- 1The Second Clinical Medical School of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Oncology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, China
- 3Department of Image, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, China
- 4Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine Syndrome, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 6State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Small bowel adenocarcinoma (SBA), particularly duodenal adenocarcinoma (DA), is a rare gastrointestinal cancer with a dismal prognosis. Data on SBA treatments are limited, and the therapeutic strategy remains uncertain. Currently, chemotherapy is the most used treatment; however, it has a poor median progression-free survival (mPFS) of no more than five months in the second-line setting. We report a case with DA that responded well to the immune checkpoint inhibitor (ICI) tislelizumab plus irinotecan in the second-line treatment. To our knowledge, this is the first report of administering ICIs plus chemotherapy to SBA. Despite the absence of microsatellite instability-high (MSI-H) and high tumor mutational burden (TMB), the patient with TP53/KRAS mutation achieved a significantly long PFS of 17 months, and the benefit is still ongoing. The mechanism of this remarkable efficacy might be associated with an increase in tumor immunogenicity after chemotherapy. The current study presents a promising effect of ICIs plus chemotherapy on SBA, affirming the need to investigate the clinical value of this combination in SBA and the underlying mechanism behind it.
Introduction
Small bowel adenocarcinoma (SBA) is a rare gastrointestinal cancer with a poor prognosis, consisting of 50% duodenal, 30% jejunal, and 20% ileal adenocarcinoma (1). Although there were around 22.7 cases/million in 2004 (2), the incidence of SBA is increasing, with a prevalence in patients over the age of 50 and in men (3). The five-year life expectancy for SBA ranges from 14%–30% (4, 5), whereas the therapeutic options for advanced SBA remain inconclusive. Available data supported chemotherapy as first-line treatment, with a median progression-free survival (mPFS) of six to 11 months (6–8). The optimal mPFS for second-line chemotherapy was only five months (9, 10). Immunotherapy combined with chemotherapy appears to be the cornerstone of treatment for various cancer; however, the efficacy of this combination on SBA has yet to be investigated.
Here, we report the first case of previously treated duodenal adenocarcinoma (DA) with a significant response to second-line tislelizumab in combination with irinotecan. The patient with microsatellite stability (MSS) status, a low tumor mutation burden (TMB), and a TP53/KRAS mutation progressed after three months of first-line oxaliplatin-based chemotherapy (XELOX). However, the patient then responded effectively to the combination of immune checkpoint inhibitors (ICIs) and chemotherapy. Our study aims to present the therapeutic potential of ICIs plus chemotherapy in SBA and discuss this combination’s underlying mechanism.
Current treatments for SBA
Chemotherapy
There is a dearth of evidence from phase III randomized controlled trials on the SBA treatment. The current therapeutic strategies are mainly derived from phase II studies or retrospective analyses. Oxaliplatin-based regimens (XELOX and FOLFOX) seem to be the most used and effective therapy in the first-line treatment, with an mPFS of six to 11 months and median overall survival (OS) of 15 to 22 months (6–8). In the single agent setting, a retrospective study demonstrated an mPFS of six months and an mOS of 11 months for gemcitabine (11). Triplet chemotherapy regimens, like FAM, CAPIRINOX, and FOLFIRINOX, were also evaluated with a dismal median OS ranging from 8 to 13 months (12, 13). For second-line therapy, an irinotecan-based regimen, FOLFIRI, was recommended with an mPFS of three to five months (9, 10). Taxane-based regimens are other options for second-line treatment, with an mPFS of 3.8 months (14).
Immunotherapy
The immunotherapy role in SBA is under evaluation. Pembrolizumab is an ideal choice for previously treated patients with MSI-H SBA. Marabelle A’s study included 19 MSI-H patients, and the results showed that pembrolizumab had an ORR of 42.1% and an mPFS of 9.2 months (15). Similar results were observed in studies by Pedersen, K.S (16). and Cardin, D.B (17). However, the mPFS for patients with MSI-L/MSS was only 2.8 months. In Marabelle A’s study (15), only one patient with MSS exhibited a confirmed partial response but correlated with high TMB. These findings suggested that predictive biomarkers may be important for administering immunotherapy in SBA.
Anti-vascular therapy
A phase II study reported that the mPFS of the XELOX regimen combined with bevacizumab was 8.7 months in first-line treatment (18). Despite the lack of statistical comparison, the mPFS of XELOX plus bevacizumab is comparable to that of XELOX alone, as reported by the same institution (6). However, another retrospective multicenter study reported an mPFS of 15 months in 10 metastatic duodenal and jejunal adenocarcinoma patients treated with bevacizumab plus platinum (19). Notably, among patients treated with bevacizumab-based regimens, the mPFS of six patients with high vascular endothelial growth factor-A (VEGF-A) expression was significantly higher than four patients with low VEGF-A expression, implying that VEGF-A expression might act as a predictor for bevacizumab efficacy.
Target therapy
It is known that the effect of the anti-epidermal growth factor receptor (EGFR) in colorectal cancer (CRC) depends on the KRAS mutation status. Theoretically, approximately 50% of SBA might be treated with anti-EGFR monoclonal antibodies. A case series demonstrated that anti-EGFR might play a role in SBA patients with wild-type KRAS. In the study, two KRAS wild-type patients had a partial response to cetuximab plus irinotecan, and one showed a complete response (20). In contrast, a phase II study reported unsatisfactory results. Among eight non-mutant KRAS SBA patients, panitumumab was administered; however, no clinical responses were observed (21). Moreover, 13 SBA patients with uncertain KRAS status were enrolled in a retrospective multicenter investigation. Cetuximab plus chemotherapy was administrated in first- or second-line treatment, and an ORR of 55% was observed (22). Nevertheless, the mPFS of these patients was only 5.5 months, whereas published data showed that SBA patients treated with XELOX or FOLFOX alone could achieve prolonged PFS. More research is required to identify anti-EGFR agents’ efficacy in SBA.
Case presentation
The patient was a 50-year-old male. In November 2020, the patient was admitted to a local hospital with a stomachache and a tarry stool. Electronic gastroscopy found a huge ring-shaped mass (Figure 1A) in the duodenal bulb, and a poorly differentiated adenocarcinoma was confirmed by biopsy. Subsequently, the patient came to our hospital seeking surgical treatment. Positron emission tomography/computed tomography (PET/CT) indicated a significant uneven thickening of the duodenal bulb’s intestinal wall (47 × 40 mm) and multiple lymph node metastases (Figures 1B, C). The margins of the tumor were indistinguishable from surrounding organs (pancreatic head, gallbladder, and liver). The tumor stage was diagnosed as T4N2M0. The gastrointestinal surgeon assessed the tumor as unresectable and referred the patient to our department. We performed a Next-generation sequencing test of circulating tumor DNA to obtain the molecular profile because the patient refused to perform a biopsy again. Results of ctDNA suggested the presence of TP53 p.S12F KRAS p.G12D mutations, TMB 2.51 Mut/MB (low), and MSS (Supplementary ctDNA results).
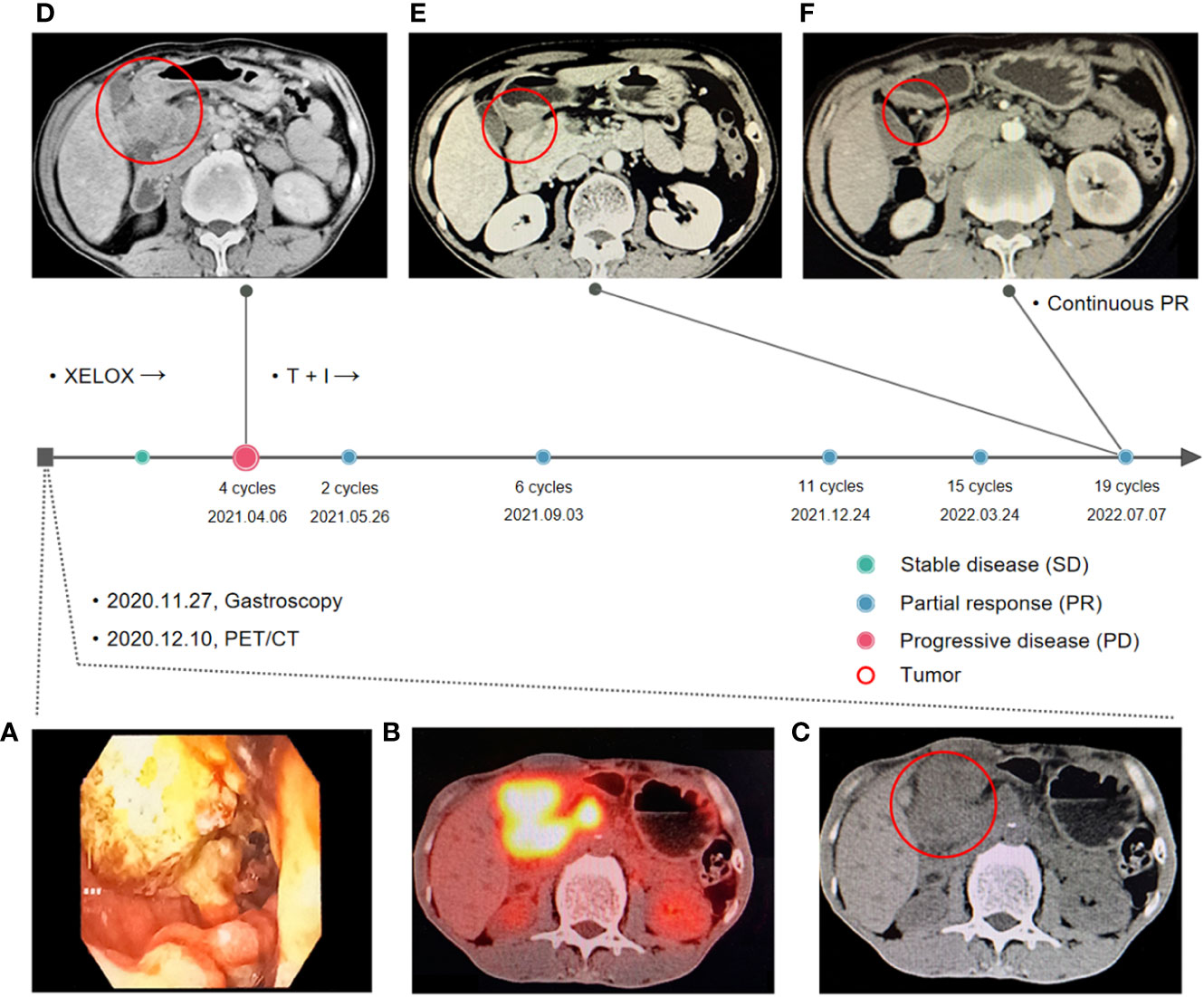
Figure 1 Treatment timeline. (A) Gastroscopy report at first visit: A large annular mass was observed in the duodenal bulb, extending to the upper part of the descending part. (B, C) Positron emission tomography/computed tomography (PET/CT) before the first-line treatment: a significant uneven thickening of the intestinal wall of duodenal bulb (47 × 40 mm) and multiple lymph node metastases. (D) Computed tomography after four sessions of XELOX treatment. The tumor progressed and fused with surrounding lymph nodes after four cycles of XELOX (65 × 63 mm). (E, F) Computed tomography after two and 19 sessions of tislelizumab in combination with irinotecan.
According to published data for advanced SBA, oxaliplatin-based regimens were the most frequently used in first-line treatment with a mPFS of six to 11 months. Therefore, from December 22, 2020, to February 24, 2021, four cycles of the XELOX regimen (oxaliplatin 195 mg day 1, capecitabine 1.5 g bid day 1–14) were administered regularly. After two XELOX cycles, the tumor shrank slightly (35 × 28 mm, Supplementary Figures S1A, B), and the response evaluation criteria in solid tumors 1.1 (RECIST 1.1) indicated stable disease. However, at the end of March, the patient appeared with tarry stool again and was admitted to the gastroenterology department at a local hospital for symptomatic treatment. On April 6, 2021, a chest and abdominal contrast-enhanced CT suggested that the tumor progressed and fused with surrounding lymph nodes (65 × 63 mm, Figure 1D).
In second-line chemotherapy for SBA, available data suggested that the prognosis was poor regardless of the chemotherapy regimen. On the contrary, patients may benefit from immunotherapy and those sensitive to ICIs could achieve significantly longer survival. Therefore, immunotherapy was considered to be used in the second-line treatment, and chemotherapy was also administered due to the patient having no positive biomarkers associated with immunotherapy. On April 14, 2021, the patient was administered tislelizumab, an immune checkpoint inhibitor, in combination with irinotecan. The giant nodules in the intestinal wall and the lymph nodes shrunk significantly after two sessions of tislelizumab in combination with irinotecan (Supplementary Figures S1C, D). After 19 therapy sessions, the giant nodules in the intestinal wall disappeared, and the lymph nodes shrunk significantly (Figures 1E, F). Until September 14, 2022, the patient has received 19 cycles of combination therapy of tislelizumab and irinotecan and three cycles of tislelizumab maintenance therapy.
Currently, there is no evidence about chemotherapy combined with ICIs for SBA. Despite the absence of MSS and low TMB, the patient responded well to immunotherapy combined with chemotherapy for 17 months, and the response is still ongoing. No serious adverse events occurred during the treatment. Compared to FOLFIRI regimens with an mPFS of five months in the second-line setting, this combination has achieved great success, which might be mainly attributed to the synergistic effect of immunotherapy and chemotherapy. However, the current study is only one case. The efficacy of the combination of chemotherapy and ICIs in SBA treatment should be further investigated.
The rationale for combining immunotherapy and chemotherapy in SBA
It was difficult to make a decision on the second-line therapy for the patient. First, the ORR of FOLFIRI in SBA was only 21% and the mPFS was 3.2 months (10), while FOLFIRI may be a better option compared with other regimens. Second, in the first-line therapy, the patient quickly developed resistance to fluorouracil and oxaliplatin, suggesting that it may be inappropriate to use fluorouracil in the second-line treatment. Third, SBA patients with positive biomarkers were sensitive to ICIs and likely to achieve significantly longer survival, but those with MSS/low TMB can hardly benefit from single ICIs (15). The effect of immunotherapy in combination with chemotherapy on SBA has not been reported, although this combination appears to be a cornerstone in the treatment of various cancers. It is well known that regardless of the status of MSS and TMB, ICIs combined with chemotherapy can significantly improve the prognosis of several gastrointestinal malignancies. Chemotherapy not only directly kills tumor cells but also produces a synergistic effect for ICIs by promoting immune recognition and countering immunosuppressive elements (23). On one side, tumor-specific antigens and damage-associated molecular patterns (DAMPs) released by chemotherapy-induced cell death can stimulate the maturation of the antigen-presentation cells and upregulate antigen presentation. In contrast, chemotherapy could modulate suppressive tumor immune microenvironment (TIME) by eliminating immune suppressor cells (regulatory T cells (24) and myeloid-derived suppressor cells (25, 26)) and repolarizing tumor-associated macrophage from M2-like to M1-like phenotype. Therefore, immunotherapy combined with chemotherapy was selected as the patient’s second-line treatment.
TP53/KRAS mutations: Potential immunotherapy biomarkers?
In our case, the patient without MSI-H and high TMB but with co-mutation of TP53/KRAS achieved great tumor regression after being treated with irinotecan plus tislelizumab. TP53 and KRAS mutations have been found to exert remarkable effects on TIME in lung cancer, including increasing PD-L1 expression, facilitating T cell infiltration, and augmenting tumor immunogenicity (27). Retrospective analyses suggested TP53/KRAS co-mutation might serve as a predictive marker for ICI response in non-small cell lung cancer (27, 28). Thus, we investigated whether TP53/KRAS mutations play the same role in gastrointestinal tumors.
Therefore, we assessed the effects of TP53/KRAS mutations on TIME, transcriptome, and proteome in gastrointestinal tumors based on The Cancer Genome Atlas (TCGA) database. Seven types of tumors were evaluated, esophageal carcinoma (ESCA), stomach adenocarcinoma (STAD), liver hepatocellular carcinoma (LIHC), cholangiocarcinoma (CHOL), pancreatic adenocarcinoma (PAAD), colon adenocarcinoma (COAD), rectum adenocarcinoma (READ). We found that in STAD and COAD, TP53/KRAS mutation groups were associated with the “cold” tumor phenotype (a tumor that is unlikely to benefit from ICIs) (29). In the mutation group, immune cells (CD8+ T cell and regulatory T cell) were less infiltrated (Figures 2A, B), and the expression of CD8A and PL-L1 was lower than in the wild-type TP53/KRAS group (Figure 3). Besides, reduced PD-L1 protein expression was also associated with TP53 mutation in STAD. Although co-mutation appeared to be associated with a “hot” tumor phenotype in ESCA, the evidence was too weak due to insufficient sample size. Apart from this, no significant difference was detected. Unfortunately, these results did not support our hypothesis that TP53 and KRAS mutations can serve as predictive biomarkers for ICI response in patients with gastrointestinal tumors. From a different perspective, however, this finding suggested that the patient’s significant benefit was more likely to be associated with the combination of immunotherapy and chemotherapy.
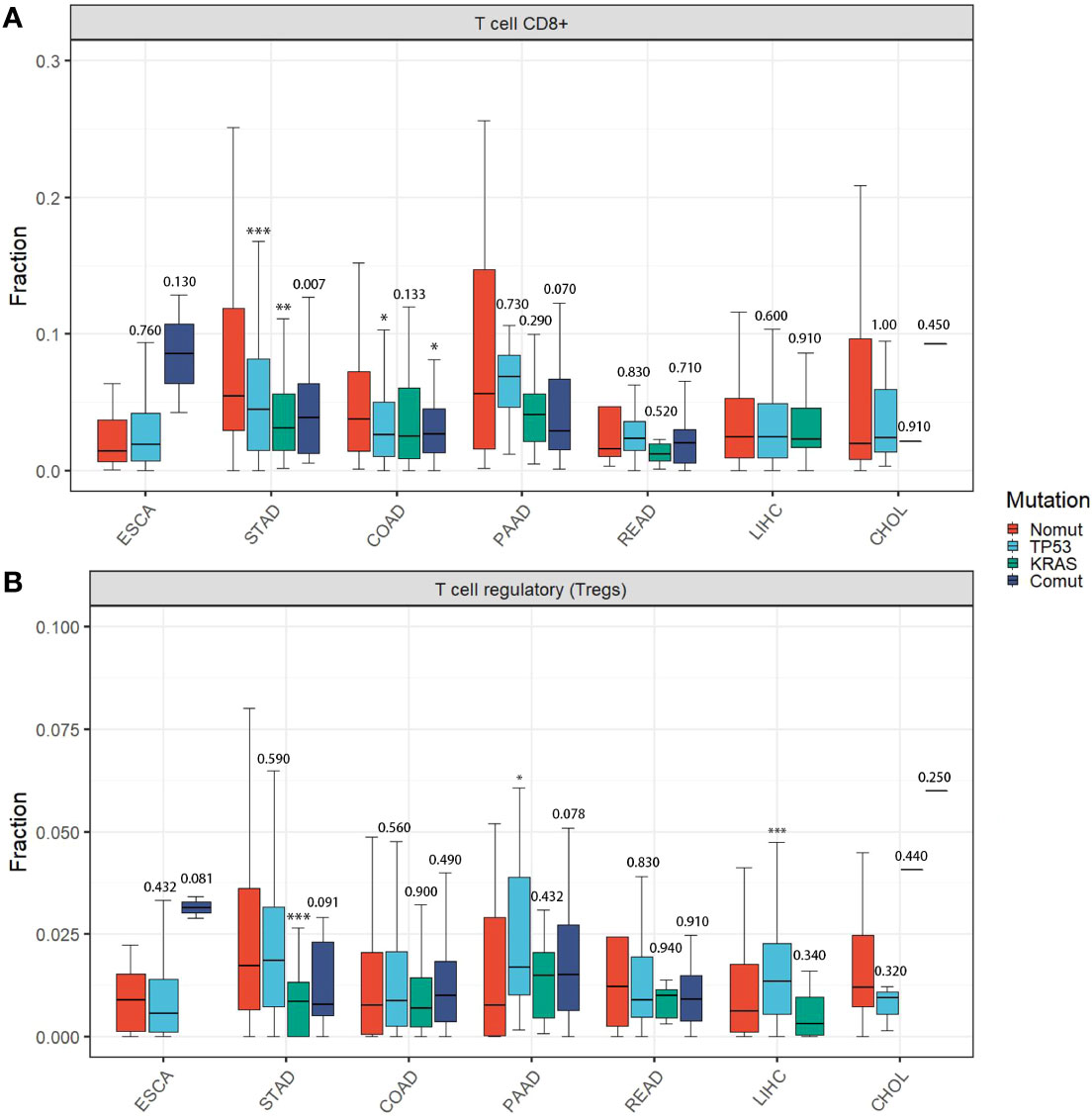
Figure 2 Immune cell infiltration among different TP53/KRAS mutation groups in gastrointestinal tumors. (A) T cell CD8+. (B) T cell regulatory (Tregs). P-values represented TP53/KRAS mutation groups compared to non-mutant group. (Wilcox test. *, P < 0.05; **, P < 0.01; ***, P < 0.001).
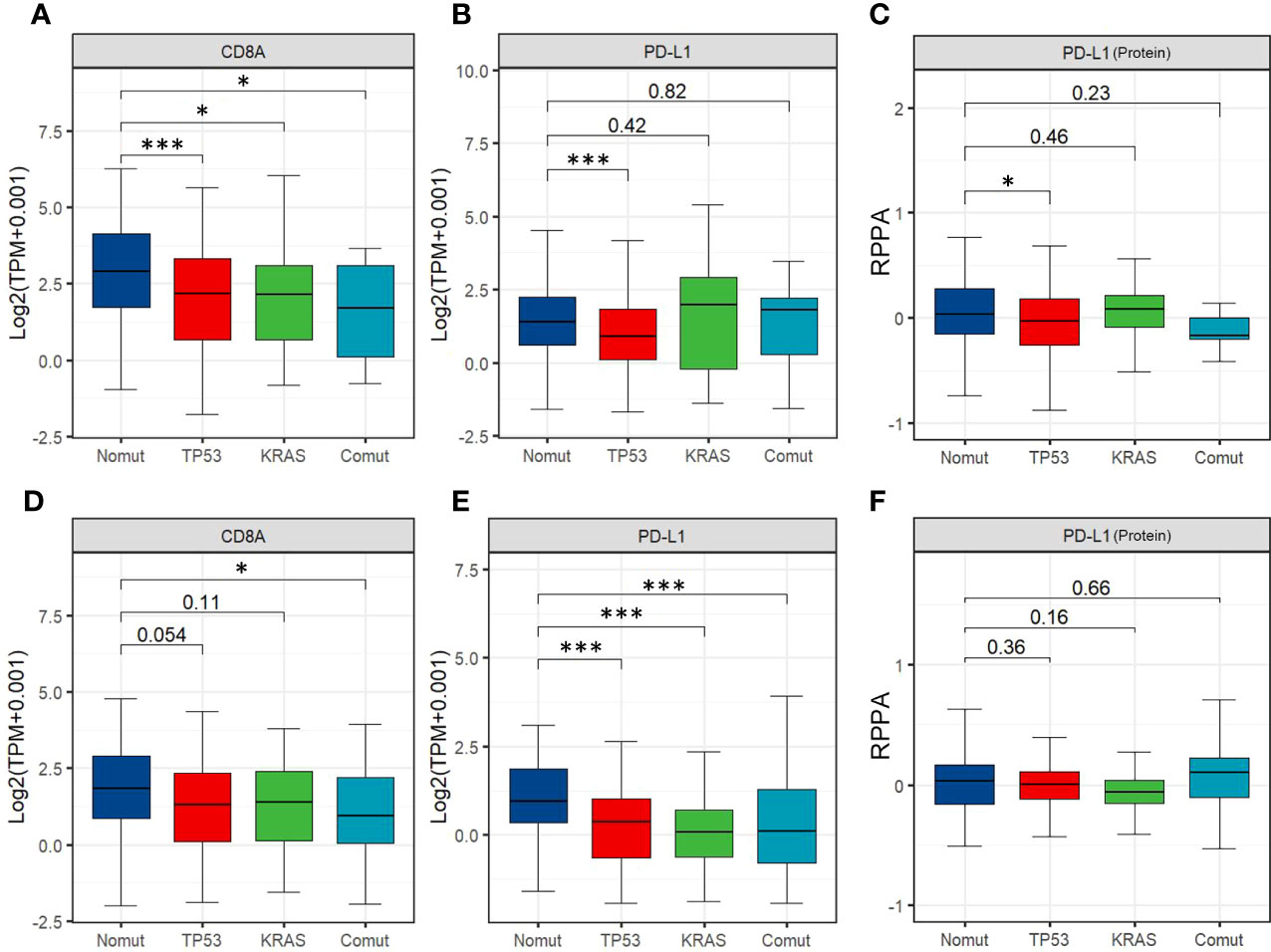
Figure 3 (A, B) Expression of CD8A and PD-L1 among STAD'sTP53/ KRAS mutation groups. (C) Expression of PD-L1 protein among different TP53/ KRAS mutation groups in STAD. (D, E) Expression of CD8A and PD-L1 among different TP53/ KRAS mutation groups in COAD. (F) Expression of PD-L1 protein among different TP53/ KRAS mutation groups in COAD. (Wilcox test. *, P<0.05; ***, P<0.001).
Conclusion
The combination of ICI and chemotherapy should be considered for patients with advanced SBA, particularly duodenal adenocarcinoma.
Data availability statement
Accession numbers for bioinformatics analysis have been provided in Supplementary Table 1. The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conception/Design: XC and H-BZ. Provision of study material or patients: XC and RZ. Collection and/or assembly of data: XC, XQ, and Y-CQ. Data analysis and interpretation: W-ZL, Y-SY and Y-JZ. Manuscript writing: XC and RZ. Final approval of manuscript: L-RL and YL. All authors have read and approved the submitted version of the manuscript.
Acknowledgments
We thanks to the patient and his family.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1046513/full#supplementary-material
Supplementary Figure 1 | Computed tomography after six (A, B), 11 (C, D) and 15 (E, F) sessions of tislelizumab in combination with irinotecan.
References
1. Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, et al. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg (2007) 142:229–35. doi: 10.1001/archsurg.142.3.229
2. Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the united states: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg (2009) 249:63–71. doi: 10.1097/SLA.0b013e31818e4641
3. Pedersen KS, Raghav K, Overman MJ. Small bowel adenocarcinoma: Etiology, presentation, and molecular alterations. J Natl Compr Canc Netw (2019) 17:1135–41. doi: 10.6004/jnccn.2019.7344
4. Lepage C, Bouvier AM, Phelip JM, Hatem C, Vernet C, Faivre J. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut (2004) 53:549–53. doi: 10.1136/gut.2003.026401
5. Overman MJ. Rare but real: management of small bowel adenocarcinoma. In: Am soc clin oncol educ book (2013) Alexandria: American Society of Clinical Oncology. p. 189–93. doi: 10.14694/EdBook_AM.2013.33.189
6. Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of vater. J Clin Oncol (2009) 27:2598–603. doi: 10.1200/jco.2008.19.7145
7. Zhang L, Wang LY, Deng YM, Wang FH, Feng F, Chen YC, et al. Efficacy of the FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma: a three-center study from China. J buon (2011) 16:689–96.
8. Xiang XJ, Liu YW, Zhang L, Qiu F, Yu F, Zhan ZY, et al. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs (2012) 23:561–6. doi: 10.1097/CAD.0b013e328350dd0d
9. Locher C, Malka D, Boige V, Lebray P, Elias D, Lasser P, et al. Combination chemotherapy in advanced small bowel adenocarcinoma. Oncology (2005) 69:290–4. doi: 10.1159/000089678
10. Zaanan A, Gauthier M, Malka D, Locher C, Gornet JM, Thirot-Bidault A, et al. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer (2011) 117:1422–8. doi: 10.1002/cncr.25614
11. Aydin D, Sendur MA, Kefeli U, Umut Unal O, Tastekin D, Akyol M, et al. Evaluation of prognostic factors and treatment in advanced small bowel adenocarcinoma: report of a multi-institutional experience of Anatolian society of medical oncology (ASMO). J buon (2016) 21:1242–9.
12. McWilliams RR, Foster NR, Mahoney MR, Smyrk TC, Murray JA, Ames MM, et al. North central cancer treatment group N0543 (Alliance): A phase 2 trial of pharmacogenetic-based dosing of irinotecan, oxaliplatin, and capecitabine as first-line therapy for patients with advanced small bowel adenocarcinoma. Cancer (2017) 123:3494–501. doi: 10.1002/cncr.30766
13. Gibson MK, Holcroft CA, Kvols LK, Haller D. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin c for metastatic small bowel adenocarcinoma. Oncologist (2005) 10:132–7. doi: 10.1634/theoncologist.10-2-132
14. Aldrich JD, Raghav KPS, Varadhachary GR, Wolff RA, Overman MJ. Retrospective analysis of taxane-based therapy in small bowel adenocarcinoma. Oncologist (2019) 24:e384–6. doi: 10.1634/theoncologist.2018-0573
15. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol (2020) 38:1–10. doi: 10.1200/jco.19.02105
16. Pedersen KS, Foster NR, Overman MJ, Boland PM, Kim SS, Arrambide KA, et al. ZEBRA: A multicenter phase II study of pembrolizumab in patients with advanced small-bowel adenocarcinoma. Clin Cancer Res (2021) 27:3641–8. doi: 10.1158/1078-0432.Ccr-21-0159
17. Cardin DB, Gilbert J, Whisenant JG, Ayers GD, Jalikis F, Dahlman KB, et al. Safety and efficacy of avelumab in small bowel adenocarcinoma. Clin Colorectal Cancer (2022) 21:236–43. doi: 10.1016/j.clcc.2022.03.003
18. Gulhati P, Raghav K, Shroff RT, Varadhachary GR, Kopetz S, Javle M, et al. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: A single-center, open-label, phase 2 study. Cancer (2017) 123:1011–7. doi: 10.1002/cncr.30445
19. Amano T, Iijima H, Shinzaki S, Tashiro T, Iwatani S, Tani M, et al. Vascular endothelial growth factor-a is an immunohistochemical biomarker for the efficacy of bevacizumab-containing chemotherapy for duodenal and jejunal adenocarcinoma. BMC Cancer (2021) 21:978. doi: 10.1186/s12885-021-08724-5
20. Santini D, Fratto ME, Spoto C, Russo A, Galluzzo S, Zoccoli A, et al. Cetuximab in small bowel adenocarcinoma: A new friend? Br J Cancer (2010) 103:1305; author reply 1306. doi: 10.1038/sj.bjc.6605898
21. Gulhati P, Raghav K, Shroff R, Varadhachary G, Javle M, Qiao W, et al. Phase II study of panitumumab in RAS wild-type metastatic adenocarcinoma of small bowel or ampulla of vater. Oncologist (2018) 23:277–e26. doi: 10.1634/theoncologist.2017-0568
22. Dell'Aquila E, Zeppola T, Stellato M, Pantano F, Scartozzi M, Madaudo C, et al. Anti-EGFR therapy in metastatic small bowel adenocarcinoma: Myth or reality? Clin Med Insights Oncol (2020) 14:1179554920946693. doi: 10.1177/1179554920946693
23. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer (2022) 21:28. doi: 10.1186/s12943-021-01489-2
24. Noordam L, Kaijen MEH, Bezemer K, Cornelissen R, Maat L, Hoogsteden HC, et al. Low-dose cyclophosphamide depletes circulating naïve and activated regulatory T cells in malignant pleural mesothelioma patients synergistically treated with dendritic cell-based immunotherapy. Oncoimmunology (2018) 7:e1474318. doi: 10.1080/2162402x.2018.1474318
25. Zhang Y, Bush X, Yan B, Chen JA. Gemcitabine nanoparticles promote antitumor immunity against melanoma. Biomaterials (2019) 189:48–59. doi: 10.1016/j.biomaterials.2018.10.022
26. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell (2015) 28:690–714. doi: 10.1016/j.ccell.2015.10.012
27. Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res (2017) 23:3012–24. doi: 10.1158/1078-0432.Ccr-16-2554
28. Shi Y, Lei Y, Liu L, Zhang S, Wang W, Zhao J, et al. Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer. Cancer Med (2021) 10:2216–31. doi: 10.1002/cam4.3649
Keywords: small bowel adenocarcinoma, duodenal adenocarcinoma, ICIS, immunotherapy, chemotherapy
Citation: Chen X, Zhou R, Li Y, Qu X, Qu Y-c, Li W-z, Ye Y-s, Liu L-r, Zhu Y-j and Zhang H-b (2022) Case report: A case of duodenal adenocarcinoma achieving significantly long survival treating with immune checkpoint inhibitors and chemotherapy without positive biomarkers. Front. Immunol. 13:1046513. doi: 10.3389/fimmu.2022.1046513
Received: 16 September 2022; Accepted: 22 November 2022;
Published: 02 December 2022.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
O. Graciela Scharovsky, National University of Rosario, ArgentinaRuiqing Ma, Aerospace Center Hospital, China
Erica Torchiaro, IRCCS Candiolo Cancer Institute, Italy
Nguyen Minh Duc, Pham Ngoc Thach University of Medicine, Vietnam
Copyright © 2022 Chen, Zhou, Li, Qu, Qu, Li, Ye, Liu, Zhu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-bo Zhang, haibozh@gzucm.edu.cn
†These authors have contributed equally to this work
 Xian Chen
Xian Chen Rui Zhou
Rui Zhou Yong Li
Yong Li Xin Qu2
Xin Qu2 Yan-chun Qu
Yan-chun Qu Wen-zhu Li
Wen-zhu Li Yan-juan Zhu
Yan-juan Zhu Hai-bo Zhang
Hai-bo Zhang