- 1School of Medical Sciences, Universiti Sains Malaysia, Kota Bharu, Malaysia
- 2Section of Gastroenterology and Hepatology, Department of Medicine, Medical College of Georgia, Georgia Regents University, Augusta, GA, USA
Constipation and fecal incontinence (FI) are common complaints predominantly affecting the elderly and women. They are associated with significant morbidity and high healthcare costs. The causes are often multi-factorial and overlapping. With the advent of new technologies, we have a better understanding of their underlying pathophysiology which may involve disruption at any levels along the gut–brain–microbiota axis. Initial approach to management should always be the exclusion of secondary causes. Mild symptoms can be approached with conservative measures that may include dietary modifications, exercise, and medications. New prokinetics (e.g., prucalopride) and secretagogues (e.g., lubiprostone and linaclotide) are effective and safe in constipation. Biofeedback is the treatment of choice for dyssynergic defecation. Refractory constipation may respond to neuromodulation therapy with colectomy as the last resort especially for slow-transit constipation of neuropathic origin. Likewise, in refractory FI, less invasive approach can be tried first before progressing to more invasive surgical approach. Injectable bulking agents, sacral nerve stimulation, and SECCA procedure have modest efficacy but safe and less invasive. Surgery has equivocal efficacy but there are promising new techniques including dynamic graciloplasty, artificial bowel sphincter, and magnetic anal sphincter. Despite being challenging, there are no short of alternatives in our toolbox for the management of constipation and FI.
Introduction
Both constipation and fecal incontinence (FI) are common symptoms facing primary care physicians and gastroenterologists alike. Predominantly affecting the elderly and women (1, 2), these symptoms are associated with significant morbidity, impaired quality of life, and associated with high health expenditures (3, 4). Constipation can be broadly classified as functional constipation (FC), dyssynergic defecation (DD), and constipation-predominant irritable bowel syndrome (IBS-C). These sub-categories are defined according to the Rome III criteria (5, 6). It must be noted that these sub-categories are not mutually exclusive and evidence suggest that overlap frequently exists. FI is involuntary loss of rectal contents (including liquid or solid stool or gas) and can be subcategorized into passive incontinence (loss of stool without the urge to defecate), urge incontinence (inability to postpone defecation urge), and fecal seepage (involuntary loss of small amounts of stool) (7).
Constipation affects between 2 and 28% of adults (8) with comparable figures in children but mainly affecting boys rather than girls (9, 10). FI affects approximately 8.3% of non-institutionalized adults (11) and at least 30% of residents in nursing homes (12). These figures are likely to be underestimated because of several barriers, including misconceptions, embarrassment, and social stigma. With a growing aging population world-wide, it is expected that both conditions will be seeing an upward trend in the future. The underlying pathophysiology has not been fully understood and treatment options remain limited in refractory cases. With advent of new technologies and molecular techniques, there are steady strides in the understanding of their pathophysiology as well as treatment options. The current review aims to provide an update on the pathophysiology and current management options of these two common conditions.
Constipation
Pathophysiology
The colon serves as a conduit for transporting formed stools into the anorectum for evacuation when socially acceptable. The functions are coordinated through neurotransmitters (acetylcholine, nitric oxide, serotonin, calcitonin gene-related peptide), colonic reflexes, learned behaviors, and gut microbiota. These functions can be disrupted at any levels along the gut–brain–microbiota axis.
Neuropathy or myopathy can result in slow-transit constipation (Figure 1), which may be localized or is part of a more generalized form of dysmotility and pseudo-obstruction syndrome. With colonic manometry, the phasic motor activity may exhibit significant impairment in response to a meal (13) and upon waking in the morning. The periodic rectal motor activity (PRMA) may be increased (14) and this will retard colonic propulsion. There is some evidence for hormonal involvement which may explain the female predominance (15). Of interest, methanogenic flora has been found to be significantly associated with constipation (16, 17) and its elimination with antibiotics has been shown to improve symptoms (18).
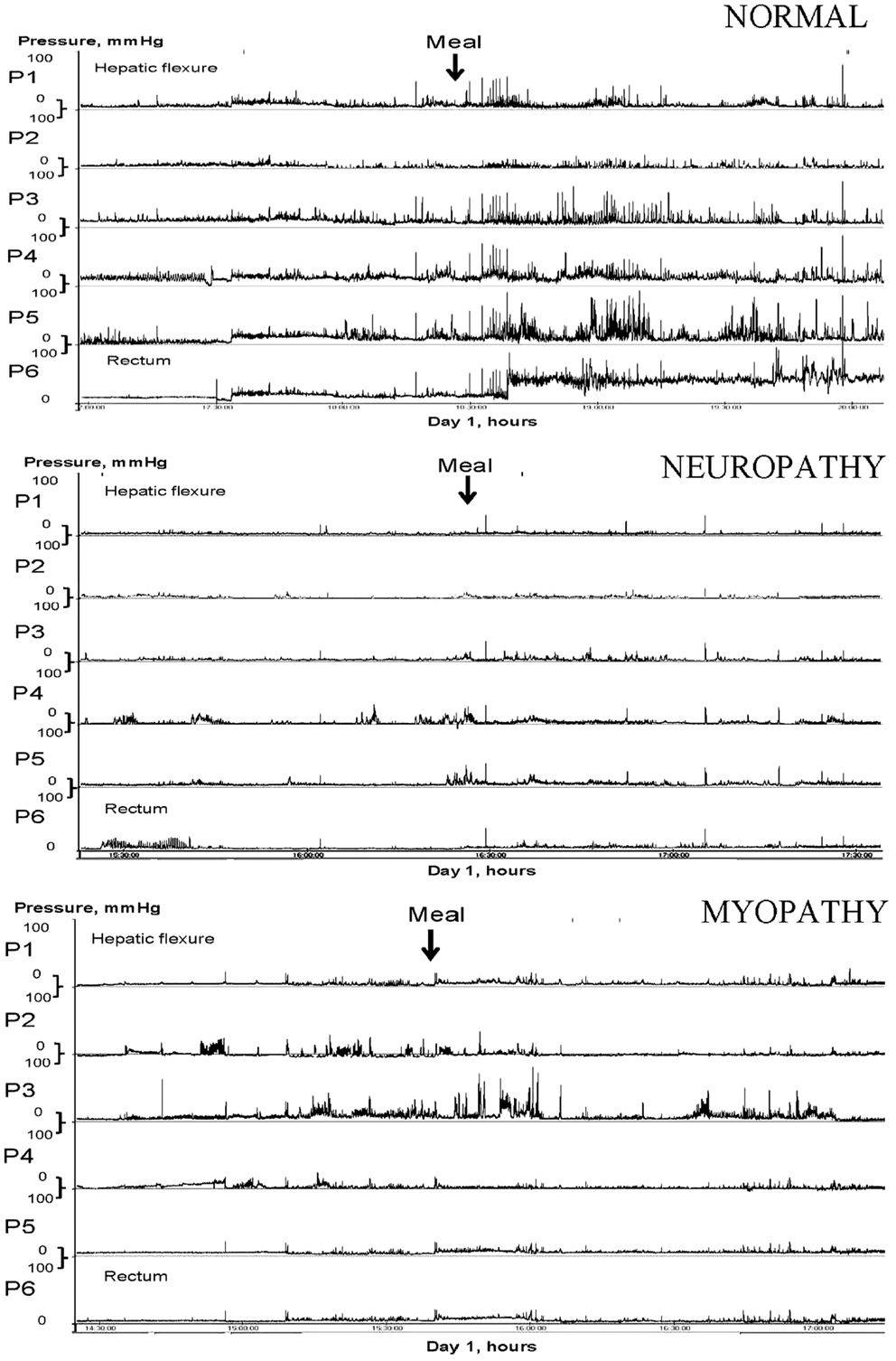
Figure 1. Colonic manometry tracings in response to a meal. Traces for normal, neuropathy, and myopathy changes in slow-transit constipation are shown.
On the other hand, DD is often an acquired form of behavioral disorder in adulthood and a third arises during childhood (19). The paradoxical anal contraction during bearing down is a result of poor rectoanal muscles coordination (Figure 2). Rectal hyposensitivity and abnormal rectal compliance are frequently associated with DD (20) and their improvement with biofeedback suggests they are consequences rather than causative (21). More recently, puborectalis muscle has been shown to play an important role in preserving fecal continence (22) and also in sensorimotor response that coincides with the desire to defecate (23).
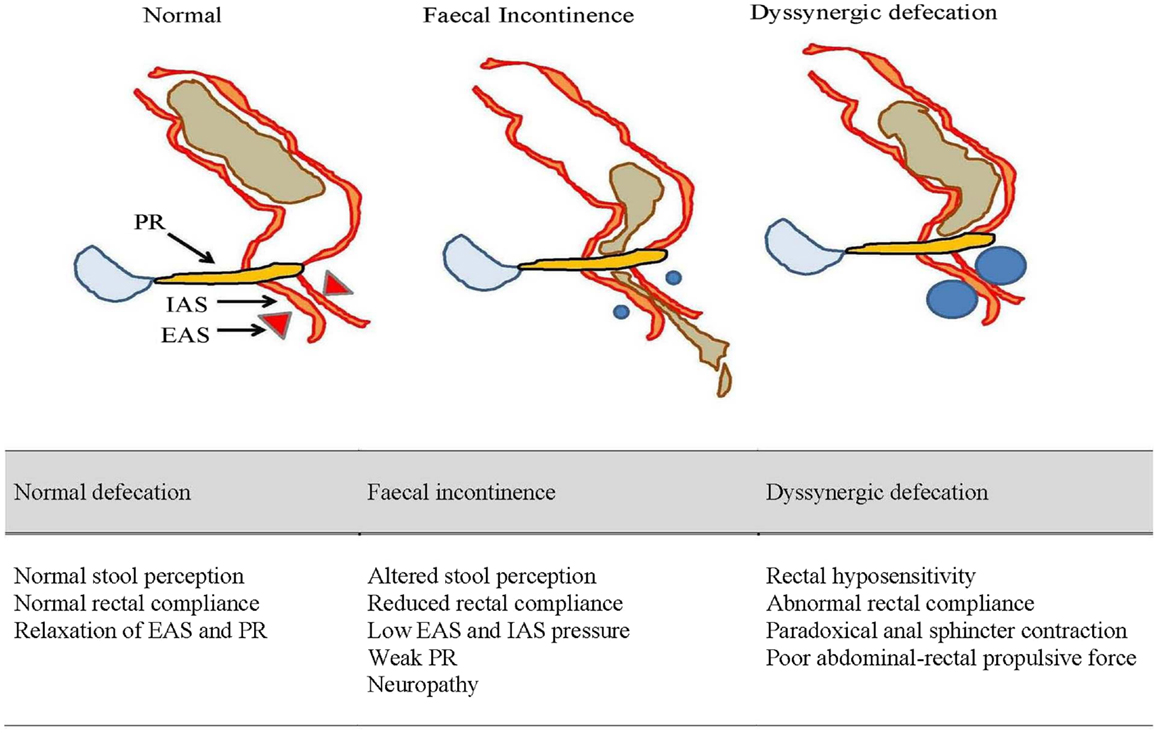
Figure 2. An illustrated summary of normal defecation and physiological disruptions that underlie fecal incontinence and dyssynergic defecation. EAS, external anal sphincter; IAS, internal anal sphincter; PR, puborectalis muscle.
The pathophysiology of IBS-C is more complex and data suggest it is almost indistinguishable from FC since abdominal pain is not exclusive to IBS-C alone. Abnormal colonic transit (24), visceral hypersensitivity (25), psychological factors including stress, anxiety, and depression (26), and small intestinal bacterial overgrowth (SIBO) (27) have all been implicated with IBS-C but recent evidence indicates that serotonin dysregulation is probably the key. Both FC and IBS-C exhibit elevated levels of 5-hydroxytryptamine (5-HT) in the mucosa and reduced concentrations of platelet-depleted plasma (PDP) 5-HT after meals (28, 29). It has been shown by Shekhar et al. that patients with IBS-C tend to be at the sensitive end and FC at the insensitive end of the visceral sensitivity following meal (30).
General Management of Constipation
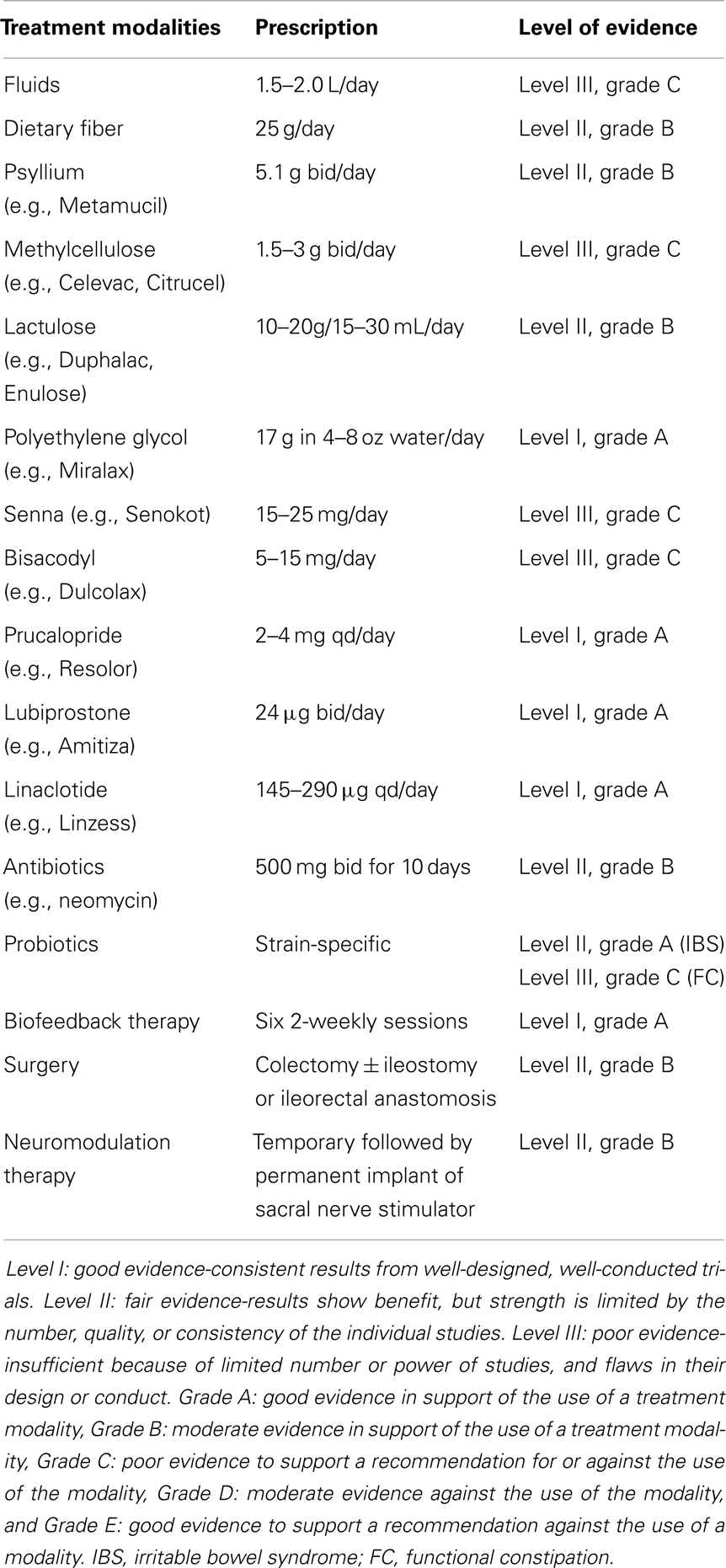
Underlying secondary causes of constipation should be evaluated and treated first. Besides endoscopy and blood tests, exclusion of organic disorders may require specialized tests that include high-resolution or high-definition anorectal manometry, 3D-endoanal ultrasound, pelvic MRI, colonic manometry, and electrophysiological tests. Drugs that may cause constipation should be looked for and stopped. Generous amount of fluids (between 1.5 and 2.0 L/day), fiber intake (25 g/day), and exercise to improve gut transit are general advice commonly given to patients but evidence are lacking (31, 32) (Table 1). Laxatives are frequently prescribed by physicians and many patients can buy them over-the-counter. Examples of laxatives include bulking agents (e.g., psyllium), stool softeners (e.g., docusate), stimulants (e.g., senna), osmotic laxatives (e.g., polyethylene glycol), and enemas (e.g., phosphate). There are no firm recommendations on the use of laxatives in most guidelines largely due to their lack of efficacy and safety concerns (33) (Table 1). Polyethylene glycol is perhaps the exception having good data on its efficacy and safety (33).
New Drugs in the Toolbox for the Management of Constipation
Prokinetics (e.g., prucalopride) and secretagogues (e.g., lubiprostone, linaclotide) are new agents that can restore colonic function in constipation (Table 1). Prokinetics are 5-HT4 agonists that accelerate colonic transit time and also gastric emptying time. An earlier version of 5-HT4 agonist, tegaserod, was shown to be effective in clinical trials (34), but the drug had been withdrawn from the market due to its coronary and cerebrovascular side effects. Safer drugs including mosapride (35) and renzapride (36) are in the pipeline but highly selective 5-HT4 agonist, prucalopride, has been shown in clinical trials to be effective and with little adverse events (37). Available as 2 or 4 mg qd, prucalopride is approved for use in Europe and Asia but not in the US. Velusetrag and naronapride are other high selective 5-HT4 agonists (37).
By promoting intestinal secretion, secretagogues produce softer stools but also accelerate intestinal transit. Lubiprostone acts on the type 2 chloride channels (CIC-2) that leads to active secretion of chloride into the luminal tract and it has been shown to improve small bowel and colonic transit time, however, gastric emptying appears to be delayed (38, 39). Given as 24 μg bid for 3 weeks, lubiprostone (Amitiza®; Sucampo Pharmaceuticals, Bethesda, MA, USA) has been shown in a number of randomized controlled trials (RCTs) to be effective in improving bowel movement, stool consistency, and also reduced bloating (33, 40). Linaclotide, another secretagogue, has a different mechanism of action where it acts on the guanylate cyclase-C (GC-C) that leads to a rise in the cyclic guanosine monophosphate and subsequently chloride and bicarbonate secretion into the lumen (41, 42). Similarly, a number of RCTs have proven the efficacy and safety of linaclotide (Linzess®; Ironwood Pharmaceuticals, Cambridge, MA, USA) given as 145 or 290 μg qd for 12 weeks (43). Linaclotide is currently approved in the US and Europe but lubiprostone is only approved in the US.
Management of Difficult and Refractory Constipation
Biofeedback is the treatment of choice for DD as shown in clinical trials (44, 45) (Table 1). It involves neuromuscular conditioning to improve rectoanal coordination and sensory training to improve stool awareness and rectal compliance. On average, four to six sessions are needed with an interval of 2 weeks, with each session lasting approximately an hour (46). With completion of training, periodic reinforcements are needed to sustain its efficacy over long-term. A trained and experienced staff and highly motivated patient are crucial to the success for this form of therapy. If present, SIBO especially methane-producing bacteria should be treated with antibiotics (18, 47). There may be a role for probiotics as part of the treatment paradigm especially in IBS (48, 49). There is also evidence that intestinal transit time in constipated patients can be reduced with probiotics (50). The benefits for probiotics are at most modest and many experts agree that probiotics do not have a firm recommendation in the management of constipation as yet (51, 52).
Patients with slow-transit constipation due to an underlying neuropathy are often refractory to any form of medical therapy and surgery should be considered in such case (53, 54). Colectomy and ileostomy or ileorectal anastomosis is usually required except in certain cases of segmental involvement especially among children (55, 56). Surgery will not improve abdominal pain or dyssynergia and may develop diarrhea and or FI following the operation (57). In selected patients, neuromodulation therapy or sacral nerve stimulation (SNS) has emerged as an alternative to surgery (Table 1). It requires a temporary placement of percutaneous lead to assess for any treatment response before permanent implantation. A recent meta-analysis has shown that initial evaluation was successful in 42–100% of patients with constipation and in those who had permanent SNS, 87% of patients achieved successful improvement in symptoms, quality of life, and satisfaction scores (58). Mechanisms underlying the success of SNS are still unclear.
Fecal Incontinence
Pathophysiology
Fecal incontinence is a multi-factorial disorder and some of the risk factors have included obstetric trauma (59, 60), anal trauma or surgery (61), pelvic radiotherapy for cancer (62), smoking (60, 63), obesity (63), diabetes (64), and also certain neurological conditions (65). The greater prevalence among females is attributed to maternal injuries sustained during childbirth and in late-onset FI, is due to changes in the pelvic floor from menopause, aging, and pudendal neuropathy (11, 66). Moreover, aging females tend to have lower anal resting pressure and shorter balloon expulsion time (22) (Figure 2). Hormones can also influence the strength and vigor of pelvic muscles (67, 68).
Besides external and internal anal sphincters, puborectalis also plays an important role in the control of continence (69) and injuries to these muscles and the supplying nerves, often in combinations, will result in FI. Unrecognized progressive neuropathy may explain why most women who sustained an obstetrical trauma in their 20s or 30s present with FI only in their 50s. Several studies have shown that neuropathic injury is a recognized cause for FI (70–72) and this is especially so among women with sphincter defects (73). There has been little progress in techniques to investigate for possible neuropathy other than needle electromyography (EMG) and pudendal nerve terminal latency (PNTL) introduced three decades ago. Both have significant limitations that prevent widespread clinical use (74). Most importantly, the involvement of spino-rectal and spino-anal pathway cannot be evaluated in a comprehensive manner with either EMG or PNTL. Recently, the availability of magnetic stimulation of the peripheral spinal roots may change this paradigm (65, 75).
Rectal compliance and sensation may also affect continence (Figure 2). A loss of rectal compliance will allow small volume of stool to generate high intra-rectal pressure and thereby overwhelm the anal resistance (76). This is made worst if the anorectal sensation is also impaired leading to excessive accumulation of stool (77). DD may also allow fecal seepage (78) especially in the elderly or children with fecal impaction.
General Management of FI
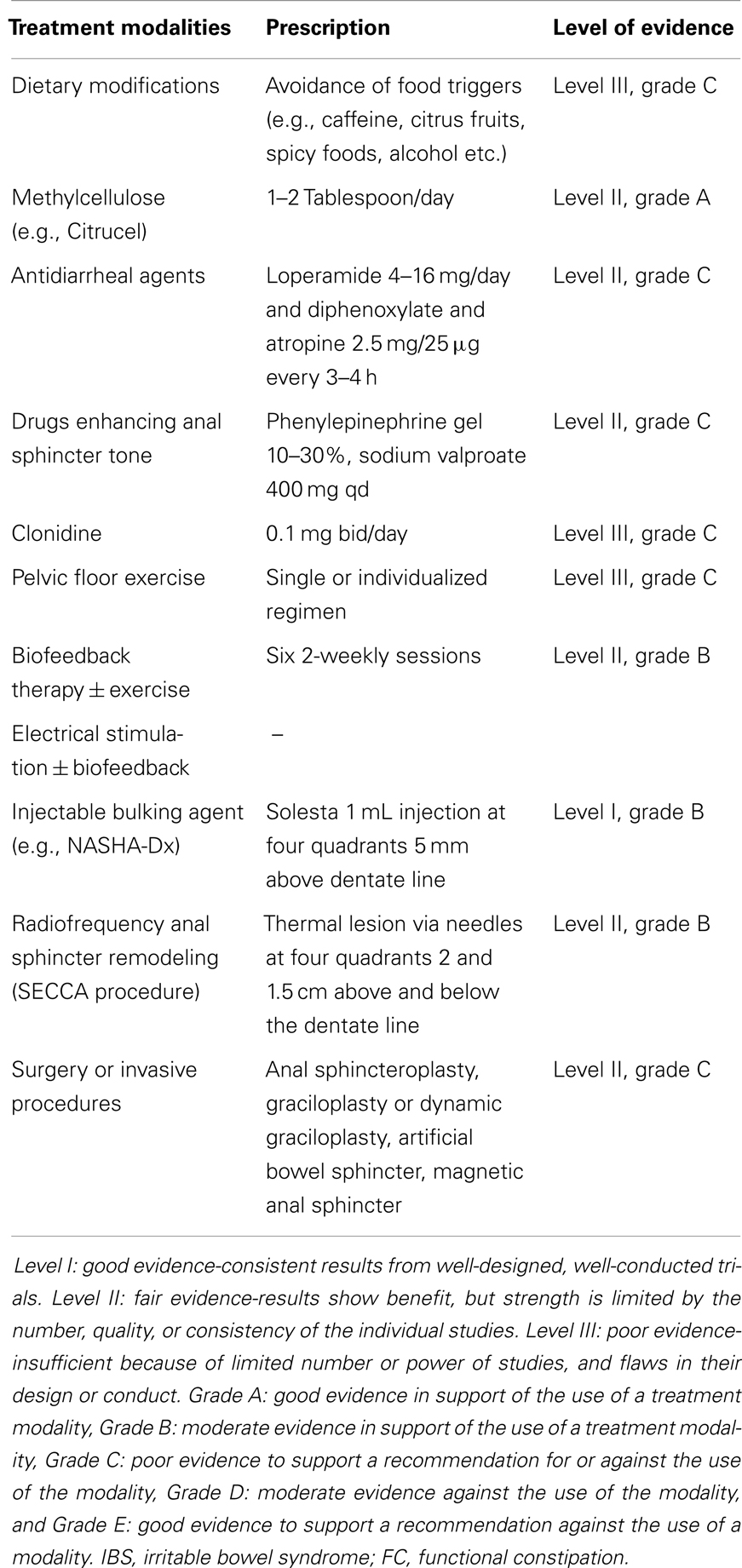
As with approach to constipation, secondary causes of FI should be evaluated and treated. Those with mild symptoms may respond to dietary modifications, medications, and exercises. Avoidance of food triggers including caffeine, citrus fruits, spicy foods, alcohol, or dairy products in those with intolerance may help but evidence is lacking (7) (Table 2). Opinions also differ with regards to the role of dietary fiber even though there is some suggestions that methylcellulose may be better tolerated (79). Antidiarrhoeal agents including loperamide and diphenoxylate can provide short-term relief (80). Drugs that can enhance the sphincter tone, for example phenylepinephrine and sodium valproate may be useful in the passive form of FI (80). More recently, clonidine, an alpha-2 agonist, was shown to improve FI in a pilot study (63) but subsequent RCT failed to show any benefits (81).
Pelvic Floor Exercise, Biofeedback, and Transcutaneous Electrical Stimulation Therapy
Pelvic floor muscle training or anal sphincter exercise can re-train the striated muscles, i.e., external anal sphincter and puborectalis but there is no consensus on the best regimen. It can be a single regimen for all patients, e.g., 10 squeezes of 5 s each 5 times/day or it can be individualized. Exercise can also be combined with biofeedback therapy which has been shown to be twice as effective as exercise alone in a study (82) (Table 2). There are considerable variations in protocols across different centers but to be successful, emphasis is the same, i.e., education, practice, motivation, and good patient–therapist interaction. Long-term follow-up at 12 months suggests a continued response and a lower fecal incontinence severity index (FISI) scores among the biofeedback-treated patients (83). Transcutaneous electrical stimulation, either alone or in combination with biofeedback, has been shown to be useful (82) but the numbers are relatively small and further studies are needed.
Minimal or Less Invasive Treatments
In those FI patients previously failing conservative therapy, surgery is the last option until recently when minimal or less invasive treatments are available. These include injectable bulking agents, neuromodulation therapy in the form of permanent implant of sacral nerve stimulator, and radiofrequency anal sphincter remodeling (SECCA procedure) (Table 2; Figure 3).
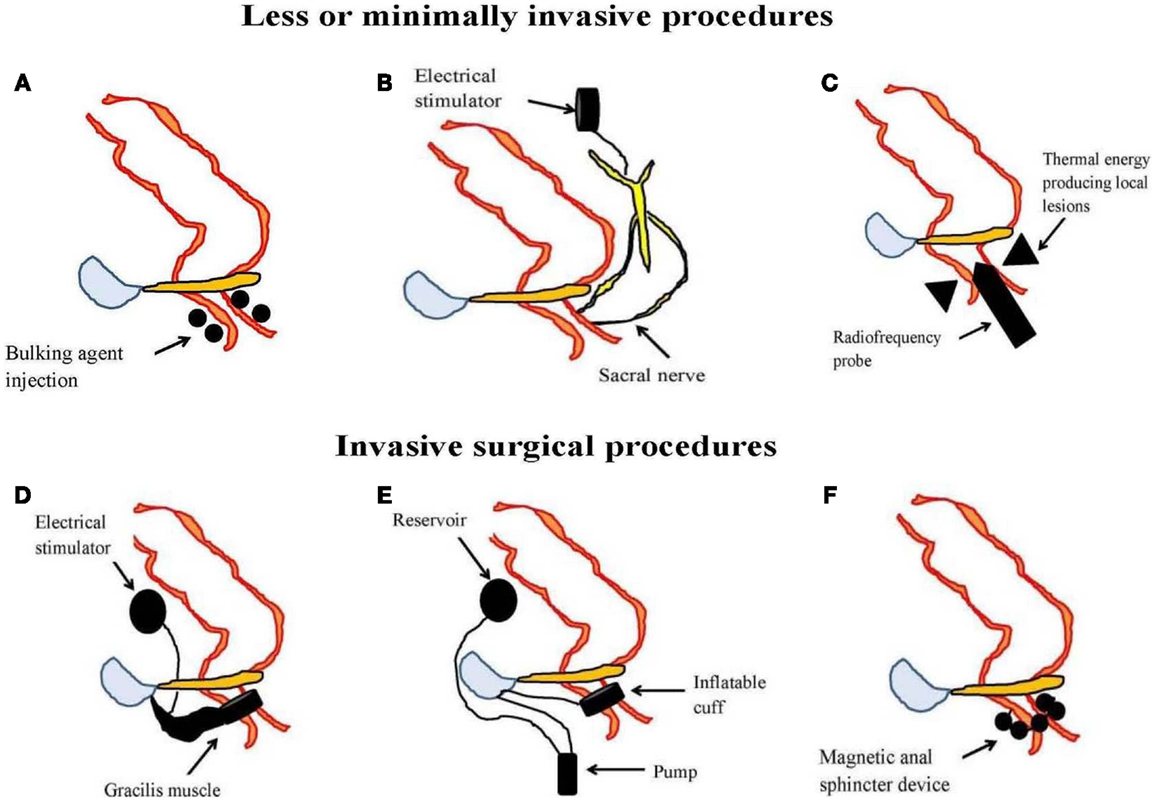
Figure 3. An illustration of less or minimally invasive procedures [(A) injectable bulking agent, (B) sacral nerve stimulation, and (C) radiofrequency anal sphincter remodeling or SECCA procedure] and invasive surgical procedures [(D) dynamic graciloplasty, (E) artificial bowel sphincter, and (F) magnetic anal sphincter] used in the management of fecal incontinence.
Bulking agents are biomaterial that can be injected into the submucosa of the anal canal to augment the anal sphincter and hence preserves continence. A number of different biomaterials, for example autologous fat, Teflon, bovine glutaraldehyde cross-linked collagen, porcine dermal collagen, dextranomer microspheres in non-animal stabilized hyaluronic acid (NASHA-Dx), etc. have been tried but with variable results. Among them, NASHA-Dx has been evaluated extensively for its efficacy and safety in RCTs (84, 85). It was shown to be more effective than sham injection with good tolerability and safety (85). Long-term data at 24-month have been reported to be efficacious, safe, and durable with significant improvement in incontinence scores and quality of life scores (86). The pre-filled NASHA-Dx injection (for example, Solesta®; Salix Pharmaceuticals, Raleigh, USA) at four quadrants 5 mm above the dentate line with 1 mL each can be given as outpatient without anesthesia. A single re-treatment can be offered to patients having persistent FI after a month.
In 1995, Matzel et al. first reported the successful use of SNS in patients with FI and without sphincter defects (87). In a recent meta-analysis, it was shown that SNS, compared to conservative management, significantly improved the weekly incontinence episodes with minimal complication rates of 15% (88). A successful outcome of therapy is typically reported as 50% reduction of incontinence episodes from baseline. Besides being effective, short-term and longer term outcome at 5 years for SNS have been reported to be 42.6% based on intention-to-treat analysis (89). Sphincter defects does not contradict the placement of SNS despite initial concerns although complicated pelvic floor disorders (including rectal resections, pelvic radiotherapy, spinal lesions, double incontinence, and anal sphincter atrophy) are less likely to respond. The mechanisms that underlie the success of SNS are not entirely clear but may involve improvement in sphincter pressure (88), rectal sensitivity (90), modulation of colonic motility (91), or alterations in corticoanal excitability (92, 93).
The SECCA procedure (Mederi Therapeutics Inc., Norwalk, USA) delivers temperature-controlled radiofrequency energy to the anorectal junction resulting in tissue damage, remodeling, scarring, and contraction in order to narrow the anal canal (94). So far, data on its efficacy have been variable and at best modest since most patients continued to have moderate FI (95, 96).
Surgery and Other Invasive Procedures
This is the last resort after failure of conservative and less invasive approach but recent systematic review appears to be inconclusive on the efficacy of surgical options in FI. If all else fails, colostomy is considered to be the very final option. Some of the surgical techniques described in literatures have included anal sphincteroplasty, graciloplasty, artificial bowel sphincter, and magnetic anal sphincter devices (Table 2; Figure 3). Of these, anal sphincteroplasty, which repairs or creates a new functional sphincter fashioned from adjacent skeletal muscles, has disappointing results in clinical studies including long-term outcome (97–100). Graciloplasty utilizes the gracilis muscle to form a new sphincter and may have an electrical stimulator implanted in the abdominal wall (dynamic graciloplasty) to maintain the sphincter tone (101). The success has been variable and only a few reports are available (102–104). Artificial bowel sphincter is an inflatable cuff that acts as a sphincter and it can be deflated when the patient desires to defecate. Again, the success has been variable from reported studies and it is associated with high complication rates that eventually require removal of the device (105–107). Magnetic anal sphincter involves placement of interlinked titanium beads having internal magnetic cores that encircles the external anal sphincter (108). To date, the efficacy data of this procedure appear promising (109–111) but more studies are needed before it will see wider clinical use.
Conclusion
Both constipation and FI are common gastrointestinal complaints having multi-factorial origins. Commonly affecting elderly and women, these disorders are associated with significant impairment in quality of life and high healthcare costs. Availability of new technologies and molecular techniques allow a better understanding of their underlying pathophysiology that may involve any levels along the gut–brain–microbiota axis. Many patients with mild symptoms of constipation and FI may respond to conservative approach that consists of diet modifications, exercise, biofeedback therapy, and medications. More refractory cases of constipation would require neuromodulation therapy and surgery. Likewise, in refractory FI, less invasive approach can be tried first including injectable bulking agents, SNS, and SECCA before moving on to more invasive surgical approach that includes graciloplasty, artificial bowel sphincter, or magnetic anal sphincter.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol (2011) 106:1582–91. doi: 10.1038/ajg.2011.164
2. Leung FW, Rao SSC. Fecal incontinence in the elderly. Gastroenterol Clin North Am (2009) 38:503–11. doi:10.1016/j.gtc.2009.06.007
3. Dunivan GC, Heymen S, Palsson OS, von Korff M, Turner MJ, Melville JL, et al. Fecal incontinence in primary care: prevalence, diagnosis, and health care utilization. Am J Obstet Gynecol (2010) 202:.e1–6. doi:10.1016/j.ajog.2010.01.018
4. Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm (2013) 19:755–64.
5. Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology (2006) 130:1510–8. doi:10.1053/j.gastro.2005.11.064
6. Whitehead WE, Wald A, Diamant NE, Enck P, Pemberton JH, Rao SS. Functional disorders of the anus and rectum. Gut (1999) 45(Suppl 2):II55–9. doi:10.1136/gut.45.2008.ii55
7. Rao SSC. Diagnosis and management of fecal incontinence. American college of gastroenterology practice parameters committee. Am J Gastroenterol (2004) 99:1585–604. doi:10.1111/j.1572-0241.2004.40105.x
8. Stewart WF, Liberman JN, Sandler RS, Woods MS, Stemhagen A, Chee E, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol (1999) 94:3530–40. doi:10.1111/j.1572-0241.1999.01642.x
9. van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol (2006) 101:2401–9. doi:10.1111/j.1572-0241.2006.00771.x
10. van Ginkel R, Reitsma JB, Büller HA, van Wijk MP, Taminiau JAJM, Benninga MA. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology (2003) 125:357–63. doi:10.1016/S0016-5085(03)00888-6
11. Whitehead WE, Borrud L, Goode PS, Meikle S, Mueller ER, Tuteja A, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology (2009) 137:512–7. doi:10.1053/j.gastro.2009.04.054
12. Leung FW, Schnelle JF. Urinary and fecal incontinence in nursing home residents. Gastroenterol Clin North Am (2008) 37:697–707. doi:10.1016/j.gtc.2008.06.005
13. Rao SSC, Ozturk R, Stessman M. Investigation of the pathophysiology of fecal seepage. Am J Gastroenterol (2004) 99:2204–9. doi:10.1111/j.1572-0241.2004.40387.x
14. Rao SS, Sadeghi P, Batterson K, Beaty J. Altered periodic rectal motor activity: a mechanism for slow transit constipation. Neurogastroenterol Motil (2001) 13:591–8. doi:10.1046/j.1365-2982.2001.00292.x
15. Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: “idiopathic slow transit constipation”. Gut (1986) 27:41–8. doi:10.1136/gut.27.1.41
16. Attaluri A, Jackson M, Valestin J, Rao SSC. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol (2010) 105:1407–11. doi:10.1038/ajg.2009.655
17. Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol (2007) 102:837–41. doi:10.1111/j.1572-0241.2007.01072.x
18. Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci (2006) 51:1297–301. doi:10.1007/s10620-006-9104-6
19. Rao SSC, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol (2004) 99:2405–16. doi:10.1111/j.1572-0241.2004.40453.x
20. Gladman MA, Lunniss PJ, Scott SM, Swash M. Rectal hyposensitivity. Am J Gastroenterol (2006) 101:1140–51. doi:10.1111/j.1572-0241.2006.00604.x
21. Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology (2005) 129:86–97. doi:10.1053/j.gastro.2005.05.015
22. Lee YY, Erdogan A, Rao SSC. High resolution and high definition anorectal manometry and pressure topography: diagnostic advance or a new kid on the block? Curr Gastroenterol Rep (2013) 15:360. doi:10.1007/s11894-013-0360-2
23. Cheeney G, Remes-Troche JM, Attaluri A, Rao SSC. Investigation of anal motor characteristics of the sensorimotor response (SMR) using 3-D anorectal pressure topography. Am J Physiol Gastrointest Liver Physiol (2011) 300:G236–40. doi:10.1152/ajpgi.00348.2010
24. Törnblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simrén M. Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol (2012) 107:754–60. doi:10.1038/ajg.2012.5
25. Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology (1995) 109:40–52. doi:10.1016/0016-5085(95)90267-8
26. Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil (2011) 17:131–9. doi:10.5056/jnm.2011.17.2.131
27. Simrén M, Barbara G, Flint HJ, Spiegel BMR, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut (2013) 62:159–76. doi:10.1136/gutjnl-2012-302167
28. Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology (2006) 130:34–43. doi:10.1053/j.gastro.2005.09.031
29. Costedio MM, Coates MD, Brooks EM, Glass LM, Ganguly EK, Blaszyk H, et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am J Gastroenterol (2010) 105:1173–80. doi:10.1038/ajg.2009.683
30. Shekhar C, Monaghan PJ, Morris J, Issa B, Whorwell PJ, Keevil B, et al. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology (2013) 145:749–57. doi:10.1053/j.gastro.2013.07.014
31. Markland AD, Palsson O, Goode PS, Burgio KL, Busby-Whitehead J, Whitehead WE. Association of low dietary intake of fiber and liquids with constipation: evidence from the national health and nutrition examination survey. Am J Gastroenterol (2013) 108:796–803. doi:10.1038/ajg.2013.73
32. Schnelle JF, Leung FW, Rao SSC, Beuscher L, Keeler E, Clift JW, et al. A controlled trial of an intervention to improve urinary and fecal incontinence and constipation. J Am Geriatr Soc (2010) 58:1504–11. doi:10.1111/j.1532-5415.2010.02978.x
33. Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut (2011) 60:209–18. doi:10.1136/gut.2010.227132
34. Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev (2007) 4:CD003960. doi:10.1002/14651858.CD003960.pub3
35. Mansour NM, Ghaith O, El-Halabi M, Sharara AI. A prospective randomized trial of mosapride vs. placebo in constipation-predominant irritable bowel syndrome. Am J Gastroenterol (2012) 107:792–3. doi:10.1038/ajg.2012.26
36. Scarpellini E, Tack J. Renzapride: a new drug for the treatment of constipation in the irritable bowel syndrome. Expert Opin Investig Drugs (2008) 17:1663–70. doi:10.1517/13543784.17.11.1663
37. Shin A, Camilleri M, Kolar G, Erwin P, West CP, Murad MH. Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther (2013) 39:239–53. doi:10.1111/apt.12571
38. Chan WW, Mashimo H. Lubiprostone increases small intestinal smooth muscle contractions through a prostaglandin E receptor 1 (EP1)-mediated pathway. J Neurogastroenterol Motil (2013) 19:312–8. doi:10.5056/jnm.2013.19.3.312
39. Camilleri M, Bharucha AE, Ueno R, Burton D, Thomforde GM, Baxter K, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol (2006) 290:G942–7. doi:10.1152/ajpgi.00264.2005
40. Lembo AJ, Johanson JF, Parkman HP, Rao SS, Miner PB, Ueno R. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci (2011) 56:2639–45. doi:10.1007/s10620-011-1801-0
41. Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology (2007) 133:761–8. doi:10.1053/j.gastro.2007.06.067
42. Bryant AP, Busby RW, Bartolini WP, Cordero EA, Hannig G, Kessler MM, et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci (2010) 86:760–5. doi:10.1016/j.lfs.2010.03.015
43. Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol (2013) 11:1084.e–92.e. doi:10.1016/j.cgh.2013.04.032
44. Rao SSC, Seaton K, Miller M, Brown K, Nygaard I, Stumbo P, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol (2007) 5:331–8. doi:10.1016/j.cgh.2006.12.023
45. Rao SSC, Valestin J, Brown CK, Zimmerman B, Schulze K. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. Am J Gastroenterol (2010) 105:890–6. doi:10.1038/ajg.2010.53
46. Rao SSC. Dyssynergic defecation and biofeedback therapy. Gastroenterol Clin North Am (2008) 37:569–86. doi:10.1016/j.gtc.2008.06.011
47. Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci (2011) 56:1612–8. doi:10.1007/s10620-011-1590-5
48. Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut (2010) 59:325–32. doi:10.1136/gut.2008.167270
49. Quigley EMM. Probiotics in the management of functional bowel disorders: promise fulfilled? Gastroenterol Clin North Am (2012) 41:805–19. doi:10.1016/j.gtc.2012.08.005
50. Miller LE, Ouwehand AC. Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World J Gastroenterol (2013) 19:4718–25. doi:10.3748/wjg.v19.i29.4718
51. Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol (2010) 16:69–75. doi:10.3748/wjg.v16.i1.69
52. Korterink JJ, Ockeloen L, Benninga MA, Tabbers MM, Hilbink M, Deckers-Kocken JM. Probiotics for childhood functional gastrointestinal disorders: a systematic review and meta-analysis. Acta Paediatr (2014) 103(4):365–72. doi:10.1111/apa.12513
53. Reshef A, Alves-Ferreira P, Zutshi M, Hull T, Gurland B. Colectomy for slow transit constipation: effective for patients with coexistent obstructed defecation. Int J Colorectal Dis (2013) 28:841–7. doi:10.1007/s00384-012-1498-3
54. Thakur A, Fonkalsrud EW, Buchmiller T, French S. Surgical treatment of severe colonic inertia with restorative proctocolectomy. Am Surg (2001) 67:36–40.
55. Di Lorenzo C, Flores AF, Reddy SN, Hyman PE. Use of colonic manometry to differentiate causes of intractable constipation in children. J Pediatr (1992) 120:690–5. doi:10.1016/S0022-3476(05)80229-X
56. Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg (1991) 214:403–11. doi:10.1097/00000658-199110000-00005
57. Zutshi M, Hull TL, Trzcinski R, Arvelakis A, Xu M. Surgery for slow transit constipation: are we helping patients? Int J Colorectal Dis (2007) 22:265–9. doi:10.1007/s00384-006-0189-3
58. Thomas GP, Dudding TC, Rahbour G, Nicholls RJ, Vaizey CJ. Sacral nerve stimulation for constipation. Br J Surg (2013) 100:174–81. doi:10.1002/bjs.8944
59. Lunniss PJ, Gladman MA, Hetzer FH, Williams NS, Scott SM. Risk factors in acquired faecal incontinence. J R Soc Med (2004) 97:111–6. doi:10.1258/jrsm.97.3.111
60. Bharucha AE, Fletcher JG, Melton LJ, Zinsmeister AR. Obstetric trauma, pelvic floor injury and fecal incontinence: a population-based case-control study. Am J Gastroenterol (2012) 107:902–11. doi:10.1038/ajg.2012.45
61. Levin A, Cohen MJ, Mindrul V, Lysy J. Delayed fecal incontinence following surgery for anal fissure. Int J Colorectal Dis (2011) 26:1595–9. doi:10.1007/s00384-011-1284-7
62. Barraclough LH, Routledge JA, Farnell DJJ, Burns MP, Swindell R, Livsey JE, et al. Prospective analysis of patient-reported late toxicity following pelvic radiotherapy for gynaecological cancer. Radiother Oncol (2012) 103:327–32. doi:10.1016/j.radonc.2012.04.018
63. Bharucha AE, Seide BM, Zinsmeister AR. The effects of clonidine on symptoms and anorectal sensorimotor function in women with faecal incontinence. Aliment Pharmacol Ther (2010) 32:681–8. doi:10.1111/j.1365-2036.2010.04391.x
64. Rey E, Choung RS, Schleck CD, Zinsmeister AR, Locke GR, Talley NJ. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol (2010) 105:412–9. doi:10.1038/ajg.2009.594
65. Tantiphlachiva K, Attaluri A, Valestin J, Yamada T, Rao SSC. Translumbar and transsacral motor-evoked potentials: a novel test for spino-anorectal neuropathy in spinal cord injury. Am J Gastroenterol (2011) 106:907–14. doi:10.1038/ajg.2010.478
66. Bohle B, Belvis F, Vial M, Maestre Y, Pera M, Castillo M, et al. Menopause and obstetric history as risk factors for fecal incontinence in women. Dis Colon Rectum (2011) 54:975–81. doi:10.1097/DCR.0b013e31821c404a
67. Haadem K, Ling L, Fernö M, Graffner H. Estrogen receptors in the external anal sphincter. Am J Obstet Gynecol (1991) 164:609–10. doi:10.1016/S0002-9378(11)80032-3
68. Knudsen UB, Laurberg S, Danielsen CC. Age-related changes in the striated anal sphincter in female rats. A quantitative morphometric study. Scand J Gastroenterol (1991) 26:347–52. doi:10.3109/00365529109025053
69. Parks AG. Royal society of medicine, section of proctology; meeting 27 November 1974. President’s address. Anorectal incontinence. Proc R Soc Med (1975) 68:681–90.
70. Rao SSC, Coss-Adame E, Tantiphlachiva K, Attaluri A, Remes-Troche JM. Translumbar and transsacral magnetic neuro-stimulation for the assessment of neuropathy in fecal incontinence. Dis Colon Rectum (2013) (in press).
71. Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet (1984) 2:546–50. doi:10.1016/S0140-6736(84)90766-9
72. Tetzschner T, Sørensen M, Lose G, Christiansen J. Anal and urinary incontinence in women with obstetric anal sphincter rupture. Br J Obstet Gynaecol (1996) 103:1034–40. doi:10.1111/j.1471-0528.1996.tb09557.x
73. Brouwer R, Duthie G. Sacral nerve neuromodulation is effective treatment for fecal incontinence in the presence of a sphincter defect, pudendal neuropathy, or previous sphincter repair. Dis Colon Rectum (2010) 53:273–8. doi:10.1007/DCR.0b013e3181ceeb22
74. Remes-Troche JM, Rao SSC. Neurophysiological testing in anorectal disorders. Expert Rev Gastroenterol Hepatol (2008) 2:323–35. doi:10.1586/17474124.2.3.323
75. Remes-Troche JM, Tantiphlachiva K, Attaluri A, Valestin J, Yamada T, Hamdy S, et al. A bi-directional assessment of the human brain-anorectal axis. Neurogastroenterol Motil (2011) 23(240–8):e117–8. doi:10.1111/j.1365-2982.2010.01619.x
76. Rao SS, Read NW, Davison PA, Bannister JJ, Holdsworth CD. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology (1987) 93:1270–5.
77. Read NW, Abouzekry L, Read MG, Howell P, Ottewell D, Donnelly TC. Anorectal function in elderly patients with fecal impaction. Gastroenterology (1985) 89:959–66.
78. Rao SSC, Tuteja AK, Vellema T, Kempf J, Stessman M. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol (2004) 38:680–5. doi:10.1097/01.mcg.0000135929.78074.8c
79. Sze EHM, Hobbs G. Efficacy of methylcellulose and loperamide in managing fecal incontinence. Acta Obstet Gynecol Scand (2009) 88:766–71. doi:10.1080/00016340902993320
80. Omar MI, Alexander CE. Drug treatment for faecal incontinence in adults. Cochrane Database Syst Rev (2013) 6:CD002116. doi:10.1002/14651858.CD002116.pub2
81. Bharucha AE, Fletcher JG, Camilleri M, Edge J, Carlson P, Zinsmeister AR. Effects of clonidine in women with fecal incontinence. Clin Gastroenterol Hepatol (2013). doi:10.1016/j.cgh.2013.06.035
82. Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev (2012) 7:CD002111. doi:10.1002/14651858.CD002111.pub3
83. Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum (2009) 52:1730–7. doi:10.1007/DCR.0b013e3181b55455
84. Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev (2013) 2:CD007959. doi:10.1002/14651858.CD007959.pub3
85. Graf W, Mellgren A, Matzel KE, Hull T, Johansson C, Bernstein M. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet (2011) 377:997–1003. doi:10.1016/S0140-6736(10)62297-0
86. La Torre F, de la Portilla F. Long-term efficacy of dextranomer in stabilized hyaluronic acid (NASHA/Dx) for treatment of faecal incontinence. Colorectal Dis (2013) 15:569–74. doi:10.1111/codi.12155
87. Matzel KE, Stadelmaier U, Hohenfellner M, Gall FP. Electrical stimulation of sacral spinal nerves for treatment of faecal incontinence. Lancet (1995) 346:1124–7. doi:10.1016/S0140-6736(95)91799-3
88. Tan E, Ngo N-T, Darzi A, Shenouda M, Tekkis PP. Meta-analysis: sacral nerve stimulation versus conservative therapy in the treatment of faecal incontinence. Int J Colorectal Dis (2011) 26:275–94. doi:10.1007/s00384-010-1119-y
89. Maeda Y, Lundby L, Buntzen S, Laurberg S. Outcome of sacral nerve stimulation for fecal incontinence at 5 years. Ann Surg (2013). doi:10.1097/SLA.0b013e31829d3969
90. Vaizey CJ, Kamm MA, Turner IC, Nicholls RJ, Woloszko J. Effects of short term sacral nerve stimulation on anal and rectal function in patients with anal incontinence. Gut (1999) 44:407–12. doi:10.1136/gut.44.3.407
91. Patton V, Wiklendt L, Arkwright JW, Lubowski DZ, Dinning PG. The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br J Surg (2013) 100:959–68. doi:10.1002/bjs.9114
92. Sheldon R, Kiff ES, Clarke A, Harris ML, Hamdy S. Sacral nerve stimulation reduces corticoanal excitability in patients with faecal incontinence. Br J Surg (2005) 92:1423–31. doi:10.1002/bjs.5111
93. Harris ML, Singh S, Rothwell J, Thompson DG, Hamdy S. Rapid rate magnetic stimulation of human sacral nerve roots alters excitability within the cortico-anal pathway. Neurogastroenterol Motil (2008) 20:1132–9. doi:10.1111/j.1365-2982.2008.01153.x
94. Felt-Bersma RJ, Szojda MM, Mulder CJ. Temperature-controlled radiofrequency energy (SECCA) to the anal canal for the treatment of faecal incontinence offers moderate improvement. Eur J Gastroenterol Hepatol (2007) 19:575–80. doi:10.1097/MEG.0b013e32811ec010
95. Takahashi-Monroy T, Morales M, Garcia-Osogobio S, Valdovinos MA, Belmonte C, Barreto C, et al. SECCA procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum (2008) 51:355–9. doi:10.1007/s10350-007-9169-0
96. Frascio M, Mandolfino F, Imperatore M, Stabilini C, Fornaro R, Gianetta E, et al. The SECCA procedure for faecal incontinence: a review. Colorectal Dis (2014) 16(3):167–72. doi:10.1111/codi.12403
97. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum (2012) 55:482–90. doi:10.1097/DCR.0b013e3182468c22
98. Zutshi M, Tracey TH, Bast J, Halverson A, Na J. Ten-year outcome after anal sphincter repair for fecal incontinence. Dis Colon Rectum (2009) 52:1089–94. doi:10.1007/DCR.0b013e3181a0a79c
99. Johnson E, Carlsen E, Steen TB, Backer Hjorthaug JO, Eriksen MT, Johannessen H-O. Short- and long-term results of secondary anterior sphincteroplasty in 33 patients with obstetric injury. Acta Obstet Gynecol Scand (2010) 89:1466–72. doi:10.3109/00016349.2010.519019
100. Mevik K, Norderval S, Kileng H, Johansen M, Vonen B. Long-term results after anterior sphincteroplasty for anal incontinence. Scand J Surg (2009) 98:234–8.
101. Edden Y, Wexner SD. Therapeutic devices for fecal incontinence: dynamic graciloplasty, artificial bowel sphincter and sacral nerve stimulation. Expert Rev Med Devices (2009) 6:307–12. doi:10.1586/erd.09.10
102. Tillin T, Gannon K, Feldman RA, Williams NS. Third-party prospective evaluation of patient outcomes after dynamic graciloplasty. Br J Surg (2006) 93:1402–10. doi:10.1002/bjs.5393
103. Wexner SD, Baeten C, Bailey R, Bakka A, Belin B, Belliveau P, et al. Long-term efficacy of dynamic graciloplasty for fecal incontinence. Dis Colon Rectum (2002) 45:809–18. doi:10.1007/s10350-004-6302-1
104. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis (2004) 6:470–6. doi:10.1111/j.1463-1318.2004.00714.x
105. Darnis B, Faucheron J-L, Damon H, Barth X. Technical and functional results of the artificial bowel sphincter for treatment of severe fecal incontinence: is there any benefit for the patient? Dis Colon Rectum (2013) 56:505–10. doi:10.1097/DCR.0b013e3182809490
106. Wong MTC, Meurette G, Stangherlin P, Lehur P-A. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum (2011) 54:773–9. doi:10.1007/DCR.0b013e3182182689
107. Wexner SD, Jin HY, Weiss EG, Nogueras JJ, Li VKM. Factors associated with failure of the artificial bowel sphincter: a study of over 50 cases from Cleveland clinic Florida. Dis Colon Rectum (2009) 52:1550–7. doi:10.1007/DCR.0b013e3181af62f8
108. Wong MTC, Meurette G, Wyart V, Glemain P, Lehur P-A. The artificial bowel sphincter: a single institution experience over a decade. Ann Surg (2011) 254:951–6. doi:10.1097/SLA.0b013e31823ac2bc
109. Lehur P-A, McNevin S, Buntzen S, Mellgren AF, Laurberg S, Madoff RD. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum (2010) 53:1604–10. doi:10.1007/DCR.0b013e3181f5d5f7
110. Barussaud M-L, Mantoo S, Wyart V, Meurette G, Lehur P-A. The magnetic anal sphincter in faecal incontinence: is initial success sustained over time? Colorectal Dis (2013) 15:1499–503. doi:10.1111/codi.12423
Keywords: constipation, fecal incontinence, anorectal disorders, pathophysiology, management
Citation: Lee YY (2014) What’s new in the toolbox for constipation and fecal incontinence? Front. Med. 1:5. doi: 10.3389/fmed.2014.00005
Received: 01 February 2014; Paper pending published: 25 February 2014;
Accepted: 13 March 2014; Published online: 24 March 2014.
Edited by:
Francesco Marotta, ReGenera Research Group for Aging Intervention, ItalyReviewed by:
Francesco Marotta, ReGenera Research Group for Aging Intervention, ItalyClaudio Romano, University of Messina, Italy
Copyright: © 2014 Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yeong Yeh Lee, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kota Bharu, Kelantan 16150, Malaysia e-mail:anVzdG5sZWV5eUBnbWFpbC5jb20=
 Yeong Yeh Lee
Yeong Yeh Lee