- 1Department of Physical Medicine and Rehabilitation, Harvard Medical School, Boston, MA, United States
- 2The Dean Center for Tickborne Illness, Spaulding Research Institute, Spaulding Rehabilitation Hospital, Boston, MA, United States
- 3Invisible International, Cambridge, MA, United States
- 4Massachusetts General Hospital, Department of Pediatric Infectious Diseases, Boston, MA, United States
- 5Stroke Biological Recovery Laboratory, Spaulding Research Institute, Spaulding Rehabilitation Hospital, Boston, MA, United States
- 6Massachusetts General Hospital, Institute for Health Professions, School of Nursing, Charlestown, MA, United States
- 7William F. Connell School of Nursing, Boston College, Chestnut Hill, MA, United States
Context: Persistent fatigue, pain, and neurocognitive impairment are common in individuals following treatment for Lyme borreliosis (LB). Poor sleep, depression, visual disturbance, and sensory neuropathies have also been reported. The cause of these symptoms is unclear, and widely accepted effective treatment strategies are lacking.
Objectives: To identify symptom clusters in people with persistent symptoms previously treated for LB and to examine the relationship between symptom severity and perceived disability.
Methods: This was a retrospective chart review of individuals with a history of treatment of LB referred to The Dean Center for Tick-Borne Illness at Spaulding Rehabilitation Hospital between 2015 and 2018 (n = 270) because of persistent symptoms. Symptoms and functional impairment were collected using the General Symptom Questionnaire-30 (GSQ-30), and the Sheehan Disability Scale. Clinical tests were conducted to evaluate for tick-borne co-infections and to rule out medical disorders that could mimic LB symptomatology. Exploratory factor analysis was performed to identify symptom clusters.
Results: Five symptom clusters were identified. Each cluster was assigned a name to reflect the possible underlying etiology and was based on the majority of the symptoms in the cluster: the neuropathy symptom cluster, sleep-fatigue symptom cluster, migraine symptom cluster, cognitive symptom cluster, and mood symptom cluster. Symptom severity for each symptom cluster was positively associated with global functional impairment (p < 0.001).
Conclusion: Identifying the interrelationship between symptoms in post-treatment LB in a cluster can aid in the identification of the etiological basis of these symptoms and could lead to more effective symptom management strategies.
Key Message: This article describes symptom clusters in individuals with a history of Lyme borreliosis. Five clusters were identified: sleep-fatigue, neuropathy, migraine-like, cognition, and mood clusters. Identifying the interrelationship between symptoms in each of the identified clusters could aid in more effective symptom management through identifying triggering symptoms or an underlying etiology.
Introduction
Lyme borreliosis (LB) is caused by various tick-borne genospecies of the spirochete bacteria Borrelia burgdorferi sensu lato (1, 2) and is a multisystem, multi-stage disease. LB is the most common vector-borne illness in the US, and the number of cases has increased steadily over the last 25 years (3). Transmission of the B. burgdorferi spirochete from infected ticks to its human host begins with the translocation of B. burgdorferi from the gut to the salivary glands of infected ticks while feeding on its human host (4). At the time of initial presentation of LB, erythema migrans (commonly described as a “bullseye” rash or an expanding, homogeneously red rash), is observed in <60% of infected patients within 7–10 days at the site of the tick bite (5–8). The rash usually resolves within weeks, even in the absence of antibacterial therapy. Additional signs of acute disseminated LB include fever, fatigue, muscle and joint pain, headache, and lymphadenopathy (5). Notably, other tickborne infections such as Anaplasma and Ehrlichia can manifest with similar flu-like symptoms and when co-existing with B.burgdorferi, increase the severity of the presentation. If erythema migrans is absent at the onset of infection and the flu-like symptoms are presumed to be related to non-specific viral infection (9), LB can go undiagnosed and untreated for weeks, months, or even years. Importantly, the spirochete can enter the bloodstream and disseminate, often affecting the heart, joints, and nervous system (5). Lyme neuroborreliosis (LNB) is reported to occur in 10–15% of LB patients although this may be an underestimate, as Borrelia burgdorferi has been shown to disseminate to the central nervous system (CNS) very early in the course of acute disseminated infection with minimal if any clinical evidence of CNS involvement (10). Furthermore, a latent neuroborreliosis can exist for quite some time without significant symptoms, then present with late CNS involvement many months to years after initial infection and less characteristic symptoms (11). Symptoms may include facial paralysis and other cranial neuropathies, headache, neck stiffness, fatigue, paresthesias, meningeal signs, depression, anxiety disorders, peripheral nervous system problems, encephalitis or encephalomyelitis, chronic meningitis, and stroke secondary to cerebral vasculitis (11–18).
A subset of individuals with Lyme borreliosis go on to experience persistent or relapsing-remitting symptoms including fatigue, pain, and neurocognitive difficulties after treatment; an illness referred to as post-treatment Lyme disease syndrome (PTLDS). These chronic symptoms are of sufficient severity to impact quality of life and physical functioning (15, 19–23). The current definition of PTLDS, developed by the Infectious Diseases Society of America (IDSA), is clinician-documented Lyme borreliosis treated with standard antibiotic regimens, with onset of fatigue, widespread musculoskeletal pain, or cognitive difficulties within 6 months of Lyme disease diagnosis and with continuous or relapsing symptoms persisting for at least 6 months after treatment has ended (24). Risk factors for the development of persistent symptoms include a delay in diagnosis and treatment, the severity of the initial infection, incomplete recovery at 4-months post-treatment, and a history of relapse (15, 21, 22, 25, 26).
While mounting scientific evidence in the last decade points to potential persistence of the bacterium Borrelia after antibiotic treatment, in vitro and in vivo (27–32), there exists ongoing confusion and controversy in the literature around PTLDS symptoms, including their etiology and management. The benefits of additional antibiotic therapy for PTLDS have been debated. Significant gains in certain domains have been reported in open label prospective studies utilizing extended antibiotic courses (33, 34), as well as two of the four randomized controlled trials of regimens containing intravenous ceftriaxone (20, 35–37); however, the authors of both randomized controlled trials that found gains in select domains with intravenous ceftriaxone therapy ultimately concluded that their studies did not support general use of IV ceftriaxone for PTLDS (35, 36). Although statistically significant improvements were seen in certain domains, their conclusions were based on risks of treatment as well as—in one trial—the lack of sustained benefit in cognitive improvement after completion of therapy (36), or—in the other—the benefit being limited to sustained improvement in a single domain, fatigue, which despite being a primary outcome measure, was deemed a “nonspecific” symptom (35). Issues surrounding the design of the randomized controlled trials and interpretation of their results have been debated (38–40). Importantly, studies to date have not led to comprehensive consensus guidelines for diagnosis and management of PTLDS. This underscores the need to more fully characterize its varied symptoms with the aim of better understanding potential underlying mechanisms which, in turn, can help inform management decisions.
While continuous or remitting fatigue, musculoskeletal pain, and/or cognitive difficulties are predominant, patients with persistent symptoms following LB treatment frequently report a variety of other symptoms including poor sleep, depression, visual disturbance, and sensory neuropathies that can be similarly burdensome and may affect fitness and function (41, 42). The cause of these persistent symptoms is not known, although several mechanisms have been proposed, including the direct neurotoxic effects of the spirochete, neuroinflammation, or autoimmunity (4, 43–48).
A symptom cluster is defined as a group of two or more symptoms that co-occur and are interrelated (49). The identification of symptom clusters has been used extensively in chronic conditions, including cancer (50, 51), inflammatory bowel disease (52), chronic obstructive pulmonary disease (53), and multiple sclerosis (54), in which symptoms seldom occur individually. Identifying the interrelationship between symptoms in a cluster can aid in more effective symptom management. For instance, symptoms may cluster together through a shared etiology such as neuroinflammation (55) or because they share the same triggering symptom (56). Identifying symptom clusters in individuals who report persistent symptoms following treatment for LB could aid in more effective symptom management through identifying triggering symptoms or an underlying etiology. The purpose of this study was to identify symptom clusters in individuals with persistent symptoms following treatment for LB and to examine the relationship between symptom severity and perceived disability in this population.
Materials and Methods
Cases
A retrospective chart review was conducted to examine symptoms and disability in individuals with a history of treatment for LB who were referred to The Dean Center for Tick-Borne Illness at Spaulding Rehabilitation Hospital in Boston. At the Dean Center, all patients completed symptom and disability surveys, which were incorporated into their medical chart. In addition, all patients underwent a complete blood count (CBC) and chemistry, tests of kidney, liver, thyroid function, and HgBA1c to rule out disorders that could mimic post-treatment Lyme borreliosis symptomatology (i.e., hypothyroidism, anemia, diabetes, etc.). Patients had serological testing for co-infections that are known or postulated to be tick-borne (i.e., Babesia, Anaplsama, Ehrlichia, Rickettsia, and Bartonella), either through their referring physician or at our center, and those with evidence of infection were treated according to established clinical protocols. The analysis of co-infection data have been omitted from this report and will be the subject of a separate study. Between 2015 and 2018, two-hundred and seventy adults (≥18 years) were identified by medical chart review. The Institutional Review Board approved this retrospective chart review, and data was de-identified prior to analysis. Responsible Conduct of Research (National Institutes of Health; Massachusetts General Hospital) and the Health Insurance Portability and Accountability Act of 1996 Privacy Rule were observed.
Self-Report Symptoms and Functional Impairment
At the time of the first clinic encounter, each patient completed the 30-item General Symptom Questionnaire-30 (GSQ-30) which assesses symptom burden over the past 2 weeks (57) on a 0 to 4 scale where 0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, and 4 = very much. Patients also indicated whether any of the symptoms impaired their work, social, or family functioning, and if yes, which symptom was the most impairing. The GSQ-30 has shown excellent validity and internal consistency (57). The Sheehan Disability Scale (SDS) was also administered at the same time. The SDS is a widely used assessment of function in three domains: work/school, social life/leisure activities, and family life/home responsibilities (58). Each domain is scored using a 0–10 scale where 0 = not at all and 10 = extremely. The three domains are summed into a single-dimensional measure of global functional impairment with a range from 0 (no impairment) to 30 (highly impaired).
Statistical Analyses
All statistical analyses were performed using IBM SPSS version 24.0 (IBM) and R version 3.6.1. Descriptive statistics and frequency distributions were calculated for demographic and clinical characteristics. We used the standard Cronbachs α coefficient to determine reliability. We used exploratory factor analysis (EFA) with principal axis factoring to identify factors or “symptom clusters.” The key concept of EFA is that multiple items on the GSQ have similar patterns of responses across individuals because they are all associated with a latent (i.e., not directly measured) variable. Principal axis factoring with oblique rotation (Varimax) was used as the factor model with squared multiple correlations used to establish communalities. The Kaiser-Meyer-Olkin test, a measure of how suited our dataset was for EFA, verified the sampling adequacy for the analysis; KMO = 0.922. The number of factors was determined using a scree plot and the total percentage of variance explained by each factor with an eigenvalue greater than the average eigenvalue. A factor loading ≥0.4 was used to identify significant factors, with at least two items loaded in each cluster (59). As symptoms are complex and could be cross-loaded on more than one factor, the decision to retain the symptom on one factor was based on the significance of the loading and the conceptual and clinical relevance of the symptom. Each factor orsymptom cluster was assigned a name to reflect the possible underlying etiology. Three items were removed from the EFA due to insufficient variation in the occurrence of these symptoms: shortness of breath, feeling feverish, and sweats, and/or chills.
Results
Two-hundred and seventy adult cases were identified, of which 67.8% were female, with a mean age of 49 ± 14.8 years (Range 18–88) and 16.1 ± 1.4 years of education. For employment status, 12.7% were on disability or unemployed, 8.5% were retired, 6.8% were students, 2.5% were homemakers, and 69.6% were employed. The mean time since LB diagnosis and treatment was 10 ± 8.2 years (Range 1–43 years, median = 8 years).
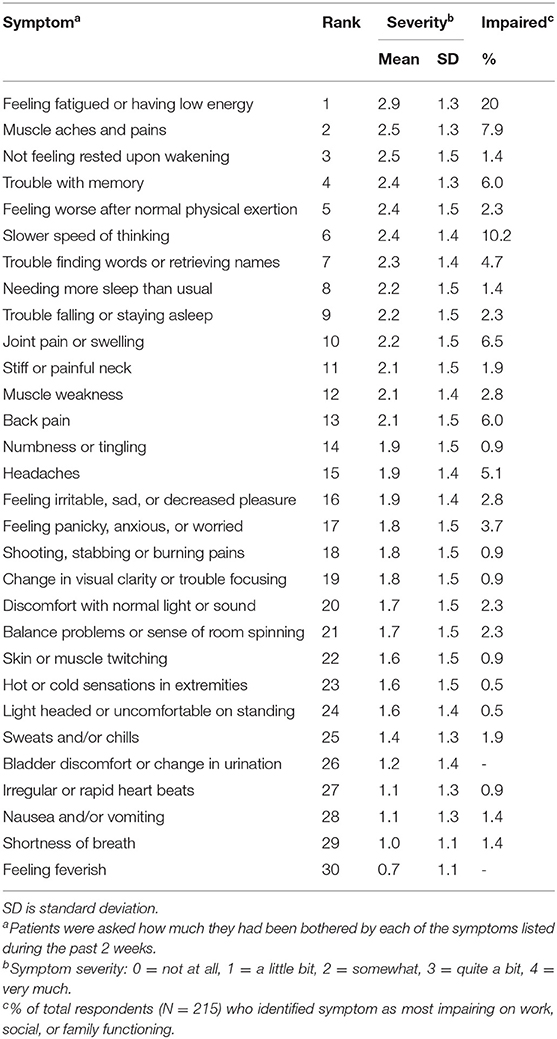
Table 1 shows the mean symptom severity scores and symptom rankings based on responses to each of the 30 questions on the GSQ-30. Potential scores ranged from 0 (Not at all) to 4 (Very much). The mean symptom severity score range for the total study population was 0.7 ± 1.1 for “feeling feverish” to 2.9 ± 1.3 for “feeling fatigued or having low energy.” Over 80% (n = 220) of patients reported “yes” when asked whether any of the symptoms impaired work, social or family function. The top five symptoms identified as the greatest cause of impaired work, social, or family function, making up over 50% of respondents, were feeling fatigued or having low energy, slower speed of thinking, muscle aches or pains, joint pain or swelling, and trouble with memory (Table 1).
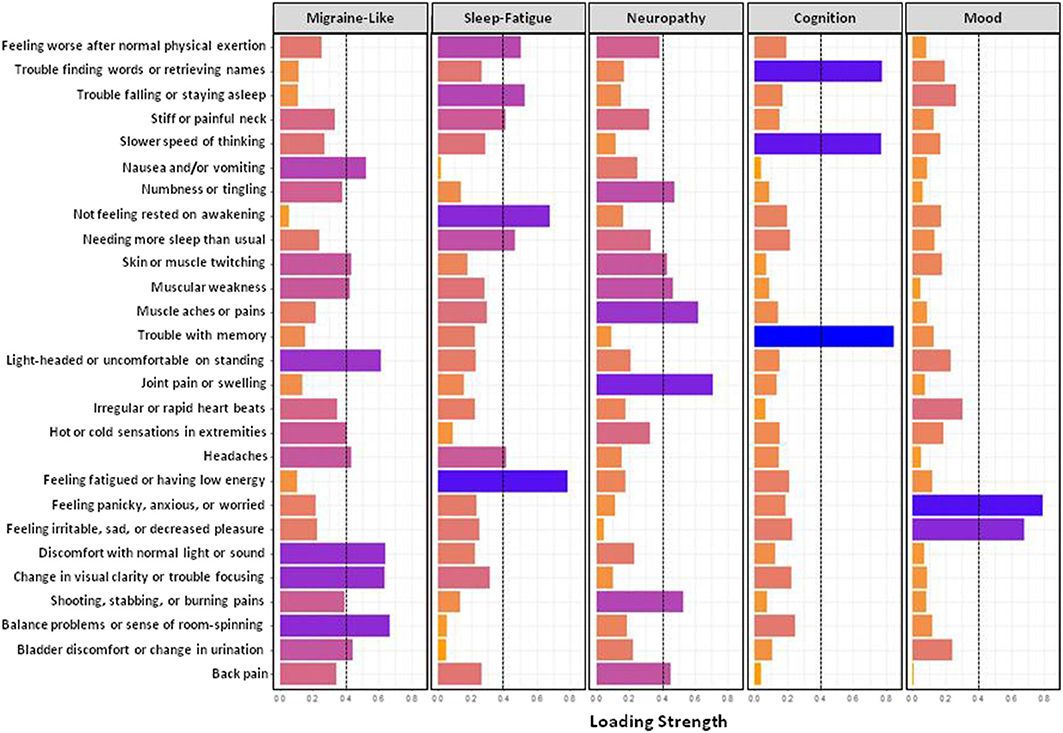
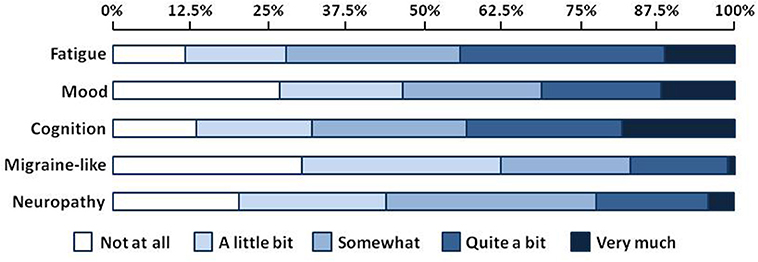
Figure 1 shows results from the exploratory factor analysis for responses on the GSQ-30 symptom survey in all patients. The six items in factor 1 (balance problems, discomfort with normal light and sound, nausea and/or vomiting, etc.) were called the migraine-like symptom cluster. The six items in factor 2 (feeling fatigued or having low energy, needing more sleep than usual, etc.) were called the sleep-fatigue symptom cluster. The eight symptoms in Factor 3 (i.e., muscle aches and pain, numbness and tingling, shooting, stabbing and burning pains, etc.) were called the neuropathy symptom cluster. The three symptoms in factor 4- trouble with memory, slower speed of thinking, and trouble finding words or retrieving names- were called the cognitive symptom cluster. Finally, the two items in factor 5, feeling panicky, anxious, or worried and feeling irritable, sad, or decreased pleasure, were called the mood symptom cluster. Figure 2 shows the percentage of patients who reported being bothered by symptoms in each of the five symptom clusters ranging from “not at all” to “very much.” Approximately 45% of patients reported that they were troubled quite a bit or very much by fatigue or cognitive difficulties. Mood symptoms were the next most troubling, with approximately 30% of patients reporting that they were bothered quite a bit of very much by these symptoms. Although migraine-like and neuropathic symptoms were the least troublesome, they were still troubling for approximately 20% of patients.
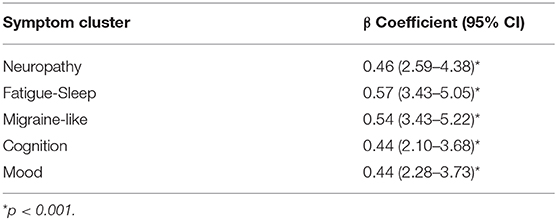
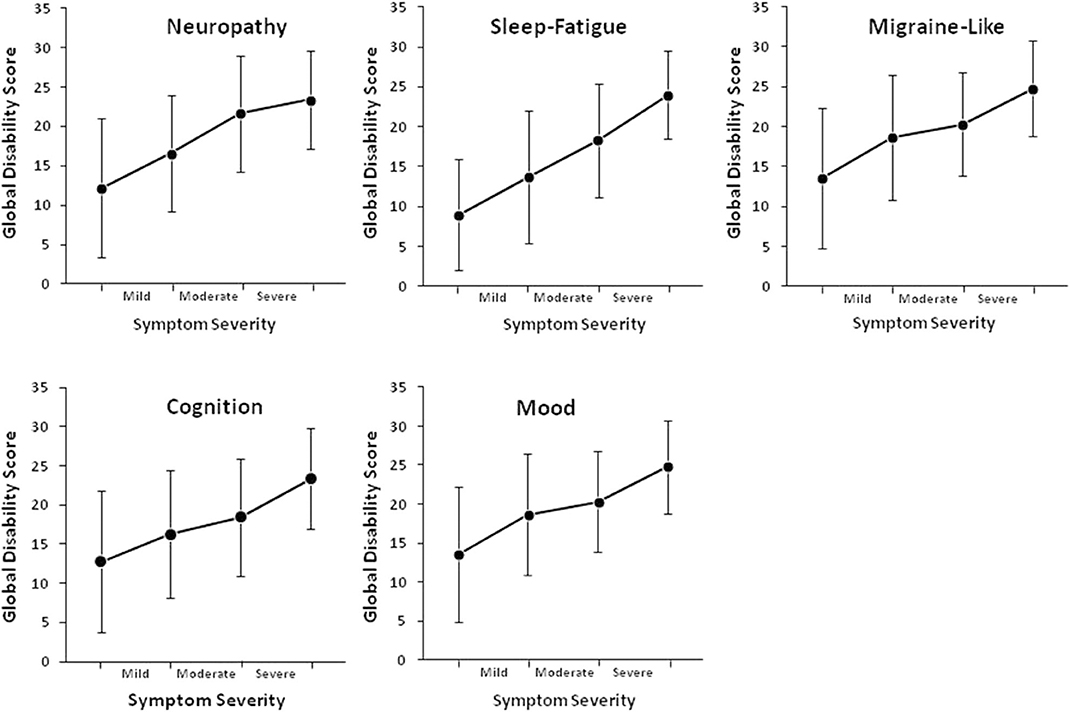
SDS data was available for 220 patients. Mean scores on the SDS work/school, social life/ leisure activities, and family life/home responsibilities domains were 5.7 ± 3.5, 6.5 ± 3.0, and 6.2 ± 3.1, respectively. The mean Global Functional Impairment score was 18.2 ± 8.9. Increasing symptom severity for each symptom cluster was linearly associated with greater global disability (p < 0.001, Table 2). Functional impairment increased when the severity of fatigue, cognitive, mood, and migraine-like symptoms increased from moderate to severe, and when neuropathy symptoms increased from mild to moderate (Figure 3).
Discussion
This is the first study to examine symptom clusters in a large cohort of individuals with persistent symptoms following treatment for Lyme borreliosis. The identification of symptom clusters may help us to identify mechanisms, and allow us to correlate clusters to specific infectious agents. We identified five symptom clusters, which we named the neuropathy, sleep-fatigue, migraine-like, cognitive, and mood symptom clusters. The sleep-fatigue symptom cluster included three items related to sleep quality: needing more sleep than usual, not feeling rested upon awakening, and trouble falling or staying asleep. Prior studies have reported poorer sleep in individuals with PTLDS compared to healthy controls (41, 60, 61), and self-reported fatigue and perceived poor sleep quality frequently co-occur in other chronic conditions, including cancer (62), diabetes (60, 63), and chronic fatigue syndrome (64). While there are frequent reports of sleep disruption in LB, only one study has examined both patient-reported and objectively measured sleep outcomes in this population. Greenberg et al. compared self-reported and polysomnographic assessment of sleep quality in LB patients and in matched healthy controls (65). Compared to healthy controls, LB patients reported greater difficulty falling asleep, more restless sleep, and increased daytime sleepiness (65). Objective sleep assessment revealed an increase in onset latency (time to fall asleep), decreased sleep efficiency (time in actual sleep divided by time attempting to sleep), and higher frequency of awakenings (65). Poor sleep quality could be the triggering symptom in this cluster since inadequate sleep would likely increase fatigue. Alternatively, individuals with high levels of fatigue, in particular, daytime sleepiness, could resort to daytime napping, which, in turn, could lead to fragmented and non-refreshing sleep at night. Further characterization of sleep deficits in people with persistent symptoms post LB are needed to develop strategies aimed at improving sleep and possibly fatigue in LB patients with documented sleep deficits.
The majority of the symptoms in the neuropathy symptom cluster were related to musculoskeletal pain and weakness, paresthesia, and hot and cold sensation in the extremities, symptoms common in small fiber neuropathy (SFN). In a recent study, Novak et al. examined SFN in individuals with persistent LB symptoms and found abnormal epidermal nerve fiber density (ENFD) in 90%, abnormal sweat gland nerve fiber density (SGNFD) in 50%, and both ENFD and SGNFD in 40% (42). Consistent with these sensorimotor deficits, many LB patients have been shown to have a reduced vibration threshold in their extremities. In the study by Rebman et al., approximately 30% of LB patients had scores below the age-adjusted cutoffs for vibration threshold in upper and lower extremities (41). Despite evidence of somatosensory deficits in the lower extremities, there have been no studies to date that have performed a detailed examination of gait and balance deficits in LB. Although the mechanism whereby LB causes SFN is not known, several mechanisms of neuronal injury have been proposed, including the direct neurotoxic effects of the spirochete, neuroinflammation, or autoimmunity (4, 43, 46, 48).
Neuroinflammatory processes may also underlie the symptoms found in the migraine symptom cluster, which included items related to visual disturbances, sensitivity to light or sound, balance problems, or being lightheaded or uncomfortable while standing. Indeed, we labeled this symptom cluster the migraine-like cluster because these symptoms are common in migraine. The overlap between migraine symptoms and those of post-treatment LB suggests that they may share a common mechanism. Migraine is a chronic neurological disorder that affects the central and peripheral nervous systems (66). Altered activity of the thalamic and thalamo-cortical areas contribute to aberrant sensory processing inherent in migraine, while MRI studies have demonstrated altered connectivity in a number of brain regions, including the cerebellum, hypothalamus, and brain stem (66). Recently the neurogenic inflammatory mediator calcitonin gene-related peptide (CGRP) has been implicated in the etiology of migraine (67). CGRP is produced by neurons in the CNS and the peripheral nervous system, where it acts as a vasodilator and inflammatory mediator acting via NF-κB (68–70). CGRP is released from neurons in response to a variety of environmental stimuli, including infectious agents such as B. burgdorferi (71). Consistent with its role in the etiology of migraine, individuals with acute migraine have elevated circulating levels of CGRP (72), and newly developed therapeutic monoclonal antibodies that inactivate circulating CGRP have proven efficacy in the treatment and prevention of migraine (67). Whether the same tools used to understand the cause of migraine could be applied to identify the cause of migraine-like symptoms in LB remains to be seen but could be a fruitful avenue for future investigation.
Items in the cognition symptom cluster included trouble with memory, trouble finding words or retrieving names, and slower speed of thinking. Self-reported cognitive deficits are frequently reported in LB and in other neurological and inflammatory conditions, including cancer, rheumatoid arthritis, and multiple sclerosis. However, self-reported cognitive deficits do not always correlate with performance on neuropsychological tests. Berende et al. found no association between self-reported cognitive difficulties in over two hundred LB patients and performance on objective tests of episodic memory, working memory / attention, verbal fluency, information-processing speed, and executive function (73). Less than 3% of participants had cognitive deficits based on neuropsychological testing, a rate comparable to the general population. Their findings were similar to the study by Kaplan et al. who similarly found no association between subjective cognitive difficulties and performance on tests of memory, attention, and executive functioning in 129 individuals with physician documented LB (74). Touradji et al. found that while over 90% of 124 LB patients reported cognitive difficulties, only 26% showed evidence of mild cognitive deficits in memory and processing speed (75). In contrast, Tager et al. reported significantly more objective cognitive deficits and psychiatric disturbances in children who developed new-onset cognitive complaints after Lyme disease compared with matched healthy controls (76). Objective cognitive deficits on neuropsychological evaluation, which included disturbances in visual and auditory processing and attention as well as in working memory and mental tracking, were still found after controlling for anxiety, depression and fatigue (76). Similarly, Keilp et al. observed statistically significant differences in several cognitive tests including tests of verbal comprehension, attention, executive function, working memory, and processing speed between patients with a history of LB and healthy controls (77). Like the Touradji study, Keilp et al. noted that the observed cognitive deficits were mild. Discordance between self-report and objectively measured cognitive function is not unique to LB and may reflect limitations in current neuropsychological testing which do not incorporate “real-world” demands on cognitive function. In the real world setting, cognitive tasks are frequently performed concurrently with motor tasks (i.e., walking while talking). While dual-tasking (i.e., performing cognitive and motor tasks concurrently) poses little problem for people with intact cognitive and sensorimotor function, it can be problematic for individuals with cognitive or motor deficits such as older adults and those with neurological conditions such as diabetes, stroke, or multiple sclerosis in which balance and cognitive deficits frequently co-exist (78–82). Given the reported sensorimotor deficits in LB patients, testing performance on cognitive tasks with a concurrent motor task may be a better indicator of cognitive decline than performance on a cognitive task administered alone under laboratory conditions. Further work is needed to fully understand the scope of neurocognitive problems in LB patients and to identify neural pathways that contribute to these deficits.
Finally, the two items in the mood symptom cluster- feeling panicky, anxious, or worried and feeling irritable, sad, or having decreased pleasure- are common symptoms of anxiety and depression. Symptoms of irritability and depression have been documented in LB (83–89) although it is unclear whether these symptoms are of sufficient severity to meet criteria for clinical anxiety/depression (89). A prior population-based retrospective cohort study did not show increased rates of depression in individuals with a history of LB and persistent symptoms compared to those without symptoms (19).
There are several limitations to the current study, including its retrospective, cross-sectional design. Data used in the analyses were extracted from the medical record and therefore lacked consistently documented clinical and demographic information that would typically be collected in a prospective research study. Although all patients seen at the clinic had a history of treatment for LB and were referred because of lingering symptoms, their charts lacked several pieces of information needed to determine whether they met the criteria for a diagnosis of PTLDS. Future studies should include a detailed analysis of the infectious origins of symptoms in these patients (i.e., multiplex PCR analysis combined with serology), which would allow us to determine whether specific clusters correlate to a particular infectious organism, or allow differential diagnoses. Because only clinical data from the initial clinic visit was used to create the symptom clusters, the stability of these clusters over time is not known. Future studies are needed to identify shared or distinct mechanisms, including distinct infectious organisms, that underlie these symptom clusters, which will aid in the development of new treatment strategies.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Spaulding Rehabilitation Hospital Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
NZ, CM, EB, and LW had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. LW and EB conducted the statistical analyses. LW and NZ take responsibility for the study's concept and design. LW, NZ, CM, and EB participated in the drafting of the manuscript. NZ and DC obtained funding. QW and RO provided administrative, technical, or material support. All authors contributed to the acquisition, analysis or interpretation of the data and all contributed to critical revision of the manuscript for important intellectual content.
Funding
This work was supported by funds from the Department of Physical Medicine and Rehabilitation, Harvard Medical School, Boston, Massachusetts, and by a generous donation from Mark and Eileen Lovell.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Christopher and Brandi Dean, Paul and Maria Selian, Michael and Lisa Bronner, Mark and Eileen Lovell, Ross Zafonte, and Glenn Bock for their support in completing this study.
References
1. Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. (2015) 29:187–210. doi: 10.1016/j.idc.2015.02.010
2. Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. (2016) 2:16090. doi: 10.1038/nrdp.2016.90
3. Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. (2014) 59:676–81. doi: 10.1093/cid/ciu397
4. Rupprecht TA, Koedel U, Fingerle V, Pfister HW. The pathogenesis of Lyme neuroborreliosis: from infection to inflammation. Mol Med. (2008) 14:205–12. doi: 10.2119/2007-00091.Rupprecht
5. Johnson KO, Nelder MP, Russell C, Li Y, Badiani T, Sander B, et al. Clinical manifestations of reported Lyme disease cases in Ontario, Canada: 2005-2014. PLoS ONE. (2018) 13:e0198509. doi: 10.1371/journal.pone.0198509
6. Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. (1977) 20:7–17. doi: 10.1002/art.1780200102
7. Bingham PM, Galetta SL, Athreya B, Sladky J. Neurologic manifestations in children with Lyme disease. Pediatrics. (1995) 96:1053–6.
8. Berger BW. Dermatologic manifestations of Lyme disease. Rev Infect Dis. (1989) 11(Suppl. 6):S1475–81. doi: 10.1093/clinids/11.Supplement_6.S1475
9. Aucott JN, Seifter A. Misdiagnosis of early Lyme disease as the summer flu. Orthop Rev. (2011) 3:e14. doi: 10.4081/or.2011.e14
10. Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, et al. Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA. (1992) 267:1364–7. doi: 10.1001/jama.1992.03480100070033
11. Pachner AR, Steere AC. CNS manifestations of third stage Lyme disease. Zentralbl Bakteriol Mikrobiol Hyg A. (1986) 263:301–6. doi: 10.1016/S0176-6724(87)80081-0
12. Back T, Grunig S, Winter Y, Bodechtel U, Guthke K, Khati D, et al. Neuroborreliosis-associated cerebral vasculitis: long-term outcome and health-related quality of life. J Neurol. (2013) 260:1569–75. doi: 10.1007/s00415-013-6831-4
13. Monteventi O, Steinlin M, Regenyi M, Roulet-Perez E, Weber P, Fluss J. Pediatric stroke related to Lyme neuroborreliosis: data from the swiss neuropaediatric stroke registry and literature review. Eur J Paediatr Neurol. (2018) 22:113–21. doi: 10.1016/j.ejpn.2017.10.010
14. Pearson S. On the radar: Lyme neuroborreliosis, an infectious cause of cerebral vasculitis. QJM. (2015) 108:755. doi: 10.1093/qjmed/hcv043
15. Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, et al. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med. (1994) 121:560–7. doi: 10.7326/0003-4819-121-8-199410150-00002
16. Wittwer B, Pelletier S, Ducrocq X, Maillard L, Mione G, Richard S. Cerebrovascular events in Lyme neuroborreliosis. J Stroke Cerebrovasc Dis. (2015) 24:1671–8. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.056
17. Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med. (1990) 323:1438–44. doi: 10.1056/NEJM199011223232102
18. Dersch R, Sommer H, Rauer S, Meerpohl JJ. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. J Neurol. (2016) 263:17–24. doi: 10.1007/s00415-015-7923-0
19. Shadick NA, Phillips CB, Sangha O, Logigian EL, Kaplan RF, Wright EA, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. (1999) 131:919–26. doi: 10.7326/0003-4819-131-12-199912210-00003
20. Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. (2001) 345:85–92. doi: 10.1056/NEJM200107123450202
21. Nowakowski J, Nadelman RB, Sell R, McKenna D, Cavaliere LF, Holmgren D, et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med. (2003) 115:91–6. doi: 10.1016/S0002-9343(03)00308-5
22. Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. Lyme disease: an infectious and postinfectious syndrome. J Rheumatol. (1994) 21:454–61.
23. Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res. (2013) 22:75–84. doi: 10.1007/s11136-012-0126-6
24. Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. (2006) 43:1089–134. doi: 10.1086/508667
25. Kalish RA, Kaplan RF, Taylor E, Jones-Woodward L, Workman K, Steere AC. Evaluation of study patients with Lyme disease, 10-20-year follow-up. J Infect Dis. (2001) 183:453–60. doi: 10.1086/318082
26. Eikeland R, Mygland A, Herlofson K, Ljostad U. Risk factors for a non-favorable outcome after treated European neuroborreliosis. Acta Neurol Scand. (2013) 127:154–60. doi: 10.1111/j.1600-0404.2012.01690.x
27. Embers ME, Hasenkampf NR, Jacobs MB, Tardo AC, Doyle-Meyers LA, Philipp MT, et al. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to borrelia burgdorferi by tick feeding. PLoS ONE. (2017) 12:e0189071. doi: 10.1371/journal.pone.0189071
28. Crossland NA, Alvarez X, Embers ME. Late disseminated Lyme disease: associated pathology and spirochete persistence posttreatment in rhesus macaques. Am J Pathol. (2018) 188:672–82. doi: 10.1016/j.ajpath.2017.11.005
29. Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother. (2015) 59:4616–24. doi: 10.1128/AAC.00864-15
30. Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, et al. Persistence of borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE. (2012) 7:e29914. doi: 10.1371/journal.pone.0029914
31. Feng J, Wang T, Shi W, Zhang S, Sullivan D, Auwaerter PG, et al. Identification of novel activity against borrelia burgdorferi persisters using an FDA approved drug library. Emerg Microbes Infect. (2014) 3:e49. doi: 10.1038/emi.2014.53
32. Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable borrelia burgdorferi following antibiotic treatment in mice. PLoS ONE. (2014) 9:e86907. doi: 10.1371/journal.pone.0086907
33. Clarissou J, Song A, Bernede C, Guillemot D, Dinh A, Ader F, et al. Efficacy of a long-term antibiotic treatment in patients with a chronic Tick Associated Poly-organic Syndrome (TAPOS). Med Mal Infect. (2009) 39:108–15. doi: 10.1016/j.medmal.2008.11.012
34. Donta ST. Tetracycline therapy for chronic Lyme disease. Clin Infect Dis. (1997) 25(Suppl 1):S52–6. doi: 10.1086/516171
35. Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. (2003) 60:1923–30. doi: 10.1212/01.WNL.0000071227.23769.9E
36. Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. (2008) 70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d
37. Berende A, ter Hofstede HJ, Vos FJ, van Middendorp H, Vogelaar ML, Tromp M, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. (2016) 374:1209–20. doi: 10.1056/NEJMoa1505425
38. Perronne C. Critical review of studies trying to evaluate the treatment of chronic Lyme disease. Presse Med. (2015) 44:828–31. doi: 10.1016/j.lpm.2015.06.002
39. Delong AK, Blossom B, Maloney EL, Phillips SE. Antibiotic retreatment of Lyme disease in patients with persistent symptoms: a biostatistical review of randomized, placebo-controlled, clinical trials. Contemp Clin Trials. (2012) 33:1132–42. doi: 10.1016/j.cct.2012.08.009
40. Fallon BA, Petkova E, Keilp JG, Britton CB. A reappraisal of the u.s. Clinical trials of post-treatment Lyme disease syndrome. Open Neurol J. (2012) 6:79–87. doi: 10.2174/1874205X01206010079
41. Rebman AW, Bechtold KT, Yang T, Mihm EA, Soloski MJ, Novak CB, et al. The clinical, symptom, and quality-of-life characterization of a well-defined group of patients with posttreatment Lyme disease syndrome. Front Med. (2017) 4:224. doi: 10.3389/fmed.2017.00224
42. Novak P, Felsenstein D, Mao C, Octavien NR, Zubcevik N. Association of small fiber neuropathy and post treatment Lyme disease syndrome. PLoS ONE. (2019) 14:e0212222. doi: 10.1371/journal.pone.0212222
43. Coughlin JM, Yang T, Rebman AW, Bechtold KT, Du Y, Mathews WB, et al. Imaging glial activation in patients with post-treatment Lyme disease symptoms: a pilot study using [(11)C]DPA-713 PET. J Neuroinflamm. (2018) 15:346. doi: 10.1186/s12974-018-1381-4
44. Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, Lackner AA, et al. Interaction of the Lyme disease spirochete borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am J Pathol. (2008) 173:1415–27. doi: 10.2353/ajpath.2008.080483
45. Ramesh G, Borda JT, Gill A, Ribka EP, Morici LA, Mottram P, et al. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J Neuroinflamm. (2009) 6:23. doi: 10.1186/1742-2094-6-23
46. Ramesh G, Didier PJ, England JD, Santana-Gould L, Doyle-Meyers LA, Martin DS, et al. Inflammation in the pathogenesis of Lyme neuroborreliosis. Am J Pathol. (2015) 185:1344–60. doi: 10.1016/j.ajpath.2015.01.024
47. Ramesh G, Philipp MT. Pathogenesis of Lyme neuroborreliosis: mitogen-activated protein kinases Erk1, Erk2, and p38 in the response of astrocytes to borrelia burgdorferi lipoproteins. Neurosci Lett. (2005) 384:112–6. doi: 10.1016/j.neulet.2005.04.069
48. Ramesh G, Santana-Gould L, Inglis FM, England JD, Philipp MT. The Lyme disease spirochete borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J Neuroinflamm. (2013) 10:88. doi: 10.1186/1742-2094-10-88
49. Kwekkeboom KL. Cancer symptom cluster management. Semin Oncol Nurs. (2016) 32:373–82. doi: 10.1016/j.soncn.2016.08.004
50. Levkovich I, Cohen M, Alon S, Kuchuk I, Nissenbaum B, Evron E, et al. Symptom cluster of emotional distress, fatigue and cognitive difficulties among young and older breast cancer survivors: the mediating role of subjective stress. J Geriatr Oncol. (2018) 9:469–75. doi: 10.1016/j.jgo.2018.05.002
51. Kwekkeboom KL, Tostrud L, Costanzo E, Coe CL, Serlin RC, Ward SE, et al. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J Pain Symptom Manage. (2018) 55:1286–95. doi: 10.1016/j.jpainsymman.2018.01.008
52. Conley S, Proctor DD, Jeon S, Sandler RS, Redeker NS. Symptom clusters in adults with inflammatory bowel disease. Res Nurs Health. (2017) 40:424–34. doi: 10.1002/nur.21813
53. Lim KE, Kim SR, Kim HK, Kim SR. Symptom clusters and quality of life in subjects with COPD. Respir Care. (2017) 62:1203–11. doi: 10.4187/respcare.05374
54. Shahrbanian S, Duquette P, Kuspinar A, Mayo NE. Contribution of symptom clusters to multiple sclerosis consequences. Qual Life Res. (2015) 24:617–29. doi: 10.1007/s11136-014-0804-7
55. Kwekkeboom K, Zhang Y, Campbell T, Coe CL, Costanzo E, Serlin RC, et al. Randomized controlled trial of a brief cognitive-behavioral strategies intervention for the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. Psychooncology. (2018) 27:2761–9. doi: 10.1002/pon.4883
56. Wood LJ, Weymann K. Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care. (2013) 7:54–9. doi: 10.1097/SPC.0b013e32835dabe3
57. Fallon BA, Zubcevik N, Bennett C, Doshi S, Rebman AW, Kishon R, et al. The general symptom questionnaire-30 (gsq-30): a brief measure of multi-system symptom burden in Lyme disease. Front Med. (2019) 6:283. doi: 10.3389/fmed.2019.00283
58. Sheehan DV, Mancini M, Wang J, Berggren L, Cao H, Duenas HJ, et al. Assessment of functional outcomes by sheehan disability scale in patients with major depressive disorder treated with duloxetine versus selective serotonin reuptake inhibitors. Hum Psychopharmacol. (2016) 31:53–63. doi: 10.1002/hup.2500
59. Wong ML, Cooper BA, Paul SM, Levine JD, Conley YP, Wright F, et al. Differences in symptom clusters identified using ratings of symptom occurrence vs. Severity in lung cancer patients receiving chemotherapy. J Pain Symptom Manage. (2017) 54:194–203. doi: 10.1016/j.jpainsymman.2017.04.005
60. Li H, Sereika SM, Marsland AL, Conley YP, Bender CM. Symptom clusters in women with breast cancer during the first 18 months of adjuvant therapy. J Pain Symptom Manage. (2019) 59:233–41. doi: 10.1016/j.jpainsymman.2019.10.002
61. Weinstein ER, Rebman AW, Aucott JN, Johnson-Greene D, Bechtold KT. Sleep quality in well-defined Lyme disease: a clinical cohort study in maryland. Sleep. (2018) 41:zsy035. doi: 10.1093/sleep/zsy035
62. Sullivan CW, Leutwyler H, Dunn LB, Cooper BA, Paul SM, Levine JD, et al. Stability of symptom clusters in patients with breast cancer receiving chemotherapy. J Pain Symptom Manage. (2018) 55:39–55. doi: 10.1016/j.jpainsymman.2017.08.008
63. Li H, Sereika SM, Marsland AL, Conley YP, Bender CM. Impact of chemotherapy on symptoms and symptom clusters in postmenopausal women with breast cancer prior to aromatase inhibitor therapy. J Clin Nurs. (2019) 28:4560–71. doi: 10.1111/jocn.15047
64. Jackson ML, Bruck D. Sleep abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: a review. J Clin Sleep Med. (2012) 8:719–28. doi: 10.5664/jcsm.2276
65. Greenberg HE, Ney G, Scharf SM, Ravdin L, Hilton E. Sleep quality in Lyme disease. Sleep. (1995) 18:912–6.
66. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. (2018) 17:174–82. doi: 10.1016/S1474-4422(17)30435-0
67. Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. (2019) 394:1765–74. doi: 10.1016/S0140-6736(19)32504-8
68. Kee Z, Kodji X, Brain SD. The role of calcitonin gene related peptide (CGRP) in neurogenic vasodilation and its cardioprotective effects. Front Physiol. (2018) 9:1249. doi: 10.3389/fphys.2018.01249
69. Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. (2009) 9:9–14. doi: 10.1016/j.coph.2008.12.007
70. Geppetti P, Capone JG, Trevisani M, Nicoletti P, Zagli G, Tola MR. CGRP and migraine: neurogenic inflammation revisited. J Headache Pain. (2005) 6:61–70. doi: 10.1007/s10194-005-0153-6
71. Huang J, Stohl LL, Zhou X, Ding W, Granstein RD. Calcitonin gene-related peptide inhibits chemokine production by human dermal microvascular endothelial cells. Brain Behav Immun. (2011) 25:787–99. doi: 10.1016/j.bbi.2011.02.007
72. Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. (1994) 117 (Pt 3):427–34. doi: 10.1093/brain/117.3.427
73. Berende A, Agelink van Rentergem J, Evers AWM, Ter Hofstede HJM, Vos FJ, Kullberg BJ, et al. Cognitive impairments in patients with persistent symptoms attributed to Lyme disease. BMC Infect Dis. (2019) 19:833. doi: 10.1186/s12879-019-4452-y
74. Kaplan RF, Trevino RP, Johnson GM, Levy L, Dornbush R, Hu LT, et al. Cognitive function in post-treatment Lyme disease: do additional antibiotics help? Neurology. (2003) 60:1916–22. doi: 10.1212/01.WNL.0000068030.26992.25
75. Touradji P, Aucott JN, Yang T, Rebman AW, Bechtold KT. Cognitive decline in post-treatment Lyme disease syndrome. Arch Clin Neuropsychol. (2019) 34:455–65. doi: 10.1093/arclin/acy051
76. Tager FA, Fallon BA, Keilp J, Rissenberg M, Jones CR, Liebowitz MR. A controlled study of cognitive deficits in children with chronic Lyme disease. J Neuropsychiatry Clin Neurosci. (2001) 13:500–7. doi: 10.1176/jnp.13.4.500
77. Keilp JG, Corbera K, Gorlyn M, Oquendo MA, Mann JJ, Fallon BA. Neurocognition in post-treatment Lyme disease and major depressive disorder. Arch Clin Neuropsychol. (2019) 34:466–80. doi: 10.1093/arclin/acy083
78. Paul L, Ellis BM, Leese GP, McFadyen AK, McMurray B. The effect of a cognitive or motor task on gait parameters of diabetic patients, with and without neuropathy. Diabet Med. (2009) 26:234–9. doi: 10.1111/j.1464-5491.2008.02655.x
79. Wajda DA, Motl RW, Sosnoff JJ. Correlates of dual task cost of standing balance in individuals with multiple sclerosis. Gait Posture. (2014) 40:352–6. doi: 10.1016/j.gaitpost.2014.04.209
80. Wajda DA, Motl RW, Sosnoff JJ. Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J Neurol Sci. (2013) 335:160–3. doi: 10.1016/j.jns.2013.09.021
81. Wang XQ, Pi YL, Chen BL, Wang R, Li X, Chen PJ. Cognitive motor intervention for gait and balance in parkinson's disease: systematic review and meta-analysis. Clin Rehabil. (2016) 30:134–44. doi: 10.1177/0269215515578295
82. Wang XQ, Pi YL, Chen BL, Chen PJ, Liu Y, Wang R, et al. Cognitive motor interference for gait and balance in stroke: a systematic review and meta-analysis. Eur J Neurol. (2015) 22:555–e37. doi: 10.1111/ene.12616
83. Barr WB, Rastogi R, Ravdin L, Hilton E. Relations among indexes of memory disturbance and depression in patients with Lyme borreliosis. Appl Neuropsychol. (1999) 6:12–8. doi: 10.1207/s15324826an0601_2
84. Elkins LE, Pollina DA, Scheffer SR, Krupp LB. Psychological states and neuropsychological performances in chronic Lyme disease. Appl Neuropsychol. (1999) 6:19–26. doi: 10.1207/s15324826an0601_3
85. Fallon BA, Nields JA. Lyme disease: a neuropsychiatric illness. Am J Psychiatry. (1994) 151:1571–83. doi: 10.1176/ajp.151.11.1571
86. Hassett AL, Radvanski DC, Buyske S, Savage SV, Gara M, Escobar JI, et al. Role of psychiatric comorbidity in chronic Lyme disease. Arthritis Rheum. (2008) 59:1742–9. doi: 10.1002/art.24314
87. Doshi S, Keilp JG, Strobino B, McElhiney M, Rabkin J, Fallon BA. Depressive symptoms and suicidal ideation among symptomatic patients with a history of Lyme disease vs two comparison groups. Psychosomatics. (2018) 59:481–9. doi: 10.1016/j.psym.2018.02.004
88. Kaplan RF, Meadows ME, Vincent LC, Logigian EL, Steere AC. Memory impairment and depression in patients with Lyme encephalopathy: comparison with fibromyalgia and nonpsychotically depressed patients. Neurology. (1992) 42:1263–7. doi: 10.1212/WNL.42.7.1263
Keywords: lyme borreliosis, symptom cluster, disability, fatigue, neurocognitive impairment
Citation: Zubcevik N, Mao C, Wang QM, Bose EL, Octavien RN, Crandell D and Wood LJ (2020) Symptom Clusters and Functional Impairment in Individuals Treated for Lyme Borreliosis. Front. Med. 7:464. doi: 10.3389/fmed.2020.00464
Received: 11 February 2020; Accepted: 13 July 2020;
Published: 21 August 2020.
Edited by:
Christian Perronne, Assistance Publique Hopitaux De Paris, FranceReviewed by:
Amir Khadir, Integrated Center for Health and Social Services Lanaudière, CanadaAlexis Lacout, Groupe Elsan, France
Copyright © 2020 Zubcevik, Mao, Wang, Bose, Octavien, Crandell and Wood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa J. Wood, d29vZGxsQGJjLmVkdQ==
 Nevena Zubcevik
Nevena Zubcevik Charlotte Mao
Charlotte Mao Qing Mei Wang1,5
Qing Mei Wang1,5 Rose Nadlyne Octavien
Rose Nadlyne Octavien Lisa J. Wood
Lisa J. Wood