- 1Institute of Biodiversity, Animal Health & Comparative Medicine, College of Medical, Veterinary & Life Sciences, University of Glasgow, Glasgow, United Kingdom
- 2Department of Control of Neglected Tropical Diseases, World Health Organization, Geneva, Switzerland
- 3Oshun Partnership, London, United Kingdom
Achieving zero human deaths from dog-mediated rabies has been set as a global target for 2030. However, the COVID-19 pandemic has disrupted essential health services across the world, with disproportionate impacts on Neglected Tropical Diseases. Through a mixed-method study using stakeholder questionnaires and in-depth interviews, we examined the scale and nature of disruption from the first year of the pandemic to rabies control programs, and reflected on lessons for the future. Study participants included practitioners and policymakers working in government, academia, international organizations, and the pharmaceutical industry across 48 countries, mainly in Africa and Asia. Mass dog vaccination, essential to rabies control, was most heavily impacted and in 2020, was carried out as planned in just 5% of surveyed countries. Access to post-exposure prophylaxis (PEP) also decreased due to fear of COVID-19 infection and difficulties in reaching health care centers. Dog vaccination and PEP delivery suffered from disruptions to the importation and distribution of vaccines. School closures affected rabies awareness activities and, when public events moved online, they could not reach the most disadvantaged groups. Surveillance, already weak, was severely disrupted by movement restrictions which, together with reduced demand for PEP, exacerbated under-reporting. Participants reported growing complaints around free-roaming dogs, with numbers likely to have increased in some settings. In some countries, dog rabies outbreaks and human rabies cases were already ascribed to the pandemic, but further impacts are likely still to be realized. Meanwhile, decreased demand for PEP from COVID-19 constraints could lead to reduced procurement in future. In the wake of post-COVID-19 demands on health services, there is an opportunity for veterinary services to show leadership in progressing the Zero by 30 agenda, particularly in scaling up mass dog vaccination within and across countries, as well as potential to make better use of community-based vaccinators. Countries must further secure stable procurement of dog and human vaccines, classifying them as essential goods prioritized for import and where needed, through sharing of stocks. Dedicated telemedicine services also show promise, for example through fostering participatory disease surveillance, including Integrated Bite Case Management, and delivering up-to-date instructions on the closest sources of PEP.
1 Introduction
Rabies is one of the oldest human diseases of animal origin known to humankind (1). All mammals can be infected with the rabies virus but, domestic dogs – because of their widespread presence in most areas of the world and their proximity to humans – are usually the main source of human infections. Worldwide, nearly all human rabies cases are caused by a bite or scratch from a rabid dog (2).
In 1885, the history of rabies reached a turning point when the vaccine developed by Louis Pasteur saved the life of the young Joseph Meister, the first person known to have survived this incurable and fatal disease. More than a century of progress in laboratory research, field epidemiology, studies in the socio-ecology of rabies, and applied research to improve the delivery of human and dog vaccination has led to the elimination of dog-mediated rabies in many areas of the world, mainly in the Global North (3) but also in Central and South America (4). At present, an estimated 59,000 people still die of rabies every year, especially in Asia and Africa (5).
In 2015, the World Health Organization (WHO), the World Organisation for Animal Health (OIE), the Food and Agriculture Organization of the United Nations (FAO), and the Global Alliance for Rabies Control (GARC) developed the first comprehensive global strategic plan for the elimination of dog-mediated human rabies deaths by 2030 (“Zero by 30”) (6). This strategy applies the One Health approach, which acknowledges the interlinkages between human, animal, and environmental health and aims at developing integrated strategies for efficient use of resources and, ultimately, shared benefits. It aims at supporting countries in the development of national and regional plans for rabies elimination that include the following key areas of action: expanding and consolidating mass dog vaccination, increasing access to affordable post-exposure prophylaxis for exposed individuals, and closely engaging with local communities to ensure their uptake. Collecting reliable data through effective surveillance is fundamental to assessing the burden of rabies, targeting high-risk populations, and monitoring progress towards elimination (7). In 2018, these institutions further joined forces in leading the United Against Rabies Forum, a platform for global, regional, national and local stakeholders to coordinate and accelerate responses to dog-mediated rabies (8).
As a Neglected Tropical Disease (NTD), rabies also falls within the scope of the WHO’s 2021-2030 roadmap for the elimination of 20 too-long ignored, yet fully preventable, diseases of poverty that disproportionately affect remote, rural, or underserved communities in the Global South (9). In such a crucial moment – the beginning of a decade-long, intense effort – the emergence of SARS-CoV-2 has swamped societies and put national health systems under immense pressure (10). As NTD interventions are implemented at the community level, these control programs have been particularly affected. According to a model-based assessment of the impact on the achievement of the 2030 goals for seven NTDs (not including rabies), most programs are expected to be able to recover from a 1-year interruption. However, longer delays as we are witnessing, will inevitably necessitate more intensive remedial strategies and will increase their burden (11). Of all NTD interventions, programs that relied on mass treatment/preventive chemotherapy were reportedly the most frequent and severely affected by the pandemic (12).
With regards to rabies, there is limited literature on the effect of the pandemic. In the Peruvian city of Arequipa where transboundary spread led to the re-emergence of rabies in 2015, modeling suggested that reduced dog vaccinations and decreased surveillance during the pandemic lead to an observed rise in cases and spread to a nearby city (13). Modelling from Haiti suggested that restarting dog vaccination in 2021 compared to 2022 would avert almost 300 human rabies deaths and prevent over 6,000 human rabies exposures over the next five years (14). Concerns were reported about chronic shortages of human rabies vaccines in Pakistan being exacerbated by the pandemic (15). While in November 2020, the first rabies death was recorded in Bhutan since 2016 (a 3-year-old girl) - stressing the need for timely post-exposure vaccination, even during these exceptional times (16).
Drawing from the observations and experiences of people directly engaged in rabies control programs around the world, this study provides a global, cross-sectoral assessment of the scale and nature of COVID-19 disruptions to current and planned efforts to eliminate dog-mediated human rabies.
2 Materials and Methods
2.1 Study Design
This mixed-method study consists of a quantitative and qualitative survey, and qualitative interviews.
The survey questionnaire, in English, comprised 26 closed-ended questions (1 Likert scale, 7 single-choice, 8 yes/no, and 10 multi-choice) and 35 open-ended questions (25 calls to expand and comment on the previous close-ended question and 10 independent questions). The questionnaire was drafted by DN, an anthropologist, reviewed for content and intelligibility by the rest of the team, and prepared in Google Forms. The topics covered were mass dog vaccination and veterinary services, access and delivery of post-exposure prophylaxis, surveillance, awareness activities, and issues intersecting human and veterinary health. Because of the range of respondents and varied progress in rabies control across settings, the questions were intentionally generic. For comparison of the early COVID-19 scenario with the pre-pandemic situation, a precise timeline (the past 5 years) was given only for the three questions about the number of animal cases, animal bite patients, and human rabies deaths in 2020 surveillance data. For other events and activities which may have occurred irregularly and at different times, the responses were taken to refer to the last time(s) these occurred before being affected by the pandemic. The full completion of the questionnaire took 20 to 40 minutes, depending on the detail provided in answer to open-ended questions.
Respondents were further invited to a subsequent, in-depth interview aimed at 1) clarifying and expanding on the survey answers, 2) exploring the nuances from each local context, and 3) allowing the interviewee to bring up unaddressed issues.
2.2 Data Collection
Using convenience sampling, the invitation to the survey was emailed to a list of global, regional, national, and local rabies stakeholders from the network of the United Against Rabies Forum and rabies practitioners known by the study authors. No exclusion criteria were applied. Recipients were asked to forward the invitation to colleagues in their sector and/or country. Responses were accepted for five weeks, from early February to early March 2021. The only personal information requested was the work sector and the email address.
Respondents who expressed interest in being interviewed were contacted, in chronological order, to schedule online, one-to-one meetings. The criteria used to schedule interviews were 1) availability within the first week of March 2021, 2) being at ease discussing in English, and 3) diversity of countries and sectors (although we do not claim country and sector representativeness). Upon permission, all the interviews were audio-recorded. Interviews lasted from 45 to 120 minutes.
2.3 Analysis
All the received questionnaires were included in the analysis, with some adjustments. Four questionnaires were not country-based, but provided a broader international perspective (e.g. from the United Nations Children’s Fund and the pharmaceutical sector). We excluded these from the country-based analyses, instead of using their inputs for qualitative analysis. Second, Taiwan (Province of People’s Republic of China) was evaluated separately from mainland China, given major epidemiological differences. Third, Bhutan, Brunei Darussalam, and Taiwan all provided high numbers of questionnaires relative to their populations (respectively, 13, 5, and 2). We therefore aggregated quantitative responses from these states while keeping qualitative responses disaggregated for further analysis.
Surveyed countries were divided into categories based on the presence of dog-mediated rabies (9) and country investment in dog rabies control (Figure 1A). Countries with routine national dog vaccination programs maintained for at least five years were classified as having rabies control in progress, but dog-mediated rabies not yet eliminated.
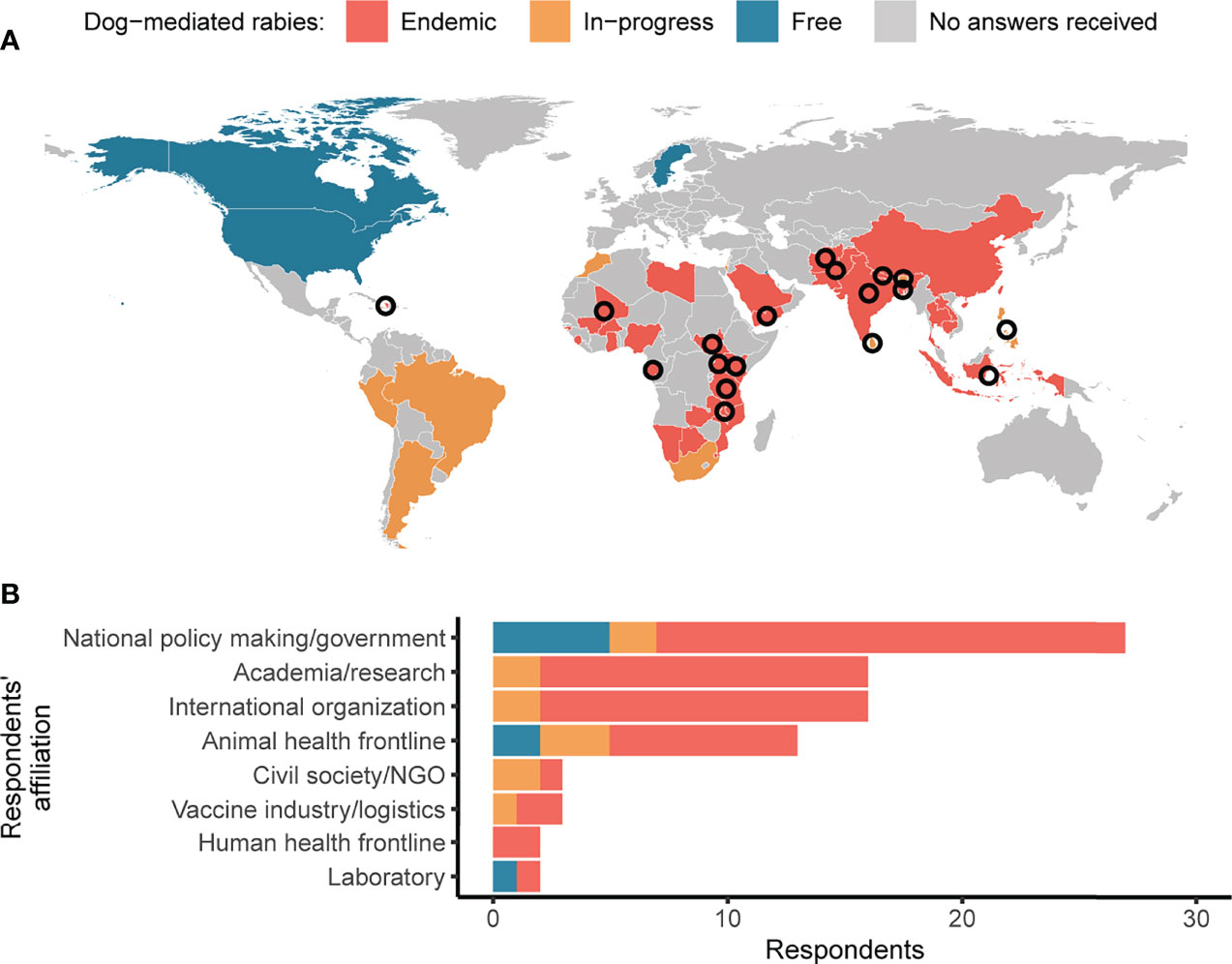
Figure 1 Survey responses and interviews across different countries and sectors. (A) Map of survey responses received from countries with endemic dog-mediated rabies (red), with rabies control programs in-progress (orange) and that are free from dog-mediated rabies (blue). Countries not visible on the map at this scale include Brunei Darussalam, Israel, Kuwait, and Singapore. Interviews were carried out with respondents from countries indicated by the circles. (B) Survey responses by sector, with shading as per the map.
The harmonized data was consolidated and cleaned for comparison across the responses. Figures and maps were created using the R computing language (v4.0.02). The interviews were initially transcribed with Amberscript®, an online software for automatic transcription, then checked manually, anonymized, and edited into verbatim transcripts.
The qualitative answers from the questionnaires and transcripts were analyzed with Nvivo12®. Codes were created (and defined) inductively, applied, and adjusted iteratively during several rounds of data reading. Several rounds were necessary to maximize reflection, minimize errors (because only one author, DN, conducted the qualitative analysis), and develop themes across both qualitative data sources (qualitative survey answers and interview transcripts).
2.4 Ethics
This study was deemed to constitute routine public health surveillance that did not require independent ethics review, but followed ethical standards and guidelines as outlined by WHO (17). Participants were informed prior to starting the questionnaire as to the purpose of the survey and that data would be used for a scientific publication. Respondents agreed to participate in this study when undertaking the questionnaire and consent was collected for scheduling and starting interviews. Informed consent was obtained for the collection and analysis of audio-recorded information. Recordings were retained by WHO.
3 Results
3.1 Overall Impact on Rabies Activities
We received and accepted 103 questionnaires and, at the end of the data analysis process described in 2.3, we worked on 82 questionnaires from 48 countries. Most responses came from endemic countries (n=62, 76%) across Africa and Asia (n=65 altogether, 79%) (Figure 1A), and people working in the national government, academia, international organizations, and the animal health frontline (n=72 altogether, 88%) (Figure 1B). Nineteen people were interviewed; 15 interviewees talked about individual countries, while four provided comparative information about two to four countries.
According to the study participants, the pandemic hit rabies control activities hard both in endemic and in-progress countries, but two main differences were observed. First, the impacts were easier to assess in the in-progress countries than in the endemic countries where surveillance systems were frailer. Second, in endemic countries which were just beginning their journey to the Zero by 30 goal, “the momentum that was gaining was lost,” while in-progress countries experienced an unfortunate step back.
The question on whether funds for rabies control were reduced or diverted to COVID-19 response divided respondents; 42 (51%) replied negatively and 39 (48%) positively. While respondents in endemic countries and in-progress countries were roughly equally distributed, seven out of the eight respondents from rabies-free countries observed no disruption to their national rabies budget. Respondents across the three country categories estimated a 25% to 50% reduction in funding. Five of those who reported no budget cuts specified that the financial resources allocated to rabies control were so low and unpredictable even before COVID-19, that no visible change occurred because of the pandemic. One respondent noted that, besides being intentionally moved to COVID-19 control, rabies funds also declined because of the government’s decision to suspend the garbage collection tax (which local authorities usually use for dog vaccination, among other purposes) to reduce pressure on families during the pandemic.
In most interviews, respondents from all sectors reported that the first resources to be cut or re-directed to the management of COVID-19 – or of other co-occurring emergencies – were those for the procurement of animal rabies vaccines and the implementation of dog vaccination campaigns. Dog vaccination campaigns often rely on unpredictable financial support from external donors or civil society, and these suffered from a sudden shift in priorities when the pandemic started. Where rabies control relies on government support, funding was often concentrated only upon delivering post-exposure prophylaxis to bitten individuals, with other rabies control activities neglected. In some countries, participants observed that, both in the human and the animal health sectors, the rabies budget was not intentionally cut, but rather remained unspent due to the impossibility of performing rabies control activities because of lockdowns and movement restrictions. It was unclear whether these savings would remain available for rabies activities in the future. One participant from the pharmaceutical sector claimed that all energies and funds were focused on the manufacturing of the COVID-19 vaccine. From a global perspective, many countries reduced the quantity of human and dog vaccines they procured during 2020, as well as their forecast for 2021 and beyond.
The return to a pre-COVID-19 situation was not expected to happen before 2022, assuming that rabies would receive at least the same amount of political and financial commitment as prior to the pandemic. The recovery will also depend on other co-occurring disease outbreaks reported by respondents (e.g. African swine fever in the Philippines, screw-worm fly disease in Yemen), food security issues, the production and distribution of COVID-19 vaccines, and the strategic planning of all the human and animal vaccination campaigns interrupted by the pandemic. The effectiveness of post-COVID-19 rabies control efforts will also depend on how much countries truly adopt a One Health approach. One respondent observed that the pandemic had neither a positive nor a negative impact on the discussion about One Health at the policymaking level, because “it’s just COVID era.”
3.2 Disruption to Rabies Activities
Each of the three pillars of the integrated strategy for dog-mediated human rabies elimination was affected by the pandemic, although to differing extents.
In the first year of the pandemic, dog vaccinations were carried out as planned in just two countries (4%) (Figure 2A). Disruptions included delays of at least six months, prolonged duration, increased costs and failure to reach targets, with the cancellation of vaccination in some areas that were planned for. Several respondents pointed out that vaccination campaigns can be planned only at specific times of the year (mainly depending on school holidays and season), causing further delays should they restart. One rabies-free country decided not to perform the usual targeted vaccination of dogs in areas at risk of incursions.
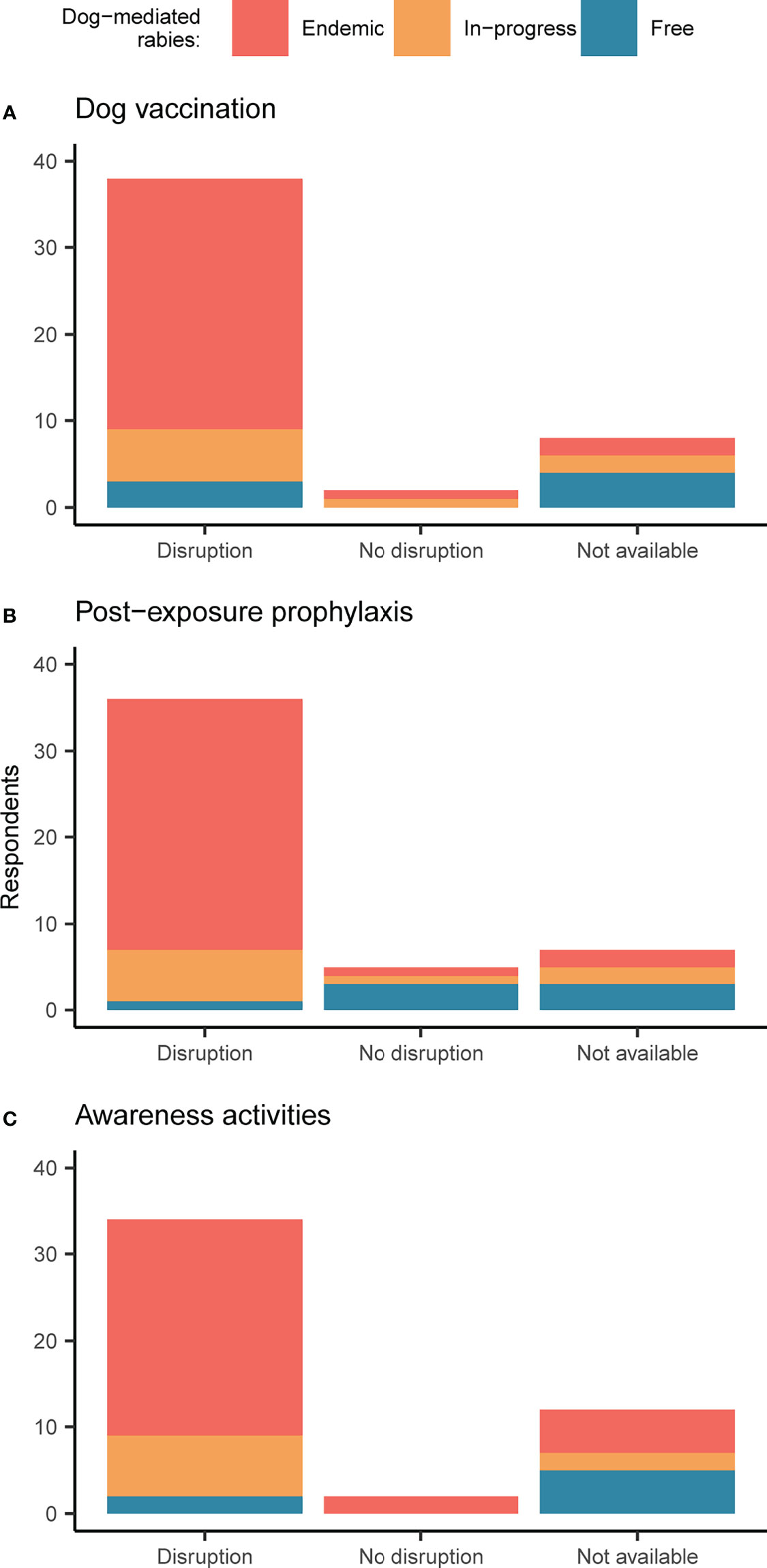
Figure 2 Impacts of COVID-19 on rabies control activities. Respondent perceptions regarding impacts on (A) mass dog vaccination, (B) post-exposure prophylaxis, and (C) awareness activities targeting children. Bars are shaded according to responses by country, with endemic countries in red, countries with rabies control in-progress in orange, and countries that are free from dog-mediated rabies in blue. If multiple responses were available for a country, these were aggregated, and in the rare cases where there was no consensus, information was considered not available.
In the few countries that have mass dog vaccination campaigns along their borders (i.e., India and Bhutan; Thailand, Lao PDR, and Cambodia), this activity was completely cancelled due to pandemic travel restrictions, mandatory quarantines, and increased costs. In Eastern Africa, an important meeting on cross-border rabies control coordination planned for April 2020, in preparation for countries applying to Gavi’s vaccine investment strategy, was cancelled. In South Asia, the international cooperation program to be launched by FAO was suspended.
Access to post-exposure prophylaxis was impacted by the pandemic in 79% of the surveyed countries (Figure 2B). According to one respondent, vaccine demand halved in South Asia. In an endemic country with chronic shortages of post-exposure vaccines where bite victims must travel to the neighboring country for access, the closure of borders reportedly had a dramatic effect. Nevertheless, other endemic countries – 6 out of 32 (19%) – reported no negative changes compared to pre-COVID-19.
Awareness activities for children, mainly carried out at schools, survived the first year of the pandemic in only two countries (4%), both of which were endemic (Figure 2C). In the experience of a large NGO that runs rabies control programs in several Asian and African countries, the worst impact of the pandemic was on education.
3.3 Reasons for Disruption
3.3.1 Mass Dog Vaccination
About a quarter (n=13/59) of respondents who detailed the reasons for disruption to dog vaccinations observed only one cause, while most reported multiple; two (n=12, 20%), three (n=11, 19%), four (n=13, 22%), or five (n=9, 15%).
Most respondents (n=46, 56%) – across all country categories – claimed that restrictions on the movement of personnel were the main hindrance, especially when high-risk COVID-19 areas coincided with endemic rabies areas (Figure 3A). Additionally, it was difficult to organize vaccination campaigns in adherence to local COVID-19 safety guidelines (n=30, 37%), particularly campaigns usually carried out by Non-Governmental Organizations (NGOs) rather than the government. Other reasons for disruption included dog owners being afraid of gathering for dog vaccinations (28, 34%) and, especially in in-progress countries, dog vaccines being limited or unavailable (n=24, 29%) and vaccinators being quarantined or reassigned to COVID-19 response (n=21, 26%). Other issues mentioned by respondents included: budget constraints; animal officers losing their contracts due to staff cuts; government veterinarians moving to the private sector due to a lack of budget for salaries; children – those who often bring dogs for vaccination – being forbidden to leave their house; and, people being afraid of dogs spreading COVID-19 at vaccination points. Indeed, one respondent talked about the new challenge of respecting “double distancing; human-human and animal-human.” Interestingly, some participants observed that at the beginning of the pandemic, people’s fear of dogs’ vulnerability to COVID-19 increased the demand for vaccination, including rabies. Finally, the need for mobile phone applications to record dog vaccination participants, personal protective equipment, hand sanitizers, extra personnel to manage queues, and, in one example, isolation cabins, all increased the cost of dog vaccinations.

Figure 3 Reasons for disruptions to rabies control and prevention activities. Respondents’ stated reasons affecting (A) mass dog vaccination, (B) health-seeking behavio, (C) provisioning of post-exposure prophylaxis, and (D) rabies surveillance. Bars are shaded according to responses by country, with endemic countries in red, countries with rabies control in-progress in orange, and countries that are free from dog-mediated rabies in blue. Results are presented individually (by study participant) rather than aggregated by country. As indicated by the asterisks in the figure, “COVID-19 safety” refers to the difficulty for organizers in adhering to pandemic control guidelines, while “Fears” refers to concerns about leaving home and crowding.
Dog vaccination was affected differently in rural versus urban settings. Some respondents said that, especially early in the pandemic, concerns about the impact of COVID-19 were higher in urban than in rural communities. Echoing this view, another participant observed that “COVID-19 may be bad, but rabies is far scarier.” So, in rabies-aware communities, rural dog owners were willing to participate in dog vaccinations, also thanks to persuasion from community leaders. Yet, vaccinators – often travelling from towns – were reluctant to work in rural areas fearing poor social distancing. Besides these concerns, the main challenge was the difficulty or costs for urban vaccinators to reach rural communities due to travel bans, towns being quarantined, and safety risks when travelling to remote areas. In one endemic country, because of the risk of hijackings, central static points replaced mobile clinics. Several participants shared their enthusiasm for dog vaccination programs based on local, lay animal vaccinators – meaning trained community animal health workers – instead of external professionals. It was argued that this would reduce transport-related challenges and costs and, in the current situation, COVID-19 spread, while increasing sustainable community engagement. Only one respondent said that, despite the challenges, rural areas were prioritized anyway, because of the high numbers of cases.
In urban settings, vaccinators were easily available and rabies awareness remained as high as usual, but people’s fear of coronavirus eroded community support and COVID-19-related logistical issues made dog vaccination challenging. For example, central static points were difficult to organize in towns due to the lack of large spaces where social distancing could be guaranteed. Moreover, stay-at-home orders and limits on gatherings were often strict in cities, reducing the number of dogs that could be vaccinated each day. Nevertheless, some positive aspects were reported. For example, as people spent more time at home, they had the time to bring their dogs for vaccination and, one respondent noted, they even took advantage of dog vaccinations to have a reason to go out. In the most affluent neighborhoods, static drive-through clinics were set up to vaccinate dogs without owners leaving the car and respecting social distancing. Additionally, vaccination services in private animal clinics – where available – were strengthened in some instances, even though only well-off dog owners could afford them. Finally, catch-vaccinate-release – which is used in some countries with many unowned dogs and where people have issues handling these dogs – was easier to perform than before the pandemic, because of reduced traffic and pedestrians.
The pandemic affected the production and supply of animal rabies vaccines. One-third of respondents reported delays (n=28, 34%) and reductions (n=25, 30%) in import, both caused by the slowdown of international trade and delays at customs, which impacted procurement orders by governments (also through the OIE Vaccine Bank), private suppliers and NGOs. In-progress countries especially reported issues with in-country vaccine distribution (n=25, 30%). Seven respondents (9%) from endemic countries observed a decrease in vaccine production, mainly because of budget constraints. Rabies-free countries faced less severe delays in vaccine importation. As a lesson for future emergencies, one respondent stressed the importance of classifying human and dog vaccines as essential goods when prioritizing import. Several participants also emphasized the need for the government, private distributors, and NGOs to coordinate and share their stocks nationally and internationally, to ensure doses do not go unused or expire – as two participants reported happening during the pandemic. One respondent lamented that, before and during the pandemic, dog vaccines in urban areas remained unused, because only people in rural areas care about dog vaccination.
3.3.2 Post-Exposure Prophylaxis
One-third (n=15/50) of respondents reported changes in health-seeking behavior for post-exposure prophylaxis, attributing only one reason for this, while most respondents identified two (n=12, 24%), three (n=13, 25%), or four (n=7, 14%) coinciding causes.
People’s fear of attending clinics because of the risk of COVID-19 infection was the most reported cause (n=34, 41%), in endemic and in-progress countries (Figure 3B). Other obstacles included difficulties in reaching clinics because of reduced public transportation – and reluctance to share private transportation – (n=22, 27%) and reduced financial means (n=15, 18%) and, in parallel, ease of access to local remedies (n=9, 11%). According to, respectively, 24 (29%) and 14 (17%) of the participants, bite victims delayed going to clinics and interrupted their vaccination schedules.
For urban residents, strict stay-at-home orders and sudden clinic closures hampered access to PEP, but bite victims in rural areas were disproportionately affected by the pandemic. In some countries, even before COVID-19, post-exposure vaccines were available only in major towns, which became harder and more expensive to reach during lockdowns and under movement restrictions. In one endemic country with an ongoing refugee crisis, where people in refugee camps often need airlifting to find a clinic with post-exposure vaccines, the pandemic exacerbated inequalities in access to care. When lockdowns started and public transportation decreased, access to healthcare worsened for rural migrants who got stuck in the cities where they were working, but where they could not benefit from Universal Health Care schemes. Additionally, respondents reported that a lower awareness about the need for medical assistance among people in rural areas was a factor contributing to bite neglect and the use of traditional healing during the pandemic.
From the perspective of the health provider, there were disruptions in the delivery of post-exposure prophylaxis. One-third (n=17/59) of respondents reported one reason for this, while most observed two (n=14, 24%), three (n=15, 26%), four (n=4, 7%), or five (n=6, 10%) reasons for disruption to the provision of health care to bitten individuals.
Shortage of human vaccines – both because of supply issues and financial constraints, in almost equal measure – was identified as the main obstacle to the provisioning of post-exposure prophylaxis by 54% (n=44) of respondents, mainly in endemic countries (Figure 3C). Postponement of Gavi’s planned vaccine investment strategy for rabies was a major concern for some respondents, worried about the need to address chronic shortages in the poorest countries. The second reason, mentioned by 49% (n=40) of respondents, was staff shortages – both because of quarantine and illness and, to a lesser extent, redeployment – and was mostly reported from in-progress countries. Other obstacles were closure or conversion of clinics (n=21, 26%), especially in in-progress countries – in one of which, all primary health care services were closed for five months – and, mainly in endemic countries, the availability of post-exposure vaccines only in the private sector (n=16, 20%). In a war-torn, endemic country, where post-exposure vaccines used to be available in private clinics, none could be found during the pandemic.
In two in-progress countries, toll-free numbers and telemedicine were implemented to assist people at home, preventing them from visiting hospitals when unnecessary. In some endemic countries, media reported on stories of bite victims who delayed seeking medical assistance, to increase public awareness about adequate bite management and denounce the lack of vaccines in health care facilities. Especially during lockdowns, it was difficult for people to know where to find open clinics and to travel to them, especially because, due to the fear of COVID-19, people tended to avoid big hospitals, preferring smaller clinics that were either closed, had no vaccines in stock, or could not administer cost-saving intradermal regimens. One interviewee stressed the value of a hotline through which bite victims, especially from rural areas, can be directed towards the most convenient health care facility with vaccines in stock.
3.3.3 Awareness Activities
Figure 2C showed the impact of the pandemic on awareness activities for children, resulting from prolonged school closures and stay-at-home orders. In one endemic country, the usual sponsorship for in-school rabies education was cut. In another, where rabies prevention is part of the national curricula, online lessons were organized, but were usually accessible only to urban children with a computer at home and a good internet connection. Whether through online or in-person lessons, one respondent observed that the experience acquired in teaching COVID-19 prevention to school children may be useful for rabies as well.
The 2020 World Rabies Day events for the general public were also affected. Half (n=41) of survey respondents reported disruption. According to 21 people (26%), in-person activities were held but with lower attendance, while 11 (13%) said that in-person activities were completely cancelled. One-third (n=26) shared a more positive experience, with new or re-organized online events. According to one respondent, the fact that events were online improved attendance by health workers. Another maintained that “the COVID-19 lockdown resulted in many – especially veterinarians – looking for a reason to do something meaningful” and this became evident on World Rabies Day 2020.
A respondent from the Philippines, recalling the strong vaccination hesitancy that started after a problematic dengue vaccine campaign in 2017, stressed the importance of investing adequate resources in the design of health communication campaigns, both for COVID-19 and human and dog rabies.
3.4 Surveillance
3.4.1 Disruption to Surveillance
One-quarter (n=14/61) of respondents who detailed the reasons for disruption to surveillance mentioned only one reason. Most people observed two (n=16, 26%) and three (n=16, 26%) co-occurring causes, while some reported four (n=11, 18%) or five (n=4, 7%).
The main hindrance – across all country categories – were restrictions on the movement of the field surveillance staff (n=43, 52%), which led to cases being missed or investigated late (Figure 3D). Other reasons, especially in in-progress countries, were lack of staff (n=35, 43%) and the difficulty of carrying out surveillance in adherence to COVID-19 safety guidelines (n=24, 29%). Other issues were budget cuts (n=21, 26%) and scarcity of equipment for sample collection and testing (n=20, 24%).
Qualitative data offered additional insights. The rabies surveillance workforce decreased because of staff cuts, salary reductions, and personnel being moved to food security tasks. Several respondents described a vicious cycle: as access to and delivery of post-exposure prophylaxis declined, so fewer dog bite cases were reported, bite reports were not sent to investigators, who could not perform diagnostics or investigations, resulting in incidents remaining unaddressed. Furthermore, the transport of samples from remote areas was slower than usual. As observed by one participant, this apparent drop in animal bites and demand for post-exposure prophylaxis could lead to a downward adjustment in the procurement of human rabies vaccines for the next few years.
Most respondents (n=45, 55%) claimed that laboratory capacity was not reduced or diverted, especially in either endemic or rabies-free countries. In contrast, this was a much-lamented issue by respondents from in-progress countries. A few respondents noticed the positive impact of the pandemic in building and expanding laboratory capacity – in terms of equipment and staff skills – that had the potential to provide future benefits for rabies diagnostics.
3.4.2 Trends in Animal Cases, Animal Bite Patients, and Human Rabies Deaths
One-third of respondents did not express their opinion about trends in animal cases, bites to humans, and human rabies deaths during the first year of the pandemic. Those who responded often highlighted the weakness of the surveillance system even before the pandemic, and the high risk of declining trends not being genuine. Additionally, one participant noted that the effects of the pandemic on these trends would be felt only in 2022 and beyond, because drops in dog herd immunity (arising from disruptions in dog vaccination campaigns) would take time to become evident.
The question on animal rabies cases depicted a very fragmented scenario, with 21 (26%) respondents reporting a decrease, 18 (22%) an increase, and 17 (21%) a stable situation. In contrast, animal bites to humans and human rabies cases seemed to have decreased according, respectively, to 27 (33%) and 26 (32%) of the respondents. It was observed that this may be caused either by an actual reduction in contact between people and free-roaming dogs during lockdowns, or reduced presentations by bite victims to health facilities. Importantly, one participant pointed out that, if stay-at-home orders really protected people from exposure to bites, this hardly applied to rural and impoverished urban areas, where a fully indoor lockdown was not always possible. Another mentioned the risk of rabies misdiagnosis due to health providers being under excessive stress. Eighteen (22%) and 19 (23%) participants claimed that, respectively, animal bites to humans and human rabies cases remained stable and only nine (11%) and eight (10%) respondents reported increases from surveillance data.
That said, from two in-progress and one endemic country, we collected accounts of human rabies cases directly linked to the pandemic. In Bhutan, a young girl bitten in her village by an unknown dog, was not brought to the hospital by her parents as her wound was minor and strict movement restrictions were in place. Her village on the border with India was both rabies endemic and had high COVID-19 transmission. Cross-border mass dog vaccination campaigns routinely performed pre-pandemic were interrupted when the border was closed in Spring 2020. In the Philippines, a man gave up his search for an open bite clinic in the town he travelled to and went back to his village, where he subsequently developed rabies and died. In Uganda, “the lack of resources [for dog vaccination] has always been a problem, but nothing has contributed to the escalation of the rabies problem like COVID-19” and the doubling of the cost of dog vaccines that followed. Since December 2020, a human fatality and recurrent canine rabies outbreaks have been ascribed to suspended dog vaccinations. Similar reports came from Tanzania, where no dog vaccines were available to respond to a sudden rise in dog rabies cases.
3.5 Changes in the Dog Population and Human-Dog Interactions
About one-third of respondents – across all country categories – reported increased numbers in dogs (n=32, 39%) and a worsening in dogs’ health (n=29, 35%) (Figure 4A). Several added that, due to the shortage of food from eateries and people who feed dogs in the community staying at home, some community dogs had become feral, formed packs, and expanded their range. Another third (n=24, 29%) observed no changes. Some respondents reported a decrease in the dog population (n=16, 20%) – particularly in in-progress countries – and increased dog aggressiveness (n=13, 16%). This trend was explained as a result of starvation, lower reproductive rates, and dog owners restraining their free-roaming dogs at home during lockdowns.
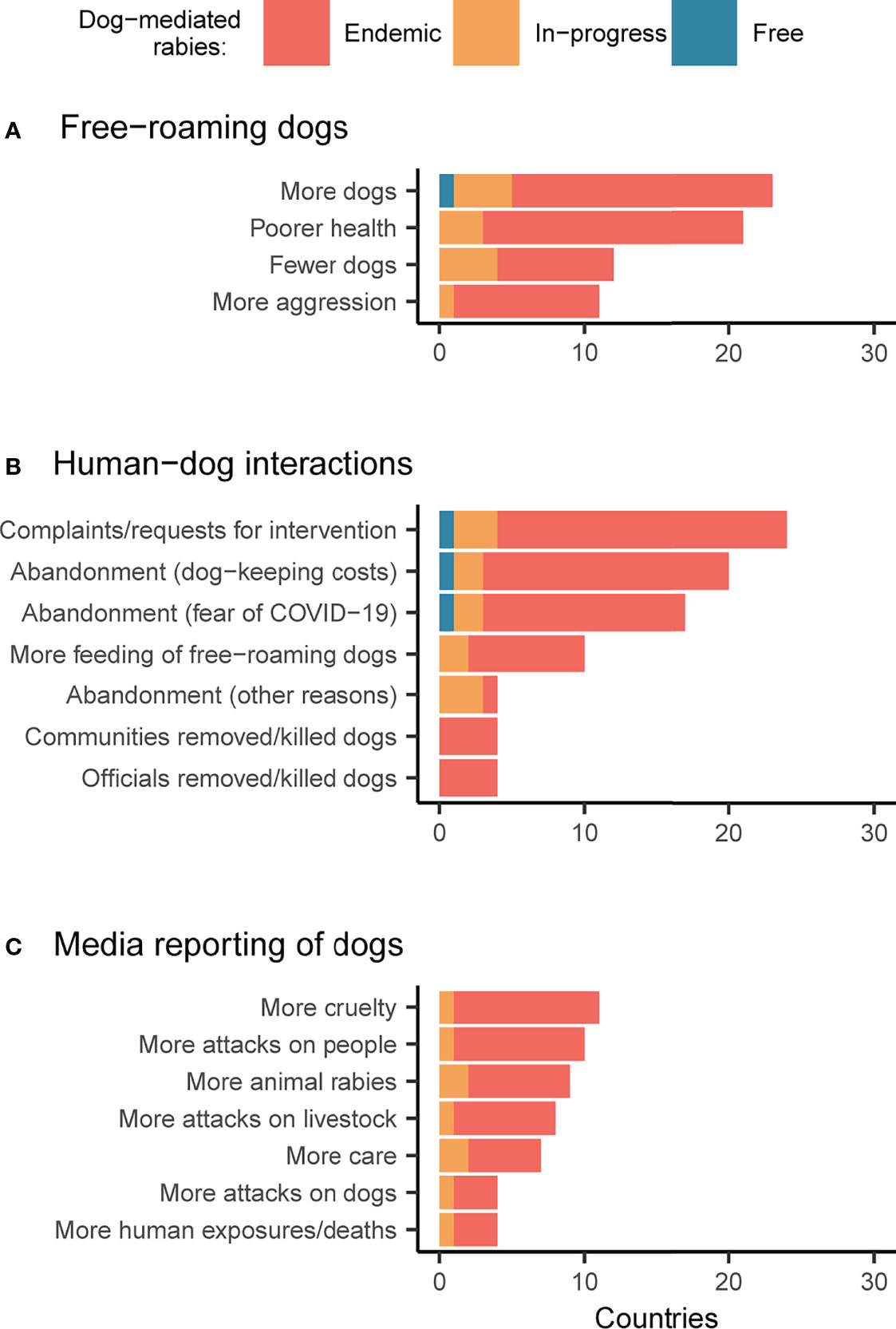
Figure 4 Changes in dog populations during the pandemic. Respondent perceptions regarding changes to (A) free-roaming dog populations and behavior, (B) human-dog interactions, and (C) media reporting on dogs (comparing the pre- and during- COVID-19 scenario). Bars are shaded according to responses by country, with endemic countries in red, countries with rabies control in-progress in orange, and countries that are free from dog-mediated rabies in blue.
Regarding people’s interactions with dogs, most respondents (n=48, 59%) noticed an increase in dog abandonment that they considered to be due to financial constraints caused by pandemic-driven job loss and fear of animals spreading COVID-19, in equal measure (Figure 4B). Other reasons for abandonment were natural calamities affecting dog owners (e.g. typhoons and floods) and behavioral changes in pet dogs during lockdowns. No matter the reason, increased abandonment was observed especially in endemic countries. One-third (n=27, 33%) of participants reported a rise in complaints about the presence of free-roaming dogs and subsequent requests for interventions (e.g. sterilization, removal, culling, etc.) to local authorities. Another third (n=20, 24%) noticed, on the contrary, an increase in people feeding free-roaming dogs, out of personal initiative or persuasion from the government, religious organizations, or animal welfare associations, especially in urban areas of South Asia. Nevertheless, one respondent commented that “dogs are worshipped, so people feed them, but that’s all they do.” Several respondents, across all country categories, observed increased dog adoption and some expressed their concern about the possibility of this resulting in high abandonment in future. In one European rabies-free country, the illegal import of dogs was particularly high in 2020, probably because of the increased demand for dogs to buy or adopt. While in one endemic country, a respondent mentioned an increase in dog meat consumption.
The question about how the media reported free-roaming dogs during the pandemic revealed divergent responses (Figure 4C). Half (n=41) of respondents noticed no changes. The other half observed, in almost equal measure, more cases of cruelty towards dogs (n=14, 17%), more attacks by dogs on humans (n=12, 15%), and on livestock (n=10, 12%), and a more caring attitude towards dogs (n=10, 12%). Attacks on humans and livestock occasionally triggered frustration and retaliatory measures against dogs. One respondent remarked on the key role played in their country by the media in keeping public attention to rabies high, but also pointed out the importance, and the struggle, of maintaining engagement despite emerging issues, such as anti-microbial resistance.
4 Discussion
This study explored how the first year of the COVID-19 pandemic affected canine rabies control and dog-mediated human rabies elimination efforts in 48 countries, mostly in Asia and Africa. Given the broad range of issues, and the descriptive nature of the data, we summarize and discuss our key findings through the exposition of three overarching themes identified during data analysis. Our aim is to reflect and share ideas as to how the experience of the pandemic can support rabies stakeholders in improving their strategy to be more efficient, resilient, and sustainable in the future (18).
4.1 Rabies Does Not Circulate in a Bubble
The different impacts of the pandemic across different components of rabies control illustrate how an integrated One Health approach is needed for effective control and prevention of rabies. Yet, it also shows how this can make the strategy vulnerable to social, political, economic, and ecological disruptions.
The first indicator of this interconnectedness is the fact that – especially with regards to dog vaccination and surveillance – multiple co-occurring reasons caused their disruption during the first year of the pandemic. This demonstrates how crucial an integrated, whole-of-society approach to rabies is, needing active engagement with all relevant stakeholders.
The way COVID-19 has influenced the human-dog relationship (not only in the surveyed countries, but around the world) (19) and the possible short- and medium-term consequences of this on rabies transmission and control provide a further example. During the first year of the pandemic, free-roaming dogs likely increased, mainly due to abandonment because of decreased family income, misconceptions about animal transmission (widely documented early in the pandemic) (20), behavioral issues in “lockdown dogs” (mainly studied in the Global North) (21, 22), and natural calamities affecting dog owners.
Surveillance data indicate an apparent decline in animal bites and human rabies cases in many countries during the first year of the pandemic, although this did not occur everywhere (see the case of South Africa in Box 1). This decline may be explained by decreased contact between people and free-roaming dogs during lockdowns and school closures. Nevertheless, an alternative explanation is provided by under-reporting, which would be consistent with literature reporting increased dog bites – especially in children – since the beginning of the pandemic and especially during lockdowns (27–29). If this is the case, bites not captured by surveillance in 2020 are likely to have mainly occurred among those who could not spend lockdown periods indoors (e.g. people living in the streets, in informal settlements, in itinerant pastoralist communities) or who live in remote or rural areas with poorer access to health care. If, as feared by some (30–32), dog abandonment increases post-COVID-19, there may be increased risks of bites and, particularly where dog vaccinations have been discontinued, increased rabies exposures.
Box 1 One Health Teams with Strong Veterinary Capacity Made the Difference in South Africa.
In South Africa, a rabies endemic country, a significant increase in the number of confirmed dog and human rabies cases was reported in 2020 and 2021 (23–25). The uneven geographic distribution of these cases demonstrates the reach that veterinary-led rabies programs need to have into communities (26). In the areas where close-knit One Health teams existed in pre-pandemic times and veterinary services remained in place despite COVID-19 and pressures from other animal disease outbreaks, surveillance and response mechanisms were quickly activated: mass dog vaccinations were strengthened, availability of post-exposure vaccines was ensured, and targeted awareness campaigns were promptly organized. This allowed rabies outbreaks to be quelled. In the areas where mass dog vaccination had been patchy, “veterinary services fell apart during COVID-19” (26) and dog rabies outbreaks were much harder to control, despite the considerable resources invested. Together with the changes in people’s health-seeking behaviors that occurred during the pandemic, this resulted in human losses. South Africa shows that countries can cope with a 2-year reduction, or even interruption, of rabies control activities if they have a functioning One Health system and routine mass dog vaccination in place.
The growing dog population trend mainly observed in endemic countries has worrying implications for the near future. In a post-COVID-19 scenario, when countries will have competing priorities and limited financial resources, it is to be expected that dog vaccination will not receive the necessary attention. The gap in implementation may also lead to other approaches being adopted, for example dog culling, which is sometimes perceived as a rapid and accessible form of rabies control. However, indiscriminate dog culling is known to be ineffective because vaccinated dogs may be killed, people may hide or move their unvaccinated dogs to protect them, and community trust and engagement can be severely affected. In some endemic countries, dog culling was already being undertaken (routinely or sporadically) before COVID-19 and, during the pandemic, further requests were reported. Increasing numbers of hard-to-catch unowned dogs will only make vaccination efforts more time-consuming and expensive. Many communities in rabies endemic areas also depend on livestock for their livelihoods. If dog attacks on livestock have increased during the pandemic, people’s perception of dogs and dog-related issues, including rabies control, may also be affected.
As exemplified by the transboundary movement of rabid dogs across the Bhutanese-Indian border and the increased illegal import of dogs in Sweden in early 2020, countries cannot work alone towards dog rabies control and elimination. Key areas for future collaboration that emerged from this study are cross-border dog vaccination programs (33) – acknowledging rabies as a border security issue – and sharing of dog and human vaccines between neighboring states during emergencies.
4.2 Communities Need Local Solutions
Movement restrictions were the primary reason for disruptions to dog vaccination, surveillance, and rabies awareness campaigns in 2020 and were the second most reported challenge for health-seeking by bite victims. Even though other factors co-occurred, the distance between vaccinators and dogs, investigators and bite incidents, educators and communities, and bite patients and life-saving vaccines proved harmful and, in some cases, lethal.
With dog vaccination, the possibility of entrusting properly trained community vaccinators (34) and the use of thermotolerant vaccines and locally-made passive cooling devices (35) emerged as potentially game-changing strategies for future rabies control. These strategies were recommended by the participants in this study not only to address pandemic disruptions, but to overcome broader challenges, such as the limited workforce. In many endemic countries, with insufficient veterinarians and weak cold chains, local vaccinators are an essential resource and can increase capacity and reach into communities, while reducing staff and transport costs, engaging communities and ensuring timely vaccination of new dogs.
The pandemic also highlighted the dependence of most rabies endemic countries on the import of both dog and human vaccines. Centralized vaccine production allows for stricter adherence to international quality standards, but there are major benefits to regional or national self-reliance for vaccine production if quality assurance, safety, and effectiveness can be achieved (36). In-country distribution challenges could, again, be minimized by the distribution of thermostable vaccines and shared supplies between countries in emergencies. Meanwhile, support from the OIE Vaccine Bank (37) and Gavi, the Vaccine Alliance (38), remains essential. Moreover, actions that are being taken to improve the inequities in vaccine supplies highlighted by COVID-19 should be co-opted in the longer term to benefit rabies control and prevention as well.
Participatory disease surveillance (39), which involves close collaboration between at-risk communities and human and animal health surveillance authorities, can also potentially alleviate the obstacle of centralized surveillance staff being unavailable or unable to quickly reach communities. Research on rapid, efficient but cheap diagnostic tests to be used directly in the field, to bypass the problem of sample shipment to central laboratories, is progressing (40–42).
In-person rabies awareness activities, both for the general population and schoolchildren, were disrupted by stay-at-home orders and school closures. Online events were organized to replace them and, in some countries, proved more successful than in-person activities in engaging with human and animal health workers. Yet, online activities could not reach children with limited computer or internet access, especially in rural areas but also in impoverished urban neighborhoods. Paper-based materials may be more reliable – for use both in schools and at home – and a better investment to simultaneously reach children with fun activities, and their relatives, with basic information on both dog vaccination and post-exposure prophylaxis. Adding key hotline numbers could enhance these materials for communities and be displayed in key locations (e.g. schools, health care centers, meeting halls, etc.).
4.3 Rural and Urban Settings Require Different Approaches
Rabies control and prevention were impacted by the pandemic in different ways depending on their rural or urban location. Despite variation among countries and the impossibility of drawing generalizable conclusions, the differences are worth discussing.
In rural communities where rabies awareness and engagement with local leaders tend to be high, dog vaccination was most severely affected by travel bans and COVID-19 safety standards. This supports the inclusion of community-based vaccinators in rabies control plans, and the continuation of strategies that ensure sustained community engagement and empowerment. In urban areas, the lack of large spaces was the main impediment, but innovative vaccination methods were piloted. Furthermore, the catch-vaccinate-release method worked particularly well because of quieter roads and, whenever possible, private vaccination was often strengthened. It seems that cities are well suited for (and perhaps require) multi-method vaccination strategies, with methods adapted to suit particular groups where they are most effective (e.g. catch-vaccinate-release out of peak hours, fixed and roaming static points during the day – 43, etc.) and – when available - with engagement with the private veterinary sector.
In relation to post-exposure prophylaxis, urban residents mainly faced the problem of finding open clinics, whilst rural residents also had to deal with travel restrictions, limited public transportation, and reduced financial resources, leading to underestimation of risk, delays in access to care, and use of ineffective remedies. Not only in exceptional times like the pandemic, but also in normal circumstances, the set-up of dedicated animal bite and rabies hotlines seems advantageous on several fronts. First, phone calls are a fast and cheap way for bite victims to receive immediate and standardized risk assessment (assuming network services/coverage), and up-to-date instructions on their nearest source of post-exposure prophylaxis. Second, hotlines work as a starting point for Integrated Bite Case Management, with immediate benefits of increasing detection of animal and human rabies cases, and targeted distribution of post-exposure vaccines. Third, hotlines provide an additional communication channel for participatory disease surveillance. Fourth, they can potentially strengthen the One Health approach to rabies, if jointly managed by staff from both the human and animal health sectors. However, further research is necessary to explore the feasibility and costs of this promising intervention (44).
4.4 Limitations
This study has several limitations. First, it was based on a convenience sample of known stakeholders engaged in rabies control efforts. Even though we received a relatively high number of responses that were quite well distributed across countries and work sectors, our convenience sample is not representative and does not allow for reliable generalization. Second, the fact that the questionnaire was only in English limited the participation of non-English speakers. Third, for most countries (n=33, 69%) only one opinion was collected, while for the others we had several questionnaires and interviews available. Fourth, in the questions that asked respondents to compare pre- and during- COVID-19 scenarios, the recall bias is to be considered. Fifth, due to chronically poor rabies surveillance and variable levels of dog vaccination in most rabies endemic countries, the 3-tier classification system that we used is intended as tentative. Sixth, even though the manual cleaning of the survey answers was carried out systematically and the entire process was repeated twice to minimize error, it involved some subjectivity. Similarly, coding was done by only one person, and reflexivity and subjectivity were an integral part of the process.
5 Conclusions
Although difficult to quantify, the impact of the first year of the COVID-19 pandemic on rabies and rabies control efforts appears significant. All the components of the current One Health-grounded strategy to eliminate dog-mediated human rabies were affected, but dog vaccination was the most severely disrupted. Considering the number of years necessary for dog vaccination programs to mature and scale up, it is recommended that, as soon as possible, efforts should be reinstated and intensified. Areas of particular attention, and possible innovation, include:
- Mobilization of political will and resources towards achieving the Zero by 30 goal, especially in the animal health sector;
- Creation of a rabies-specific budget that can be dedicated to long-term animal and human vaccine procurement;
- Classification of animal and human vaccines as essential goods
- Design of mechanisms to support national and international animal and human vaccine sharing among rabies stakeholders;
- Identification of the most cost-effective, local, and sustainable methods of meeting the needs and challenges of different communities, particularly in having their dogs vaccinated, for example through the use of community-based vaccinators;
- Implementation of cross-border dog vaccination campaigns;
- Development of telemedicine systems such as hotlines to increase fast and hassle-free access to PEP, support Integrated Bite Case Management, and build participatory disease surveillance;
- Design of practical rabies awareness packages that target children and their parents.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below:
DOI: 10.5281/zenodo.5918372
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
BA-R, DN, KH and SB: Conceptualization. DN, KC, KH, SB, and SC: Study design and methodology. DN: Investigation. DN and KH: Analysis. KH and RS: Visualization. DN: Writing – original draft. BA-R, KC, KH, SB and SC: Writing – review & editing. All authors read and approved the final manuscript.
Funding
This work was supported by the World Health Organization, the European Union’s Horizon 2020 research and innovation program under a Marie Skłodowska-Curie grant (751267) to DN, and a Wellcome grant (207569/Z/17/Z) to KH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The pharmaceutical company MSD Animal Health provides dog rabies vaccines to rabies research programs in Tanzania, that KH and SC work on.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the people that made this study possible by sharing their observations and reflections with us. The following people (in alphabetical order) allowed us to acknowledge their contribution: Alasdair Iain MacGregor King, Anna Gertrud Katarina Gyllenhammar, Bassirou Bonfoh, Bavukile Kunene, Bernardo Cassamá, Chendu Dorji, Christine Fehlner-Gardiner, Conrad Martin Freuling, Emily Kavosa Mudoga, Felicia Owusu-Antwi, Felix Lankester, Fernando Rodrigues, Grace Sabo Nok Kia, Guigma Victor Yacinthe, Gyanendra Gongal, Hanan Mohammed Abuabaid, Hervé Bourhy, Ibrahim Dominic Manu, Jakob Zinsstag, Jeetendra Man Shrestha, Joshua Waiswa, Karen Reed, Kennedy Lushasi, Kevin Odindo Miheso, Kyaw Thu, Linous Munsimbwe, Luiz Carlos Monteiro Jr., Luna Gongal, Mathilde Sopi Tetchi, Nien-Nung Lin, Raihan Zuhairah Hj Zulkifli, Rauna Ndinelao Athingo, Samir J. Desai, Sarah Ilio Jayme, Sarah Schmidt, Shamsudeen Faisal Fagbo, Shivani Pradhan, Sonam Jamtsho, Suthida Muangnoicharoen Hearn, Tahir Yaqub, Waqas Ahmad, and Yasser Mohammed Al-Eryani.
References
1. Tarantola A. Four Thousand Years of Concepts Relating to Rabies in Animals and Humans, its Prevention and Its Cure. Trop Med Infect Dis (2017) 2(2):5. doi: 10.3390/tropicalmed2020005
3. Fooks AR, Banyard AC, Horton DL, Johnson N, Mcelhinney LM, Jackson AC. Current Status of Rabies and Prospects for Elimination. Lancet (2014) 384(9951):1389–99. doi: 10.1016/S0140-6736(13)62707-5
4. González-Roldán JF, Undurraga EA, Meltzer MI, Atkins C, Vargas-Pino F, Gutiérrez-Cedillo V, et al. Cost-Effectiveness of the National Dog Rabies Prevention and Control Program in Mexico, 1990–2015. PloS Negl Trop Dis (2021) 15(3):e0009130. doi: 10.1371/journal.pntd.0009130
5. Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Estimating the Global Burden of Endemic Canine Rabies. PloS Negl Trop Dis (2015) 9(4):e0003709. doi: 10.1371/journal.pntd.0003709
6. World Health Organization, Food and Agriculture Organization of the United Nations, World Organisation for Animal Health. (in: Global Elimination of Dog-Mediated Human Rabies: Report of the Rabies Global Conference, 10-11 December 2015, Geneva, Switzerland, Geneva: WHO (2016).
7. World Health Organization, Food and Agriculture Organization of the United Nations, World Organisation for Animal Health. Zero by 30: The Global Strategic Plan to End Human Deaths From Dog-Mediated Rabies by 2030. Geneva: WHO (2019).
8. Food and Agriculture Organization of the United Nations, World Organisation for Animal Health, World Health Organization. United Against Rabies Forum: Zero by 30: One Health in Action. Geneva: WHO (2021).
9. World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030. Geneva: WHO (2021).
10. Capolongo S, Gola M, Brambilla A, Morganti A, Mosca EI, Barach P. COVID-19 and Healthcare Facilities: A Decalogue of Design Strategies for Resilient Hospitals. Acta Biomed (2020) 91(9):50–60. doi: 10.23750/abm.v91i9-S.10117
11. NTD Modelling Consortium. The Potential Impact of Programmes Interruptions Due to COVID-19 on 7 Neglected Tropical Diseases: A Modelling-Based Analysis (2020). doi: 10.21955/gatesopenres.1116665.1
12. World Health Organization. Neglected Tropical Diseases: Impact of COVID-19 and WHO’s Response – 2021 Update. Geneva: WHO (2021).
13. Raynor B, Diaz EW, Shinnick J, Zegarra E, Monroy Y, Mena C, et al. The Impact of the COVID-19 Pandemic on Rabies Reemergence in Latin America: The Case of Arequipa, Peru. PloS Negl Trop Dis (2021) 15(5):e0009414. doi: 10.1371/journal.pntd.0009414
14. Kunkel A, Jeon S, Joseph HC, Dilius P, Crowdis K, Meltzer MI, et al. The Urgency of Resuming Disrupted Dog Rabies Vaccination Campaigns: A Modeling and Cost-Effectiveness Analysis. Sci Rep (2021) 11:12476. doi: 10.1038/s41598-021-92067-5
15. Siddiqui A, Ahmed A, Tanveer M, Arshad A. The Crux of Pakistan’s Prolonged Rabies Vaccine Shortage: A Rising Mortal Threat in the COVID-19 Pandemic. J Med Virol (2021) 93(9):5221–22. doi: 10.1002/jmv.27024
16. Lhendup K, Dorji T. Probable Rabies in a Child in a Bhutanese Town Bordering India, 2020. SAGE Open Med Case Rep (2021) 9:1–5. doi: 10.1177/2050313X211019786
17. World Health Organization. WHO Guidelines on Ethical Issues in Public Health Surveillance. Geneva: WHO (2017).
18. Ehrenberg JP, Zhou XN, Fontes G, Rocha EMM, Tanner M, Utzinger J. Strategies Supporting the Prevention and Control of Neglected Tropical Diseases During and Beyond the COVID-19 Pandemic. Infect Dis Poverty (2020) 9(1):86. doi: 10.1186/s40249-020-00701-7
19. Morgan L, Protopopova A, Dupont Birkler RI, Itin-Shwartz B, Sutton GA, Gamliel A, et al. Human–Dog Relationships During the COVID-19 Pandemic: Booming Dog Adoption During Social Isolation. Humanit Soc Sci Commun (2020) 7(155):1–11. doi: 10.1057/s41599-020-00649-x
20. Parry NMA. COVID-19 and Pets: When Pandemic Meets Panic. Forensic Sci Int (2020) 2:100090. doi: 10.1016/j.fsir.2020.100090
21. Hargrave C. COVID-19: Implications of Self−Isolation and Social Distancing for the Emotional and Behavioural Health of Dogs. Companion Animal (2020) 25(4):1–8. doi: 10.12968/coan.2020.0032
22. Christley RM, Murray JK, Anderson KL, Buckland EL, Casey RA, Harvey ND, et al. Impact of the First COVID-19 Lockdown on Management of Pet Dogs in the UK. Animals (2020) 11(1):5. doi: 10.3390/ani11010005
23. Dixon CA, Mistry RD. Dog Bites in Children Surge During Coronavirus Disease-2019: A Case for Enhanced Prevention. J Pediatr (2020) 225:231–2. doi: 10.1016/j.jpeds.2020.06.071
24. Tulloch JSP, Minford S, Pimblett V, Rotheram M, Christley RM, Westgarth C. Paediatric Emergency Department Dog Bite Attendance During the COVID-19 Pandemic: An Audit at a Tertiary Children’s Hospital. BMJ Paediatr Open (2021) 5:e001040. doi: 10.1136/bmjpo-2021-001040
25. Parente G, Gargano T, Di Mitri M, Cravano S, Thomas E, Vastano M, et al. Consequences of COVID-19 Lockdown on Children and Their Pets: Dangerous Increase of Dog Bites Among the Paediatric Population. Children (2021) 8(8):620. doi: 10.3390/children8080620
26. Dogs Trust. The Impact of COVID-19 Lockdown Restrictions on Dogs & Dog Owners in the UK. London: Dogs Trust (2020).
27. Holland KE, Owczarczak-Garstecka SC, Anderson KL, Casey RA, Christley RM, Harris L, et al. “More Attention Than Usual”: A Thematic Analysis of Dog Ownership Experiences in the UK During the First COVID-19 Lockdown. Animals (2021) 11(1):240. doi: 10.3390/ani11010240
28. Applebaum JW, Tomlinson CA, Matijczak A, McDonald SE, Zsembik BA. The Concerns, Difficulties, and Stressors of Caring for Pets During COVID-19: Results From a Large Survey of U.S. Pet Owners. Animals (2020) 10(10):1882. doi: 10.3390/ani10101882
29. Mauti S, Léchenne M, Mbilo C, Nel L, Zinsstag J. Rabies. In: Kardjadj M, Diallo A, Lancelot R, editors. Transboundary Animal Diseases in Sahelian Africa and Connected Regions. New York City: Springer (2019).
30. Duamor CT, Hampson K, Lankester F, Sambo M, Kreppel K, Wyke S, et al. Use of Lay Vaccinators in Animal Vaccination Programmes: A Scoping Review. PloS Negl Trop Dis (2021) 15(8):e0009691. doi: 10.1371/journal.pntd.0009691
31. Lugelo A, Hampson K, Bigambo M, Kazwala R, Lankester F. Controlling Human Rabies: The Development of an Effective, Inexpensive and Locally Made Passive Cooling Device for Storing Thermotolerant Animal Rabies Vaccines. Trop Med Infect Dis (2020) 5(3):130. doi: 10.3390/tropicalmed5030130
32. World Health Organization, MI4A. Global Market Study. Human Rabies Vaccines. Geneva: WHO (2020).
33. Mace J, Renaudin S, Dieuzy-Labaye I, Dehove A. Vaccine Banks for Controlling Dog-Mediated Rabies. Rev Sci Tech (2018) 37(2):511–8. doi: 10.20506/rst.37.2.2819
34. Wentworth D, Hampson K, Thumbi SM, Mwatondo A, Wambura G, Chng NR. A Social Justice Perspective on Access to Human Rabies Vaccines. Vaccine (2019) 37(1):A3–5. doi: 10.1016/j.vaccine.2019.01.065
35. Smolinski MS, Crawley AW, Olsen JM, Jayaraman T, Libel M. Participatory Disease Surveillance: Engaging Communities Directly in Reporting, Monitoring, and Responding to Health Threats. JMIR Public Health Surveill (2017) 3(4):e62. doi: 10.2196/publichealth.7540
36. Mauti S, Léchenne M, Naïssengar S, Traoré A, Kallo V, Kouakou C, et al. Field Postmortem Rabies Rapid Immunochromatographic Diagnostic Test for Resource-Limited Settings With Further Molecular Applications. J Vis Exp (2020) 160:1–29. doi: 10.3791/60008
37. Rasolonjatovo FS, Guis H, Rajeev M, Dacheux L, Arivony Nomenjanahary L, Razafitrimo G, et al. Enabling Animal Rabies Diagnostic in Low-Access Areas: Sensitivity and Specificity of a Molecular Diagnostic Test From Cerebral Tissue Dried on Filter Paper. PloS Negl Trop Dis (2020) 14(3):e0008116. doi: 10.1371/journal.pntd.0008116
38. Mananggit MR, Manalo DL, Saito N, Kimitsuki K, Garcia AMG, Lacanilao PMT, et al. Lateral Flow Devices for Samples Collected by Straw Sampling Method for Postmortem Canine Rabies Diagnosis. PloS Negl Trop Dis (2021) 15(12):e0009891. doi: 10.1371/journal.pntd.0009891
39. Department of Health of the Republic of South Africa, National Institute for Communicable Diseases. Rabies Prevention Advisory. (2021). Available at: https://www.nicd.ac.za/wp-content/uploads/2021/12/An-update-for-Veterinary-services-Animal-Welfare-and-Volunteers_final29112021.docxWRJW.pdf
40. Department of Health of the Republic of South Africa, Centre for Emerging Zoonotic and Parasitic Diseases. An Update on Rabies in South Africa. Communicable Dis Communiqué (2021) 20(12).
41. Department of Health of the Republic of South Africa, Centre for Emerging Zoonotic and Parasitic Diseases. An Update on Rabies in South Africa. Communicable Dis Communiqué (2021) 20(8).
42. United Against Rabies Forum. Rabies, One Health and COVID-19 Webinar. Available at: https://www.youtube.com/watch?v=gCEkS5u8ggk.
43. Mazeri S, Burdon Bailey JL, Mayer D, Chikungwa P, Chulu J, Grossman PO, et al. Using Data-Driven Approaches to Improve Delivery of Animal Health Care Interventions for Public Health. Proc Natl Acad Sci USA (2021) 118(5):e2003722118. doi: 10.1073/pnas.2003722118
Keywords: COVID-19 pandemic, dog-mediated human rabies elimination, post-exposure prophylaxis, One Health, mass dog vaccination, rabies dog rabies control
Citation: Nadal D, Abela-Ridder B, Beeching S, Cleaveland S, Cronin K, Steenson R and Hampson K (2022) The Impact of the First Year of the COVID-19 Pandemic on Canine Rabies Control Efforts: A Mixed-Methods Study of Observations About the Present and Lessons for the Future. Front. Trop. Dis 3:866811. doi: 10.3389/fitd.2022.866811
Received: 31 January 2022; Accepted: 20 April 2022;
Published: 05 July 2022.
Edited by:
Monique Sarah Léchenne, Swiss Tropical and Public Health Institute (Swiss TPH), SwitzerlandReviewed by:
Stephanie Salyer, Africa Centres for Disease Control and Prevention, EthiopiaJulie Cleaton, Centers for Disease Control and Prevention (CDC), United States
Victoria J. Brookes, The University of Sydney, Australia
Copyright © 2022 Nadal, Abela-Ridder, Beeching, Cleaveland, Cronin, Steenson and Hampson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deborah Nadal, Deborah.Nadal@glasgow.ac.uk
 Deborah Nadal
Deborah Nadal Bernadette Abela-Ridder
Bernadette Abela-Ridder Sarah Beeching
Sarah Beeching Sarah Cleaveland
Sarah Cleaveland Katy Cronin
Katy Cronin Rachel Steenson
Rachel Steenson Katie Hampson
Katie Hampson