- 1Botswana Sexual and Reproductive Health Initiative, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana
- 2Usher Institute, University of Edinburgh, Edinburgh, United Kingdom
- 3MRC Centre for Reproductive Health and Centre for Global Health, University of Edinburgh, Edinburgh, United Kingdom
Introduction: Uninterrupted access to HIV and sexual and reproductive health (SRH) services is essential, particularly in high HIV prevalence settings, to prevent unintended pregnancy and vertical HIV transmission. Understanding the challenges that COVID-19 and associated social distancing measures (SDMs) posed on health service access is imperative for future planning.
Methods: This cross-sectional study was conducted in Botswana between January–February 2021. A web-based questionnaire was disseminated on social media as part of the International Sexual Health and REproductive Health (I-SHARE) Survey. Respondents answered questions on SRH, before and during COVID-19 SDMs. Subgroup analysis and comparison of descriptive data was performed for people living with HIV (PLWH).
Results: Of 409 participants, 65 were PLWH (80% female, 20% male). During SDMs, PLWH found it more difficult to access condoms and treatment for HIV and STIs; attend HIV appointments; and maintain adherence to antiretroviral therapy. Compared to HIV-negative women, a higher proportion of women living with HIV used condoms as their primary method of contraception (54% vs. 48%), and had lower use of long-acting reversible contraception (8% vs. 14%) and dual contraception (8% vs. 16%).
Discussion: Mirroring global trends, COVID-19 disrupted HIV and SRH service access in Botswana. However, in high HIV-prevalence settings, disruption may more severely impact population health with disproportionate effects on women. Integration of HIV and SRH services could build health system capacity and resilience, reduce missed opportunities for delivering SRH services to PLWH and limit the consequences of future restrictions that may cause health system disruption
Introduction
The COVID-19 pandemic has put immense pressure on health systems globally. In resource-limited, high HIV prevalence settings within Africa, services operate closer to the limits of their capacity and are less resilient to system shock events (1). This risks services becoming stretched and overwhelmed. To limit the effects of the pandemic, the Botswana Government announced a “state of public emergency” and a range of measures including lockdown movement restrictions, border closures, social distancing and isolation recommendations, and alcohol bans were introduced (2). Previous epidemics and associated restrictions have resulted in unanticipated, indirect consequences on wider population health and wellbeing; during the West Africa 2013–2016 Ebola epidemic, HIV and SRH care was disrupted, reducing HIV testing, contraception access and antenatal care visits (3, 4), whilst the Zika outbreak in Latin America in 2015 led to increased demand for contraception and online abortion medications (5, 6). Modelling estimated that COVID-19–related disruptions could increase new HIV infections and AIDS-related deaths by 10% globally over 2 years (7), and UNFPA predictions suggested that restricted access to SRH services could lead to up to 1.4 million unintended pregnancies (8). A systematic review found the indirect effects of COVID-19 did indeed significantly reduce access to contraception, abortion and HIV/STI testing globally (9). In Burkina Faso and Kenya, while contraception use since the onset of COVID-19 rose, an appreciable proportion of women cited COVID-19 as a reason for discontinuing contraception (10) and in Burkina Faso, rates of unintended pregnancy, stillbirth and abortion increased (11). These COVID-19 related impacts could have profound consequences in high HIV prevalence settings.
Botswana has the third highest HIV prevalence in the world (12); 19.9% of adults and 24.8% of women aged 15–49 are living with HIV (13). Large-scale investment in infrastructure for HIV diagnosis and treatment (14), alongside universal free antiretroviral therapy (ART), led to Botswana becoming the third country to meet the UNAIDS 95–95–95 target: 95% of people living with HIV (PLWH) aware of their status, 95% of diagnosed PLWH on ART and 95% of those on ART virally suppressed, by 2025 (15). However, HIV remains a leading cause of morbidity and mortality in Botswana and challenges persist in maintaining success (16). These challenges include: responding to donor funding cuts resulting from Botswana's transition to upper-middle income country (17, 18); improving access to other essential health services including sexual and reproductive health (SRH) for PLWH (19); improving equitable HIV service provision for women and other disproportionately affected groups (e.g., sex-workers and adolescents) (13, 20, 21); and adapting service provision during the COVID-19 pandemic.
Providing comprehensive SRH services to women living with HIV (WLWH) has additional specific benefits: preventing unintended pregnancy reduces risk of vertical HIV transmission by enabling the planning and safer conception of desired pregnancies (22); more frequent cervical cancer screening is required due to the increased risk of cervical cancer (23, 24); and sexually transmitted infections (STIs) are more prevalent among WLWH and may increase the likelihood of HIV transmission (25). Men living with HIV also have increased need for SRH services, relying on consistent access to condoms and ART medication to reduce vertical transmission of HIV. Providing unrestricted, high quality and person-centred SRH services to WLWH is essential to both providing holistic HIV care and maintaining 95–95–95 target coverage.
Further requirements for COVID-19-related restrictions or restrictions under future pandemics remain a significant possibility; understanding the impact of restrictions on HIV service will help minimise the effects of future measures and improve health system resilience. This study aims to assess the effect of COVID-19 social distancing measures on sexual health behaviour, and HIV and SRH service access for PLWH in Botswana.
Methods
This cross-sectional study was conducted in Botswana between 17th January and 22nd February 2021 as part of the International Sexual Health and Reproductive Health (I-SHARE) Survey, a multi-country, online survey aiming to examine the impact of COVID-19 on SRH (26). Detailed study methods are described elsewhere (26).
The Botswana in-country team were responsible for local survey adaptation, translation into Setswana, ethical approval and survey dissemination in Botswana. The survey was disseminated on the social media platforms Facebook and Instagram, in both English and Setswana. Facebook location targeting was used to target the advertisement to people living in Botswana. People aged ≥18 years and residing in Botswana were eligible to complete the survey. Participants were asked to confirm this information and indicate informed consent by ticking a box before being allowed to proceed with the survey. Participants were asked questions on socio-demographics; sexual behaviour and relationships; access to SRH services including contraception, HIV diagnosis and management; access to pregnancy care; intimate partner violence (IPV); and mental health. For the purposes of this analysis “social distancing measures” (SDMs) were defined as measures taken by the Botswana government to contain the spread of COVID-19 on or after 2nd April 2020, the date on which the initial “state of public emergency” was announced (2).
Participants were not required to answer every question and could stop the survey at any point, resulting in variable response rates across questions. Percentages and the absolute number of positive responses (n) for questions are displayed in the text whilst full sample sizes for each question are given in the Tables. Descriptive analyses relating to sexual behaviours, access to contraceptives, IPV and mental health were performed and, where appropriate, these were restricted to individuals who self-reported positive HIV status. Data were analysed using Stata 17. Responses were anonymised and no identifiable information was collected. No compensation or incentives were given for participation. Botswana national resources for IPV, psychological support and SRH services were provided upon completion of the survey. Ethics approval for this study was provided by the Botswana Health Research Development Committee.
Results
Demographics
Socioeconomic and demographic characteristics of respondents are shown in Table 1. In Botswana, 409 participants (female 82%, male 18%) completed the survey. Sixty-five (16%) self-reported positive HIV status (female 80%, male 20%), 334 (82%) reported negative HIV status and 10 (2%) stated they would “prefer not to say”. The median age was 30 years for PLWH and 27 years for those HIV negative. Of PLWH, the majority identified as cisgender (99%, n = 64); heterosexual (57%, n = 36); reported black ethnicity (95%, n = 62) and identified as Christian (86%, n = 56). Educational level, rural/urban residence, ethnicity, relationship status and religious belief were similar across HIV status. Compared to HIV-negative participants, a higher proportion of PLWH reported earning less than BWP5,000 (434 USD) per month (59%, n = 38 vs. 45%, n = 148) and reported partial/total loss of income during SDMs (59%, n = 38 vs. 46%, n = 154).
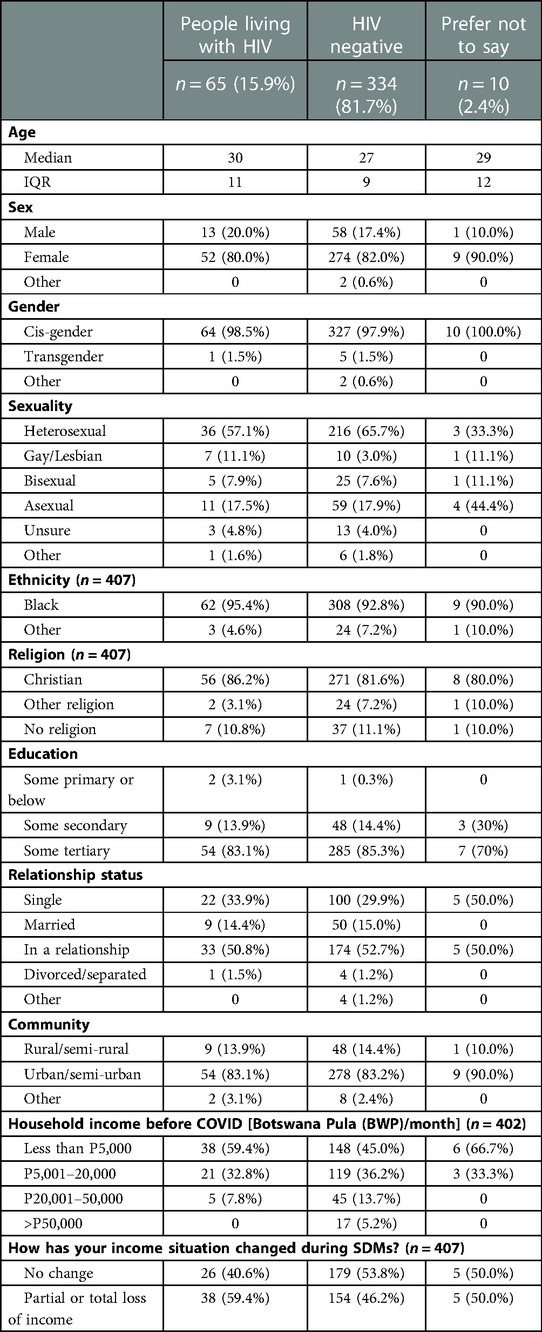
Table 1. Demographic characteristics of participants of the 2021 Botswana I-SHARE study, an online cross-sectional survey.
Compared to HIV-negative respondents, a higher proportion of PLWH reported ever having transactional sex (“exchanging sex for money, material goods, favours, drugs or shelter”) (13%, n = 7 vs. 5%, n = 17) and ever being subjected to physical or sexual IPV (including sexual assault & coercion) (15.4%, n = 10 vs. 12.5%, n = 43).
Access to HIV/STI treatment and consultation during SDMs
Of the 399 respondents, 16% (n = 63) reported seeking HIV/STI treatment during COVID-19 SDMs. Of these 63, 13% (n = 8) reported COVID-19 SDMs prevented them from accessing treatment (Table 2). Reasons provided for being unable to access HIV/STI treatment included: unavailability of services, inability to access transport or leave the house, and long queues at health services.
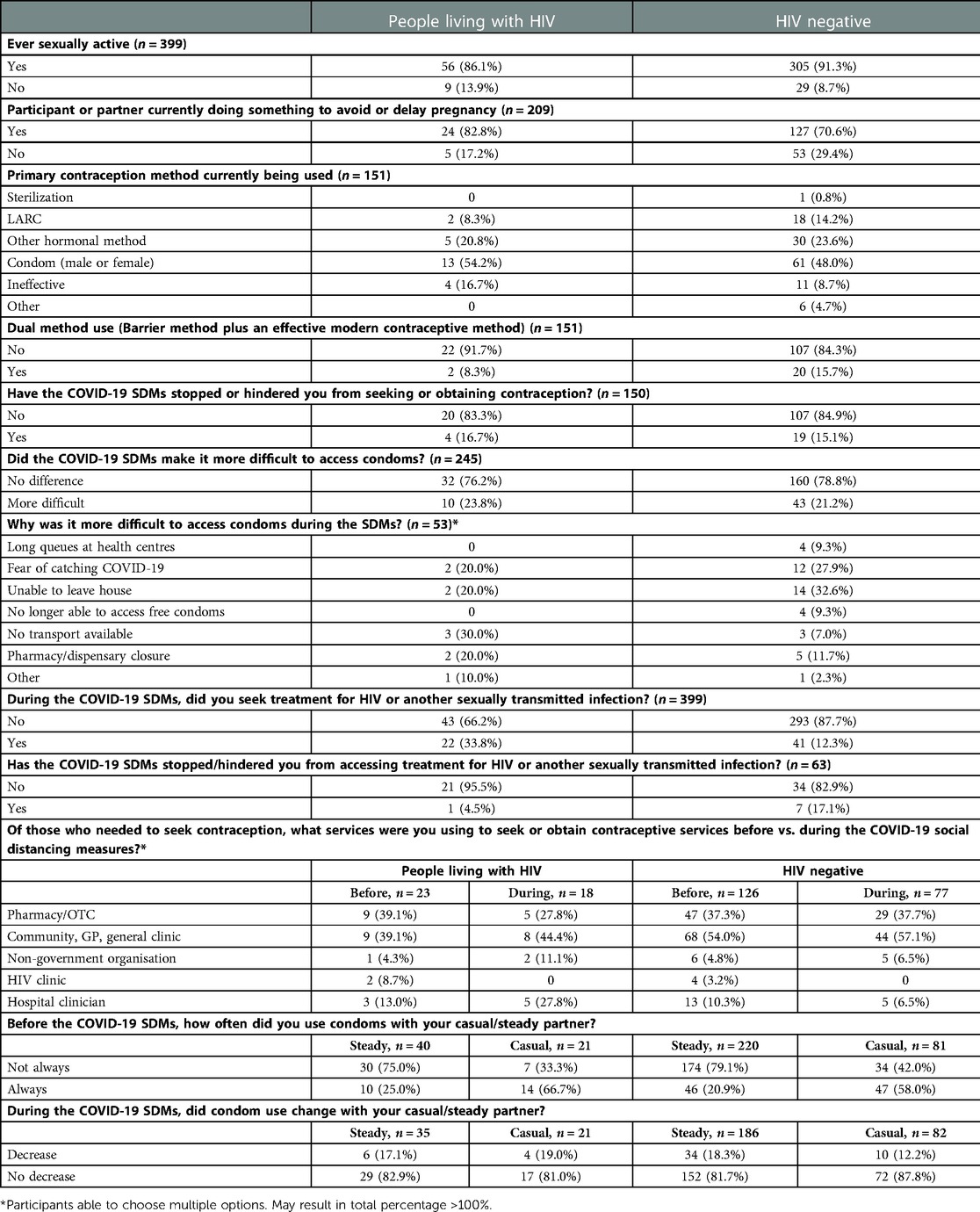
Table 2. Comparative use and patterns of access to essential sexual health services before and during social distancing measures by HIV status, for respondents of the Botswana I-SHARE study.
Forty-five percent of PLWH (n = 24) said that during SDMs, they worried they would be prevented from accessing ART and 18% (n = 9) felt SDMs made ART adherence more difficult or impossible (Table 3). Twenty-eight percent of PWLH (n = 17) reported their HIV treatment/care appointments had been cancelled during SDMs, whilst 20% (n = 13) decided to delay/miss their appointments due to: fears of acquiring COVID-19 (31%), lack of transport (23%), inability to leave the house (15%), lack of clinician availability (8%), and long clinic queues (8%). Ten percent of PLWH (n = 5) reported COVID-19 SDMs “forced disclosure” of their HIV status.
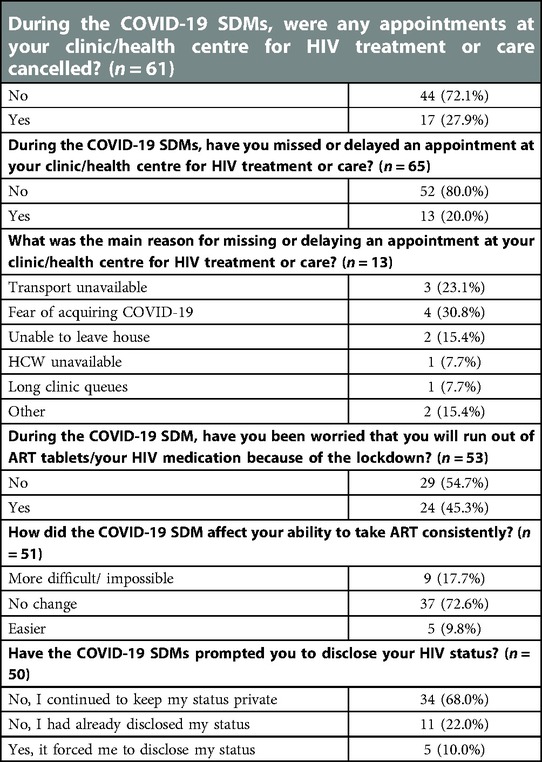
Table 3. The impact of COVID-19 social distancing measures on access to essential HIV treatment and services for PLWH responding to the Botswana I-SHARE study.
Contraception use and access to SRH services during COVID-19 SDMs
More WLWH reported “currently doing something to avoid or delay pregnancy” (e.g., contraception, traditional methods etc.) than HIV-negative women (83%, n = 24 vs. 71%, n = 127) (Table 2). Amongst those actively avoiding pregnancy, WLWH were more likely to be using condoms (male or female) as their primary method of contraception (54%, n = 13 vs. 48%, n = 61), and had lower use of long-acting reversible methods of contraception (LARCs: IUD and implant) (8%, n = 2 vs. 14%, n = 18) and dual contraception (i.e., a barrier method in addition to an effective method of contraception) (8%, n = 2 vs. 16%, n = 20) than women without HIV.
During SDMs, similar proportions of people living with and without HIV reported encountering problems accessing condoms (24%, n = 10 vs. 21%, n = 43) and their regular method(s) of contraception (17%, n = 4 vs. 15%, n = 19) (Table 3). Reasons women gave for not being able to access their regular method of contraception during SDMs were broadly similar across women living with and without HIV, including: fear of acquiring COVID-19; inability to leave the house; long queues at providers; and shop/pharmacy closures. Prior to SDMs, PLWH (n = 23) most commonly obtained contraception from pharmacies (n = 9, 39%) and general/community clinics (n = 9, 39%) and were less likely to seek contraception from hospitals (n = 3, 13%). During SDMs, fewer PLWH reported needing to seek contraception (n = 18) and patterns of access changed, with PLWH more likely to acquire contraception from hospitals (n = 5, 28%) and less likely to use pharmacies (n = 5, 28%) when compared to before the SDMs. Contraception access from HIV clinics reduced during SDMs from 9% to 0%.
Discussion
Our findings suggest that COVID-19 SDMs may have affected many aspects of comprehensive HIV care in Botswana. PLWH reported finding it more difficult to access condoms and other methods of contraception; access treatment for HIV and STIs; attend HIV care appointments; and maintain adherence to ART. The impact of the COVID-19 SDMs is likely to be multifactorial; lockdowns and travel restrictions may have impacted the ability of PLWH to attend healthcare facilities, whilst reduced clinician availability and clinic closures may have caused routine HIV appointments to be cancelled, and ART and contraceptive medication refills to be delayed.
The reduced access to HIV and STI treatment and barriers to accessing testing in Botswana during COVID-19 SDMs demonstrated in this study suggests that COVID-19 restrictions could have set back national HIV prevention and treatment progress. Participants reported that COVID-19 SDMs directly challenged clinic attendance and ART adherence, and condom use was found to be lower during SDMs. Further research would be required to assess whether this impacted viral suppression and ART resistance due to treatment breaks and HIV transmission.
Almost half of PWLH expressed fears of running out of ART and 10% were forced to disclose their HIV status during the COVID-19 SDMs. PLWH also reported being unable to access contraception and missing HIV clinic appointments due to fear of catching COVID-19. This highlights the importance of good public health messaging in times of crisis. Disseminated information must be easily understood, trusted and delivered in culturally sensitive ways to avoid fear and misinformation affecting population health decisions and outcomes.
More WLWH reported use of contraception than HIV-negative women during SDMs but WLWH were more likely to rely on condoms as their only method and were less likely to use LARCs or dual methods. The proportion of WLWH who reported condom use as their primary contraceptive method was lower than the 64.2% reported in the 2017 Botswana Demographic Survey (27), however this still represents a much greater proportion than the 17.5% reported by modern contraception users across sub-Saharan Africa (28). Given this reliance on condoms, it is concerning that in Botswana, a high prevalence HIV setting, more people were unable to access condoms during COVID-19 SDMs than the global I-SHARE average (23.8% vs. 8.7%) (29). Some Botswana participants reported that they were unable to access condoms due to affordability. Maintaining universal contraceptive provision that is free at the point of access remains a highly cost-effective intervention, especially in populations with high HIV prevalence (30), and supply infrastructure must be varied and resilient to ensure uninterrupted access. Patterns of contraceptive access changed during COVID-19 SDMs: more PLWH but fewer HIV-negative individuals obtained contraception from hospitals, whilst fewer PLWH obtained contraceptives from their HIV clinic. PLWH may be more likely to attend other essential hospital appointments for management of co-morbidities; offering good contraceptive services at as many interactions with healthcare providers as possible, especially during times of restricted service access, minimises missed opportunities for service delivery.
A lower proportion of WLWH reported using LARCs compared to HIV-negative women. Although we did not explore factors influencing contraceptive decision-making and method choice in this study, this may be due to lingering healthcare provider concerns about potential drug-drug interactions (DDIs) between hormonal contraception and ART and the safety of IUD use among WLWH, as well a provider-emphasis on barrier contraception use for PLWH to prevent HIV transmission (31, 32). However, widespread adoption of ART regimens with lower DDI risk (33, 34) alongside updated guidelines, no longer preclude the use of any contraceptive method due to HIV status, and the safety of IUD use among WLWH has been established. Health providers and WLWH need to be educated on these changes to increase the use of the highly effective long-acting contraceptive options in PLWH. Additionally, women using LARCs are less prone to having their method of contraception disrupted due to failures of supply chains or clinic closures, improving system resilience, especially during periods of health system stress.
The reductions in HIV treatment, condom, and contraception access reported in our study during COVID-19 SDMs highlight the need for increased resilience and capacity in healthcare infrastructure for PLWH. Integration of HIV and other essential health services, to deliver comprehensive healthcare to PWLH, is supported by the African Union and other key multinational actors, due to the potential to increase cost-efficiency and reduce the number of missed opportunities for delivering essential SRH care (35, 36). A recent meta-analysis incorporating 114 studies from across Africa demonstrated services that integrated HIV care with another service significantly improved HIV care cascade outcomes, including higher HIV testing and counselling; faster and higher rates of ART initiation; improved rates of viral suppression; and better retention of PLWH in health services (37). Integration has also been shown to reduce the likelihood of SRH supply stockout (38, 39); improve service access and convenience (40, 41); and reduce waiting times and end-user fees (42).
For integration to be successful, services must be properly funded to prevent overloading of the health system (42, 43) and changes should be adapted to the setting, taking a “bottom-up” approach, so community engagement and local ownership is developed to build capacity and long-term success (38, 40, 44). The variety of barriers encountered by PLWH implies there is no single solution to improving service access in times of stress. If implemented prior to the onset of the COVID-19 pandemic, integration of HIV and SRH services may have partially negated some of the negative effects caused by SDMs, however a varied package of improvements is required to build sustainable health system resilience.
Botswana has the highest HIV prevalence within the international I-SHARE study, providing invaluable insight into COVID-19-related impacts on contraception and SRH service access in a high HIV prevalence setting. The I-SHARE study had a number of limitations including sampling bias against those unable to access the online survey; the use of cross-sectional sampling preventing causal inferences; and recall bias due to the length of time that had passed since the initiation of COVID-19 SDMs in Botswana and study commencement. Common limitations are explored further in the I-SHARE study protocol (26). Another limitation of the Botswana survey was the lack of representation of under-18s, as ethical approval required participants to be 18 or older. The effects of COVID-19 on HIV and SRH care for adolescents in Botswana who have an increased need for SRH services and already face increased barriers to accessing services, could be even greater than what we see here among our adult participants. Our study was primarily descriptive and not designed to assess statistical significance of associations. Given the relatively small sample sizes, we would have been underpowered to show significance of associations when comparing sexual health and health seeking behaviours between people living with and without HIV. A lack of biological data collection limits our ability to correlate PLWH's experience of the barriers they faced with data on HIV treatment adherence and viral suppression. Rates of question completion varied greatly throughout the survey which may have led to the introduction of attrition bias. Finally, differences in the level of HIV service provision and the type, timing and enforcement of SDMs, limit our ability to use our finding to make inferences about other surrounding countries. More research is required to investigate the impact of COVID-19 SDMs in other high HIV-prevalence settings.
In Botswana, COVID-19 SDMs reduced access to HIV and SRH services, mirroring global trends. In a resource limited, high HIV-prevalence setting, additional barriers to SRH access may have more severe impacts on population health. In addition, as HIV rates are higher amongst women in Botswana, interruptions to HIV care disproportionately impact women's health, and propagates gender and socioeconomic inequalities. Uninterrupted access to HIV and SRH services for PLWH is essential, as even a short break in the supply of ART or contraceptives could lead to HIV transmission or unintended pregnancy. Integration of HIV and SRH services should be prioritised to build health system capacity and resilience in Botswana. This will reduce the number of missed opportunities for delivering SRH services to PLWH and limit the harm of future COVID-19 SDMs or other situations resulting in health system disruption.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Botswana Health Research Development Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AM, RR, SE and IM: conceptualised the analysis and analysed the data. BB, LT and NM: were involved with translation of the questionnaire and implementation of the surveys. SE and IM: prepared the first draft of the manuscript with equal contribution. RR, AM and CM: contributed to subsequent drafts. All authors critically reviewed and approved the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank all participants who responded to the I-SHARE survey and our NGO partners (Botswana Gender Based Violence Prevention & Support Centre; Lesbians, Gays and Bisexuals of Botswana; Postnatal Mental Health Society of Botswana and Botswana Family Welfare Association) for disseminating the survey and sharing the I-SHARE Botswana social media posts to their social media platforms.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Karamagi HC, Titi-Ofei R, Kipruto HK, Seydi ABW, Droti B, Talisuna A, et al. On the resilience of health systems: a methodological exploration across countries in the WHO African region. PLoS One. (2022) 17(2):e0261904. doi: 10.1371/journal.pone.0261904
2. Government of Botswana. Emergency powers (COVID-19) regulations, 2020. Botswana: Government of Botswana. (2020).
3. Parpia AS, Ndeffo-Mbah ML, Wenzel NS, Galvani AP. Effects of response to 2014–2015 ebola outbreak on deaths from malaria, HIV/AIDS, and Tuberculosis, West Africa. Emerg Infect Dis. (2016) 22(3):433. doi: 10.3201/eid2203.150977
4. Camara BS, Delamou A, Diro E, Béavogui AH, el Ayadi AM, Sidibé S, et al. Effect of the 2014/2015 ebola outbreak on reproductive health services in a rural district of Guinea: an ecological study. Trans R Soc Trop Med Hyg. (2017) 111(1):22–9. doi: 10.1093/trstmh/trx009. Available at: https://academic.oup.com/trstmh/article/111/1/22/3074506 (cited 2022 Feb 23).28340207
5. Wenham C, Arevalo A, Coast E, Corrêa S, Cuellar K, Leone T, et al. Zika, abortion and health emergencies: a review of contemporary debates. Global Health. (2019) 15(1):49. doi: 10.1186/s12992-019-0489-3
6. Aiken ARA, Scott JG, Gomperts R, Trussell J, Worrell M, Aiken CE. Requests for abortion in Latin America related to concern about zika virus exposure. N Engl J Med. (2016) 375(4):396–8. doi: 10.1056/NEJMc1605389
7. Stover J, Glaubius R, Teng Y, Kelly S, Brown T, Hallett TB, et al. Modeling the epidemiological impact of the UNAIDS 2025 targets to end AIDS as a public health threat by 2030. PLoS Med. (2021) 18(10):e1003831. doi: 10.1371/journal.pmed.1003831
8. UNFPA. Impact of COVID-19 on family planning: What we know one year into the pandemic. New York: UNFPA. (2021).
9. Mukherjee TI, Khan AG, Dasgupta A, Samari G. Reproductive justice in the time of COVID-19: a systematic review of the indirect impacts of COVID-19 on sexual and reproductive health. Reprod Health. (2021) 18(1):1–25. doi: 10.1186/s12978-021-01286-6
10. Karp C, Wood SN, Guiella G, Gichangi P, Bell SO, Anglewicz P, et al. Contraceptive dynamics during COVID-19 in sub-Saharan Africa: longitudinal evidence from Burkina Faso and Kenya. BMJ Sex Reprod Health. (2021) 47(4):252–60. doi: 10.1136/bmjsrh-2020-200944
11. Druetz T, Cooper S, Bicaba F, Bila A, Shareck M, Milot DM, et al. Change in childbearing intention, use of contraception, unwanted pregnancies, and related adverse events during the COVID-19 pandemic: results from a panel study in rural Burkina Faso. PLOS Global Public Health. (2022) 2(4):e0000174. doi: 10.1371/journal.pgph.0000174
13. UNAIDS. AIDSinfo | Botswana country factsheet (2021). Available at: https://aidsinfo.unaids.org/ (cited February 2, 2022).
14. Marsh K, Eaton JW, Mahy M, Sabin K, Autenrieth CS, Wanyeki I, et al. Global, regional and country-level 90–90–90 estimates for 2018: assessing progress towards the 2020 target. AIDS. (2019) 33(Suppl 3):S213. doi: 10.1097/QAD.0000000000002355
15. Mine M, Stafford K, Laws R, Marima R, Lekone P, Ramaabya D, et al. Botswana Achieved the joint united nations programme on HIV/AIDS (UNAIDS) 95-95-95 targets: results from the fifth Botswana HIV/AIDS impact survey (BAIS V), 2021. Poster session presented at: aIDS 2022—the 24th international AIDS conference. (2022); Monreal, QC
17. Ministry of Health and Wellness: Republic of Botswana., UNFPA, UNAIDS. Evaluation of the SRH/HIV linkages project in Botswana 2016. Gaborone (2016).
18. UNFPA. Assessment of the government of Botswana/united nations population fund 6th country programme 2017–2021. Gaborone: UNFPA (2021).
19. UNFPA Botswana. Integration of sexual and reproductive health rights and HIV services in Botswana. Gaborone: UNFPA Botswana (2018). Available at: https://botswana.unfpa.org/sites/default/files/pub-pdf/INTERGRATION%20OF%20SEXUAL%20REPRODUCTION.pdf (cited November 19, 2021).
20. Ministry of Health: Republic of Botswana. 2012 Mapping, size estimation & behavioral and biological surveillance survey (BBSS) of HIV/STI among select high-risk sub-populations in Botswana. Gaborone: Ministry of Health: Republic of Botswana (2013).
21. Khalifa A, Stover J, Mahy M, Idele P, Porth T, Lwamba C. Demographic change and HIV epidemic projections to 2050 for adolescents and young people aged 15–24. Global Health Action. (2019) 12(1). doi: 10.1080/16549716.2019.1662685.
22. Providing Contraceptive Services in the Context of HIV Treatment Programmes Implementation Tool HIV Treatment and Reproductive Health (2019). Available at: http://apps.who.int/bookorders (cited November 22, 2022).
23. Okoye JO, Ofodile CA, Adeleke OK, Obioma O. Prevalence of high-risk HPV genotypes in sub-saharan Africa according to HIV status: a 20-year systematic review. Epidemiol Health. (2021) 43. doi: 10.4178/epih.e2021039
24. Mbulawa ZZA, Coetzee D, Williamson AL. Human papillomavirus prevalence in South African women and men according to age and human immunodeficiency virus status. BMC Infect Dis. (2015) 15(1):1–11. doi: 10.1186/s12879-015-1181-8
25. Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. (2011) 87(3):183. doi: 10.1136/sti.2010.047514
26. Michielsen K, Larrson EC, Kågesten A, Erausquin JT, Griffin S, van de Velde S, et al. International sexual health and REproductive health (I-SHARE) survey during COVID-19: study protocol for online national surveys and global comparative analyses. Sex Transm Infect. (2021) 97(2):88–92. doi: 10.1136/sextrans-2020-054664. Available at: https://sti.bmj.com/content/97/2/88 (cited January 18, 2022).33082232
27. Statistics Botswana. 2017 Botswana demographic survey report (2018). Available at: www.statsbots.org.bw (cited January 20, 2022).
28. Boadu I. Coverage and determinants of modern contraceptive use in sub-Saharan Africa: further analysis of demographic and health surveys. Reprod Health. (2022) 19(1):1–11. doi: 10.1186/s12978-022-01332-x
29. Erausquin JT, Tan RKJ, Uhlich M, Francis JM, Kumar N, Campbell L, et al. The International Sexual Health And Reproductive Health Survey (I-SHARE-1): A Multi-Country Analysis of Adults from 30 Countries Prior to and During the Initial COVID-19 Wave. medRxiv. (2021). Available at: https://pubmed.ncbi.nlm.nih.gov/34704103/ (cited January 18, 2022).
30. Sadler S, Tosh J, Pennington R, Rawdin A, Squires H, Romero C, et al. A cost-effectiveness analysis of condom distribution programmes for the prevention of sexually transmitted infections in England on JSTOR. J Epidemiol Community Health. (2017) 71(9):897–904. doi: 10.1136/jech-2017-209020. Available at: https://www.jstor.org/stable/26383958 (cited February 4, 2022).28679537
31. Tseng A, Hills-Nieminen C. Drug interactions between antiretrovirals and hormonal contraceptives. Expert Opin Drug Metab Toxicol. (2013) 9(5):559–72. doi: 10.1517/17425255.2013.772579. Available at: https://pubmed.ncbi.nlm.nih.gov/23425052/ (cited April 12, 2022).23425052
32. Scarsi KK, Darin KM, Chappell CA, Nitz SM, Lamorde M. Drug–drug interactions, effectiveness, and safety of hormonal contraceptives in women living with HIV. Drug Saf. (2016) 39(11):1053–72. doi: 10.1007/s40264-016-0452-7
33. Tittle V, Bull L, Boffito M, Nwokolo N. Pharmacokinetic and pharmacodynamic drug interactions between antiretrovirals and oral contraceptives. Clin Pharmacokinet. (2015) 54(1):23–34. doi: 10.1007/s40262-014-0204-8. Available at: https://pubmed.ncbi.nlm.nih.gov/25331712 / (cited April 8, 2022).25331712
34. Song IH, Borland J, Chen S, Wajima T, Peppercorn AF, Piscitelli SC. Dolutegravir has No effect on the pharmacokinetics of oral contraceptives with norgestimate and ethinyl estradiol. Ann Pharmacother. (2015) 49(7):784–9. doi: 10.1177/1060028015580637. Available at: https://pubmed.ncbi.nlm.nih.gov/25862012/ (cited April 8, 2022).25862012
35. Sweeney S, Obure CD, Terris-Prestholt F, Darsamo V, Michaels-Igbokwe C, Muketo E, et al. The impact of HIV/SRH service integration on workload: analysis from the Integra initiative in two African settings. Hum Resour Health. (2014) 12(1):42. doi: 10.1186/1478-4491-12-42
36. Obure CD, Guinness L, Sweeney S, Initiative I, Vassall A. Does integration of HIV and SRH services achieve economies of scale and scope in practice? A Cost Function Analysis of the Integra Initiative. Sex Transm Infect. (2016) 92(2):130–4. doi: 10.1136/sextrans-2015-052039
37. Bulstra CA, Hontelez JAC, Otto M, Stepanova A, Lamontagne E, Yakusik A, et al. Integrating HIV services and other health services: a systematic review and meta-analysis. PLoS Med. (2021) 18(11):e1003836. doi: 10.1371/journal.pmed.1003836
38. Mutisya R, Wambua J, Nyachae P, Kamau M, Karnad SR, Kabue M. Strengthening integration of family planning with HIV/AIDS and other services: experience from three Kenyan cities. Reprod Health. (2019) 16(Suppl 1):16. doi: 10.1186/s12978-019-0715-8.30736803
39. Close MA, Barden-O’Fallon J, Mejia C. Quality of family planning services in HIV integrated and non-integrated health facilities in Malawi and Tanzania. Reprod Health. (2019) 16(Suppl 1):16. doi: 10.1186/s12978-019-0712-y
40. Milford C, Scorgie F, Rambally Greener L, Mabude Z, Beksinska M, Harrison A, et al. Developing a model for integrating sexual and reproductive health services with HIV prevention and care in KwaZulu-Natal. South Africa. Reprod Health. (2018) 15(1). doi: 10.1186/s12978-018-0633-1
41. Mutemwa R, Mayhew S, Colombini M, Busza J, Kivunaga J, Ndwiga C. Experiences of health care providers with integrated HIV and reproductive health services in Kenya: a qualitative study. BMC Health Serv Res. (2013) 13(1). doi: 10.1186/1472-6963-13-18. Available at: https://pubmed.ncbi.nlm.nih.gov/23311431/ (cited November 23, 2021).23311431
42. Narasimhan M, Yeh PT, Haberlen S, Warren CE, Kennedy CE. Integration of HIV testing services into family planning services: a systematic review. Reprod Health. (2019) 16(Suppl 1):16. doi: 10.1186/s12978-019-0714-9
43. Kimani J, Warren CE, Abuya T, Ndwiga C, Mayhew S, Vassall A, et al. Use of HIV counseling and testing and family planning services among postpartum women in Kenya: a multicentre, non-randomised trial. BMC Womens Health. (2015) 15(1):1–11. doi: 10.1186/s12905-015-0262-6. Available at: https://bmcwomenshealth.biomedcentral.com/articles/10.1186/s12905-015-0262-6 (cited November 22, 2021).25608736
Keywords: COVID—19, SARS—cov—2, HIV—human immunodeficiency virus, family planning (FP), Botswana, Africa, sexual and reproductive health
Citation: Ensor S, Mechie I, Ryan R, Mussa A, Bame B, Tamuthiba L, Moshashane N and Morroni C (2023) Measuring the impact of COVID-19 social distancing measures on sexual health behaviours and access to HIV and sexual and reproductive health services for people living with HIV in Botswana. Front. Glob. Womens Health 4:981478. doi: 10.3389/fgwh.2023.981478
Received: 29 June 2022; Accepted: 22 February 2023;
Published: 8 March 2023.
Edited by:
Supriya Dinesh Mehta, University of Illinois at Chicago, United StatesReviewed by:
Gathari Ndirangu Gichuhi, Jhpiego, United StatesChido Dziva Chikwari, University of London, United Kingdom
© 2023 Ensor, Mechie, Ryan, Mussa, Bame, Tamuthiba, Moshashane and Morroni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imogen Mechie SW1vZ2VuLm1lY2hpZUBuaHMubmV0
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Contraception and Family Planning, a section of the journal Frontiers in Global Women's Health
 Samuel Ensor
Samuel Ensor Imogen Mechie
Imogen Mechie Rebecca Ryan
Rebecca Ryan Aamirah Mussa
Aamirah Mussa Bame Bame
Bame Bame Lefhela Tamuthiba1
Lefhela Tamuthiba1 Chelsea Morroni
Chelsea Morroni