- 1Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Gastroenterology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Molecular and Medical Pharmacology, University of California, Los Angeles, CA, United States
- 4Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 5Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 6Department of Oncology, Huang Gang Central Hospital, Huanggang, China
- 7Department of Endocrinology, Huang Gang Central Hospital, Huanggang, China
Objectives: As of June 1, 2020, coronavirus disease 2019 (COVID-19) has caused a global pandemic and resulted in over 370,000 deaths worldwide. Early identification of COVID-19 patients who need to be admitted to the intensive care unit (ICU) helps to improve the outcomes. We aim to investigate whether absolute eosinophil count (AEC) can predict ICU transfer among elderly COVID-19 patients from general isolation wards.
Methods: A retrospective study of 94 elderly patients older than 60 years old with COVID-19 was conducted. We compared the basic clinical characteristics and levels of inflammation markers on admission to general isolation wards and the needs for ICU transfer between the eosinopenia (AEC on admission <20 cells/μl) and non-eosinopenia (AEC ≥20 cells/μl) groups.
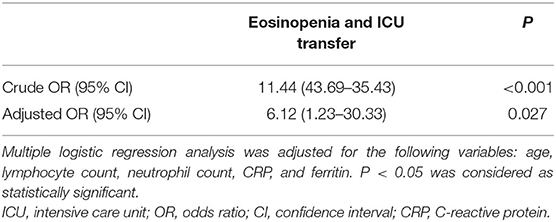
Results: There was a significantly higher ICU transfer rate in the eosinopenia group than in the non-eosinopenia group (51 vs. 9%, P < 0.001). Multivariate analysis revealed that eosinopenia was associated with an increased risk of ICU transfer in elderly COVID-19 patients [adjusted odds ratio (OR) 6.12 (95% CI, 1.23–30.33), P = 0.027] after adjustment of age, lymphocyte count, neutrophil count, C-reactive protein (CRP), and ferritin levels. The eosinopenia group had higher levels of CRP, ferritin, and cytokines [interleukin-2 receptor (IL-2R), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α)] than the non-eosinophil group (P < 0.001). The area under the curve of AEC on admission for predicting ICU transfer among elderly COVID-19 patients was 0.828 (95% CI, 0.732–0.923). The best cut-off value of AEC was 25 cells/μl with a sensitivity of 91% and a specificity of 71%, respectively.
Conclusion: Absolute eosinophil count on admission is a valid predictive marker for ICU transfer among elderly COVID-19 patients from general isolation wards and, therefore, can help case triage and optimize ICU utilization, especially for health care facilities with limited ICU capacity.
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been spreading worldwide with over 6 million cases and more than 370,000 deaths by June 1, 2020 (1, 2). A study of 34,249 COVID-19 patients in Spain found that patients aged ≥60 years accounted for 68% of hospitalizations, 70% of intensive care unit (ICU) admissions, and 94% of mortalities (3). It is implied that elderly COVID-19 patients are more likely to have critical progression, which poses a major challenge in health care facilities with limited ICU capacity (4, 5).
Currently, early discrimination of elderly COVID-19 patients with ICU requirement remains challenging in clinics. Recently, the traditional inflammation markers, such as C-reactive protein (CRP) (6), ferritin (7), and cytokines (8), are considered as predictive markers for COVID-19 severity. However, these inflammation indicators are usually expensive and time-consuming (9). Thus, a cost-effective, convenient, and fast predictive marker for severe elderly patients with COVID-19 is highly demanded during this pandemic.
Eosinophils are multifunctional cells involved in inflammatory response triggered by diverse stimuli (10). It is already known that acute bacterial infection would result in eosinopenia (11). Some studies have found that eosinopenia on ICU admission could be a useful biomarker for children mortality (12), 28-day mortality (13), unexpected ICU re-admission, and post-ICU mortality (14). However, there is no evidence showing that eosinopenia and ICU admission of COVID-19 are closely relevant. Therefore, our study aims to evaluate the value of absolute eosinophil count (AEC) on admission as a predictive marker for ICU transfer among elderly COVID-19 patients from general isolation wards.
Methods
Study Design
This retrospective study included COVID-19 elderly patients aged ≥60 years from four general wards at the Sino-French New City Branch of Tongji Hospital in Wuhan between February 10, 2020 and February 20, 2020. Exclusion criteria included: (1) patients who had taken corticosteroids before admission, (2) patients who died within 24 h after admission, and (3) patients with a history of allergies and parasitic infections. The protocol of our study was approved by the Tongji Hospital Ethics Committee.
Criteria of ICU Transfer for COVID-19
The criteria of ICU transfer for elderly COVID-19 patients included: (1) severe dyspnea that responds inadequately to initial emergency therapy, (2) changes in mental status (confusion, lethargy, coma, etc.), (3) persistent or worsening hypoxemia and/or severely worsening respiratory acidosis despite supplemental oxygen and non-invasive ventilation, and (4) the need for invasive mechanical ventilation and/or hemodynamic instability.
Data Collection
Clinical data were extracted from electronic medical records, including age, sex, comorbidities, and blood routine and blood biochemistry values on admission. Records of inflammation indicators, such as CRP, ferritin, and cytokines [interleukin-2 receptor (IL-2R), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α)], were also collected on admission.
Laboratory Procedures
The levels of IL-2R, IL-8, IL-10, and TNF-α in serum were measured according to an automatic procedure of a solid-phase two-site chemiluminescent immunometric assay via the IMMULITE 1000 Analyzer (Siemens, Munich, Germany). The level of IL-6 was measured using the electrochemiluminescence method (Roche Diagnostics, Basel, Switzerland).
AEC Profiling
Peripheral blood samples of our enrolled patients were assayed for full blood panel count including AEC on the Sysmex XS-800i (Sysmex Shanghai Ltd., Shanghai, China) as per the manufacturer's protocol. In accordance with the normal range of AEC in the Chinese population (15), we defined eosinopenia as AEC <20 cells/μl and non-eosinopenia as AEC ≥20 cells/μl. According to AEC on admission, the patients were divided into the eosinopenia group and the non-eosinopenia group.
Statistical Analysis
Continuous variables were described as means (±standard deviation, SD) or medians (interquartile ranges, IQRs) according to their distributions checked by Kolmogorov–Smirnov-test. Student's t-test or Mann–Whitney U-test was used to compare continuous variables. Categorical variables were compared by chi-square or Fisher's exact-tests. Variables with P-value <0.10 on univariate analysis were tested in multivariate analysis. The odds ratio (OR) along with the 95% confidence interval (CI) was reported. The receiver operating characteristics (ROC) curve and the area under the curve (AUC) were calculated for AEC in predicting ICU transfer of patients on admission. The best eosinophil cut-off value was decided depending on the Youden index. All statistical analyses were conducted using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA) 16.0 and STATA 15 (StataCorp, College Station, TX, USA). A two-tailed P-value <0.05 was considered to be statistically significant.
Results
Patient Characteristics
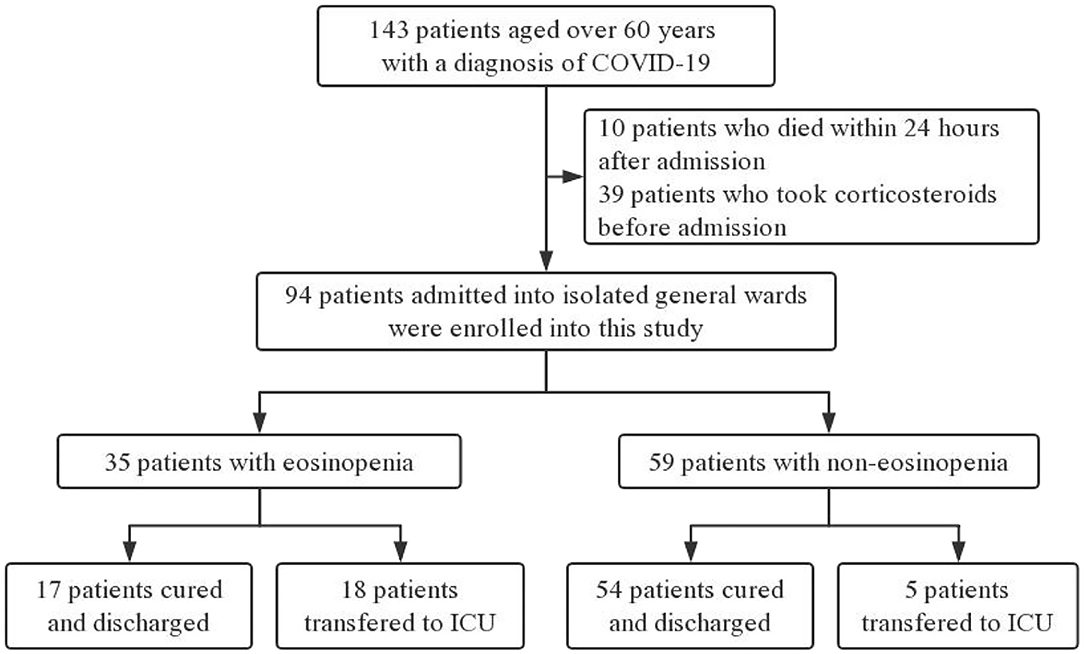
Electronic medical records of 143 COVID-19 patients older than 60 years old were collected by two physicians from four general isolation wards. After excluding 39 patients who had taken corticosteroids before admission and 10 patients who died within 24 h after admission, 94 participants were enrolled for further analysis (Figure 1).
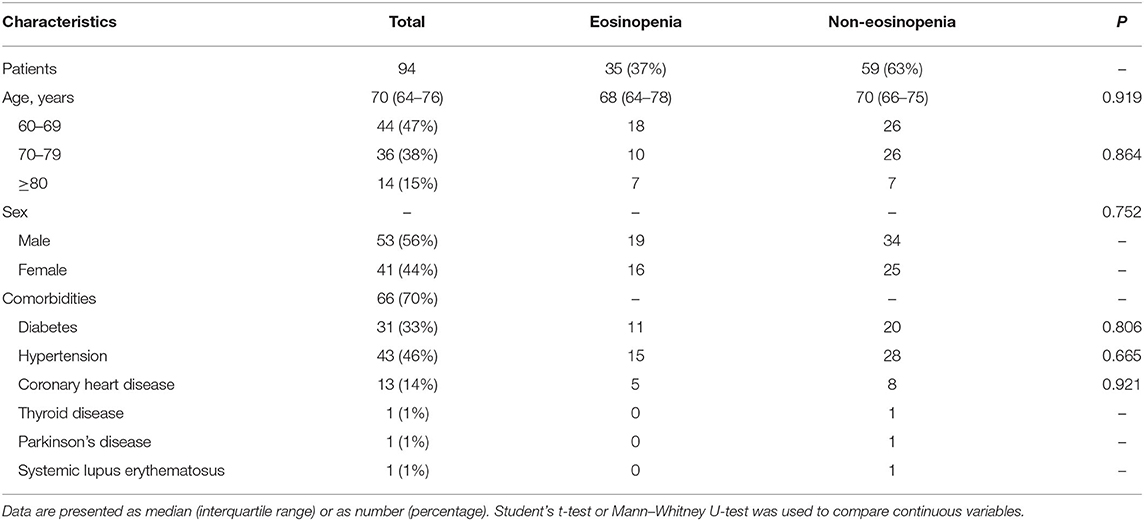
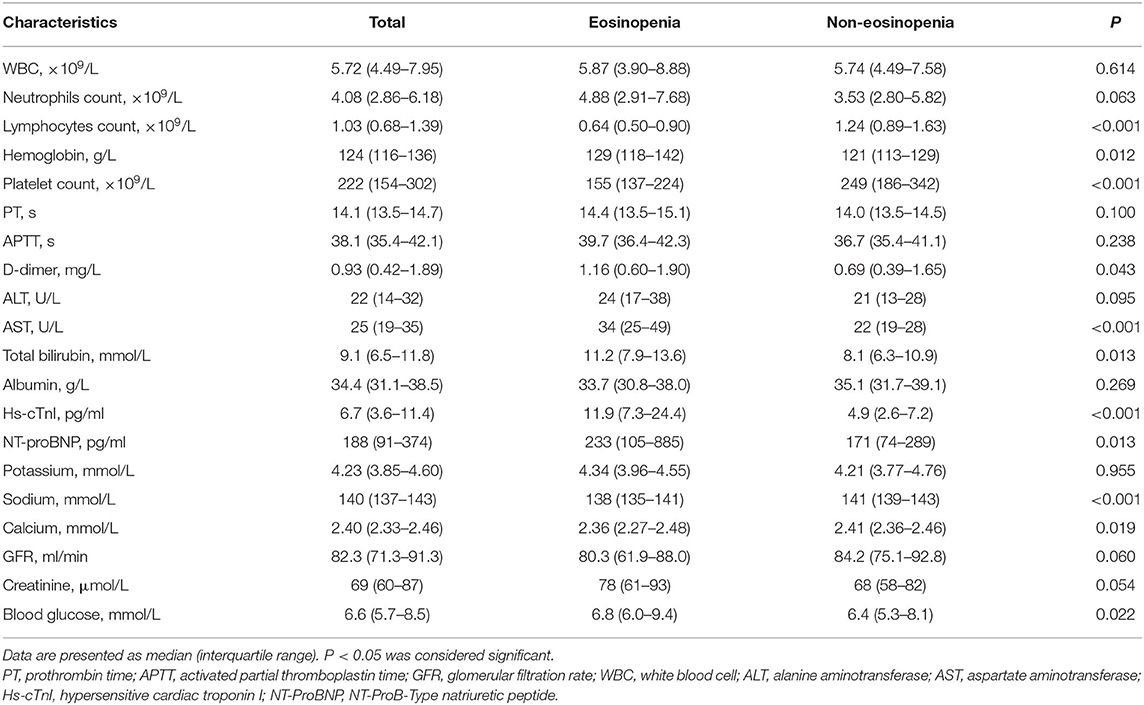
The median age of the 94 patients was 70 years (IQR 65–76 years), ranging from 60 to 88 years (60–69 years, 47%; 70–79 years, 38%; >80 years, 15%). There were more males than females in this cohort (56 vs. 44%). Comorbidities were presented in nearly three quarters of these elderly patients, with hypertension being the most common comorbidity, followed by diabetes and coronary heart disease. There were no significant differences in age, sex, and comorbidity between the eosinopenia group (35 patients) and the non-eosinopenia group (59 patients) (P > 0.05; Table 1). However, the levels of lymphocyte count, platelet count, aspartate aminotransferase, hypersensitive cardiac troponin I, and serum sodium were significantly different between the two groups (P < 0.001; Table 2).
Association Between Eosinopenia and ICU Transfer of Elderly COVID-19 Patients
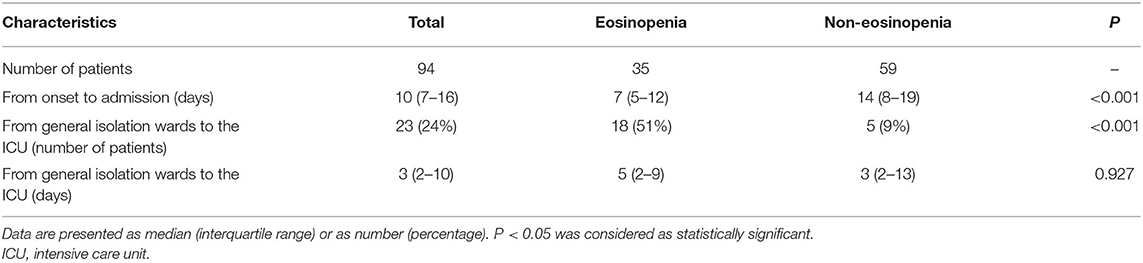
The median duration from illness onset to hospital admission was 10 days (IQR 7–16 days) for all patients, with 7 days (IQR 5–12 days) for the eosinopenia group and 14 days (IQR 8–19 days) for the non-eosinopenia group, respectively. There was a significantly higher ICU transfer rate in the eosinopenia group than in the non-eosinopenia group (51 vs. 9%, P < 0.001; Table 3).
In univariate analysis, a significant inverse association between eosinopenia and ICU transfer of elderly COVID-19 patients was revealed [crude OR 11.44 (95% CI, 3.69–35.43), P < 0.001; Table 3]. Furthermore, the inverse association remained significant [adjusted OR 6.12 (95% CI, 1.23–30.33), P = 0.027] by multivariate analysis after adjustment for age, lymphocyte count, neutrophil count, CRP, and ferritin levels (Table 4).
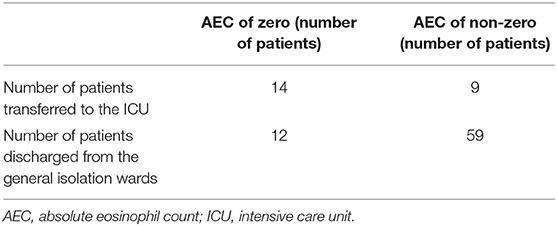
Surprisingly, 61% (14 out of 23) of patients transferred to the ICU demonstrated AEC at zero, whereas this phenomenon applied to only 17% (12 out of 71) of patients discharged from the general isolation wards (Table 5).
Association Between Eosinopenia and Traditional Inflammation Indicators
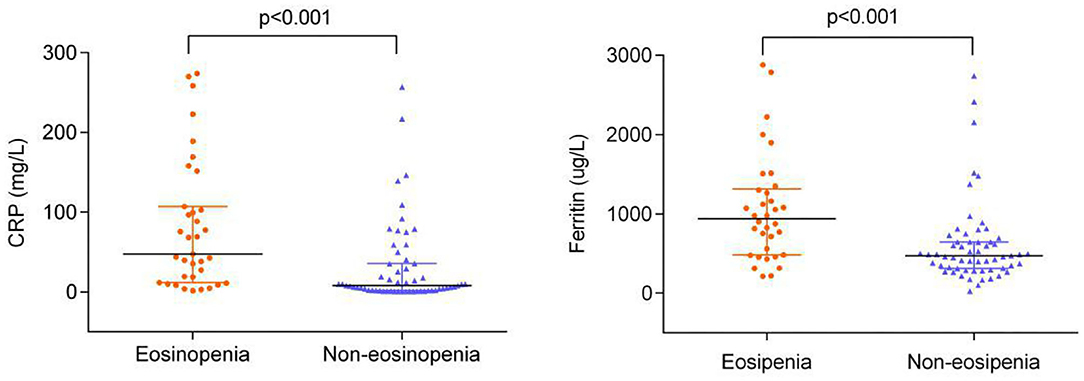
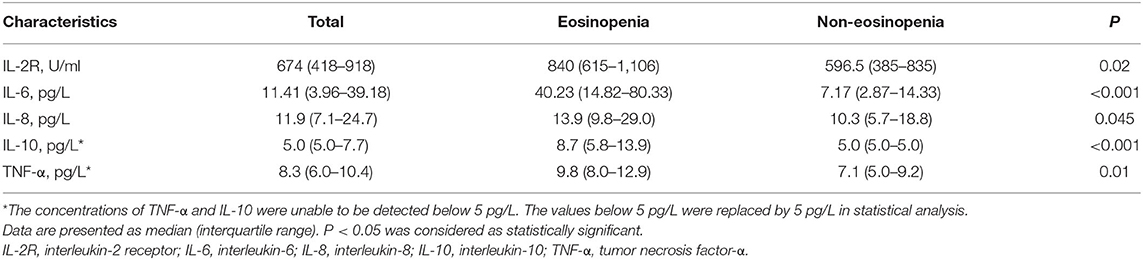
The levels of CRP and ferritin were much higher in the eosinopenia group than in the non-eosinopenia group with significant differences (P < 0.001; Figure 2). Additionally, 76 elderly patients in this cohort were tested for serum cytokines. We found that IL-2R, IL-6, IL-8, IL-10, and TNF-α levels were significantly higher in the eosinopenia group than in the non-eosinopenia group (P < 0.05; Table 6).
AEC in Predicting ICU Transfer Among Elderly COVID-19 Patients
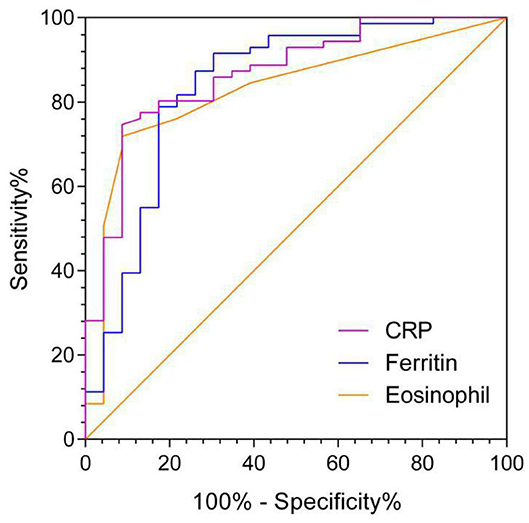
The AUC of AEC on admission in predicting ICU transfer among elderly COVID-19 patients was 0.828 (95% CI, 0.732–0.923), whereas the AUCs of CRP and ferritin were 0.867 (95% CI, 0.784–0.951) and 0.838 (95% CI, 0.730–0.947), respectively, with no significant difference in the pairwise comparison of ROC curves (P > 0.05; Figure 3 and Table 7). The best cut-off value of AEC was 25 cells/μl, yielding a sensitivity of 91%, a specificity of 71%, a positive predicted value of 51%, and a negative predicted value of 98%, respectively.
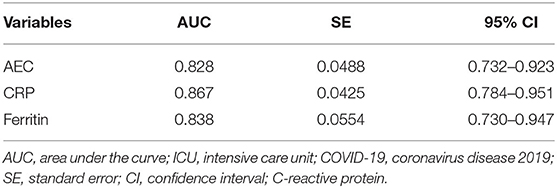
Table 7. The AUC of variables in predicting ICU transfer among elderly COVID-19 patients on admission.
Discussion
We conducted the present study to evaluate the association between eosinopenia and ICU transfer prediction of elderly COVID-19 patients from general isolation wards. Our results showed that the eosinopenia group had a significantly higher ICU transfer rate in the eosinopenia group than in the non-eosinopenia group (51 vs. 9%, P < 0.001). In univariate analysis and multivariate analysis, the strong inverse association between eosinopenia and ICU transfer of elderly COVID-19 patients remained significant. Moreover, the AUC of AEC in predicting ICU transfer for elderly COVID-19 patients was equivalent to that of CRP and ferritin. Collectively, AEC could be a valid predictive marker of ICU transfer for elderly COVID-19 patients from general isolation wards.
The mechanism of eosinopenia has not been completely understood in COVID-19. Eosinophils were observed in the infiltrating immune cells of the lungs as revealed by minimal autopsy of dead COVID-19 patients (16). It is assumed that eosinopenia is the redistribution result of circulating eosinophils to the infection locus due to the chemotactic effects of increased cytokines (17). Cytokine storm was observed in severe COVID-19 patients with high levels of various serum cytokines, such as IL-2, IL-7, IL-10, TNF-α, granulocyte-colony stimulating factor (G-CSF), interferon gamma-induced protein 10 (IP-10; CXCL10), and monocyte chemotactic protein-1 (MCP-1) (18–21). We demonstrated that concentrations of classic inflammation indicators, including CRP, ferritin, and several serum cytokines (IL-2R, IL-6, IL-8, IL-10, and TNF-α), were significantly higher in the eosinopenia group than in the non-eosinopenia group. Surprisingly, AEC was zero in more than half (61%) of the patients who eventually transferred to the ICU in this study. This phenomenon described by Shaaban et al. (9) was called “the almost zero eosinophil effect,” probably because of the overwhelming effects of cytokine storm caused by severe infection. The above evidences suggest that eosinopenia in COVID-19 patients may be due to cytokine storm.
The association between AEC and COVID-19 has been explored by limited studies. Eosinopenia was observed in COVID-19 patients with a high percentage up to 47–66% (22, 23). Moreover, compared with mild COVID-19 patients, severe patients had much lower AEC (24–26). Similarly, eosinopenia was detected in 37% of our COVID-19 patients, which was highly correlated with ICU transfer as revealed by multivariate analysis. Therefore, it is suggested that AEC could be a valid predictive marker for severe elderly COVID-19 patients.
Additionally, our results showed that the AUC of AEC on admission for predicting ICU transfer among elderly COVID-19 patients was 0.828, nearly equivalent to that of CRP and ferritin. Compared with the expensive and time-consuming CRP and ferritin, AEC is a cost-effective, convenient, and fast predictive marker, which is helpful for health care facilities to allocate limited ICU resources more reasonably.
Our study has some limitations. Firstly, it is a retrospective study that would provide limited evidence. Secondly, it is a single-center study including only those patients older than 60 years old, but not patients of all ages. Thirdly, the evaluation of AEC is in a single time point on admission without subsequent dynamic changes due to systemic corticosteroids treatment. Hence, a multicenter study with a larger cohort is required for further validation of our conclusion.
Conclusions
We demonstrated that AEC on general admission is a valid predictive marker for ICU transfer among elderly COVID-19 patients from general isolation wards and, therefore, can help case triage and optimize ICU utilization. Additionally, AEC is cost-effective, convenient, and fast compared with the expensive and time-consuming CRP and ferritin, making it an appropriate alternative for health care facilities with limited ICU capacity.
Data Availability Statement
The original contributions generated in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JH and ZZ designed the study and drafted the manuscript. GA and LC collected and summarized all epidemiological and clinical data. SL, XZ, CZ, and CG contributed to the statistical analysis. DL and ZZ revised the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81770168).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all patients and health-care workers who were involved in the present study. We also thank Yunsheng Fang, Meng Gao, Chuanyi Lu (University of California, San Francisco, CA, UCSF), and Junhui Hu (University of California, Los Angeles, CA, UCLA) for revision of the manuscript.
Abbreviations
AEC, absolute eosinophil count; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ICU, intensive care unit; CRP, C-reactive protein; Hs-cTnI, hypersensitive cardiac troponin I; NT-ProBNP, NT-ProB-type natriuretic peptide; IL-2R, interleukin-2 receptor; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; IQR, interquartile range; TNF-α, tumor necrosis factor-α; PT, prothrombin time; APTT, activated partial thromboplastin time; GFR, glomerular filtration rate; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ROC, receiver operating characteristics; AUC, area under the curve; OR, odds ratio; 95% CI, 95% confidence interval; PPV, positive predicted value; NPV, negative predicted value; G-CSF, granulocyte-colony stimulating factor; IP-10, interferon gamma-induced protein 10; MCP-1, monocyte chemotactic protein-1; SARS, systemic inflammatory response syndrome.
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. World Health Organization. Coronavirus Disease (COVID-19). Situation report-−133. WHO. Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4 (accessed June 1, 2020).
3. Bonanad C, García-Blas S, Tarazona-Santabalbina FJ, Díez-Villanueva P, Ayesta A, Sanchis Forés J, et al. Coronavirus: the geriatric emergency of 2020. Joint document of the section on geriatric cardiology of the Spanish Society of Cardiology and the Spanish Society of Geriatrics and Gerontology. Revista espanola de cardiologia (English ed). (2020) 73:569–76. doi: 10.1016/j.rec.2020.05.001
4. Li L, Gong S, Yan J. Covid-19 in China: ten critical issues for intensive care medicine. Crit Care. (2020) 24:124. doi: 10.1186/s13054-020-02848-z
5. Vergano M, Bertolini G, Giannini A, Gristina GR, Livigni S, Mistraletti G, et al. Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource-limited circumstances: the Italian perspective during the COVID-19 epidemic. Crit Care. (2020) 24:165. doi: 10.1186/s13054-020-02891-w
6. Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. (2020) 92:856–62. doi: 10.1002/jmv.25871
7. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. (2020) 58:1021–8. doi: 10.1515/cclm-2020-0369
8. Hou H, Zhang B, Huang H, Luo Y, Wu S, Tang G, et al. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clin Exp Immunol. (2020) 201:76–84. doi: 10.1111/cei.13450
9. Shaaban H, Daniel S, Sison R, Slim J, Perez G. Eosinopenia: is it a good marker of sepsis in comparison to procalcitonin and C-reactive protein levels for patients admitted to a critical care unit in an urban hospital? J Crit Care. (2010) 25:570–5. doi: 10.1016/j.jcrc.2010.03.002
10. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. (2006) 24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720
11. Choi J, Oh JY, Lee YS, Hur GY, Lee SY, Shim JJ, et al. The association between blood eosinophil percent and bacterial infection in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2019) 14:953–9. doi: 10.2147/COPD.S197361
12. Kim YH, Park HB, Kim MJ, Kim HS, Lee HS, Han YK, et al. Prognostic usefulness of eosinopenia in the pediatric intensive care unit. J Korean Med Sci. (2013) 28:114–9. doi: 10.3346/jkms.2013.28.1.114
13. Abidi K, Belayachi J, Derras Y, Khayari ME, Dendane T, Madani N, et al. Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med. (2011) 37:1136–42. doi: 10.1007/s00134-011-2170-z
14. Yip B, Ho KM. Eosinopenia as a predictor of unexpected re-admission and mortality after intensive care unit discharge. Anaest Intensive Care. (2013) 41:231–41. doi: 10.1177/0310057X1304100130
15. Wu X, Zhao M, Pan B, Zhang J, Peng M, Wang L, et al. Complete blood count reference intervals for healthy Han Chinese adults. PLoS ONE. (2015) 10:e0119669. doi: 10.1371/journal.pone.0119669
16. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. (2020) 49:411–7. doi: 10.3760/cma.j.cn112151-20200312-00193
17. Bass DA, Gonwa TA, Szejda P, Cousart MS, DeChatelet LR, McCall CE. Eosinopenia of acute infection: production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. (1980) 65:1265–71. doi: 10.1172/JCI109789
18. Yang Y, Shen C, Li J, Yuan J, Yang MH, Wang FX, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. [Preprint]. (2020). doi: 10.1101/2020.03.02.20029975
19. Gong J, Dong H, Xia SQ, Huang YZ, Wang DK, Zhao Y, et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. medRxiv. [Preprint]. (2020). doi: 10.1101/2020.02.25.20025643
20. Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. (2020) 17:541–3. doi: 10.1038/s41423-020-0401-3
21. Chen H, Xuan B, Yan Y, Zhu X, Shen C, Zhao G, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. [Preprint]. (2020).
22. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020) 75:1730–41. doi: 10.1111/all.14238
23. Yun H, Sun Z, Wu J, Tang A, Hu M, Xiang Z. Laboratory data analysis of novel coronavirus (COVID-19) screening in 2510 patients. Clin Chim Acta. (2020) 507:94–7. doi: 10.1016/j.cca.2020.04.018
24. Sun Y, Dong Y, Wang L, Xie H, Li B, Chang C, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun. (2020) 112:102473. doi: 10.1016/j.jaut.2020.102473
25. Sun S, Cai X, Wang H, He G, Lin Y, Lu B, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. (2020) 507:174–80. doi: 10.1016/j.cca.2020.04.024
Keywords: COVID-19, eosinophils, prediction, severity, intensive care unit
Citation: Huang J, Zhang Z, Liu S, Gong C, Chen L, Ai G, Zhu X, Zhang C and Li D (2020) Absolute Eosinophil Count Predicts Intensive Care Unit Transfer Among Elderly COVID-19 Patients From General Isolation Wards. Front. Med. 7:585222. doi: 10.3389/fmed.2020.585222
Received: 20 July 2020; Accepted: 05 October 2020;
Published: 04 November 2020.
Edited by:
Zhiliang Hu, Nanjing Second Hospital, ChinaReviewed by:
Luiz Ricardo Berbert, Rio de Janeiro State Federal University, BrazilPedro Xavier-Elsas, Federal University of Rio de Janeiro, Brazil
Copyright © 2020 Huang, Zhang, Liu, Gong, Chen, Ai, Zhu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dengju Li, bGlkZW5nanVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jinjin Huang1†
Jinjin Huang1† Chen Gong
Chen Gong Liping Chen
Liping Chen Dengju Li
Dengju Li