- Hematology Department, The 960th Hospital of The People’s Liberation Army (PLA) Joint Logistics Support Force, Jinan, China
Introduction: Chimerism is closely correlated with disease relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, chimerism rate is dynamic changes, and the sensitivity of different chimerism requires further research.
Methods: To investigate the predictive value of distinct chimerism for relapse, we measured bone marrow (BM), peripheral blood (PB), and T-cell (isolated from BM) chimerism in 178 patients after allo-HSCT.
Results: Receiver operating characteristic (ROC) curve showed that T-cell chimerism was more suitable to predict relapse after allo-HSCT compared with PB and BM chimerism. The cutoff value of T-cell chimerism for predicting relapse was 99.45%. Leukemia and myelodysplastic syndrome (MDS) relapse patients’ T-cell chimerism was a gradual decline from 2 months to 9 months after allo-HSCT. Higher risk of relapse and death within 1 year after allo-HSCT. The T-cell chimerism rates in remission and relapse patients were 99.43% and 94.28% at 3 months after allo-HSCT (P = 0.009), 99.31% and 95.27% at 6 months after allo-HSCT (P = 0.013), and 99.26% and 91.32% at 9 months after allo-HSCT (P = 0.024), respectively. There was a significant difference (P = 0.036) for T-cell chimerism between early relapse (relapse within 9 months after allo-HSCT) and late relapse (relapse after 9 months after allo-HSCT) at 2 months after allo-HSCT. Every 1% increase in T-cell chimerism, the hazard ratio for disease relapse was 0.967 (95% CI: 0.948–0.987, P<0.001).
Discussion: We recommend constant monitoring T-cell chimerism at 2, 3, 6, and 9 months after allo-HSCT to predict relapse.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) provides the most effective therapy in hematological malignancies (HM) and is used to treat leukemia and myelodysplasia (MDS) (1, 2). Despite the enormous benefit of allo-HSCT and improved patient overall survival, disease relapse remains the primary obstacle to successful allo-HSCT. Although the majority of patients with leukemia/MDS can be cured by allo-HSCT, almost all high-risk patients will eventually relapse (3). Relapse represents the leading cause of failure in patients after allo-HSCT and the clinical prognosis for HM patients with allo-HSCT failure is poor (4). Relapse is the most frequent cause of death in transplant patients (5). Notably, deaths due to relapse account for nearly 30% of all deaths within 100 days post-transplant (6). As a consequence, an ideal strategy would be to monitor patients who are at risk of relapses early and then institute effective therapies.
Multiple studies have demonstrated that chimerism monitoring might be associated with HM relapse after allo-HSCT (6, 7). Chimerism can broadly be defined as being able to quantify donor and recipient hematopoiesis and can be conducted in T cells, peripheral blood (PB) and bone marrow (BM) (6, 8, 9). The complete donor chimerism relies on allo-HSCT and the complete reconstruction immune system in HM patients (10). Generally, posttransplant chimerism is a dynamic phenomenon and the mixed chimerism (MC) may be indicative of relapse (11). Given the relapse and poor outcomes in the disease of leukemia/MDS, it is necessary to verify the predictive value of donor chimerism. In the present study, we compare and contrast the dynamic range difference between T-cell chimerism, PB chimerism, and BM chimerism and put forward the most appropriate testing method.
Methods
Patients
After approval of the Ethical Committee of the 960th Hospital of the People’s Liberation Army, a cohort of 178 allo-HSCT patients who underwent chimerism determination was retrospectively analyzed. Chimerism determination was performed at least twice.
Evaluation of donor-host chimerism
Magnetic bead sorting
Magnetic bead sorting separates T cells by binding antibodies on magnetic beads to antigens on T cell surfaces. BM is collected in a centrifuge tube, an appropriate amount of antibody is added and shaken well, followed by the addition of pre-prepared magnetic beads, shaken again, and allowed to stand. Place the tube in the magnetic pole, and the magnetic beads will adhere to the tube’s wall, removing the unbound cell solution while binding the T cells to the magnetic beads. PBS was added and washed twice, resulting in purified T cells with over 90% purity.
STR-PCR
DNA was extracted from PB/BM/purified T cells and amplified for 18 STR markers, including THO1, D21S11, D2S1338, Penta E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Amel, VWA, D8S1179, TPOX, FGA, D6S1043, D12S391, D10S1248, and Penta D. STR-PCR conditions: pre-denaturation at 95°C for 660s, denaturation at 94°C for 20s, annealing at 59°C for 120s, extension at 72°C for 60s, 35 cycles total, and final extension at 60°C for 1500s. The peak graphs were examined to determine the results of STR typing for the reference products.
Definitions
HM relapse was defined by the reappearance of leukemic blasts in PB or relapse of BM blasts ≥5% or the development of extramedullary disease after allo-HSCT (12, 13). T-cell, PB, and BM donor chimerism were monitored at different time points after transplantation. Complete chimerism (CC) was broadly defined as >95% donor cells in the PB and BM samples and MC was defined as 5 to 95% donor cells (12, 14).
Statistical analysis
Descriptive statistics were used to summarize the clinical characteristics of patients in the study. The quantitative data were expressed as mean ± SD (standard deviation). Shapiro–Wilk and Levene test were used to analyze normality and homogeneity of variance of all experimental data. Data with normally and homogeneously distributed were analyzed using SPSS 26.0 by one-way analysis of variance (ANOVA). When all variables in the distribution failed to meet the assumptions of normality and homogeneity, Kruskal-Wallis one-way ANOVA by ranks was used for data evaluation. Significant differences were defined as p-values less than 0.05. Diagnostic accuracy of chimerism on relapse was compared using receiver operating characteristic (ROC) curves. The influence of chimerism at different periods of relapse was evaluated using the Cox proportional hazards model.
Results
Patients
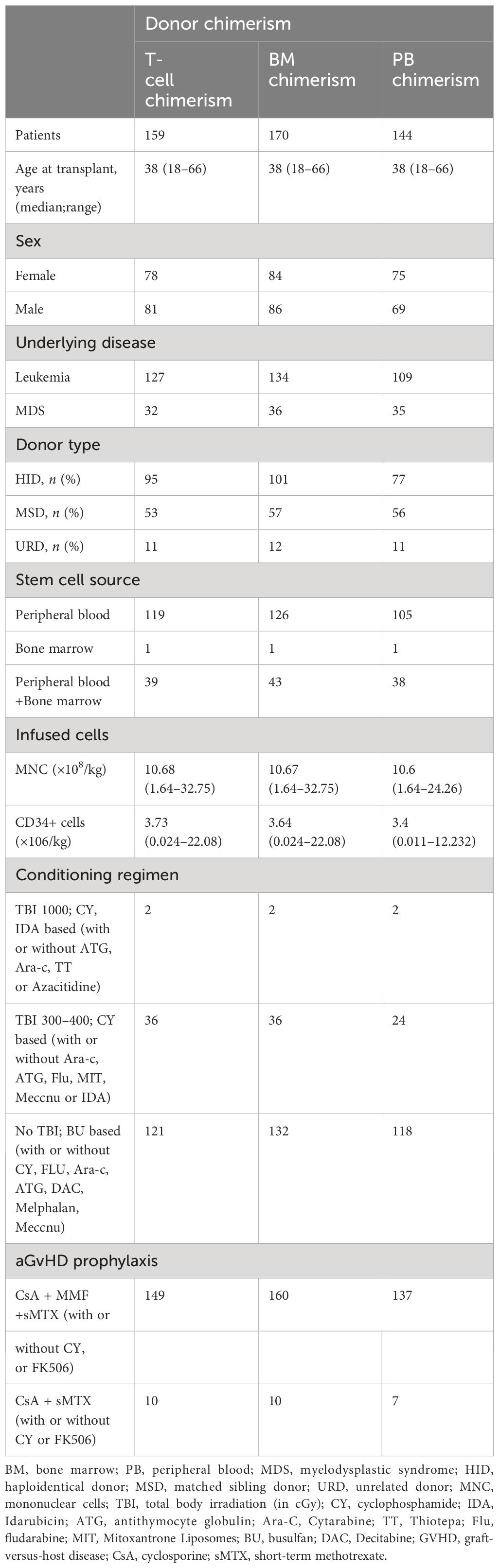
Between May 2017 and June 2023, 178 patients underwent allo-HSCT for leukemia/MDS in our institution. In case of multiple relapse, only the first relapse was considered. One hundred fifty-nine patients underwent T-cell chimerism testing. In addition, 170 patients underwent BM chimerism testing, while 144 underwent PB chimerism testing. Baseline characteristics of patients are detailed in Table 1.
The number of patients and chimerism status
In total, 178 leukemia/MDS allo-HSCT patients were entered into the study cohort. The total number of remission in leukemia/MDS was 106 and 34, respectively, whereas the number of relapses was 32 and 6 (Figure 1A). In the ROC curve, T-cell chimerism showed the highest area under the curve (AUC) value (Figure 1B). The cutoff value of the T-cell chimerism was 99.45%, the sensitivity was 88.5%, and the specificity was 86.7% (AUC = 0.879, P < 0.001). The cutoff value of the BM chimerism was 98.12%, the sensitivity was 51.9%, and the specificity was 100% (AUC = 0.707, P = 0.01). The cutoff value of the PB chimerism was 98.18%, the sensitivity was 45.5%, and the specificity was 100% (AUC = 0.739, P = 0.083).
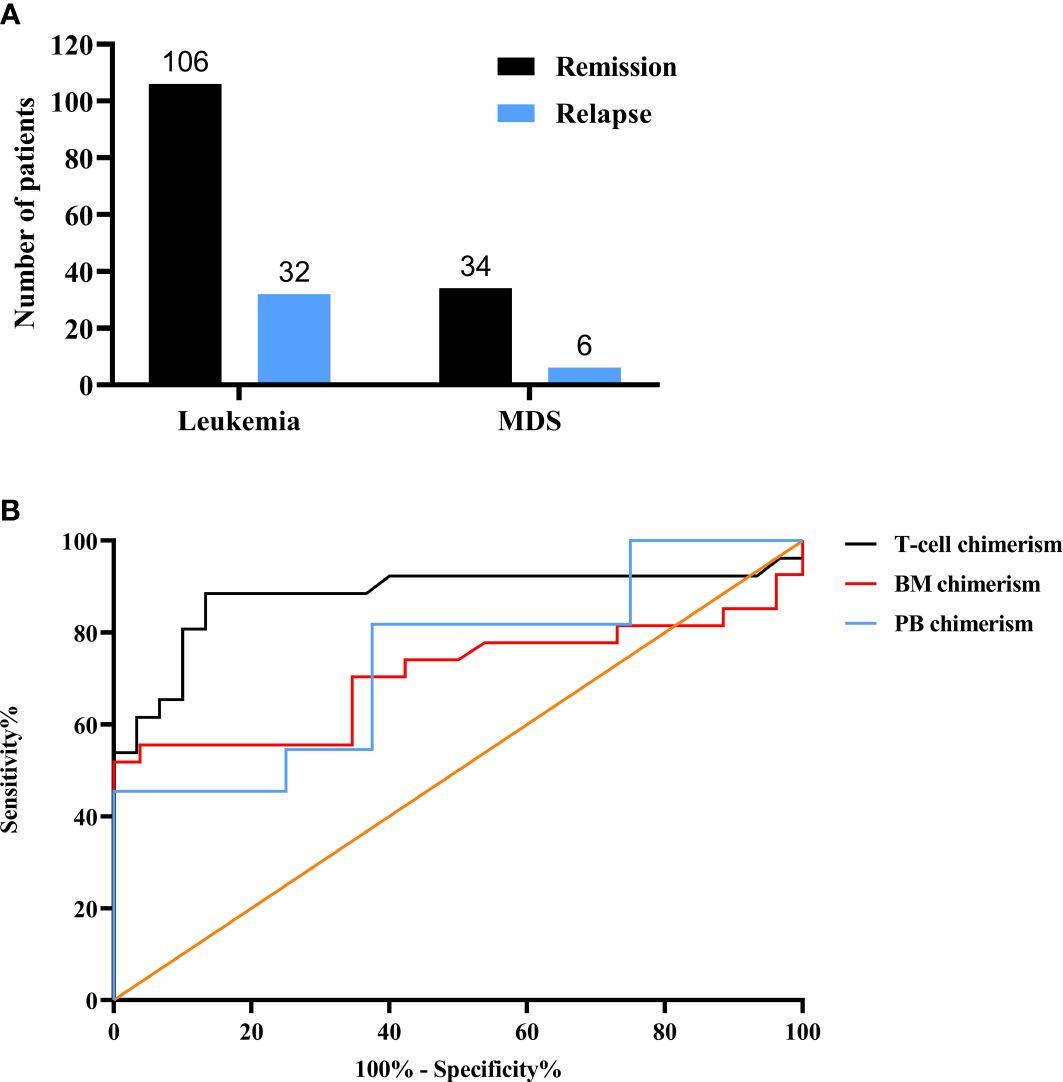
Figure 1 Chimerism status after allo-HSCT. (A) The numbers of leukemia and MDS. (B) The comparison of three different chimerism using ROC curves. MDS, myelodysplasia; ROC, receiver operating characteristic; BM, bone marrow; PB, Peripheral blood.
Comparison of donor-host chimerism in relapse patients at different time intervals after allo-HSCT
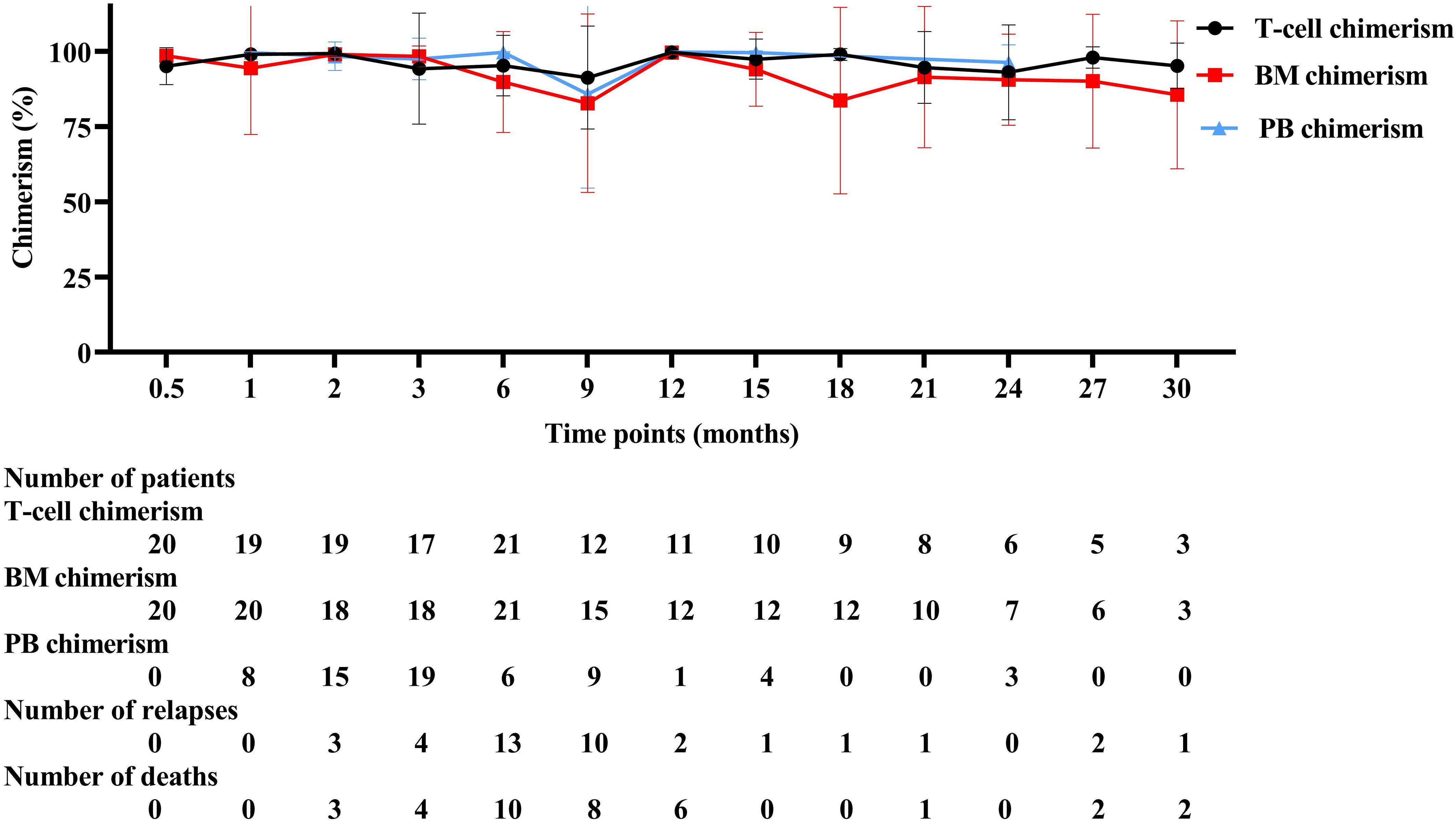
There was a high risk of relapse and mortality in relapsed patients within 12 months after allo-HSCT and three chimerism displayed a downward trend as a whole (Figure 2). Chimerism reached its highest point at 12 months after allo-HSCT. T-cell chimerism was statistically significant for 9 months and 12 months after allo-HSCT (P = 0.001). Detailed values are shown in Supplementary Table S1.
Comparison of donor-host chimerism in remission and relapse patients at different time intervals after allo-HSCT
T-cell chimerism decreased progressively from 2 months after allo-HSCT and dropped to the lowest point at 9 months (Figure 3A). T-cell chimerism of remission and relapsed patients at 3 months was 99.43% and 94.28% (P = 0.009), respectively. T-cell chimerism of remission and relapsed patients at 6 months was 99.31% and 95.27% (P = 0.013), respectively. T-cell chimerism of remission and relapsed patients at 9 months was 99.26% and 91.32% (P = 0.024), respectively. BM chimerism decreased progressively from 3 months after allo-HSCT and dropped to the lowest point at 9 months (Figure 3B). BM chimerism of remission and relapsed patients at 6 months was 96.38% and 89.82% (P = 0.044), respectively. BM chimerism of remission and relapsed patients at 9 months was 99.33% and 82.79% (P = 0.198), respectively. PB chimerism decreased progressively from 6 months after allo-HSCT and dropped to the lowest point at 9 months (Figure 3C). PB chimerism of remission and relapsed patients at 9 months was 99.58% and 85.78% (P = 0.531), respectively. Detailed values are shown in Supplementary Table S2.
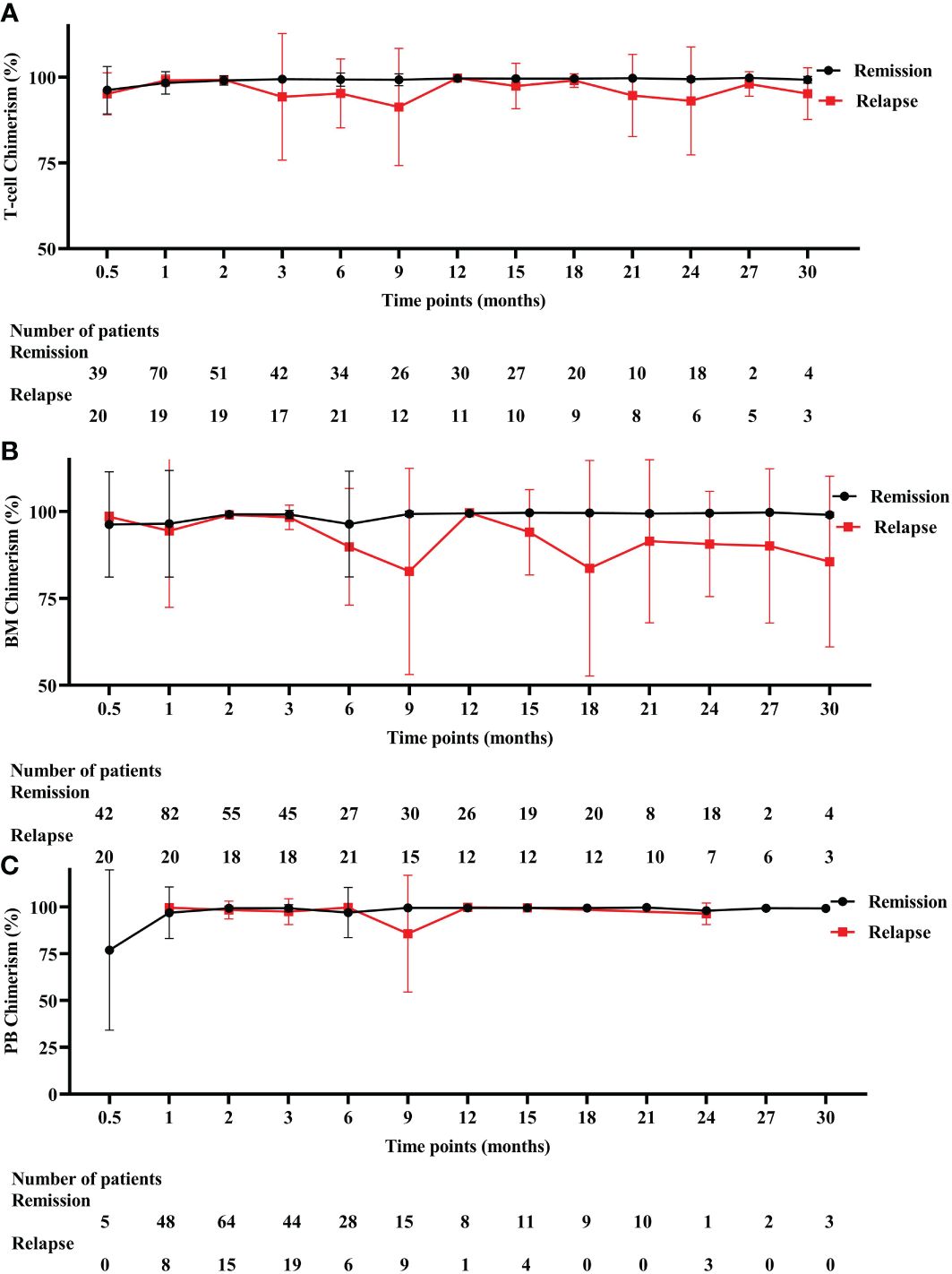
Figure 3 Three different chimerism in remission and relapsed patients. (A) T-cell chimerism. (B) BM chimerism. (C) PB chimerism. BM, bone marrow, PB, peripheral blood.
Comparison of donor-host chimerism in early relapse and late relapse patients at different time intervals after allo-HSCT
Among 38 relapse patients, T-cell chimerism of early relapse (relapse within 9 months after allo-HSCT) and late relapse (relapse after 9 months after allo-HSCT) was 99.16% and 99.76% (P = 0.036) at 2 months after allo-HSCT, respectively (Figure 4A). BM chimerism of early relapse patients showed a decreasing trend at 3 months after allo-HSCT, while the PB chimerism decreasing tendency was preserved at 6 months after allo-HSCT (Figures 4B, C). Detailed values are shown in Supplementary Table S3.
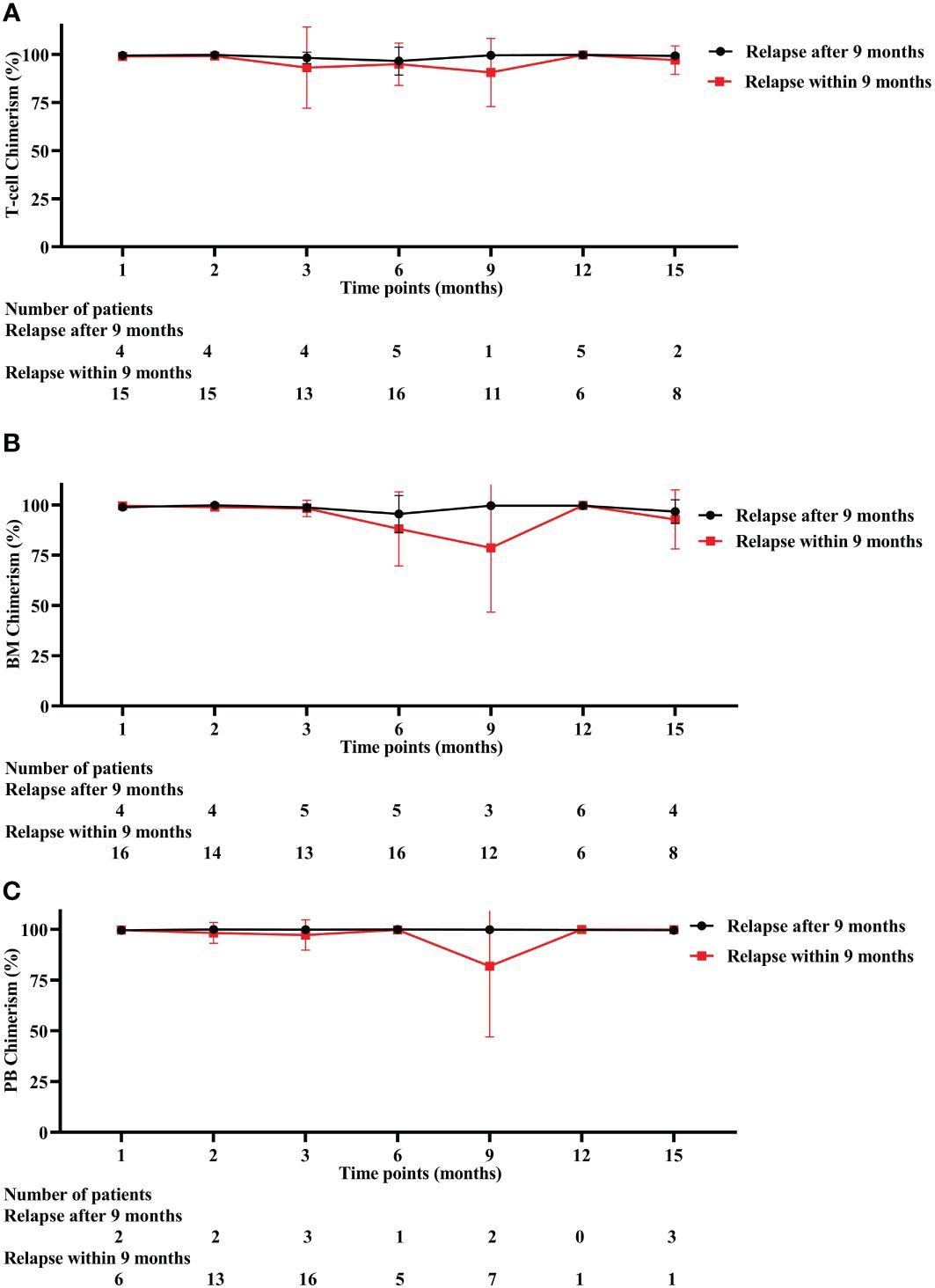
Figure 4 Three different chimerism in early and late relapse patients. (A) T-cell chimerism. (B) BM chimerism. (C) PB chimerism. BM, bone marrow, PB, peripheral blood.
Relapse risk
We created a univariate Cox model for T-cell chimerism predicting relapse.
T-cell chimerism showed that compared with the CC, MC had increased odds of relapse (Hazard Ratio = 5.377, 95% CI: 2.249–12.853, P < 0.001). In addition, every 1% increase in T-cell chimerism, the hazard ratio for disease relapse was 0.967 (95% CI: 0.948–0.987, P < 0.001).
Discussion
Due to failure to avoid relapse of disease, relapse patients after allo-HSCT had a poor prognosis and limited therapies (15). HM relapse remains the primary cause of the dismal prognosis after allo-HSCT (16, 17). Thus, precision predicting the relapse of disease after allo-HSCT to guide treatment decisions is particularly important. To predict the relapse of high-risk individuals early and take specific intervention measures in time, it is helpful for clinicians to reduce the poor outcomes of patients. Related studies have confirmed that the chimerism status was strongly associated with the risk of disease relapse (7, 18–20). Chimerism is a dynamic process and ever-changing, and MC is responsible for allo-HSCT failure (21). The most appropriate chimerism analysis is based on disease and allo-HSCT type (22). However, there is a lack of comparative analysis of different chimerism of dynamic changes. This report details the evolution of dynamic changes in T-cell chimerism, BM chimerism, and PB chimerism at different points after allo-HSCT. By examining these chimerism statuses, one can identify the chimerism that is most strongly linked to relapse, which offers valuable reference information for clinical practice.
The ROC curve is universally used as a method for assessing the accuracy of potential biomarkers by calculating the area under the ROC curve (AUC) (23–25). As shown in the ROC curve, compared with BM chimerism and PB chimerism, T-cell chimerism had the highest AUC and was more suitable for the prediction of disease relapse. Through successive tests can be able to effectively identify high-risk relapse patients, which indicates close monitoring is extremely important (26, 27). Donor cells need time to rebuild the hematopoietic system after allo-HSCT, so it is not advisable to assess the chimerism status after transplantation too early (22). In this study, the study was examined from 15 days after allo-HSCT, and the study’s duration was restricted to a 30-month follow-up period. We found that the number of relapse and death patients is continuously increasing with a decrease in chimerism rate from 2 to 9 months after allo-HSCT. The change trend of the chimerism rate is opposite to the number of relapse and death patients. The relapse of leukemia/MDS is accompanied by a drop in donor chimerism (22). Hence, these findings further support that chimerism can predict clinical relapse. Given the decrease in the number of relapsed patients after 9 months, these findings imply that 9 months following allo-HSCT may be an important turning point.
For those with relapse patients, high-risk relapse mainly occurs in 12 months after allo-HSCT. A relatively large of relapse and death was at this stage. Early relapse affects the state of T-cell chimerism after allo-HSCT. Compared with late relapse patients, early relapse patients’s T-cell chimerism rate decreased gradually from 2 months to 9 months after allo-HSCT in 38 relapse samples. Nine months after allo-HSCT as an important time point, the T-cell chimerism rate is transformed from low to high. Changes in T-cell chimerism were most pronounced at 3, 6, and 9 months after allo-HSCT in patients with early relapses, which can serve as a preventive screen for disease relapse earlier, whereas 2 months failed to detect a difference in remission and relapse patients, perhaps related to the small sample size.
It is notable that we found MC with a high probability of relapse by univariate Cox regression analysis. The relapse incidence of mixed T-cell chimerism was several times higher than complete donor chimerism. Mixed T-cell chimerism requires close monitoring and additional interventions due to its high risk of relapse. With each 1% increase in the T-cell chimerism, the probability of relapse of leukemia/MDS after allo-HSCT decreases by 3.3%.
Furthermore, as an independent predictor, MRD detection has a strong clinical correlation with disease relapse after allo-HSCT, which is useful for guiding treatment decisions. With the use of NGS and other technologies, MRD detection becomes more sensitive, overcoming the low sensitivity of chimerism in STR-PCR detection. When there are no high-specificity immunophenotypic markers, chimerism detection can be used to supplement them. The feasibility of combining the two in clinical research has been confirmed in related studies.
In conclusion, our research clarifies that, in contrast to BM and PB chimerism, continuous T-cell chimerism monitoring predicting relapse is more accurate. Therefore, we recommend that the T-cell chimerism rate should be closely monitored for 2, 3, 6, and 9 months after allo-HSCT. This would be helpful for early detection of relapse after allo-HSCT and timely interventions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethical Committee of the 960th Hospital of the People’s Liberation Army. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZL: Writing – original draft. JW: Writing – original draft. LD: Conceptualization, Writing – original draft. XL: Data curation, Writing – original draft. FK: Methodology, Writing – original draft. YZ: Formal analysis, Writing – review & editing. YH: Software, Writing – review & editing. FZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by Natural Science Foundation of Shandong Province (NO. ZR2022MH135).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1382099/full#supplementary-material
References
1. Baur R, Karl F, Böttcher-Loschinski R, Stoll A, Völkl S, Gießl A, et al. Accumulation of T-cell-suppressive PD-L1(high) extracellular vesicles is associated with GvHD and might impact GvL efficacy. J Immunother Cancer. (2023) 11(3):e006362. doi: 10.1136/jitc-2022-006362
2. Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev. (2020) 7:CD7001. doi: 10.1002/14651858.CD007001.pub5
3. Balasubramanian SK, Azmi AS, Maciejewski J. Selective inhibition of nuclear export: a promising approach in the shifting treatment paradigms for hematological neoplasms. Leukemia. (2022) 36:601–12. doi: 10.1038/s41375-021-01483-z
4. Chen DP, Chang SW, Wang PN, Hus FP, Tseng CP. Association between single nucleotide polymorphisms within HLA region and disease relapse for patients with hematopoietic stem cell transplantation. Sci Rep. (2019) 9:13731. doi: 10.1038/s41598-019-50111-5
5. Uhl FM, Chen S, O'Sullivan D, Edwards-Hicks J, Richter G, Haring E, et al. Metabolic reprogramming of donor T cells enhances graft-versus-leukemia effects in mice and humans. Sci Transl Med. (2020) 12(567):eabb8969. doi: 10.1126/scitranslmed.abb8969
6. Ciurea SO, Kothari A, Sana S, Al Malki MM. The mythological chimera and new era of relapse prediction post-transplant. Blood Rev. (2023) 57:100997. doi: 10.1016/j.blre.2022.100997
7. Llaurador G, Nicoletti E, Prockop SE, Hsu S, Fuller K, Mauguen A, et al. Donor-host lineage-specific chimerism monitoring and analysis in pediatric patients following allogeneic stem cell transplantation: influence of pretransplantation variables and correlation with post-transplantation outcomes. Transplant Cell Ther. (2021) 27:780–1. doi: 10.1016/j.jtct.2021.05.020
8. Bendjelloul M, Usureau C, Etancelin P, Saidak Z, Lebon D, Garçon L, et al. Utility of assessing CD3(+) cell chimerism within the first months after allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia. HLA. (2022) 100:18–23. doi: 10.1111/tan.14557
9. Reshef R, Hexner EO, Loren AW, Frey NV, Stadtmauer EA, Luger SM, et al. Early donor chimerism levels predict relapse and survival after allogeneic stem cell transplantation with reduced-intensity conditioning. Biol Blood Marrow Transplant. (2014) 20:1758–66. doi: 10.1016/j.bbmt.2014.07.003
10. Alho AC, Kim HT, Chammas MJ, Reynolds CG, Matos TR, Forcade E, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. (2016) 127:646–57. doi: 10.1182/blood-2015-10-672345
11. Kanaan SB, Urselli F, Radich JP, Nelson JL. Ultrasensitive chimerism enhances measurable residual disease testing after allogeneic hematopoietic cell transplantation. Blood Adv. (2023) 7:6066–79. doi: 10.1182/bloodadvances.2023010332
12. Zhang H, Fan Z, Huang F, Han L, Xu Y, Xu N, et al. Busulfan plus cyclophosphamide versus total body irradiation plus cyclophosphamide for adults acute B lymphoblastic leukemia: an open-label, multicenter, phase III trial. J Clin Oncol. (2023) 41:343–53. doi: 10.1200/JCO.22.00767
13. Elfeky R, Lucchini G, Lum SH, Ottaviano G, Builes N, Nademi Z, et al. New insights into risk factors for transplant-associated thrombotic microangiopathy in pediatric HSCT. Blood Adv. (2020) 4:2418–29. doi: 10.1182/bloodadvances.2019001315
14. Cheng AP, Cheng MP, Loy CJ, Lenz JS, Chen K, Smalling S, et al. Cell-free DNA profiling informs all major complications of hematopoietic cell transplantation. Proc Natl Acad Sci USA. (2022) 119(4):e2113476118. doi: 10.1073/pnas.2113476118
15. de Jong G, Janssen JJWM, Biemond BJ, Zeerleder SS, Ossenkoppele GJ, Visser O, et al. Survival of early posthematopoietic stem cell transplantation relapse of myeloid Malignancies. Eur J Haematol. (2019) 103:491–9. doi: 10.1111/ejh.13315
16. Schwarzbich MA, Dai H, Kordelas L, Beelen DW, Radujkovic A, Müller-Tidow C, et al. Pre-transplant serum leptin levels and relapse of acute myeloid leukemia after allogeneic transplantation. Int J Mol Sci. (2022) 23(4):2337. doi: 10.3390/ijms23042337
17. Zhang R, Wang L, Chen P, Gao X, Wang S, Li F, et al. Haematologic Malignancies with unfavourable gene mutations benefit from donor lymphocyte infusion with/without decitabine for prophylaxis of relapse after allogeneic HSCT: A pilot study. Cancer Med. (2021) 10:3165–76. doi: 10.1002/cam4.3763
18. Loke J, McCarthy N, Jackson A, Siddique S, Hodgkinson A, Mason J, et al. Posttransplant MRD and T-cell chimerism status predict outcomes in patients who received allografts for AML/MDS. Blood Adv. (2023) 7:3666–76. doi: 10.1182/bloodadvances.2022009493
19. Navarro-Bailón A, Carbonell D, Escudero A, Chicano M, Muñiz P, Suárez-González J, et al. Short tandem repeats (STRs) as biomarkers for the quantitative follow-up of chimerism after stem cell transplantation: methodological considerations and clinical application. Genes (Basel). (2020) 11(9):993. doi: 10.3390/genes11090993
20. Choi YB, Lee JW, Sung KW, Koo HH, Kim HJ, Yoo KH. Impact of day 14 peripheral blood chimerism after allogeneic hematopoietic stem cell bone transplantation on the treatment outcome of non-malignant disease. J Korean Med Sci. (2019) 34:e46. doi: 10.3346/jkms.2019.34.e46
21. Xu L, Durruthy-Durruthy R, Eastburn DJ, Pellegrino M, Shah O, Meyer E, et al. Clonal evolution and changes in two AML patients detected with A novel single-cell DNA sequencing platform. Sci Rep. (2019) 9:11119. doi: 10.1038/s41598-019-47297-z
22. Kricke S, Rao K, Adams S. The significance of mixed chimaerism and cell lineage chimaerism monitoring in paediatric patients post haematopoietic stem cell transplant. Br J Haematol. (2022) 198:625–40. doi: 10.1111/bjh.18190
23. Hegazy F, Aboelnasr E, Abuzaid M, Kim IJ, Salem Y. Comparing validity and diagnostic accuracy of clarke's angle and foot posture index-6 to determine flexible flatfoot in adolescents: A cross-sectional investigation. J Multidiscip Healthc. (2021) 14:2705–17. doi: 10.2147/JMDH.S317439
24. Li LY, Wang YY, Gao JW, Chen J, Kang M, Ying P, et al. The predictive potential of altered voxel-based morphometry in severely obese patients with meibomian gland dysfunction. Front Neurosci. (2022) 16:939268. doi: 10.3389/fnins.2022.939268
25. Marquez-Pedroza J, Cárdenas-Bedoya J, Morán-Moguel MC, Escoto-Delgadillo M, Torres-Mendoza BM, Pérez-Ríos AM, et al. Plasma microRNA expression levels in HIV-1-positive patients receiving antiretroviral therapy. Biosci Rep. (2020) 40(5):BSR20194433. doi: 10.1042/BSR20194433
26. Wang N, Xi W, Lu S, Jiang J, Wang C, Zhu Z, et al. A novel inflammatory-nutritional prognostic scoring system for stage III gastric cancer patients with radical gastrectomy followed by adjuvant chemotherapy. Front Oncol. (2021) 11:650562. doi: 10.3389/fonc.2021.650562
Keywords: chimerism, allo-HSCT, relapse, leukemia, MDS
Citation: Li Z, Wang J, Deng L, Liu X, Kong F, Zhao Y, Hou Y and Zhou F (2024) The predictive value of T-cell chimerism for disease relapse after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 15:1382099. doi: 10.3389/fimmu.2024.1382099
Received: 05 February 2024; Accepted: 25 March 2024;
Published: 11 April 2024.
Edited by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaReviewed by:
Marco Andreani, Bambino Gesù Children’s Hospital (IRCCS), ItalyYang Cao, Huazhong University of Science and Technology, China
Huidong Guo, Peking University People’s Hospital, China
Copyright © 2024 Li, Wang, Deng, Liu, Kong, Zhao, Hou and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zhou, zhoufang1@medmail.com.cn
†These authors have contributed equally to this work
 Zhipeng Li
Zhipeng Li Jing Wang†
Jing Wang† Fang Zhou
Fang Zhou