- 1Department of Rheumatology and Immunology, Changzheng Hospital, The Second Military Medical University, Shanghai, China
- 2Department of Rheumatology and Immunology, Shanghai Guanghua Hospital, Shanghai, China
- 3Department of Rheumatology and Immunology, Shanghai Tongji Hospital, Shanghai, China
- 4Department of Rheumatology and Immunology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 5Department of Rheumatology and Immunology, Huaxi Hospital, Sichuan University, Chengdu, China
- 6Institute of Health and Biomedical Innovation, Queensland University of Technology, Princess Alexandra Hospital, Woolloongabba, QLD, Australia
- 7Translational Research Institute, Queensland University of Technology, Princess Alexandra Hospital, Woolloongabba, QLD, Australia
Objectives: Cigarette smoking has been shown in European populations to be associated with rheumatoid arthritis (RA) susceptibility. This study aims to examine the association of smoking with RA in the Han Chinese population.
Methods: 718 Han Chinese RA patients and 404 healthy controls were studied. The associations of cigarette smoking (current, former or ever vs. never smokers, and pack-years of exposure) with RA, anti-cyclic citrullinated peptide antibody (ACPA) positive RA, IgM rheumatoid factor (RF) positive RA, and baseline radiographic erosions (modified van der Heijde–Sharp scores) were assessed. The interaction between smoking and the HLA-DRB1 shared epitope (SE) in RA was also examined.
Results: In this study, 11 (1.53%) cases and 6 (1.49%) controls were former smokers (p = 0.95), while 95 (13.23%) cases and 48 (11.88%) controls were current smokers (p = 0.52). Trends toward associations between smoking status (ever vs. never) with RA-overall (p = 0.15, OR = 1.44), ACPA-positive RA (p = 0.24, OR = 1.37), RF-positive RA (p = 0.14, OR = 1.46), or the presence of radiographic erosions (p = 0.66, OR = 1.28) were observed although individually here were not statistically significant. There was no evidence of statistical interaction between smoking status (ever vs. never) and SE for all RA, ACPA-positive RA, ACPA-negative RA, RF-positive RA, RF-negative RA (p = 0.37, 0.50, 0.24, 0.26, and 0.81 respectively), and the 95% CI for the attributable proportion for all interactions included 0.
Conclusion: This is the first study to examine the association of cigarette smoking with RA in the Han Chinese population. This study shows a trend toward an interaction between smoking and SE carriage influencing the risk of RA, though findings were not statistically significant. It is possible that in the presence of universal exposure to heavy air pollution the effect of smoking on RA risk may be obscured.
Introduction
Rheumatoid arthritis (RA) is a complex immune-mediated disease that is thought to arise due to the interacting effects of environmental and genetic factors. Several studies performed in populations of European ancestry have suggested that smoking is an environmental risk factor increasing the risk of RA (1–3). Carriage of the HLA-DRB1 shared epitope (SE) is the most important genetic risk factor for RA, accounting for approximately 40% of genetic susceptibility to the disease (4). Several studies have reported that smoking interacts with HLA-DRB1 in increasing the risk of anti-cyclic citrullinated peptide antibody (ACPA)-positive, but not ACPA-negative, RA (5–7). This suggests that the pathogenesis of ACPA-positive RA might differ from that of ACPA-negative disease (8), although the extensive sharing of genetic associations between the two subtypes of RA (9) indicates that they have marked similarity of genetic architecture.
The studies showing that cigarette smoking was associated with RA and ACPA risk have almost exclusively involved populations of Caucasian ancestry. Evidence of association of smoking specifically with ACPA-positive RA has thus far been restricted to studies of European-descent ethnic groups. Studies in Koreans and in African-Americans have found that while smoking increased the risk of developing RA-overall, the association was not restricted to ACPA-positive disease (10, 11). Smoking rates in Chinese are very high, with 52.7% of males being ever smokers. While smoking rates in women are lower (about 2.4%) they are increasing (12). Therefore, it is of great relevance to investigate the role of smoking in the Han Chinese population with RA.
Materials and Methods
Patients and Controls
Among these 718 cases, 191 were recruited from Shanghai Changzheng Hospital, 349 from Shanghai Guanghua Hospital, 49 from Shanghai Tongji Hospital, 28 from Peking Union Medical College Hospital, and 91 Huaxi Hospital. All patients were diagnosed in accordance with the American College of Rheumatology 1987 classification criteria (13). 404 healthy controls (blood donors on no prescription medications) were recruited from Shanghai. All human studies have been approved by the Research Ethical Committee of Second Military Medical University, and all patients and controls gave informed written consent for their participation in the studies. Patient charts were reviewed to collect the patients’ demographic information, disease duration, and smoking history before the disease onset. Questionnaires were designed to collect the controls’ demography and smoking information. We defined who ever smoked cigarette as smokers. We also defined ≥10 pack-years as heavy smoker when assessing smoking cumulative effect. EDTA preserved blood was collected from all cases and controls for DNA extraction, and serum samples from all cases. Radiographs of the hands and wrists were obtained at recruitment and scored by two independent experienced radiologists using the modified van der Heijde–Sharp score in 517 of 718 cases. Patients with available scores were classified as having either erosive disease (Sharp erosion score > 0) or non-erosive disease (Sharp erosion score = 0) (14).
IgG ACPA titers were measured by a commercially available second generation (CCP2) enzyme-linked immunosorbent assay (ELISA, EUROIMMUN, Lubeck, Germany) and were considered to be positive at a cut of value >5 IU/ml in accordance to the instructions of manufacturer. IgM rheumatoid factor (RF) was measured by ELISA (EUROIMMUN, Lubeck, Germany) and considered to be positive when the titers were ≥20 RU/ml.
Sequence-specific primer-polymerase chain reaction method was used to determine carriage of HLA-DRB1*01, HLA-DRB1*04, and HLA-DRB1*10 alleles (15), and HLA-DRB1*0101, *0102, *0401, *0404, *0405, *0408, and *1001 were defined as SE.
Statistical Analysis
Differences of gender, smoking, and SE carriage between groups were investigated using the chi-squared test, and Student’s t-test was used to examine the differences of age between cases and controls. Logistic regression was used to examine the association of smoking (ever smokers vs. never smokers, <10 pack-years and never vs. ≥10 pack-years) with affection status, ACPA carriage, RF-IgM positive disease, and baseline radiographic erosions, adjusting for SE status (positive or negative), age, and gender given the differences in these characteristics between cases and controls.
Logistic regression analysis was used to calculate the association of smoking–SE interaction with all patients, carriage of ACPA or RF, and radiographic erosions (16). The attributable proportion (AP) due to interaction (where an AP = 0 corresponds to no interaction and an AP = 1.0 corresponds to “complete” interaction) and corresponding 95% confidence intervals were calculated (17). A p-value of <0.05 was considered statistically significant. All analyses were conducted using SPSS13.0 (Chicago, IL, USA).
Results
Characteristics of cases and controls are shown in Table 1. Cases and controls were well matched for gender (77.44 vs. 75.74% female, p = 0.52), but RA cases were older than controls (53.19 vs. 44.56 years, p < 0.01). The mean disease duration from onset of symptoms in cases was 7.54 (8.14) years. HLA-DRB1 SE positivity was much higher in RA cases than in controls (40.11 vs. 19.06%, odds ratio 15.8, p = 4.3 × 10−84). The differences of SE subtypes between two groups were shown in Table 2. 566 (78.83%) and 574 (79.94%) cases were ACPA and RF-positive, respectively. 462 cases (89.36%) had baseline radiographic erosions. 11 (1.53%) cases and 6 (1.49%) controls were former smokers while 95 (13.23%) cases and 48 (11.88%) controls were current smokers. Smoking status was not associated with affection status (p = 0.95 and p = 0.52 for former and current smokers, respectively), even among heavy smokers (defined as ≥10 pack-years; 13.79 vs. 11.39%, p = 0.25).
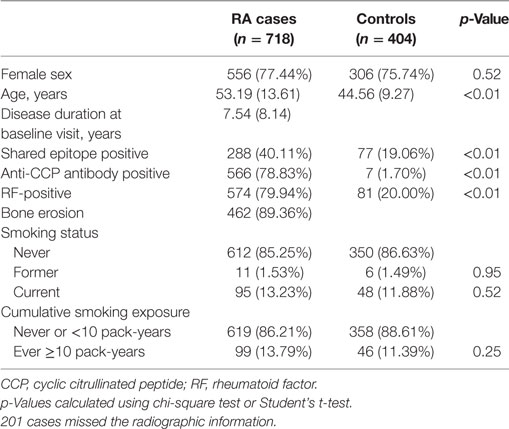
Table 1. Characteristics of Chinese Han rheumatoid arthritis (RA) cases and healthy controls; means (±SD) or %.
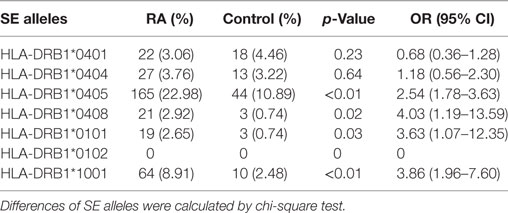
Table 2. Differences of shared epitope (SE) alleles between rheumatoid arthritis (RA) and healthy control.
In sex–age–SE adjusted analysis (Table 3), there was no association between smoking status (Table 3, A, former and current vs. never) with either RA-overall (p = 0.89 and p = 0.13, respectively), ACPA-positive RA (p = 0.93 and p = 0.20, respectively), RF-positive RA (p = 0.87 and p = 0.12, respectively), or the presence of radiographic erosions (p = 0.81 and p = 0.68, respectively). There was also no association between smoking status (Table 3, B, ever vs. never) with RA-overall (p = 0.15), ACPA-positive RA (p = 0.24), RF-positive RA (p = 0.14), or the presence of radiographic erosions (p = 0.66). Cumulative smoking (Table 3, C, cumulative exposure vs. never) was also not associated with RA-overall (p = 0.15), ACPA-positive RA (p = 0.25), RF-positive RA (p = 0.12), or radiographic erosions (p = 0.55).
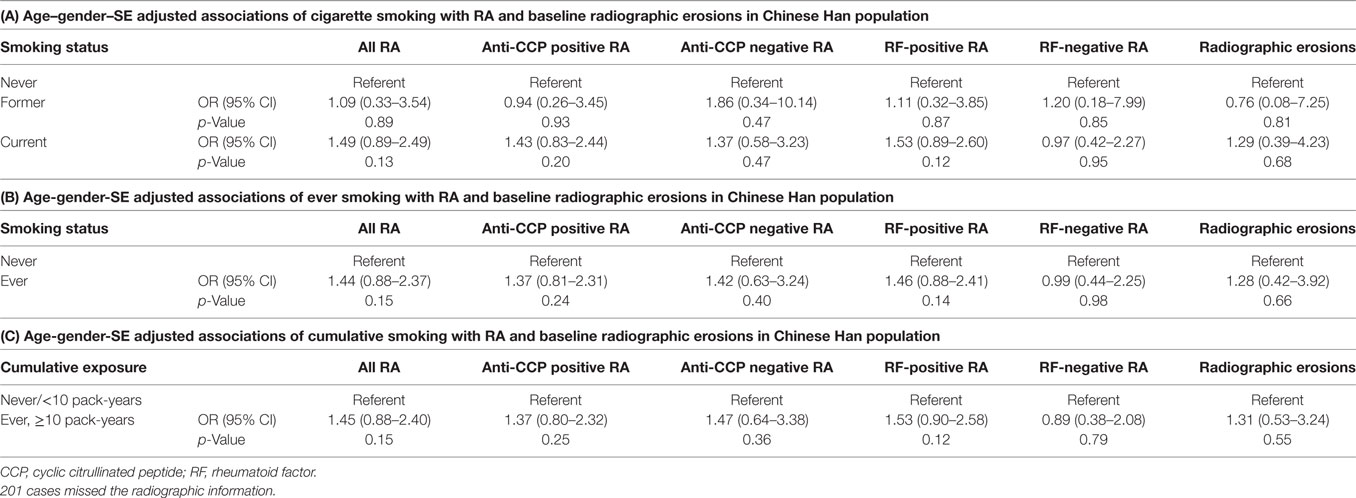
Table 3. Age–gender–SE adjusted associations of cigarette smoking (smoking status and cumulative smoking exposure) with rheumatoid arthritis (RA) and baseline radiographic erosions in Chinese Han population.
The assessment of interaction between HLA-DRB1 SE (positive or negative) and smoking (ever or never) in RA was shown in Table 4. After adjustment for age and gender, and considering never smoking/SE-negative as the reference, ORs (95% CI) of ever smoking/SE-negative, never smoking/SE-positive, and ever smoking/SE-positive in all RA were 1.52 (0.88–2.60), 2.82 (2.01–3.95), and 3.46 (1.50–7.95), respectively (p = 0.13, <0.01, and <0.01, respectively). In ACPA-positive RA, for the same categories, the ORs (95% CI) were 1.41 (0.79–2.49), 2.86 (2.02–4.06), and 3.62 (1.53–8.55), respectively (p = 0.24, <0.01, and <0.01, respectively). In ACPA-negative RA, again for the same categories, ORs (95% CI) RA were 1.70 (0.69–4.19), 3.11 (1.83–5.29), and 2.07 (0.66–10.37), respectively (p = 0.25, <0.01, and 0.17, respectively). In RF-positive RA, for the same categories, ORs (95% CI) in RF-positive RA were 1.66 (0.91–2.82), 2.92 (2.07–4.14), and 3.25 (1.36–7.75), respectively (p = 0.10, <0.01, and 0.008, respectively), and in RF-negative RA were 0.86 (0.32–2.27), 3.10 (1.81–5.30), and 3.97 (1.88–12.88), respectively (p = 0.25, <0.01, and 0.17, respectively). There was no evidence of statistical interaction between smoking status (never, ever) and SE for all RA, ACPA-positive RA, ACPA-negative RA, RF-positive RA, RF-negative RA (p = 0.37, 0.50, 0.24, 0.26, and 0.81 respectively), and the 95% CI for the AP for all interactions included 0.
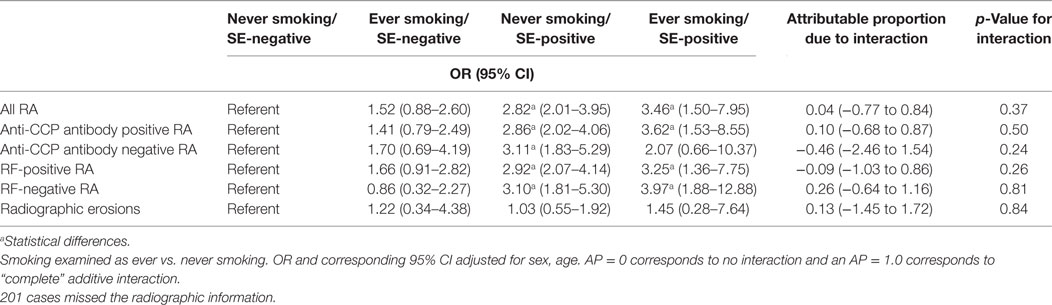
Table 4. Assessment of interaction between HLA-DRB1 shared epitope (SE) (positive or negative) and smoking (ever or never) in rheumatoid arthritis (RA) risk in Chinese Han population.
Assessment of interaction between HLA-DRB1 SE (positive or negative) and cumulative smoking exposure (≥10 pack-years, <10 pack-years or never smoker was defined as other) in RA was shown in Table 5. After adjustment for age and gender and considering never smoking/SE-negative as the reference, ORs (95% CI) of heavy smoking/SE-negative, other/SE-positive, heavy smoking/SE-positive in all RA were 1.46 (0.85–2.53), 2.77 (1.99–3.86), and 3.81 (1.53–9.78) (p = 0.17, <0.01, and <0.01, respectively). In ACPA-positive RA, these ORs (95% CI) were 1.36 (0.76–2.43), 2.83 (2.01–3.99), and 3.89 (1.50–10.09), respectively (p = 0.30, <0.01, and <0.01, respectively) and in ACPA-negative RA were 1.61 (0.65–3.96), 2.98 (1.76–5.05), and 3.24 (0.75–13.92), respectively (p = 0.30, <0.01, and 0.12, respectively). Considering RF-positive RA, for the same categories ORs (95% CI) were 1.58 (0.89–2.79), 2.85 (2.02–4.01), and 3.83 (1.47–9.98), respectively (p = 0.12, <0.01, and <0.01, respectively), and in RF-negative RA were 0.67 (0.29–2.12), 3.13 (1.85–5.30), and 3.67 (0.99–13.67), respectively (p = 0.63, <0.01, and 0.05, respectively). No statistical interaction between cumulative smoking exposure and SE was observed among all RA cases or in ACPA-positive or negative, or RF-positive or negative cases (p = 0.61, 0.81, 0.69, 0.62, and 0.75), and the 95% CI for the AP these analyses always included 0.
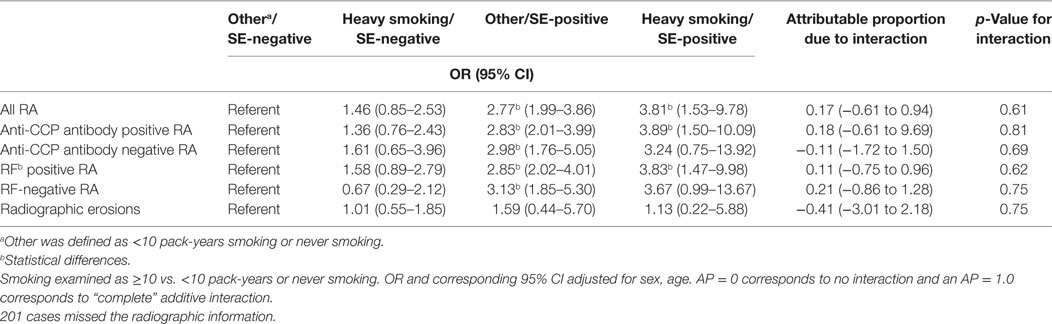
Table 5. Assessment of interaction between HLA-DRB1 shared epitope (SE) (positive or negative) and cumulative smoking exposure (≥10 pack-years, <10 pack-years, or never smoking) in rheumatoid arthritis (RA) risk in Chinese Han population.
As shown in Tables 4 and 5, ORs of ever smoking/SE-negative, never smoking/SE-positive, and ever smoking/SE-positive for radiographic erosions were 1.22 (0.34–4.38), 1.03 (0.55–1.92), and 1.45 (0.28–7.64), respectively, and for heavy smoking/SE-negative, other/SE-positive, and heavy smoking/SE-positive were 1.01 (0.55–1.85), 1.59 (0.44–5.70), and 1.13 (0.22–5.88), respectively (all p > 0.05). There was no smoking (cumulative smoking)–SE interaction for radiographic erosions (p = 0.84 and 0.75, respectively), and the 95% CI for the AP for all these analyses also included 0.
Discussion
To the best of our knowledge, this is the first study to evaluate the association of cigarette smoking with RA in the Han Chinese population. The results of our study showed that while smoking was not significantly associated with RA, serological positive RA, or radiographic erosions in Chinese patients trends toward associations were noticed in nearly all analyses. Based on previous studies (5–7), Klareskog et al. hypothesized that smoking might trigger citrullination in the lungs of the individuals who carry the HLA-DRB1 SE, thereby providing a substrate for immune activation that finally causes ACPA-positive RA (8). However, epidemiology studies from Korean, Japan, and China have previously not shown any association between smoking, HLA-DRB1, and ACPA-positive RA (9, 18, 19). In this study, we have recruited 718 cases and 404 healthy controls, and logistic regression analysis can remove this bias caused by age and gender (16). In our study, smoking (either ever compared with never smokers, or cumulative dose) was non-significantly associated with both risk of RA-overall, and among ACPA-positive and -negative and RF-positive and RF-negative cases, and presence of baseline erosions. We do not see a formal interaction between smoking and SE carriage in the development of ACPA-positive disease. However, greater increases in risk were seen for SE-positive smokers compared with either SE-negative smokers or SE-positive non-smokers for RA-overall, ACPA-positive and -negative RA, and RF-positive and -negative RA, considering either ever compared with never smokers or cumulative smoking exposure. These findings, while individually not statistically significant, are consistent with and support the presence of interaction between smoking, SE carriage, and the risk of RA. The similarity of findings of the effects of smoking and SE carriage between ACPA-positive and -negative RA suggests that the interaction is not restricted to ACPA-positive disease, as originally suggested.
Based on previous work, there were several significant differences in the role of SE between Han Chinese and Caucasians. First, the prevalence of SE in Han Chinese is much lower than in Caucasians (Han 40% vs. Caucasians 70%) (5–7). Second, the SE subtypes found in Han Chinese and European-descent RA cases were different. European-descent RA cases have two main subtypes, HLA-DRB1*0401 and HLA-DRB1*0405, while Chinese have one main subtype, HLA-DRB1*0405. The prevalence of HLA-DRB1*0401 among Han Chinese and Asians is much lower than that in Caucasians (20). Interethnic HLA-DRB1 subtype differences like this may explain the differences in our findings compared with those previously reported in European populations.
Another potential explanation for the different results in our study compared with previous studies is the proportion of smokers among RA cases. In studies that involving American or European subjects, more than half of the RA patients and more than 40% healthy people were ever smokers (2, 5). Smoking rates among Chinese women are much lower, with only 2.4% women being ever smokers (12). This is consistent with the low rate of smoking among females in our study. This would lead to lower power to identify an association between smoking and RA and to investigate interactions between smoking, HLA-DRB1, and RA.
It is also possible that smoking effects were obscured by high exposure to other environmental agents. For example, personal smoking effects could have been obscured by passive smoking, which we did not assess but which is a major public health problem in China. We also hypothesize that air pollution could play a role. While in Europe and USA air pollution was not associated with RA (21, 22), the very heavy air pollution found in major Chinese cities is a possible contributing factor. Indirect evidence only exists to support this hypothesis. First, a new study (23) found association between airway insults and peptide citrullinations in lungs suggesting that smoking-induced inflammation, rather than smoking itself, contributes to an increase in citrullination in lungs. Second, in the Korea population, whose genetic background and SE constitution are similar with Chinese, but where air pollution is not as marked (24), the effect of smoking on RA risk is observed (9). Third, the prevalence of RA was higher in China than that in South Korea [0.37% (25) vs. 0.27% (26)]. This phenomenon may be due to air pollution exposure being more prevalent than smoking. Fourth, smoking (27) as well as fine atmospheric particulates (PM2.5) (24) were reported to be associated with Oxidative Stress, so we speculate that high concentration of PM2.5 may obscure the effect of smoking. Clarifying the relationship between PM2.5 and RA will help us learn more about the pathogenesis of RA.
In conclusion, this study provides support to the existence of interaction between smoking and HLA-DRB1 in the pathogenesis of RA. However, the effect is not as strong as reported in other East Asian countries or in European or North American studies. This may be related to statistical power, or an alternative explanation that greater exposure to other potential environmental factors may act as triggers for the disease, such as passive smoking and industrial air pollution, in China. Further studies will be required to investigate the potential role for these factors in RA.
Ethics Statement
This study was carried out in accordance with the recommendations of the Second Military Medical University Ethics Committee with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Second Military Medical University Ethics Committee.
Author Contributions
HX and MB designed this study; HX organized this study and was the correspondence author; JY and DH carried on this research, they collected samples, demographic data, and finished the lab job, JY also finished the statistic work; LJ, QG, SH, FC, XZ, and YL collected samples and demographic data; HX, MB, and JY wrote the paper. JY and DH contributed equally to this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, HM, and the handling editor declared their shared affiliation, and the handling editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
The authors would like to thank the following people for contributing to this report: all participating patients and controls; for recruiting patients: Tao Yue, Guanghua Hospital; Xiaolei Fan, Guanghua Hospital.
Funding
HX was supported by Ministry of Science and Technology of the People’s Republic of China (973 program of China 2014CB541800) and National Natural Science Foundation of China (grant 81020108029) and Shanghai Shen Kang Hospital Development Center (grant SHDC12012117). DH is funded by the National Natural Science Foundation of China (grant 81273979) and Shanghai clinical base construction of traditional Chinese medicine (ZY3-LCPT-1).
References
1. Vessey MP, Villard-Mackintosh L, Yeates D. Oral contraceptives, cigarette smoking and other factors in relation to arthritis. Contraception (1987) 35(5):457–64. doi: 10.1016/0010-7824(87)90082-5
2. Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum (1999) 42(5):910–7. doi:10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D
3. Reckner Olsson A, Skogh T, Wingren G. Comorbidity and lifestyle, reproductive factors, and environmental exposures associated with rheumatoid arthritis. Ann Rheum Dis (2001) 60(10):934–9. doi:10.1136/ard.60.10.934
4. MacKay K, Eyre S, Myerscough A, Milicic A, Barton A, Laval S, et al. Whole-genome linkage analysis of rheumatoid arthritis susceptibility loci in 252 affected sibling pairs in the United Kingdom. Arthritis Rheum (2002) 46(3):632–9. doi:10.1002/art.10147
5. Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum (2006) 54(1):38–46. doi:10.1002/art.21575
6. Pedersen M, Jacobsen S, Garred P, Madsen HO, Klarlund M, Svejgaard A, et al. Strong combined gene-environment effects in anti-cyclic citrullinated peptide-positive rheumatoid arthritis: a nationwide case-control study in Denmark. Arthritis Rheum (2007) 56(5):1446–53. doi:10.1002/art.22597
7. Morgan AW, Thomson W, Martin SG; Yorkshire Early Arthritis Register Consortium, Carter AM; UK Rheumatoid Arthritis Genetics Consortiumet al. Reevaluation of the interaction between HLA-DRB1 shared epitope alleles, PTPN22, and smoking in determining susceptibility to autoantibody-positive and autoantibody-negative rheumatoid arthritis in a large UK Caucasian population. Arthritis Rheum (2009) 60(9):2565–76. doi:10.1002/art.24752
8. Klareskog L, Malmstrom V, Lundberg K, Padyukov L, Alfredsson L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin Immunol (2011) 23(2):92–8. doi:10.1016/j.smim.2011.01.014
9. Jiang L, Yin J, Ye L, Yang J, Hemani G, Liu AJ, et al. Novel risk loci for rheumatoid arthritis in Han Chinese and congruence with risk variants in Europeans. Arthritis Rheumatol (2014) 66(5):1121–32. doi:10.1002/art.38353
10. Bang SY, Lee KH, Cho SK, Lee HS, Lee KW, Bae SC. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum (2010) 62(2):369–77. doi:10.1002/art.27272
11. Mikuls TR, Sayles H, Yu F, Levan T, Gould KA, Thiele GM, et al. Associations of cigarette smoking with rheumatoid arthritis in African Americans. Arthritis Rheum (2010) 62(12):3560–8. doi:10.1002/art.27716
12. He P, Takeuchi T, Yano E. An overview of the China National Tobacco Corporation and State Tobacco Monopoly Administration. Environ Health Prev Med (2013) 18(1):85–90. doi:10.1007/s12199-012-0288-4
13. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum (1988) 31(3):315–24. doi:10.1002/art.1780310302
14. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol (1999) 26(3):743–5.
15. Zetterquist H, Olerup O. Identification of the HLA-DRB1*04, -DRB1*07, and -DRB1*09 alleles by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Hum Immunol (1992) 34(1):64–74. doi:10.1016/0198-8859(92)90086-3
16. Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol (2005) 20(7):575–9. doi:10.1007/s10654-005-7835-x
17. Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology (1996) 7(3):286–90. doi:10.1097/00001648-199605000-00012
18. Terao C, Ohmura K, Kochi Y, Ikari K, Maruya E, Katayama M, et al. A large-scale association study identified multiple HLA-DRB1 alleles associated with ACPA-negative rheumatoid arthritis in Japanese subjects. Ann Rheum Dis (2011) 70(12):2134–9. doi:10.1136/annrheumdis-2011-200353
19. Xue Y, Zhang J, Chen YM, Guan M, Zheng SG, Zou HJ. The HLA-DRB1 shared epitope is not associated with antibodies against cyclic citrullinated peptide in Chinese patients with rheumatoid arthritis. Scand J Rheumatol (2008) 37(3):183–7. doi:10.1080/03009740701874444
20. Okada Y, Kim K, Han B, Pillai NE, Ong RT, Saw WY, et al. Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum Mol Genet (2014) 23(25):6916–26. doi:10.1093/hmg/ddu387
21. Gan RW, Deane KD, Zerbe GO, Demoruelle MK, Weisman MH, Buckner JH, et al. Relationship between air pollution and positivity of RA-related autoantibodies in individuals without established RA: a report on SERA. Ann Rheum Dis (2013) 72(12):2002–5. doi:10.1136/annrheumdis-2012-202949
22. Hart JE, Kallberg H, Laden F, Bellander T, Costenbader KH, Holmqvist M, et al. Ambient air pollution exposures and risk of rheumatoid arthritis: results from the Swedish EIRA case-control study. Ann Rheum Dis (2013) 72(6):888–94. doi:10.1136/annrheumdis-2012-201587
23. Lugli EB, Correia RE, Fischer R, Lundberg K, Bracke KR, Montgomery AB, et al. Expression of citrulline and homocitrulline residues in the lungs of non-smokers and smokers: implications for autoimmunity in rheumatoid arthritis. Arthritis Res Ther (2015) 17:9. doi:10.1186/s13075-015-0520-x
24. Bae S, Pan XC, Kim SY, Park K, Kim YH, Kim H, et al. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect (2010) 118(4):579–83. doi:10.1289/ehp.0901077
25. Xiang YJ, Dai SM. Prevalence of rheumatic diseases and disability in China. Rheumatol Int (2009) 29(5):481–90. doi:10.1007/s00296-008-0809-z
26. Sung YK, Cho SK, Choi CB, Bae SC. Prevalence and incidence of rheumatoid arthritis in South Korea. Rheumatol Int (2013) 33(6):1525–32. doi:10.1007/s00296-012-2590-2
Keywords: smoking, rheumatoid arthritis, anti-CCP antibodies, shared epitope, Han Chinese
Citation: Yin J, He D, Jiang L, Cheng F, Guo Q, Huang S, Zeng X, Liu Y, Brown MA and Xu H (2017) Influence of Cigarette Smoking on Rheumatoid Arthritis Risk in the Han Chinese Population. Front. Med. 4:76. doi: 10.3389/fmed.2017.00076
Received: 04 January 2017; Accepted: 26 May 2017;
Published: 15 June 2017
Edited by:
Burkhard Franz Leeb, State Hospital Stockerau, AustriaReviewed by:
Maximilian F. Konig, Massachusetts General Hospital, United StatesMarkus Gaugg, KH der Elisabethinen Klagenfurt GmbH, Austria
Harsono The-Hien Mai, Lower Austrian State Hospital Stockerau, Austria
Copyright: © 2017 Yin, He, Jiang, Cheng, Guo, Huang, Zeng, Liu, Brown and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huji Xu, eHVodWppQHNtbXUuZWR1LmNu
†These authors have contributed equally to this work.
 Jian Yin
Jian Yin Dongyi He
Dongyi He Lei Jiang1
Lei Jiang1 Yi Liu
Yi Liu Matthew A. Brown
Matthew A. Brown Huji Xu
Huji Xu