- 1Department of Pathology, University of Jos, Jos, Nigeria
- 2Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
Background: It is a well-documented fact that world-wide cancer incidence and mortality remains high in Human Immunodeficiency Virus (HIV) infected population despite potent antiretroviral therapy. With the current capture of HIV status of cancer patients in our cancer registry at Jos Nigeria, this study aims to assess the effect of HIV on cancer mortality outcomes.
Methodology: We conducted a 2-year retrospective cohort study of cancer registry data from Jos, north central Nigeria. The cancers were grouped into cervical, breast, liver, hematologic, colonic, AIDS defining, prostate and others in this study. Patients were followed up to determine their patient time contribution from time at initiation of cancer treatment to death or the end of study period. Those lost to follow-up were censored at date of their last known follow-up in clinic.
Results: Out of 930 cancer cases evaluated, 52 (5.6%) were HIV positive, 507 (54.5%) were HIV negative and 371 (39.9%) did not know their HIV status. After 525,223 person- days of follow-up, there were 232 deaths leading to a crude mortality rate of 4.3 per 10,000 person-days. Median survival probability for both HIV-infected and HIV uninfected patients were equal (1,013 days). Unadjusted hazard of death was associated with greater age, HR 0.99 (95% CI: 0.98,0.99, p = 0.002); hepatitis virus, HR 2.40 (95% CI: 1.69,3.43, p = 0.001); liver cancer, HR 2.25 (95% CI:1.11,4.55, p = 0.024); prostate cancer, HR 0.17 (95% CI: 0.06,0.393, p = 0.001). In an adjusted model, only prostate cancer AHR 0.23 (95% CI: 0.12, 0.42, p < 0.001) and liver cancer AHR 2.45 (95% CI: 1.78, 5.51, p < 0.001) remained significantly associated with death regardless of HIV status.
Conclusion: Having liver cancer increases risk for mortality among our cancer patients. Screening, early detection and treatment are therefore key to improving dismal outcomes.
Introduction
Globally, of the total 9.6 million cancer deaths worldwide, 50.6% was accounted for by lung, colo-rectum, stomach, liver and breast cancers (1). Out of the 18.1 million world cancer incidence cases reported in 2018, lungs, breast, colorectal, prostate and stomach cancers accounted for 46.1% (1). According to Globocan 2018, there are 115,950 new cancer cases with 70,327 deaths due to cancer in Nigeria (2). Cancers of the breast, cervix uteri, prostate, non-Hodgkin lymphoma, liver, colon, ovary, rectum, leukemia and stomach are reported to be the top 10 commonest cancers in Nigeria in 2018 (2).
The absence of a reliable and quality cancer incidence data in low and middle income countries (LMICs) is well-documented bringing to bear the need for urgent and stringent actions at addressing this (2). Entrenched in the National Cancer Control plan of Nigeria (2018–2022) is the need to improve the existing cancer registration process in Nigeria through adequate funding and increasing the number of staffs of the various cancer registries (3). According to the National Agency for the control of AIDS (NACA) 2018, the national prevalence rate of HIV in Nigeria is 1.4% among adults aged 15–49 years (4). Though this rate has been reducing from the previous estimates of 2.8% the size of Nigeria's population means 1.9 million people were living with HIV in 2018 (4). Several published literature alludes to the fact that there is increased incidence of “HIV-associated cancers” amongst HIV population consisting of both the Acquired Immune Deficiency Syndrome(AIDS)- defining cancers (Kaposi sarcoma, aggressive B-cell non-Hodgkin lymphoma and cervical cancer) and non-AIDS-defining cancers (anal, liver, oral cavity/pharynx, lungs, and Hodgkin lymphoma) (5, 6). Thus, people infected with HIV are 19 times more likely to be diagnosed with anal cancer, 3 times as likely to be diagnosed with liver cancer, 2 times as likely to be diagnosed with lung cancer, about 2 times as likely to be diagnosed with oral cavity/pharynx cancer, and about 8 times more likely to be diagnosed with Hodgkin lymphoma compared with the general population (7). Survival outcomes are worse in HIV- associated than in the non-HIV cancer patients (5–7). The high population of people living with HIV in Jos, Nigeria and its known association with cancers necessitated this study which is to access the effect of HIV on cancer mortality.
Methods
Study Setting and Design
We conducted a 2-year retrospective cohort study from January 1, 2016 to March 31, 2018 using data on cancer patients from the population-based Jos cancer registry maintained by the Jos University Teaching Hospital (JUTH) in Jos, Plateau state Nigeria. Plateau state is located in Nigeria's middle belt with an area of 26,899 square kilometers and an estimated population of about 3 million people in 2019 (8). The registry receives cancer data from various health institutions in all the 17 local Government areas of the state. We also included admission and clinic records of these patients. Patients were followed starting at initiation of cancer treatment to occurrence of the primary outcome (death) or the end of study period. Those lost to follow-up were censored at date of their last known follow-up in clinic. We excluded patients who were pregnant and those with incomplete data.
Data Collection and Measures
Variables collected included HIV sero-status which is usually not part of the evaluated protocol at diagnosis of cancer but information on HIV status of the subject is usually elicited as known or not known and further categorized as positive or negative for those with known HIV sero status from previous HIV screening and confirmation. Cancer topography, hepatitis B and C status, mortality data, demographics (age, sex) and family history of cancer were obtained. The cancers were grouped into cervical, breast, liver, hematologic, colonic, AIDS defining, prostate and others in this study.
Socio-Demographic Variables Measured
Sex was either male or female; age was analyzed as both continuous and categorized variable; family history of cancer was made into a binary variable as present or absent.
Clinical Variables Measured
The main exposure variable was HIV sero-status defined by HIV antibody testing. Cancer topography was grouped into cervical, breast, liver, hematologic, colonic, AIDS defining, prostate and others in this study. Histologic tumor type was based on hematoxylin and eosin stain microscopic pathology report. The primary outcome variable was all-cause mortality and was obtained from clinic record sources and the cancer registry for those whose clinic records were absent.
Statistical Analysis
The analytical focus of this study was to determine all-cause probability of death. The overall mortality rate was estimated using total number of death events and the cumulative person time follow-up from initiation of cancer treatment to death. Differences in proportions and means of observable measures between the HIV-infected vs. HIV-uninfected patients were assessed using Chi-square test for proportions and t-test for difference of means. Cox proportional hazard analysis (unadjusted) was done where hazard ratio (HR) and 95% Confidence Intervals (CIs) were used as point and interval estimates of the effects of apriorically selected variables such as age, sex, cancer topography(cervical, breast, colonic, hematological, prostate, AIDS defining, liver and others), HIV infection and Hepatitis infection on all-cause mortality in the cancer patients. Cervical cancer though AIDS defining, was separated from others because of its high prevalence. Adjusted analysis was then conducted where only significant values with a hazard ratio not including 1 were used in the final cox model. All statistical tests were 2-sided with type 1 error set at 0.05 for statistical significance. Sex, HIV infection and Hepatitis infection were all treated as binary variables for determining predictors of death. To estimate the hazard of death following initiation of cancer treatment, we used time from initiation of cancer treatment in days as time covariate and death as failure event. Kaplan-Meier graphs estimating the probability of survival following initiation of cancer treatment by HIV status was plotted. The Log-rank test was used to assess differences in the probability of survival between the HIV positive and HIV negative cancer groups with p-value 0.05 signifying significant difference in survival. Statistical analysis was performed with STATA version 11.0 college station, Texas, USA.
Results
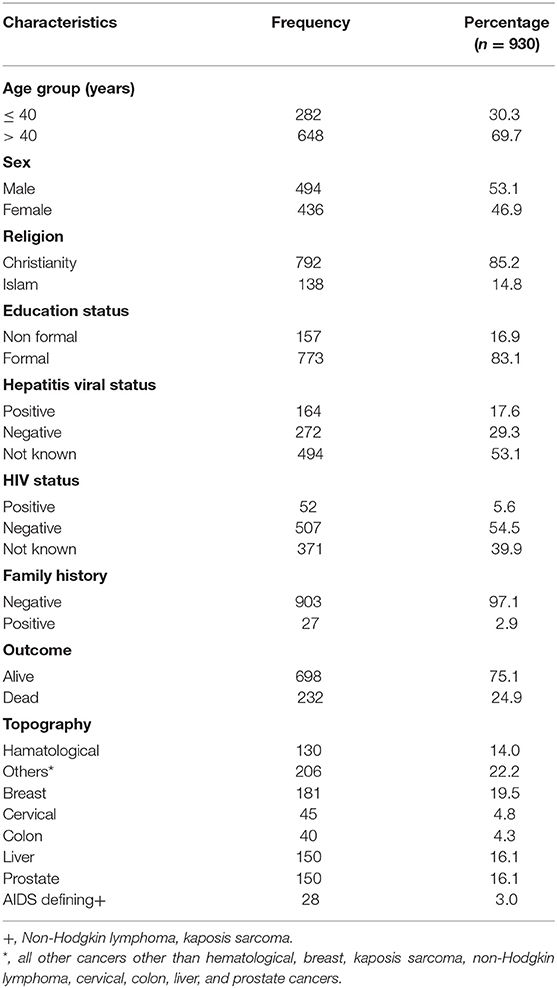
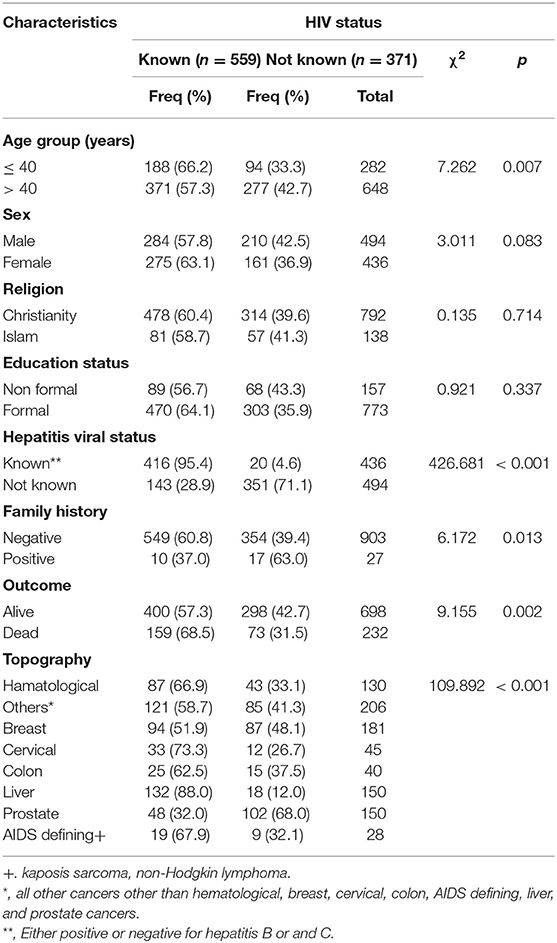
Out of 930 cancer cases evaluated, 52 (5.6%) were HIV positive, 507 (54.5%) were HIV negative and 371 (39.9%) did not know their HIV status. Thus, a total of 559 (60.1%) had their HIV status known either as positive or negative while testing for HIV after cancer diagnosis (Tables 1–3).
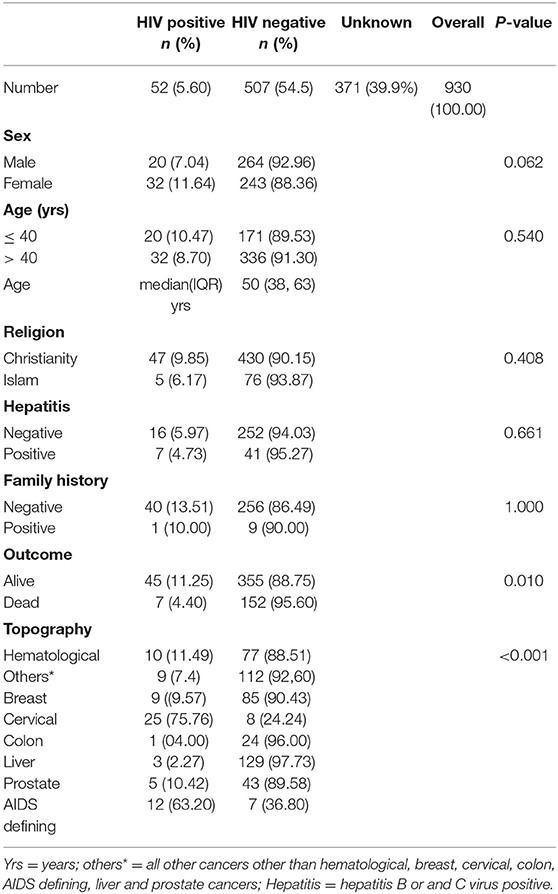
Table 3. A bivariate analysis of demographic/clinical characteristics data of cancer patients documented at the cancer registry from 01/01/2016 to 03/30/2018 by HIV status.
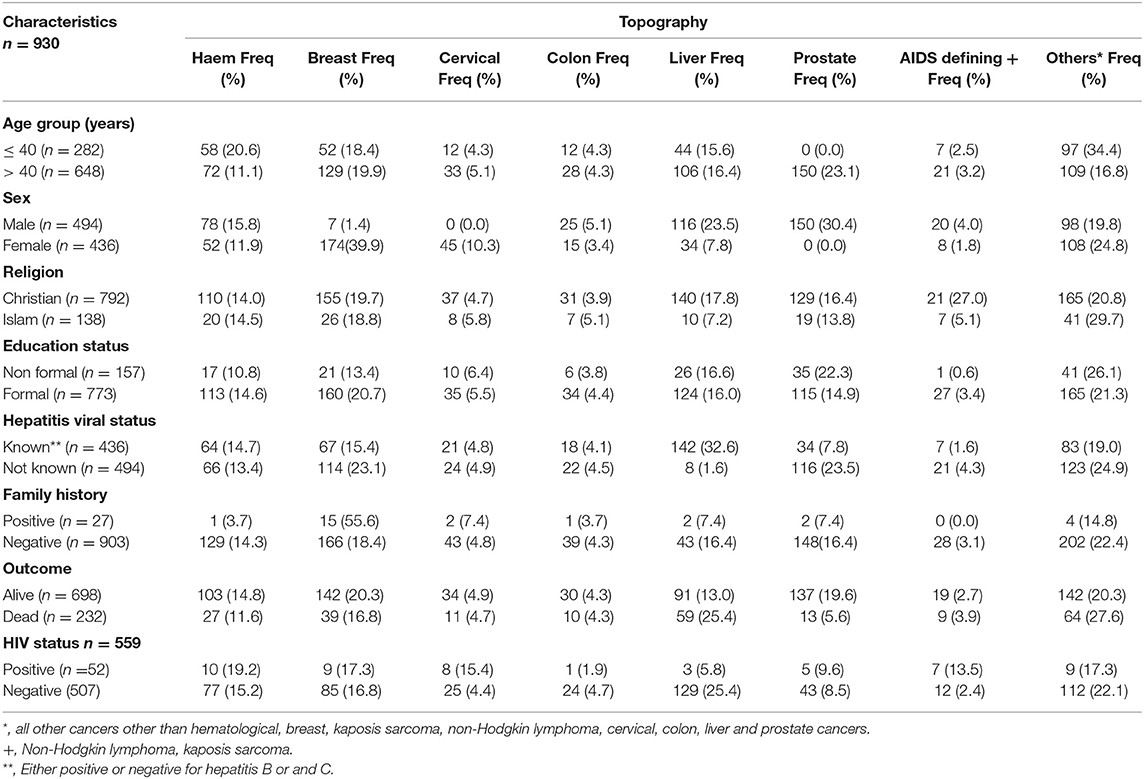
The commonest cancers irrespective of HIV status were breast cancer 181 (19.5%), liver cancer 150 (16.1%), prostate cancer 150 (16.1%), and cervical cancer 45 (4.8%). Proportions for other cancers known to be associated with HPV, EBV, and HHV8 were: oropharngeal cancers 15 (1.6%), Non-Hodgkin Lymphoma 10 (1.1%), and Karposis sarcoma 9 (0.9%), respectively, out of which over 70% occurred in HIV positive patients (Tables 1–3). Out of a total of 930 cancer cases evaluated 648 (69.7%) were of age group > 40 years and 494 (53.1%) were males (Tables 3 and 4). Median age of the participants was 50, 25 and 75% interquartile range is 38 and 63. After 525,223 person- days of follow-up, there were 232 deaths leading to a crude mortality rate of 4.3 per 10,000 person-days. Median survival probability for both HIV-infected and HIV uninfected patients were equal (1,013 days) (Figure 1). Unadjusted hazard of death was associated with greater age, HR 0.99 (95% CI: 0.98,0.99, p = 0.002); hepatitis virus, HR 2.40 (95% CI: 1.69,3.43, p = 0.001); liver cancer, HR 2.25 (95% CI: 1.11,4.55, p = 0.024); prostate cancer, HR 0.17 (95% CI: 0.06,0.39, p = 0.001) (Table 5). In an adjusted model, only liver cancer AHR 2.45 (95% CI: 1.17, 5.51, p < 0.001) and prostate cancer AHR 0.23 (95% CI: 0.12, 0.42, p < 0.001) using others as reference significantly predicted death regardless of HIV status (Table 5). The log-rank test for Kaplan-Meier graph was not significantly different between those with and without HIV infection (P = 0.072).
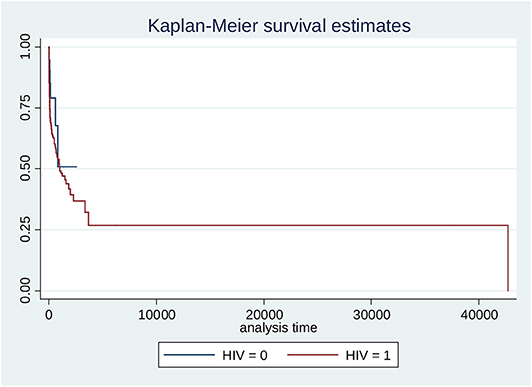
Figure 1. Kaplan Meier estimation of survival stratified by HIV status (HIV positive = black/0; HIV negative = red/1). Log-rank test for equality of survivor functions: chi2 = 3.23; Pr>chi2 = 0.0722.
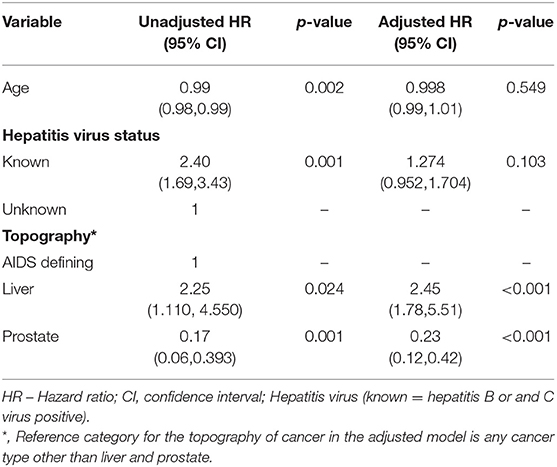
Table 5. Unadjusted and Adjusted analysis of characteristics of cancer patients using cox- proportional hazards.
Adjusted Cox Model
In an adjusted model, prostate cancer AHR 0.23 (0.12, 0.42, p < 0.001) and liver cancer AHR 2.45 (95% CI: 1.78, 5.51, p < 0.001) using others as reference significantly predicted death positively and negatively, respectively, regardless of HIV status (Table 5).
Kaplan-Meier Survival Graph for Separate HIV Groups (Figure 1)
The log-rank test for Kaplan-Meier graph was not significantly different between those with and without HIV infection (P = 0.072). Median survival probability for HIV-infected and HIV-uninfected group was 1,013 days each (Figure 1).
Discussion
In this 2-year retrospective cohort study, the significant proportion of cancer patients observed with unknown HIV status 371 (35.6%) could be attributed to the fact that testing for HIV status of cancer patients was not considered as a standard of care in the management of cancer patients in the various health institutions in our setting. It is now being advocated that healthcare providers should request for HIV testing for all cancer patients being managed as HIV has been well-documented to have a significant impact on ultimate survival outcomes of cancer patients (8–19). All those cases identified in this study with known HIV status had indicated their status in the course of cancer care and recorded in the registry as such.
Probability of survival among the HIV positive and HIV negative cohorts was not significantly different (Pr = 0.072). This is contrary to most reports on mortality after cancer diagnosis which showed higher mortality among HIV seropositive cancer patients than their HIV sero-negative counterparts (16–20). In a study on the contribution of HIV infection to mortality among cancer patients in Uganda, a more than 2-fold increased risk of death among the HIV-infected cancer patients compared to HIV- uninfected cancer patients was reported. Though in their study only five major cancers were considered and patients were at least 18 years of age (21). Most of the patients in this study were of age group > 40 years consistent with the age group with frequent age associated cancers as reported in other similar studies (22–26). Our relatively equal probability of survival between HIV positive and HIV negative cohorts could be attributed to the possible diffusing effect of unknown HIV status in this study as some of them could have been sero- positive. Also is the relatively low sample size of those with known HIV positive status.
Our study showed that patients with liver cancer were about three times more likely to die than those with other cancers regardless of HIV status. This might be due to inadequate screening facilities and challenges faced with early diagnosis of liver pathology especially in low and middle income countries (LMICs). However, surgical interventions having the possibility of prolonging lives at early stage diagnosis are also not readily available in this setting. Most patients with liver cancer thus present at advanced stages due to illiteracy, poverty and reliance on the readily available and cheap local traditional remedies (27, 28). There is also increased prevalence of risk factors of liver cancer in our communities such as aflatoxin, hepatitis B and C (27, 28). Probability of death due to prostate cancer however was low as fatality due to prostate cancer is generally known to be relatively rare.
Limitations of this study includes use of secondary observational data, small size of the HIV cohort, lack of important information that could impact survival such as actual mode of cancer treatment, cancer stage and grade and the unavailability of important HIV clinical data such as CD4 count and viral load.
In spite these limitations, our study has several strengths, to our knowledge this is the first study to determine effect of HIV on cancer mortality outcomes in our sub-region. This study has also revealed the fact that most of our patients are not aware of their HIV status. This underscores the need to increase HIV screening uptake in our population and the need to institute HIV screening as part of standard of care in cancer patients. Results of this study could serve as baseline for future large prospective studies that could be replicated in other regions of the country for generalizability.
Conclusion
Cancers have remained a vital cause of mortality in our setting with high HIV population thus necessitating screening and early detection to improve outcomes. Having liver cancer increases risk for mortality among our cancer patients. Hence, screening for premalignant lesions, early detection and treatment of cancers generally are therefore key to improving dismal outcomes. However, the contributions of HIV infection to cancer mortality still need to be further explored particularly if HIV testing is institutionalized as part of the standard of care for cancer patients and by the linkage of necessary HIV clinic data to the cancer registry data.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Jos University Teaching Hospital's Ethical committee. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
OS: conceptualized the study. JM, TA, CA, and LH: made technical inputs in the conceptualization and design of the study. OS, JM, CE, AS, and LH: produced the first draft of the manuscript. AS, RM, LH, and CE: contributed in further interpretation of findings and editing of the final draft of the manuscript. All co-authors contributed in revising the manuscript and approved the final version for submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work and publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U54CA221205 and by the Fogarty International Center of the National Institutes of Health under Award Number D43TW009575. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Thanks to Mr C. Wambuda, Mrs N. G. Nyango and Mrs R. J. Gwamson, Mr Chidi Okpara, Miss Ju Gye, and Mrs Florence Oyerimba of the Jos cancer registry for their support.
References
1. Nigeria source: Globocan (2018). Available online at: https://gco.iarc.fr/today/data/factsheets/populations/566-nigeria-fact-sheets.pdf (accessed September 26, 2019).
2. The Global Cancer Burden in 2018-World Cancer Congress (2018). Available online at: https://www.worldcancercongress.org/sites/congress/files/atoms/files/T3-121.pdf (accessed September 26, 2019).
3. National Cancer Control Plan 2018-2022 (2019). Available online at: https://www.iccp-portal.org/system/files/plans/NCCP_Final%20%5B1%5D.pdf (accessed September 26, 2019).
4. National Agency for Control of AIDS, Nigeria Prevalence Rate (2018). Available online at: https://naca.gov.ng/nigeria-prevalence-rate/ (accessed Septemper 26, 2019).
5. Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. (2017) 4:e495–504. doi: 10.1016/S2352-3018(17)30125-X
6. Fink VI, Jenkins CA, Castilho JL, Person AK, Shepherd BE, Grinsztejn B, et al. Survival after cancer diagnosis in a cohort of HIV-positive individuals in Latin America. Infect Agent Cancer. (2018) 8:13–6. doi: 10.1186/s13027-018-0188-3
7. Achenbach C, Cole S, Casper C, Kitahata M, Willig J, Mugavero M, et al. Mortality after cancer diagnosis among HIV-infected individuals in the CFAR network of integrated clinical systems (CNICS). Infect Agents Cancer. (2010) 5:A56. doi: 10.1186/1750-9378-5-S1-A56
8. National Bureau of Statistics Federal Republic of Nigeria. Population Census official Gazette FGP 71/52007/2,500(OL24); Legal Notice on Publication of the Details of the Breakdown of the National and State Provisional Totals 2006 Census (2006) Available online at: http://www.nigerianstat.gov.ng/connection/pop2006.pdf (accessed December 12, 2019).
9. Grover S, Desir F, Jing Y, Bhatia RK, Trifiletti DM, Swisher-McClure S, et al. Reduced cancer survival among adults with HIV and AIDS-defining illnesses despite no difference in cancer stage at diagnosis. JAIDS. (2018) 79:421–9. doi: 10.1097/QAI.0000000000001842
10. Gotti D, Raffetti E, Albini L, Sighinolfi L, Maggiolo F, Di Filippo E, et al. Survival in HIV-infected patients after a cancer diagnosis in the cART Era: results of an italian multicenter study. PLoS ONE. (2014) 9:e94768. doi: 10.1371/journal.pone.0094768
11. Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. (2011) 103:753–62. doi: 10.1093/jnci/djr076
12. Brickman C, Palefsky J. Cancer in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr HIV AIDS Rep. (2015) 12:388–96. doi: 10.1007/s11904-015-0283-7
13. Lewden C, Salmon D, Morlat P, Bévilacqua S, Jougla E, Bonnet F, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. (2005) 34:121–30. doi: 10.1093/ije/dyh307
14. Coghill AE, Engels EA. Are cancer outcomes worse in the presence of HIV infection? Cancer Epidemiol Biomark Prev. (2015) 24:1165–6. doi: 10.1158/1055-9965.EPI-15-0454
15. Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. (2008) 148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005
16. Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. (2009) 23:2337–45. doi: 10.1097/QAD.0b013e3283319184
17. Bonnet F, Burty C, Lewden C, Costagliola D, May T, Bouteloup V, et al. Changes in cancer mortality among HIV-infected patients: the mortalite 2005 survey. Clin Infect Dis. (2009) 48:633–9. doi: 10.1086/596766
18. Adamu PI, Adamu MO, Okagbue HI, Opoola L, Bishop SA. Survival analysis of cancer patients in north eastern nigeria from 2004. - 2017 - a kaplan - meier method. Open Access Maced J Med Sci. (2019). 7:643–50. doi: 10.3889/oamjms.2019.109
19. Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV cohort study. Br J Cancer. (2010) 103:416–22. doi: 10.1038/sj.bjc.6605756
20. Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess mortality among HIV-infected individuals with cancer in the United States. Cancer Epidemiol Biomark Prev. (2017) 26:1027–33. doi: 10.1158/1055-9965.EPI-16-0964
21. Coghill AE, Newcomb PA, Madeleine MM, Richardson BA, Mutyaba I, Okuku F, et al. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS. (2013) 27:2933–42. doi: 10.1097/01.aids.0000433236.55937.cb
22. Morlat P, Roussillon C, Henard S, Salmon D, Bonnet F, Cacoub P, et al. Cancer-related causes of death among HIV-infected patients in France in 2010: evolution since 2000. PLoS ONE. (2015) 10:e129550. doi: 10.1371/journal.pone.0129550
23. Zhang J, Huang R, Feng WL, Kong YN, Xu F, Zhao L, et al. Influence of occupation and education level on breast cancer stage at diagnosis, and treatment options in China: a nationwide, multicenter 10-year epidemiological study. Medicine. (2017) 96:e6641. doi: 10.1097/MD.0000000000006641
24. Vanthomme K, Vandenheede H, Hagedoorn P. Socioeconomic disparities in lung cancer mortality in Belgian men and women (2001-2011): does it matter who you live with? BMC Public Health. (2016) 2016:493. doi: 10.1186/s12889-016-3139-1
25. WHO. key Facts on STIs (2019). Available online at: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed August 7, 2019).
26. Milano AF, Singer RB. The cancer mortality risk project - cancer mortality risks by anatomic site: part 1 - introductory overview; part ii - carcinoma of the colon: 20-year mortality follow-up derived from 1973-2013 (NCI) seer*stat survival database. J Insur Med. (2017) 47:65–94. doi: 10.17849/insm-47-02-65-94.1
27. Oreagba IA, Oshikoya KA, Amachree M. Herbal medicine use among urban residents in lagos, Nigeria. BMC Complement Altern Med. (2011) 11:117. doi: 10.1186/1472-6882-11-117
Keywords: cancer registry, human immunodeficiency virus, mortality, cancer, Nigeria
Citation: Silas OA, Musa J, Afolaranmi TO, Sagay AS, Evans CT, Achenbach CJ, Hou L and Murphy RL (2020) Predictors of Mortality From a Population-Based Cancer Registry Data in Jos, Nigeria: A Resource-Limited Setting. Front. Med. 7:227. doi: 10.3389/fmed.2020.00227
Received: 16 December 2019; Accepted: 04 May 2020;
Published: 04 June 2020.
Edited by:
Antonio Recchiuti, Università degli Studi G. d'Annunzio Chieti e Pescara, ItalyReviewed by:
Maria Contaldo, University of Campania Luigi Vanvitelli, ItalyFabio Verginelli, University “G. d'Annunzio” of Chieti-Pescara, Italy
Copyright © 2020 Silas, Musa, Afolaranmi, Sagay, Evans, Achenbach, Hou and Murphy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olugbenga Akindele Silas, ZHJnYmVuZ2FAeWFob28uY29t
 Olugbenga Akindele Silas
Olugbenga Akindele Silas Jonah Musa1
Jonah Musa1 Tolulope Olumide Afolaranmi
Tolulope Olumide Afolaranmi Robert Leo Murphy
Robert Leo Murphy