- 1Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, United States
- 2Center for Family Health Research Zambia, Ndola, Zambia
- 3Center for Family Health Research Zambia, Lusaka, Zambia
- 4Parasitic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, GA, United States
- 5Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine and Hubert Department of Global Health, Rollins School of Public Health, Laney Graduate School, Emory University, Atlanta, GA, United States
Background: The World Health Organization estimates that 56 million women and girls live with female genital schistosomiasis (FGS) in sub-Saharan Africa. FGS is often confused with symptoms of other genital abnormalities, and gold standard diagnosis with colposcopy is infeasible in most health facilities. Schistosomiasis haematobium is endemic in Zambia, yet routine screening or diagnostic efforts for FGS remain unavailable. Our study aimed to develop and pilot test a feasible FGS screening algorithm to implement in Zambian government clinics.
Methodology/Principal Findings: We recruited 499 women from a longitudinal cohort of HIV-negative adult women in Lusaka and Ndola, Zambia. We used demographic, risk factor, and symptom data collected from standardized surveys, gynecological exams, and laboratory tests to develop a screening algorithm for FGS among a derivation cohort (n=349). After cross-validation using 5-fold iterative resampling, the algorithm was applied in a holdout sample of the cohort (n=150). The prevalence of FGS (ascertained by expert review) was 23.4% in the study population. The screening algorithm included childhood and travel exposure to rivers and streams; testing positive for visual inspection of the cervix with acetic acid; hematuria; reporting less than the median average age at sexual debut (<17 years); when asked what diseases can be transmitted via freshwater exposure, reporting ‘none’; being born outside of Lusaka or Copperbelt Province; and reporting occupation as ‘Housekeeper’. The screening algorithm had reasonable discrimination in the derivation cohort (area under the curve [AUC]=0.69, 95% confidence interval [CI]: 0.66-0.79, p-value<0.001). Using a score cut off ≥ 2 the risk algorithm in the derivation cohort had 77% sensitivity, 48% specificity, 35% positive predictive value, and 85% negative predictive value.
Conclusions/Significance: Given the prevalence of FGS and associated morbidities, improved screening for FGS is imperative. We developed a simple screening algorithm to improve the diagnosis and treatment of FGS among adult women in Zambian government clinics.
Introduction
Female genital schistosomiasis (FGS) is a common disease manifestation caused by infection with Schistosoma haematobium (1). Upwards of 260 million people worldwide have a Schistosoma (haematobium, japonicum or mansoni) infection and nearly 56 million women and girls are living with FGS in sub-Saharan Africa alone (2). FGS is caused by an inflammatory reaction to schistosome eggs trapped in body tissue, which leads to fibrosis and scarring of the female genital tract (3). FGS is one of the most neglected sexual and reproductive health diseases in sub-Saharan Africa and has been linked to numerous reproductive sequelae, including infertility (4), ectopic pregnancies (5), and adverse birth outcomes (6). FGS is also linked to increased susceptibility to human papillomavirus (HPV) (1) and human immunodeficiency virus (HIV) infection (7, 8) and progression to AIDS among HIV-infected individuals (9). Therefore, timely identification and treatment of FGS would not only reduce the debilitating burden of FGS, but may lead to improvements in birth outcomes, reduced genital abnormalities, and reduced susceptibility to HIV and other sexually transmitted infections (STIs). Proper diagnosis and treatment of FGS may also help to reduce stigma in women improperly thought to have HIV or STIs.
In Zambia, schistosomiasis (mainly S. haematobium) is endemic, with prevalence estimates ranging from 5-40% (10, 11). The mass provision of praziquantel to children 5-14 remains an effective public health initiative; however, adult praziquantel coverage is disparately low at 14%, compared to 54% coverage of school-aged children (12). Among Zambian women in the capital city, we found from 1994-2012 the prevalence of schistosome antibodies was 51% (13), necessitating a shift in thinking of schistosomiasis as a disease of children and rural areas. In the recently published Zambian Ministry of Health Elimination of Neglected Tropical Disease National Masterplan, 2019-2023, however, there are no guidelines for FGS identification in adults (14).
FGS is difficult to diagnose from symptomology alone, as it is often confused with other genital abnormalities. While diagnosis of FGS by experts during colposcopy, the gold standard diagnostic method, has been improved by the WHO pocket atlas (http://pocketatlas.org) and published criteria exist to identify lesions, colposcopy is expensive and requires extensive training, putting it out of reach for many resource-poor settings (2). Much work is needed to improve clinical FGS screening and diagnostics in feasible, affordable, and sustainable ways.
Our objective in this study was to develop and pilot test a screening algorithm to screen for FGS among women that is feasible and affordable for use in Zambian government clinics. To do so, we recruited participants from an established cohort of women at high-risk for STIs and other genital abnormalities in urban Zambia (15) to undergo colposcopy to identify FGS. We then developed a screening algorithm using survey and clinical data and assessed the screening algorithm performance against gold standard findings by colposcopy. The screening algorithm was validated internally using five-fold cross-validation and then applied to a subset of the cohort.
Methods
Ethics statement
This study received ethical approval from the Emory University Institutional Review Board (IRB), University of Zambia Biomedical Research Ethics Committee and the Zambia National Health Research Authority. All eligible participants provided written informed consent before enrollment into the study. Data were anonymized prior to analysis.
Population
Women were recruited from an established longitudinal cohort of HIV-negative in Lusaka and Ndola, Zambia. Eligibility criteria included being a female sex worker or single mother, 18-45 years of age, and being HIV-negative. This cohort is at high-risk for HIV and other sexually transmitted infections (STIs) (15). Recruitment strategies have been described previously (15). Briefly, an estimated 30-40 cohort members attend the study sites each day, and the members were recruited to participate in this investigation by convenience sampling. Participants who were attending the clinic for their annual or quarterly visits were provided with a description of this substudy. Participants who agreed to participate were compensated to cover the travel and time spent participating. The target sample size given time and resource constraints and anticipated clinic attendance was 520 participants. Between February 2021 and August 2021, 349 women were recruited into the derivation cohort. Between September 2021 and December 2021, 150 women were recruited into the temporal validation cohort. To prevent overfitting and to confirm whether a tool is clinically useful, the performance of the tool should be based on a cohort different from that used to develop the model (16). This splitting of a single cohort into development and validation sets by time is regarded as an approach that lies midway between internal and external validation (17). The sample size for the derivation and validation cohort were selected to allow a 70% to 30% percent split, respectively.
Survey, genital exam, and laboratory measures
All study procedures took place at the Center for Family Health Research Zambia (CFHRZ) research sites in Lusaka and Ndola, Zambia. In both the derivation and temporal validation cohorts, demographic, risk factor, and symptom data were collected via standardized surveys. Survey questions were identified through a literature review and drawn from our previous work describing risk factors for genital abnormalities in Zambia (13, 15, 18, 19). Surveys were programmed into SurveyCTO and were administered by nurses using tablets. The specific subset of survey questions that were included in the algorithm are included in the (Online Supplement).
Genital exams were performed by trained physicians and nurses to assess for inflammation, contact bleeding, discharge, ulceration, and adenopathy. We conducted laboratory testing of participant samples including phlebotomy for rapid HIV and rapid plasma reagin (RPR) serologies; microscopy of nurse-collected vaginal swab wet mounts to diagnose Trichomonas vaginalis (TV), bacterial vaginosis (BV) and vaginal Candida albicans (VCA); and nurse-collected endocervical swabs for GeneXpert (Cepheid, Sunnyvale USA) testing for Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT). Women also provided urine samples for hematuria testing and detection of S. haematobium eggs by trained laboratory technicians using standard operating procedures.
Colposcopy procedures
Women underwent colposcopic examination during which images of the cervix were taken for FGS diagnosis and visual inspection with acetic acid (VIA) tests were conducted. Co-investigators and ObGyns Drs. Vwalika [WHO FGS pocket atlas contributor (2)] and Inambao are trained in colposcopy, FGS identification via images taken during colposcopy, and visual inspection with acetic acid (VIA) testing. They trained four CFHRZ research doctors and nurses in these methods. These doctors and nurses performed colposcopy, took photographs of the cervix, and performed VIA tests. Drs. Vwalika and Inambao reviewed all images taken during colposcopy for FGS diagnosis.
Colposcopic exams were conducted on non-menstruating women by inserting an autoclaved (sterilized) bivalve speculum. A noted limitation of colposcopy is that it only captures lesions in the lower reproductive tract; and thus, vaginal walls were not inspected. The mucosal and vulval surfaces were inspected and photographs of the cervix and vaginal mucosa were taken prior to VIA. Images were also taken after a cervical wash with acetic acid. During diagnosis of FGS from images taken during colposcopy (using the Bovie Colpo-Master™ CS-105LEDI Swing Arm Colposcope equipped with Continuous Zoom, with zoom ratio 1:6.7 (0.67x-4.5x) and magnification ranged from 3.9x - 27x), investigators recorded whether there was the presence of any indicators of FGS (i.e., grainy sandy patches, homogenous yellow sandy patches, abnormal blood vessels, rubbery papules) and where each of these indicators were located by cervical quadrant. To reduce bias, gynecological exams, laboratory, and survey findings were not known to the investigators interpreting the images. No specific reviewer agreement was evaluated in this investigation; however, a follow-up study evaluating treatment resolution among the same staff found 81% agreement between reviewers (20).
Treatments and referrals
Women who were FGS+ by colposcopy or who had eggs or blood in their urine were treated for free at our research facility with praziquantel at the WHO-recommended single dosage of 40 mg/kg (2). Women diagnosed with STIs or BV were treated for free at our research facility per Zambian National Guidelines (21). Women who were HIV, VIA, or HPV positive were referred for care per National Guidelines (21, 22).
Data management
Survey data were collected on tablets using SurveyCTO. Clinical data were collected directly on tablets using the SurveyCTO application or were collected using paper forms that were then data entered into the SurveyCTO application. Survey data were imported into an MS Access database on a weekly basis for long-term storage, quality control, and data cleaning. Additional data quality checks were performed using Statistical Analysis Software (SAS) version 9.4 (Cary, NC).
Data analyses
Analyses are conducted with Statistical Analysis Software (SAS) version 9.4 (Cary, NC).
Our outcome of interest was FGS diagnosed by images taken during colposcopy. Demographic, behavioral, and symptom data are described for the derivation cohort (Table 1). Counts and percentages were described overall and by FGS-status. Chi-square tests evaluated whether the differences in the distribution of these data by FGS-status were statistically significant.
Bivariate logistic regression models identified variables associated with the outcome of interest. These variables were included in multivariable logistic regression models if they were statistically significantly associated (p-value < 0.2) with the outcome in bivariate analyses, survived backward elimination (p-value < 0.2), and were considered feasible measures in a Zambian clinic setting.
We selected 0.20 as our cut-off to reduce overfitting the model to a small number of variables. Variable multi-collinearity was assessed by minimizing variables with a high variable inflation factor. Crude and adjusted prevalence odds ratios (cPOR, aPOR), 95% confidence intervals (CIs), and p-values were calculated. Score values for individual variables in the final model were obtained by dividing each variable’s estimated model coefficient by the lowest coefficient among all variables and rounding to the nearest integer. To assess model calibration, we used the Hosmer-Lemeshow Goodness-of-Fit testing using the LACKFIT option in SAS.
To further evaluate overfitting of the algorithm to our specific data set, we deploy internal cross validation, a process that uses subsets of the data to iteratively train and test the performance of the model (23). A general form of cross-validation is known as k-fold cross-validation, where data are split into k different subsets. The number of folds (k) is selected by the researcher; however, 5 or 10 are most chosen. In standard 5-fold cross-validation methods. Variables associated with the outcome (p-value < 0.2) in bivariate analysis were included in initial multivariate models excluding 1/5th of the data. The final model was derived by backward elimination and a model coefficient-weighted score was created from the variables retained in the final model. The scores derived were then tested in the remaining 1/5th of the data. This process was repeated five times such that each 1/5th was withheld and then tested in turn. Then the same process was applied to five 80% training sets and 20% test sets. The sets were sampled at random after setting a seed to allow for reproducibility. Significance testing of the area under the receiver operating curve (ROC) was performed for each fold, where a p-value less than alpha indicates that we reject the null hypothesis that the true (population) Area under the ROC curve is 0.5. To check for overfitting, we would assess whether the p-value for each fold was consistently greater than the prespecified alpha (i.e. 0.05).
The area under the receiver operating curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the risk algorithm was calculated using score cut-offs defined as the median score +/- 1 point. The AUC was calculated using standard methods (24) for the screening algorithm applied to the derivation cohort, after 5-fold cross validation (average AUC of the 5 different models is presented), and applied to the temporal validation cohort. These measures were calculated for the risk algorithm applied to both the derivation and temporal validation cohorts.
Results
From February 2021 to December 2021, we studied 538 women at high risk of genital abnormalities who were enrolled in standing cohorts at CFHRZ research sites in Lusaka and Ndola, Zambia. The final study group after the exclusion of 39 (7.2%) women who were missing data on FGS was 499.
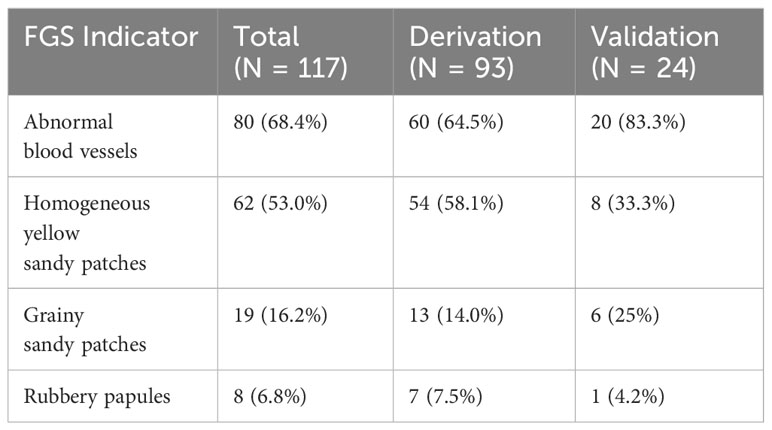
There were 117 cases (23.4%) of FGS in the entire cohort, indicated most frequently by abnormal blood vessels (n = 80, 68.4%), followed by homogeneous yellow sandy patches (n = 62, 53.0%), grainy sandy patches (n = 19, 16.2%) and by rubbery papules (n = 8, 6.8%). The breakdown of FGS indicators by derivation and validation groups is presented in Table 1. Women in the derivation cohort (n=349) were recruited from February 2021 to August 2021, and in the validation cohort (n=150) from September 2021 to December 2021. Among the 349 women in the derivation cohort, the prevalence of FGS was 26.6% (n=93). Among the 150 women in the temporal validation cohort, the prevalence of FGS was lower at 16.0% (n=24).
We have included descriptive statistics overall and by FGS status for the derivation cohort in Table 2. Though we examined far more covariates in association with FGS, we include just the primary demographic characteristics and those that were significant at an alpha = 0.2 in the bivariate analysis. The specific survey items for these significant items are included in the (Online Supplement). The average age in the derivation cohort was 27.1 years (SD = 4.7). Most participants were single-never married (76.8%) and 42.5% had secondary education.
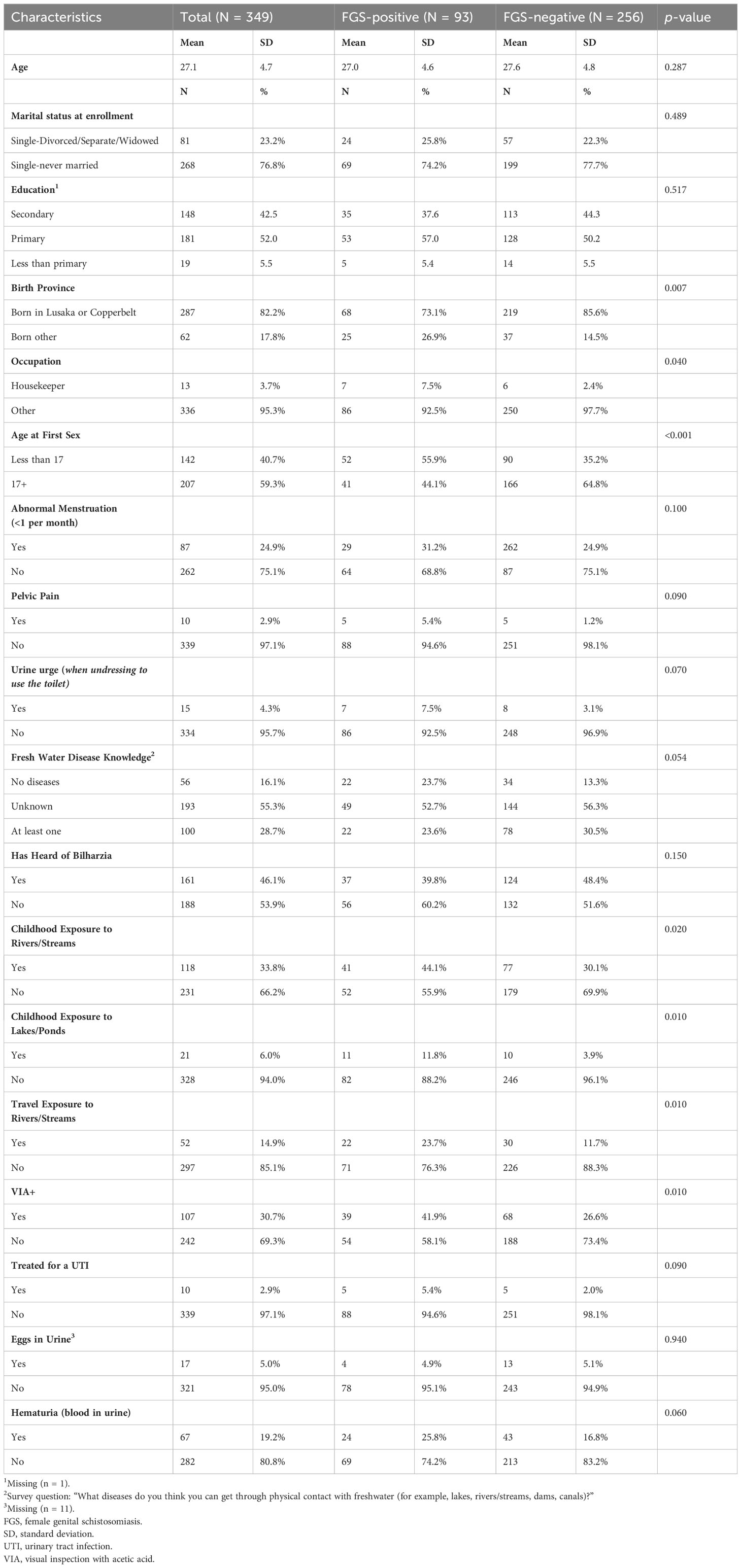
Table 2 Baseline characteristics and associations with FGS in urban Zambian women (derivation cohort N = 349).
Most of our sample was born in Lusaka or Copperbelt province, with only 17.8% being born outside. Lusaka and Copperbelt provinces account for 69% of Zambia’s total urban population (25); therefore, grouping these two locations together reflects a proxy for urbanicity. A greater proportion of those who were FGS positive (26.9% vs. 14.5%) were born outside of these two provinces (p = 0.007). Though housekeeping only represented 3.7% of the overall employment, 7.5% of those who were FGS positive reported housekeeping as their occupation compared to 2.5% of those not diagnosed with FGS (p = 0.040). The other survey items for occupation included ‘vendor in the market’, ‘hairdresser’, ‘receptionist/secretary’, ‘sex worker’, and ‘other’. Housekeeping, albeit, a small proportion of the overall employment sector for this sample, likely introduces a unique exposure to freshwater (i.e. laundry, cleaning, etc.) (26) compared to the other professions. Two different measurements of age were included in the bivariate analysis, including continuous age (presented in Table 2) and tertile categories. Age was not significantly different across groups (mean = 27.0 in the FGS positive group and mean = 27.6 in the FGS negative group), regardless of variable type; however, age at first sex was significantly different across groups, with 55.9% of people living with FGS reporting being less than 17 at first sex compared to 35.2% of those not living with FGS (p < 0.001). The delineation between having a sexual debut at 16.9 to 17.1 is unlikely to be substantively different, however, this statistical significance against FGS status may be capturing risk factors that coincide with younger age at sexual debut, including lower education and rurality (27).
Although not statistically significant, participants living with FGS also reported more frequent urogenital symptoms, including abnormal menstruation (defined as having a period less than once per month), pelvic pain, the urge to urinate when undressing to use the bathroom, receiving treatment for a urinary tract infection (UTI), and blood in urine (lab detected). Screening positive for precancerous lesions when undergoing visual inspection of the cervix with acetic acid (VIA+) was significantly associated with FGS (p = 0.01). The prevalence of positive VIA+ screen was 30.7% in the derivation group (41.9% among those with FGS and 26.6% among those without). All participants who had a positive screen for precancerous lesions were linked to follow-up care.
When evaluating freshwater exposure and knowledge, participants with FGS were more likely to report childhood exposure to rivers/streams (p = 0.020) or lakes/ponds (p = 0.010), as well as travel exposure to rivers/streams (p = 0.010). Those living with FGS were less likely to have heard of schistosomiasis (‘bilzharia or snail fever’) (39.8% vs. 48.4%, NS) and were less likely to name a disease that could be transmitted by freshwater exposure (p = 0.002).
The risk algorithm in the derivation cohort (Table 3) included having childhood exposure to rivers and streams (2 points), travel exposure to rivers and streams (1 point), testing positive for visual inspection of the cervix with acetic acid (1 point), hematuria (1 point), reporting less than the median average age at first sex (<17 years) (2 points), when asked what diseases can be transmitted via freshwater exposure, reporting ‘none’ (2 points), born outside of Lusaka or Copperbelt Province (1 points), and reporting their occupation as ‘Housekeeper’ (3 points). The Hosmer-Lemeshow Goodness-of-Fit test indicated good calibration (p-value=0.77).
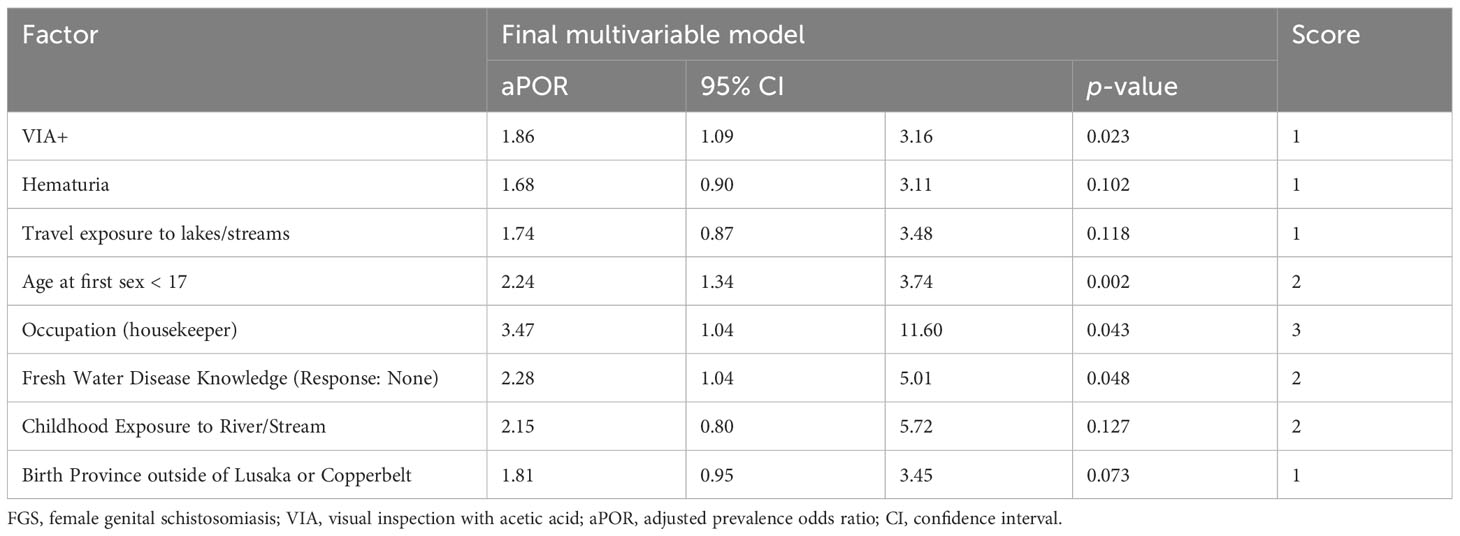
Table 3 Screening algorithm comprised of factors associated with FGS in high-risk Zambian women (derivation cohort N = 349).
The risk algorithm had reasonable discrimination in the derivation cohort (AUC=0.68, 95% CI: 0.62-0.75, p-value < 0.001) (Table 4). The internal cross-validation models had slightly lower average discrimination (AUC=0.62, 95% CI: 0.48-0.76, p-value=0.15).

Table 4 Results of 5-fold cross validation of screening algorithm comprised of factors associated with FGS in high-risk Zambian women (derivation cohort N = 349).
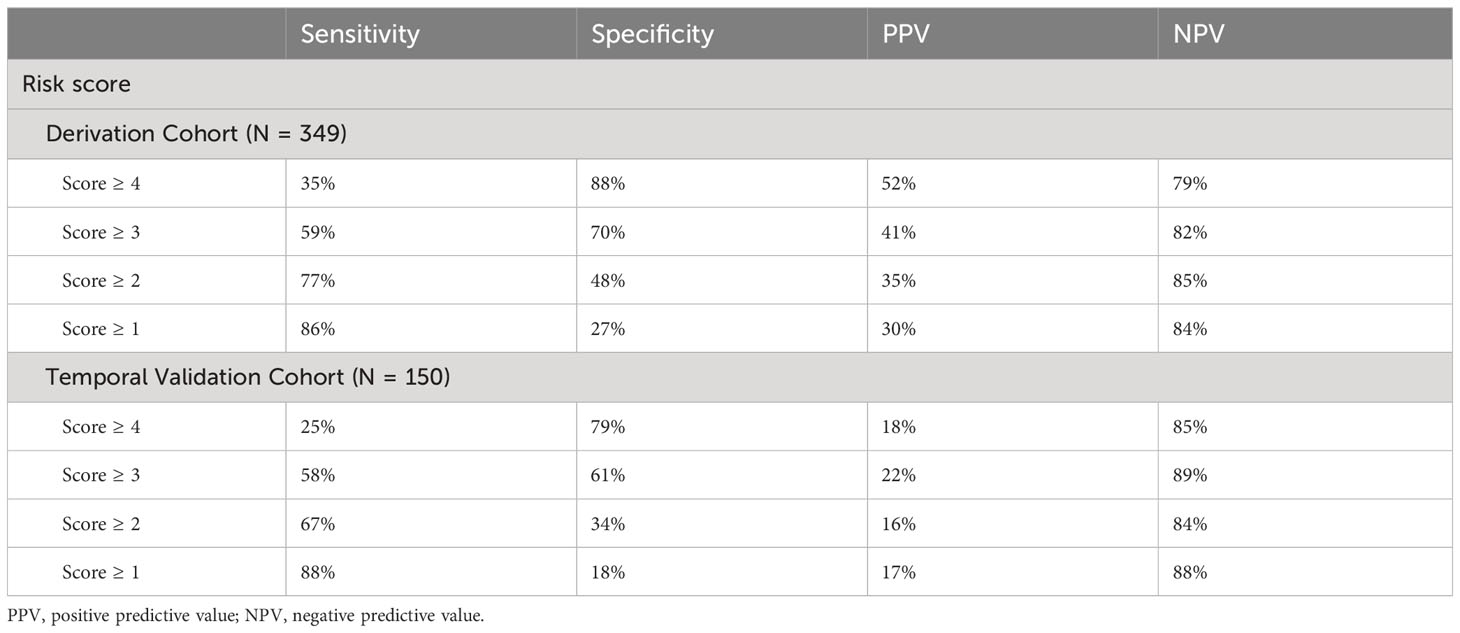
Using a score cut-off of ≥ 2, the risk algorithm in the derivation cohort had 77% sensitivity, 48% specificity, 35% PPV, and 85% NPV (Table 5). For a cut-off of 3, the sensitivity, specificity, PPV, and NPV scores were 59%, 70%, 41%, and 82% respectively. When applied to the temporal validation cohort, and a score cut off of ≥ 2, the risk algorithm had a sensitivity of 67%, specificity of 34%, PPV of 16%, and NPV of 84%.
Discussion
We evaluated the performance of an algorithm to detect female genital schistosomiasis in a population of women in Zambia. This simple screening algorithm is comprised of measures (demographic, symptoms, and laboratory data) that can be gleaned from a checklist that is both cost-effective and likely to improve referral for diagnosis and treatment of FGS among adult women in areas where access to colposcopy is limited. The risk algorithm demonstrated reasonable fit and discrimination in the derivation cohort, and we found reasonable sensitivity (77%) when the risk score was set to less than or greater than the median (cut off ≥ 2). We detected a relatively high rate of false negatives (23%) among those with FGS and a higher rate of false positives (52%) among those without FGS. The estimates (AUC: 0.58-0.68) were robust across each round of our 5-fold internal cross-validation, and the wider variance of each round is not surprising given that each one only comprised 20% of the derivation data.
Sensitivity is often prioritized in screening tests for serious and treatable diseases, particularly when the risks of false positives are low, and the costs/risks of further testing are reasonable (28). In practice, we can see how the algorithm would detect a high rate of false positives. For example, with a score cut of two or more, anyone receiving treatment for a UTI or anyone who was born outside of Lusaka or Copperbelt would screen positive for FGS. Similarly, with a cut-off of three or more, all housekeepers would screen positive for FGS. We can imagine that this would capture a large section of the population, regardless of true underlying schistosome exposure. However, in the case of FGS, the risk of receiving a false positive result may be relatively low, as schistosome infections can be effectively treated with praziquantel. The costs and availability of further testing, including colposcopy and biopsy, should also be factored in when deciding whether to implement the algorithm as a screening tool. In urban areas like Lusaka and Ndola, further testing could mirror cervical cancer screening programs, where women who screen VIA+ at government facilities are referred to larger hospitals where colposcopy directed biopsies are available, and providers are trained to conduct these procedures. To consider the possibility of using this as a screening tool for any pelvic exam, we also conducted an analysis that does not incorporate the VIA screening. Results from this analysis are included in the (Online Supplement).
Additionally, the PPV was consistently low across the testing and validation groups, suggesting that while the algorithm may be a useful screening tool, it may not be suitable as a diagnostic tool in this population. Unlike sensitivity and specificity, the NPV and PPV are directly related to the prevalence of the outcome in the population, with PPV going up when the tool is applied to a population with a higher prevalence of disease. As an alternative, the tool could be used to increase the pre-test probability of disease or the prevalence of disease in the population referred for subsequent diagnostic testing.
Our findings also highlight the concerningly high prevalence of FGS in adult women living in urban areas of Zambia. It is interesting to note that many of these women did not report traveling or living outside of urban areas, indicating the possibility of urban/peri-urban transmission of the disease, as has been reported near other cities (29). More research is needed to examine the role of urbanization, migration, and climate change in the transmission of schistosomiasis in endemic countries, as recent modeling studies demonstrate that climate change is likely to alter freshwater suitability for hosting parasites. It is projected that the locales in Zambia where schistosomiasis is endemic will be expanding, further altering how we define who is at risk of Schistosoma infection (30). Similarly, we also want to consider the 30% of individuals who were diagnosed with FGS but were missed by the screening algorithm. This is a particularly important group, as their risk profile may differ from what prior research suggests and was left unaccounted for in the development of the algorithm.
The results of this study suggest that our algorithm performs reasonably well within the validation sample. However, because this validation was only conducted in a subset of the same cohort from which the algorithm was developed, the protocol for care was consistent across the training and validation groups. To improve the generalizability of our findings, we need to expand our evaluation of the algorithm to a truly external cohort, where sampling, recruitment and implementation are indistinguishable from the cohort by which the algorithm was developed. We are working to improve our algorithm by testing stored serum for schistosome-specific antibody levels, which if successful, will become rather useful when antibody testing is more accessible. Although antibody positivity does not differentiate between current and former infection, because FGS can remain following successful treatment of schistosomiasis, knowledge about whether a woman had ever been infected would likely contribute to the accuracy of the algorithm. Additionally, it is necessary to conduct further testing with a true external cohort in a less controlled environment. A proposed avenue for future research is to test the algorithm’s performance in a sample of women recruited at Zambian government clinics (e.g., among those receiving HIV/STI testing and care). This will provide a more realistic assessment of its performance in a diverse and less controlled setting.
Additionally, praziquantel’s impact on symptom resolution among women with FGS is an important topic that has received less attention than FGS detection. Of those that have monitored long-term treatment outcomes, egg excretion stops in as soon as two weeks (31) following a single dose of praziquantel, and women remained egg negative for up to six months (32), indicating successful treatment of an active infection. However, only a subset of these women achieved marked improvements in urogenital abnormalities (e.g., lesions and growths). Women with cocirculating HPV infections, who are older, or who have more advanced lesions, appear to have more persistent urogenital abnormalities (31, 32). Overall, we require a better understanding of treatment outcomes by the duration, dose, and frequency of praziquantel as well as by age, duration and severity of infection. We recently completed a study to evaluate factors associated with the resolution/non-resolution of FGS lesions after treatment with praziquantel in a subset of women in this study and found that most women (60%) experienced decreased FGS severity and 23% experienced complete lesion resolution (20).
Lastly, the prevalence of FGS was high among our sample, which is comprised of women at high risk for HIV infection. The mechanism between FGS and HIV acquisition and transmission risk has not been fully elucidated, though there is a large amount of observational data supporting the association between FGS and HIV risk (8, 13). Some pre-clinical and human studies have explored possible mechanisms. To support this effort, our team has collected and banked two high vaginal swabs, two cervicovaginal lavage samples, and one endocervical swab from each participant. Some studies propose that the optimal vaginal microbiota combined with a preserved cervicovaginal epithelium provides an effective barrier of defense against HIV-1 acquisition (33, 34). Others have hypothesized that levels of certain cervicovaginal cytokines may be elevated in women with FGS and may relate to the risk of HIV-1 acquisition (35). We are working to evaluate whether FGS positivity is associated with disrupted vaginal microbiota and with elevated cytokines. Though likely infeasible to implement as a screening tool in government clinics, these measures may help contribute to understanding the causal link between FGS and HIV. More generally, we suspect that screening, testing, and treatment for FGS would be cost effective measures to take to reduce HIV infection among women who are considered at high risk.
Conclusions
To our knowledge, this is the first of such algorithms to be developed for use in Zambian government clinic settings; it could improve screening for FGS and the subsequent diagnosis and treatment. Currently, in Zambia, no routine screening or diagnostic efforts for FGS are taking place, in large part due to the difficulties of diagnosis with colposcopy. Our findings confirmed a high prevalence of FGS (n=24%) in our sample of young, urban adult women, indicating that such screening tools are urgently needed, even in urban settings where transmission of S. haematobium has historically not been of major concern. This study supports opportunities for identifying a screening FGS algorithm that is feasible to implement in Zambian government clinics without the need for expensive colposcopy equipment. To our knowledge, there currently exists no screening methods or protocols for FGS that are easy to implement or are cost-effective. We acknowledge that the sensitivity of our algorithm would result in substantial false-positive detection if deployed; though the benefits of treating truly positive cases could outweigh the risks, as we have seen leveraged in the presumptive treatment of school-age children. Additional testing of this screening tool in external government clinic settings in Zambia and other countries where S. haematobium is endemic would be useful.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Emory University Institutional Review Board, University of Zambia Biomedical Research Ethics Committee, Zambia National Health Research Authority. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ER: Writing – original draft, Writing – review & editing. SM: Writing – review & editing. CK: Writing – review & editing. WK: Writing – review & editing. BV: Writing – review & editing. MI: Writing – review & editing. KM: Writing – review & editing. CC: Writing – review & editing. WS: Writing – review & editing. VM: Writing – review & editing. CH: Writing – review & editing. RP: Writing – original draft, Writing – review & editing. AT: Writing – review & editing. KB: Writing – review & editing. SA: Writing – review & editing. KW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Coalition for Operational Research on Neglected Tropical Diseases to K. M. W., which is funded by The Task Force for Global Health primarily by the Bill & Melinda Gates Foundation; by the US Agency for International Development through its Neglected Tropical Diseases Program; and with UK aid from the British people. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This research was funded in part by NIDA grant 5T32DA050552. The results and opinions expressed therein represent those of the authors and do not necessarily reflect those of NIH or NIDA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2023.1308129/full#supplementary-material
Supplementary Table 1 | Select survey measurements and relevant data manipulations for analysis.
Supplementary Table 2 | Baseline characteristics in urban Zambian women by derivation and validation samples (Total = 499).
Supplementary Table 3 | Screening algorithm comprised of factors associated with FGS in high-risk Zambian women, excluding VIA+ (derivation cohort N = 349).
Supplementary Table 4 | Screening algorithm performance to identify FGS in high-risk Zambian women (excluding VIA+).
References
1. Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol (2012) 28(2):58–65. doi: 10.1016/j.pt.2011.10.008
2. World Health Organization. Female Genital Schistosomiasis: a pocket atlas for clinical health-care professionals. Geneva, Switzerland: World Health Organization (2015). Available at: https://www.who.int/publications/i/item/9789241509299.
3. Engels D, Hotez PJ, Ducker C, Gyapong M, Bustinduy AL, Secor WE, et al. Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull World Health Organ. (2020) 98(9):615–24. doi: 10.2471/BLT.20.252270
4. Kjetland EF, Kurewa EN, Mduluza T, Midzi N, Gomo E, Friis H, et al. The first communitybased report on the effect of genital Schistosoma haematobium infection on female fertility. Fertil Steril. (2010) 94(4):1551–3. doi: 10.1016/j.fertnstert.2009.12.050
5. Friedman JF, Mital P, Kanzaria HK, Olds GR, Kurtis JD. Schistosomiasis and pregnancy. Trends Parasitol (2007) 23(4):159–64. doi: 10.1016/j.pt.2007.02.006
6. Freer JB, Bourke CD, Durhuus GH, Kjetland EF, Prendergast AJ. Schistosomiasis in the first 1000 days. Lancet Infect Dis (2018) 18(6):e193–203. doi: 10.1016/S1473-3099(17)30490-5
7. Sturt A, Webb E, Francis S, Hayes R, Bustinduy A. Beyond the barrier: Female Genital Schistosomiasis as a potential risk factor for HIV-1 acquisition. Acta Trop (2020) 209:105524. doi: 10.1016/j.actatropica.2020.105524
8. Sturt AS, Webb EL, Phiri CR, Mudenda M, Mapani J, Kosloff B, et al. Female genital schistosomiasis and HIV-1 incidence in Zambian women: A retrospective cohort study. Open Forum Infect Dis (2021) 8(7):ofab349. doi: 10.1093/ofid/ofab349
9. Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis (2005) 192(11):1956–61. doi: 10.1086/497696
10. Mwanakasale V, Vounatsou P, Sukwa TY, Ziba M, Ernest A, Tanner M. Interactions between Schistosoma haematobium and human immunodeficiency virus type 1: the effects of coinfection on treatment outcomes in rural Zambia. Am J Trop Med Hyg (2003) 69(4):420–8.
11. Modjarrad K, Vermund SH, Freedman DO, Njobvu L, Redden DT, Zulu I. Prevalence and predictors of intestinal helminth infections among human immunodeficiency virus type 1- infected adults in an urban African setting. Am J Trop Med Hyg (2005) 73(4):777–82. doi: 10.4269/ajtmh.2005.73.777
12. WHO. Schistosomiasis and soil-transmitted helmintiases: number of people treated in 2016. (2017). pp. 749–60, (Geneva, Switzerland: Weekly Epidemiologic Record), Report No.: 92. (2017). pp. 749–60.
13. Wall KM, Kilembe W, Vwalika B, Dinh C, Livingston P, Lee YM, et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. Bustinduy AL, editor. PloS Negl Trop Dis (2018) 12(12); e0006902. doi: 10.1371/journal.pntd.0006902
14. Zambia Ministry of Health. Elimination of Neglected Tropical Disease National Masterplan, 2019-2023 . Available at: https://www.afro.who.int/sites/default/files/2019-06/Zambia%20NTD%20Master%20Plan%20%202019-2023.pdf.
15. Kilembe W, Inambao M, Sharkey T, Wall KM, Parker R, Himukumbwa C, et al. Single mothers and female sex workers in Zambia have similar risk profiles. AIDS Res Hum Retroviruses (2019) 35(9):814–25. doi: 10.1089/aid.2019.0013
16. Itaya T, Murakami Y, Ota A, Shimomura R, Fukushima T, Nishigaki M. Temporal validation of an assessment tool that predicts a possibility of home discharge for patients with acute stroke. J Stroke Cerebrovasc Dis (2022) 31(1):106188. doi: 10.1016/j.jstrokecerebrovasdis.2021.106188
17. Altman DG, Vergouwe Y, Royston P, Moons KGM. Prognosis and prognostic research: validating a prognostic model. BMJ (2009) 338(may28 1):b605–5. doi: 10.1136/bmj.b605
18. Malama K, Price MA, Sagaon-Teyssier L, Parker R, Wall KM, Tichacek A, et al. Evolution of condom use among a 5-year cohort of female sex workers in Zambia. AIDS Behav (2022) 26(2):470–7. doi: 10.1007/s10461-021-03403-9
19. Connolly S, Wall KM, Parker R, Kilembe W, Inambao M, Visoiu AM, et al. Sociodemographic factors and STIs associated with Chlamydia trachomatis and Neisseria gonorrhoeae infections in Zambian female sex workers and single mothers. Int J STD AIDS (2020) 31(4):364–74. doi: 10.1177/0956462419894453
20. Kabengele C, Mwangelwa S, Kilembe W, Vwalika B, Inambao M, Moonga V, et al. Female genital schistosomiasis lesion resolution post-treatment with praziquantel in zambian adults. Am J Trop Med Hyg (2024) 9:tpmd230552. doi: 10.4269/ajtmh.23-0552. Online ahead of print.
21. Zambia Ministry of Health. Standard Treatment Guidelines, Essential Medicines List, Essential Laboratory Supplies for Zamiba. 5th edition. Lusaka, Zambia: Zambia National Formulary Committee (2020).
22. Zambia Ministry of Health. Zambia Consolidated Guidelines for Treatment and Prevention of HIV infection. Lusaka, Zambia: Zambia Ministry of Health (2020).
23. Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: A review. JAMA Psychiatry (2020) 77(5):534. doi: 10.1001/jamapsychiatry.2019.3671
24. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology (2010) 21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2
26. Krauth SJ, Musard C, Traoré SI, Zinsstag J, Achi LY, N’Goran EK, et al. Access to, and use of, water by populations living in a schistosomiasis and fascioliasis co-endemic area of northern Côte d’Ivoire. Acta Trop (2015) :149:179–85. doi: 10.1016/j.actatropica.2015.05.019
27. Amo-Adjei J, Tuoyire DA. Timing of sexual debut among unmarried youths age 15-24 years in Sub-Saharan Africa. J Biosoc Sci (2018) 50(2):161–77. doi: 10.1017/S0021932017000098
28. Burger EA, Pedersen K, Sy S, Kristiansen IS, Kim JJ. Choosing wisely: a model-based analysis evaluating the trade-offs in cancer benefit and diagnostic referrals among alternative HPV testing strategies in Norway. Br J Cancer. (2017) 117(6):783–90. doi: 10.1038/bjc.2017.248
29. Klohe K, Koudou BG, Fenwick A, Fleming F, Garba A, Gouvras A, et al. A systematic literature review of schistosomiasis in urban and peri-urban settings. Cantacessi C, editor. PloS Negl Trop Dis (2021) 15(2):e0008995. doi: 10.1371/journal.pntd.0008995
30. De Leo GA, Stensgaard AS, Sokolow SH, N’Goran EK, Chamberlin AJ, Yang GJ, et al. Schistosomiasis and climate change. BMJ (2020), m4324. doi: 10.1136/bmj.m4324
31. Richter J, Poggensee G, Kjetland EF, Helling-Giese G, Chitsulo L, Kumwenda N, et al. Reversibility of lower reproductive tract abnormalities in women with Schistosoma haematobium infection after treatment with praziquantel — An interim report. Acta Trop (1996) 62(4):289–301. doi: 10.1016/S0001-706X(96)00030-7
32. Downs JA, Kabangila R, Verweij JJ, Jaka H, Peck RN, Kalluvya SE, et al. Detectable urogenital schistosome DNA and cervical abnormalities 6 months after single-dose praziquantel in women with Schistosoma haematobium infection. Trop Med Int Health (2013) 18(9):1090–6. doi: 10.1111/tmi.12154
33. Sturt AS, Webb EL, Himschoot L, Phiri CR, Mapani J, Mudenda M, et al. Association of female genital schistosomiasis with the cervicovaginal microbiota and sexually transmitted infections in Zambian women. Open Forum Infect Dis (2021) 8(9):ofab438. doi: 10.1093/ofid/ofab438
34. McKinnon LR, Achilles SL, Bradshaw CS, Burgener A, Crucitti T, Fredricks DN, et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses (2019) 35(3):219–28. doi: 10.1089/aid.2018.0304
Keywords: female genital schistosomiasis (FGS), risk score, screening, neglected tropical disease, reproductive health
Citation: Rogers EQ, Mwangelwa S, Kabengele C, Kilembe W, Vwalika B, Inambao M, Mumba K, Chanda C, Secor WE, Musale V, Himukumbwa C, Parker R, Tichacek A, Bougouma K, Allen S and Wall KM (2024) Developing and validating a screening tool for female genital schistosomiasis in urban Zambia. Front. Trop. Dis 4:1308129. doi: 10.3389/fitd.2023.1308129
Received: 05 October 2023; Accepted: 22 December 2023;
Published: 27 February 2024.
Edited by:
Margaret Gyapong, University of Health and Allied Sciences, GhanaReviewed by:
Celine Nguefeu Nkenfou, University of Yaounde I, CameroonVerner Orish, University of Health and Allied Sciences, Ghana
Copyright © 2024 Rogers, Mwangelwa, Kabengele, Kilembe, Vwalika, Inambao, Mumba, Chanda, Secor, Musale, Himukumbwa, Parker, Tichacek, Bougouma, Allen and Wall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erin Q. Rogers, Erin.rogers@emory.edu
 Erin Q. Rogers
Erin Q. Rogers Sepo Mwangelwa
Sepo Mwangelwa Chishiba Kabengele
Chishiba Kabengele William Kilembe
William Kilembe Bellington Vwalika
Bellington Vwalika Mubiana Inambao2
Mubiana Inambao2 William Evan Secor
William Evan Secor Vernon Musale
Vernon Musale Kristin M. Wall
Kristin M. Wall