- 1Department of Pharmaceutical Sciences, Sunyani Technical University, Sunyani, Ghana
- 2Department of Pharmacy Practice, Faculty of Pharmacy and Pharmaceutical Sciences, College of Health, KNUST, Kumasi, Ghana
- 3School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
The emergence of multidrug-resistant Acinetobacter baumannii is a major concern to healthcare providers and facilities in many parts of the world. This bacterial pathogen is commonly implicated in hospital-acquired infections, particularly in critically ill patients admitted to the intensive care unit (ICU). The extensive use of antibiotics, particularly in ICUs, and the lack of proper infection control interventions in many hospitals have led to an increased emergence of multidrug-resistant A. baumannii. Infections due to multidrug-resistant A. baumannii are associated with prolonged hospital stays and high morbidity and mortality, particularly among hospitalized ICU patients. The lack of antibiotic stewardship programmes in many healthcare facilities has exacerbated the burden of A. baumannii infections in many parts of Africa. This review discusses the prevalence and antibiotic-resistance pattern of the multidrug-resistant A. baumannii, and the possible ways to address or minimise its emergence in healthcare settings in Africa.
Introduction
Acinetobacter baumannii is a Gram-negative, non-fermentative, strictly aerobic, non-fastidious, non-motile, oxidase-negative, catalase-positive, and ubiquitous bacterial pathogen that is usually isolated from natural and healthcare environments (1). It is an opportunistic pathogen, and one of the six most significant multidrug-resistant (resistant to at least one agent in more than three classes of antibiotics) ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) pathogens, described by the Infectious Disease Society of America as a global problematic nosocomial threat (2, 3). This bacterium is implicated in various infections, commonly pneumonia, bacteraemia, meningitis, respiratory tract, and urinary tract infections, particularly among immunocompromised and mechanically ventilated intensive care unit (ICU) patients (4). The incidence of such infections has increased in the last decade, with an associated mortality rate of between 30% and 75% in many parts of the world (4–7). Several predisposing factors, such as burns, premature birth, prolonged hospital stay (particularly in ICUs), mechanical ventilation, indwelling foreign devices, and extensive exposure to antimicrobial therapy (especially broad-spectrum antibiotics), have been associated with multidrug-resistant A. baumannii (MDRAB) infections (4, 8, 9). The intrinsic and acquired resistance of A. baumannii to antibacterial agents and its propensity to cause outbreaks of hospital-acquired infections, especially in ICUs, is a major health concern (10–12). The increasing prevalence of MDRAB has led to limited therapeutic options. Because of its resistance to multiple antibiotic classes, particularly the carbapenems and third-generation cephalosporins, as well as being implicated in life-threatening infections, MDRAB is currently listed as one of the highly prioritized pathogens in the World Health Organization’s “Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery and Development of New Antibiotics” (13). Despite the impact of MDRAB being recognized in many healthcare settings, there is a paucity of surveillance data in most countries, especially those in Africa, owing to their sparse healthcare resources (14). This review discusses MDRAB infections and possible ways to control their emergence in healthcare settings in Africa.
Antibiotic resistance mechanisms of A. baumannii
Like other Gram-negative bacteria, A. baumanii has several mechanisms of resistance that enable it to escape the bactericidal and bacteriostatic effects of antibiotics. These include expression of efflux pumps that export antibiotics from the cell (efflux mechanisms), alterations of outer membrane proteins (reduction of porin permeability), and the production of hydrolytic enzymes, such as extended spectrum β-lactamases (ESBLs) and carbapenemases (15). However, the production of β-lactamases, especially of carbapenem-hydrolysing enzymes, is the most important resistance mechanism (16). The Ambler class A β-lactamases, such as Vietnam extended-spectrum β-lactamases (VEBs), Guiana extended-spectrum types (GES), class B metallo-β-lactamases (MBLs), including the imipenemase metallo-β-lactamases (IMPs), Verona integron-encoded metallo-β-lactamases (VIMs), German imipenemases (GIMs), New Delhi metallo-β-lactamases (NDMs), and Seoul imipenemases (SIMs), as well as the class D oxacillinases, such as OXA-23, -24, -51, and 58, have been commonly implicated in β-lactam resistance, particularly to carbapenems (17–19). The expression of MBLs located on mobile genetic elements poses a significant risk of clinical outbreaks because it increases ease of dissemination of the organism (20, 21).
In Africa, a number of studies have reported that A. baumannii resistance is mediated mainly by the production of OXA-23, -24, -51, and -58 and NDM-1 (22–24). The blaOXA-23-like gene is widely reported across many parts of Africa, including in South Africa, Libya, Senegal, Tunisia, Algeria, Egypt, and Nigeria (25–28). GES-11 producing A. baumannii isolates have also been reported in Tunisia (19). Other resistance mechanisms, such as the expression of efflux pumps (TetA, TetB, and AdeABC) (29), in combination with a loss of or reduction in outer membrane proteins (OMPs), have contributed to resistance to multiple antibiotics (tetracycline, fluoroquinolones, β-lactams, and aminoglycosides) in this bacterium (30), in turn posing a challenge to clinicians in their treatment of infections caused by A. baumannii in many healthcare facilities in Africa (31). A summary of studies from Africa and other parts of the world investigating A. baumannii’s resistance to antibiotics, its mechanism of actions, and the protein/genes involved in these processes is provided in Table 1.
Global epidemiology of MDRAB
In many parts of the world, infections caused by MDRAB have increased among patients admitted to medical wards, burns units, surgical wards, and especially ICUs (47, 48). Increasing levels of global travel have led to the spread of multidrug-resistant A. baumannii at the local, national, and international levels (49). The spread of this bacterial pathogen between different healthcare settings commonly occurs with the transfer or movement of colonized individuals or patients. For instance, the presence of the Vietnam extended-spectrum-β-lactamases (VEB-1)-producing A. baumannii clone was detected in 55 hospitals in northern and south-eastern France. There are also reports that this clonal strain spread from healthcare settings in the Mediterranean region to those in south-west Germany and, additionally, of the circulation of the European clonal types I and II in healthcare settings in Italy (17, 43, 50). The first detection of A. baumannii-producing OXA-48 β-lactamase was associated with outbreaks across hospitals in New York and neighbouring regions in the US. The transmission and outbreaks of MDRAB have largely been attributed to the movement of contaminated equipment and the transfer of patients and personnel between healthcare facilities (51).
The increase in the number of patients travelling abroad for medical care has also facilitated the intercontinental spread of this resistant pathogen, particularly from one country to another, thus posing a threat to global public health. For instance, in Morocco, A. baumannii harbouring the blaOXA-58 gene was first identified in a cluster of A. baumannii clones that were previously detected in France in an outbreak of hospital infections in 2003 (37), which was also reported in Iran in transferred patients receiving medical care (1). In addition, NDM-1-producing A. baumannii isolated from patients in Jimma Specialized University Hospital in Ethiopia (22) had previously been detected in patients receiving medical care in France, and was also implicated in the first outbreak of infection caused by NDM-1-producing A. baumannii in Europe (52). The patients infected by NDM-1-producing had previously been hospitalized in Algeria, Tunisia, and Egypt, and may have also carried the variant from Africa to Europe (53). Studies from countries including Algeria, Egypt, Tunisia, Libya, and Morocco have reported the presence of carbapenem-resistant A. baumannii, especially the oxacillinases variant, in many hospitals (54–56), with OXA-23 as the main OXA-type carbapenemase (25, 27, 28). The OXA-23-producing strain has also been implicated in outbreaks of infections that occurred between 2010 and 2011 in Aga Khan University Hospital in Kenya (57). The fact that the hydrolytic enzyme activity mediated by OXA-23 is commonly found among resistant bacterial pathogens isolated from healthcare settings also suggests that all strains of MDRAB originate from the same genetic lineage or pool (54).
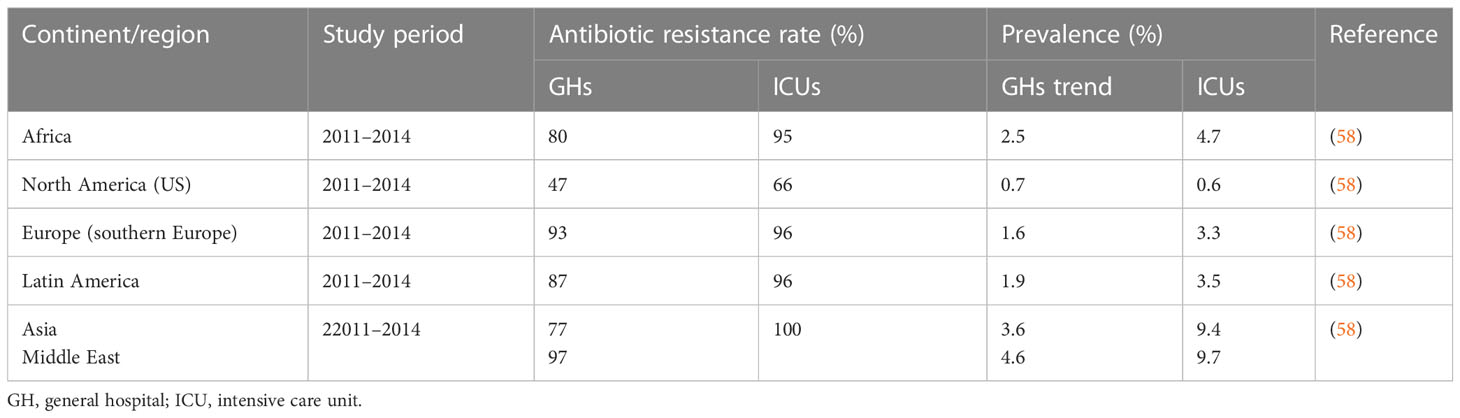
A systematic review on regional differences and trends in the antimicrobial susceptibility of A. baumannii reported A. baumannii prevalence of 0.7%, 1.6%, 1.9%, 2.5%, 3.6%, and 4.6% of all infections in hospital settings from the US, Europe, Latin America, Africa, Asia, and the Middle East, respectively (58). Rates of multidrug resistance (MDR) ranging between 77% and 87% in Africa, Asia, and Latin America, and of 47% in North America and > 93% in the Middle East and Europe, have also been reported. It was observed that MDR rates were higher in ICUs than in conventional wards (58). A high prevalence of the MDRAB pathogen in hospital settings has serious health and economic implications for both developed and developing countries. For example, a study of the cost of management of infections conducted in three tertiary care hospitals in the US found that the average cost of treating MDRAB infections ($11,359) was higher than the average cost ($7,049) of treating infections caused by susceptible pathogens owing to the need for prolonged hospitalization among the former group. The increased cost associated with a prolonged hospital stay was largely attributable to microbiological investigations and the use of more expensive antibiotic therapies as a consequence of the antibiotic resistance developed by the pathogen (59). Although MDRAB has emerged as a global problem, its impact is particularly serious in low- and middle-income regions such as Africa, straining already limited healthcare resources in this continent (Table 2) (60–62).
Multidrug-resistant A. baumannii prevalence in Africa
A. baumannii is ubiquitous in nature and can be isolated from various sites in healthcare settings, with ICU patients noted as being high-risk for colonization or infection by this pathogen. Invasive procedures, the extensive use of antibiotics, and a lack of adherence to strict infection control measures, especially when patients require persistent care, are major risk factors for transmission of the resistant pathogen in ICUs (63). In Africa, MDRAB is implicated in 4.7% of all ICU infections. Its prevalence in Africa is higher than the prevalences of 3.5%, 3.3%, and 0.6% reported in Latin America, Europe, and North America, respectively, but lower than the prevalences of 9.4% and 9.7% reported in Asia and the Middle East, respectively (58). Antibiotic resistance rates ranging from 31.8% to 92.1%, from 8.8% to 88.8%, from 28.8% to 91.6%, from 30% to 90.3%, and from 12.2% to 89.9% for cephalosporins, carbapenems, fluoroquinolones, aminoglycosides, and β-lactam/inhibitor combinations (piperacillin/tazobactam), respectively, have also been reported in a number of studies (58, 64–66). The relatively high prevalence of A. baumannii with multiple antibiotic resistance in Africa is commonly associated with increased morbidity, mortality, and high healthcare costs for patients (63).
There is a paucity of data on the prevalence of MDRAB in healthcare facilities for many African countries. However, several studies conducted in East and West African countries, such as Nigeria, have indicated that the prevalence of this pathogen ranges between 8.5% and 9.0% in hospital settings (Table 3). High antibiotic resistance rates of 100% for amikacin and ciprofloxacin, 90.9% for ceftriaxone and ceftazidime, and 81.8%, 72.2%, 72.7%, and 63.6% for piperacillin, gentamicin, imipenem, and meropenem, respectively, have been reported (38). The high prevalence of multidrug-resistant bacterial pathogens, including MDRAB, has been mainly attributed to suboptimal antibiotic stewardship protocols in many hospitals in Nigeria (38, 72). In Ghana, a nationwide surveillance study found that MDRAB accounted for 1.56% of all bacterial infections and for 2.0% of infections with Gram-negative bacilli (67). Resistance rates of 100% to ampicillin, tetracycline, and cotrimoxazole in a neonatal intensive care unit have also been reported (73), consistent with a study by Agyepong and colleagues that reported 100% resistance to all major antibiotics (ampicillin, trimethoprim/sulfamethoxazole, cefuroxime, cefotaxime, ertapenem, meropenem, and amikacin) used for the treatment of various infections in a Ghanaian teaching hospital (74). A study in Tanzania among children admitted to the national hospital with bloodstream infections reported that MDRAB accounted for 3.97% all bacterial nosocomial infections and 5.88% of Gram-negative bacterial infections (75). Approximately a decade later, in the same country, Manyahi and colleagues, in a study to determine the predominance of multidrug-resistant bacterial pathogens in surgical site infections, reported that the prevalence of infections caused by MDRAB was twofold higher (10.2%) (68). However, it should be noted that the two patient samples differed in terms of age (i.e., infants versus all age groups), number (1,787 versus 100 patients) and sites of infection. In the same study, Manyahi and colleagues recorded antibiotic resistance rates of 100% to ampicillin, cefotaxime, ceftriaxone, amoxicillin–clavulanic acid, and chloramphenicol, and of 86% to ceftazidime and gentamicin (68). A study from a national laboratory of public health in Gabon reported that MDRAB accounted for 3.2% of all bacterial infections (69). A similar figure (4.2%) was recorded in a reference hospital in Cameroun among children with sickle cell disease and various forms of bacterial infections (76). Furthermore, in Benin, in the first national surveillance study on nosocomial infections and anti-infective therapy, the prevalence of MDRAB was found to be 1.0%, and reported antibiotic resistance rates were 100% for ampicillin, amoxacillin/clavulanate, and ceftazidime, and 80%, 75%, and 62% for tetracycline, gentamycin, and trimethoprim–sulfamethoxazole, respectively (70). The growing prevalence of MDRAB is a serious threat, especially in low-resource economies, including sub-Saharan Africa, where newly efficacious antibiotics tend to be unavailable or unaffordable (77, 78).
In South Africa, a 7-year study intended to support the preauthorization of antibiotics was carried out in an academic complex hospital with a population of infected patients ranging between 155 and 453 per year. Analysis of microbiological samples revealed an MDRAB resistance rate of 53%–60%. This study also found that the prevalence of infection attributable to A. baumanii in ICU patients was 2.2% to 2.4% in 2009 and 2010, but that it dropped to 1.6% in 2014 (79). The decline was attributed to the reinforcement of infection prevention practices in the hospital in response to recognition of the potential threat posed by the pathogen (79). Other studies, including that by Reddy and coworkers, reported a prevalence of 9.3% among hospitalized patients, particularly those on mechanical ventilation, and critically ill patients in the paediatric intensive care unit of a children’s hospital in South Africa (66). The 9.3% prevalence rate of MDRAB recorded by Reddy et al. (66) was lower than the 15% prevalence rate previously reported by Ntusi and colleagues among South African HIV patients (268 out of 1,784 patients). The higher prevalence observed by Ntusi and colleagues is possibly due to the reduced immune status of the HIV patients (64). Antibiotic resistance rates of 88.7%, 80.9%, 77.3%, and 75.3% for aminoglycosides, penicillins, β-lactamase inhibitor cephalosporins, and carbapenems, respectively, were also reported by Reddy et al. (66). High resistance rates of 100% for ampicillin, amoxicillin, cefuroxime, cefuroxime axetil, cefoxitin, cefotaxime, and nitrofurantoin and more than 67% for ceftazidime, cefepime, imipemem, meropenem, ciprofloxacin, and gentamicin were also reported in a study among patients with A. baumannii infections in healthcare facilities in the Tshwane region (41).
In Morocco, MDRAB accounted for 6.94% of all infections and 9.6% of all Gram-negative bacilli infections between 2003 and 2016. The figure of 6.94% is higher than the figure of 2.5% reported for Africa as a whole (58), indicating an increasing prevalence of MDRAB in this country. Antibiotic resistance rates increased from 23% to 76%, from 63% to 86%, from 41% to 52%, and from 68% to 87% for imipenem, ceftazidime, amikacin, and ciprofloxacin, respectively (65, 80). In Libya, in a 1-year study conducted among hospitalized patients in a tertiary teaching hospital, MDRAB was implicated in 20% of all Gram-negative bacterial device-associated hospital infections. The same study recorded a 56.3% multidrug resistance rate for A. baumannii (71). An assessment of the antibiotic resistance capability of A. baumannii in five hospitals in Algiers recorded a multidrug resistance rate of 93.6%, and the antibiotic resistance rate ranged from 93.6% to 98.3% for cephalosporins, and was 75.2% for imipenem (55). The high prevalence of multidrug-resistant bacterial pathogens, including MDRAB, in many parts of Africa has been largely attributed to antibiotic abuse or misuse due to suboptimal antibiotic use policies and guidelines in healthcare settings (71).
Clinical significance and economic impacts of multidrug-resistant A. baumannii
The ability of MDRAB to survive under harsh environmental conditions over a long period (81) and its ability to cause nosocomial outbreaks (12) pose a significant threat to healthcare settings in Africa (28). Several factors, including resistance to broad spectrum of antibiotics, desiccation, and disinfectants could account for the pathogen’s survival in hospital environments (81), in turn making it challenging to control the spread of this antibiotic-resistant bacterium. The challenges inherent in controlling the spread of, and treating infections caused by, MDRAB have seriously impacted the economies of African countries, which must contend with the loss of productivity associated with significant morbidity, mortality, and excess healthcare costs brought about by the spread of MDRAB (82, 83). Prolonged hospitalization and the use of more expensive alternative antibiotics increases the healthcare costs associated with patient management (84, 85). Colistin, which hitherto has been reserved for use as a last resort in the event of carbapenem resistance, is associated with a higher toxicity risk and adverse drug reactions (7, 86).
Although MDRAB is negatively impactful, there is unsettled controversy regarding determination of mortality associated with the resistant pathogen independent of the underlying patients’ disease conditions, as studies have reported varying results in many parts of the world (87, 88). Some studies conducted in South Africa have reported mortality rates of 26.5% among HIV patients and of more than 50% in neonatal and paediatric units, with increased morbidity associated with MDRAB infections in tertiary hospitals (64, 79). Although the economic impact of morbidity and mortality caused by MDRAB infections in Africa remains unclear, it has been found that MDRAB stretches already limited economic resource and imposes a burden on healthcare logistics (63).
Control of antibiotic-resistant A. baumannii spread in healthcare settings
The survival of A. baumanii in hospital environments, due to its resistance to a wide range of antibiotics, poses a challenge to the control of its spread (89, 90). In Africa, inadequate active surveillance, coupled with a lack of effective epidemiological studies, hinder the implementation of robust infection control practices (63, 91). A review by Essack et al. (92) on antimicrobial resistance in the WHO African region highlighted the policy package to combat antimicrobial resistance in member African countries. The review found that, although several countries have implemented pilot surveillance projects, no African country, with the exception of South Africa has a national surveillance system, as prescribed by the WHO, that adequately records data on the use of antimicrobials and resistance patterns. South Africa is host to a national laboratory-based surveillance programme on selected bacterial and fungal pathogens (92). This review highlights the need to adopt comprehensive surveillance policy that provides information on regional microbial resistance, and encourages the undertaking of epidemiological studies, the scaling up of national antimicrobial stewardship plans, and increased research into new medicines and diagnostic testing. To combat the increasing prevalence of MDR pathogens, such as MDRAB, in Africa, it is important to enforce the implementation of infection control and antibiotic stewardship programmes, as well as to improve laboratory capacity, as recommended by the World Health Assembly’s 2014 resolution 67.25, set out in the WHO’S “Global action plan on antimicrobial resistance” (93).
In hospital settings, indirect transmission or cross-transmission of bacterial pathogens, including MDRAB, mainly occurs when contaminated gloves, dressings, and needles used by healthcare workers are not changed between patients. Other contaminated sources, such as infusion pumps, mattresses, pillows, shower units, tables, and suction and resuscitation equipment, have been implicated in direct transmission between patients and healthcare workers and through surface contact between an object and susceptible host (94–96). Studies conducted in many African countries have identified non-compliance with hand hygiene practice among healthcare professionals as a major route for the transmission of pathogens (65, 97, 98). A study by Asare and colleagues in a tertiary hospital ICU in Ghana found that compliance with hand hygiene practices before and after patient contact was between 15.4% and 38.5% for physicians, and between 14.1% and 9.9% for nurses. Enforcing hand hygiene practice among healthcare professionals has been a major challenge and, thus, the study recommended the incorporation of effective education programmes into the curriculum of health professionals as a means of improving adherence, with the aim of preventing the transmission of antibiotic-resistant pathogens in healthcare settings (99). In addition, other studies have recommended the thorough disinfection of potentially contaminated environments and medical equipment by using sodium hypochlorite, as well as bathing patients with chlorohexidine, as a means of minimizing the spread of antibiotic-resistant pathogens (95, 100). The enforcement of contact precautions and isolation of infected patients, especially in ICUs, has also been reported to prevent outbreaks (97, 100, 101).
Antibiotic stewardship programmes are strategies intended to control antibiotic resistance globally. The adoption and implementation of stewardship programmes by all WHO countries is critical in monitoring the appropriate use of antibiotics to control the emergence and spread of antibiotic-resistant pathogens, particularly, clinically relevant bacterial pathogens, including A. baumannii, in healthcare facilities worldwide (63, 102). Some of the strategies in these programmes involve the use of a microbiology-informed antibiotic therapy to help minimize the escalation of resistance. In general, microbial susceptibility profile data in a geographical location or hospital setting and the development of rapid but low-cost diagnostics techniques for resource-constrained countries may become necessary for the control of resistant bacterial strains, including A. baumannii (103). In most African countries, inadequate functional laboratory facilities, coupled with low levels of continuous training and awareness of the burden of antimicrobial resistance among healthcare professionals, is a major challenge to the effective prevention and control of the spread of multidrug-resistant bacteria. A study conducted in Nigeria to determine the awareness of multidrug-resistant bacteria among 486 healthcare professionals, comprising doctors, pharmacists, medical laboratory scientists, nurses, pharmacy assistants, and midwives from different hospitals, reported that 30 medical laboratory scientists (MLSs) interviewed in the study had never screened isolates for ESBLs, AmpC, or carbapenemase enzymes. In addition, it revealed that none of the MLSs, despite having over 5 years’ experience, had the expertise to screen phenotypically for these enzymes, suggesting a lack of professional enhancement training in many healthcare settings (104). A lack of trained personnel and inadequate in-service training on effective infection control practices have also been reported by other authors to be major barriers to containing the spread of multidrug-resistant pathogens in many developing countries, particularly in Africa (96). To overcome this challenge, stringent measures aimed at improving healthcare services, such as the continuous professional training of personnel in how to carry out effective and reliable laboratory investigations, and research capacity building for care workers, particularly among staff in the ICUs, is imperative if the prevalence of MDRAB in healthcare settings in Africa is to be reduced (63, 104).
Conclusion
A. baumannii has become a predominant pathogen associated with hospital-acquired infections and has been implicated in several outbreaks in many parts of the world, including in Africa. The emergence of MDR in many healthcare settings, particularly ICUs, coupled with the limited therapeutic options, is of great concern to clinical practice. Increasing epidemiological and surveillance studies to ascertain the magnitude of the problem is crucial to the implementation of effective control strategies to combat the growing prevalence of MDRAB in Africa.
Author contributions
NA, FF, and AO together conceptualized the study, contributed to the review of published scholarly articles, and drafted the manuscript. All authors read, edited, and gave approval for the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MDRAB, Multidrug-resistant Acinetobacter baumannii; MDR, Multidrug resistance; ICU, Intensive Care Unit; WHO, World Health Organization.
References
1. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev (2008) 21:538–82. doi: 10.1128/CMR.00058-07
2. Navidinia M. The clinical importance of emerging ESKAPE pathogens in nosocomial infections. J Paramed Sci (2016) 7:43–57. doi: 10.22037/jps.v7i3.12584
3. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! an update from the infectious diseases society of America. Clin Infect Dis (2009) 48:1–12. doi: 10.1086/595011
4. Antunes L, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis (2014) 71:292–301. doi: 10.1111/2049-632X.12125
5. Brotfain E, Borer A, Koyfman L, Saidel-Odes L, Frenkel A, Gruenbaum SE, et al. Multidrug resistance acinetobacter bacteremia secondary to ventilator-associated pneumonia risk factors and outcome. J Intensive Care Med (2016) 10:1–7. doi: 10.1177/0885066616632193
6. Garnacho-Montero J, Gutiérrez-Pizarraya A, Díaz-Martín A, Cisneros-Herreros JM, Cano ME, Gato E, et al. Acinetobacter baumannii in critically ill patients: Molecular epidemiology, clinical features and predictors of mortality. Enfermedades Infecciosas y Microbiol Clin (2016) 34:551–8. doi: 10.1016/j.eimc.2015.11.018
7. Hakyemez IN, Kucukbayrak A, Tas T, Yikilgan AB, Akkaya A, Yasayacak A, et al. Nosocomial acinetobacter baumannii infections and changing antibiotic resistance. Pakistan J Med Sci (2013) 29:1245–50. doi: 10.12669/pjms.295.3885
8. Visca P, Seifert H, Towner KJ. Acinetobacter infection–an emerging threat to human health. IUBMB Life (2011) 63:1048–54. doi: 10.1002/iub.534
9. Munoz-Price LS, Weinstein RA. Acinetobacter infection. New Engl J Med (2008) 358:1271–81. doi: 10.1056/NEJMra070741
10. Molter G, Seifert H, Mandraka F, Kasper G, Weidmann B, Hornei B, et al. Outbreak of carbapenem-resistant acinetobacter baumannii in the intensive care unit: a multi-level strategic management approach. J Hosp Infect (2016) 92:194–8. doi: 10.1016/j.jhin.2015.11.007
11. Kempf M, Rolain J-M. Emergence of resistance to carbapenems in acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents (2012) 39:105–14. doi: 10.1016/j.ijantimicag.2011.10.004
12. Karah N, Sundsfjord A, Towner K, Samuelsen Ø. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of acinetobacter baumannii. Drug Resist Updates (2012) 15:237–47. doi: 10.1016/j.drup.2012.06.001
13. WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: World Health Organization (2017).
14. Perez F, Villegas MV. The role of surveillance systems in confronting the global crisis of antibiotic-resistant bacteria. Curr Opin Infect Dis (2015) 28:375–83. doi: 10.1097/QCO.0000000000000182
15. Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol (2014) 12:35–48. doi: 10.1038/nrmicro3155
16. Doi Y, Murray GL, Peleg AY. Seminars in respiratory and critical care medicine. (2015) 36(1):85–98. doi: 10.1055/s-0034-1398388
17. Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant acinetobacter baumannii. J Antimicrob Chemother (2010) 65:233–8. doi: 10.1093/jac/dkp428
18. Lee K, Kim M-N, Choi TY, Cho S-E, Lee S, Whang DH, et al. Wide dissemination of OXA-type carbapenemases in clinical acinetobacter spp. isolates from south Korea. Int J antimicrob Agents (2009) 33:520–4. doi: 10.1016/j.ijantimicag.2008.10.009
19. Chihi H, Bonnin R, Bourouis A, Mahrouki S, Besbes S, Moussa MB, et al. GES-11-producing acinetobacter baumannii clinical isolates from Tunisian hospitals: Long-term dissemination of GES-type carbapenemases in north Africa. J Global Antimicrob Resist (2016) 5:47–50. doi: 10.1016/j.jgar.2016.03.005
20. Farajnia S, Azhari F, Alikhani MY, Hosseini MK, Peymani A, Sohrabi N. Prevalence of PER and VEB type extended spectrum betalactamases among multidrug resistant acinetobacter baumannii isolates in north-West of Iran. Iranian J Basic Med Sci (2013) 16:751–5.
21. Bush K. Proliferation and significance of clinically relevant β-lactamases. Ann New York Acad Sci (2013) 1277:84–90. doi: 10.1111/nyas.12023
22. Pritsch M, Zeynudin A, Messerer M, Baumer S, Liegl G, Schubert S, et al. First report on bla NDM-1-producing acinetobacter baumannii in three clinical isolates from Ethiopia. BMC Infect Dis (2017) 17:180–7. doi: 10.1186/s12879-017-2289-9
23. Mesli E, Berrazeg M, Drissi M, Bekkhoucha SN, Rolain J-M. Prevalence of carbapenemase-encoding genes including new Delhi metallo-β-lactamase in acinetobacter species, Algeria. Int J Infect Dis (2013) 17:739–43. doi: 10.1016/j.ijid.2013.02.024
24. Al-Agamy MH, Khalaf NG, Tawfick MM, Shibl AM, El Kholy A. Molecular characterization of carbapenem-insensitive acinetobacter baumannii in Egypt. Int J Infect Dis (2014) 22:49–54. doi: 10.1016/j.ijid.2013.12.004
25. Olaitan AO, Berrazeg M, Fagade OE, Adelowo OO, Alli JA, Rolain JM. Emergence of multidrug-resistant acinetobacter baumannii producing OXA-23 carbapenemase, Nigeria. Int J Infect Dis (2013) 17:e469–70. doi: 10.1016/j.ijid.2012.12.008
26. Bakour S, Olaitan AO, Ammari H, Touati A, Saoudi S, Saoudi K, et al. Emergence of colistin-and carbapenem-resistant acinetobacter baumannii ST2 clinical isolate in Algeria: first case report. Microb Drug Resist (2015) 21:279–85. doi: 10.1089/mdr.2014.0214
27. Mugnier PD, Poirel L, Naas T, Nordmann P. Worldwide dissemination of the blaOXA-23 carbapenemase gene of acinetobacter baumannii1. Emerg Infect Dis (2010) 16:35–40. doi: 10.3201/eid1601.090852
28. Nogbou N-D, Phofa DT, Nchabeleng M, Musyoki AM. Investigating multi-drug resistant acinetobacter baumannii isolates at a tertiary hospital in Pretoria, south Africa. Indian J Med Microbiol (2021) 39:218–23. doi: 10.1016/j.ijmmb.2021.03.005
29. Pannek S, Higgins PG, Steinke P, Jonas D, Akova M, Bohnert JA, et al. Multidrug efflux inhibition in acinetobacter baumannii: comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-β-naphthylamide. J Antimicrob Chemother (2006) 57:970–4. doi: 10.1093/jac/dkl081
30. Mardani M. Pan resistant acinetobacter baumannii: Is there any available alternative therapy? Arch Clin Infect Dis (2011) 6:65.
31. Alawami AA, Al Haji H, Hamied MI, Chouchani C, El Salabi AA. New treatment options of blood stream infections caused by multidrug-resistant acinetobacter baumannii in some Libyan patients. Microb Drug Resist (2022) 28:99–101. doi: 10.1089/mdr.2020.0493
32. Agyepong N, Govinden U, Owusu-Ofori A, Allam M, Ismail A, Pedersen T, et al. Whole-genome sequences of two multidrug-resistant acinetobacter baumannii strains isolated from patients with urinary tract infection in Ghana. Microbiol Resource Announcements (2019) 8:e00270–00219. doi: 10.1128/MRA.00270-19
33. Ayibieke A, Kobayashi A, Suzuki M, Sato W, Mahazu S, Prah I, et al. Prevalence and characterization of carbapenem-hydrolyzing class d β-lactamase-producing acinetobacter isolates from Ghana. Front Microbiol (2020) 11:587398. doi: 10.3389/fmicb.2020.587398
34. Naomi W, Winnie M, Duffton M, Victor M, Fredrick O, Stephen N. Carbapenemase genes among multidrug resistant acinetobacter baumannii in the critical care unit of Kenyatta national hospital, Kenya. East Afr Med J (2018) 95:2196–202.
35. Mathlouthi N, Areig Z, Al Bayssari C, Bakour S, Ali El Salabi A, Ben Gwierif S, et al. Emergence of carbapenem-resistant pseudomonas aeruginosa and acinetobacter baumannii clinical isolates collected from some Libyan hospitals. Microb Drug Resist (2015) 21:335–41. doi: 10.1089/mdr.2014.0235
36. Mathlouthi N, El Salabi AA, Jomàa-Jemili MB, Bakour S, Al-Bayssari C, Zorgani AA, et al. Early detection of metallo-β-lactamase NDM-1-and OXA-23 carbapenemase-producing acinetobacter baumannii in Libyan hospitals. Int J antimicrob Agents (2016) 48:46–50. doi: 10.1016/j.ijantimicag.2016.03.007
37. Natoubi S, Barguigua A, Zerhouni N, Baghdad N, Timinouni M, Hilali A, et al. Letter to the Editor: First report of an OXA-58 carbapenemase-producing acinetobacter baumannii isolated fromurinary tract infection, in Morocco. Afr J Urol (2017) 23:66–7. doi: 10.1016/j.afju.2016.05.008
38. Odewale G, Adefioye O, Ojo J, Adewumi F, Olowe O. Multidrug resistance of acinetobacter baumannii in ladoke akintola university teaching hospital, osogbo, Nigeria. Eur J Microbiol Immunol (2016) 6:238–43. doi: 10.1556/1886.2015.00018
39. Kumburu HH, Sonda T, Mmbaga BT, Alifrangis M, Lund O, Kibiki G, et al. Patterns of infections, aetiological agents, and antimicrobial resistance at a tertiary care hospital in northern Tanzania. Trop Med Int Health (2017) 22:454–64. doi: 10.1111/tmi.12836
40. Camara M, Saad Bouh Boye C, Mboup S, Gaye-Diallo A, Toure-Kane C, Diop-Ndiaye H, et al. Molecular epidemiology of carbapenem-resistant acinetobacter baumannii isolates in a Senegalese university teaching hospital. J Adv Microbiol (2022) 22(3):73–82. doi: 10.9734/jamb/2022/v22i330449
41. Lowings M, Ehlers MM, Dreyer AW, Kock MM. High prevalence of oxacillinases in clinical multidrug-resistant acinetobacter baumannii isolates from the tshwane region, south Africa–an update. BMC Infect Dis (2015) 15:521–31. doi: 10.1186/s12879-015-1246-8
42. Ahmed S, Alp E, Ulu-Kilic A, Dinc G, Aktas Z, Ada B, et al. Spread of carbapenem-resistant international clones of acinetobacter baumannii in Turkey and Azerbaijan: a collaborative study. Eur J Clin Microbiol Infect Dis (2016) 35:1463–8. doi: 10.1007/s10096-016-2685-x
43. D'arezzo S, Capone A, Petrosillo N, Visca P. Epidemic multidrug-resistant acinetobacter baumannii related to European clonal types I and II in Rome (Italy). Clin Microbiol infect (2009) 15:347–57. doi: 10.1111/j.1469-0691.2009.02668.x
44. Banerjee T, Mishra A, Das A, Sharma S, Barman H, Yadav G. High prevalence and endemicity of multidrug resistant acinetobacter spp. in intensive care unit of a tertiary care hospital, varanasi, India. J Pathog (2018) 1–8:2018. doi: 10.1155/2018/9129083
45. Al-Tamimi M, Albalawi H, Alkhawaldeh M, Alazzam A, Ramadan H, Altalalwah M, et al. Multidrug-resistant acinetobacter baumannii in Jordan. Microorganisms (2022) 10:849. doi: 10.3390/microorganisms10050849
46. Villalón P, Valdezate S, Medina-Pascual MJ, Rubio V, Vindel A, Saez-Nieto JA. Clonal diversity of nosocomial epidemic acinetobacter baumannii strains isolated in Spain. J Clin Microbiol (2011) 49:875–82. doi: 10.1128/JCM.01026-10
47. Ahmed NH, Hussain T, Biswal I. Antimicrobial resistance of bacterial isolates from respiratory secretions of ventilated patients in a multi-specialty hospital. Avicenna J Med (2015) 5:74–8. doi: 10.4103/2231-0770.160233
48. Shahid A, Muzammil S, Rasheed F, Aslam B, Ali MA, Haider SZ, et al. Emergence of armA mediated aminoglycoside resistance in multidrug-resistant acinetobacter baumannii in Pakistani hospitals. Pakistan J Zool (2021) 53:2507. doi: 10.17582/journal.pjz/20210520200559
49. Johnson AP, Woodford N. Global spread of antibiotic resistance: the example of new Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol (2013) 62:499–513. doi: 10.1099/jmm.0.052555-0
50. Naas T, Coignard B, Carbonne A, Blanckaert K, Bajolet O, Bernet C, et al. On behalf of the French nosocomial infection early warning, investigation and surveillance network. VEB-1 extended-spectrum ßlactamase–producing acinetobacter baumannii, France. Emerg Infect Dis (2006) 12:1214–22. doi: 10.3201/eid1708.051547
51. Alsan M, Klompas M. Acinetobacter baumannii: an emerging and important pathogen. J Clin Outcomes Manage (2010) 17:363–9. doi: 10.1016/j.micpath.2018.12.023
52. Decousser J, Jansen C, Nordmann P, Emirian A, Bonnin R, Anais L, et al. Outbreak of NDM-1-producing acinetobacter baumannii in France, January to may 2013. Eurosurveillance (2013) 18:20547. doi: 10.2807/1560-7917.ES2013.18.31.20547
53. Bonnin R, Poirel L, Cuzon G, Nordmann P. ECCMID conference. Germany: European Society of Clinical Microbiology and Infectious Diseases Berlin (2013).
54. Mathlouthi N, Ben lamine Y, Somai R, Bouhalila-Besbes S, Bakour S, Rolain J-M, et al. Incidence of OXA-23 and OXA-58 carbapenemases coexpressed in clinical isolates of acinetobacter baumannii in Tunisia. Microb Drug Resist (2017) 2016:1–6. doi: 10.1089/mdr.2016.0306
55. Khorsi K, Messai Y, Hamidi M, Ammari H, Bakour R. High prevalence of multidrug-resistance in acinetobacter baumannii and dissemination of carbapenemase-encoding genes bla OXA-23-like, bla OXA-24-like and bla NDM-1 in Algiers hospitals. Asian Paci J Tropical Med (2015) 8:438–46. doi: 10.1016/j.apjtm.2015.05.011
56. El-Sayed MAE-G, Amin MA, Tawakol WM, Loucif L, Bakour S, Rolain J-M. High prevalence of blaNDM-1 carbapenemase-encoding gene and 16SrRNA armA methyltransferase among acinetobacter baumannii clinical isolates. Egypt: Antimicrobial Agents and Chemotherapy:AAC (2015) p. 04412–4.
57. Huber CA, Sartor AL, McOdimba F, Shah R, Shivachi P, Sidjabat HE, et al. Outbreaks of multidrug-resistant acinetobacter baumannii strains in a Kenyan teaching hospital. J Global Antimicrob Resist (2014) 2:190–3. doi: 10.1016/j.jgar.2014.03.007
58. Lob SH, Hoban DJ, Sahm DF, Badal RE. Regional differences and trends in antimicrobial susceptibility of acinetobacter baumannii. Int J Antimicrob Agents (2016) 47:317–23. doi: 10.1016/j.ijantimicag.2016.01.015
59. Lemos E, Hoz F, Alvis N, Einarson T, Quevedo E, Castaneda C, et al. Impact of carbapenem resistance on clinical and economic outcomes among patients with acinetobacter baumannii infection in Colombia. Clin Microbiol Infect (2014) 20:174–80. doi: 10.1111/1469-0691.12251
60. Rivera G, Bulnes J, Castillo C, Ajenjo MC, Garcia P, Labarca J. Extensively drug-resistant acinetobacter baumannii isolated in a university hospital: Role of inter-hospital transmission. J Infect Developing Countries (2016) 10:96–9. doi: 10.3855/jidc.6713
61. Kim Y, Kim S, Kim Y, Hong K, Wie S, Park Y, et al. Carbapenem-resistant acinetobacter baumannii: diversity of resistant mechanisms and risk factors for infection. Epidemiol infect (2012) 140:137. doi: 10.1017/S0950268811000744
62. Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
63. Ulu-Kilic A, Ahmed S, Alp E, Doğanay M. Challenge of intensive care unit-acquired infections and acinetobacter baumannii in developing countries. OA Crit Care (2013) 1:2–7. doi: 10.13172/2052-9309-1-1-382
64. Ntusi NB, Badri M, Khalfey H, Whitelaw A, Oliver S, Piercy J, et al. ICU-Associated acinetobacter baumannii colonisation/infection in a high HIV-prevalence resource-poor setting. PloS One (2012) 7:52452–9. doi: 10.1371/journal.pone.0052452
65. Uwingabiye J, Frikh M, Lemnouer A, Bssaibis F, Belefquih B, Maleb A, et al. Acinetobacter infections prevalence and frequency of the antibiotics resistance: comparative study of intensive care units versus other hospital units. Pan Afr Med J (2016) 23(1):1–8. doi: 10.11604/pamj.2016.23.191.7915
66. Reddy D, Morrow BM, Argent AC. Acinetobacter baumannii infections in a south African paediatric intensive care unit. J Trop Pediatr (2015) 61:182–7. doi: 10.1093/tropej/fmv017
67. Opintan JA, Newman MJ, Arhin RE, Donkor ES, Gyansa-Lutterodt M, Mills-Pappoe W. Laboratory-based nationwide surveillance of antimicrobial resistance in Ghana. Infect Drug Resist (2015) 8:379–85. doi: 10.2147/IDR.S88725
68. Manyahi J, Matee MI, Majigo M, Moyo S, Mshana SE, Lyamuya EF. Predominance of multi-drug resistant bacterial pathogens causing surgical site infections in muhimbili national hospital, Tanzania. BMC Res Notes (2014) 7:500–7. doi: 10.1186/1756-0500-7-500
69. Rerambiah LK, Ndong J-C, Massoua PMM, Medzegue S, Elisee-Ndam M, Mintsa-Ndong A, et al. Antimicrobial profiles of bacterial clinical isolates from the gabonese national laboratory of public health: data from routine activity. Int J Infect Dis (2014) 29:48–53. doi: 10.1016/j.ijid.2014.01.015
70. Ahoyo TA, Bankolé HS, Adéoti FM, Gbohoun AA, Assavèdo S, Amoussou-Guénou M, et al. Prevalence of nosocomial infections and anti-infective therapy in Benin: results of the first nationwide survey in 2012. Antimicrob Resist Infect Control (2014) 3:17–23. doi: 10.1186/2047-2994-3-17
71. Zorgani A, Abofayed A, Glia A, Albarbar A, Hanish S. Prevalence of device-associated nosocomial infections caused by gram-negative bacteria in a trauma intensive care unit in Libya. Oman Med J (2015) 30:270. doi: 10.5001/omj.2015.54
72. Nwadike VU, Ojide CK, Kalu EI. Multidrug resistant acinetobacter infection and their antimicrobial susceptibility pattern in a nigerian tertiary hospital ICU. Afr J Infect Dis (2014) 8:14–8.
73. Acquah SE, Quaye L, Sagoe K, Ziem JB, Bromberger PI, Amponsem AA. Susceptibility of bacterial etiological agents to commonly-used antimicrobial agents in children with sepsis at the tamale teaching hospital. BMC Infect Dis (2013) 13:1–14. doi: 10.1186/1471-2334-13-89
74. Agyepong N, Govinden U, Owusu-Ofori A, Essack SY. Multidrug-resistant gram-negative bacterial infections in a teaching hospital in Ghana. Antimicrob Resist Infect Control (2018) 7:37–45. doi: 10.1186/s13756-018-0324-2
75. Blomberg B, Manji KP, Urassa WK, Tamim BS, Mwakagile DS, Jureen R, et al. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infect Dis (2007) 7:43–57. doi: 10.1186/1471-2334-7-43
76. Yanda ANA, Nansseu JRN, Awa HDM, Tatah SA, Seungue J, Eposse C, et al. Burden and spectrum of bacterial infections among sickle cell disease children living in Cameroon. BMC Infect Dis (2017) 17:211–8. doi: 10.1186/s12879-017-2317-9
77. Heyman G, Cars O, Bejarano M-T, Peterson S. Access, excess, and ethics–towards a sustainable distribution model for antibiotics. Upsala J Med Sci (2014) 119:134–41. doi: 10.3109/03009734.2014.904958
78. Holloway K, Mathai E, Gray A. Surveillance of antimicrobial resistance in resource-constrained settings–experience from five pilot projects. Trop Med Int Health (2011) 16:368–74. doi: 10.1111/j.1365-3156.2010.02696.x
79. Swe-Han KS, Mlisana KP, Pillay M. Analysis of clinical and microbiological data on acinetobacter baumannii strains assist the preauthorization of antibiotics at the patient level for an effective antibiotic stewardship program. J Infect Public Health (2017) 698:1–9. doi: 10.1016/j.jiph.2017.01.014
80. Alouane T, Uwingabiye J, Lemnouer A, Lahlou L, Laamarti M, Kartti S, et al. First whole-genome sequences of two multidrug-resistant acinetobacter baumannii strains isolated from a Moroccan hospital floor. Genome Announcements (2017) 5:1–2. doi: 10.1128/genomeA.00298-17
81. Obeidat N, Jawdat F, Al-Bakri AG, Shehabi AA. Major biologic characteristics of acinetobacter baumannii isolates from hospital environmental and patients' respiratory tract sources. Am J Infect Control (2014) 42:401–4. doi: 10.1016/j.ajic.2013.10.010
82. WHO. Antimicrobial resistance: global report on surveillance. France: World Health Organization (2014).
83. Sipahi OR. Economics of antibiotic resistance. Expert Rev Anti-infect Ther (2008) 6:523–39. doi: 10.1586/14787210.6.4.523
84. Mendelson M, Whitelaw A, Nicol M, Brink A. Wake up, south Africa! the antibiotic'horse'has bolted. SAMJ: South Afr Med J (2012) 102:607–8. doi: 10.7196/SAMJ.5759
85. Ferri M, Ranucci E, Romagnoli P, Giaccone V. Antimicrobial resistance: a global emerging threat to public health systems. Crit Rev Food Sci Nutr (2017) 57:2857–76. doi: 10.1080/10408398.2015.1077192
86. Balkan II, Dogan M, Durdu B, Batirel A, Hakyemez IN, Cetin B, et al. Colistin nephrotoxicity increases with age. Scand J Infect Dis (2014) 46:678–85. doi: 10.3109/00365548.2014.926021
87. Kwon KT, Oh WS, Song J-H, Chang H-H, Jung S-I, Kim S-W, et al. Impact of imipenem resistance on mortality in patients with acinetobacter bacteraemia. J Antimicrob Chemother (2007) 59:525–30. doi: 10.1093/jac/dkl499
88. Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J, et al. Epidemiology and impact of imipenem resistance in acinetobacter baumannii. Infect Control Hosp Epidemiol (2009) 30:1186–92. doi: 10.1086/648450
89. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A syst review BMC Infect Dis (2006) 6:130–8. doi: 10.1186/1471-2334-6-130
90. Garnacho-Montero J, Dimopoulos G, Poulakou G, Akova M, Cisneros JM, De Waele J, et al. Task force on management and prevention of acinetobacter baumannii infections in the ICU. Intensive Care Med (2015) 41:2057–75. doi: 10.1007/s00134-015-4079-4
91. Tambo E, Ai L, Zhou X, Chen J-H, Hu W, Bergquist R, et al. Surveillance-response systems: the key to elimination of tropical diseases. Infect Dis Poverty (2014) 3:17–27. doi: 10.1186/2049-9957-3-17
92. Essack S, Desta A, Abotsi R, Agoba E. Antimicrobial resistance in the WHO African region: current status and roadmap for action. J Public Health (2017) 39:8–13. doi: 10.1093/pubmed/fdw015
93. Shallcross LJ, Davies SC. The world health assembly resolution on antimicrobial resistance. J Antimicrob Chemother (2014) 69:2883–5. doi: 10.1093/jac/dku346
94. Fournier PE, Richet H, Weinstein RA. The epidemiology and control of acinetobacter baumannii in health care facilities. Clin Infect Dis (2006) 42:692–9. doi: 10.1086/500202
95. Morgan DJ, Liang SY, Smith CL, Johnson JK, Harris AD, Furuno JP, et al. Frequent multidrug-resistant acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol (2010) 31:716–21. doi: 10.1086/653201
96. Samuel S, Kayode O, Musa O, Nwigwe G, Aboderin A, Salami T, et al. Nosocomial infections and the challenges of control in developing countries. Afr J Clin Exp Microbiol (2010) 11:102–9. doi: 10.4314/ajcem.v11i2.53916
97. Gavaldà L, Soriano AM, Cámara J, Gasull R, Arch O, Ferrer M, et al. Control of endemic extensively drug-resistant acinetobacter baumannii with a cohorting policy and cleaning procedures based on the 1 room, 1 wipe approach. Am J Infect Control (2016) 44:520–4. doi: 10.1016/j.ajic.2015.11.036
98. Huang C-H, Lee C-L, Lin AC-M, Chen W-Y, Teng P-C, Lee S-H, et al. Different strains of acinetobacter baumannii spreading in an intensive care unit. J Acute Med (2011) 1:5–10. doi: 10.1016/j.jacme.2011.07.003
99. Asare A, Enweronu-Laryea CC, Newman MJ. Hand hygiene practices in a neonatal intensive care unit in Ghana. J Infect Developing Countries (2009) 3:352–6.
100. Chung YK, Kim J-S, Lee SS, Lee J-a, Kim H-S, Shin K-s, et al. Effect of daily chlorhexidine bathing on acquisition of carbapenem-resistant acinetobacter baumannii (CRAB) in the medical intensive care unit with CRAB endemicity. Am J Infect Control (2015) 43:1171–7. doi: 10.1016/j.ajic.2015.07.001
101. Apisarnthanarak A, Pinitchai U, Warachan B, Warren DK, Khawcharoenporn T, Hayden MK. Effectiveness of infection prevention measures featuring advanced source control and environmental cleaning to limit transmission of extremely-drug resistant acinetobacter baumannii in a Thai intensive care unit: An analysis before and after extensive flooding. Am J infect control (2014) 42:116–21. doi: 10.1016/j.ajic.2013.09.025
102. Ha DR, Haste NM, Gluckstein DP. The role of antibiotic stewardship in promoting appropriate antibiotic use. Am J Lifestyle Med (2019) 13:376–83. doi: 10.1177/1559827617700824
103. Yamamoto N, Hamaguchi S, Akeda Y, Santanirand P, Kerdsin A, Seki M, et al. Clinical specimen-direct LAMP: A useful tool for the surveillance of bla OXA-23-Positive carbapenem-resistant acinetobacter baumannii. PloS One (2015) 10:133204–14. doi: 10.1371/journal.pone.0133204
Keywords: antibiotic resistance, intensive care unit, multidrug-resistant Acinetobacter baumannii, prevalence, healthcare settings
Citation: Agyepong N, Fordjour F and Owusu-Ofori A (2023) Multidrug-resistant Acinetobacter baumannii in healthcare settings in Africa. Front. Trop. Dis 4:1110125. doi: 10.3389/fitd.2023.1110125
Received: 28 November 2022; Accepted: 06 February 2023;
Published: 28 February 2023.
Edited by:
Denis Sereno, Institut de Recherche Pour le Développement (IRD), FranceReviewed by:
Luther King Abia Akebe, University of KwaZulu-Natal, South AfricaCopyright © 2023 Agyepong, Fordjour and Owusu-Ofori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas Agyepong, agyanicho96@gmail.com
 Nicholas Agyepong
Nicholas Agyepong Francis Fordjour
Francis Fordjour Alex Owusu-Ofori
Alex Owusu-Ofori