- 1Department of Radiation Oncology, Azienda Sanitaria Universitaria Integrata di Trieste (ASUITS), Trieste, Italy
- 2Department of Medical Physics, Azienda Sanitaria Universitaria Integrata di Trieste (ASUITS), Trieste, Italy
- 3Department of Surgery, University of Trieste, Trieste, Italy
- 4Department of Physics, University of Trieste, Trieste, Italy
- 5Department of Medical Direction, Azienda Sanitaria Universitaria Integrata di Trieste (ASUITS), Trieste, Italy
Introduction: Failure Mode Effects and Criticalities Analysis (FMECA) represents a prospective method for risk assessment in complex medical practices. Our objective was to describe the application of FMECA approach to intraoperative electron beam radiotherapy (IOERT), delivered using a mobile linear accelerator, for the treatment of early breast cancer as an anticipated boost.
Materials and methods: A multidisciplinary Working Group, including several different professional profiles, was created before the beginning of clinical practice in 2012, with the purpose of writing the Flow Chart and applying the FMECA methodology to IOERT procedure. Several criticalities were identified a priori in the different steps of the procedure and a list of all potential failure modes (FMs) was drafted and ranked using the risk priority number (RPN) scoring system, based on the product of three parameters: severity, occurrence, and detectability (score between 1 and 5). The actions aimed at reducing the risk were then defined by the Working Group and the risk analysis was repeated in 2014 and in 2016, in order to assess the improvement achieved.
Results: Fifty-one FMs were identified, which represented the issues prospectively investigated according to the FMECA methodology. Considering a set threshold of 30, the evaluated RPNs show that 33 out of 51 FMs are critical; 6 are included in the moderate risk class (RPN: 31–40); 16 in the intermediate risk class (RPN: 41–50), and 11 in the high risk class (RPN: >50).
Discussion: The most critical steps concerned the surgical procedure and IOERT set-up. The introduction of the corrective actions into the clinical practice achieved the reduction of the RPNs in the re-analysis of the FMECA worksheet after 2 and 4 years, respectively.
Conclusion: FMECA proved to be a useful tool for prospective evaluation of potential failures in IOERT and contributed to optimize patient safety and to improve risk management culture among all the professionals of the Working Group.
Introduction
Adjuvant radiotherapy (RT) after breast conservative surgery is currently considered the standard treatment for early breast cancer and plays an important role to reduce local recurrences (LR) and to improve disease-free and overall survival (1).
Taking into consideration that around 85% of ipsilateral breast cancer recurrences occur in the tumor bed, a local dose escalation is commonly used in addition to whole breast irradiation (2). Three randomized trials have demonstrated that the addition of a boost to the tumor bed reduces further the incidence of LR (3, 4).
Intraoperative electron beam radiotherapy (IOERT) in the treatment of early-stage breast cancer was introduced into the clinical practice at the end of the 1990s, when dedicated mobile linear accelerators (linacs) became available (5). It can be used both as elective RT (partial breast irradiation) in selected patients (6) and as an anticipated boost (7). In this case, it shortens the total radiation treatment time by 1–1.5 weeks and improves the precision of dose delivery to the tumor bed (3, 7).
Our Center acquired a mobile electron linac MOBETRON by IntraOp dedicated to IOERT, and our clinical activity started at the end of June 2012.
The risk assessment performed before the start of clinical activity was integrated with the prospective method FMECA (Failure Mode Effects and Criticalities Analysis). Two and four years later, an analysis of all the relevant criticalities was performed in order to improve quality.
One possible approach to prevent failure mode (FM) in IOERT session consists in fact in the identification and prevention of possible hazards a priori (8, 9).
The aim of this study is to present the results of the method elaborated by our Working Group and the application of FMECA prospective approach to IOERT procedure.
Materials and Methods
A multidisciplinary Working Group was created before the beginning of clinical practice with IOERT in 2012, according to the Guidelines for Quality Assurance in Intraoperative Radiation Therapy by the Superior Institute of Health (10), including several different professional profiles: a Surgeon, a Radiation Oncologist, a Physicist, an Anesthesiologist, the Chief of the RT technicians, and the Nurse Responsible for the Operating Block. The Group was coordinated by a facilitator, a Medical Doctor from the Medical Direction of the Hospital, qualified in clinical risk management, skilled in risk analysis, but not expert in radiation therapy.
At first, the Procedure text was elaborated, according to the Joint Commission International (JCI) method (11), followed in our Hospital, with the contribution of all the members of the Working Group, who periodically met with the facilitator.
The description of the Flow Chart of IOERT (Table 1), included in the Procedure text, made up the platform of the FMECA investigation.
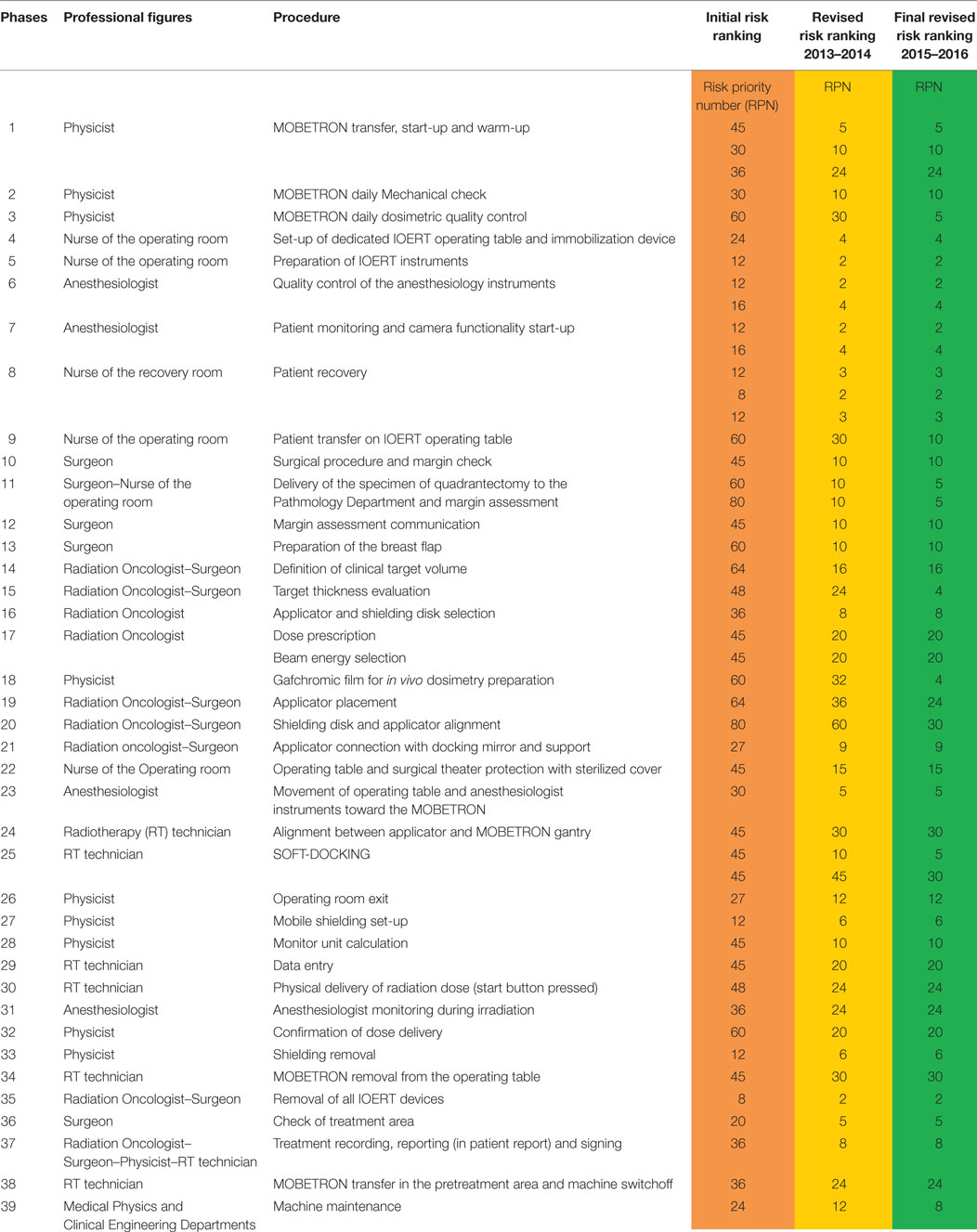
Table 1. FLOW CHART: processes identified in intraoperative electron beam radiotherapy (IOERT) treatment in Trieste.
Each member of the Working Group was asked to identify a priori the criticalities he/she might meet in the process steps concerning his/her specific activity. In this way, a list of all potential FMs occurring in each process step was drafted.
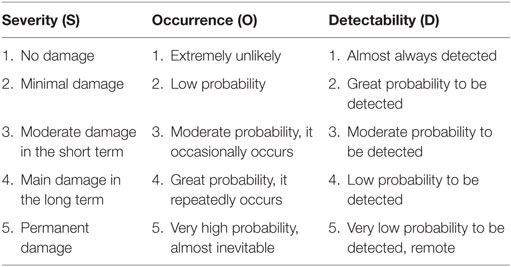
FMECA leads to quantitative data: for every step of IOERT procedure a score is assigned. The score ranges from 1 to 5 and includes three pre-established parameters:
– severity (S),
– occurrence (O),
– detectability (D).
In clinical practice, FMECA consists in the identification of different steps of the examined procedure (12). For each step, possible critical situations are identified and a risk priority number (RPN) is assigned. RPN value is obtained by multiplying S, O, and D parameters (RPN = S × O × D) (score between 1 and 5 assigned following rules) (Table 2).
The higher the value obtained, the more likely the risk that an accident occurs (“failure”) during the procedure and the higher the probability of relevant consequences are.
Based on calculated RPN, four different “risk classes” are identified:
– low risk (RPN ≤30),
– moderate risk (RPN 31–40),
– intermediate risk (RPN 41–50),
– high risk (RPN >50).
Thus, the identification of the critical steps of a procedure leads to modifications, even of substantial nature, of behavior and actions in order to reduce errors occurrence as much as possible. Decreasing this hazard also leads to the reduction of possible damage both for health personnel and patients.
The risk analysis was completed by asking the members of the team to evaluate the RPN of each FM.
Every 2 years since the beginning of IOERT clinical activity, the risk analysis was repeated by the Working Group in order to assess the improvement achieved.
Results
Our activity with IOERT as an anticipated boost, followed by conventional or hypofractionated external beam RT, in the treatment of early breast cancer started in June 2012.
Ninety-two cases have been treated up to April 2016 (46 by the end of 2014 and an additional 40 by the end of 2016).
The first FMECA analysis was performed before the start of clinical activity; the risk analysis was then repeated at the end of 2014 and again at the end of 2016.
In the Flow Chart, the IOERT process was subdivided into 39 steps, in which the different professional figures are involved, from the start-up of the Mobetron to the end of the operation, the switch off of the machine and the draft of the IOERT report (Table 1).
An Excel worksheet was created, inserting in rows: process step, professional figures involved, FM, potential effects of failure, potential causes of failure, initial risk ranking with the RPN, and corrective actions. In the re-analysis of the process—2 and 4 years later—the final RPN was elaborated and the risk reduction (RR) (preliminary RPN—final RPN) was also calculated in order to assess the weight of the corrective measures.
In this worksheet, 51 FMs were identified, which represented the issues prospectively investigated according to the FMECA method.
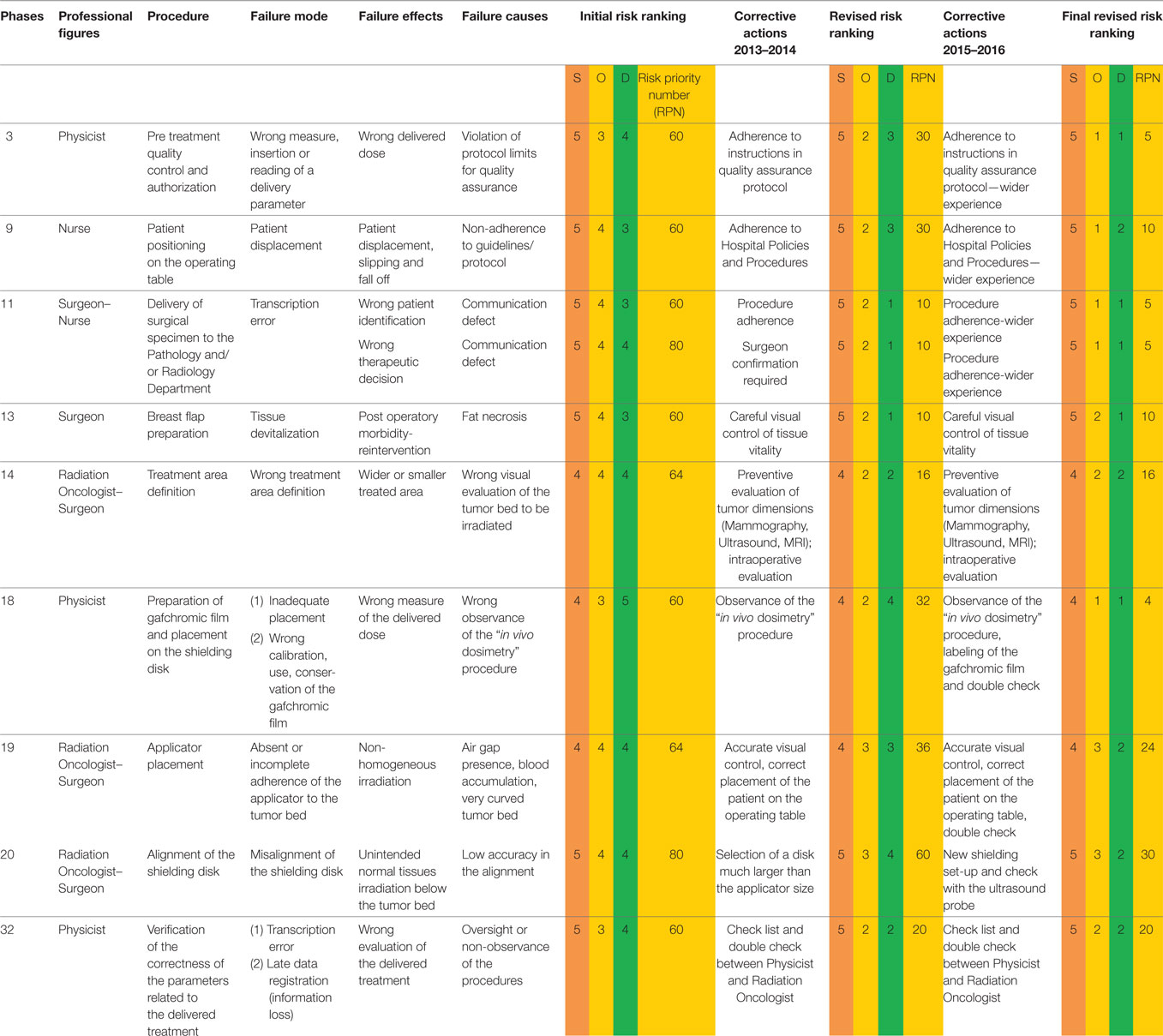
Considering a set threshold of 30, the evaluated RPNs show that 33 out of 51 FMs are critical; 6 are included in the moderate risk class (RPN: 31–40); 16 in the intermediate risk class (RPN: 41–50), and 11 in the high risk class (RPN: >50).
Failure modes included in the high risk class (Table 3) enabled us to pinpoint the main criticism of the whole IOERT procedure and so we were able to make procedural changes in order to reduce hazards. The data highlight the fact that several critical steps concern the surgical procedures and IOERT set-up.
Discussion
The analysis of adverse events in RT is a recent topic; either retrospective or prospective approaches can be employed. In the former group, the root cause analysis is the most widely used, aimed at identifying the root causes of near misses or adverse events; in the latter group, several methodologies are available, such as process mapping, value stream mapping, fault tree analysis and failure mode effects and criticality analysis (FMECA) (12). A proactive approach allows to study the whole process or part of it, independently from the occurrence of an adverse event, to implement the corrective actions and to evaluate the benefits obtained; it is, therefore, more suitable to a complex process, such as IOERT.
Analyzing the high risk class steps of our procedure in chronological order (Table 3), the first phase with a high risk score (RPN = 60) concerns the Physicist who is in charge of the program of quality control (FMECA step No. 3).
During the pre-clinical quality control, the FM was due to the incorrect measurement and/or reading of the pre-established parameters, which might cause the delivery of a wrong dose to the patient. The precise observation of the Quality Control Protocol at first (RR: 30; 50%) and then a wider experience of the operator (RR: 25; 83.3%) could avoid this serious occurrence.
The second criticality involves the nurses of the Operating Theater (step No. 9): it concerns the incorrect positioning of the patient on the operating table with the danger of slipping or falling off (RPN = 60). The adherence to the well-known Policies and Procedures of our Hospital (RR: 30; 50%) and the training of the nursing staff acquired over time (RR: 20; 66.7%) could prevent these accidents.
In the FMECA analysis, a high score was also assigned to the phase No. 11, which involves both the operating room Nurses and the Surgeon; it regards the delivery of the specimen of quadrantectomy to the Pathology Department and the margins assessment with a macroscopic evaluation performed by the Pathologist.
Assuming that the patient identification (step No. 11a; RPN = 60) has been properly done during the “time out” phase (before the surgical procedure begins) (final RPN: 5), the main debated issues are as follows: the way to communicate sensitive data (by phone), the amount of time to obtain the results, and the right evaluation of the Pathologist report. Time delay for surgical specimen examination may vary from 15 to 30 min due to the time for mammographic/ultrasound evaluation of the specimen, sample processing, and caseload of the Pathologists.
A wrong identification of the margin (step No. 11b; RPN = 80) could cause an incorrect choice of the surgical procedure; the risk of local failure in fact is reported to be higher in case of positive or close margins (13) and, in this case, the Surgeon can widen them during the same surgical procedure.
We identified and selected a group of Pathologists specialized and dedicated to breast tumors, with the aim to reduce this risk ranking. As regards sensitive data communication, a specific procedure was established: data need to be communicated by phone from a physician to another physician and the colleague who receives the communication has to print the online report in order to have an official written document (final RPN: 5).
Step No. 13 involves the Surgeon (RPN = 60) and the correct preparation of the breast flap in order to prevent that a devitalized tissue could cause necrosis and postoperative morbidity. An accurate visual control during the surgical procedure could decrease the risk (RR: 50; 83.3%).
Step No. 14 regards the definition of the clinical target volume (CTV) (RPN = 64). This represents a critical point involving the Radiation Oncologist and the Surgeon: underdosing of the target and/or unintended normal tissues irradiation can occur (14). The exact evaluation of the dimensions of the tumor on preoperative diagnostic imaging and an accurate intraoperative definition of the CTV determined a significant RPN reduction from 64 to 16 (RR: 48; 75%).
Another relevant criticality (step No. 18) involves the Physicist and the preparation of the gafchromic film for in vivo dosimetry (RPN = 60). A wrong calibration of the gafchromic film or an inadequate placement of the film on top of the internal shield can cause a wrong evaluation of the dose delivered (14). These risks were prevented first of all by following the “in vivo dosimetry” Procedure, elaborated by the Physicist, with an RPN reduction from 60 to 32 (RR: 28; 46.7%), and then by labeling the gafchromic film and employing a double check in the procedure with a further reduction of RPN from 32 to 4 (RR: 28; 87.5%).
The next critical score (step No. 19) is related to the inaccurate placement of the applicator in the tumor bed (RPN = 64).
Absent or incomplete adherence of the applicator to the tumor bed, determined by air gap presence, blood accumulation, or very curved tumor bed, can cause a non-homogeneous irradiation (14). Corrective actions, such as an accurate visual control and the correct placement of the patient on the operating table, reduced the risk ranking from 64 to 36 (RR: 28; 43.8%) after 2 years and from 36 to 24 in the next 2 years (RR: 12; 33.3%) employing a double check.
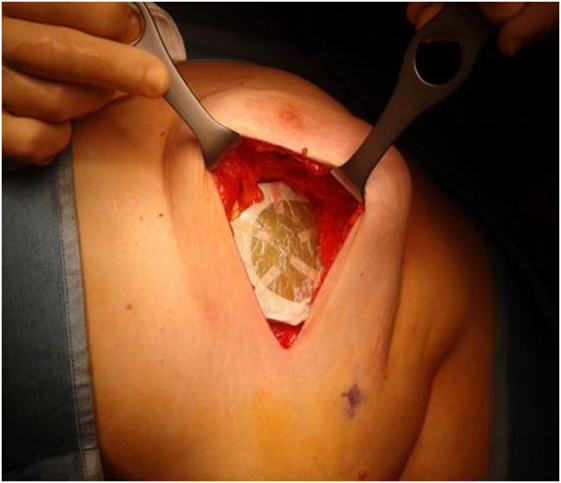
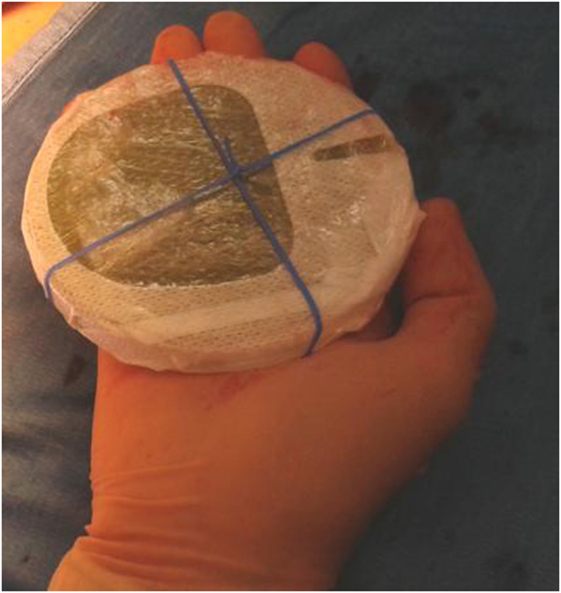
The highest step score (No. 20) (RPN = 80) was attributed to the misalignment of the shielding disk, used to protect the normal tissues underneath the target volume, such as the lung and the heart (for the left breast). The disk is positioned by the Surgeon between the residual breast and the pectoralis fascia, its size depending on the collimator diameter chosen for the treatment (Figure 1).
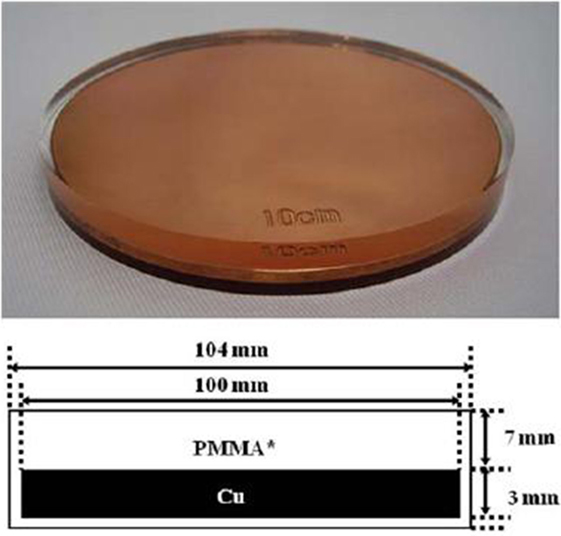
In most of the cases, we employed an 8-cm-diameter shielding disk provided by the IntraOp, made up of three stacked layers: a 5 mm polymethyl methacrylate (PMMA), a 3 mm copper, and a 2 mm PMMA layer (Figure 2).
The low accuracy in the alignment of the disk would cause the delivery of an excess of dose to the underlying normal tissues.
The selection of a plate much larger than the applicator size was the first corrective action.
Furthermore, to avoid shielding disk misalignment, the Surgeon and the Radiation Oncologist decided to design a new shielding set-up, implementing an elastic band fixed by stitches to the pectoralis fascia, thus preventing the disk slipping (14) (Figure 3).
The optimized set-up described above was introduced into the clinical practice, and a RR from 80 to 60 (RR: 20; 25%) was observed in the first re-analysis of the procedure in 2014.
In 2015, intraoperative ultrasound (IOUS) measures were employed either to better define the target thickness or to check the position of the shielding disk. IOUS proved to be accurate in evaluating both target thickness and shielding alignment, halving the RPN (RR from 48 to 24 in the former case and from 60 to 30 in the latter case).
The last high risk step in the FMECA worksheet (RPN = 60) concerns the Physicist and the verification a posteriori of the parameters of the delivered dose (step No. 32). The constant use of the check list and a double check by the Physicist and the Radiation Oncologist contributed to decrease the RPN from 60 to 20 (RR: 40; 66.7%).
Only two studies published in the literature examined the importance of FMECA applied to IOERT procedure: in the Italian experience a dedicated mobile Linac was used (8), while in the Spanish analysis the irradiation was performed with a fixed conventional Linac (9). In both studies, FMECA proved to be an excellent tool to evaluate patient safety and allowed to reduce risks and improve quality in IOERT procedure. Ciocca et al. (8) found that the highest risk was associated with the alignment of the shielding disc, as we observed in this study (Table 3). López-Tarjuelo et al. (9) found that the highest ranked RPNs were related not only to incorrect protection assembly but also to incorrect transmission and programming of treatment parameters. In our analysis, incorrect monitor unit calculation and data entry (step No. 28 and No. 29) (Table 1) had an initial intermediate risk score, which decreased to a low score after the introduction of the corrective actions, while only the verification a posteriori of the delivered dose (step No. 32) was initially classified in the high risk class, as reported above.
Conclusion
Intraoperative radiation treatment as an anticipated boost proved to be very effective in the treatment of early breast cancer.
This approach presents several advantages:
– No topographic miss,
– More favorable radiobiology of a single dose (α/β),
– Shorter radiation time (<1–2 weeks),
– Good dose distribution,
– Complete skin sparing,
– Minimal toxicity in the long-term follow-up.
On the other side, it reveals some drawbacks:
– Uncertainty of the final pathologic report and
– Lack of definition of the resection margins.
To date, every published interim analysis showed lower local recurrence rates than standard treatment schedules (7). In our experience, no local or regional recurrences were detected, with a median follow-up of 28 months, and the incidence and severity of the side effects were acceptable; no grade 3 or greater side effects were observed.
The FMECA has provided a prospective systematic method to discover potential failures in IOERT procedure: evaluating not only the frequency of FM but also their severity and detectability, it has given a more complete assessment of the risk. It contributes, therefore, to optimize patient safety right from the start of our clinical activity and to improve risk management culture among all the professionals involved in the Working Group.
The IOERT procedure was standardized and the application of FMECA allowed us to define the safest pathway for the patient.
Its use, still rather limited, should be strongly encouraged in RT Centers treating patients with IOERT.
Author Contributions
The right sequence of the names is as follows: CV, MS, MU, LT, AP, and MB (corresponding Author). CV, MS, and MB elaborated the test; MU and LT analyzed the patient data; and AP gave management support.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomized trials. Lancet (2011) 378:1707–16. doi:10.1016/S0140-6736(11)61629-2
2. Botteri E, Bagnardi V, Rotmensz N, Gentilini O, Disalvatore D, Bazolli B, et al. Analysis of local and regional recurrences in breast cancer after conservative surgery. Ann Oncol (2010) 21:723–8. doi:10.1093/annonc/mdp386
3. Lemanski C, Azria D, Thezenas S, Gutowski M, Saint-Aubert B, Rouanet P, et al. Intraoperative radiotherapy given as a boost for early breast cancer: long-term clinical and cosmetic results. Int J Radiat Oncol Biol Phys (2006) 64:1410–5. doi:10.1016/j.ijrobp.2005.10.025
4. Bartelink H, Maingon P, Poortmans P, Weltens C, Fourquet A, Jager J, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol (2015) 16:47–56. doi:10.1016/S1470-2045(14)71156-8
5. Orecchia R, Ciocca M, Tosi G, Franzetti S, Luini A, Gatti G, et al. Intraoperative electron beam radiotherapy (ELIOT) to the breast: a need for a quality assurance programme. Breast (2005) 14:541–6. doi:10.1016/j.breast.2005.08.038
6. Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol (2017) 7(2):73–9. doi:10.1016/j.prro.2016.09.007
7. Fastner G, Sedlmayer F, Merz F, Deutschmann H, Reitsamer R, Menzel C, et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: long term results of an ISIORT pooled analysis. Radiother Oncol (2013) 108:279–86. doi:10.1016/j.radonc.2013.05.031
8. Ciocca M, Cantone MC, Veronese I, Cattani F, Pedroli G, Molinelli S, et al. Application of failure mode and effects analysis to intraoperative radiation therapy using mobile electron linear accelerators. Int J Radiat Oncol Biol Phys (2012) 82:e305–11. doi:10.1016/j.ijrobp.2011.05.010
9. López-Tarjuelo J, Bouché-Babiloni A, Santos-Serra A, Morillo-Macías V, Calvo FA, Kubyshin Y, et al. Failure mode and effect analysis oriented to risk-reduction interventions in intraoperative electron radiation therapy: the specific impact of patient transportation, automation, and treatment planning availability. Radiother Oncol (2014) 113:283–9. doi:10.1016/j.radonc.2014.11.012
10. Istituto Superiore di Sanità. Linee guida per la garanzia di qualità nella radioterapia intraoperatoria. A cura di Antonella Rosi, e Vincenza Viti (2003). 68 p. Rapporti ISTISAN 03/1 IT
11. Joint Commission International. Joint Commission International Accreditation Standards for Hospitals. 5th ed. (2013). Available from: https://www.jointcommission.org/
12. Scorsetti M, Signori C, Lattuada P, Urso G, Bignardi M, Navarria P, et al. Applying failure mode effects and criticality analysis in radiotherapy: lessons learned and perspectives of enhancement. Radiother Oncol (2010) 94:367–74. doi:10.1016/j.radonc.2009.12.040
13. Gruppo di Lavoro AIRO per la Patologia Mammaria. La radioterapia dei tumori della mammella: Indicazioni e criteri guida (2013). Available from: http://vecchio.radioterapiaitalia.it/cont__169.phtml
Keywords: risk assessment, intraoperative electron beam radiotherapy, quality assurance, FMECA, patient safety
Citation: Vidali C, Severgnini M, Urbani M, Toscano L, Perulli A and Bortul M (2017) FMECA Application to Intraoperative Electron Beam Radiotherapy Procedure As a Quality Method to Prevent and Reduce Patient’s Risk in Conservative Surgery for Breast Cancer. Front. Med. 4:138. doi: 10.3389/fmed.2017.00138
Received: 13 April 2017; Accepted: 02 August 2017;
Published: 28 August 2017
Edited by:
Joachim Paul Hasebrook, Steinbeis University Berlin, GermanyReviewed by:
Frits Van Merode, Maastricht University Medical Centre, NetherlandsHaitham Mutlak, University Hospital Frankfurt, Germany
Copyright: © 2017 Vidali, Severgnini, Urbani, Toscano, Perulli and Bortul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina Bortul, bS5ib3J0dWxAZm1jLnVuaXRzLml0
 Cristiana Vidali1
Cristiana Vidali1 Licia Toscano
Licia Toscano Marina Bortul
Marina Bortul