severe form with greater regularity interfering with daily life is defined as premenstrual syndrome (PMS). The predominant and extreme psychological form of PMS is conceptualised as premenstrual dysmorphic disorder (PMDD). In the latest Diagnostic and Statistical Manual for mental disorders—fifth edition text revision (DSM)–5-TR, PMDD is diagnosed when “a patient, in most of her menstrual cycles during the past one year, has at least five symptoms such as affective lability, irritability, depressed mood, anxiety (at least one of these four), loss of interest, fatigue, feeling emotionally overwhelmed, and physical symptoms” (1). These symptoms must be present a week before the onset of menstrual flow and improve within a few days after, following a cyclical pattern from menarche to menopause. The symptoms must occur only during the luteal phase in most cycles during the last 1 year and include a cluster of affective, somatic, and cognitive symptoms causing significant distress, interfering with work, school, or usual social activities, and lower quality of life. These disorders are treatable—selective serotonin reuptake inhibitors (SSRIs) such as sertraline, paroxetine, fluoxetine, and escitalopram have been shown to treat both the psychiatric as well as physical symptoms (2); other medications that have shown benefit include quetiapine (3) (as an adjunct to an SSRI), oral contraceptives (4), and calcium supplementation (5). Among non-pharmacological treatments, evidence suggests that cognitive behaviour therapy may be helpful (6). In a study by Hylan et al. (7), it was estimated that women have approximately 481 menstrual cycles during their lifespan, and women with PMDD have approximately 6.4 days of severe symptoms during each menstrual cycle, spending over 3,000 days in the premenstrual phase. However, studies have found widely variable prevalence rates for PMS (8, 9) (∼4%–80%) and PMDD (10, 11) (up to 10%). Several etio-pathological theories have been proposed and found inconclusive. Given its self-remitting and cyclical nature, the efficacy of medical and psychological interventions is contentious. The validity of PMDD stands arguable among clinicians and researchers alike since the 1980s. This review used the gold standard Robins and Guze's (12) five phases of validating a psychiatric diagnosis (clinical description, laboratory study, exclusion of other disorders, follow-up study, and family study) to critically examine the published literature on PMDD for identifying important knowledge gaps and setting the research agenda to enhance the understanding of the prevalence and associated biopsychosocial factors through a neurodevelopmental lens.
1.1. Clinical description
Ancient medical literature described menstrually related physical and psychological problems approximately 4 millennia ago. Interestingly, proponents of either school of physical symptoms-predominant or psychological neurosis were dogmatically leading to biases.
1.1.1. Psychologising bias
Kahun Gynaecological Papyrus (c.1800 BC) illustrated menstrual-related symptoms such as musculoskeletal aches, discomfort, and menorrhagia and attributed them to the females having a “womb” (13). The womb was ascribed to physical symptoms until Thomas Sydenham proposed “emotional experiences” in women as “hysteria” (suffocation of the womb) in the 17th century (13). In the late 19th century, Sigmund Freud hypothesised hysteria as a neurotic clinical entity. During this period, there was a prevailing notion that all women were pathologically emotional, causing discrimination, marginalisation, and devoid of electoral rights, among many violations (14).
1.1.2. Medicalising bias
The discovery of female sex hormones in the 1950s paved the way for scientific understanding of premenstrual nervous tension. Greene and Dalton (15) studied physical symptoms and renamed it PMS. During the 14 days of the luteal phase, the progesterone levels supersede the oestrogen levels. Progesterone provides negative feedback to the anterior pituitary, initially causing a sharp fall in the levels of follicle-stimulating hormone (FSH) and luteinising hormone (LH) in the late luteal phase. At this stage, the corpus luteum regresses, leading to a sharp decrease in its production of 17-beta-oestradiol and progesterone. The rapid changes in the progesterone levels during the luteal phase of the menstrual cycle have an impact on serotonin and may result in premenstrual symptoms, despite the presence of normal ovarian function (16). Owing to this theory, it was formerly termed late luteal phase dysphoric disorder (LLPDD) and included in the Appendix A (proposed diagnostic category for further study) of DSM-IIIR in 1987 (17). Medicalising PMDD geared the research towards investigating the efficacy of progesterone and its congeners; however, these interventions have been found to be ineffective and overlooked the potential role of psychotropic and psychological interventions for a long time.
The guidelines on PMS provided by the Royal College of Obstetricians and Gynaecologists (RCOG 2016) urged the integration of biological and psychological constructs to define the illness characteristics without unintentionally pathologising the menstrual cycle or stigmatising an entire gender (18).
1.1.3. Diagnostic guidelines
In DSM-IV, LLPD disorder was renamed “premenstrual dysphoric disorder” (PMDD) due to an empirical evidence indicating premenstrual onset and early follicular phase offset in the menstrual cycle and included it in Appendix III (diagnosis for further study) of DSM-IV (19). The DSM-IV work group recommended the prospective use of standardised rating instruments to determine the true prevalence of PMDD (19). The work group proposed that incorporating prospective daily ratings could improve the accuracy of diagnosis by confirming the specific timing of symptom onset and offset in relation to the menstrual phase. This approach would also help prevent the inappropriate inclusion of women experiencing milder symptoms or premenstrual worsening of existing affective disorders (19).
The American College of Obstetricians and Gynaecologists (ACOG) requires the presence of at least one affective symptom (e.g., anger outbursts, anxiety, confusion, depression, irritability, or social withdrawal) and one somatic symptom (e.g., abdominal bloating, breast tenderness or swelling, headache, joint or muscle pain, swelling of extremities, or weight gain) for a diagnosis of PMS. In contrast, the DSM-IV criteria require only the presence of somatic symptoms.
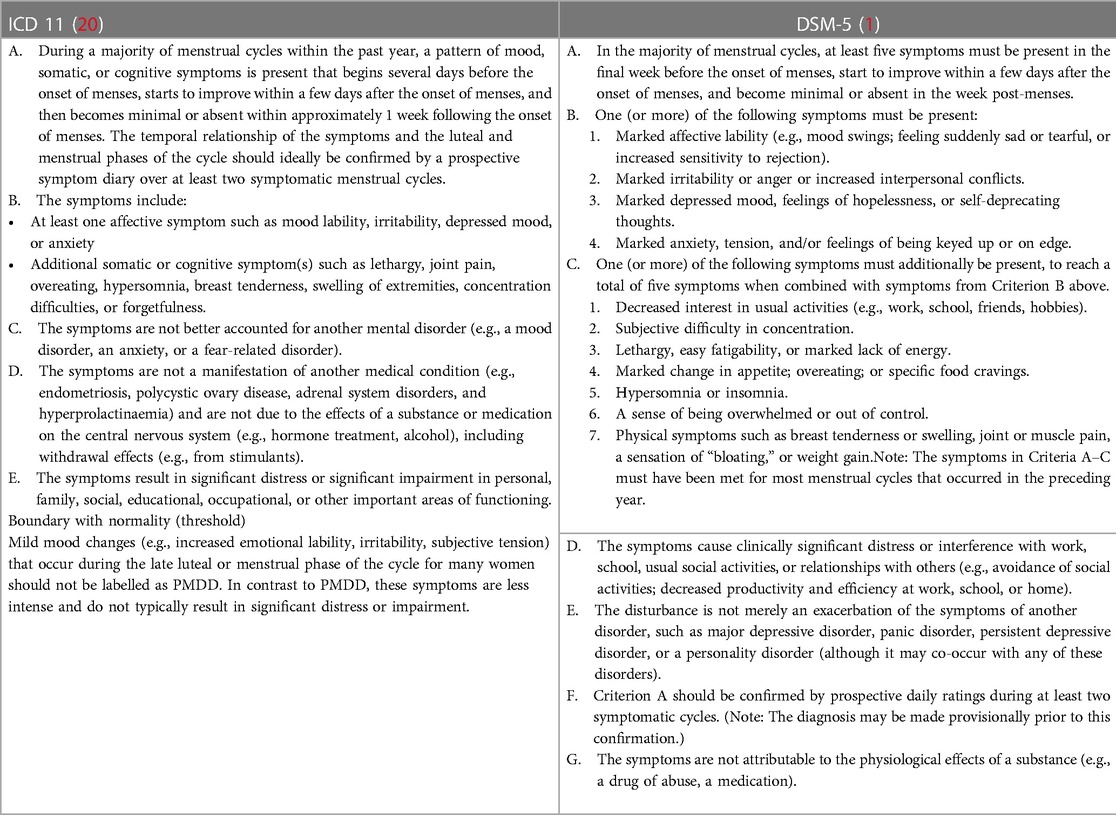
After DSM-5 recognised PMDD as an independent diagnosis, the World Health Organization (WHO) added it to the International Statistical Classification of Diseases and Related Health Problems, Eleventh Revision (ICD-11) with code GA34.41, under diseases of the genitourinary system (20). PMDD is cross-listed in the sub-grouping of depressive disorders due to the prominence of mood symptomatology. Given the debilitating nature of this illness, both traditional classificatory systems (DSM and ICD) have designated it as an independent diagnostic entity (Table 1).
1.1.4. Assessment tools
The screening tools for premenstrual symptoms, including both adult and adolescent versions, are widely used in clinical practice (21, 22). The structured clinical interview for DSM-IV-TR PMDD (SCID-PMDD) is a diagnostic interview schedule developed in 2013 (23), which includes five scales (24–28) for self-monitoring of PMDD during the prospective daily ratings over at least two menstrual cycles. Among them, the Daily Record of Severity of Problems (DRSP) based on the DSM-IV criteria for PMDD is the most commonly used (24). Carolina Premenstrual Assessment Scoring System (C-PASS) is based on the DSM-5 criteria for PMDD in four diagnostic dimensions (symptoms, severity, cyclicity, and chronicity). The C-PASS assessment tool is sensitive to predict sub-threshold PMDD, i.e., women with a menstrual-related mood disorder (MRMD) who experience distress and impairment sufficient to warrant treatment but do not meet the full DSM-5 criteria for PMDD (28). They have high internal consistency of 0.8–0.9 (Table 2). The ICD-11 provided guidance on establishing the boundary with normality for the exclusion of mild premenstrual mood changes; nevertheless, culturally-adapted and standardised tools are yet to be developed.
1.1.5. Prevalence of PMDD
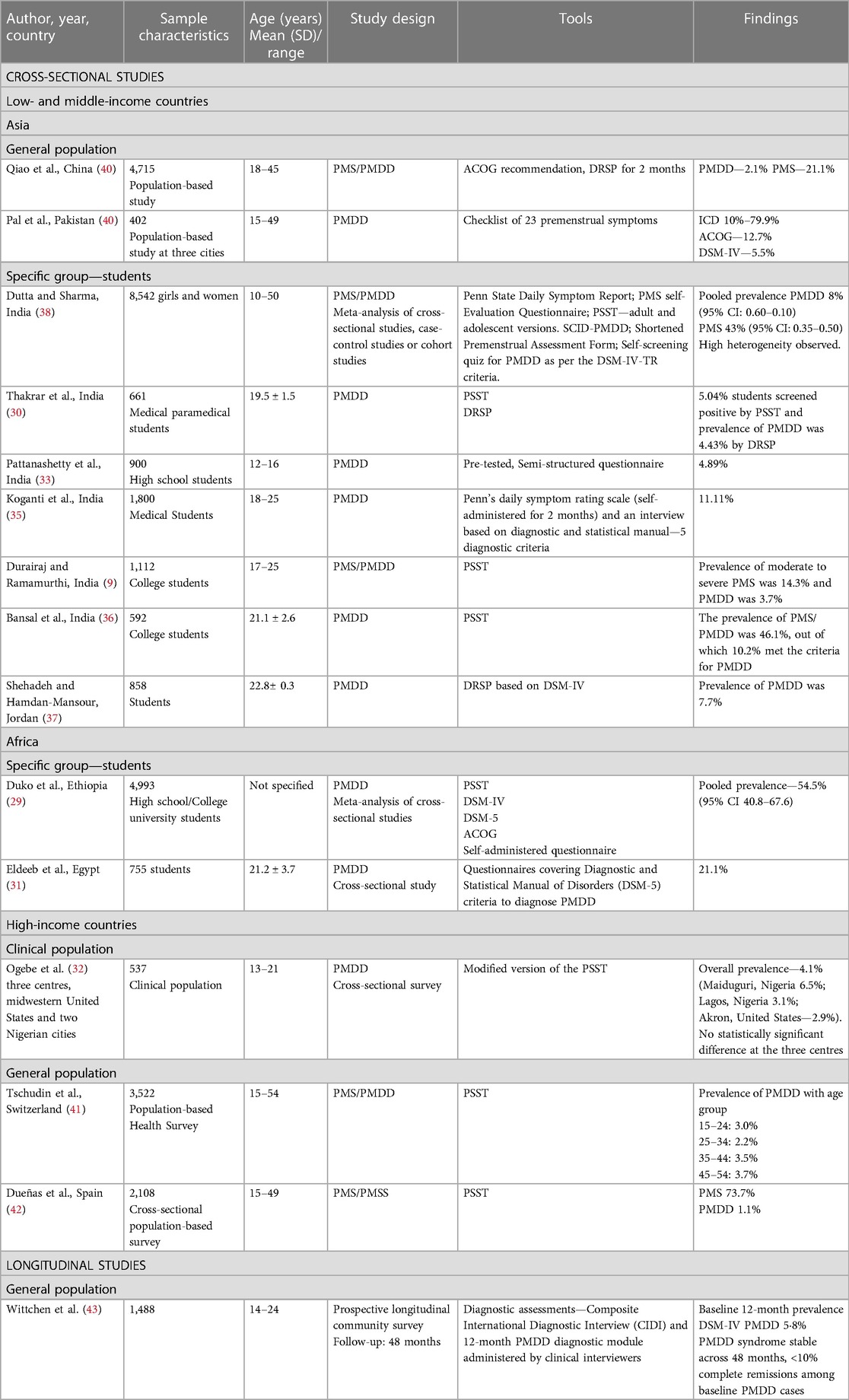
The majority of global prevalence studies have predominantly focused on PMS since the 1950s. There has been a limited number of country-wide prevalence studies conducted on PMDD in the last two decades only, and most of these studies are cross-sectional observations (Table 3). Ten out of 14 cross-sectional studies are conducted among adolescents and young women (9, 29–37), and only five studies examined the prevalence in middle-aged women (38–42). A cross-sectional study found a prevalence rate ranging from 2.2% to 3.7% across all reproductive-age groups and a greater incidence rate in women of age 45–54 years, indicating that PMDD is a disorder spanning from menarche to menopause (41).
There is a prevailing belief that PMDD is a disorder mostly observed in developed, high-income countries and criticised as a cultural syndrome of urbanisation. Contrary to that, Ogebe et al. (32) noticed greater reports of PMDD in two Nigerian cities as opposed to the United States. Studies conducted in high-income countries reported the prevalence rate of PMDD to vary from 3% to 4.1%, while the prevalence rate of PMDD in low- and middle-income countries (LAMIC) ranged from 3.7% to 11%. The greater prevalence observed in LAMIC indicates the need to thoroughly examine the socio-economic determinants of PMDD, such as literacy, economic decline, migration, public health policies, and laws protecting women against violence and discrimination.
In the general population, the prevalence rate of PMDD ranges from 2.1% to 79.9%, depending on the assessment tools (39, 40). In a specific group (student population), it was found that the prevalence rate ranges from 3.7% to 10.2% (9, 36), while in the clinical population, it ranges from 2.9% to 4.1% (32).
1.2. Laboratory studies
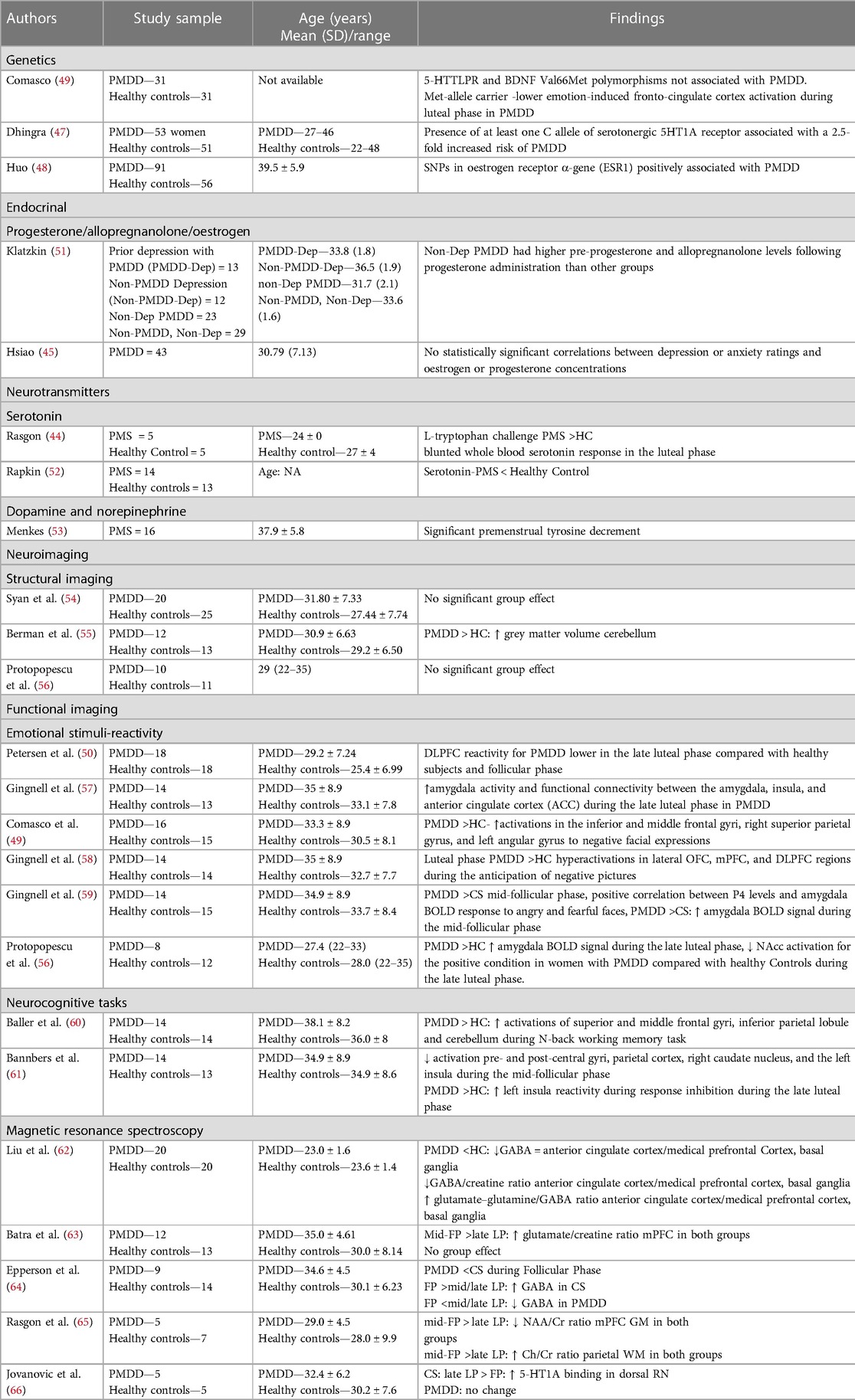
Neurotransmitter levels and hormonal changes are extensively studied in PMS but are limited for PMDD. Tryptophan challenge and tyrosine depletion tests concluded lower serotonin, dopamine, and norepinephrine levels during the luteal phase (44). However, the severity of PMS was correlated with the depletion of tryptophan only, implicating a hypo-serotonergic theory and the potential therapeutic role of SSRIs in PMS and PMDD. PMDD symptoms were not associated with oestrogen and progesterone levels (45). Allopregnanolone is a neurosteroid and an anxiolytic metabolite of progesterone that acts at the GABA-A receptor. Lower baseline allopregnanolone and a more marked increase in allopregnanolone levels were reported in women with PMDD after administering progesterone. However, no similar changes were observed in women with depression or in healthy subjects. The rapid efficacy of SSRIs in PMDD has been attributed to their ability to increase allopregnanolone levels in the brain, enhancing GABA-A receptor function and alleviating anxiety (46). Single nucleotide polymorphisms (SNPs) in the serotonergic 5HT1A receptor (47) and oestrogen receptor α-gene (ESR1) (48) have been found to be associated with PMDD. Met-allele carriers of brain-derived neurotropic factor (BDNF) (Val66Met SNP) had shown impaired fronto-cingulate cortex activation during the luteal phase (49).
There are no major structural brain changes in women with PMDD (Table 4). Functional imaging studies have reported increased amygdalar activity in the limbic region and decreased activity in prefrontal cortical structures, such as the anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), and dorsolateral prefrontal cortex (DLPFC), more pronounced during the late luteal phase. These findings are inconsistent. The reversal of hypo-reactivity in the DLPFC during the follicular phase implies that the prefrontal hypoactivity is transitory and excessive during the late luteal phase, which requires replication studies (50). White matter integrity has not yet been studied. Although patients with PMDD have higher cerebellar grey matter volume and metabolism as well as altered serotonergic and GABAergic neurotransmission, it is better distinguished by differentiating amygdalar and fronto-cortical function in response to emotional stimuli. There is a need for further structural, chemical, and functional brain signatures in order to gain a comprehensive understanding of the complex, emotional, behavioural, physical, and cognitive symptoms. Currently, they are understudied and inconsistent.
1.2.1. Risk factors and protective factors
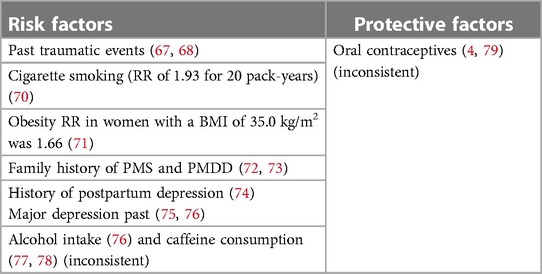
In the absence of consistent laboratory markers, a few factors were identified to pose a greater risk for PMDD such as prior traumatic events, a history of mental disorders, peripartum depression, obesity, smoking, alcohol use, and heavy drinking (67–76) (Table 5). There is little research regarding protective variables, and factors such as caffeine intake and oral contraceptive pills are still inconsistently discussed (4, 77–79).
1.2.2. Link to postpartum depression
Oestrogen and progesterone affect a variety of biological processes, brain networks, and mood-related behaviours; therefore, alterations in their levels may result in depressive symptoms. Women who are sensitive to hormonal changes may experience both PMS and postpartum depression (PPD) due to the sudden reduction in hormone levels that occurs during the luteal period as well as after delivery. The prevalence of PMDD symptoms was shown to be significantly higher in women with PPD compared with those without PPD, with a medium effect size (80). A meta-analysis of seven retrospective studies found a strong positive association between PMDD and PPD (81). A prospective cohort study found that higher severity of depressive symptoms in the first month following childbirth significantly predicted the incidence of PMDD during the first year of the postpartum period, implicating that PPD can be a risk factor for PMDD. However, replication studies are needed to substantiate this finding.
1.2.3. Link to climacteric phase
Women with a PMDD history exhibit significantly more severe depressive features than those without PMDD during their perimenopausal phase (80). It indicates that PMDD has a trajectory to develop into climacteric depression in women. However, longitudinal studies are required to investigate this matter.
1.2.4. Link to personality disorders
Women with PMDD exhibit less compulsive, rather more passive/aggressive, borderline/cycloid, and depressive and manic symptoms (82). One study found them to have higher obsessional personality features in the absence of a definitive diagnosis of a personality disorder (83). Another study found a higher risk of avoidant personality disorder, but only in women who are aged 30 years or older (84). Ducasse et al. (85) found that independent of the time of the menstrual cycle, women with PMS or PMDD have an impulsive-aggressive personality style. The association between trait anger and both PMS and PMDD was observed to be independent of all other personality traits. A higher level of anger is considered to pose a higher risk of experiencing both PMS and PMDD.
1.2.5. Suicidal risk
Women with PMDD and PMS are at seven times the odds of suicide attempt and almost four times as likely to exhibit suicidal ideation compared with women without premenstrual disturbances (86). A routine assessment of suicide risk for women experiencing moderate-to-severe premenstrual disturbances is warranted, and psychosocial treatments targeting suicidality must be provided to improve their wellbeing.
1.3. Exclusion of other disorders
The broad presentation of PMDD frequently includes co-occurring physical symptoms (87). It is important to investigate for any abnormalities of thyroid, gynaecological problems, and anaemia that can cause physical and psychological symptoms as observed in PMDD.
When the menstrual cycle coincides with the periodicity of epilepsy, the exacerbation is known as catamenial epilepsy (88). It is seen in 10%–70% of reproductive-age women with both focal and generalised epilepsy (89). The aberrant interaction between ovarian hormones and the central nervous system (CNS) has been proposed as a potential mechanism linking menstrual cycle-related disorders such as catamenial epilepsy and PMDD (90). Nowosielski et al. (87) found that women with PMS experience a twofold increased risk of sexual dissatisfaction and increased sexual pain when compared with women without PMS. More studies are required to examine the prevalence and patterns of various sexual dysfunctions associated with PMDD.
Criterion C of ICD-11 and criterion E of DSM-5 TR state that the disturbance should not be a mere exacerbation of the symptoms of another mood or anxiety disorder. Due to inadequate awareness of PMDD, most patients present, during the symptomatic phase of comorbid depressive or anxiety illness, with a history of mood symptoms worsening during the premenstrual phase with a potential retrospective falsification coloured with dysphoric mood. In such cases, a prospective observation is the only prudent way to ascertain PMDD. The clinicians must wait for the remission of symptoms and examine the daily subjective record of PMDD symptoms to identify premenstrual worsening during the two consecutive months of a remitted phase of comorbid illness. The major distinguishing symptoms of PMDD are irritability and affect lability rather than a low mood or anxiety. Serotonin reuptake inhibitors exhibit a different profile in PMDD, including a short onset of action, thus implying that this effect is possibly mediated by different serotonergic synapses from those that are involved in the antidepressant and anti-anxiety activities of these medications (91).
In attention deficit hyperkinetic disorder (ADHD), emotional dysregulation with premenstrual worsening has been recognised as a diagnostic criterion (DSM-5TR), an overlapping feature with PMDD. Dorani et al. (80) found that 45.5% of women with ADHD have a diagnosis of PMDD.
Wittchen et al. (43) concluded that women with PMDD had an eightfold increase in the risk of bipolar disorder (BD). A study titled Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) found that women with comorbid PMDD experienced a worse course of illness in the form of an earlier age at the onset for BD, increased number of episodes, more severe mood symptoms in the perinatal period, higher comorbidities (anxiety spectrum, ADHD, and substance use disorders), and higher rates of rapid cycling (92). A systematic review of 17 studies concluded that women with PMS or PMDD had more frequent diagnoses of BD-I or BD-II than those without PMS or PMDD. Women with BD-II and cyclothymia are more commonly diagnosed with PMS or PMDD. Women with both BD and PMS were found to have increased severity of manic symptoms, particularly irritability, anger, lability of mood, and sleep deprivation. This suggests that PMDD may induce or perpetuate mania in individuals with BD. In addition, a worse therapeutic response and more frequent relapses were observed in BD patients with comorbid PMDD (93). These alarming findings insinuate that PMDD and BD might have shared pathophysiological processes.
1.4. Follow-up study
To date, one longitudinal study (43) (Table 2) conducted over 48 months of the prospective investigation reported that only 10% of baseline PMDD patients have remission of symptoms, and the rest continued to have features of PMDD, suggesting the stability of diagnosis.
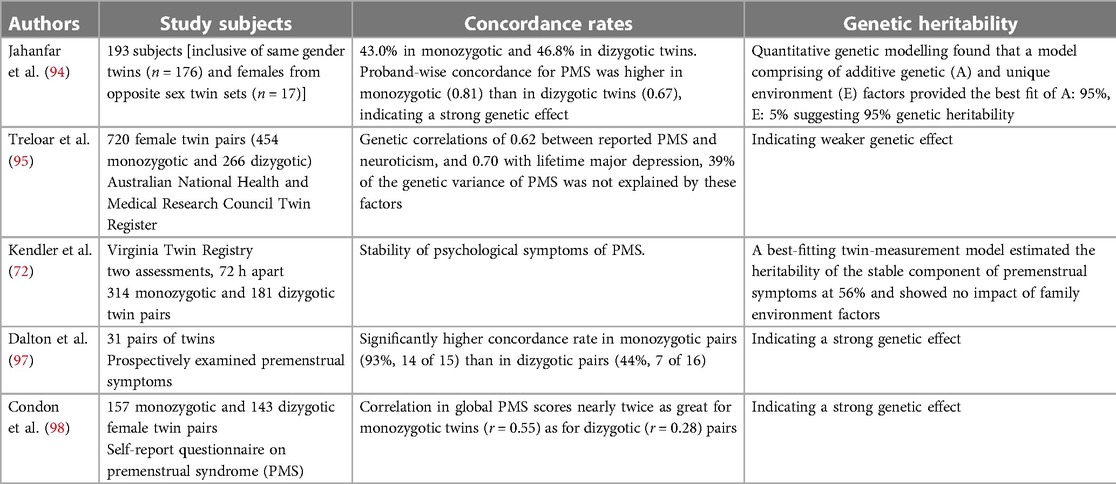
1.5. Family study
Genetic vulnerabilities are indicated through family research. Studies on families, notably those involving twins, point to connected heritable factors. Table 6 (94–98) summarises the findings from several studies on the heritability of PMS using twin samples. There is no family study available regarding PMDD, and its heritability is unknown. PMS family studies collectively suggest that PMS has a strong genetic component, with higher proband-wise concordance in monozygotic twins compared with dizygotic twins (94, 97). Additive genetic influences were identified, and a genetic correlation was found between PMS and neuroticism and lifetime major depression (95).
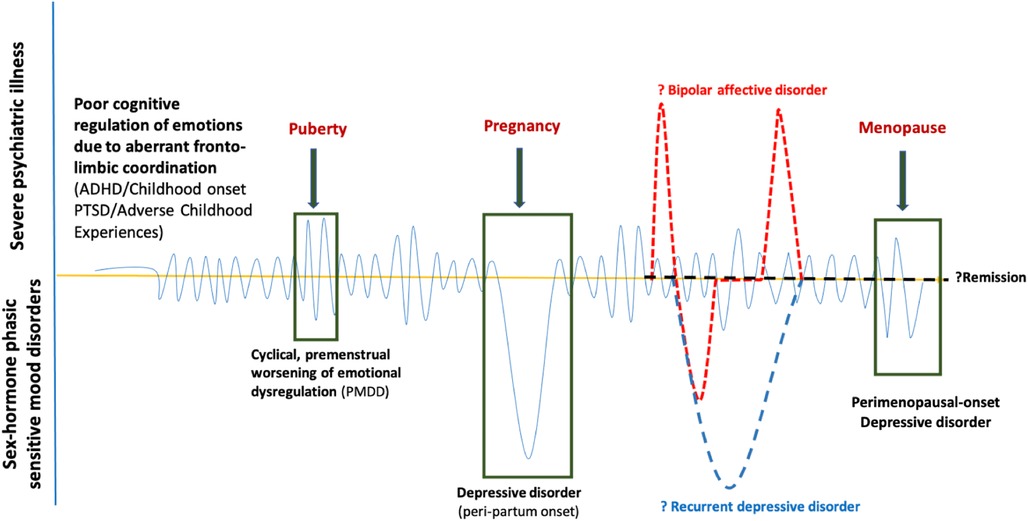
1.6. Is PMDD a neurodevelopmental disorder?
Dorani et al. (80) found that the prevalence of PMDD, PPD, and climacteric mood symptoms (Cohen's d: 3.71) were high in women with ADHD compared with the general population. ADHD may be an early risk factor for the development of PMDD and BD (99), with a shared neurobiological and genetic underpinning. “Fronto-limbic disconnection” can be hypothesised to understand the continuum of childhood-onset dysfunctional emotional brain networks (100), either genetic (ADHD) or acquired (traumatic or adverse childhood experiences), expressed as poorer emotion regulation with depressive, anxiety, and behavioural features of PMDD during the gonadal hormonal rapid fluctuations phases (luteal, pregnancy and perimenopausal) (Figure 1). It is yet unknown how much this childhood-onset dysfunctional emotional brain network remains as a personality trait marker or elevates the risk for bipolar disorder, recurrent depressive disorder, or remits in due course, reiterating the need for more longitudinal studies to understand the origin and evolution of the disease process in the developing brain in children at-risk for PMDD.
2. Discussion
The recognition of PMDD has gone far beyond the debate of whether it is an idiom of distress or a cultural syndrome. Through the application of Robins and Guze's diagnostic validation exercise, we found that the diagnostic guidelines yielded diagnostic stability (12). The validated screening and diagnostic assessment tools demonstrate favourable psychometric properties and require more cultural adaptations. The laboratory findings require replication studies. More follow-up studies and family studies of PMDD are needed. Despite the shortcomings of not having scientifically robust biological validators, as of today, it is a psychobiological illness similar to depressive disorder but has a cyclical course warranting the need for animal models, genetic risks, changes in neurotransmitters and neural network activity during the active symptomatic and remitted phases. PMDD is a disabling disorder as it reduces the work efficiency of women during their most productive years of life. There is evidence suggesting that it might precede peripartum and perimenopausal depressive disorders. Therefore, obstetricians, gynaecologists, and mental health professionals must closely monitor and intervene to prevent or alleviate subsequent depressive episodes. Accurate assessment and diagnosis of PMDD require rigorous training of community workers such as midwives and nurses and primary care physicians. The extent to which individuals seek assistance for PMDD varies worldwide due to factors such as age, subjective perception, and retrospective vs. prospective reporting (retrospective reporting is more prone to false positives and overdiagnosis, whereas a minimum of 2 months of prospective daily ratings is more associated with drop-out from clinical consultations), cultural context, awareness of the illness, and internalised stigma.
Only one 4-year longitudinal study found that only 5% of PMDD women had remission, while the remaining participants continued to suffer from PMDD symptoms with 4.4 odds of increased suicidal attempts, 7.3 odds of having more than three comorbidities, and the highest odds of 8.1 for bipolar disorder II (43). Women with ADHD had an elevated prevalence of hormone-related mood symptoms PMDD, PPD, and perimenopausal mood symptoms throughout their lives. It is suggestive of a plausibly aberrant cognitive control of mood developmentally. We generated a neurodevelopmental hypothesis based on observational/descriptive studies, and there are no biological studies conducted yet to support this hypothesis.
More research must probe into understanding the life course of PMDD, aiming to identify and intervene in the early stages, optimistically when someone is at-risk (early presentation of ADHD, adverse childhood experiences, initial traumatic experiences) to mitigate the duration, severity, and frequency of PMDD episodes.
Recent evidence suggests the crucial role of oestradiol and progesterone in modulating the neuronal network activity associated with emotion processing and attention and reward functions in susceptible women by regulating the synthesis of important neurotransmitters such as serotonin, noradrenaline, dopamine, glutamate, and GABA (101). Despite the suggestive role of gonadal hormones in PMDD, the clinical trials found limited efficacy of oral contraceptive pills (containing oestrogen and progesterone/drospirenone) confounded with high placebo rates (102), thus identified as the second-line drug for PMDD (103). This suggests that gonadal hormones may not have a direct implication in the development of PMDD, but rather they could potentially be involved through other biological mechanisms. The emergence of trials involving SSRIs or serotonin–norepinephrine reuptake inhibitors (SNRIs) can be attributed to the influence of gonadal hormones on serotonergic and norepinephrine changes. Serotonergic drugs were found to be modestly efficacious, with daily or intermittent dosing and minimal adverse effects (104). SSRI administration exclusively during the luteal phase may be a more effective treatment option, considering its self-remitting nature. Either continuous or luteal phase-only, SSRI administration has been regarded as the first line of treatment for PMDD (103). Psychological treatments such as cognitive-behavioural intervention are more efficacious in reducing the mood and behavioural symptoms of PMDD when compared with SSRI, while the latter was more efficacious in reducing the physical symptoms of PMDD (105). Emotion-focused group therapy (EFGT) with components of strengthening emotion regulation skills, increasing positive interactions, and breaking down negative cycles of interaction had been studied for PMDD women. EFGT was found to improve self-compassion and sexual function and reduce the components of pain perception and couple burnout (106). There are a variety of alternative and complementary medicine treatments under evaluation such as nutraceuticals, acupuncture, and yoga, Vitex agnus-castus (107), Hypericum perforatum (108, 109), Crocus sativus (110), Elsholtzia splendens, and Ginkgo biloba (111, 112). Neuromodulation techniques can be explored to strengthen the connectivity between prefrontal control and limbic structures to improve the symptoms and prevent future episodes.
Current diagnostic classificatory systems have rightfully acknowledged the glaringly high prevalence, distress, and dysfunction associated with PMDD. While prevalence studies reported the presence of PMDD in women from menarche to menopause, no study has mentioned regarding the typical age at the onset of illness. Furthermore, the naturalistic course and outcome of PMDD symptoms are unclear from the existing literature. This review highlighted the paucity of observational studies to understand the life trajectories of PMDD that have been limiting the clinician's judgment in diagnosing the illness and posing a dilemma on what to expect for the future course of PMDD and appropriate treatment duration. This review might resolve the clinician's dilemma of diagnosing the illness with the recommended 2 months of prospective daily symptom ratings. PMDD is a unique diagnostic entity, neither a variant of depression nor an anxiety disorder. Besides the biological validators of each symptom and syndrome-level PMDD, more research on ecological validators of PMDD is required to delineate it from underlying personality traits and acute cyclical psychological reaction to any physical stress.
Author contributions
SN has conceptualised the work, interpreted the published literature, and drafted the manuscript for important intellectual content. YN has acquired the published articles, reviewed them, and drafted the manuscript. KK conceptualised the work, reviewed the manuscript, and provided intellectual content. SG conceptualised the work, reviewed the manuscript, and provided intellectual content. SN agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor GD declared a past co-authorship with the author SN.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing (2013). p. 150–1.
2. Freeman EW, Sondheimer SJ. Premenstrual dysphoric disorder: recognition and treatment. Prim Care Companion J Clin Psychiatry. (2003) 5:30–9. doi: 10.4088/pcc.v05n0106
3. Jackson C, Pearson B, Girdler S, Johnson J, Hamer RM, Killenberg S, et al. Double-blind, placebo-controlled pilot study of adjunctive quetiapine SR in the treatment of PMS/PMDD. Hum Psychopharmacol. (2015) 30:425–34. doi: 10.1002/hup.2494
4. de Wit AE, de Vries YA, de Boer MK, Scheper C, Fokkema A, Janssen CAH, et al. Efficacy of combined oral contraceptives for depressive symptoms and overall symptomatology in premenstrual syndrome: pairwise and network meta-analysis of randomized trials. Am J Obstet Gynecol. (2021) 225:624–33. doi: 10.1016/j.ajog.2021.06.090
5. Abdi F, Ozgoli G, Rahnemaie FS. A systematic review of the role of vitamin D and calcium in premenstrual syndrome. Obstet Gynecol Sci. (2019) 62:73. doi: 10.5468/ogs.2019.62.2.73
6. Lustyk MKB, Gerrish WG, Shaver S, Keys SL. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. (2009) 12:85–96. doi: 10.1007/s00737-009-0052-y
7. Hylan TR, Sundell K, Judge R. The impact of premenstrual symptomatology on functioning and treatment-seeking behavior: experience from the United States, United Kingdom, and France. J Womens Health Gend Based Med. (1999) 8:1043–52. doi: 10.1089/jwh.1.1999.8.1043
8. Direkvand-Moghadam A, Sayehmiri K, Delpisheh A, Sattar K. Epidemiology of premenstrual syndrome (PMS)—a systematic review and meta-analysis study. J Clin Diagn Res. (2014) 8:106–9. doi: 10.7860/JCDR/2014/8024.4021
9. Durairaj A, Ramamurthi R. Prevalence, pattern and predictors of premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) among college girls. OBGYN. (2019) 5:93–8. doi: 10.21276/obgyn.2019.5.2.6
10. Budarapu S, Sadam H, Harshitha K, Nageswari DM, Reddy HK, Dhanekula G. A study to assess the prevalence of premenstrual syndrome and premenstrual dysphoric disorder and various coping strategies used by students in a Womens Medical College from South India. Int J Contemp Med Res. (2018) 5(11):K1–5. doi: 10.21276/ijcmr.2018.5.11.18
11. Di Giulio G, Reissing ED. Premenstrual dysphoric disorder: prevalence, diagnostic considerations, and controversies. J Psychosom Obstet Gynaecol. (2006) 27:201–10. doi: 10.1080/01674820600747269
12. Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. (1970) 126:983–6. doi: 10.1176/ajp.126.7.983
13. King S. Premenstrual Syndrome (PMS) and the Myth of the Irrational Female. In: Bobel S, Winkler IT, Fahs B, Hasson KA, Kissling EA, Roberts TA, editors. The palgrave handbook of critical menstruation studies. Singapore: Palgrave Macmillan (2020) p. 287–302. Chapter 23.
14. Freud S. The standard edition of the complete psychological works of Sigmund Freud. Oxford: Macmillan (1964). In James Strachey, Anna Freud, Alix Strachey, Alan Tyson. London: Hogarth Press (1974).
15. Greene R, Dalton K. The premenstrual syndrome. Br Med J. (1953) 1(4818): 1007–14. doi: 10.1136/bmj.1.4818.1007
16. Rapkin AJ, Akopians AL. Pathophysiology of premenstrual syndrome and premenstrual dysphoric disorder. Menopause Int. (2012) 18:52–9. doi: 10.1258/mi.2012.012014
17. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing (1980).
18. Management of premenstrual syndrome: green-top guideline No. 48. BJOG. (2017) 124:e73–105.27900828
19. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing (2000).
20. International Classification of Diseases, Eleventh Revision (ICD-11), World Health Organization (WHO) (2019/2021). https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1526774088
21. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Women’s Mental Health. (2003) 6:203–9. doi: 10.1007/s00737-003-0018-4
22. Steiner M, Peer M, Palova E, Freeman EW, Macdougall M, Soares CN. The premenstrual symptoms screening tool revised for adolescents (PSST-A): prevalence of severe PMS and premenstrual dysphoric disorder in adolescents. Arch Womens Ment Health. (2011) 14:77–81. doi: 10.1007/s00737-010-0202-2
23. Accortt EE, Bismark A, Schneider T, Allen J. Diagnosing premenstrual dysphoric disorder: the reliability of a structured clinical interview. Arch Women’s Mental Health. (2011) 14:265–7. doi: 10.1007/s00737-011-0209-3
24. Endicott J, Nee J, Harrison W. Daily record of severity of problems (DRSP): reliability and validity. Arch Womens Ment Health. (2006) 9:41–9. doi: 10.1007/s00737-005-0103-y
25. Feuerstein M, Shaw WS. Measurement properties of the calendar of premenstrual experience in patients with premenstrual syndrome. J Reprod Med. (2002) 47:279–89. 12012879.12012879
26. Steiner M, Streiner DL. Validation of a revised visual analog scale for premenstrual mood symptoms: results from prospective and retrospective trials. Can J Psychiatry. (2005) 50:327–32. doi: 10.1177/070674370505000607
27. Steiner M, Haskett RF, Carroll BJ. Premenstrual tension syndrome: the development of research diagnostic criteria and new rating scales. Acta Psychiatr Scand. (1980) 62:177–90. doi: 10.1111/j.1600-0447.1980.tb00605.x
28. Eisenlohr-Moul TA, Girdler SS, Schmalenberger KM, Dawson DN, Surana P, Johnson JL, et al. Toward the reliable diagnosis of DSM-5 premenstrual dysphoric disorder: the Carolina premenstrual assessment scoring system (C-PASS). Am J Psychiatry. (2017) 174:51–9. doi: 10.1176/appi.ajp.2016.15121510
29. Duko B, Mekuriaw B, Molla A, Ayano G. The prevalence of premenstrual dysphoric disorder among adolescents in Ethiopia: a systematic review and meta-analysis. Ir J Med Sci. (2021) 190:419–27. doi: 10.1007/s11845-020-02275-7
30. Thakrar P, Bhukar K, Oswal R. Premenstrual dysphoric disorder: prevalence, quality of life and disability due to illness among medical and paramedical students. J Affect Disord Rep. (2021) 4:100112. doi: 10.1016/j.jadr.2021.100112
31. Eldeeb SM, Eladl AM, Elshabrawy A, Youssef AM, Ibrahim MH. Prevalence, phenomenology and personality characteristics of premenstrual dysphoric disorder among female students at Zagazig University, Egypt. Afr J Prim Health Care Fam Med. (2021) 13:e1–9. doi: 10.4102/phcfm.v13i1.2924
32. Ogebe O, Abdulmalik J, Bello-Mojeed MA, Holder N, Jones HA, Ogun OO, et al. A comparison of the prevalence of premenstrual dysphoric disorder and comorbidities among adolescents in the United States of America and Nigeria. J Pediatr Adolesc Gynecol. (2011) 24:397–403. doi: 10.1016/j.jpag.2011.07.009
33. Pattanashetty NO, Mugali J, Hs N. Prevalence of premenstrual dysphoric disorder among high school girls of Gadag district, Karnataka, India—a school-based cross-sectional study. Kerala J Psychiatry. (2021) 34:90–5. doi: 10.30834/KJP.34.2.2021.273
34. Geta TG, Woldeamanuel GG, Dassa TT. Prevalence and associated factors of premenstrual syndrome among women of the reproductive age group in Ethiopia: systematic review and meta-analysis. PLoS One. (2020) 15:e0241702. doi: 10.1371/journal.pone.0241702
35. Koganti CT, Bobba NS. A study on the prevalence of premenstrual dysphoric disorder in medical students. Acad J Med. (2020) 3:74–7. doi: 10.47008/ajm.2020.3.1.15
36. Bansal D, Raman R, Rao TSS. Premenstrual dysphoric disorder: ranking the symptoms and severity in Indian college students. J Psychosexual Health. (2019) 1:159–63. doi: 10.1177/2631831819827183
37. Shehadeh JH, Hamdan-Mansour AM. Prevalence and association of premenstrual syndrome and premenstrual dysphoric disorder with academic performance among female university students. Perspect Psychiatr Care. (2018) 54:176–84. doi: 10.1111/ppc.12219
38. Dutta A, Sharma A. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in India: a systematic review and meta-analysis. Health Promot Perspect. (2021) 11:161–70. doi: 10.34172/hpp.2021.20
39. Qiao M, Zhang H, Liu H, Luo S, Wang T, Zhang J, et al. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample in China. Eur J Obstet Gynecol Reprod Biol. (2012) 162:83–6. doi: 10.1016/j.ejogrb.2012.01.017
40. Pal SA, Dennerstein L, Lehert P. Premenstrual symptoms in Pakistani women and their effect on activities of daily life. J Pak Med Assoc. (2011) 61:763–8. 22355998
41. Tschudin S, Bertea PC, Zemp E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch Womens Ment Health. (2010) 13:485–94. doi: 10.1007/s00737-010-0165-3
42. Dueñas JL, Lete I, Bermejo R, Arbat A, Pérez-Campos E, Martínez-Salmeán J, et al. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in a representative cohort of Spanish women of fertile age. Eur J Obstet Gynecol Reprod Biol. (2011) 156:72–7. doi: 10.1016/j.ejogrb.2010.12.013
43. Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. (2002) 32:119–32. doi: 10.1017/S0033291701004925
44. Rasgon N, McGuire M, Tanavoli S, Fairbanks L, Rapkin A. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. (2000) 73:144–9. doi: 10.1016/S0015-0282(99)00452-5
45. Hsiao CC, Liu CY, Hsiao MC. No correlation of depression and anxiety to plasma estrogen and progesterone levels in patients with premenstrual dysphoric disorder. Psychiatry Clin Neurosci. (2004) 58:593–9. doi: 10.1111/j.1440-1819.2004.01308.x
46. Eser D, Schüle C, Baghai TC, Romeo E, Uzunov DP, Rupprecht R. Neuroactive steroids and affective disorders. Pharmacol Biochem Behav. (2006) 84:656–66. doi: 10.1016/j.pbb.2006.05.020
47. Dhingra V, Magnay JL, O’Brien PMS, Chapman G, Fryer AA, Ismail KMK. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. (2007) 110:788–92. doi: 10.1097/01.AOG.0000284448.73490.ac
48. Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. (2007) 62:925–33. doi: 10.1016/j.biopsych.2006.12.019
49. Comasco E, Hahn A, Ganger S, Gingnell M, Bannbers E, Oreland L, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. (2014) 35:4450–8. doi: 10.1002/hbm.22486
50. Petersen N, Ghahremani DG, Rapkin AJ, Berman SM, Liang L, London ED. Brain activation during emotion regulation in women with premenstrual dysphoric disorder. Psychol Med. (2018) 48:1795–802. doi: 10.1017/S0033291717003270
51. Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. (2006) 31:1208–19. doi: 10.1016/j.psyneuen.2006.09.002
52. Rapkin AJ, Edelmuth E, Chang LC, Reading AE, McGuire MT, Su TP. Wholeblood serotonin in premenstrual syndrome. Obstet Gynecol. (1987) 70:533–7.3627623
53. Menkes DB, Coates DC, Fawcett JP. Acute tryptophan depletion aggravates premenstrual syndrome. J Affect Disord. (1994) 32:37–44. doi: 10.1016/0165-0327(94)90059-0
54. Syan SK, Smith M, Frey BN, Remtulla R, Kapczinski F, Hall GBC, et al. Resting state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J Psychiatry Neurosci. (2018) 43:298–316. doi: 10.1503/jpn.170175
55. Berman SM, London ED, Morgan M, Rapkin AJ. Elevated Gray Matter Volume of the Emotional Cerebellum in Women with Premenstrual Dysphoric Disorder Disorder. J Affect Disord. (2013) 146:266–71. doi: 10.1016/j.jad.2012.06.038
56. Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. (2008) 108:87–94. doi: 10.1016/j.jad.2007.09.015
57. Gingnell M, Ahlstedt V, Bannbers E, Wikström J, Sundström-Poromaa I, Fredrikson M. Social stimulation and corticolimbic reactivity in premenstrual dysphoric disorder: a preliminary study. Biol Mood Anxiety Disord. (2014) 4:3. doi: 10.1186/2045-5380-4-3
58. Gingnell M, Bannbers E, Wikström J, Fredrikson M, Sundström-Poromaa I. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. (2013) 23:1474–83. doi: 10.1016/j.euroneuro.2013.08.002
59. Gingnell M, Morell A, Bannbers E, Wikström J, Sundström Poromaa I. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. (2012) 62:400–6. doi: 10.1016/j.yhbeh.2012.07.005.1674820009075604
60. Baller EB, Wei SM, Kohn PD, Rubinow DR, Alarcón G, Schmidt PJ, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. (2013) 170:305–14. doi: 10.1176/appi.ajp.2012.12030385.1334
61. Bannbers E, Gingnell M, Engman J, Morell A, Comasco E, Kask K, et al. The effect of premenstrual dysphoric disorder and menstrual cycle phase on brain activity during response inhibition. J Affect Disord. (2012) 142(1-3):347–50. doi: 10.1016/j.jad.2012.04.006
62. Liu B, Wang G, Gao D, Gao F, Zhao B, Qiao M, et al. Alterations of GABA and glutamate-glutamine levels in premenstrual dysphoric disorder: a 3T proton magnetic resonance spectroscopy study. Psychiatry Res. (2015) 231(64):70. doi: 10.1016/j.pscychresns.2014.10.020
63. Batra NA, Seres-Mailo J, Hanstock C, Seres P, Khudabux J, Bellavance F, et al. Proton magnetic resonance spectroscopy measurement of brain glutamate levels in premenstrual dysphoric disorder. Biol Psychiatry. (2008) 63:1178–84. doi: 10.1016/j.biopsych.2007.10.007
64. Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical gamma -aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. (2002) 59:851–8. doi: 10.1001/archpsyc.59.9.851
65. Rasgon NL, Thomas MA, Guze BH, Fairbanks LA, Yue K, Curran JG, et al. Menstrual cycle-related brain metabolite changes using 1H magnetic resonance spectroscopy in premenopausal women: a pilot study. Psychiatry Res. (2001) 106(1):47–57. doi: 10.1016/s0925-4927(00)00085-8.
66. Jovanovic H, Cerin A, Karlsson P, Lundberg J, Halldin C, Nordström AL. A PET study of 5-HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res. (2006) 148(2-3):185–93. doi: 10.1016/j.pscychresns.2006.05.002.
67. Perkonigg A, Yonkers KA, Pfister H, Lieb R, Wittchen HU. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J Clin Psychiatry. (2004) 65:1314–22. doi: 10.4088/JCP.v65n1004
68. Wittchen HU, Perkonigg A, Pfister H. Trauma and PTSD—an overlooked pathogenic pathway for premenstrual dysphoric disorder? Arch Womens Ment Health. (2003) 6:293–7. doi: 10.1007/s00737-003-0028-2
69. Cao S, Jones M, Tooth L, Mishra G. Does premenstrual syndrome before pregnancy increase the risk of postpartum depression? Findings from the Australian longitudinal study on women’s health. J Affect Disord. (2021) 279:143–8. doi: 10.1016/j.jad.2020.09.130
70. Bertone-Johnson ER, Hankinson SE, Johnson SR, Manson JE. Cigarette smoking and the development of premenstrual syndrome. Am J Epidemiol. (2008) 168:938–45. doi: 10.1093/aje/kwn194
71. Bertone-Johnson ER, Hankinson SE, Willett WC, Johnson SR, Manson JE. Adiposity and the development of premenstrual syndrome. J Womens Health (Larchmt). (2010) 19:1955–62. doi: 10.1089/jwh.2010.2128
72. Kendler KS, Silberg JL, Neale MC, Kessler RC, Heath AC, Eaves LJ. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. (1992) 22:85–100. doi: 10.1017/S0033291700032761
73. van den Akker OB, Eves FF, Stein GS, Murray RM. Genetic and environmental factors in premenstrual symptom reporting and its relationship to depression and a general neuroticism trait. J Psychosom Res. (1995) 39:477–87. doi: 10.1016/0022-3999(94)00152-U
74. Breaux C, Hartlage S, Gehlert S. Relationships of premenstrual dysphoric disorder to major depression and anxiety disorders: a re-examination. J Psychosom Obstet Gynaecol. (2000) 21:17–24. doi: 10.3109/01674820009075604
75. Warner P, Bancroft J, Dixson A, Hampson M. The relationship between perimenstrual depressive mood and depressive illness. J Affect Disord. (1991) 23:9–23. doi: 10.1016/0165-0327(91)90031-M
76. Bancroft J, Rennie D, Warner P. Vulnerability to perimenstrual mood change: the relevance of a past history of depressive disorder. Psychosom Med. (1994) 56:225–31. doi: 10.1097/00006842-199405000-00008
77. del MarFernández M, Saulyte J, Inskip HM, Takkouche B. Premenstrual syndrome and alcohol consumption: a systematic review and meta-analysis. BMJ Open. (2018) 8:e019490. doi: 10.1136/bmjopen-2017-019490
78. Purdue-Smithe AC, Manson JE, Hankinson SE, Bertone-Johnson ER. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. (2016) 104:499–507. doi: 10.3945/ajcn.115.127027
79. Rossignol AM. Caffeine-containing beverages and premenstrual syndrome in young women. Am J Public Health. (1985) 75:1335–7. doi: 10.2105/AJPH.75.11.1335
80. Dorani F, Bijlenga D, Beekman ATF, van Someren EJW, Kooij JJS. Prevalence of hormone-related mood disorder symptoms in women with ADHD. J Psychiatr Res. (2021) 133:10–5. doi: 10.1016/j.jpsychires.2020.12.005
81. Pereira D, Pessoa AR, Madeira N, Macedo A, Pereira AT. Association between premenstrual dysphoric disorder and perinatal depression: a systematic review. Arch Womens Ment Health. (2022) 25:61–70. doi: 10.1007/s00737-021-01177-6
82. Parry BL, Ehlers CL, Mostofi N, Phillips E. Personality traits in LLPDD and normal controls during follicular and luteal menstrual-cycle phases. Psychol Med. (1996) 26:197–202. doi: 10.1017/S0033291700033833
83. Sassoon SA, Colrain IM, Baker FC. Personality disorders in women with severe premenstrual syndrome. Arch Womens Ment Health. (2011) 14:257–64. doi: 10.1007/s00737-011-0212-8
84. De Ronchi D, Muro A, Marziani A, Rucci P. Personality disorders and depressive symptoms in late luteal phase dysphoric disorder. Psychother Psychosom. (2000) 69:27–34. doi: 10.1159/000012363
85. Ducasse D, Jaussent I, Olié E, Guillaume S, Lopez-Castroman J, Courtet P. Personality traits of suicidality are associated with premenstrual syndrome and premenstrual dysphoric disorder in a suicidal women sample. PLoS One. (2016) 11:e0148653. doi: 10.1371/journal.pone.0148653
86. Prasad D, Wollenhaupt-Aguiar B, Kidd KN, de Azevedo Cardoso T, Frey BN. Suicidal risk in women with premenstrual syndrome and premenstrual dysphoric disorder: a systematic review and meta-analysis. J Womens Health. (2021) 30:1693–707. doi: 10.1089/jwh.2021.0185
87. Nowosielski K, Drosdzol A, Skrzypulec V, Plinta R. Sexual satisfaction in females with premenstrual symptoms. J Sex Med. (2010) 7:3589–97. doi: 10.1111/j.1743-6109.2010.01927.x
88. Herzog AG. Catamenial epilepsy: definition, prevalence pathophysiology and treatment. Seizure. (2008) 17:151–9. doi: 10.1016/j.seizure.2007.11.014
89. Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia. (1993) 34:827–31. doi: 10.1111/j.1528-1157.1993.tb02097.x
90. Logothetis J, Harner R, Morrell F, Torres F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology. (1959) 9:352–60. doi: 10.1212/WNL.9.5.352
91. Landén M, Eriksson E. How does premenstrual dysphoric disorder relate to depression and anxiety disorders?: theoretical review: premenstrual dysphoria, anxiety and depression. Depress Anxiety. (2003) 17:122–9. doi: 10.1002/da.10089
92. Slyepchenko A, Minuzzi L, Frey BN. Comorbid premenstrual dysphoric disorder and bipolar disorder: a review. Front Psychiatry. (2021) 12:719241. doi: 10.3389/fpsyt.2021.719241
93. Cirillo PC, Passos RBF, Bevilaqua MCDN, López JRRA, Nardi AE. Bipolar disorder and premenstrual syndrome or premenstrual dysphoric disorder comorbidity: a systematic review. Braz J Psychiatry. (2012) 34:467–79. doi: 10.1016/j.rbp.2012.04.010
94. Jahanfar S, Lye MS, Krishnarajah IS. The heritability of premenstrual syndrome. Twin Res Hum Genet. (2011) 14:433–6. doi: 10.1375/twin.14.5.433
95. Treloar SA, Heath AC, Martin NG. Genetic and environmental influences on premenstrual symptoms in an Australian twin sample. Psychol Med. (2002) 32:25–38. doi: 10.1017/S0033291701004901
96. Kendler KS, Karkowski LM, Corey LA, Neale MC. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. (1998) 155:1234–40. doi: 10.1176/ajp.155.9.1234
97. Dalton K, Dalton ME, Guthrie K. Incidence of the premenstrual syndrome in twins. Br Med J (Clin Res Ed). (1987) 295:1027–8. doi: 10.1136/bmj.295.6605.1027-a
98. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. (1993) 162:481–6. doi: 10.1192/bjp.162.4.481
99. Salvi V, Ribuoli E, Servasi M, Orsolini L, Volpe U. ADHD and bipolar disorder in adulthood: clinical and treatment implications. Medicina (Kaunas). (2021) 57:466. doi: 10.3390/medicina57050466
100. Kebets V, Favre P, Houenou J, Polosan M, Perroud N, Aubry JM, et al. Fronto-limbic neural variability as a transdiagnostic correlate of emotion dysregulation. Transl Psychiatry. (2021) 11(1):545. doi: 10.1038/s41398-021-01666-3
101. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. (2015) 9:37. doi: 10.3389/fnins.2015.00037
102. Freeman EW, Halbreich U, Grubb GS, Rapkin AJ, Skouby SO, Smith L, et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. (2012) 85:437–45. doi: 10.1016/j.contraception.2011.09.010
103. Carlini SV, Deligiannidis KM. Evidence-based treatment of premenstrual dysphoric disorder: a concise review. J Clin Psychiatry. (2020) 81:19ac13071. doi: 10.4088/JCP.19ac13071
104. Freeman EW, Rickels K, Yonkers KA, Kunz NR, McPherson M, Upton GV. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstetrics & Gynecology. (2001) 98:737–44. doi: 10.1016/s0029-7844(01)01530-7.
105. Kleinstäuber M, Witthöft M, Hiller W. Cognitive-behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta-analysis. J Clin Psychol Med Settings. (2012) 19:308–19. doi: 10.1007/s10880-012-9299-y
106. Shareh H, Ghodsi M, Keramati S. Emotion-focused group therapy among women with premenstrual dysphoric disorder: a randomized clinical trial. Psychother Res. (2022) 32:440–55. doi: 10.1080/10503307.2021.1980239
107. Cerqueira RO, Frey BN, Leclerc E, Brietzke E. Vitex agnus castus for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. (2017) 20:713–9. doi: 10.1007/s00737-017-0791-0
108. Hicks SM, Walker AF, Gallagher J, Middleton RW, Wright J. The significance of “nonsignificance” in randomized controlled studies: a discussion inspired by a double-blinded study on St. John’s wort (Hypericum perforatum L.) for premenstrual symptoms. J Altern Complement Med. (2004) 10:925–32. doi: 10.1089/acm.2004.10.925
109. Canning S, Waterman M, Orsi N, Ayres J, Simpson N, Dye L. The efficacy of Hypericum perforatum (St John’s wort) for the treatment of premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. CNS Drugs. (2010) 24:207–25. doi: 10.2165/11530120-000000000-00000
110. Agha-Hosseini M, Kashani L, Aleyaseen A, Ghoreishi A, Rahmanpour H, Zarrinara AR, et al. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: a double-blind, randomised and placebo-controlled trial. BJOG. (2008) 115:515–9. doi: 10.1111/j.1471-0528.2007.01652.x
111. Carlini SV, di Scalea TL, McNally ST, Lester J, Deligiannidis KM. Management of premenstrual dysphoric disorder: a scoping review. Int J Womens Health. (2022) 14:1783–801. doi: 10.2147/IJWH.S297062
Keywords: premenstrual dysphoric disorder, menstrual, validity, Robins and Guze, attention deficit hyperactive disorder, postpartum, bipolar, depression
Citation: Naik SS, Nidhi Y, Kumar K and Grover S (2023) Diagnostic validity of premenstrual dysphoric disorder: revisited. Front. Glob. Womens Health 4:1181583. doi: 10.3389/fgwh.2023.1181583
Received: 7 March 2023; Accepted: 4 October 2023;
Published: 27 November 2023.
Edited by:
Geetha Desai, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaReviewed by:
Preethi Veerappa Reddy, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaGirish Babu, SDM College of Medical Sciences and Hospital, India
Krishna Prasad M, National Institute of Mental Health and Neurosciences (NIMHANS), India
© 2023 Naik, Nidhi, Kumar and Grover. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shalini S. Naik ZHJzaGFsaW5pLmFuamlAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Shalini S. Naik
Shalini S. Naik Yadav Nidhi
Yadav Nidhi Krishan Kumar
Krishan Kumar Sandeep Grover
Sandeep Grover