- 1Department of Dermatology and Venereology, First Affiliated Hospital of Kunming Medical University, Kunming, China
- 2Yunnan Provincial Hospital of Infectious Disease/Yunnan AIDS Care Center (YNACC), Anning, China
- 3Department of Medical Imaging, First Affiliated Hospital of Kunming Medical University, Kunming, China
- 4Department of Dermatology and Venereology, Jining Second People's Hospital, Jining, China
Sweet's syndrome and eosinophilic folliculitis are aseptic inflammatory dermatitis mainly because of infiltrated neutrophils and eosinophils on skin, respectively. These diseases rarely overlap or coexist in the same patient, especially co-occur in HIV infected patient. Here, we report a rare case of an AIDS patient who developed eosinophilic folliculitis and Sweet's syndrome within 1 month of initial antiretroviral therapy, presumably due to immune reconstitution inflammatory syndrome. The CD4+ T cell counts increased dramatically from 70 to 249 cells/μL within a period of 1 month. Interestingly, the patient was rapidly and strikingly responsive to thalidomide, which has anti-inflammatory, immune regulation, inhibition of neutrophil chemotaxis etc. Moreover, we focused our attention on discussing the clinical, pathological, and possible pathogenic aspects of the rare overlap of HIV complicated with neutrophilic and eosinophilic dermatosis.
Background
Eosinophilic folliculitis (EF) has been regarded as a significant marker of advanced AIDS (1), reportedly occurs in 9–10% HIV infected patients (2, 3). HIV-associated eosinophilic folliculitis (HIV-EF) was first reported in 1986 by Soeprono and Schinella (4), which is a variant of eosinophilic pustular folliculitis (EPF) (known as Ofuji disease) (5) and characterized by pruritic, erythematous, follicular papules distributed on face, trunk, and limbs. Histopathology of HIV-EF lesion shows eosinophil infiltration in the epithelium of the follicular infundibulum. The specific etiology and pathogenesis of HIV-EF is still unclear. Recently, more reports have suggested HIV-EF is associated with immune reconstitution inflammatory syndrome (IRIS) after commencing anti-retroviral therapy (ART) (6–9), especially when patients with a low baseline CD4 cells count increased rapidly after ART. Maybe due to immune reconstitution recognized antigens from past or ongoing infections (8). Furthermore, accumulated evidence confirmed that Th2 shift and produced cytokines/chemokines play a role in Ofuji disease and a possible pathogenic mechanism of HIV-EF (10), especially interleukin (IL)-4, IL-5, Eotaxin and intercellular adhesion molecule 1 (ICAM-1), which could promote activity, proliferation, and recruitment of eosinophils (11).
Another pro-inflammatory neutrophilic dermatoses (ND) with the predominance of mature neutrophils infiltrate diffusely in the papillary and upper reticular dermis on histopathology, is Sweet's syndrome (also known as acute febrile neutrophilic dermatosis). It can manifest with fever, neutrophilia, tender and painful skin lesions like pseudovesicular nodules and plaques on the face, neck, and upper extremities (12). It was initially proposed by British dermatologist Sweet in 1964 (13). The etiologies and pathogenesis may be multifactorial. It may be associated with tumor antigens (especially hematologic malignancies and underlying malignancy), drugs (granulocyte-colony stimulating factor), infections (bacterial, viral) etc. that can induce cytokine cascade (12). It is classified as classical, drug-induced, and malignancy-associated Sweet's syndrome (14). It has been increasingly reported with co-occurring immunodeficiencies (15, 16). However, Sweet's syndrome has not been well-established associations with HIV. Scattered cases had reported that Sweet's syndrome was associated with IRIS (6, 8, 9). In addition, the immunohistochemistry and serological tests suggested that Th1 (17) and Th17 cells (18) secreted pro-inflammatory cytokines (IL-2, INF-γ, and IL-17) played a dominant role in the pathogenesis of Sweet's syndrome, which could activate and recruit neutrophils to the site of inflammation. Besides, other neutrophil recruiters and activators, such as TNF-α, IL-6, and IL-8 are potential cytokine candidates in the pathogenesis of Sweet's syndrome (14, 19, 20). And some authors have speculated that IL-6 may also be a potential target for the treatment of ND (21).
Both eosinophilic folliculitis and Sweet's syndrome are chronic recurrent and have no specific treatment. But they are sensitive to anti-inflammatory drugs such as glucocorticoids and Non-steroidal anti-inflammatory drugs (NSAIDs) (5, 12). Here, we first reported a case of occurrence of eosinophilic folliculitis and Sweet's syndrome after ART. And first attempted successful treatment with thalidomide and discuss the clinical, pathological, and possible pathogenic aspects of the rare overlap of these three diseases.
Case Presentation
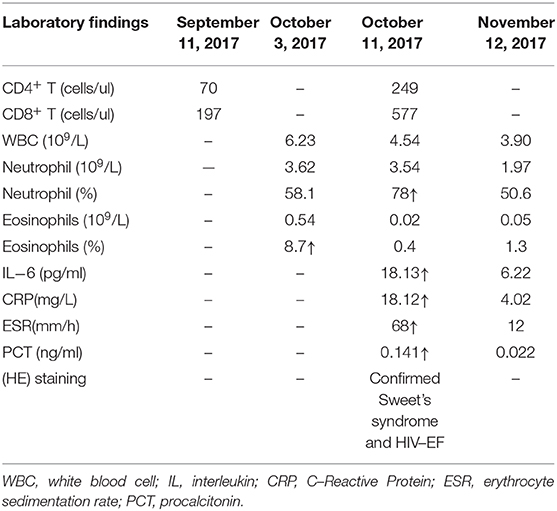
A 47-year-old Chinese woman confirmed HIV-seropositive for 6 years had a history of 3 years of irregular ART, and stopped taking any antiviral medications for the next 3 years because of non-compliance. In September 11 2017, as the CD4+ T cells counts was only 70 cells/μL and initiation of ART (tenofovir, lamivudine, and dolutegravir) was started. However, 3 weeks after the onset of ART, there were dense, red follicular papules with itching involving the face, neck and upper trunk, ranging from 2 to 5 mm in diameter (Figure 1a). Routine examination of blood showed white blood cell count of 6.23 × 109/L, the percentage of neutrophils and eosinophils were 58.10% and 8.7%, respectively. The counts of neutrophils and eosinophils were 3.62 × 109/L and 0.54 × 109/L, respectively. She hadn't received any treatment for skin lesions but continued ART.
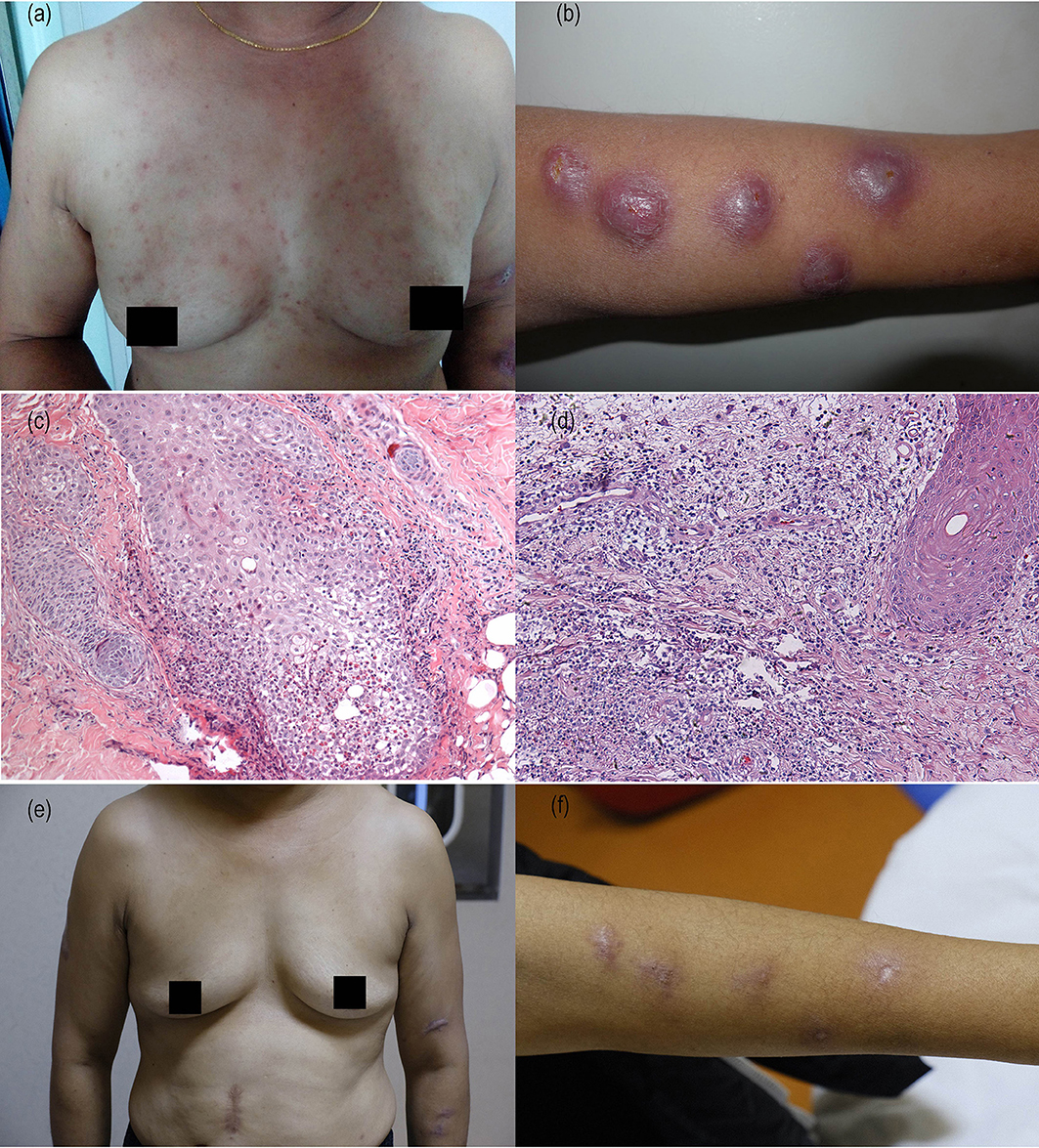
Figure 1. Clinical and histopathological images of the patient before and after treatment. (a) Neck and upper chest with erythema and red papules. (b) Left forearm with raised, pseudovesicular cysts, and infiltrated plaques. (c) Hematoxylin–eosin (HE) staining of skin biopsy from papule at back of neck showing edema of hair follicle epithelial cells in the dermis, surrounded by eosinophils, lymphocytes, and neutrophils inflammatory infiltration. And neutrophils and eosinophils were seen migrated into the hair follicle epithelium (original magnification ×100). (d) HE staining of skin biopsy from left forearm plaque showing obvious edema in the superficial dermis, neutrophils, and lymphocytes infiltrated diffusely in the superficial and middle layers of dermis, and nuclear sedimentation were present significantly (×100). (e) Neck and upper chest papules resolved completely. (f) Forearm with scars formation.
Then, she had persistent fever for 1 week and the highest temperature was 39°C. She then developed painful, raised, infiltrated plaques, and pseudovesicular on her left forearm (Figure 1b). Re-examination of blood (Table 1) revealed a white blood cell count of 4.54 × 109/L, with 78% neutrophils and 0.4% eosinophils. The neutrophil count was 3.54 × 109/L, the eosinophils count was 0.02 × 109/L; ESR was 68 mm/h, C reactive protein (CRP) was 18.12 mg/L, procalcitonin (PCT) was 0.141 ng/mL and IL-6 was 18.13 pg/ml. The CD4+ T lymphocytes counts increased sharply from 70 to 249 cells/μL (Table 1).
She underwent dermatopathology biopsies in two sites from her left forearm plaque and the back of neck papules. The pathological examination of the papules confirmed the diagnosis of eosinophilic folliculitis, edema of hair follicle epithelial cells in the dermis, surrounded by eosinophils, lymphocytes, and neutrophils inflammatory infiltration. And neutrophils and eosinophils were seen migrated into the hair follicle epithelium (Figure 1c). Pathological examination of the left forearm biopsy confirmed the diagnosis of Sweet's syndrome with obvious edema in the superficial dermis, neutrophils and lymphocytes infiltrated diffusely in the superficial and middle layers of dermis, and nuclear sedimentation were present significantly (Figure 1d). No obvious abnormality was found in the examination of lung, liver, renal etc. and without any other systemic manifestations.
Considering the CD4+ T cells count increased sharply, the IL-6 and C-reactive protein in peripheral blood were increased. Combined with clinical manifestations and pathological examination results, the patient was diagnosed as AIDS complicated with Sweet's syndrome and eosinophilic folliculitis. We speculate that the patient's symptoms were attributable to IRIS. The patient received thalidomide 100 mg/day combined with ART. On follow-up half month later, the follicular papules on face, neck and trunk were improved and about 1 month after the onset of treatment the plaques were also improved significantly (Figures 1e,f). The patient developed scars at the site of plaque on the forearm after treatment and are present till now. Re-examination laboratory tests showed CRP was 4.02 mg/L, PCT was 0.022 ng/mL and IL-6 was 6.22 pg/ml, all of which returned to their normal levels. The blood tests returned to normal (Table 1). ART was continued during the treatment of thalidomide. There is no sign of relapsing pruritic papules and plaque up till now.
Discussion
Anecdotally, Sweet's syndrome and eosinophilic folliculitis co-occurring with immunodeficiencies is very rare. It may be related through an overlap in immunopathogenesis. There are different postulated pathogeneses for them, but the patient suffered Sweet's syndrome followed by EF successively within 1 month of initial ART, accompanied by a sharp increase in CD4+ T cells. It indicated that her immunity state switched from disease of predominant Th2 activation—EF—to disease of predominant Th1 activation—Sweet's syndrome. There seems to be a shift of Th2 cytokines production and a decline in Th1 cytokines as CD4+ T cells decreased with AIDS progression (22), which may first contribute to EF, and then developed Sweet's syndrome with increase in Th1 cytokines secretion. In addition, rapid recovery of effector memory CD4+T cells in the first phase of CD4 cell recovery (23) also restored immunity to previously non-pathogenic infectious state (8, 14), possibly resulting in Sweet's syndrome and eosinophilic folliculitis. It still needs to be explored whether the rapidly increased and newly distributed CD4+ T cells could directly or indirectly regulate neutrophils and eosinophils and inflammatory agents and may contribute to Sweet's syndrome and EF.
ART restores the Th1/Th2 cell balance, which allows the adaptive immune system to overcome some dermatosis. However, new appearance or paradoxical worsening of existing dermatosis may attribute to dramatic immune restoration because of IRIS which occurs in about 15–25% of patients in the early stage of ART (24). IRIS is the excessive inflammatory response to the existing pathogens or underlying antigens in the early stage of ART. The increased levels of IL-6, TNF-a, and CRP can be potential biomarkers for IRIS (25, 26). The rapidly increased of circulating CD4 cells leads to the increase of Th1 and Th2 cells and their secreted cytokines (IL-4, IL-5, IL-6, IL-8, TNF-a ect) (22), which may promote the occurrence and development of Sweet's syndrome and eosinophilic folliculitis. Taken together, we speculate that the presence of Sweet's syndrome and EF after ART may be closely related to IRIS. Although it has been reported that ART drug abacavir can cause Sweet's syndrome (27). We affirmed that the patient suffered Sweet's syndrome was not caused by ART drugs, because the patient's skin lesions improved when the ART was not stopped during the treatment of thalidomide. At the same time, comprehensive laboratory tests have also ruled out malignancy related Sweet's syndrome.
There are many methods of treatment for HIV-EF and Sweet's syndrome, co-treatment included anti-inflammatory agents of glucocorticoids and NSAIDs. Thalidomide is a strong non-steroid anti-inflammatory drug and immunomodulatory which seems to have been forgotten or abandoned due to the side effects of “seal-leg deformity” since 1961 (28). Thalidomide is contraindicated in pregnant females because of its adverse teratogenic side effects. Prolonged administration of thalidomide can cause peripheral neuropathy, drowsiness and fatigue (29). In addition, venous thrombosis, neutropenia and cardiovascular side effects have also been reported in clinical practice (30). Therefore, baseline electrophysiological examinations, coagulation function, absolute neutrophil count, and cardiac function should be monitored carefully during thalidomide treatment.
Recently, it has been approved and used in the field of dermatology, such as leprosy nodular erythema, vascular lupus erythematosus, and refractory inflammatory bowel disease (29). Although thalidomide had been reported for treatment of HIV/AIDS-related oral ulcers (30) or Kaposi's sarcoma (31). But there have been no reports concerning thalidomide treatment for AIDS associated Sweet's syndrome or EF. Only one report on thalidomide had achieved good curative effect without relapse after ineffective systemic corticosteroids therapy in Sweet's syndrome associated with myelodysplasia (32).
Thalidomide could inhibit TNF-a, IL-1β, and IL-6 production (33, 34). This benefits not only by inhibiting SS and EF associated cytokines (IL-4, IL-5, IL-6, IL-8, TNF-a etc.), but also by inhibiting IRIS induced release of inflammatory cytokines (TNF-a, IL-6 etc.). More importantly, pro-inflammatory cytokine TNF-a could not only mediate the recruitment and activation of eosinophils by expressing adhesion molecules such as ICAM-1, but also activates and chemotactic neutrophils directly (35). Therefore, the use of TNF-a inhibitor infliximab or etanercept has been effective in the treatment of EF and Sweet's syndrome (36, 37). In addition, TNF-α induces viral replication via NF-κB-dependent transcriptional pathway (38), the anti-TNF activity of thalidomide may reduce HIV viral replication (39), which also benefits HIV infected patients. In this case, it significantly improved HIV associated with eosinophilic and neutrophilic dermatoses lesions, which means another weapon to be considered in the novel therapeutic strategies.
To our knowledge, this was the first report of AIDS patient co-incidental occurrence of Sweet's syndrome and eosinophilic folliculitis. Moreover, it was the first to report on the efficacy of old drug thalidomide for treatment of HIV-EF and Sweet's syndrome. This case provides a new perspective for the dialectical understanding of pharmaceutical value of thalidomide rather than its complete negation. Thalidomide may be an effective and safe treatment strategy for HIV patients with eosinophilic and (or) neutrophilic dermatitis.
Ethics Statement
The studies involving human participants were reviewed and approved by the studies involving human participants were reviewed and approved by The Ethics committe of First Affiliated Hospital of Kunming Medical University. The patients/participants provided their written informed consent to participate in this study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
R-JD, Y-YL, and Y-JZ contributed conception and design of the study. S-ZH performed the statistical analysis. SS wrote the first draft of the manuscript. PU wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
This work was partially supported by the Science and Technology Innovation Team of Sexually Transmitted Diseases of Kunming Medical University (2018HC005), the Joint Special Fund of Science and Technology Department of Yunnan—Kunming Medical University [2017FE467-(005)], the Medical Leadership Foundation of Health and Family Planning Commission of Yunnan Province, China (L-201613), the National Natural Science Foundation of China (81860553).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the patients and medical staffs participated in this study.
References
1. Meyer T, Lopez-Navarro N, Herrera-Acosta E, Gallego E, Bosch RJ, Herrera E. Human immunodeficiency virus (HIV)-associated eosinophilic folliculitis and follicular mucinosis in a black woman. Int J Dermatol. (2010) 49:1308–10. doi: 10.1111/j.1365-4632.2009.04257.x
2. Yokobayashi H, Sugaya M, Miyagaki T, Kai H, Suga H, Yamada D, et al. Analysis of serum chemokine levels in patients with HIV-associated eosinophilic folliculitis. J Eur Acad Dermatol Venereol. (2013) 27:e212–6. doi: 10.1111/j.1468-3083.2012.04592.x
3. Uthayakumar S, Nandwani R, Drinkwater T, Nayagam AT, Darley CR. The prevalence of skin disease in HIV infection and its relationship to the degree of immunosuppression. Br J Dermatol. (1997) 137:595–8. doi: 10.1111/j.1365-2133.1997.tb03793.x
4. Soeprono FF, Schinella RA. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. Report of three cases. J Am Acad Dermatol. (1986) 14:1020–2. doi: 10.1016/S0190-9622(86)70126-6
5. Ofuji S, Ogino A, Horio T, Oseko T, Uehara M. Eosinophilic pustular folliculitis. Acta Dermato-Venereol. (1970) 50:195–203.
6. Rajendran PM, Dolev JC, Heaphy MR Jr, Maurer T. Eosinophilic folliculitis: before and after the introduction of antiretroviral therapy. Arch Dermatol. (2005) 141:1227–31. doi: 10.1001/archderm.141.10.1227
7. Huiras E, Preda V, Maurer T, Whitfeld M. Cutaneous manifestations of immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS. (2008) 3:453–60. doi: 10.1097/COH.0b013e328302cefe
8. Bouscarat F, Maubec E, Matheron S, Descamps V. Immune recovery inflammatory folliculitis. AIDS. (2000) 14:617–8. doi: 10.1097/00002030-200003310-00019
9. Springinsfeld G, Roth B, Martinot M, Tortel MC, Batard ML. Immune reconstitution inflammatory syndrome associated with eosinophilic pustular folliculitis in an HIV-infected patient. Med Malad Infect. (2011) 41:49–50. doi: 10.1016/j.medmal.2010.09.003
10. Amerio P, Verdolini R, Proietto G, Feliciani C, Toto P, Shivji G, et al. Role of Th2 cytokines, RANTES and eotaxin in AIDS-associated eosinophilic folliculitis. Acta Dermato Venereol. (2001) 81:92–5. doi: 10.1080/00015550152384191
11. Akuthota P, Weller PF. Eosinophils and disease pathogenesis. Semin Hematol. (2012) 49:113–9. doi: 10.1053/j.seminhematol.2012.01.005
12. Cohen PR. Sweet's syndrome–a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. (2007) 2:34. doi: 10.1186/1750-1172-2-34
13. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. (1964) 76:349–56. doi: 10.1111/j.1365-2133.1964.tb14541.x
14. Heath MS, Ortega-Loayza AG. Insights into the pathogenesis of sweet's syndrome. Front Immunol. (2019) 10:414. doi: 10.3389/fimmu.2019.00414
15. Abbott JK, Gelfand EW. Common variable immunodeficiency: diagnosis, management, and treatment. Immunol Allergy Clin North Am. (2015) 35:637–58. doi: 10.1016/j.iac.2015.07.009
16. Elbuluk N, Martiniuk F, Levis WR. Erythema nodosum leprosum, Sweet's syndrome, and human immunodeficiency virus may be related through an overlap in immunopathogenesis. Int J Dermatol. (2010) 49:1344–5. doi: 10.1111/j.1365-4632.2009.04066.x
17. Giasuddin AS, El-Orfi AH, Ziu MM, El-Barnawi NY. Sweet's syndrome: is the pathogenesis mediated by helper T cell type 1 cytokines? J Am Acad Dermatol. (1998) 39:940–3. doi: 10.1016/S0190-9622(98)70266-X
18. Lauridsen HM, Pellowe AS, Ramanathan A, Liu R, Miller-Jensen K, McNiff JM, et al. Tumor necrosis factor-α and IL-17A activation induces pericyte-mediated basement membrane remodeling in human neutrophilic dermatoses. Am J Pathol. (2017) 187:1893–906. doi: 10.1016/j.ajpath.2017.04.008
19. Marzano AV, Cugno M, Trevisan V, Fanoni D, Venegoni L, Berti E, et al. Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin Exp Immunol. (2010) 162:100–7. doi: 10.1111/j.1365-2249.2010.04201.x
20. Marzano AV, Cugno M, Trevisan V, Lazzari R, Fanoni D, Berti E, et al. Inflammatory cells, cytokines and matrix metalloproteinases in amicrobial pustulosis of the folds and other neutrophilic dermatoses. Int J Immunopathol Pharmacol. (2011) 24:451–60. doi: 10.1177/039463201102400218
21. Marzano AV, Ortega-Loayza AG, Heath M, Morse D, Genovese G, Cugno M. Mechanisms of inflammation in neutrophil-mediated skin diseases. Front Immunol. (2019) 10:1059. doi: 10.3389/fimmu.2019.01059
22. Klein SA, Dobmeyer JM, Dobmeyer TS, Pape M, Ottmann OG, Helm EB, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. (1997) 11:1111–8. doi: 10.1097/00002030-199709000-00005
23. Smiatacz T. Immune mechanisms in HIV infection and their role in antiretroviral therapy. Przeglad Epidemiologiczny. (2003) 57:309–16.
24. Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. (2006) 42:418–27. doi: 10.1086/499356
25. Morlese JF, Orkin CM, Abbas R, Burton C, Qazi NA, Nelson MR, et al. Plasma IL-6 as a marker of mycobacterial immune restoration disease in HIV-1 infection. AIDS. (2003) 17:1411–3. doi: 10.1097/00002030-200306130-00025
26. Narendran G, Andrade BB, Porter BO, Chandrasekhar C, Venkatesan P, Menon PA, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS ONE. (2013) 8:e63541. doi: 10.1371/journal.pone.0063541
27. Del Giudice P, Vandenbos F, Perrin C, Bernard E, Marq L, Dellamonica P. Sweet's syndrome following abacavir therapy. J Am Acad Dermatol. (2004) 51:474–5. doi: 10.1016/j.jaad.2003.09.033
28. Neri P. The Pandora's box of thalidomide analogs and their substrates. Blood. (2019) 134:105–6. doi: 10.1182/blood.2019001420
29. Chen M, Doherty SD, Hsu S. Innovative uses of thalidomide. Dermatol Clin. (2010) 28:577–86. doi: 10.1016/j.det.2010.03.003
30. Ramirez-Amador VA, Esquivel-Pedraza L, Ponce-de-Leon S, Reyes-Teran G, Gonzalez-Guevara M, Ponce-de-Leon S, et al. Thalidomide as therapy for human immunodeficiency virus-related oral ulcers: a double-blind placebo-controlled clinical trial. Clin Infect Dis. (1999) 28:892–4. doi: 10.1086/515222
31. Little RF, Wyvill KM, Pluda JM, Welles L, Marshall V, Figg WD, et al. Activity of thalidomide in AIDS-related Kaposi's sarcoma. J. Clin. Oncol. (2000) 18:2593–602. doi: 10.1200/JCO.2000.18.13.2593
32. Browning CE, Dixon JE, Malone JC, Callen JP. Thalidomide in the treatment of recalcitrant Sweet's syndrome associated with myelodysplasia. J Am Acad Dermatol. (2005) 53(2 Suppl 1):S135–8. doi: 10.1016/j.jaad.2004.12.041
33. Kumar V, Chhibber S. Thalidomide: an old drug with new action. J Chemother. (2011) 23:326–34. doi: 10.1179/joc.2011.23.6.326
34. Casal JJ, Bollini M, Lombardo ME, Bruno AM. Thalidomide analogues: tumor necrosis factor-alpha inhibitors and their evaluation as anti-inflammatory agents. Eur J Pharmaceut Sci. (2016) 83:114–9. doi: 10.1016/j.ejps.2015.12.017
35. Ferrante A, Hauptmann B, Seckinger P, Dayer JM. Inhibition of tumour necrosis factor alpha (TNF-alpha)-induced neutrophil respiratory burst by a TNF inhibitor. Immunology. (1991) 72:440–2.
36. Hasegawa A, Kobayashi N, Fukumoto T, Asada H. A case of eosinophilic pustular folliculitis with response to infliximab. J Am Acad Dermatol. (2012) 67:e136–7. doi: 10.1016/j.jaad.2011.10.023
37. Yamauchi PS, Turner L, Lowe NJ, Gindi V, Jackson JM. Treatment of recurrent Sweet's syndrome with coexisting rheumatoid arthritis with the tumor necrosis factor antagonist etanercept. J Am Acad Dermatol. (2006) 54(3 Suppl 2):S122–6. doi: 10.1016/j.jaad.2005.11.1089
38. Pomerantz RJ, Feinberg MB, Trono D, Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. (1990) 172:253–61. doi: 10.1084/jem.172.1.253
39. Vergara TRC, Samer S, Santos-Oliveira JR, Giron LB, Arif MS, Silva-Freitas ML, et al. Thalidomide is associated with increased t cell activation and inflammation in antiretroviral-naive HIV-infected individuals in a randomised clinical trial of efficacy and safety. EBioMed. (2017) 23:59–67. doi: 10.1016/j.ebiom.2017.08.007
Keywords: AIDS, thalidomide, sweet's syndrome, eosinophilic folliculitis, IRIS
Citation: Dong R-J, Huang S-Z, Upadhyay P, Shrestha S, Zhai Y-J and Li Y-Y (2020) Thalidomide in the Treatment of Sweet's Syndrome and Eosinophilic Folliculitis Associated With Immune Reconstitution Inflammatory Syndrome. Front. Med. 6:343. doi: 10.3389/fmed.2019.00343
Received: 18 September 2019; Accepted: 27 December 2019;
Published: 21 January 2020.
Edited by:
Ivan V. Litvinov, McGill University, CanadaReviewed by:
Hassanin Al-Aasam, Luebeck University of Applied Sciences, GermanyFeras M. Ghazawi, University of Ottawa, Canada
Copyright © 2020 Dong, Huang, Upadhyay, Shrestha, Zhai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Ye Li, eXllbGkyMDAwQDEyNi5jb20=
 Rong-Jing Dong1
Rong-Jing Dong1 Samip Shrestha
Samip Shrestha Yu-Ye Li
Yu-Ye Li