- 1Department of Pathophysiology, Hospital de Clínicas, School of Medicine, Universidad de la República, Montevideo, Uruguay
- 2Advanced Bioimaging Unit, Institut Pasteur Montevideo and Universidad de la República, Montevideo, Uruguay
Although oxygen (O2) is essential for aerobic life, it can also be an important source of cellular damage. Supra-physiological levels of O2 determine toxicity due to exacerbated reactive oxygen species (ROS) production, impairing the homeostatic balance of several cellular processes. Furthermore, injured cells activate inflammation cascades, amplifying the tissue damage. The lung is the first (but not the only) organ affected by this condition. Critically ill patients are often exposed to several insults, such as mechanical ventilation, infections, hypo-perfusion, systemic inflammation, and drug toxicity. In this scenario, it is not easy to dissect the effect of oxygen toxicity. Translational investigations with animal models are essential to explore injuring stimuli in controlled experimental conditions, and are milestones in understanding pathological mechanisms and developing therapeutic strategies. Animal models can resemble what happens in critical care or anesthesia patients under mechanical ventilation and hyperoxia, but are also critical to explore the effect of O2 on lung development and the role of hyperoxic damage on bronchopulmonary dysplasia. Here, we set out to review the hyperoxia effects on lung pathology, contributing to the field by describing and analyzing animal experimentation's main aspects and its implications on human lung diseases.
Introduction
Aerobic respiration is a vital process in mammalian cells in which adequate oxygen (O2) delivery is essential. Nevertheless, supra-normal levels of O2 can damage cellular constituents and thus trigger cell injury and death (1, 2). The lung is the first organ affected by hyperoxia, but increasing evidence indicates that high blood concentration of O2 (hyperoxemia) can also determine harmful systemic consequences (3). Acute lung injury is featured by diffuse alveolar damage with interstitial and alveolar edema due to increased alveolar-capillary permeability to liquid, proteins and inflammatory cells (4). Hyperoxia is a frequent iatrogenic consequence of oxygen therapy, which can induce pulmonary damage and maximize mechanical ventilation associated acute lung injury, leading to severe consequences on gas exchange and respiratory mechanics (2, 5, 6). Moreover, in the last few years clinical reports have shown an increased mortality in patients with hyperoxia in intensive care units (7, 8). The lungs of critically ill patients are often exposed to several insults, such as mechanical stress, infections, hypo-perfusion, systemic inflammation and drug toxicity. In these scenarios it is not easy to clearly dissect the effect of oxygen toxicity. Translational investigations with animal models are essential to exploring injuring stimuli in controlled experimental conditions, and are milestones in the understanding of pathological mechanisms and the development therapeutic strategies. Here, we set out to review the animal models used to investigate hyperoxia and its effects on different pathological situations such as acute lung injury, chronic respiratory diseases and impairment of pulmonary development, among others.
Methods
An electronic search of Pubmed was made to identify the eligible studies, involving those published until 2020, combining the following keywords: hyperoxia; lung injury; animal models. All articles and cross-referenced studies were screened for appropriate information and reviewed by the authors. Inclusion criteria included original experimental and review articles. Publications not written in English or Spanish were excluded.
Oxygen Toxicity Mechanism
The homeostatic balance of cellular processes can be disrupted when exposed to supra-physiological concentrations of O2, due to exacerbated reactive oxygen species (ROS). Production of ROS is directly proportional to tissue O2 concentration, as they are intermediate metabolites produced during aerobic metabolism. Mitochondrial ROS generation begins with superoxide anion (O2•−) the main sources of which are complex I and II electron transfer. Further reactions lead to hydrogen peroxide (H2O2), hydroxyl radical (OH•−) and peroxynitrite anion (ONOO−) formation, all of which are highly reactive molecules able to damage intracellular components, proteins, lipids and nucleic acids. Antioxidant cell defense mechanisms, including superoxide dismutase, catalase and peroxidase enzymes, and non-enzymatic compounds (low molecular weight scavengers, proteins, glutathione), are overwhelmed when mitochondrial ROS production increases in the presence of hyperoxia (9–11). Injured cells activate inflammation cascades and cytokines such as interleukin-1, interleukin-6, and interleukin-8, which have an important role on amplifying the tissue damage by attracting and activating neutrophils, macrophages and other inflammatory cells, causing increased vascular permeability and secondary ROS production. After secondary injury, both endothelial and epithelial cells are damaged, therefore alveolar-capillary barrier integrity is lost, leading to interstitial edema (2, 12, 13).
Animal Models
Since O2 was described by Lavoisier on 18th century, pioneer investigators observed that animals breathing under high O2 atmosphere suffered severe lung inflammation and died within a few hours. In an article published in 1849, Lorrain Smith reported that hyperbaric hyperoxia on mice, rats, guinea pigs and birds, resulted in convulsions and lung congestion leading to death (14). During the 20th century, experiments on different species (rabbits, cats, dogs, monkeys, mice and rats), reported a lethal toxicity of oxygen. In general, these animals developed progressive respiratory distress and died from respiratory failure in between 3 and 6 days (12). The injury level and the exposure time needed to induce death vary notably among animal species. Mortality studies show that a fraction of 0.7 inspired O2 (FIO2) was the upper limit beyond which toxic effects are clinically relevant (15, 16).
Large Animal Models of Hyperoxic Acute Lung Injury (HALI)
Primates were used to model human disease since they are evolutionarily closer to humans than other mammals. HALI was observed in baboons, with progressive pulmonary damage characterized by destruction of endothelial and alveolar type I cells, hypertrophy of type II cells, interstitial edema and neutrophil accumulation (17). In terms of recovery, monkeys who survived a hyperoxic environment and who were allowed to reach full recovery showed normal lung histology suggesting that sub-lethal HALI has a reversible pattern (18). Oxygen toxicity was also proposed as a second hit injury. Studies in baboons revealed a synergistic effect of hyperoxia and bacterial pneumonia on lung injury (19), however, experiments with hyperoxia exposure after oleic acid infusion suggested that previous lung injury does not change the response to hyperoxia (20). Paradoxically, rabbits pre-treated with oleic acid were found to develop a delayed oxygen toxicity and prolonged survival, compared with a non-pre-treated hyperoxic group (21, 22). Furthermore, a potential benefit of hyperoxia was proposed by Milstein et al., who observed in rabbits that hyperoxia induces changes in microcirculatory flow in a reversible manner with a slight impact on macro-hemodynamic parameters (23).
Large Animal Models of Bronchopulmonary Dysplasia (BPD)
The effect of a high oxygen dose during the early stages of life is known to have deleterious effects on lung development. Experimentation on animal models has been essential to understanding the role of hyperoxia and the underlying mechanisms of BPD, a chronic lung disease in which the development of the respiratory system is severely affected. Rabbit models helped to elucidate some important features of the BPD in preterm born infants. The transcriptome analysis on preterm pups shows that the main pathways altered by hyperoxia are related with reactive oxygen species production, inflammation, and lung and vascular development (24). Other reports observed that the gestational age and the FiO2 are determinant factors for BPD, inducing characteristic histological changes and decreasing survival rates (25). Moreover, Jiménez et al. have reported that preterm rabbits exposed to up to seven days of hyperoxia show early structural changes in the lung vasculature and alveoli development compromised with severe consequences on respiratory physiology (26). Nonetheless, short term noninvasive ventilation with 100% O2 as a strategy for preserving spontaneous breathing was shown to be beneficial in preterm rabbits (27). Additionally, therapeutic studies on large animal models have contributed to proposed therapeutic strategies, such as the pharmacological effect of statins on preventing the arterial remodeling and BPD induced by hyperoxia in a rabbit model (28) and the protective effect of the proton-pump inhibitor Omeprazole in a rabbit model of neonatal hyperoxic lung injury by inducing Cytochrome P4501A1 (CYP1A1) activation (29). Moreover, larger preterm animals such as lambs, baboons, and pigs (30–33) have been studied to model human neonatal disease, in which hyperoxia has a key role.
Small Animal Models of Hyperoxic Acute Lung Injury (HALI)
Following the 3Rs (Replacement, Reduction, and Refinement) recommendations in the bioethics principles (34), and considering that large animal models are often costly and time-consuming, small animals have been used for alternative models for biological research.
Rat Models
Rats breathing 24 h in a 100% oxygen atmosphere showed an increase of stress response gene HO-1 (heme oxygenase-1) in lung tissue homogenates (35), and at 48 h the pulmonary surfactant was altered, leading to a decrease in lung compliance after 60 h of hyperoxia (36). Moreover, in a short exposure of 90 min to hyperoxia, inflammatory cells and biomarkers of oxidative stress were increased and histology revealed lung injury in a dose dependent manner (37). Furthermore, experiments in rats revealed that lung injury after 60 h of hyperoxia could be paradoxically prevented by the free radical nitric oxide when it was added to the hyperoxic gas mixture (38).
Mice Models
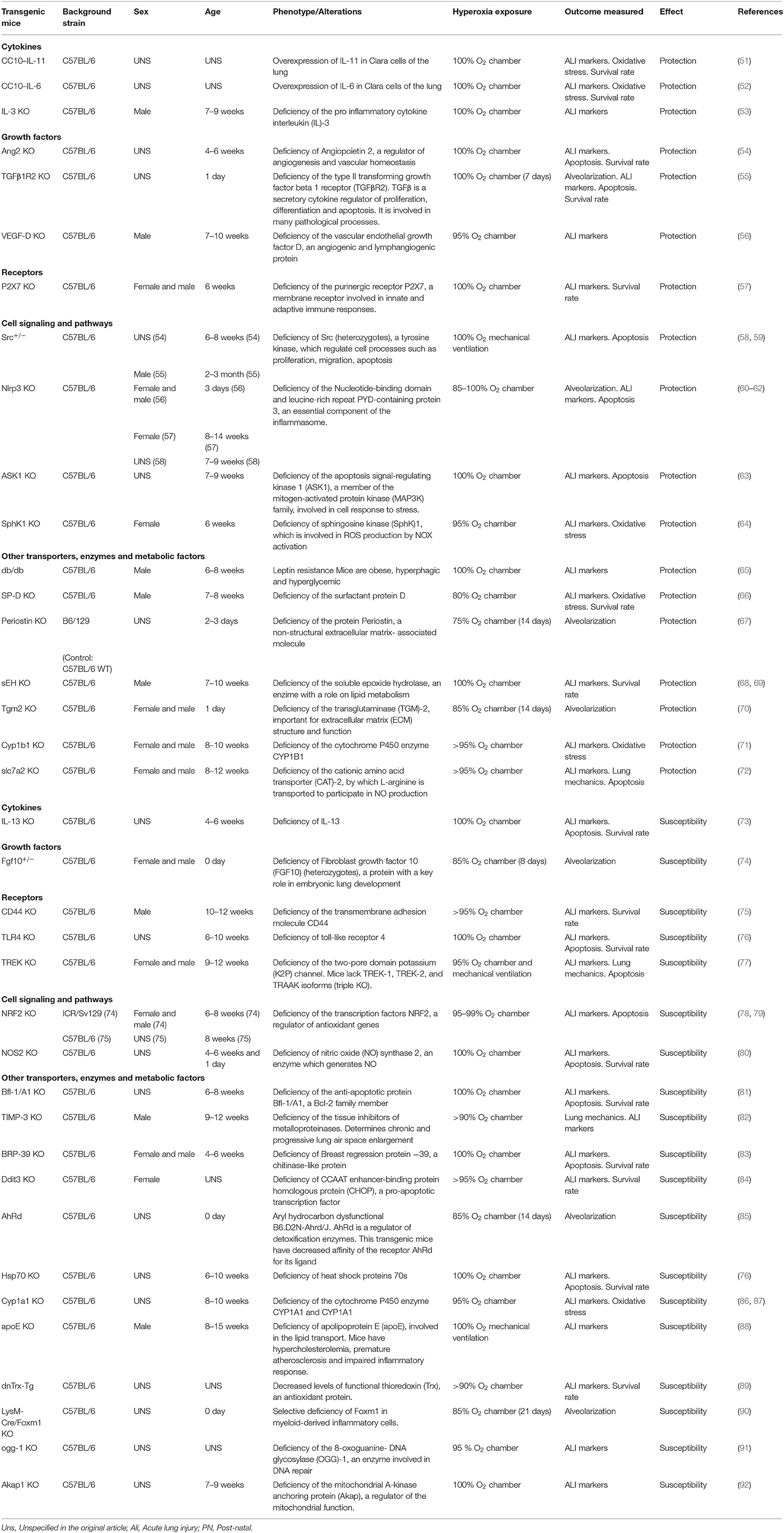
At present, mice models are the most used to study hyperoxia due to the wide availability of transgenic strains, essentials to explore the pathophysiology of HALI. The susceptibility to hyperoxia was shown to vary between mice and rats and within different mice strains. More than 40 years ago, Tierney et al. observed that mice exposed to an enriched oxygen atmosphere for several days have an increased mortality rate when compared to rats (39). Recent research demonstrated that the susceptibility to oxygen toxicity in mice depends on three main factors, (1) sex: the adult C57BL/6 WT male mice had more lung injury and inflammatory edema after hyperoxia exposure (40–42). Also, neonatal male C57BL/6 WT are more susceptible to oxygen toxicity in terms of inflammatory response and impairment in lung development (43–45). Nevertheless, a different sex susceptibility can be observed in other strains. In genetic analysis, Prows et al. showed that in adult mice resulted from the cross of sensitive (C57BL/6) and resistant (29X1/SvJ) progenitor strains, females have increased susceptibility to hyperoxia compared with males (46, 47). Considering the strain and the sex differences in terms of susceptibility is imperative when analyzing the effects of hyperoxic damage. (2) age: the survival rate is inversely correlated with age, and (3) strain: C3H/HeJ and 129X1/SvJ mice are resistant to hyperoxic damage, whereas C57BL/6J background confers a consistent sensitivity to the strain (47–49). In terms of genetic research, Prows et al. demonstrated that the quantitative trait loci Shali-1 and Shali-2 have strong effects on survival times after hyperoxia (46, 50). Experimental hyperoxia on transgenic mice have helped us to understand the pathophysiology of HALI and to elucidate mediators, signaling pathways and cell death mechanisms involved. Some important processes of oxygen toxicity include cytokine (IL-3) and grow factors (TGFß, VEGF, Ang2) release, activation of transmembrane receptor (P2X7) and intracellular pathways including inflammasome, kinases cascades, and oxygen reactive species processes. Many transgenic mice strains have been used to explore hyperoxia, showing different susceptibility patterns, in Table 1 the main reports are listed, describing gene alteration, background strain, sex, age, method of exposure to hyperoxia, outcomes measured and susceptibility pattern (Table 1).
Hyperoxia and Second Hit Models of HALI in Small Rodents
In addition to the described for large animals (20–22), small rodents have also been used to study the role of hyperoxic insult superimposed on a preexisting damage, enhancing its effect (a second hit). Rats exposed continuously to 100% oxygen are more susceptible to toxic acute lung injury with a marked increase in mortality rate (39). Also, hamsters breathing on 70% O2 atmosphere for 72 h after bleomycin instillation showed more pulmonary fibrosis and higher mortality (15). Hakkinen et al. observed similar effect on rats with bleomycin and cyclophosphamide induced lung injury followed by exposition to 80% oxygen for 6 days (93). Moreover, rats have been essential for research on lung mechanical stress and its effects on respiratory physiology. The characterization of the mechanical ventilation induced lung injury (VILI) determined a paradigm shift in the management of critically ill patients. Furthermore, the hyperoxic acute lung injury was proposed to be an additional mechanism on VILI. High tidal volume (Vt) ventilation of 20 ml/kg plus high FiO2 for 2 h was shown to cause significantly more pulmonary edema and neutrophil migration on Sprague-Dawley rats. The mechanical stress can induce chemoattractant MIP-2 (macrophage inflammatory protein-2), which could have a role on this inflammatory response to VILI and HALI (94). Additionally, it was observed in a rat model that the synergistic effect of VILI and HALI impaired alveolar type II cell adhesion due to changes in adhesion proteins by RhoA signaling activation (95). In terms of susceptibility, aged Wistar rats had a more severe lung damage and diaphragmatic dysfunction in a model of short term mechanical ventilation and hyperoxia when compared with adult rats (96). Furthermore, the enhanced effect of hyperoxia on VILI was also widely studied in mice as is discussed in the acute exposure section of this review. Second hit impact of hyperoxia was additionally demonstrated in a rat model of fulminant sepsis, in which increasing FiO2 directly affected mortality rates in a dose dependent manner (97). Experiments in adult mice also observed increased inflammatory injury in with 100% hyperoxia for 48 h after acute lung injury induced by intra-tracheal administration of lipopolysaccharide (LPS) and staphylococcal enterotoxin B (98). These models are relevant for translational analysis, considering that most critically ill patients exposed to high doses of oxygen by mechanical ventilation have some pulmonary or systemic additional disease, resulting in a multi factorial insult to the lungs in which hyperoxia has an important role.
Small Animal Models of Bronchopulmonary Dysplasia (BPD)
Modeling the effects of hyperoxia on the neonatal in mice and rats has many advantages, e.g., they have relatively short gestation times and short lifetimes and are less expensive than larger animals. However, perhaps the most important benefit is that mice and rats are delivered at term in the saccular stage of lung development, like preterm infants that develop BPD. Development of the lung is typically described to occur over five stages: embryonic, pseudo-glandular, canalicular, saccular, and alveolar stages in overlapping periods (99–101). Term human babies (38 week-old) are born in the alveolar phase, whereas preterms between 24–36 weeks are born in the saccular stage. On the other hand, the saccular phase of rats and mice starts on the embryonic day 20 and 17, respectively, and both end in the postnatal day 5, where the alveolar phase starts. Comparison of developmental stages among different animal species can be found in recent state-of-the-art reviews (30, 32, 101, 102). Hyperoxia has been widely used to model BPD as it determines an impaired alveolarization, increased collagen deposition, and interstitial thickness, associated with changes in lung function. Cell proliferation is inhibited, whereas inflammatory cell activation and its products are exacerbated. Proinflammatory cytokines, such as TNF-α, IL-6, and IL-1, play a key role in hyperoxic oxidative lung damage by altering essential growth factors and thus have been proposed as therapeutic targets (103–105). Prolonged exposition to high FiO2 in sealed chambers for several days is the usual model to induce BPD and evaluate the effect of hyperoxia on the impairment of lung development. The dose needed to develop BPD in murine models are frequently very high (FiO2 >0.8), which does not precisely correlate with the dose used usually in patients. Interestingly, lower FiO2 was demonstrated to cause structural alteration in lung tissue, and a dose dependent effect was observed (106–108). Moreover, Yee et al. reported a model closer to the clinical course in human preterm neonates using concentrations from 0.4 to 1 for PN days 1–4, followed by 8 weeks of room air recovery (107). The duration of exposure is also a critical factor in the effect of hyperoxia in lung development. In many studies, continue exposure from PN day 0–28 was used to reproduce histopathological changes similar to BPD (103, 109, 110). Nevertheless, a shorter exposure also induces a marked impairment in lung tissue development (111–114). Consistently, in vitro studies have demonstrated robust changes in cellular metabolism with alteration of oxidative phosphorylation triggered by only 4 h of hyperoxia (114).
Effects of Hyperoxia on Pulmonary Vasculature on Neonatal Models
Hyperoxia is known to alter growth factor signaling, extracellular matrix (ECM) assembly, cell proliferation, apoptosis, and vascular development (100, 115). Experiments in animals have shown that hyperoxia impairs angiogenesis by downregulating essential growth factors such as vascular endothelial growth factor (VEGF) (104, 116–120). Additionally, the role of factors such as hypoxia-inducible factor (117, 121, 122) has been studied in animal models and helped to identify potential therapeutic targets. Transforming growth factor-β (TGF-β) plays a pivotal role during lung development and angiogenesis by regulating endothelial cell growth, differentiation and migration, and ECM production. Recent findings on mice models of hyperoxia reported the contribution of two endoglin isoforms (L- and S-endoglin) to TGF-β downstream signaling and its role on angiogenesis and BPD development (123). Moreover, the vascular consequences of hyperoxia exposure during lung development can cause long term alterations that persist into adulthood. Studies in rats demonstrated that hyperoxia alters the reactivity in pulmonary arteries (124), leading to an impairment of the contractile properties of the right ventricle (125). Pulmonary hypertension signs such as arterial thickness and right ventricle hypertrophy were observed in mice with post-natal hyperoxia, among other systemic vascular structural effects (126–128).
Hyperoxia and Second Hit Models of BDP in Small Rodents
Hyperoxic insult has also been studied as a second hit factor on BPD. The combination of prenatal infections or hypoxia with post-natal noxious stimuli such as mechanical ventilation and hyperoxia are known to be key pathogenic factors. Using a mice model, Gortner et al. combined prenatal grow restriction by hypoxia with post-natal hyperoxia (129, 130) to induce BPD. Interestingly, when hypoxia in short episodes occurs after hyperoxia, the structural alteration is exacerbated (129). Perinatal inflammation induced by LPS, followed by prolonged exposure to hyperoxia produces BPD and was used as a model to study anti-inflammatory therapeutic strategies (131, 132).
Discussion
Chronic Exposure
As a mechanistic approach, experimental animals breathing in a high oxygen atmosphere in a chronic scenario has been widely used. The main method for chronic hyperoxic exposition consists in rooming the animals in closed and sealed chambers with an oxygen enriched atmosphere for several hours or days. This model has been used in many reports with mice and rats, assessing different features of oxygen toxicity. Thereby, exposing rodents to 72 h of a pure oxygen atmosphere in a chamber has been shown to induce lung edema and alveolar leakage with increased oxidative stress markers and recruitment of inflammatory cells and mediators, such as IL1-ß, TNF α and IL-6. Moreover, prolonged exposure causes lung mechanics alterations, increasing airway resistance and diminishing lung compliance; also histological evidence of injury has been consistently observed (53, 57, 72, 77, 92). The extended exposure in a plastic chamber requires an inexpensive system which could be hand crafted, additionally, it is a technically simple and high fidelity reproducible model. It is a suitable method to characterize different phases of acute lung injury and also to observe persistent structural, biochemical and inflammatory changes that can be translated into human diseases such as chronic obstructive pulmonary disease (COPD) with permanent oxygen dependency. Nevertheless, it does not simulate the effect of an acute high fraction of inspiratory oxygen that is frequently delivered to critically ill patients under mechanical ventilation. For this purpose, short exposure models (with and without mechanical ventilation) have been reported and are reviewed in the following section.
Acute Exposure
The exposure to supra-normal concentration of oxygen in an acute scenario usually involves mechanical ventilation (MV). MV entails profound changes on respiratory physiology by applying positive pressure to the lungs and subjecting the parenchyma to abnormal stretching stress, which, if excessive, could lead to ventilator-induced lung injury (VILI), with structural alteration, impaired gas exchange and activation of the inflammatory cascades (133). Hyperoxia added to MV with high tidal volumes (Vt) establishes a scenario with high risk of acute lung injury and systemic inflammation. Animal models of acute hyperoxia are helpful to evaluate the harmful effects of oxygen and MV, resembling what happens in critical care or anesthesia patients. Hence, rodent models have been used to characterize HALI plus VILI lung injury. The ventilatory pattern with large Vt (20 ml/k) and 100% oxygen was observed to induce more pulmonary edema and inflammatory infiltration than the same Vt without hyperoxia in a rat model (94). Pre-exposure to a high oxygen environment was also shown to aggravate lung injury produced by mechanical ventilation in adult mice (134). Also, the potential anti-inflammatory effect of pluripotent stem cells was proposed to ameliorate hyperoxia-augmented VILI through the inhibition of Src-dependent signaling pathway (59). Interestingly, Wagner et al. observed that 4 h of low Vt mechanical ventilation with 100% O2 did not increase lung injury after blunt chest trauma and cigarette smoking in a mouse model, but paradoxically, decreased nitrosative stress (135). Furthermore, hyperoxia without mechanical ventilation was seen to be harmful in acute experimental conditions, namely, mice with 2 h-exposition to 60% O2 showed pulmonary genotoxicity that could be prevented by the halogenated volatile anesthetic Isofluorane by decreasing superoxide anion generation (136). Moreover, in a rat model Nagato et al. observed that 90 min of hyperoxia itself determines lung injury with typical histological changes, immune cells infiltration and excessive oxidative stress (37). All those findings taken together suggest that short term hyperoxia - which is often tolerated in perioperative and severe ill patients - could be as harmful as long term hyperoxia, and should be avoided.
Modeling the Disease
Critically ill adults and newborn babies are often exposed to several insults, including oxygen toxicity. Translational investigation with animal models is essential to explore injuring stimuli in controlled experimental conditions. Nevertheless, some aspects of animal biology differ markedly from human functional mechanisms. Moreover, the models often do not entirely resemble what happens in human pathology and can give an oversimplified explanation of the mechanisms. Many animal models lack the complexity of multifactorial insults, the interaction of mechanisms, and the individual differences that converge in human disease. To address this point, the second hit injury models previously described have been proposed to study the hyperoxic effect in a scenario closer to human disease. Large animal models can be anatomically similar to humans, appropriate to test some therapeutic strategies (i.e., Mechanical ventilation, non-invasive support), and more accessible to technical instrumentation, but are expensive, time demanding, and offer fewer reagent and transgenic models compared with rodents. Models are not good or bad; the point is to choose a model that can test our scientific hypothesis. Animal models of hyperoxia are fundamental to understanding lung diseases and the development of therapeutic strategies.
In summary, hyperoxia is a frequent harmful consequence of oxygen therapy, leading to lung injury and exacerbating the effect of other insults. Translational investigation with animal models is essential to study the toxicity of oxygen in a complete system and explore the mechanisms involved, safety levels, and potential therapeutic targets. In this narrative review, we contribute to the research field by describing and analyzing the main aspects of animal models of hyperoxia and its implications on human pathology.
Author Contributions
LA and LQ carried out the database search and wrote the manuscript. LA, LM, and JH reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
LM was supported by Comisión Sectiorial de Investigación Científica (CSIC) grant I+D-591 2018 #85 and the Chan Zuckerberg Initiative.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are indebted to Prof. David Jameson for his valuable comments and proof reading.
References
1. Chandel NS, Budinger GRS. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. (2007) 42:165–74. doi: 10.1016/j.freeradbiomed.2006.10.048
2. Dias-Freitas F, Metelo-Coimbra C, Roncon-Albuquerque R Jr. Molecular mechanisms underlying hyperoxia acute lung injury. Respir Med. (2016) 119:23–8. doi: 10.1016/j.rmed.2016.08.010
3. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Westerloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. (2015) 19:284. doi: 10.1186/s13054-015-0996-4
4. Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. (2019) 5:18. doi: 10.1038/s41572-019-0069-0
5. Bailey TC, Martin EL, Zhao L, Veldhuizen RA. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J Appl Physiol 1985. (2003) 94:975–82. doi: 10.1152/japplphysiol.00619.2002
6. Sinclair SE, Altemeier Wa Fau - Matute-Bello G, Matute-Bello G Fau - Chi EY, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. (2004) 32:2496–501. doi: 10.1097/01.CCM.0000148231.04642.8D
7. Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. (2016) 316:1583–9. doi: 10.1001/jama.2016.11993
8. Chu DK, Kim LHY, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. (2018) 391:1693–705. doi: 10.1016/S0140-6736(18)30479-3
9. Larosa V, Remacle C. Insights into the respiratory chain and oxidative stress. Biosci Rep. (2018) 38:BSR20171492. doi: 10.1042/BSR20171492
10. Suomalainen A, Battersby BJ. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol. (2018) 19:77–92. doi: 10.1038/nrm.2017.66
11. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. (2015) 97:55–74. doi: 10.1016/j.ejmech.2015.04.040
12. Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. (2013) 58:123–41. doi: 10.4187/respcare.01963
13. Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. (2008) 13:6653–61. doi: 10.2741/3179
14. Smith JL. The pathological effects due to increase of oxygen tension in the air breathed. J Physiol. (1899) 24:19–35. doi: 10.1113/jphysiol.1899.sp000746
15. Tryka AF, Skornik WA, Godleski JJ, Brain JD. Potentiation of bleomycin-induced lung injury by exposure to 70% oxygen. morphologic assessment. Am Rev Respir Dis. (1982) 126:1074–9.
16. Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Ann Rev Physiol. (1986) 48:721–31. doi: 10.1146/annurev.ph.48.030186.003445
17. Fracica PJ, Knapp MJ, Piantadosi CA, Takeda K, Fulkerson WJ, Coleman RE, et al. Responses of baboons to prolonged hyperoxia: physiology and qualitative pathology. J Appl Phisiol. (1991) 71:2352–62. doi: 10.1152/jappl.1991.71.6.2352
18. Kaplan HP, Robinson FR, Kapanci Y, Weibel ER. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. I. Clinical and light microscopic studies. Lab Invest. (1969) 20:94–100.
19. Coalson JJ, King RJ, Winter VT, Prihoda TJ, Anzueto AR, Peters JI, et al. O2- and pneumonia-induced lung injury. I. Pathological and morphometric studies. J Appl Physiol 1985. (1989) 67:346–56. doi: 10.1152/jappl.1989.67.1.346
20. de los Santos R, Coalson JJ, Holcomb JR, Johanson WG Jr. Hyperoxia exposure in mechanically ventilated primates with and without previous lung injury. Exp Lung Res. (1985) 9:255–75. doi: 10.3109/01902148509057527
21. Smith G, Winter PM, Wheelis RF. Increased normobaric oxygen tolerance of rabbits following oleic acid-induced lung damage. J Appl Physiol. (1973) 35:395–400. doi: 10.1152/jappl.1973.35.3.395
22. Winter PM, Smith G, Wheelis RF. The effect of prior pulmonary injury on the rate of development of fatal oxygen toxicity. Chest. (1974) 66(1 Suppl.):1s−4s. doi: 10.1378/chest.66.1_Supplement.1S
23. Milstein DM, Helmers R, Hackmann S, Belterman CN, van Hulst RA, de Lange J. Sublingual microvascular perfusion is altered during normobaric and hyperbaric hyperoxia. Microvasc Res. (2016) 105:93–102. doi: 10.1016/j.mvr.2016.02.001
24. Rogers LK, Salaets T, Richter J, Brady P, Jimenez J, Nagatomo T, et al. Transcriptome analysis of the preterm rabbit lung after seven days of hyperoxic exposure. PLoS ONE. (2015) 10:e0136569. doi: 10.1371/journal.pone.0136569
25. Manzano RM, Mascaretti RS, Carrer V, Haddad LB, Fernandes AR, Reyes AM, et al. A hyperoxic lung injury model in premature rabbits: the influence of different gestational ages and oxygen concentrations. PLoS ONE. (2014) 9:e95844. doi: 10.1371/journal.pone.0095844
26. Jiménez J, Richter J, Nagatomo T, Salaets T, Quarck R, Wagennar A, et al. Progressive vascular functional and structural damage in a bronchopulmonary dysplasia model in preterm rabbits exposed to hyperoxia. Int J Mol Sci. (2016) 17:1776. doi: 10.3390/ijms17101776
27. Dekker J, Hooper SB, Croughan MK, Crossley KJ, Wallace MJ, McGillick EV, et al. Increasing respiratory effort with 100% oxygen during resuscitation of preterm rabbits at birth. Front Pediatr. (2019) 7:427. doi: 10.3389/fped.2019.00427
28. Salaets T, Tack B, Jimenez J, Gie A, Lesage F, de Winter D, et al. Simvastatin attenuates lung functional and vascular effects of hyperoxia in preterm rabbits. Pediatr Res. (2019) 87:1193–200. doi: 10.1038/s41390-019-0711-2
29. Richter J, Jimenez J, Nagatomo T, Toelen J, Brady P, Salaets T, et al. Proton-pump inhibitor omeprazole attenuates hyperoxia induced lung injury. J Transl Med. (2016) 14:247. doi: 10.1186/s12967-016-1009-3
30. Albertine KH. Utility of large-animal models of BPD: chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol. (2015) 308:L983–1001. doi: 10.1152/ajplung.00178.2014
31. Yoder BA, Coalson JJ. Animal models of bronchopulmonary dysplasia. The preterm baboon models. Am J Physiol Lung Cell Mol Physiol. (2014) 307:L970–7. doi: 10.1152/ajplung.00171.2014
32. Caminita F, van der Merwe M, Hance B, Krishnan R, Miller S, Buddington K, et al. A preterm pig model of lung immaturity and spontaneous infant respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. (2015) 308:L118–29. doi: 10.1152/ajplung.00173.2014
33. Davis JM, Dickerson B, Metlay L, Penney DP. Differential effects of oxygen and barotrauma on lung injury in the neonatal piglet. Pediatr Pulmonol. (1991) 10:157–63. doi: 10.1002/ppul.1950100305
34. Prescott MJ, Lidster K. Improving quality of science through better animal welfare: the NC3Rs strategy. Lab Anim. (2017) 46:152–6. doi: 10.1038/laban.1217
35. Lee PJ, Alam J, Sylvester SL, Inamdar N, Otterbein L, Choi AMK. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. Am J Respir Cell Mol Biol. (1996) 14:556–68. doi: 10.1165/ajrcmb.14.6.8652184
36. Pace PW, Yao L-J, Wilson JX, Possmayer F, Veldhuizen RAW, Lewis JF. The effects of hyperoxia exposure on lung function and pulmonary surfactant in a rat model of acute lung injury. Exp Lung Res. (2009) 35:380–98. doi: 10.1080/01902140902745166
37. Nagato A, Silva FL, Silva AR, Bezerra FS, Oliveira ML, Bello-Klein A, et al. Hyperoxia-induced lung injury is dose dependent in Wistar rats. Exp Lung Res. (2009) 35:713–28. doi: 10.3109/01902140902853184
38. Howlett CE, Hutchison JS, Veinot JP, Chiu A, Merchant P, Fliss H. Inhaled nitric oxide protects against hyperoxia-induced apoptosis in rat lungs. Am J Physiol. (1999) 277:L596–605. doi: 10.1152/ajplung.1999.277.3.L596
39. Tierney DF, Ayers L, Kasuyama RS. Altered sensitivity to oxygen toxicity. Am Rev Respir Dis. (1977) 115 :59–65.
40. Lingappan K, Jiang W, Wang L, Couroucli XI, Moorthy B. Sex-specific differences in hyperoxic lung injury in mice: role of cytochrome P450 (CYP)1A. Toxicology. (2015) 331:14–23. doi: 10.1016/j.tox.2015.01.019
41. Lingappan K, Srinivasan C, Jiang W, Wang L, Couroucli XI, Moorthy B. Analysis of the transcriptome in hyperoxic lung injury and sex-specific alterations in gene expression. PLoS ONE. (2014) 9:e101581. doi: 10.1371/journal.pone.0101581
42. Lingappan K, Jiang W, Wang L, Couroucli XI, Barrios R, Moorthy B. Sex-specific differences in hyperoxic lung injury in mice: implications for acute and chronic lung disease in humans. Toxicol Appl Pharmacol. (2013) 272:281–90. doi: 10.1016/j.taap.2013.06.007
43. Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. (2016) 311:L481–93. doi: 10.1152/ajplung.00047.2016
44. Leary S, Das P, Ponnalagu D, Singh H, Bhandari V. Genetic strain and sex differences in a hyperoxia-induced mouse model of varying severity of bronchopulmonary dysplasia. Am J Pathol. (2019) 189:999–1014. doi: 10.1016/j.ajpath.2019.01.014
45. Cheng H, Wang H, Wu C, Zhang Y, Bao T, Tian Z. Proteomic analysis of sex differences in hyperoxic lung injury in neonatal mice. Int J Med Sci. (2020) 17:2440–8. doi: 10.7150/ijms.42073
46. Prows DR, Gibbons WJ Jr, Smith JJ, Pilipenko V, Martin LJ. Age and sex of mice markedly affect survival times associated with hyperoxic acute lung injury. PLoS ONE. (2015) 10:e0130936. doi: 10.1371/journal.pone.0130936
47. Prows DR, Hafertepen AP, Gibbons WJ Jr, Winterberg AV, Nick TG. A genetic mouse model to investigate hyperoxic acute lung injury survival. Physiol Genom. (2007) 30:262–70. doi: 10.1152/physiolgenomics.00232.2006
48. Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am J Respir Cell Mol Biol. (2002) 26:42–51. doi: 10.1165/ajrcmb.26.1.4536
49. Johnston CJ, Stripp BR, Piedbeouf B, Wright TW, Mango GW, Reed CK, et al. Inflammatory and epithelial responses in mouse strains that differ in sensitivity to hyperoxic injury. Exp Lung Res. (1998) 24:189–202. doi: 10.3109/01902149809099582
50. Prows DR, Hafertepen AP, Winterberg AV, Gibbons WJ Jr, Liu C, et al. Genetic analysis of hyperoxic acute lung injury survival in reciprocal intercross mice. Physiol Genom. (2007) 30:271–81. doi: 10.1152/physiolgenomics.00038.2007
51. Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, et al. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest. (1998) 101:1970–082. doi: 10.1172/JCI1337
52. Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, et al. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol. (2000) 22:535–42. doi: 10.1165/ajrcmb.22.5.3808
53. Huang Z, Zhang W, Yang J, Sun F, Zhou H. Interleukin-3 plays a vital role in hyperoxic acute lung injury in mice via mediating inflammation. BMC Pulm Med. (2018) 18:164. doi: 10.1186/s12890-018-0725-2
54. Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. (2006) 12:1286–93. doi: 10.1038/nm1494
55. Sureshbabu A, Syed MA, Boddupalli CS, Dhodapkar MV, Homer RJ, Minoo P, et al. Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung. Respir Res. (2015) 16:4. doi: 10.1186/s12931-014-0162-6
56. Sato T, Paquet-Fifield S, Harris NC, Roufail S, Turner DJ, Yuan Y, et al. VEGF-D promotes pulmonary oedema in hyperoxic acute lung injury. J Pathol. (2016) 239:152–61. doi: 10.1002/path.4708
57. Galam L, Rajan A, Failla A, Soundararajan R, Lockey RF, Kolliputi N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am J Physiol Lung Cell Mol Physiol. (2016) 310:L572–81. doi: 10.1152/ajplung.00417.2015
58. Li LF, Lee CS, Liu YY, Chang CH, Lin CW, Chiu LC, et al. Activation of Src-dependent Smad3 signaling mediates the neutrophilic inflammation and oxidative stress in hyperoxia-augmented ventilator-induced lung injury. Respir Res. (2015) 16:112. doi: 10.1186/s12931-015-0275-6
59. Liu YY, Li LF, Fu JY, Kao KC, Huang CC, Chien Y, et al. Induced pluripotent stem cell therapy ameliorates hyperoxia-augmented ventilator-induced lung injury through suppressing the Src pathway. PLoS ONE. (2014) 9:e109953. doi: 10.1371/journal.pone.0109953
60. Liao J, Kapadia VS, Brown LS, Cheong N, Longoria C, Mija D, et al. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat Commun. (2015) 6:8977. doi: 10.1038/ncomms9977
61. Mizushina Y, Shirasuna K, Usui F, Karasawa T, Kawashima A, Kimura H, et al. NLRP3 protein deficiency exacerbates hyperoxia-induced lethality through Stat3 Protein signaling independent of interleukin-1beta. J Biol Chem. (2015) 290:5065–77. doi: 10.1074/jbc.M114.603217
62. Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK, et al. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol. (2013) 305:C182–9. doi: 10.1152/ajpcell.00086.2013
63. Fukumoto J, Cox R Jr, Fukumoto I, Cho Y, Parthasarathy PT, et al. Deletion of ASK1 protects against hyperoxia-induced acute lung injury. PLoS ONE. (2016) 11:e0147652. doi: 10.1371/journal.pone.0147652
64. Harijith A, Pendyala S, Ebenezer DL, Ha AW, Fu P, Wang YT, et al. Hyperoxia-induced p47phox activation and ROS generation is mediated through S1P transporter Spns2, and S1P/S1P1&2 signaling axis in lung endothelium. Am J Physiol Lung Cell Mol Physiol. (2016) 311:L337–51. doi: 10.1152/ajplung.00447.2015
65. Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. (2007) 175:587–94. doi: 10.1164/rccm.200603-312OC
66. Jain D, Atochina-Vasserman E, Kadire H, Tomer Y, Inch A, Scott P, et al. SP-D-deficient mice are resistant to hyperoxia. Am J Physiol Lung Cell Mol Physiol. (2007) 292:L861–71. doi: 10.1152/ajplung.00145.2006
67. Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, et al. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS ONE. (2012) 7:e31336. doi: 10.1371/journal.pone.0031336
68. Li PS, Tao W, Yang LQ, Shu YS. Effect of soluble epoxide hydrolase in hyperoxic acute lung injury in mice. Inflammation. (2018) 41:1065–72. doi: 10.1007/s10753-018-0758-y
69. Liu LP, Li B, Shuai TK, Zhu L, Li YM. Deletion of soluble epoxide hydrolase attenuates mice hyperoxic acute lung injury. BMC Anesthesiol. (2018) 18:48. doi: 10.1186/s12871-018-0490-z
70. Mizikova I, Pfeffer T, Nardiello C, Surate Solaligue DE, Steenbock H, Tatsukawa H, et al. Targeting transglutaminase 2 partially restores extracellular matrix structure but not alveolar architecture in experimental bronchopulmonary dysplasia. FEBS J. (2018) 285:3056–76. doi: 10.1111/febs.14596
71. Veith AC, Bou Aram B, Jiang W, Wang L, Zhou G, Jefcoate CR, et al. Mice lacking the cytochrome P450 1B1 gene are less susceptible to hyperoxic lung injury than wild type. Toxicol Sci. (2018) 165:462–74. doi: 10.1093/toxsci/kfy154
72. Jin Y, Liu Y, Nelin LD. Deficiency of cationic amino acid transporter-2 protects mice from hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. (2019) 316:L598–l607. doi: 10.1152/ajplung.00223.2018
73. Bhandari V, Choo-Wing R, Homer RJ, Elias JA. Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J Immunol. (2007) 178:4993–5000. doi: 10.4049/jimmunol.178.8.4993
74. Chao CM, Yahya F, Moiseenko A, Tiozzo C, Shrestha A, Ahmadvand N, et al. Fgf10 deficiency is causative for lethality in a mouse model of bronchopulmonary dysplasia. J Pathol. (2017) 241:91–103. doi: 10.1002/path.4834
75. van der Windt GJ, Schouten M, Zeerleder S, Florquin S, van der Poll T. CD44 is protective during hyperoxia-induced lung injury. Am J Respir Cell Mol Biol. (2011) 44:377–83. doi: 10.1165/rcmb.2010-0158OC
76. Zhang Y, Zhang X, Shan P, Hunt CR, Pandita TK, Lee PJ. A protective Hsp70-TLR4 pathway in lethal oxidant lung injury. J Immunol. (2013) 191:1393–403. doi: 10.4049/jimmunol.1300052
77. Schwingshackl A, Lopez B, Teng B, Luellen C, Lesage F, Belperio J, et al. Hyperoxia treatment of TREK-1/TREK-2/TRAAK-deficient mice is associated with a reduction in surfactant proteins. Am J Physiol Lung Cell Mol Physiol. (2017) 313:L1030–46. doi: 10.1152/ajplung.00121.2017
78. Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. (2002) 26:175–82. doi: 10.1165/ajrcmb.26.2.4501
79. Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am J Respir Cell Mol Biol. (2011) 45:1161–8. doi: 10.1165/rcmb.2011-0144OC
80. Bhandari V, Choo-Wing R, Harijith A, Sun H, Syed MA, Homer RJ, et al. Increased hyperoxia-induced lung injury in nitric oxide synthase 2 null mice is mediated via angiopoietin 2. Am J Respir Cell Mol Biol. (2012) 46:668–76. doi: 10.1165/rcmb.2011-0074OC
81. He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, et al. Bcl-2–related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest. (2005) 115:1039–48. doi: 10.1172/JCI23004
82. Martin EL, McCaig LA, Moyer BZ, Pape MC, Leco KJ, Lewis JF, et al. Differential response of TIMP-3 null mice to the lung insults of sepsis, mechanical ventilation, and hyperoxia. Am J Physiol Lung Cell Mol Physiol. (2005) 289:L244–51. doi: 10.1152/ajplung.00070.2005
83. Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, et al. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. (2010) 182:918–28. doi: 10.1164/rccm.200912-1793OC
84. Lozon TI, Eastman AJ, Matute-Bello G, Chen P, Hallstrand TS, Altemeier WA. PKR-dependent CHOP induction limits hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. (2011) 300:L422–9. doi: 10.1152/ajplung.00166.2010
85. Shivanna B, Zhang W, Jiang W, Welty SE, Couroucli XI, Wang L, et al. Functional deficiency of aryl hydrocarbon receptor augments oxygen toxicity-induced alveolar simplification in newborn mice. Toxicol Appl Pharmacol. (2013) 267:209–17. doi: 10.1016/j.taap.2013.01.003
86. Lingappan K, Jiang W, Wang L, Wang G, Couroucli XI, Shivanna B, et al. Mice deficient in the gene for cytochrome P450 (CYP)1A1 are more susceptible than wild-type to hyperoxic lung injury: evidence for protective role of CYP1A1 against oxidative stress. Toxicol Sci. (2014) 141:68–77. doi: 10.1093/toxsci/kfu106
87. Wang L, Lingappan K, Jiang W, Couroucli XI, Welty SE, Shivanna B, et al. Disruption of cytochrome P4501A2 in mice leads to increased susceptibility to hyperoxic lung injury. Free Radic Biol Med. (2015) 82:147–59. doi: 10.1016/j.freeradbiomed.2015.01.019
88. Yamashita CM, Fessler MB, Vasanthamohan L, Lac J, Madenspacher J, McCaig L, et al. Apolipoprotein E-deficient mice are susceptible to the development of acute lung injury. Respiration. (2014) 87:416–27. doi: 10.1159/000358438
89. Das KC. Thioredoxin-deficient mice, a novel phenotype sensitive to ambient air and hypersensitive to hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. (2015) 308:L429–42. doi: 10.1152/ajplung.00285.2014
90. Xia H, Ren X, Bolte CS, Ustiyan V, Zhang Y, Shah TA, et al. Foxm1 regulates resolution of hyperoxic lung injury in newborns. Am J Respir Cell Mol Biol. (2015) 52:611–21. doi: 10.1165/rcmb.2014-0091OC
91. Ye Y, Lin P, Zhang W, Tan S, Zhou X, Li R, et al. DNA repair interacts with autophagy to regulate inflammatory responses to pulmonary hyperoxia. J Immunol. (2017) 198:2844–53. doi: 10.4049/jimmunol.1601001
92. Narala VR, Fukumoto J, Hernandez-Cuervo H, Patil SS, Krishnamurthy S, Breitzig M, et al. Akap1 genetic deletion increases the severity of hyperoxia-induced acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol. (2018) 314:L860–70. doi: 10.1152/ajplung.00365.2017
93. Hakkinen PJ, Morse CC, Martin FM, Dalbey WE, Haschek WM, Witschi HR. Potentiating effects of oxygen in lungs damaged by methylcyclopentadienyl manganese tricarbonyl, cadmium chloride, oleic acid, and antitumor drugs. Toxicol Appl Pharmacol. (1983) 67:55–69. doi: 10.1016/0041-008X(83)90244-2
94. Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol 1985. (2002) 93:517–25. doi: 10.1152/japplphysiol.00570.2001
95. Desai LP, Sinclair SE, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol. (2007) 293:L769–78. doi: 10.1152/ajplung.00127.2007
96. Andrade PV, dos Santos JM, Silva HC, Wilbert DD, Cavassani SS, Oliveira-Junior IS. Influence of hyperoxia and mechanical ventilation in lung inflammation and diaphragm function in aged versus adult rats. Inflammation. (2014) 37:486–94. doi: 10.1007/s10753-013-9762-4
97. Garner WL, Downs JB, Reilley TE, Frolicher D, Kargi A, Fabri PJ. The effects of hyperoxia during fulminant sepsis. Surgery. (1989) 105:747–51.
98. Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. (2005) 3:e174. doi: 10.1371/journal.pbio.0030174
99. Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol. (2014) 307:L936–47. doi: 10.1152/ajplung.00159.2014
100. Hilgendorff A, Reiss I, Ehrhardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol. (2014) 50:233–45. doi: 10.1165/rcmb.2013-0014TR
101. Nardiello C, Mizikova I, Morty RE. Looking ahead: where to next for animal models of bronchopulmonary dysplasia? Cell Tissue Res. (2017) 367:457–68. doi: 10.1007/s00441-016-2534-3
102. O'Reilly M, Thebaud B. Animal models of bronchopulmonary dysplasia. The term rat models. Am J Physiol Lung Cell Mol Physiol. (2014) 307:L948–58. doi: 10.1152/ajplung.00160.2014
103. Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. (1998) 275:L110–7. doi: 10.1152/ajplung.1998.275.1.L110
104. Kaya G, Saldir M, Polat A, Fidanci MK, Erdem A, Erdem G, et al. Evaluation of etanercept treatment in newborn rat model with hyperoxic lung injury. Fetal Pediatr Pathol. (2016) 35:327–38. doi: 10.1080/15513815.2016.1189018
105. Lee DD, Lal CV, Persad EA, Lowe C-W, Schwarz AM, Awasthi N, et al. Endothelial monocyte-activating polypeptide ii mediates macrophage migration in the development of hyperoxia-induced lung disease of prematurity. Am J Respir Cell Mol Biol. (2016) 55:602–12. doi: 10.1165/rcmb.2016-0091OC
106. Li Z, Choo-Wing R, Sun H, Sureshbabu A, Sakurai R, Rehan VK, et al. A potential role of the JNK pathway in hyperoxia-induced cell death, myofibroblast transdifferentiation and TGF-β1-mediated injury in the developing murine lung. BMC Cell Biol. (2011) 12:54. doi: 10.1186/1471-2121-12-54
107. Yee M, Chess PR, McGrath-Morrow SA, Wang Z, Gelein R, Zhou R, et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. (2009) 297:L641–9. doi: 10.1152/ajplung.00023.2009
108. Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, et al. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice*. Chest. (2003) 123:530–8. doi: 10.1378/chest.123.2.530
109. Tibboel J, Joza S, Reiss I, de Jongste JC, Post M. Amelioration of hyperoxia-induced lung injury using a sphingolipid-based intervention. Eur Respir J. (2013) 42:776–84. doi: 10.1183/09031936.00092212
110. Woyda K, Koebrich S, Reiss I, Rudloff S, Pullamsetti SS, Ruhlmann A, et al. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. EurRespir J. (2009) 33:861–70. doi: 10.1183/09031936.00109008
111. Witsch TJ, Turowski P, Sakkas E, Niess G, Becker S, Herold S, et al. Deregulation of the lysyl hydroxylase matrix cross-linking system in experimental and clinical bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. (2014) 306:L246–59. doi: 10.1152/ajplung.00109.2013
112. Popova AP, Bentley JK, Anyanwu AC, Richardson MN, Linn MJ, Lei J, et al. Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol. (2012) 303:L439–48. doi: 10.1152/ajplung.00408.2011
113. Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol. (2010) 108:1347–56. doi: 10.1152/japplphysiol.01392.2009
114. Garcia D, Carr JF, Chan F, Peterson AL, Ellis KA, Scaffa A, et al. Short exposure to hyperoxia causes cultured lung epithelial cell mitochondrial dysregulation and alveolar simplification in mice. Pediatr Res. (2020). doi: 10.1038/s41390-020-01224-5. [Epub ahead of print].
115. Madurga A, MiŽíková I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. (2013) 305:L893–905. doi: 10.1152/ajplung.00267.2013
116. Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, et al. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. (2007) 293:L1099–10. doi: 10.1152/ajplung.00217.2007
117. Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol. (2003) 285:L161–8. doi: 10.1152/ajplung.00285.2002
118. Perkett EA, Klekamp JG. Vascular endothelial growth factor expression is decreased in rat lung following exposure to 24 or 48 hours of hyperoxia: implications for endothelial cell survival. Chest. (1998) 114(1 Suppl.):52s−3. doi: 10.1378/chest.114.1_Supplement.52S
119. Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol. (1997) 16:557–67. doi: 10.1165/ajrcmb.16.5.9160838
120. Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury. Circulation. (2005) 112:2477–86. doi: 10.1161/CIRCULATIONAHA.105.541524
121. Asikainen TM, Chang L-Y, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J. (2006) 20:1698–700. doi: 10.1096/fj.06-5887fje
122. Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am J Physiol Lung Cell Mol Physiol. (2006) 291:L588–95. doi: 10.1152/ajplung.00098.2006
123. Lee Y, Lee J, Nam SK, Hoon Jun Y. S-endoglin expression is induced in hyperoxia and contributes to altered pulmonary angiogenesis in bronchopulmonary dysplasia development. Sci Rep. (2020) 10:3043. doi: 10.1038/s41598-020-59928-x
124. Su Y, Dumas de la Roque E, Smeralda G, Quignard J-F, Freund-Michel V, Courtois A, et al. Altered vasoreactivity in neonatal rats with pulmonary hypertension associated with bronchopulmonary dysplasia: implication of both eNOS phosphorylation and calcium signaling. PLoS ONE. (2017) 12:e0173044. doi: 10.1371/journal.pone.0173044
125. Patel JR, Barton GP, Braun RK, Goss KN, Haraldsdottir K, Hopp A, et al. Altered right ventricular mechanical properties are afterload dependent in a rodent model of bronchopulmonary dysplasia. Front Physiol. (2017) 8:840. doi: 10.3389/fphys.2017.00840
126. Nakanishi H, Morikawa S, Kitahara S, Yoshii A, Uchiyama A, Kusuda S, et al. Morphological characterization of pulmonary microvascular disease in bronchopulmonary dysplasia caused by hyperoxia in newborn mice. Med Mol Morphol. (2018) 51:166–75. doi: 10.1007/s00795-018-0182-2
127. Kumar VHS, Wang H, Kishkurno S, Paturi BS, Nielsen L, Ryan RM. Long-term effects of neonatal hyperoxia in adult mice. Anat Rec. (2018) 301:717–26. doi: 10.1002/ar.23766
128. Menon RT, Shrestha AK, Reynolds CL, Barrios R, Shivanna B. Long-term pulmonary and cardiovascular morbidities of neonatal hyperoxia exposure in mice. Int J Biochem Cell Biol. (2018) 94:119–24. doi: 10.1016/j.biocel.2017.12.001
129. Ratner V, Slinko S, Utkina-Sosunova I, Starkov A, Polin RA, Ten VS. Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia. Neonatology. (2009) 95:299–305. doi: 10.1159/000178798
130. Monz D, Tutdibi E, Mildau C, Shen J, Kasoha M, Laschke MW, et al. Human umbilical cord blood mononuclear cells in a double-hit model of bronchopulmonary dysplasia in neonatal mice. PLoS ONE. (2013) 8:e74740. doi: 10.1371/journal.pone.0074740
131. Nold MF, Mangan NE, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD, et al. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc Natl Acad Sci USA. (2013) 110:14384–9. doi: 10.1073/pnas.1306859110
132. Rudloff I, Cho SX, Bui CB, McLean C, Veldman A, Berger PJ, et al. Refining anti-inflammatory therapy strategies for bronchopulmonary dysplasia. J Cell Mol Med. (2017) 21:1128–38. doi: 10.1111/jcmm.13044
133. Slutsky AS. Lung injury caused by mechanical ventilation. Chest. (1999) 116(1 Suppl.):9s−15. doi: 10.1378/chest.116.suppl_1.9S-a
134. Makena PS, Luellen CL, Balazs L, Ghosh MC, Parthasarathi K, Waters CM, et al. Preexposure to hyperoxia causes increased lung injury and epithelial apoptosis in mice ventilated with high tidal volumes. Am J Physiol Lung Cell Mol Physiol. (2010) 299:L711–9. doi: 10.1152/ajplung.00072.2010
135. Wagner K, Groger M, McCook O, Scheuerle A, Asfar P, Stahl B, et al. Blunt chest trauma in mice after cigarette smoke-exposure: effects of mechanical ventilation with 100% O2. PLoS ONE. (2015) 10:e0132810. doi: 10.1371/journal.pone.0132810
Keywords: hyperoxia, animal models, translational science, lung injury, hyperoxia acute lung injury
Citation: Amarelle L, Quintela L, Hurtado J and Malacrida L (2021) Hyperoxia and Lungs: What We Have Learned From Animal Models. Front. Med. 8:606678. doi: 10.3389/fmed.2021.606678
Received: 15 September 2020; Accepted: 15 February 2021;
Published: 09 March 2021.
Edited by:
Anne Hilgendorff, Ludwig Maximilian University of Munich, GermanyReviewed by:
Trent E. Tipple, University of Oklahoma Health Sciences Center, United StatesXanthi Couroucli, Baylor College of Medicine, United States
Binoy Shivanna, Baylor College of Medicine, United States
Copyright © 2021 Amarelle, Quintela, Hurtado and Malacrida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luciano Amarelle, bGFtYXJlbGxlQGhjLmVkdS51eQ==; Leonel Malacrida, bG1hbGFjcmlkYUBoYy5lZHUudXk=
†These authors share senior authorship
 Luciano Amarelle
Luciano Amarelle Lucía Quintela
Lucía Quintela Javier Hurtado
Javier Hurtado Leonel Malacrida
Leonel Malacrida