- 1Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Science, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China
- 2Section of Rheumatology, Allergy and Immunology, Yale University School of Medicine, New Haven, CT, United States
Background: Renal impairment is a critical complication in primary Sjögren's syndrome (pSS), resulting in chronic renal disease and even death. This meta-analysis was designed to find out the relevant factors of renal involvement in pSS.
Methods: PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science were systemically searched until August 30, 2019. Studies were selected according to inclusion criteria, and data was extracted by two researchers independently. The Newcastle-Ottawa Scale was applied for quality assessment. Random- and fixed-effects models were used in this meta-analysis based on the result of the heterogeneity test. Meanwhile, a sensitivity analysis was conducted to investigate the cause of heterogeneity. Publication bias was shown in the funnel plot and evaluated further by Begg's and Egger's tests.
Results: Of the 9,989 articles identified, five articles enrolling 1,867 pSS patients were included in the final analysis, 533 with and 1,334 without renal involvement. There was no statistical significance in age and gender between these two groups. According to the meta-analysis, anti-SSB antibody, and arthralgia showed a significant association with renal involvement in pSS, the overall odds ratio (OR) values of which were 1.51 (95% CI, 1.16–1.95) and 0.59 (95% CI, 0.46–0.74), respectively. On the other hand, the overall OR values of anti-SSA antibody, rheumatoid factor, dry eyes, and labial salivary gland biopsy were just 0.90 (95% CI, 0.49–1.64), 1.05 (95% CI, 0.59–1.86), 0.60 (95% CI, 0.34–1.06), and 1.38 (95% CI, 0.98–1.95), respectively.
Conclusion: The presence of anti-SSB antibody is positively associated with renal involvement in pSS, while arthralgia is inversely associated. Large-scale prospective cohort studies are needed in the future to identify further risk factors.
Introduction
Primary Sjögren's syndrome (pSS) is a chronic autoimmune disease characterized by the involvement of exocrine glands, usually resulting in xerostomia and xeropthalmia (1), and it is also the second common autoimmune disease in middle-aged women (2). Additionally, it has been realized that pSS could have an extensive effect on many organs in the human body, among which the kidney is an important target (3). The exact prevalence of renal involvement in pSS remains unknown, considering the difference in diagnosis criteria of SS, study populations, and the definition of renal impairment. According to studies by far, it ranges from 0.3 to 33.5% (4–9). The nephropathy of pSS could be further divided into two categories: tubulointerstitial nephritis (TIN) and, less frequently, glomerular involvement (10). The presentation of TIN could be insidious without clinically significant signs or symptoms, so there is a possibility that these patients would develop terminal chronic kidney disease due to not receiving proper treatment, even though this kind of nephropathy could be alleviated after treatment in many studies (4, 11, 12). Meanwhile, glomerular involvement is usually accompanied by overt symptoms such as hypertension, evident proteinuria, and hematuria (13), which could be treated earlier after differential diagnosis. A study by Goules et al. revealed that pSS patients with glomerulonephritis showed increased mortality but a lower proportion of chronic renal failure compared with those with interstitial nephritis (8).
Therefore, we designed this meta-analysis to find out clinical factors associated with renal involvement in pSS. Based on that, for those patients with specific clinical factors, clinicians are more likely to pay attention to renal impairment and then give them appropriate treatment earlier.
Methods
This meta-analysis was conducted according to the instruction of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Literature Search
We searched PubMed (from 1948 to August 2019), EMBASE (from 1980 to August 2019), Cochrane Library (from 2000 to August 2019), Scopus (from 1960 to August 2019), and Web of Science (from 1950 to August 2019) to identify all relevant articles. These following keywords or MeSH terms were used: “Sjogren's syndrome,” “Sjogren* syndrome,” “sicca syndrome,” “SS,” “kidney,” and “renal.” There was no addition of any filter such as language, species, or article types during the search process. Take the research strategy in PubMed as an example:
#1, “Search Sjogren's syndrome [MeSH Terms]”, 12,329.
#2, “Search (sjogren* syndrome [Title/Abstract] OR sicca syndrome [Title/Abstract]),” 15,785.
#3, “Search (#1 OR #2),” 17,578.
#4, “Search (kidney [MeSH Terms]) OR (renal [Title/Abstract] OR kidney*[Title/Abstract]),” 935,287.
#5, “Search (#3 AND #4),” 1,076.
Study Selection
We imported all articles into EndNote software (version X9) for further screening. The inclusion criteria were as follows: (1) all enrolled patients in the study with a diagnosis of pSS fulfilling the European criteria or the 2002 classification criteria of the American-European Consensus Group (AECG); (2) comparing pSS patients with renal involvement and those without in terms of clinical and laboratory characteristics; and (3) studies published in English. Two researchers assessed study eligibility and completed the study selection, respectively. These two researchers would discuss any discrepancies together, and a consensus was finally reached.
Data Collection
Data was collected independently by two researchers. The following items were extracted from each study included: country, study type, time span, diagnosis of SS, the definition of renal involvement, clinical presentations of renal involvement, pathological results of renal biopsy, the mean age of participants, gender, and comparative items in the study. Discrepancies were discussed and solved by the researchers.
Quality Assessment
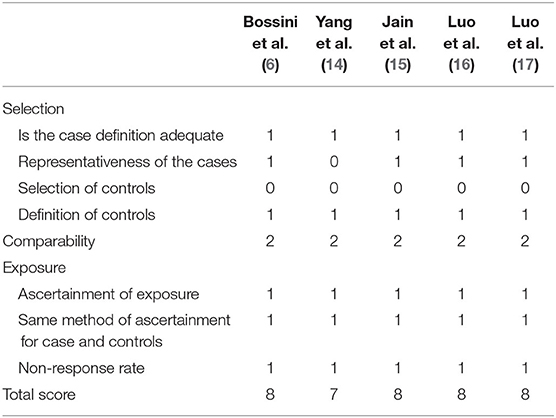
The Newcastle-Ottawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to assess the quality of the studies, in which each study was scored based on the quality of selection, comparability, and exposure. The quality of selection was further divided into four aspects including the definition of the case, the representativeness of the cases, the selection of controls, and the definition of controls. The quality of exposure involved its ascertainment, the same method of ascertainment for case and controls, and non-response rate. In total, there were eight items in this scale with a score up to nine. Two researchers completed quality assessment independently, and a third one was needed to solve any discrepancies if necessary.
Statistical Analysis
Items were included for meta-analysis if they met the following criteria: (1) analyzed in at least three articles; (2) presented in the same form; and (3) the statistical significance of which not in consensus among different articles. As for those items with significant heterogeneity test (P < 0.05), the random-effects model was adopted in forest plots, or else, the fixed-effects model was used. Meanwhile, a sensitivity analysis was conducted by leaving out each included study one by one to investigate heterogeneity further. In addition, publication bias was evaluated by funnel plots and Begg's and Egger's tests. Data analyses were conducted using Stata/SE 15.0 and Review Manager 5.3 software, and any outcome, with a two-tailed P < 0.05, was considered significant.
Results
Literature Search
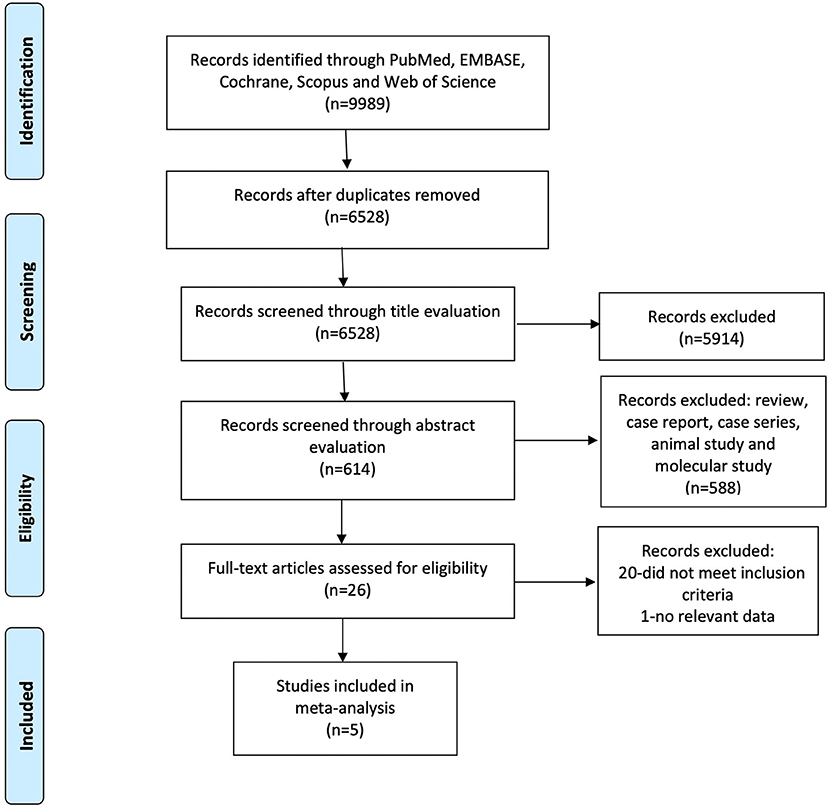
A total of 9,989 articles were found by a comprehensive search in PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science, of which 6,528 were left after the removal of duplicates. We excluded 5,914 articles unrelated to pSS-associated renal involvement after title evaluation. Among the remaining 614 articles, we screened the abstract further. Case report, case series, reviews, and molecular study were excluded. Finally, we read the full text of 26 articles for eligibility. Those studies not providing clinical data needed were excluded. Meanwhile, owing to the paucity of relevant articles or data, we did not conduct subgroup analysis considering the different subtypes of renal involvement such as TIN and glomerulonephritis. We finally included five articles for further data collection and analysis. Figure 1 shows the flowchart of article screening in this meta-analysis.
Basic Characteristics of Included Studies
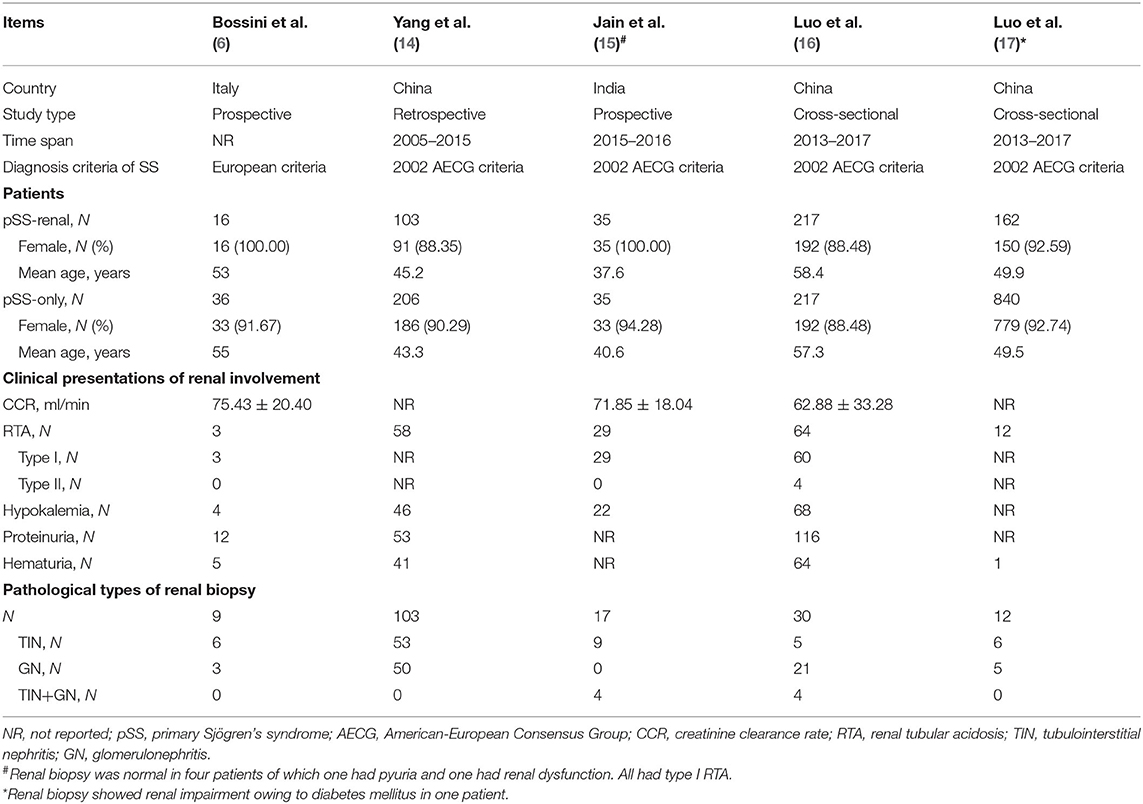
Table 1 summarizes the five included studies' essential characteristics, which were carried out in China, Italy, and India. Bossini et al. used the European criteria for SS diagnosis, while the 2002 AECG criteria were applied in the other four studies. As for the definition of renal involvement, Yang et al. only chose biopsy-proven cases, the indications of which were nephrotic range proteinuria, acute nephritic syndrome, and unexplained renal insufficiency. On the other hand, the other four studies took an overall consideration of urine analysis, serological analysis, and imaging evaluation including routine urine test, urine protein estimation, urine pH, serum creatine, serum electrolytes, and arterial blood gases. Additionally, renal function tests were performed when necessary, and kidney biopsy was applied as appropriate. Finally, 1,867 pSS patients were enrolled in these studies, 533 with and 1,334 without renal involvement.
Among these, the creatinine clearance rate was lower in patients with renal involvement. Of the five studies, renal tubular acidosis (RTA) was a critical clinical presentation, and type I RTA had a predominance, which was usually accompanied by hypokalemia. Proteinuria and hematuria were not rare in these studies. When it came to renal biopsy, according to Table 1, the result showed an inclination for TIN, even though, in the study of “Luo, 2018,” glomerulonephritis dominated (21/30). The researcher ascribed this phenomenon to the higher proportion of patients with proteinuria, which was more likely to indicate glomerular lesion and be indicative of renal biopsy. There was no statistical significance in age and gender observed in these studies. Quality assessment using the Newcastle-Ottawa Scale is illustrated in Table 2.
Factors Associated With Renal Involvement
These comparative items were analyzed in at least three studies: antinuclear antibody (ANA), anti-SSA antibody, anti-SSB antibody, rheumatoid factor (RF), dry eyes, dry mouth, arthralgia, and labial salivary gland biopsy (LSGB), the results of which were all presented in the form of positive rate. The comparative results of ANA and dry mouth were not significant in all four studies involved. Figure 2 shows the results of the meta-analysis of the remaining six items. The overall odds ratio (OR) values of anti-SSA antibody, anti-SSB antibody, RF, dry eyes, arthralgia, and LSGB were 0.90 (95% CI, 0.49–1.64), 1.51 (95% CI, 1.16–1.95), 1.05 (95% CI, 0.59–1.86), 0.60 (95% CI, 0.34–1.06), 0.59 (95% CI, 0.46–0.74), and 1.38 (95% CI, 0.98–1.95), respectively. We further conducted subgroup analysis considering the different diagnostic criteria of pSS. “Bossini, 2001” was involved with the meta-analysis of anti-SSA antibody, anti-SSB antibody, RF, and arthralgia. There were no significant discrepancies between the whole analysis and subgroup analysis, and the final conclusions remained the same.
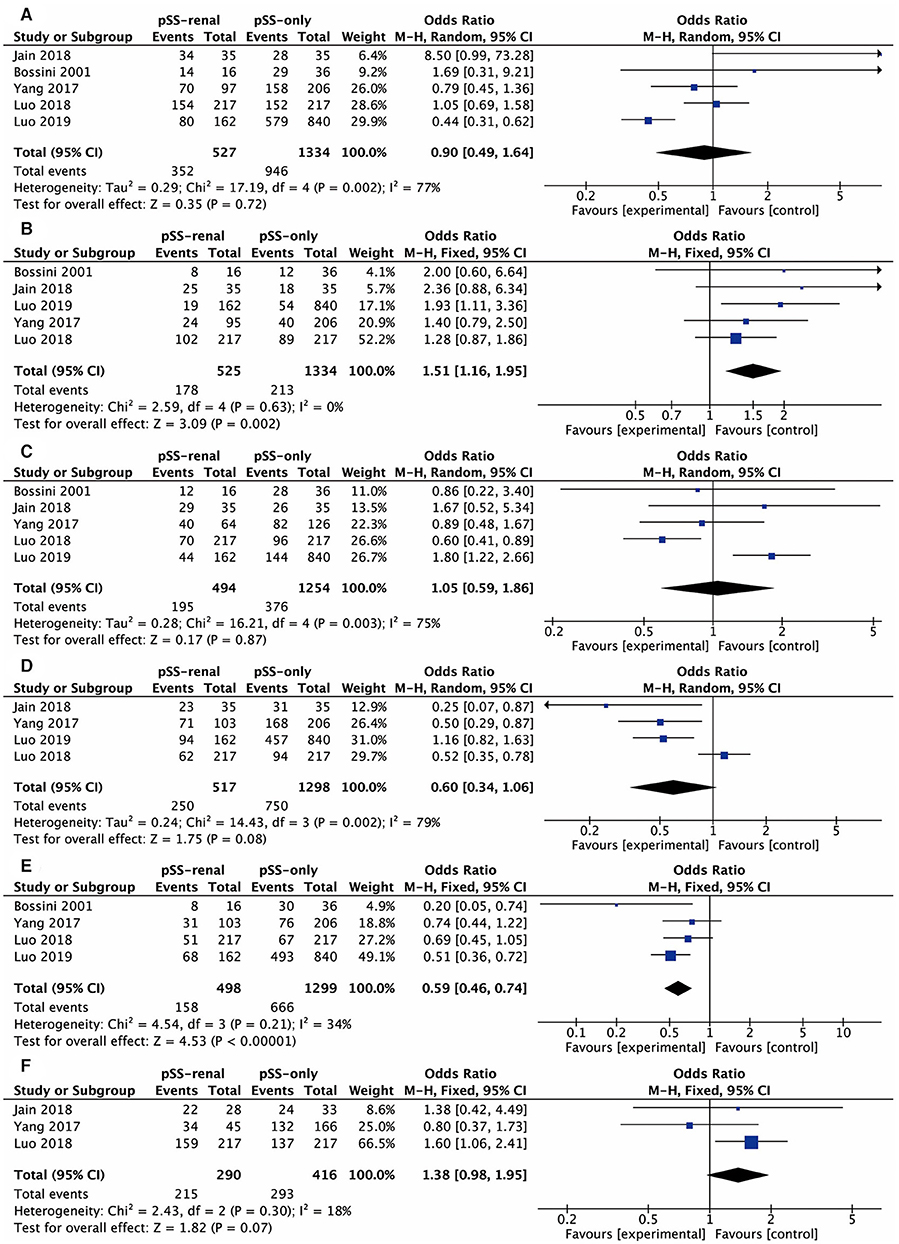
Figure 2. Forest plots of relevant factors of renal involvement in primary Sjögren's syndrome. (A) Anti-SSA antibody; (B) anti-SSB antibody; (C) rheumatoid factor; (D) dry eyes; (E) arthralgia; (F) labial salivary gland biopsy.
Sensitivity Analysis
Sensitivity analysis suggested that the result of the anti-SSB antibody was stable, and arbitrarily deleting one study would not change its heterogeneity. Nevertheless, “Luo, 2019” had an influence on heterogeneity for anti-SSA antibody (I2 = 77% vs. I2 = 40%), RF (I2 = 75% vs. I2 = 11%), and dry eyes (I2 = 79% vs. I2 = 0%). Also, “Bossini, 2001” was the major cause of heterogeneity for arthralgia (I2 = 34% vs. I2 = 0%).
Publication Bias
Figure 3 shows the results of funnel plots to assess the possibility of publication bias. Obvious asymmetry of shape was not observed in these funnel plots. In addition, Begg's and Egger's tests did not reveal statistical significance with a P-value more than 0.05, indicating no publication bias.
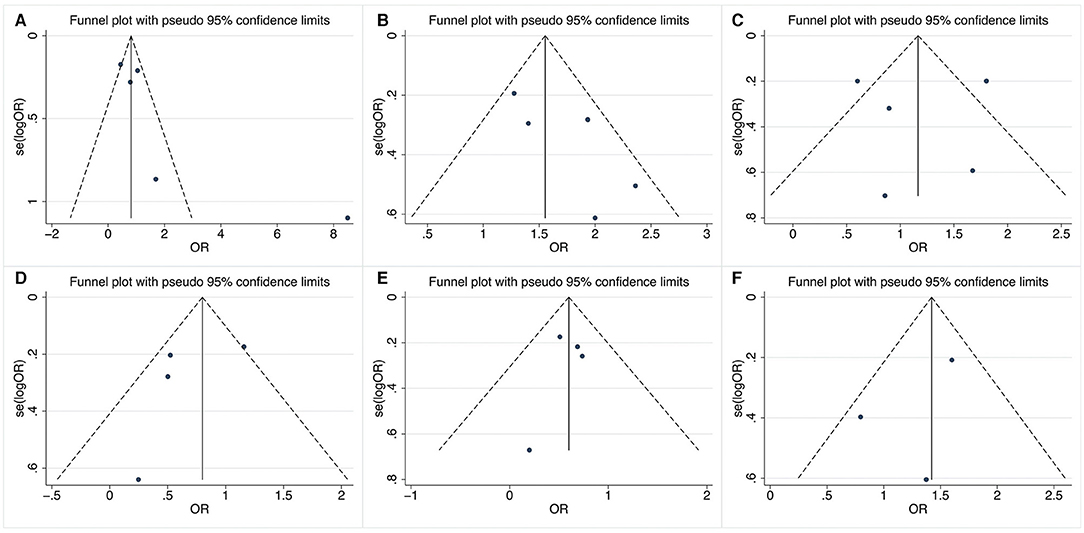
Figure 3. Funnel plots of relevant factors of renal involvement in primary Sjögren's syndrome. (A) Anti-SSA antibody; (B) anti-SSB antibody; (C) rheumatoid factor; (D) dry eyes; (E) arthralgia; (F) labial salivary gland biopsy.
Discussion
Renal involvement is a common complication in pSS with a variety of clinical presentations, which greatly affects the prognosis of patients. Ramos-Casals et al. revealed that renal failure (defined as creatinine more than 1.3 mg/dl) was found in ~24% of 198 pSS patients with renal involvement (18). Bearing the severity in mind, we conducted this meta-analysis to identify relevant factors in order to manage these patients better.
Among these comparative items, the existence of anti-SSB antibody was positively correlated with renal impairment in pSS. According to previous studies, anti-SSA/SSB antibodies were known to be related to extraglandular manifestations (19). For these pSS patients with RTA, the test of the anti-SSB antibody showed more positive results (3) and the titer of the anti-SSB antibody was higher than that in those without (20). Furthermore, in other autoimmune diseases such as systemic lupus erythematosus (SLE), the positive anti-SSB antibody test was identified to be one of the risk factors for renal recurrence in those with lupus nephritis type IV (21). Meanwhile, it was also observed to have a connection with nodal or spleen enlargement and with an increased level of gamma-globulins in SS, SLE, and progressive systemic sclerosis (22). All of these indicated immunologic disorder participating in the development of nephropathy in pSS. There were many studies showing lymphocytic infiltration as well as autoantibodies to components of renal tubules in TIN (23–26). Cryoglobulinemia and immune-complex deposition were found in glomerulonephritis (8, 27). As for these two types of renal impairment in pSS, the anti-SSB antibody did not show a predominance in any one of them (8, 11, 28, 29). The treatment of abatacept in pSS could reduce activated circulating follicular helper T cell to attenuate B cell hyperactivity, in which the titer of the anti-SSB antibody decreased as well as EULAR Sjogren's syndrome disease activity index (ESSDAI) and Clinical ESSDAI scores (30). However, Jasiek et al. found that the presence of anti-SSA and anti-SSB antibodies was related to no or poor response to the treatment of CSs and/or rituximab in pSS patients with TIN (31). More research is necessary to fully understand the relationship between the anti-SSB antibody and the pathophysiology of nephropathy in pSS.
The anti-SSA antibody was associated with the development or worse prognosis of renal impairment in some studies (31–33). However, we did not observe statistical significance in the anti-SSA antibody. The following reasons may explain this. The anti-SSA antibody was very common in pSS with a positive rate of up to 84.6% (34), while the ratio of renal involvement in pSS was relatively low. The number of patients we enrolled in the present meta-analysis is perhaps not enough to discover the exact association between the anti-SSA antibody and renal disease. Therefore, studies conducted on a larger scale are needed in the future.
pSS patients with renal involvement showed a lower prevalence in arthralgia than those without renal impairment. Articular manifestations are not rare presentations of pSS and could be simultaneous with, precede, or follow the development of sicca syndrome (35). According to previous studies, the prevalence of articular involvement ranges from 20 to 60%, and the most common symptoms are arthralgia and non-erosive polyarthropathy (36, 37). Consistent with this meta-analysis, arthralgia was more frequent in pSS without RTA compared with those with RTA (64 vs. 29%, P < 0.05) (38). Similarly, Jain et al. also found fewer articular manifestations in pSS patients with renal involvement (15). However, Fauchais et al. retrospectively analyzed pSS patients with and without articular manifestations, which were defined as arthralgia or non-erosive arthritis involving one or more peripheral joints. They found more renal involvement in pSS patients with articular manifestations than those without (23/188 vs. 11/231, P = 0.007) (36). Nevertheless, there was no statistical significance in RTA between these two groups in the study conducted by Pease and co-authors (39). The difference may be explained by the variety of populations as well as the definitions of articular and renal involvement. Considering more articular involvement and extraglandular features correlated with the positive result of RF in pSS (19, 40), it is interesting to explore the association between RF and renal disease, even though it showed no significance in this meta-analysis. In other autoimmune diseases, some studies revealed a negative relationship between RF and renal disease in systemic lupus erythematosus (41, 42).
This meta-analysis did not find an association between renal involvement and dry mouth as well as LSGB. Though the OR value reached 0.60 (95% CI, 0.34–1.06) and 1.38 (95% CI, 0.98–1.95), respectively, they remained insignificant. The pathophysiology of exocrine gland and renal disease in pSS both involved the infiltration of lymphocytes and tissue damage (1). It indicates that different and more specific mechanisms participate in the development of these two complications, respectively.
Inevitably, there are some limitations in this meta-analysis. First, we included five studies into the final analysis, and just three studies were involved with the analysis of LSGB, which would weaken the effect of meta-analysis. Second, owing to the various manifestations of renal impairment and the different detection methods, it hardly unified the definition of renal involvement in these included studies. Third, given that most of them were retrospective studies, we could not reach a causative conclusion. In addition, the authors did not provide enough information of potential confounding factors like medications, which would make our final conclusions a little less convincing and necessary to be strengthened further by well-designed studies in the future. Finally, heterogeneity was an issue always discussed in meta-analysis. The study size and enrollment population may explain the heterogeneity caused by “Bossini, 2001” for arthralgia. Besides, the patient number used in this meta-analysis was the total number of pSS patients with arthralgia or arthritis in “Bossini, 2001,” which would introduce deviation to some extent. “Luo, 2019” was the source of heterogeneity for anti-SSA antibody, RF, and dry eyes. Among these five studies, “Luo, 2019” was the largest one in study size. As for dry eyes, it was a subjective symptom and influenced by many factors such as the way of communication between researchers and patients. In addition, the detection method would impact the result of the anti-SSA antibody, which was different from that in the anti-SSB antibody (43). This also explained why the result of the anti-SSB antibody was stable in the sensitivity analysis.
Conclusion
This meta-analysis aims to find out the factors associated with renal involvement in pSS. The presence of anti-SSB antibody is positively related to nephropathy in pSS, while arthralgia is inversely related. Large-scale and well-designed prospective cohort studies are necessary in the future to identify risk factors of renal disease in pSS, which is beneficial for the further management of these special individuals.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
ML, YZ, XZ, and EH: conception and design of this study. RH, DX, and YX: literature research and data acquisition. RH, DX, JZ, QW, and XT: data analysis and interpretation. ML, YZ, XZ, and EH: supervision. Each author contributed importantly during manuscript drafting or revision and was accountable for all aspects of the work. The final version to be published was approved by all authors.
Funding
This work was supported by research grant from the Chinese National Key Technology R&D Program, Ministry of Science and Technology (grant numbers 2017YFC0907601 and 2017YFC0907605) and CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2019-I2M-2-008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate Alyssa Grimshaw and Katherine Stemmer-Frumento, the librarians in Yale University School of Medicine, New Haven, CT, USA, for their kind guidance in literature search and their support in the software.
References
1. Mavragani CP, Moutsopoulos HM. Sjogren's syndrome. Annu Rev Pathol. (2014) 9:273–85. doi: 10.1146/annurev-pathol-012513-104728
2. Kaufman I, Schwartz D, Caspi D, Paran D. Sjogren's syndrome - not just Sicca: renal involvement in Sjogren's syndrome. Scand J Rheumatol. (2008) 37:213–8. doi: 10.1080/03009740701867323
3. Aasarod K, Haga HJ, Berg KJ, Hammerstrom J, Jorstad S. Renal involvement in primary Sjogren's syndrome. QJM. (2000) 93:297–304. doi: 10.1093/qjmed/93.5.297
4. Maripuri S, Grande JP, Osborn TG, Fervenza FC, Matteson EL, Donadio JV, et al. Renal involvement in primary sjögren's syndrome: A clinicopathologic study. Clin J Am Soc Nephrol. (2009) 4:1423–31. doi: 10.2215/CJN.00980209
5. Lin DF, Yan SM, Zhao Y, Zhang W, Li MT, Zeng XF, et al. Clinical and prognostic characteristics of 573 cases of primary Sjögren's syndrome. Chin Med J. (2010) 123:3252–7. doi: 10.3760/cma.j.issn.0366-6999.2010.22.015
6. Bossini N, Savoldi S, Franceschini F, Mombelloni S, Baronio M, Cavazzana I, et al. Clinical and morphological features of kidney involvement in primary Sjögren's syndrome. Nephrol Dialysis Trans. (2001) 16:2328–36. doi: 10.1093/ndt/16.12.2328
7. Malladi AS, Sack KE, Shiboski SC, Shiboski CH, Baer AN, Banushree R, et al. Primary Sjögren's syndrome as a systemic disease: a study of participants enrolled in an international Sjögren's syndrome registry. Arthritis Care Res. (2012) 64:911–8. doi: 10.1002/acr.21610
8. Goules AV, Tatouli IP, Moutsopoulos HM, Tzioufas AG. Clinically significant renal involvement in primary Sjogren's syndrome: clinical presentation and outcome. Arthritis Rheum. (2013) 65:2945–53. doi: 10.1002/art.38100
9. Ramos-Casals M, Brito-Zeron P, Solans R, Camps MT, Casanovas A, Sopena B, et al. Systemic involvement in primary Sjogren's syndrome evaluated by the EULAR-SS disease activity index: analysis of 921 Spanish patients (GEAS-SS Registry). Rheumatology. (2014) 53:321–31. doi: 10.1093/rheumatology/ket349
10. Francois H, Mariette X. Renal involvement in primary Sjogren syndrome. Nat Rev Nephrol. (2016) 12:82–93. doi: 10.1038/nrneph.2015.174
11. Ren H, Wang W-M, Chen X-N, Zhang W, Pan X-X, Wang X-L, et al. Renal involvement and followup of 130 patients with primary Sjogren's syndrome. J Rheumatol. (2008) 35:278–84.
12. Goules A, Masouridi S, Tziofas AG, Ioannidis JPA, Skopouli FN, Moutsopoulos HM. Clinically significant and biopsy-documented renal involvement in primary Sjogren syndrome. Medicine. (2000) 79:241–9. doi: 10.1097/00005792-200007000-00005
13. Matignon M, Cacoub P, Colombat M, Saadoun D, Brocheriou I, Mougenot B, et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine. (2009) 88:341–8. doi: 10.1097/MD.0b013e3181c1750f
14. Yang HX, Wang J, Wen YB, Fei YY, Jiang MD, Zhou MY, et al. Renal involvement in primary Sjögren's syndrome: a retrospective study of 103 biopsy-proven cases from a single center in China. Int J Rheum Dis. (2018) 21:223–9. doi: 10.1111/1756-185X.13182
15. Jain A, Srinivas BH, Emmanuel D, Jain VK, Parameshwaran S, Negi VS. Renal involvement in primary Sjogren's syndrome: a prospective cohort study. Rheumatol Int. (2018) 38:2251–62. doi: 10.1007/s00296-018-4118-x
16. Luo J, Xu S, Lv Y, Huang X, Zhang H, Zhu X, et al. Clinical features and potential relevant factors of renal involvement in primary Sjogren's syndrome. Int J Rheum Dis. (2019) 22:182–90. doi: 10.1111/1756-185X.13429
17. Luo J, Huo YW, Wang JW, Guo H. High-risk indicators of renal involvement in primary Sjogren's syndrome: a clinical study of 1002 cases. J Immunol Res. (2019) 2019:3952392. doi: 10.1155/2019/3952392
18. Ramos-Casals M, Brito-Zerón P, Seror R, Bootsma H, Bowman SJ, Dörner T, et al. Characterization of systemic disease in primary Sjögren's syndrome: EULAR-SS task force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology. (2015) 54:2230–8. doi: 10.1093/rheumatology/kev200
19. Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, Del Pino-Montes J, et al. Primary Sjogren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine. (2008) 87:210–9. doi: 10.1097/MD.0b013e318181e6af
20. Takemoto F, Hoshino J, Sawa N, Tamura Y, Tagami T, Yokota M, et al. Autoantibodies against carbonic anhydrase II are increased in renal tubular acidosis associated with Sjögren syndrome. Am J Med. (2005) 118:181–4. doi: 10.1016/j.amjmed.2004.07.049
21. Wang J, Qi YY, Chen XP, Ma L, Zhang LL, Zhao Y, et al. Analysis of clinical risk factors in relapsed patients with class IV lupus nephritis. Exp Ther Med. (2018) 15:5461–8. doi: 10.3892/etm.2018.6130
22. Montecucco C, Bestagno M, Cerino A, Caporali R, Carnevale R, Longhi M, et al. Anti-SSB/La antibodies in Sjögren's syndrome and related autoimmune diseases. results of a quantitative immunoassay using a highly purified antigen. Clin Exp Rheumatol. (1989) 7:5–11.
23. Rosenberg ME, Schendel PB, McCurdy FA, Platt JL. Characterization of immune cells in kidneys from patients with Sjogren's syndrome. Am J Kidney Dis. (1988) 11:20–2. doi: 10.1016/S0272-6386(88)80169-0
24. Matsumura R, Kondo Y, Sugiyama T, Sueishi M, Koike T, Takabayashi K, et al. Immunohistochemical identification of infiltrating mononuclear cells in tubulointerstitial nephritis associated with Sjogren's syndrome. Clin Nephrol. (1988) 30:335–40.
25. Kim YK, Song HC, Kim W-Y, Yoon HE, Choi Y-J, Ki C-S, et al. Acquired gitelman syndrome in a patient with primary Sjogren syndrome. Am J Kidney Dis. (2008) 52:1163–7. doi: 10.1053/j.ajkd.2008.07.025
26. Pertovaara M, Bootorabi F, Kuuslahti M, Pasternack A, Parkkila S. Novel carbonic anhydrase autoantibodies and renal manifestations in patients with primary Sjogren's syndrome. Rheumatology. (2011) 50:1453–7. doi: 10.1093/rheumatology/ker118
27. Goules A, Geetha D, Arend LJ, Baer AN. Renal involvement in primary Sjogren's syndrome: natural history and treatment outcome. Clin Exp Rheumatol. (2019) (37 Suppl)118:123–32.
28. Kidder D, Rutherford E, Kipgen D, Fleming S, Geddes C, Stewart GA. Kidney biopsy findings in primary Sjogren syndrome. Nephrol Dial Transplant. (2015) 30:1363–9. doi: 10.1093/ndt/gfv042
29. Xia W, Gao B, Duan L, Li Y, Wen Y, Chen L, et al. Clinical significance of C4d deposition in renal tissues from patients with primary Sjogren's syndrome-a preliminary study. BMC Nephrol. (2019) 20:189. doi: 10.1186/s12882-019-1341-y
30. Verstappen GM, Meiners PM, Corneth OBJ, Visser A, Arends S, Abdulahad WH, et al. Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjogren's syndrome. Arthritis Rheumatol. (2017) 69:1850–61. doi: 10.1002/art.40165
31. Jasiek M, Karras A, Guern VL, Krastinova E, Mesbah R, Faguer S, et al. A multicentre study of 95 biopsy-proven cases of renal disease in primary Sjögren's syndrome. Rheumatology. (2017) 56:362–70. doi: 10.1093/rheumatology/kew376
32. Aktar K, Liu X, Yang X. Combination of anti-early apoptotic cell autoantibodies and anti-SSA autoantibodies in lupus nephritis. Cell Mol Biol. (2018) 64:48–54. doi: 10.14715/cmb/2018.64.13.10
33. Conti F, Ceccarelli F, Iaiani G, Perricone C, Giordano A, Amori L, et al. Association between Staphylococcus aureus nasal carriage and disease phenotype in patients affected by systemic lupus erythematosus. Arthritis Res Ther. (2016) 18:177. doi: 10.1186/s13075-016-1079-x
34. Xu D, Zhao S, Li Q, Wang YH, Zhao JL, Li MT, et al. Characteristics of Chinese patients with primary Sjögren's syndrome: preliminary report of a multi-centre registration study. Lupus. (2020) 29:45–51. doi: 10.1177/0961203319889666
35. Castro-Poltronieri A, Alarcón-Segovia D. Articular manifestations of primary Sjögren's syndrome. J Rheumatol. (1983) 10:485–8.
36. Fauchais AL, Ouattara B, Gondran G, Lalloué F, Petit D, Ly K, et al. Articular manifestations in primary Sjögren's syndrome: clinical significance and prognosis of 188 patients. Rheumatology. (2010) 49:1164–72. doi: 10.1093/rheumatology/keq047
37. Mirouse A, Seror R, Vicaut E, Mariette X, Dougados M, Fauchais AL, et al. Arthritis in primary Sjogren's syndrome: Characteristics, outcome and treatment from French multicenter retrospective study. Autoimmun Rev. (2019) 18:9–14. doi: 10.1016/j.autrev.2018.06.015
38. Siamopoulos KC, Elisaf M, Drosos AA, Mavridis AA, Moutsopoulos HM. Renal tubular acidosis in primary Sjogren's syndrome. Clin Rheumatol. (1992) 11:226–30. doi: 10.1007/BF02207962
39. Pease CT, Shattles W, Barrett NK, Maini RN. The arthropathy of Sjögren's syndrome. Br J Rheumatol. (1993) 32:609–13. doi: 10.1093/rheumatology/32.7.609
40. García-Carrasco M, Ramos-Casals M, Rosas J, Pallarés L, Calvo-Alen J, Cervera R, et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine. (2002) 81:270–80. doi: 10.1097/00005792-200207000-00003
41. Howard TW, Iannini MJ, Burge JJ, Davis J St. Rheumatoid factor, cryoglobulinemia, anti-DNA, and renal disease in patients with systemic lupus erythematosus. J Rheumatol. (1991) 18:826–30.
42. Helin H, Korpela M, Mustonen J, Pasternack A. Rheumatoid factor in rheumatoid arthritis associated renal disease and in lupus nephritis. Ann Rheum Dis. (1986) 45:508–11. doi: 10.1136/ard.45.6.508
Keywords: Sjögren's syndrome, renal, kidney, arthralgia, anti-SSB antibody
Citation: Hong R, Xu D, Hsieh E, Xiang Y, Zhao J, Wang Q, Tian X, Li M, Zhao Y and Zeng X (2020) Factors Associated With Renal Involvement in Primary Sjögren's Syndrome: A Meta-Analysis. Front. Med. 7:614482. doi: 10.3389/fmed.2020.614482
Received: 06 October 2020; Accepted: 29 October 2020;
Published: 26 November 2020.
Edited by:
Xinhua Yu, Research Center Borstel (LG), GermanyReviewed by:
Wang-Dong Xu, Southwest Medical University, ChinaMitsuhiro Takeno, Nippon Medical School, Japan
Copyright © 2020 Hong, Xu, Hsieh, Xiang, Zhao, Wang, Tian, Li, Zhao and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengtao Li, bWVuZ3Rhby5saUBjc3Rhci5vcmcuY24=; Yan Zhao, emhhb3lhbl9wdW1jaDIwMDJAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
 Ruping Hong
Ruping Hong Dong Xu1†
Dong Xu1† Jiuliang Zhao
Jiuliang Zhao Xiaofeng Zeng
Xiaofeng Zeng