- 1Geriatric Unit, San Gerardo Hospital, Monza, Italy
- 2School of Medicine and Surgery, University Milano-Bicocca, Milan, Italy
- 3National Research Council (CNR), Nuclear Medicine Department, San Raffaele Hospital (IRCCS), Milan, Italy
- 4San Raffaele Hospital (IRCCS), Milan, Italy
- 5Geriatric Unit, Department of Medical Sciences and Community Health, University of Milano, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy
- 6Department of Biomedical Sciences for Health, University of Milano, Segrate, Italy
- 7Department of Rehabilitation and Aged Care, Casa di Cura “Ancelle della Carità”, Fondazione Teresa Camplani, Cremona, Italy
- 8Geriatric Unit, Fondazione IRCCS Ca ‘Granda, Ospedale Maggiore Policlinico, Milan, Italy
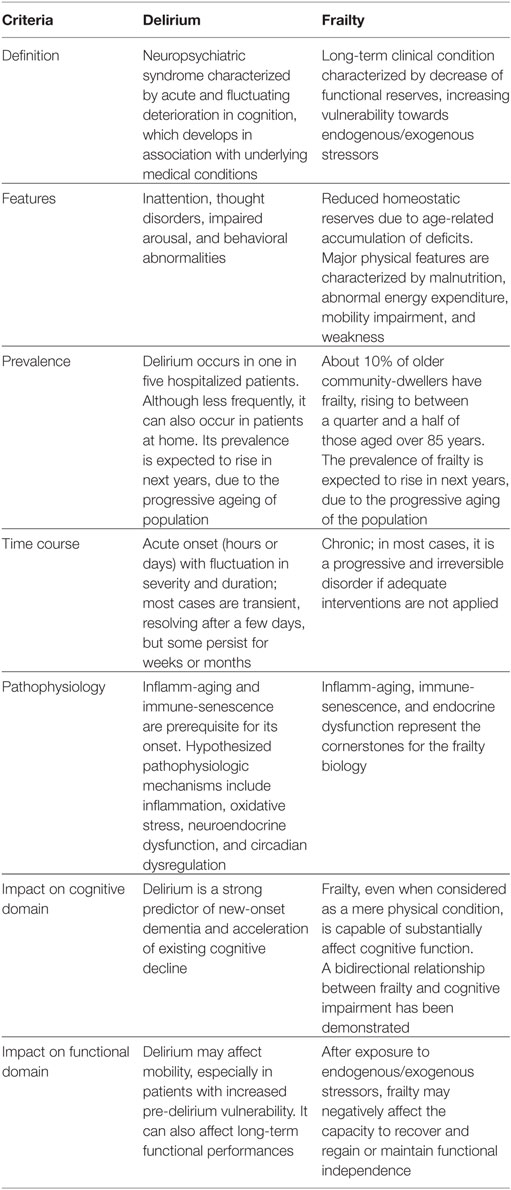
Frailty is a clinical syndrome defined by the age-related depletion of the individual’s homeostatic reserves, determining an increased susceptibility to stressors and disproportionate exposure to negative health changes. The physiological systems that are involved in the determination of frailty are mutually interrelated, so that when decline starts in a given system, implications may also regard the other systems. Indeed, it has been shown that the number of abnormal systems is more predictive of frailty than those of the abnormalities in any particular system. Delirium is a transient neurocognitive disorder, characterized by an acute onset and fluctuating course, inattention, cognitive dysfunction, and behavioral abnormalities, that complicates one out of five hospital admissions. Delirium is independently associated with the same negative outcomes of frailty and, like frailty, its pathogenesis is usually multifactorial, depending on complex inter-relationships between predisposing and precipitating factors. By definition, a somatic cause should be identified, or at least suspected, to diagnose delirium. Delirium and frailty potentially share multiple pathophysiologic mechanisms and pathways, meaning that they could be thought of as the two sides to the same coin. This review aims at summarizing the existing evidence, referring both to human and animal models, to postulate that delirium may represent the cognitive harbinger of a state of frailty in older persons experiencing an acute clinical event.
Introduction
Although frailty and delirium are intuitively associated, a clear taxonomy of their biological and clinical relationship has not provided yet in geriatric medicine.
For many years, age has been considered as one of the most powerful predictors of mortality and adverse outcomes in older people. However, growing empirical evidence and several scientific publications have clearly shown that “chronological age” is not able per se to capture with sufficient accuracy the extreme heterogeneity of the health status in older persons (1, 2). In order to promote a measure more focused on the individual’s functions and biology, the concept of frailty has received special attention over the past years. In fact, frailty has been indicated as a condition which may accurately capture the homeostatic reserves of the organism and, as such, improve the assessment of the risk profile. In other words, frailty might represent the new criterion for defining the individual as (biologically) old and replace the obsolete age concept (2). Interestingly, this change of paradigm might also support a more person-tailored approach in the design of clinical interventions.
According to a commonly accepted definition, frailty is defined as a medical syndrome characterized by a decrease of functional reserve capacities, diminished strength, and endurance. The consequence of this increased vulnerability is that a frail person is more prone than a non-frail to develop negative health-related outcomes, including decline in functional and motor performance, prolonged length of hospital stay, institutionalization, rehospitalization, and mortality (2–4). Frailty might thus be considered as the complex biological background on which multiple protective and disruptive factors interact in the determination of the clinical manifestations and negative outcomes (2, 4). From a pathophysiological perspective, it is well accepted that the physiological systems which are involved in the determination of frailty, including brain, endocrine system, immune system, and skeletal muscle, are mutually interrelated, so that when decline starts in a given system, implications may also regard the others. To support this, it has shown that the number of abnormal systems is more predictive of frailty than are the abnormalities in any particular system (4). Recently, to explore the mechanistic relationship between aging, frailty, and mortality, Rutenberg et al. developed a computational model in which possible health attributes are represented by the nodes of a complex network, with the connection showing a scale-free distribution (1). Each node can be either damaged (i.e., a deficit) or undamaged. The most connected nodes are the mortality nodes; the next most connected nodes are frailty nodes that broadly correspond to clinically or biologically significant health characteristics. According to this model, individuals die when mortality nodes are highly damaged. Nodes are damaged randomly reflecting environmental influences, intrinsic features, and their interactions (5). Through interactions, the rate of damage of an individual node increases as more of its connected neighbors are damaged. This model can explain why frail individuals are at higher risk of vulnerability and mortality than robust ones, and facilitates the initial understanding of the factors influencing the health trajectories of older individuals (1).
Delirium is a transient neurocognitive disorder, characterized by an acute onset and fluctuating course, inattention, cognitive dysfunction, and behavioral abnormalities, which develops in association with another underlying medical condition (6). Sometimes, though not invariably, delirium presents with behavioral disturbances, including sleep-wake cycle disruption, psychotic symptoms, and agitation (7). It has been shown that delirium complicates about one out of five hospital admissions (8, 9), representing a clear burden for the patient as well as for public health. Like frailty, delirium is independently associated with a number of negative outcomes, including increased length of hospital stay, elevated healthcare costs, accelerated cognitive impairment, delayed or limited recovery of functional decline, increased risk of institutionalization, and mortality (10–14). In addition, delirium may cause patient and caregiver’s emotional distress (15, 16). Although a single factor can cause it (e.g., infections), its pathogenesis is usually multifactorial (10), depending on complex inter-relationships between predisposing and precipitating factors acting on the substratum of biological vulnerability (i.e., frailty). According to this view, delirium can thus be regarded as a clinical consequence of frailty in older persons experiencing stressful events. It is also important to mention that frailty and delirium are expected to rise in their prevalence in the next years, largely due to global aging of the populations worldwide.
In this review, we will summarize the existing evidence on the relationship between the two conditions (i.e., frailty and delirium), referring both to human and animal models.
Commonalities and Differences Between Frailty and Delirium
Frailty and delirium share several commonalities but also have specific differences (Table 1). Both should be considered as multifactorial health conditions, characterized by multiple risk factors and causation which are not necessarily specific to a given organ system failure. This notion is indirectly confirmed by a growing body of evidence, from cardiology (17, 18) to infectious disease medicine (19, 20), from oncology (21, 22) to anesthesiology (23, 24), that these two conditions have a crucial role in clinical and research areas. Both frailty and delirium share many commonalities. In particular, they are both predictive of several negative health-related outcomes, most of which might be prevented by applying adapted and personalized interventions. A common biological substratum between the two conditions can also be suggested, possibly involving inflammation, endocrine and vascular systems, and oxidative stress (25). However, since both frailty and delirium find their biological roots in the aging process, it might be hypothesized that the same mechanisms responsible for the aging of the individual may become the causes of the conditions of interest when abnormally enhanced/stimulated by negative (endogenous or exogenous) stressors.
At the same time, frailty and delirium also differ in many aspects. Frailty is the long-term result of a decline in the homeostatic individual’s capacity across multiple physiological systems and it is usually considered as the endpoint of the progressive activity of corrosion exerted by chronic diseases during the normal aging process. On the contrary, delirium is an acute condition that occurs in response to a stressor (generally a medical problem) that may have a relatively rapid resolution, though sometimes it can persist weeks or even months. Delirium can be thought as an acute brain failure, reflecting the interaction between a predisposing factor (i.e., brain vulnerability) and one or more precipitating factor (i.e., the noxious insults), in which the brain is not able to compensate. Frailty may thus represent the ideal pabulum for the development of delirium, and delirium, on its side, may represent the clinical manifestation of underlying frailty in a patient suffering from an acute decompensation.
The relationship between frailty and delirium is even more complicated than above depicted. From a clinical perspective, frailty cannot be considered exclusively a pure disorder of function, though the criteria that are currently used for its definition may suggest. Indeed, there is empirical evidence that isolating physical from cognitive performance is really challenging in several cases. Some researchers have even proposed the terms of “cognitive frailty” and “reversible cognitive frailty” to describe heterogeneous cognitive conditions characterized by the simultaneous presence of both physical frailty and cognitive impairment (26). It is noteworthy that these concepts nest the idea of a reversible condition, the characteristic of dynamic mechanisms linking the physical and cognitive domains. The demonstration that cognitive impairment might reverse over time has been provided by a recent systematic review, showing that mild cognitive impairment (MCI) can return to normality with 8% reversion rate in clinical-based studies and 25% rate in population-based studies. The frequency of reversion from MCI to normality further increases to 26% when considering only studies of better quality (27).
Similar to what occurs for frailty, even delirium cannot be regarded only as an isolated mental disorder but there is evidence that it affects motor function as well. A study by Bellelli et al. compared four groups of 15 patients [with delirium alone, with dementia alone, with delirium superimposed on dementia (DSD), and with neither delirium nor dementia], finding that the mechanisms leading to the onset of delirium can also worsen motor performance (11). Other studies indirectly support such hypothesis, showing that delirium can complicate the functional recovery after adverse clinical events (13, 28). The reasons underlying this phenomenon are under study. It is possible that delirium causes motor fluctuations due to the disrupture of key central neurotransmitters (for example, related to attentive and executive functions) leading to an inability in planning and sustaining movement (11). According to Rockwood, it can also be hypothesized that the mobility impairment accompanying delirium is a reflection of the whole-system failure. Indeed, when complex systems collapse, their failure follows a cascade where highest order functions decline first. As such, the mobility impairment occurring in the course of delirium may represent a sign of a complex system close to failure. The more critical the individual’s health status is before the delirium onset, the higher will then be the likelihood that delirium will lead to mobility impairment (29).
Under a broad viewpoint, frailty reflects the life-long accumulation of deficits, thus defining the more or less state of vulnerability of the individual. Such (more or less overt) accentuated susceptibility to stressors represents the biological background where delirium might find its onset. In an optimal scenario, frailty should be detected in order to take adequate preventive countermeasures for avoiding the onset of its negative outcomes (including delirium). Nevertheless, delirium might become the condition making clinically evident for the first time a previously unknown/overlooked substratum of frailty.
Review of Studies on Frailty and Delirium in Humans
To date, only few studies have specifically focused on the relationship between frailty and delirium in older people, and even less have assessed if frailty is a predictor of delirium. In a prospective observational study in 133 elective cardiac surgery patients, frailty was assessed using three different methods, i.e., a Modified Fried Criteria (MFC), the Short Physical Performance Battery (SPPB), and a 35-itemFrailty Index (FI). The primary exposure variable was postoperative delirium, assessed using the Confusion Assessment Method (CAM) (30). A proportion of patients ranging from 35.3 to 66.2% were frail, according to the method used to define it, and 18% had postoperative delirium. After adjusting for covariates, the presence of frailty resulted in a threefold to eightfold increase in risk of postoperative delirium, independent of the severity of the cardiac disease. In another study (31), carried out in 89 patients who underwent trans-catheter aortic valve implantation (TAVI), frailty was assessed by clinical judgment and as a summary score from baseline components, including the score assigned for Mini–Mental State Examination; Basic Activities of Daily Living; Instrumental Activities of Daily Living; Mini Nutritional Assessment, and impaired mobility. Delirium was assessed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Again, frailty predicted delirium onset and conferred additional value in the prediction of mortality after TAVI but only when frailty was assessed by subjective clinical judgment. On the contrary, such association was not found when frailty was assessed using the summary score. A third small study of older non-cardiac surgical patients evaluated whether a preoperative frailty score was an independent predictor of postoperative delirium. One-third of patients were frail and 25% developed postoperative delirium. In the multivariable logistic regression, frailty score (odds ratio = 1.84; 95% confidence interval = 1.07–3.1; P = 0.028) was independently associated with the development of postoperative delirium. More recently, in a prospective cohort of older patients admitted to a specialized delirium unit, Chew et al. assessed frailty with a 20-item index derived using items from a comprehensive geriatric assessment and delirium using the CAM (32). The authors also measured the residual sub-syndromal delirium (RSSD) before discharge from the unit by using the Delirium Rating Scale-Revised-98 severity score. The functional status was measured the modified Barthel Index on admission and 12 months post-delirium. In a logistic regression model, independent predictors of RSSD were as follows: frailty (OR 4.1, 95% CI 2.1–8.2, P < 0.001), the severity of delirium symptoms on admission (OR 1.2, 95% CI 1.1–1.2), and a pre-existing dementia (OR 4.2, 95% CI 2.0–8.6) (32). Interestingly, RSSD significantly mediated the effect of baseline frailty status on functional recovery at 12 months (32).
Other studies have assessed whether the coexistence of frailty and delirium is associated with an increased risk of death (33), or if delirium was associated with higher levels of frailty, in both studies finding that it was the case (34). However, other studies did not find a significant relationship between these two conditions (35–37).
Differences in the methods used to assess frailty and delirium as well as the selected populations and the length of follow-up can explain the heterogeneity in the study results. Taken together, the data from these studies suggest that further research is urgently needed to understand the complex relationship between frailty and delirium.
A further point is whether delirium may predispose itself to frailty. Indeed, several studies have demonstrated that delirium may be a risk factor for not only for dementia or a worsening of a preexisting dementia (14, 38), but also for subsequent functional impairment (12, 13, 39). Patients with persistent delirium are also less likely to regain activity of daily living function in comparison with non-delirious patients (40, 41). Moreover, when delirium is superimposed on dementia (which may represent itself a marker of pre-existing frailty), the risk to die in the middle short term is overall increased (42). It can be therefore hypothesized that the persistent or residual effects of delirium may delay or even hamper cognitive and functional recovery, ultimately resulting in new or increasing frailty and long-term disability and institutionalization (25). Future studies should better clarify this point.
Common Biomarkers and Pathophysiological Mechanisms of Frailty and Delirium
A premise is required before describing the pathophysiological mechanisms proposed for both frailty and delirium. With aging, a number of changes occur in several interrelated physiological systems, one of the most important being the immune system. The changes in the immune system that occurs with aging are termed “immunosenescence” and may be defined as an age-associated decline in immune function that includes increased susceptibility to infections, reduced vaccination responses, and increased risk of chronic inflammatory diseases. Immunosenescence occurs in parallel with inflamm-aging, i.e., the increased presence of a low-grade chronic systemic inflammatory state typical of older age (43, 44). Inflamm-aging is characterized by increased levels of proinflammatory cytokines [e.g., interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), C-reactive protein (CRP)] and reduced concentrations of anti-inflammatory cytokines (e.g., IL-10, IL-1RA) (43). A variety of tissues (e.g., adipose tissue, muscle), organs (e.g., brain, liver), systems (e.g., immune system), and ecosystems (e.g., gut microbiota) of the body may contribute in a different manner to the onset and progression of inflamm-aging (45). Immunosenescence and inflamm-aging are particularly relevant for the pathophysiology of both delirium and frailty.
The exact pathophysiological mechanisms of delirium are not completely understood. A recent review by Maldonado suggests that at least five mechanisms are involved in delirium pathophysiology, including neuronal aging, neuroinflammation, oxidative stress, neuroendocrine dysregulation, and circadian dysregulation (46). In this review, we will focus exclusively on the three mechanisms which are thought to be more relevant for a common understanding of both delirium and frailty pathophysiology.
The neuroinflammatory hypothesis of delirium proposes that an acute peripheral inflammatory trigger (either infective, surgical, or traumatic) can provoke the activation of brain parenchymal cells, leading to an overexpression of proinflammatory cytokines and inflammatory mediators in the brain parenchyma, neuronal, and synaptic dysfunction and subsequent cognitive and behavioral symptoms of delirium (47). Importantly, brain is able to constantly monitor the presence of peripheral inflammation and how, upon exposure to specific stimuli, individuals may react to illness with a “sickness behavior,” i.e., a constellation of non-specific physiologic and behavioral signs and symptoms, including fever, malaise, fatigue, anorexia, lethargy, social withdrawal, and depressed mood (48, 49). According to the neuroinflammatory hypothesis, delirium may thus represent the CNS manifestation of a systemic disease, with an overproduction of cytokines that provoke a chain reaction in the neuronal cells of the brain (47). The immune signals and cytokines may enter the brain through two pathways (i.e., the neural pathway and the humoral pathway) where they determine the release of other proinflammatory cytokines by macrophage-like cells expressing toll-like receptors. In the neural pathway, the cytokines may activate primary afferent nerves, such as the vagus, and enter the brain through saturable transport system. In the humoral pathway, cytokines enter the brain at the level of the choroid plexus and the circumventricular organs. When enter the brain, cytokines may activate microglial cells. Microglial cells are the resident macrophages of the CNS and represent the 5–10% of all CNS cells. In the healthy brain, these are in a quiescent state, but, when they detect injured CNS cells or invading pathogens, they are able to adopt a specific phenotype with an amoeboid morphology. Phenotype modifications lead to further secretion of proinflammatory cytokines, and the expression of different cell surface receptors. With immune-senescence, microglial cells are characterized by an exaggerated proinflammatory response, acquiring a phenotype of primed less ramification, and reducing their chemotactic, phagocytic, and regulatory functions. A primed microglial phenotype is present also in chronic neurodegenerative processes where microglia cells lost their supportive role in neuroplasticity, thus favoring cognitive decline and synaptic dysfunction (50–53). Several studies, in patients of surgical and medical hospital wards, have shown that delirium is associated with significantly higher circulating levels of these inflammatory markers in comparison with non-delirium (54, 55). Importantly, cytokines can disrupt the neurotransmitter system balance, leading to reduced acetylcholine release (56) and decreased cholinesterase activity (57) and can activate the microglial cells, provoking an inflammatory response which can interfere with the connection and transmission functions of synapses (58, 59).
The oxidative stress hypothesis proposes that a number of physiologic and pathological events, such as tissue damage, hypoxia, illness, and infections, may lead to increased oxygen consumption, decreased oxygen availability, and reduced cerebral oxidative metabolism, which in turn may provoke cerebral dysfunction and associated cognitive and behavioral symptoms of delirium (47). Abnormal oxidative stress has been found in patients undergoing cardiopulmonary bypass surgery and in intensive care unit patients (60, 61). In one of these studies, Seaman has also shown that poor oxygenation is associated with cerebral dysfunction. Among a cohort of 101 patients, the authors assessed three measures of oxygenation (hemoglobin, hematocrit, pulse oximetry) and two measures of oxidative stress (sepsis, pneumonia), finding that indicators of oxidative dysfunction were more common in those who developed more frequently delirium, and this was not linked to illness severity (60). Pericytes may also be a potential target of interest in this framework. The pericytes are specific cells located at the abluminal side of the brain and muscular capillaries, which have the potential to express the inducible nitric oxide synthase (iNOS) and generate reactive oxygen and nitrogen species (RONS) (62). These studies suggest that pericytes, under specific circumstances such as an increased inflammatory status, may not only increase the production of iNOS and RONS but also by behaving as immune cells they are able to enhance the inflammatory response (63). Taken together, these data suggest that an increased oxidative stress at vascular level and in the brain parenchyma may predispose and underlie delirium development, with potential interaction between inflammation and oxidative stress.
The neuroendocrine hypothesis proposes that delirium reflects a reaction to acute stress. It is commonly accepted that stress can activate the hypothalamic–pituitary–adrenal axis: stressors activate the paraventricular nucleus of the hypothalamus resulting in the release of corticotrophin-releasing hormone, which acts on the pituitary gland, releasing adrenocorticotrophic hormone, which promotes glucorticoid release from the adrenal gland (47). Under normal circumstances, glucorticoids act to help the body in coping with the demands imposed by stress exposure, but there is evidence demonstrating that glucorticoids secreted during stress can have deleterious effects in the brain, inducing delirium and cognitive dysfunction (64, 65). Current evidence also suggests that the highly catabolic glucorticoids induce a general metabolic vulnerability in brain neurons and thus compromise their ability to survive various toxic insults (66), indirectly suggesting that the effect of increased glucorticoid secretion may be not always reversible.
The pathophysiology of frailty is complex too. With aging, the muscle undergoes several changes in its structure and composition, which are in part related to both immunosenescence and inflamm-aging. For example, proteomic studies in senescent mice have reported an increase of iron load and changes in redox homeostasis, associated with a severe loss of muscle function and loss of satellite cell recruitment (67). Importantly, these changes appear to occur in parallel with biochemical, morphological, and functional changes including a decrease of myelinated and unmyelinated fibers, ballooning, splitting, and enfolding of myelinated fibers (68) and decreased axonal neurofilaments (69). Other studies have confirmed that metabolic and structural changes are common between muscle and nerve, suggesting that both tissues may share a common signaling associated with muscle and nerve decline (67, 70). Furthermore, a release of metabolites, amino acids, and a dysregulation of myokine signaling seem to be related to “inflamm-aging” (44) with increased cytokine release.
The imbalance in the cytokine network may influence frailty either directly by promoting protein degradation or indirectly by affecting important metabolic pathways (71). A recent meta-analysis including 32 cross-sectional studies and 23,910 older subjects has shown that frailty and pre-frailty (i.e., a condition which is thought to be intermediate between the normal and the frailty status) are associated with significantly higher than normal serum inflammatory parameters, including CRP, IL-6, white blood cell, and fibrinogen levels (72). In other studies, frailty was associated also with lower serum levels of IL-12 and IL-23, two interleukins that are able to modulate the production of other interleukins (i.e., IL-17 and IL-22) in lymphocytes as well as the rapid recruitment of neutrophils in stressful conditions (73). Importantly, these changes in inflammatory patterns are consistent in frail individuals across various geographical regions and are associated with a decreased muscle strength, resistance to physical exercise and walking distance as determined by the 6-min walking test (74).
Cytokines dysregulation is also related to the lack of response to some hormones and anabolic factors (75), which typically underlie frailty development (75). These hormones include the dehydroepiandrosterone sulfate (DHEA-S), testosterone, cortisol, and insulin-like growth factor-1 (IGF-1). The DHEA, in particular, plays an important role in the maintenance of muscle mass (76) and both increased cortisol serum levels and an increased cortisol: DHEA-S ratios in the serum are associated with a decline in individual’s functional performance (77). Interestingly, the cortisol serum levels are elevated in frail individuals as compared with non-frail individuals, and are associated with both an increased muscle breakdown rate (78) and loss of bone density (79). The same role seems to be exerted by IGF-1 that is related to the maintenance of muscle mass structure and muscle strength by the way to inhibit apoptosis and to lower the oxidative stress in muscle (80).
In addition to the above-depicted mechanisms, it has recently been shown that body composition might play a dual role as source of inflammatory stimulus (through endocrine secretion of pro-inflammatory adipokines) (81) and target of the negative effects (i.e., induction of catabolic, apoptosis, autophagic muscular pathways) (82). This is of particular importance since sarcopenia (i.e., a loss of muscle mass and strength and/or reduced physical performance) is a key component of frailty, if not its central biological substrate (83). In this context, IL-6 was identified as being produced by immune cells as well as by muscle and adipose tissue, as also suggested by the fact that its expression acutely increases during muscle contraction. In addition, IL-6 induces insulin resistance which suppresses activity of various intrinsic and extrinsic modulators of muscle synthesis (84). Another pro-sarcopenic effect of inflammation is related to the generation of cortisol within tissues. Cortisol is profoundly catabolic and can be synthesized from inactive cortisone by the actions of the enzyme 11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1). The activity of the (11β-HSD1) increases with age and is induced by cytokines, including TNF-α and IL-6. Interestingly, IL-6 has been indicated as a cytokine for gerontologists due to its contribution in the pathogenesis in multiple age-related conditions (83).
Review of Studies on Frailty and Delirium in Animals
Preclinical animal models may be of particular importance for the study of both frailty and delirium, given the absence of neuropathological studies in humans on these two conditions (Figure 1).
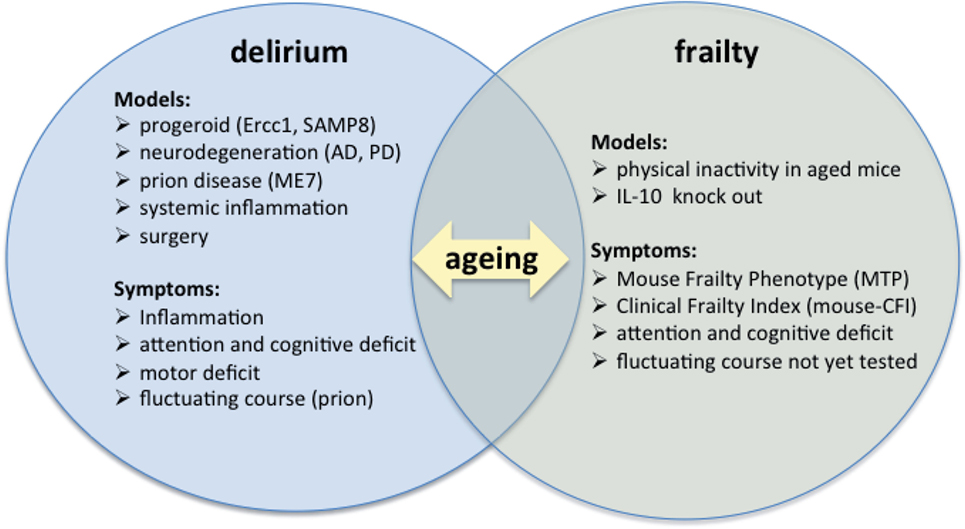
Figure 1. Preclinical models of frailty and delirium. Current studies on preclinical models of delirium and frailty must be put in the context of aging, as shown by the intersection of the two diagrams.
Animal models for delirium are substantially based on the neuroinflammatory hypothesis of delirium. A recent review by Hoogland et al. discuss the studies with animal experiments related to the effects of systemic inflammation on the microglial and inflammatory response in the brain (85). The authors identified 51 studies of which the majority was performed in mice (n = 30) or in rats (n = 19). Despite heterogeneity in the outcomes measures and in the methods used to assess microglial activation, these studies clearly showed that peripheral inflammatory stimuli can cause microglial activation. The authors also observed distinct differences in microglial activation between systemic stimulation with (supranatural doses) LPS and live or heat-killed bacteria (85). Another systematic review included not only studies with inflammatory challenge but also studies based on the effect of surgery (86). The effect of acute administration of bacterial endotoxin was reported in 29 comparative studies on normal animals (24 LPS and 5 Escherichia coli bacterial), 3 studies on progeroid model (Ercc1 mutant mouse deficient in DNA repair and the SAMP8 mouse, characterized by overproduction of amyloid precursor protein and oxidative damage), and 14 studies on disease models related with delirium (ME7 prion disease mice, Tg2576, 3_Tg, and APPswe Tg Alzheimer’s mice, Parkinson disease, basal forebrain cholinergic lesions) (87–89). Furthermore, the effects of different surgery procedures, such as clamping of the upper mesenteric artery, hepatectomy, laparotomy, splenectomy, and appendectomy, reported in 13 comparative studies were also included (10 in mice; 3 in rats) (90). It was found that, in a comparison of adult with young animals, acute peripheral challenges in old animals induce a highest inflammatory response. In particular, studies found that ageing was associated with (a) higher circulating (IL6, TNF-α, and IL10) and brain (IL1b, IL6, TNF-α) cytokines levels or transcripts, (b) increased activated microglia cells and astrocytes, and (c) sickness behavior and reduced cognitive skills with reduced performance at different tests including those evaluating to anxiety, attention or cognition, or activity (fear conditioning, water maze, novel object recognition, attentional set-shifting, social exploratory, general activity, or locomotor test). Considering the effect of surgery, the majority of studies reported an increase of brain proinflammatory cytokines (IL-1b, TNF-a, IL-6). Activation of microglia or astrocytes, increase of neuronal apoptosis, and loss of neuronal dendritic spine were also reported in some studies, confirming a tight link between primed microglial cells and neuronal plasticity (91). It should also be considered that neurodegeneration (or the presence of progeroid genetic defects) may anticipate the exaggerated inflammatory response that it is associated with a more rapid cognitive decline and disease progression. Administration of low doses of LPS (100 μg/kg) in ME7 animals, for example, induced transient working memory deficits with increased and prolonged transcription of inflammatory mediators in the brain. Results of these studies indicate that preexisting synaptic loss and microglial priming are predisposing factors for acute cognitive impairments provoked by systemic inflammation. In the same animal model, the peripheral administration of a single proinflammatory cytokine, like TNF-α in pre-symptomatic phase, is able to produce an exaggerated sickness behavior response but not neuronal death, synaptic loss, or hyperphosphorylation of tau. In a recent study, a fluctuating course of cognitive dysfunction was also reported in ME7 mice injected with 0.1 mg/kg LPS. LPS precipitated severe and fluctuating cognitive deficits in 16-week ME7 with a lower incidence or no deficits in 12-week ME7 and controls, respectively. Fluctuating impairments were associated with progressive thalamic synaptic loss and axonal pathology (14, 92).
Another relevant finding for delirium was obtained in a mouse model of lesioned basal forebrain cholinergic system, based on the administration of ribosomal toxin saporin linked to the p75 neurotrophin receptor. In this model, cognitive deficits induced by systemic LPS are restored by donepezil. However, in this model no signs of increased brain inflammation were detected, suggesting that factor other than primed microglial cells may be involved in the development of cognitive dysfunction and that neuronal vulnerability may represent predisposing factor to peripheral inflammation associated cognitive impairment (87).
Various tools for the assessment of frailty have been developed in mice, based on the assessment tools used in humans (93). Parks et al. proposed a preclinical frailty Index scale based on the assessment of deficits in activity skills, body composition, metabolic status, and vascular system (94). Whitehead and colleagues developed a mouse Clinical Frailty Index (mouse-CFI) assessment tool based on a 31 different impairments and deficits (95) and Liu et al. developed the Mouse Frailty Phenotype (MTP) scale, including grip strength, speed in walking, physical activity, and endurance (96). These tools have demonstrated to be consistent in frailty assessment in that (a) their scores increase in severity with aging in both male and female; (b) the increase in score severity which is observed with aging is similar to that observed in humans; and (c) the age-associated changes in myocytes are more prominent in animals with elevated frailty scores than in others. In a recent study (97), the mouse-MTP scale and the mouse-CFI were compared in a group of mice aged 23–24 months. Using the first tool, none of the mice was classified as frail. On the contrary, the second tool classified 16.6% of mice as frail. As indicated by the authors, similar difference in estimating the true incidence of frailty can be found when the frailty phenotype model and the frailty index tools are compared in humans: indeed, 6–16% of older adults (70–85 years old) are defined as frail with the phenotype-model tool and 22–32% with the FI (65 years and older) (98, 99). Despite the different sensitivity between the tools, however, both are able to detect frailty at preclinical level (93). Based on the known association between physical activity and frailty, Gomez-Cabrera et al. proposed physical inactivity as a mouse model of frailty (100). They adapted to animals the frailty phenotype developed for human and defined a score (the Valentia score for frailty evaluation) to be used in mice. Scores were calculated on the basis of five different components, such as weight loss (change in body weight), weakness (grip strength), poor endurance and slowness (incremental treadmill test), and low activity level (motor coordination), and were expressed with frailty scores similar to these defined for human being. Sedentary and wheel runner animals were compared in longitudinal study until the age of 28 months. Results of the study indicate that sedentary animals become frail as they get older whereas lifelong spontaneous exercise signicantly retards the onset of frailty.
Another animal model for frailty is the IL-10 (tm/tm) mouse developed by Walston (101). The lack of the anti-inflammatory cytokine interleukin-10 (IL-10) makes this animal more susceptible to the activation of inflammatory pathway activation. With aging, this mouse shows higher than normal levels of circulating IL-6, reduced muscular strength, impaired skeletal muscle ATP kinetics and cardio-vascular functions, and increased expression of gene associated with the regulation mitochondrial function and apoptosis (102).
Conclusion and Future Directions
There is initial evidence that frailty and delirium might share common pathophysiological links and are strictly interrelated from a clinical perspective. Altered inflammatory status is clearly involved in the pathophysiology of both frailty and delirium. Muscle and adipocytes may be a source of inflammatory stimulus (through endocrine secretion of pro-inflammatory adipokines) and also a target of the negative effects (i.e., induction of catabolic, apoptosis, autophagic muscular pathways) (81, 82). The inflammatory markers produced at the muscle and adipocytes level, on one side, may enter the brain through neural and/or humoral pathways, priming microglia and other neuronal cells of the brain that react with overexpression of cytokines. The primed microglia are able, under these conditions, to promote neuronal dysfunction leading to cognitive and behavioral symptoms of delirium (85).
Another pathophysiological link may be at vascular level. Pericytes are spatially isolated contractile cells on capillaries and venules throughout the body, which are designated to control cerebral blood flow physiologically, and to limit blood flow after ischemia. In skeletal muscle, pericytes are located at the interstitium, where they can express typical markers associated with capillaries. Pericytes have also adipogenic and myogenic properties, contributing to muscle fat generation and lipotoxicity. It could be hypothesized that an exaggerated activation of these cells leads to increased inflammatory response and nitrosative stress in the muscle, thus contributing to sarcopenia, a key feature of frailty. At the brain level, pericytes may contribute to enhance the inflammatory response under specific circumstances, which may represent both a predisposing and a precipitating factor for delirium occurrence in frail subjects. Pericyte alterations may also be responsible for increased permeability of the blood brain barrier microvascular endothelium, which in turn may lead to an overexpression of inflammatory markers in the brain and overactivation of microglial cells (103). Indeed, it has been demonstrated an upregulation of proteins in the cerebrospinal fluid of delirious patients within clusters related to inflammation, protease inhibitors, chromogranins/secretogranins and apolipoproteins, triggered by infections, metabolic problems and adverse drug reactions (104). Increased oxidative stress and neuroendocrine abnormalities may also occur at both levels (i.e., at the body and brain levels), igniting a chain of reactions that include overexpression of cytokines and other inflammatory markers.
The review also suggests that the complex relationship between frailty and delirium should be thought as a dynamic and continuous cross-talk between the body and the brain. Future studies should therefore try to identify biomarkers specific for body cells and brain in frail individuals with delirium. An excellent example of the cross-talk between muscle and brain should be represented by the Brain-derived neurotrophic factor (BDNF). BDNF is strongly expressed in the brain (105), where it regulates neuronal development, synaptic plasticity, and influence memory (106) but it is also expressed in skeletal muscle, where it contributes to fat oxidation and modulates myogenesis inducing satellite cell activation and skeletal muscle regeneration (107, 108).
From a clinical perspective, frailty may be considered a risk factor for delirium, although full evidence is still lacking, and delirious individuals may be regarded by default as frail individuals. Moreover, delirium may be viewed, in some cases (e.g., when it persists for long time) as a precipitating factor for worsening frailty. Specific attention will therefore be paid by clinicians both on frailty assessment, since it may allow to anticipate delirium occurrence and to the systematic screening of delirium since it may help identifying individuals at risk of subsequent worsen of frailty.
With regard to animal models, future research is needed to identify a panel of biomarkers that should be relevant both in humans with delirium and in mouse models of frailty, specifically challenged with triggers causing delirium, in order to explore new pathophysiological pathways.
Author Contributions
All authors drafted and reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rutenberg AD, Mitnitski AB, Farrell SG, Rockwood K. Unifying aging and frailty through complex dynamical networks. Exp Gerontol (2017). doi:10.1016/j.exger.2017.08.027
2. Cesari M, Marzetti E, Thiem U, Pérez-Zepeda MU, Abellan Van Kan G, Landi F, et al. The geriatric management of frailty as paradigm of “The end of the disease era”. Eur J Intern Med (2016) 31:11–4. doi:10.1016/j.ejim.2016.03.005
3. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc (2013) 14(6):392–7. doi:10.1016/j.jamda.2013.03.022
4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (2013) 381(9868):752–62. doi:10.1016/S0140-6736(12)62167-9
5. Jazwinski SM, Kim S. Metabolic and genetic markers of biological age. Front Genet (2017) 8:64. doi:10.3389/fgene.2017.00064
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed. Washington DC: American Psychiatric Publishing (2013).
7. Meagher DJ, Leonard M, Donnelly S, Conroy M, Saunders J, Trzepacz PT. A comparison of neuropsychiatric and cognitive profiles in delirium, dementia, comorbid delirium-dementia and cognitively intact controls. J Neurol Neurosurg Psychiatry (2010) 81(8):876–81. doi:10.1136/jnnp.2009.200956
8. Bellelli G, Morandi A, Di Santo SG, Mazzone A, Cherubini A, Mossello E, et al. "Delirium Day": a nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med (2016) 14(1):106. doi:10.1186/s12916-016-0649-8
9. Pendlebury ST, Lovett NG, Smith SC, Dutta N, Bendon C, Lloyd-Lavery A, et al. Observational, longitudinal study of delirium in consecutive unselected acute medical admissions: age-specific rates and associated factors, mortality and re-admission. BMJ Open (2015) 5(11):e007808. doi:10.1136/bmjopen-2015-007808
10. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet (2014) 383(9920):911–22. doi:10.1016/S0140-6736(13)60688-1
11. Bellelli G, Speciale S, Morghen S, Torpilliesi T, Turco R, Trabucchi M. Are fluctuations in motor performance a diagnostic sign of delirium? J Am Med Dir Assoc (2011) 12(8):578–83. doi:10.1016/j.jamda.2010.04.010
12. Guerini F, Frisoni G, Morghen S, Speciale S, Bellelli G, Trabucchi M. Clinical instability as a predictor of negative outcomes among elderly patients admitted to a rehabilitation ward. J Am Med Dir Assoc (2010) 11(6):443–8. doi:10.1016/j.jamda.2009.10.005
13. Morandi A, Davis D, Fick D, Turco R, Boustani M, Lucchi E, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J Am Med Dir Assoc (2014) 15(5):349–54. doi:10.1016/j.jamda.2013.12.084
14. Davis DH, Skelly DT, Murray C, Hennessy E, Bowen J, Norton S, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry (2015) 23(4):403–15. doi:10.1016/j.jagp.2014.08.005
15. Morandi A, Lucchi E, Turco R, Morghen S, Guerini F, Santi R, et al. Delirium superimposed on dementia: a quantitative and qualitative evaluation of informal caregivers and health care staff experience. J Psychosom Res (2015) 79(4):272–80. doi:10.1016/j.jpsychores.2015.06.012
16. Morandi A, Lucchi E, Turco R, Morghen S, Guerini F, Santi R, et al. Delirium superimposed on dementia: a quantitative and qualitative evaluation of patient experience. J Psychosom Res (2015) 79(4):281–7. doi:10.1016/j.jpsychores.2015.07.010
17. Afilalo J, Kim S, O’Brien S, Brennan JM, Edwards FH, Mack MJ, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol (2016) 1(3):314–21. doi:10.1001/jamacardio.2016.0316
18. Liao Y, Flaherty JH, Yue J, Wang Y, Deng C, Chen L. The incidence of delirium after cardiac surgery in the elderly: protocol for a systematic review and meta-analysis. BMJ Open (2017) 7(3):e014726. doi:10.1136/bmjopen-2016-014726
19. Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis (2014) 210(8):1170–9. doi:10.1093/infdis/jiu258
20. Berman P, Hogan DB, Fox RA. The atypical presentation of infection in old age. Age Ageing (1987) 16(4):201–7. doi:10.1093/ageing/16.4.201
21. Jeong YM, Lee E, Kim KI, Chung JE, In Park H, Lee BK, et al. Association of pre-operative medication use with post-operative delirium in surgical oncology patients receiving comprehensive geriatric assessment. BMC Geriatr (2016) 16:134. doi:10.1186/s12877-016-0311-5
22. Balducci L. Frailty: a common pathway in aging and cancer. Interdiscip Top Gerontol (2013) 38:61–72. doi:10.1159/000343586
23. Winter A, Steurer MP, Dullenkopf A. Postoperative delirium assessed by post anesthesia care unit staff utilizing the Nursing Delirium Screening Scale: a prospective observational study of 1000 patients in a single Swiss institution. BMC Anesthesiol (2015) 15:184. doi:10.1186/s12871-015-0168-8
24. Hubbard RE, Story DA. Patient frailty: the elephant in the operating room. Anaesthesia (2014) 69(Suppl 1):26–34. doi:10.1111/anae.12490
25. Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc (2011) 59(Suppl 2):S262–8. doi:10.1111/j.1532-5415.2011.03674.x
26. Solfrizzi V, Scafato E, Seripa D, Lozupone M, Imbimbo BP, D’Amato A, et al. Reversible cognitive frailty, dementia, and all-cause mortality. The Italian Longitudinal Study on Aging. J Am Med Dir Assoc (2017) 18(1):89.e1–e8. doi:10.1016/j.jamda.2016.10.012
27. Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M, Mariani C, et al. Spontaneous reversion of mild cognitive impairment to normal cognition: a systematic review of literature and meta-analysis. J Am Med Dir Assoc (2016) 17(10):943–8. doi:10.1016/j.jamda.2016.06.020
28. Adamis D, Treloar A, Gregson N, Macdonald AJ, Martin FC. Delirium and the functional recovery of older medical inpatients after acute illness: the significance of biological factors. Arch Gerontol Geriatr (2011) 52(3):276–80. doi:10.1016/j.archger.2010.04.006
29. Rockwood K, Rockwood MR, Andrew MK, Mitnitski A. Reliability of the hierarchical assessment of balance and mobility in frail older adults. J Am Geriatr Soc (2008) 56(7):1213–7. doi:10.1111/j.1532-5415.2008.01773.x
30. Jung P, Pereira MA, Hiebert B, Song X, Rockwood K, Tangri N, et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg (2015) 149(3):869–75.e1–2. doi:10.1016/j.jtcvs.2014.10.118
31. Assmann P, Kievit P, van der Wulp K, Verkroost M, Noyez L, Bor H, et al. Frailty is associated with delirium and mortality after transcatheter aortic valve implantation. Open Heart (2016) 3(2):e000478. doi:10.1136/openhrt-2016-000478
32. Chew J, Lim WS, Chong MS, Ding YY, Tay L. Impact of frailty and residual subsyndromal delirium on 1-year functional recovery: a prospective cohort study. Geriatr Gerontol Int (2017). doi:10.1111/ggi.13108
33. Nguyen TN, Cumming RG, Hilmer SN. The impact of frailty on mortality, length of stay and re-hospitalisation in older patients with atrial fibrillation. Heart Lung Circ (2016) 25(6):551–7. doi:10.1016/j.hlc.2015.12.002
34. Eeles EM, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing (2012) 41(3):412–6. doi:10.1093/ageing/afs021
35. Hempenius L, Slaets JP, van Asselt DZ, Schukking J, de Bock GH, Wiggers T, et al. Interventions to prevent postoperative delirium in elderly cancer patients should be targeted at those undergoing nonsuperficial surgery with special attention to the cognitive impaired patients. Eur J Surg Oncol (2015) 41(1):28–33. doi:10.1016/j.ejso.2014.04.006
36. Joosten E, Demuynck M, Detroyer E, Milisen K. Prevalence of frailty and its ability to predict in hospital delirium, falls, and 6-month mortality in hospitalized older patients. BMC Geriatr (2014) 14:1. doi:10.1186/1471-2318-14-1
37. Pol RA, van Leeuwen BL, Visser L, Izaks GJ, van den Dungen JJ, Tielliu IF, et al. Standardised frailty indicator as predictor for postoperative delirium after vascular surgery: a prospective cohort study. Eur J Vasc Endovasc Surg (2011) 42(6):824–30. doi:10.1016/j.ejvs.2011.07.006
38. Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain (2012) 135(Pt 9):2809–16. doi:10.1093/brain/aws190
39. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc (2011) 59(Suppl 2):S301–4. doi:10.1111/j.1532-5415.2011.03679.x
40. Kiely DK, Jones RN, Bergmann MA, Murphy KM, Orav EJ, Marcantonio ER. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci (2006) 61(2):204–8. doi:10.1093/gerona/61.2.204
41. Speciale S, Bellelli G, Lucchi E, Trabucchi M. Delirium and functional recovery in elderly patients. J Gerontol A Biol Sci Med Sci (2007) 62(1):107–8. doi:10.1093/gerona/62.1.107 author reply 108
42. Bellelli G, Speciale S, Barisione E, Trabucchi M. Delirium subtypes and 1-year mortality among elderly patients discharged from a post-acute rehabilitation facility. J Gerontol A Biol Sci Med Sci (2007) 62(10):1182–3. doi:10.1093/gerona/62.10.1182
43. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci (2000) 908:244–54. doi:10.1111/j.1749-6632.2000.tb06651.x
44. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci (2014) 69(Suppl 1):S4–9. doi:10.1093/gerona/glu057
45. Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, et al. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des (2010) 16(6):609–18. doi:10.2174/138161210790883840
46. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin (2017) 33(3):461–519. doi:10.1016/j.ccc.2017.03.013
47. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry (2013) 21(12):1190–222. doi:10.1016/j.jagp.2013.09.005
48. Dantzer R. [Neurotropic effects of cytokines: at the limits of immunology and neurobiology]. Pathol Biol (Paris) (1994) 42(9):826–9.
49. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci (2008) 9(1):46–56. doi:10.1038/nrn2297
50. Besedovsky HO, del Rey A. Central and peripheral cytokines mediate immune-brain connectivity. Neurochem Res (2011) 36(1):1–6. doi:10.1007/s11064-010-0252-x
51. Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry (2009) 65(4):304–12. doi:10.1016/j.biopsych.2008.07.024
52. Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci (2003) 4(2):103–12. doi:10.1038/nrn1032
53. Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience (2009) 158(3):1062–73. doi:10.1016/j.neuroscience.2008.07.031
54. Beloosesky Y, Hendel D, Weiss A, Hershkovitz A, Grinblat J, Pirotsky A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol A Biol Sci Med Sci (2007) 62(4):420–6. doi:10.1093/gerona/62.4.420
55. de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res (2007) 62(5):521–5. doi:10.1016/j.jpsychores.2006.11.013
56. Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, et al. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci (2008) 63(2):184–9. doi:10.1093/gerona/63.2.184
57. Cerejeira J, Nogueira V, Luís P, Vaz-Serra A, Mukaetova-Ladinska EB. The cholinergic system and inflammation: common pathways in delirium pathophysiology. J Am Geriatr Soc (2012) 60(4):669–75. doi:10.1111/j.1532-5415.2011.03883.x
58. Huat TJ, Khan AA, Pati S, Mustafa Z, Abdullah JM, Jaafar H. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci (2014) 15:91. doi:10.1186/1471-2202-15-91
59. Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature (2013) 503(7475):267–71. doi:10.1038/nature12618
60. Seaman JS, Schillerstrom J, Carroll D, Brown TM. Impaired oxidative metabolism precipitates delirium: a study of 101 ICU patients. Psychosomatics (2006) 47(1):56–61. doi:10.1176/appi.psy.47.1.56
61. Schoen J, Meyerrose J, Paarmann H, Heringlake M, Hueppe M, Berger KU. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care (2011) 15(5):R218. doi:10.1186/cc10454
62. Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res (2014) 1550:1–8. doi:10.1016/j.brainres.2014.01.004
63. Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation (2011) 8:139. doi:10.1186/1742-2094-8-139
64. Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress (1996) 1(1):1–19. doi:10.3109/10253899609001092
65. O’Keeffe ST, Devlin JG. Delirium and the dexamethasone suppression test in the elderly. Neuropsychobiology (1994) 30(4):153–6. doi:10.1159/000119154
66. Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science (1985) 229(4720):1397–400. doi:10.1126/science.4035356
67. Capitanio D, Vasso M, De Palma S, Fania C, Torretta E, Cammarata FP, et al. Specific protein changes contribute to the differential muscle mass loss during ageing. Proteomics (2016) 16(4):645–56. doi:10.1002/pmic.201500395
68. Melcangi RC, Magnaghi V, Cavarretta I, Martini L, Piva F. Age-induced decrease of glycoprotein Po and myelin basic protein gene expression in the rat sciatic nerve. Repair by steroid derivatives. Neuroscience (1998) 85(2):569–78. doi:10.1016/S0306-4522(97)00628-3
69. Parhad IM, Scott JN, Cellars LA, Bains JS, Krekoski CA, Clark AW. Axonal atrophy in aging is associated with a decline in neurofilament gene expression. J Neurosci Res (1995) 41(3):355–66. doi:10.1002/jnr.490410308
70. Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci (2014) 6:208. doi:10.3389/fnagi.2014.00208
71. Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology (2009) 55(5):539–49. doi:10.1159/000211949
72. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev (2016) 31:1–8. doi:10.1016/j.arr.2016.08.006
73. Viña J, Tarazona-Santabalbina FJ, Pérez-Ros P, Martínez-Arnau FM, Borras C, Olaso-Gonzalez G, et al. Biology of frailty: modulation of ageing genes and its importance to prevent age-associated loss of function. Mol Aspects Med (2016) 50:88–108. doi:10.1016/j.mam.2016.04.005
74. Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax (2006) 61(1):17–22. doi:10.1136/thx.2005.041996
75. O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol (2008) 252(1–2):91–110. doi:10.1016/j.cellimm.2007.09.010
76. Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res (2004) 16(3):249–52. doi:10.1007/BF03327392
77. Berkman LF, Seeman TE, Albert M, Blazer D, Kahn R, Mohs R, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol (1993) 46(10):1129–40. doi:10.1016/0895-4356(93)90112-E
78. Seene T, Alev K. Effect of glucocorticoids on the turnover rate of actin and myosin heavy and light chains on different types of skeletal muscle fibres. J Steroid Biochem (1985) 22(6):767–71. doi:10.1016/0022-4731(85)90284-5
79. Mussolino ME. Depression and hip fracture risk: the NHANES I epidemiologic follow-up study. Public Health Rep (2005) 120(1):71–5. doi:10.1177/003335490512000112
80. Arvat E, Broglio F, Ghigo E. Insulin-Like growth factor I: implications in aging. Drugs Aging (2000) 16(1):29–40. doi:10.2165/00002512-200016010-00003
81. Prins JB. Adipose tissue as an endocrine organ. Best Pract Res Clin Endocrinol Metab (2002) 16(4):639–51. doi:10.1053/beem.2002.0222
82. Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol (2013) 45(10):2288–301. doi:10.1016/j.biocel.2013.06.024
83. Cesari M, Landi F, Calvani R, Cherubini A, Di Bari M, Kortebein P, et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin Exp Res (2017) 29:81–8. doi:10.1007/s40520-016-0716-1
84. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev (2017) 36:1–10. doi:10.1016/j.arr.2017.01.006
85. Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation (2015) 12:114. doi:10.1186/s12974-015-0332-6
86. Schreuder L, Eggen BJ, Biber K, Schoemaker RG, Laman JD, de Rooij SE. Pathophysiological and behavioral effects of systemic inflammation in aged and diseased rodents with relevance to delirium: a systematic review. Brain Behav Immun (2017) 62:362–81. doi:10.1016/j.bbi.2017.01.010
87. Field RH, Gossen A, Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci (2012) 32(18):6288–94. doi:10.1523/JNEUROSCI.4673-11.2012
88. Raj DD, Jaarsma D, Holtman IR, Olah M, Ferreira FM, Schaafsma W, et al. Priming of microglia in a DNA-repair deficient model of accelerated aging. Neurobiol Aging (2014) 35(9):2147–60. doi:10.1016/j.neurobiolaging.2014.03.025
89. Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, et al. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res (2000) 885(1):25–31. doi:10.1016/S0006-8993(00)02883-3
90. Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, et al. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun (2009) 23(1):46–54. doi:10.1016/j.bbi.2008.07.002
91. Li Z, Ma L, Kulesskaya N, Võikar V, Tian L. Microglia are polarized to M1 type in high-anxiety inbred mice in response to lipopolysaccharide challenge. Brain Behav Immun (2014) 38:237–48. doi:10.1016/j.bbi.2014.02.008
92. Murray CL, Skelly DT, Cunningham C. Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1β and IL-6. J Neuroinflammation (2011) 8:50. doi:10.1186/1742-2094-8-50
93. Kane AE, Hilmer SN, Mach J, Mitchell SJ, de Cabo R, Howlett SE. Animal models of frailty: current applications in clinical research. Clin Interv Aging (2016) 11:1519–29. doi:10.2147/CIA.S105714
94. Parks RJ, Fares E, Macdonald JK, Ernst MC, Sinal CJ, Rockwood K, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci (2012) 67(3):217–27. doi:10.1093/gerona/glr193
95. Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci (2014) 69(6):621–32. doi:10.1093/gerona/glt136
96. Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci (2014) 69(12):1485–91. doi:10.1093/gerona/glt188
97. Kane AE, Huizer-Pajkos A, Mach J, Mitchell SJ, de Cabo R, Le Couteur DG, et al. A comparison of two mouse frailty assessment tools. J Gerontol A Biol Sci Med Sci (2017) 72(7):904–9. doi:10.1093/gerona/glx009
98. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc (2012) 60(8):1487–92. doi:10.1111/j.1532-5415.2012.04054.x
99. Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc (2009) 57(5):830–9. doi:10.1111/j.1532-5415.2009.02225.x
100. Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, Garcia-Garcia FJ, Olaso-Gonzalez G, Salvador-Pascual A, et al. A New Frailty Score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J Gerontol A Biol Sci Med Sci (2017) 72(7):885–91. doi:10.1093/gerona/glw337
101. Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci (2008) 63(4):391–8. doi:10.1093/gerona/63.4.391
102. Akki A, Yang H, Gupta A, Chacko VP, Yano T, Leppo MK, et al. Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr) (2014) 36(1):21–30. doi:10.1007/s11357-013-9540-0
103. Rigor RR, Beard RS, Litovka OP, Yuan SY. Interleukin-1β-induced barrier dysfunction is signaled through PKC-θ in human brain microvascular endothelium. Am J Physiol Cell Physiol (2012) 302(10):C1513–22. doi:10.1152/ajpcell.00371.2011
104. Poljak A, Hill M, Hall RJ, MacLullich AM, Raftery MJ, Tai J, et al. Quantitative proteomics of delirium cerebrospinal fluid. Transl Psychiatry (2014) 4:e477. doi:10.1038/tp.2014.114
105. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci (2001) 24:677–736. doi:10.1146/annurev.neuro.24.1.677
106. Wisse BE, Schwartz MW. The skinny on neurotrophins. Nat Neurosci (2003) 6(7):655–6. doi:10.1038/nn0703-655
107. Matthews VB, Aström MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia (2009) 52(7):1409–18. doi:10.1007/s00125-009-1364-1
Keywords: frailty, delirium, older adults, review of literature pathophysiology, geriatric syndromes
Citation: Bellelli G, Moresco R, Panina-Bordignon P, Arosio B, Gelfi C, Morandi A and Cesari M (2017) Is Delirium the Cognitive Harbinger of Frailty in Older Adults? A Review about the Existing Evidence. Front. Med. 4:188. doi: 10.3389/fmed.2017.00188
Received: 17 August 2017; Accepted: 20 October 2017;
Published: 08 November 2017
Edited by:
Emanuele Marzetti, Fondazione Policlinico Universitario Agostino Gemelli, ItalyReviewed by:
Alice Laudisio, Università Campus Bio-Medico, ItalyLuis Miguel Gutierrez Robledo, Instituto Nacional de Geriatría, Mexico
Copyright: © 2017 Bellelli, Moresco, Panina-Bordignon, Arosio, Gelfi, Morandi and Cesari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Bellelli, Z2l1c2VwcGUuYmVsbGVsbGlAdW5pbWliLml0
 Giuseppe Bellelli
Giuseppe Bellelli Rosamaria Moresco
Rosamaria Moresco Paola Panina-Bordignon4
Paola Panina-Bordignon4 Beatrice Arosio
Beatrice Arosio Cecilia Gelfi
Cecilia Gelfi Matteo Cesari
Matteo Cesari