- 1Department of Ophthalmology, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
- 2Department of Urology and Guangdong Key Laboratory of Urology, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, China
Objective: We conducted a meta-analysis to explore all the potential risk factors for non-arteritic anterior ischemic optic neuropathy (NAION) based on the published literature.
Methods: A comprehensive literature search through the online databases was performed to obtain studies concerning the risk factors of NAION up to June 2020. Pooled unadjusted odds ratios (ORs) or rate ratios (RRs) were calculated to evaluate the weight of risk factors. This study was registered in PROSPERO under the number CRD42018084960.
Results: Our meta-analysis included 49 original studies comprising of more than 10 million patients. The following risk factors were proved to be significantly associated with NAION: male gender (OR = 1.67, 95% CI: 1.50–1.85, P < 0.00001), hypertension (RR = 1.28, 95% CI: 1.20–1.37, P < 0.00001), hyperlipidemia (RR = 1.43, 95% CI: 1.26–1.62, P < 0.00001), diabetes mellitus (DM) (RR = 1.53, 95% CI: 1.36–1.73, P < 0.00001), coronary heart disease (CHD) (RR = 1.68, 95% CI: 1.24–2.27, P = 0.0008), sleep apnea (RR = 3.28, 95% CI: 2.08–5.17, P < 0.00001), factor V Leiden heterozygous (RR = 2.21, 95% CI: 1.19–4.09, P = 0.01), and medication history of cardiovascular drugs.
Conclusion: We concluded that the above risk factors were significantly related to NAION. Better understanding of these risk factors in NAION can help the direct therapeutic approaches.
Introduction
Non-arteritic anterior ischemic optic neuropathy (NAION) is the most common ischemic optic neuropathy. The incidence rate is 2.5–11.8 per 100,000 cases in men elder than 50 (1). Characterized by optic nerve ischemia mostly due to hypoperfusion of short posterior ciliary arteries (SPCAs) (2), NAION can lead to unilateral, sudden, and painless loss of vision among awake patients. Segmental or diffuse optic disc edema can be observed without evidence of arteritis (3). Although the detailed pathogenesis is unclear, NAION is probably related to systematic hypoperfusion, nocturnal hypotension, local autoregulation failure, and hypercoagulation (2). NAION is a naturally progressive disease and the contralateral eye involvement rate is 15–20% in the following 5 years (4). It has been proved that the medications, including corticosteroids, aspirin, and neurotrophic drugs, have limited and controversial efficacies (5, 6). The risk factors should be taken into thorough consideration when providing precautions and treatments for NAION.
The elder men were susceptible to NAION (1, 3). NAION-related cardiac and cerebral vascular diseases were reported: hypertension, diabetes mellitus (DM), hyperlipidemia, stroke, prothrombotic disorders, and so on (2). Other systematic and ocular factors were also researched in the previously published articles, such as smoking (1), obstructive sleep apnea (7), phosphodiesterase type-5 inhibitors (PDE5-Is) (8), depression, anemia (9), chronic obstructive pulmonary disease (COPD) (10), hypothyroidism (11), small optic disc (12), age-related macular degeneration, and glaucoma (9). Furthermore, several specific genetic polymorphisms were tested for their association with NAION, involving ACE I/D, MTHFR C677T, and factor V Leiden (13).
The two published meta-analyses reported influences of DM or sleep apnea on NAION, respectively (7, 14). However, impacts of other factors differed in literature and no cumulative conclusions were reached. Therefore, we decided to perform a large-scale systematic review and meta-analysis on all the possible NAION risk factors identified in the published studies. To the best of our knowledge, this is the first meta-analysis concentrating on multiple factors of NAION. We expect to help the clinicians comprehensively understand the risk factors of NAION and provide more evidence for the preventions and treatments.
Methods
We conducted this systematic review and meta-analysis in accordance with the meta-analysis of observational studies in epidemiology (MOOSE) guidelines. The protocol registration number of PROSPERO (https://www.crd.york.ac.uk/prospero/) was CRD42018084960.
Search Strategy and Study Selection
Original literature was searched comprehensively through electronic Pubmed, Medline, Embase, and Cochrane Library databases. The related references were also screened, including gray literature (in the website http://graylit.osti.gov/). The language of included studies was restricted to English. The last search was on June 6, 2020. The search terms were applied as follows: “non-arteritic anterior ischemic optic neuropathy” OR “non-arteritic anterior ischaemic optic neuropathy” OR “NAION” OR “NA-AION” in combination with “risk” OR “factor” OR “risk factor.” These terms were searched in all the fields of articles, not restricted to their abstracts.
The inclusion criteria were listed as follows. First, the clinical studies concerning the comparisons of risk factors between NAION and controls were taken into further consideration. Second, the risk factors should exist before the diagnosis of NAION, which was judged by carefully screening the abstracts and/or full texts. Third, the samples of case and control groups were provided directly, or odds ratios (ORs) or rate ratios (RRs) of risk factors were reported with 95% CIs. Accordingly, we excluded the animal experiments, case reports/series, abstracts, conference proceedings, repeated publications, non-published materials, reviews, and editorials.
Two investigators (B. L. and Y. Y.) did the literature search, study screening, data extraction, and eligible study quality assessment independently. The inconsistency was resolved by a third reviewer or via an open discussion.
Data Extraction and Study Quality Assessment
We collected the following data in a prepared standard form: first author, year of publication, country, ethnicity, study design, study duration, sample size, baseline information of the patient, and the number of patients (ORs or RRs with 95% CIs) in both the NAION and the control groups. The Newcastle-Ottawa Scale (NOS) (15) was applied for quality assessment of the non-randomized controlled trials (RCTs). The studies achieving seven or more stars were regarded as high quality.
Statistics Analysis
We calculated the pooled unadjusted ORs or RRs on dichotomous variables to identify the association between the risk factors and NAION. Accordingly, the mean differences (MDs) were used on the continuous variables. Heterogeneity was determined through the chi-square test based on Q and I2 values (16). No significant heterogeneity existed if the p-value was >0.10, and in this condition, the fixed-effect model was used. On the contrary, the random-effect model was applied. We also conducted the subgroup analyses according to different populations. The results were significant in our meta-analysis if a two-sided p-value was < 0.05. Inverted funnel plot visual inspection was to assess the publication bias for all the comparisons, and the Egger's test was added when the number of studies was more than 10. The data analyses were performed in software RevMan (version 5.3; Cochrane Collaboration, Oxford, UK) and STATA (version 13.0; StataCorp, College Station, TX, USA).
Results
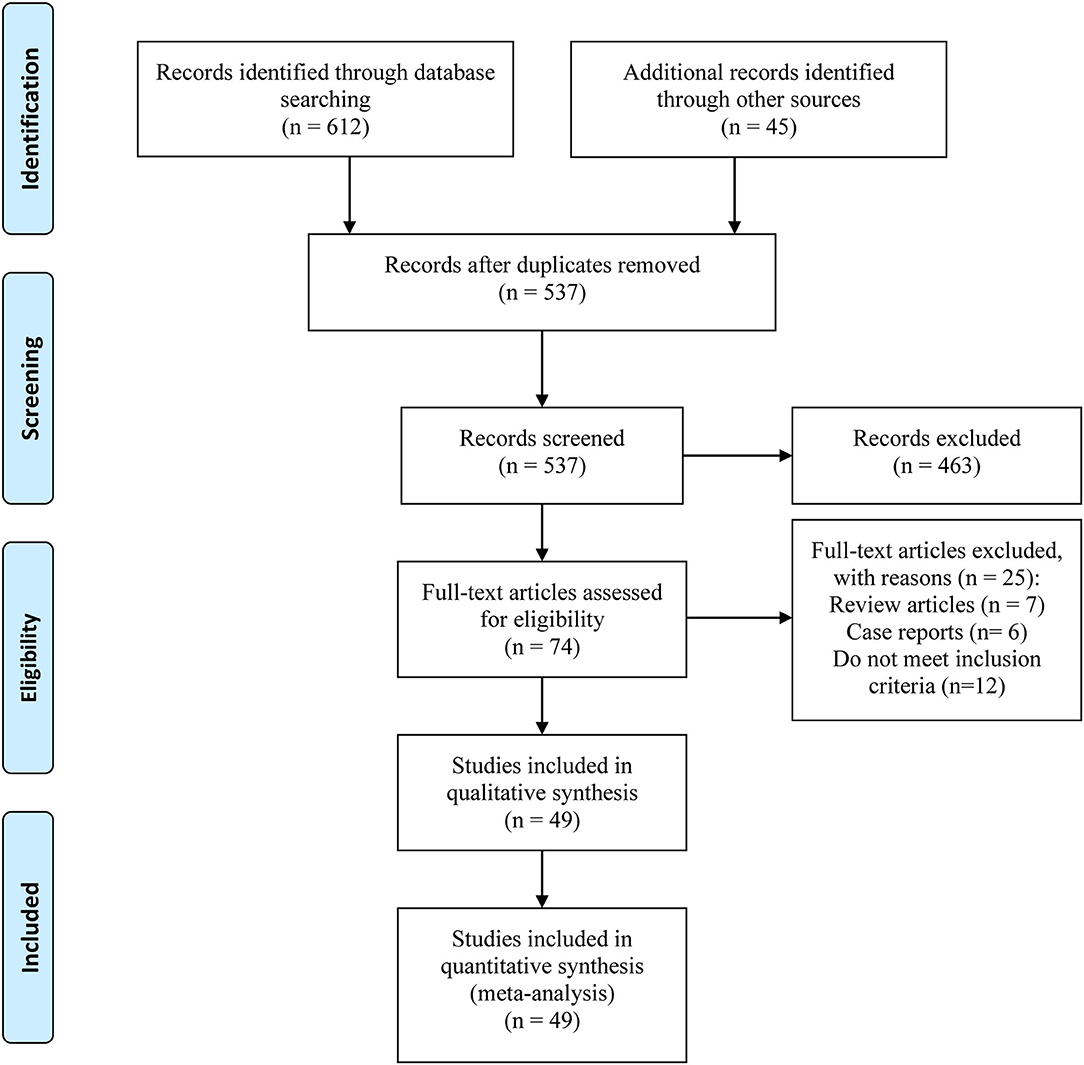
We included a total of 49 studies containing more than 10 million patients. The process of verifying the studies was in accordance with a PRISMA flow diagram (Figure 1). The basic characteristics are listed in Table 1. The studies were published between 1991 and 2019. A total of 38 studies were case-control studies (10, 11, 13, 19, 22–29, 31–35, 37, 39–43, 46–60) and 11 were retrospective cohort studies (9, 17, 18, 20, 21, 30, 36, 38, 44, 45, 61). About 12 of them were studied in the Asian populations, 24 in Europeans, and the remaining 13 in mixed ethnicities. Forty-one studies were graded higher than seven stars, while eight of them were six stars.
Figure 2 shows the relationship between NAION and age, gender, and smoking status. In three studies (9, 19, 48) investigating the influence of age on NAION, results indicated no significance (MD = 0.64 years, 95% CI: −6.50 to 7.78, P = 0.86) with heterogeneity (I2 = 93%, P < 0.00001) (Figure 2A). In eight studies (9, 13, 19, 24, 38, 48, 52, 55) concerning gender, men had significant higher incidence of NAION than women (OR = 1.67, 95% CI: 1.50–1.85, P < 0.00001) without heterogeneity (I2 = 0%, P = 0.63). In the subgroups, five studies in Europeans also demonstrated the higher susceptibility in men (OR = 1.77, 95% CI: 1.23–2.54, P = 0.002). Two studies in mixed ethnicities reached similar results (OR = 1.68, 95% CI: 1.51–1.87, P < 0.00001) (Figure 2B). No heterogeneity existed in both the subgroups. One Asian study showed no difference in NAION incidence between men and women (OR = 0.77, 95% CI: 0.35–1.70, P = 0.51). There were no obvious publication biases detected through the inverted funnel plots of these factors.
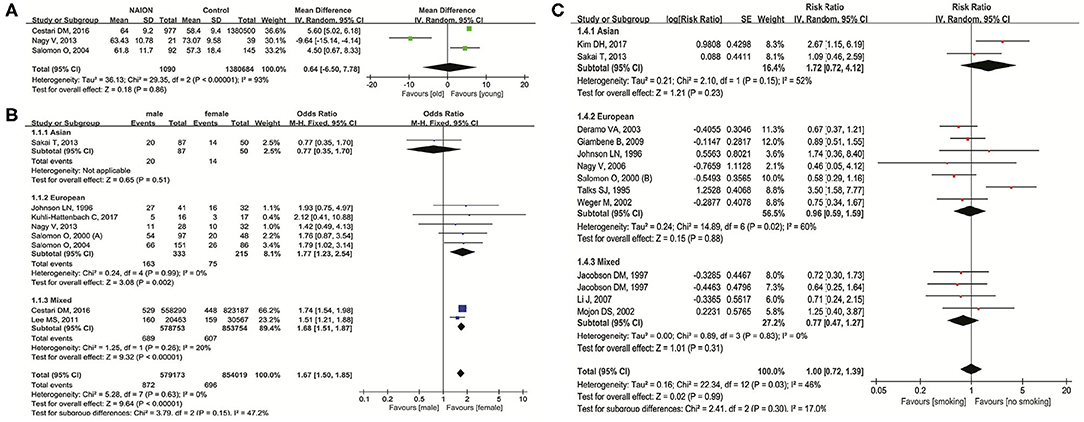
Figure 2. Meta-analysis of the association of nonarteritic anterior ischemic optic neuropathy (NAION) with age (A), gender (B), and smoking (C).
Twelve studies (10, 11, 13, 26, 41, 47, 50, 51, 54–56, 59) researched the impact of current smoking status on NAION and found no significant association (RR = 1.00, 95% CI: 0.72–1.39, P = 0.99) with slight heterogeneity (I2 = 46%, P = 0.03). In the subgroup analysis based on the ethnicities, results in all the three groups (Asian, European, and mixed) pointed out no significant association (Figure 2C). The shape of the inverted funnel plot was approximately symmetrical and the Egger's test did not show any indication of publication bias (t = 0.41, P = 0.691).
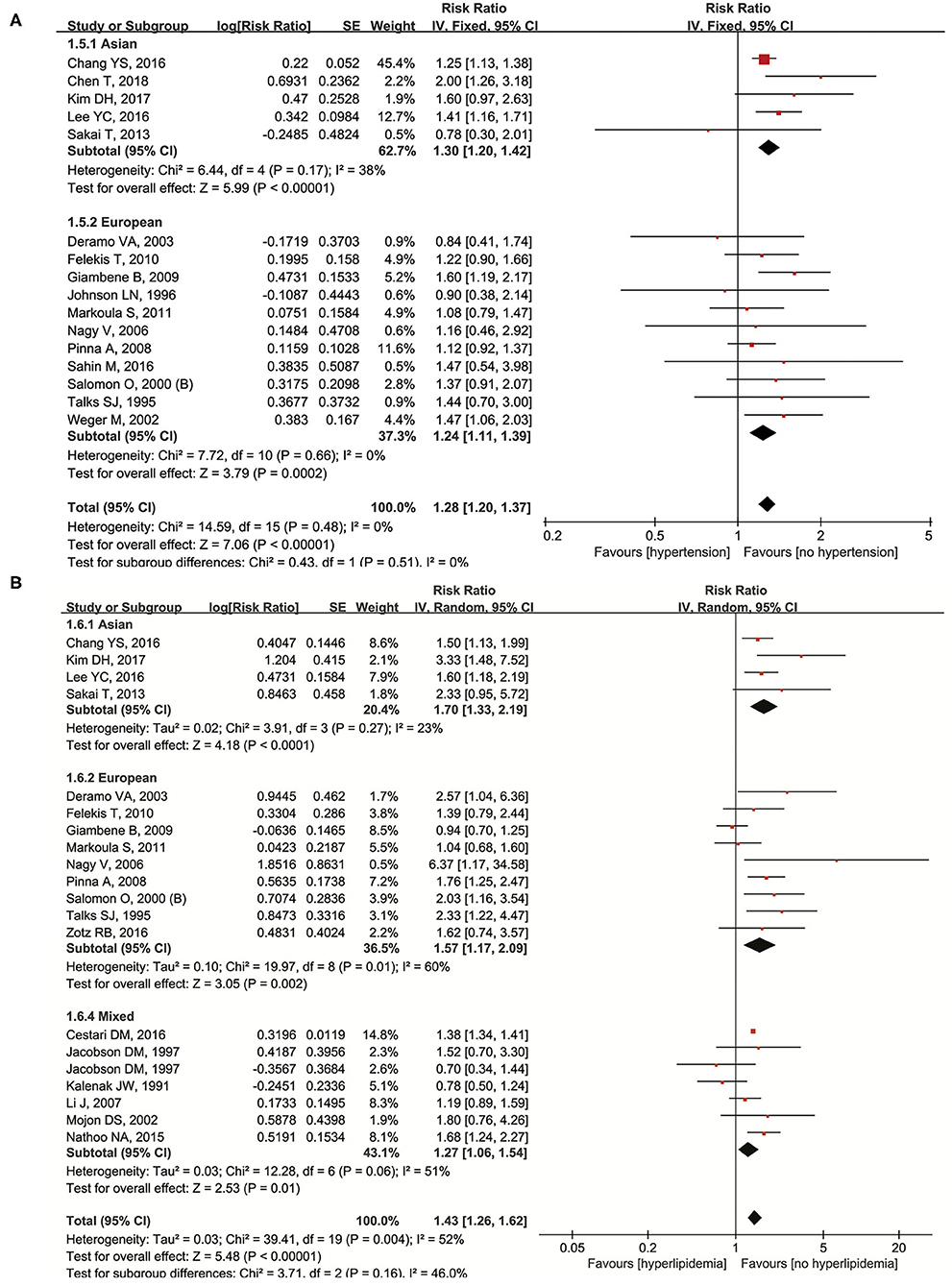
Hypertension was studied in the 16 original articles (11, 13, 22, 26, 29–31, 37, 39, 41, 43, 47, 50, 55, 56, 59). Our results showed that it was a significant risk factor of NAION (RR = 1.28, 95% CI: 1.20–1.37, P < 0.00001) with no heterogeneity (I2 = 0%, P = 0.48). In five of the studies on Asians, a significant higher incidence of NAION was found in hypertensive patients (RR = 1.30, 95% CI: 1.20–1.42, P < 0.00001) and 11 European studies achieved similar results (RR = 1.24, 95% CI: 1.11–1.39, P < 0.00001). Both the cases did not demonstrate heterogeneity (Figure 3A). No significant publication bias was detected through either the inverted funnel plot or the Egger's test (t = 0.24, P = 0.817).
We extracted and pooled data from the 19 original studies (9–11, 13, 26, 27, 30–32, 37, 39, 41, 43, 47, 51, 54, 56, 58, 59) researching hyperlipidemia. It was a significant risk factor of NAION (RR = 1.43, 95% CI: 1.26–1.62, P < 0.00001), and heterogeneity was detected (I2 = 52%, P = 0.004). According to the subgroup analysis, more NAION cases were found in the hyperlipidemia patients among the studies on Asian (RR = 1.65, 95% CI: 1.35–2.01, P < 0.00001), European (RR = 1.57, 95% CI: 1.17–2.09, P = 0.002), and mixed populations (RR = 1.27, 95% CI: 1.06–1.54, P < 0.01). Heterogeneity appeared in the European and mixed groups, while in Asians no heterogeneity was found (Figure 3B). Neither the inverted funnel plot nor the Egger's test showed publication bias (t = 0.97, P = 0.347).
Twenty-three studies (9–11, 13, 22, 26, 29–32, 37–39, 41, 43, 47, 50, 51, 54–56, 58, 59) were included for examining the relationship between DM and NAION. In our meta-analysis, diabetes significantly increased the occurrence of NAION (RR = 1.53, 95% CI: 1.36–1.73, P < 0.00001) and results had heterogeneity (I2 = 55%, P = 0.0006). The subgroup analysis was shown as below: RR was 2.12 (95% CI: 1.40–3.21, P = 0.0004) in Asians, 1.49 (95% CI: 1.01–2.18, P = 0.04) in Europeans, and 1.52 (95% CI: 1.44–1.59, P < 0.00001) in mixed populations. Heterogeneity was shown together with the forest plot (Figure 4A). The inverted funnel plot and the Egger's test showed no publication bias (t = 1.34, P = 0.203).
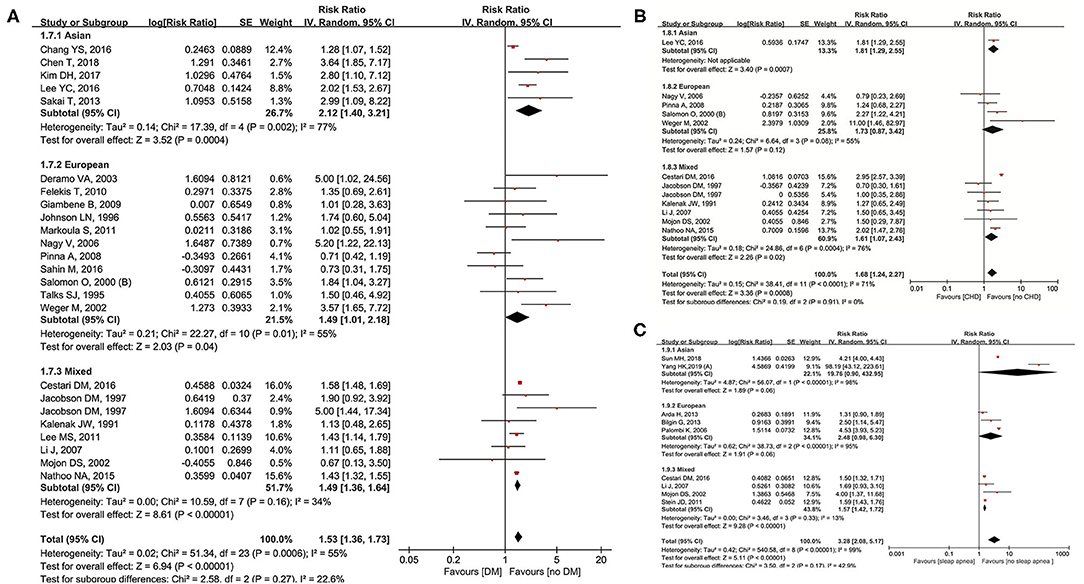
Figure 4. Meta-analysis of the association of NAION with diabetes mellitus (DM) (A), coronary heart disease (CHD) (B), and sleep apnea (C).
The data from 11 original studies (9, 10, 30, 32, 43, 47, 50, 51, 54, 58, 59) were collected for pooled results of coronary heart disease (CHD) on NAION. Our forest plot indicated CHD to be a significant risk factor with RR = 1.68 (95% CI: 1.24–2.27, P = 0.0008). Heterogeneity was also significant (I2 = 71%, P < 0.00001). In the subgroups, one Asian study (RR = 1.81, 95% CI: 1.29–2.55, P = 0.0007) and six studies on mixed ethnicities (RR = 1.61, 95% CI: 1.07–2.43, P = 0.02) found significant association, while no such relationship was detected in four of the Europeans studies (RR = 1.73, 95% CI: 0.87–3.42, P = 0.12). Heterogeneity was obvious in the European and mixed groups, while not applicable in the Asian group (Figure 4B). No significant publication bias was detected through either the inverted funnel plot or the Egger's test (t = −1.00, P = 0.346).
Nine studies (9, 10, 34–36, 38, 46, 51, 61) investigated sleep apnea and its correlation with NAION. Sleep apnea impacted the existence of NAION significantly (RR = 3.28, 95% CI: 2.08–5.17, P < 0.00001) bearing heterogeneity (I2 = 99%, P < 0.00001). Four studies on the mixed ethnicities observed positive association (RR = 1.57, 95% CI: 1.42–1.72, P < 0.00001) without heterogeneity (I2 = 13%, P = 0.33), while three of the European studies demonstrated no influence of sleep apnea on NAION (Figure 4C) in the subgroup analysis. The inverted funnel plot did not show publication bias.
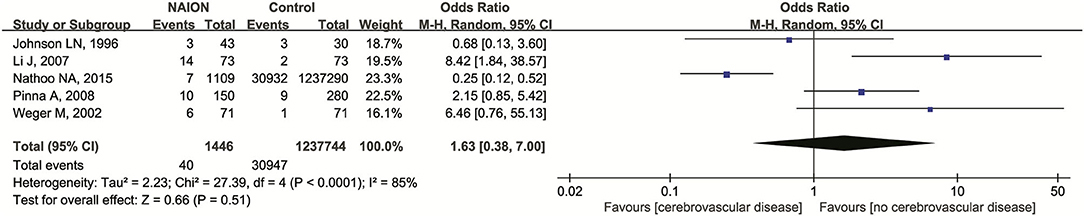
Cerebrovascular disease was investigated in a total of five studies (10, 32, 43, 50, 55). No significant association was detected after we pooled the original data (OR = 1.63, 95% CI: 0.38–7.00, P = 0.51). Heterogeneity was found significantly (I2 = 85%, P < 0.00001) (Figure 5). No publication bias was detected through the inverted funnel plot.
The laboratory biochemical markers measuring coagulative status were examined and studied (27, 40, 41, 47, 53, 56). Our meta-analysis found the following three factors to be significantly related to NAION: increased fibrinogen (RR = 1.78, 95% CI: 1.26–2.52, P = 0.001), hyperhomocysteinemia (RR = 3.14, 95% CI: 1.99–4.93, P < 0.00001), and high level of lipoprotein(a) (RR = 1.36, 95% CI: 1.09–1.71, P = 0.007) (Table 2). All the results showed no heterogeneity. Elevated VIII factor had no significant influence on NAION (RR = 1.91, 95% CI: 0.79–4.63, P = 0.15) with apparent heterogeneity (I2 = 82%, P = 0.04). There were no publication biases detected through the inverted funnel plot for all the above factors.
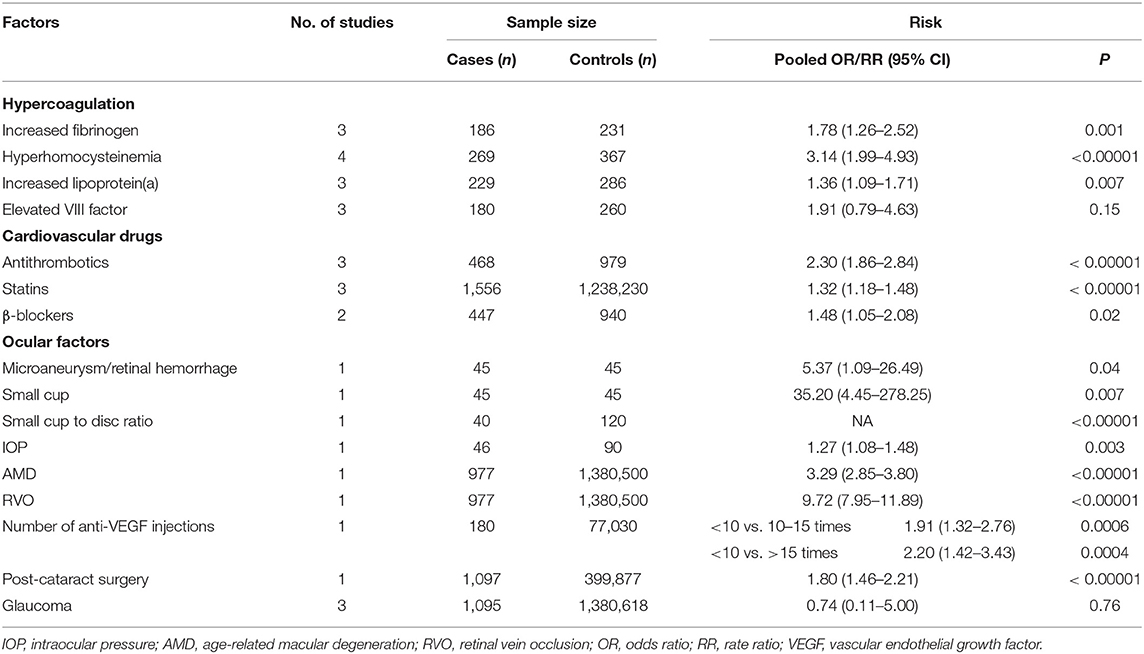
Table 2. Meta-analysis results of the association of hypercoagulation, cardiovascular drugs, and ocular risk factors with non-arteritic anterior ischemic optic neuropathy (NAION).
Data of cardiovascular drugs history were collected and pooled. Three studies (19, 30, 42) inspected the relationship of antithrombotics and NAION. Significant association was detected (OR = 2.30, 95% CI: 1.86–2.84, P < 0.00001) with no heterogeneity (I2 = 0%, P = 0.39). In detail, a significant increase in the NAION cases was found in patients taking statins (OR = 1.32, 95% CI: 1.18–1.48, P < 0.00001). Beta-blockers (β-blockers) were also investigated in the two studies with significant correlation to NAION (OR = 1.48, 95% CI: 1.05–2.08, P = 0.02) (Table 2).
We analyzed the six genotypes and gained pooled results from the nine included studies (13, 22, 27, 37, 39, 41, 47, 49, 59). Factor V Leiden heterozygous cases were more susceptible to NAION (RR = 2.21, 95% CI: 1.19–4.09, P = 0.01), while five genotypes were not significant: MTHFR 677T heterozygous (OR = 0.96, 95% CI: 0.11–8.40, P = 0.97), MTHFR 677T homozygous (OR = 1.78, 95% CI: 0.58–5.49, P = 0.31), ACE DD (OR = 0.92, 95% CI: 0.44–1.89, P = 0.81), ACE II (OR = 0.87, 95% CI: 0.59–1.30, P = 0.51), and ACE ID (OR = 1.09, 95% CI: 0.70–1.70, P = 0.69). Inverted funnel plots did not show the publication biases.
Six trials from the five studies were analyzed for the impact of 1-month use of PDE5-Is on NAION (25, 32, 33, 44, 45). Results from meta-analysis showed no significant increased trends of NAION incidence (RR = 1.16, 95% CI = 0.98–1.39, P = 0.09) with obvious heterogeneity (I2 = 57%, P = 0.04) (Figure 6). No obvious publication bias was detected through the inverted funnel plot visual inspection.
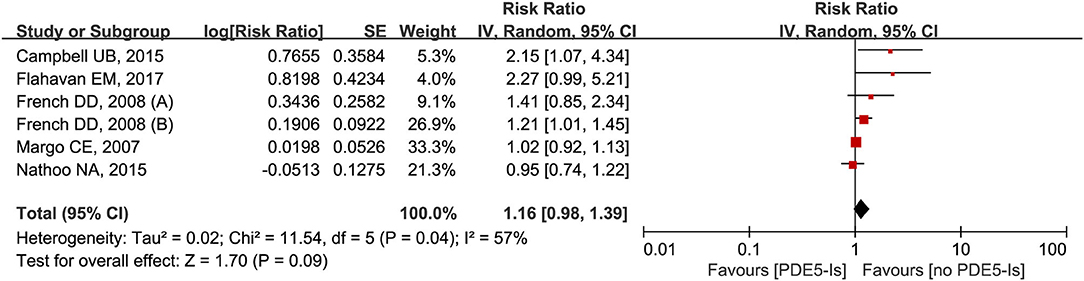
Figure 6. Meta-analysis of the association of NAION with the use of phosphodiesterase type-5 inhibitors (PDE5-Is) within 1-month.
Apart from the systematic diseases, ocular factors were also studied and recorded comprehensively in the published literature; however, they were mentioned in less than the three articles. In three studies (9, 10, 58) concerning glaucoma, no significant association was found (OR = 0.74, 95% CI: 0.11–5.00, P = 0.76) with heterogeneity (I2 = 90%, P < 0.00001). Microaneurysm/retinal hemorrhage, small optic cup, high intraocular pressure (29), small cup to disc ratio (CDR) (60), age-related macular degeneration, retinal vein occlusion (9), anti-vascular endothelial growth factor injection times (17), and post-cataract surgery (18) were all studied in one literature, which is listed in Table 2.
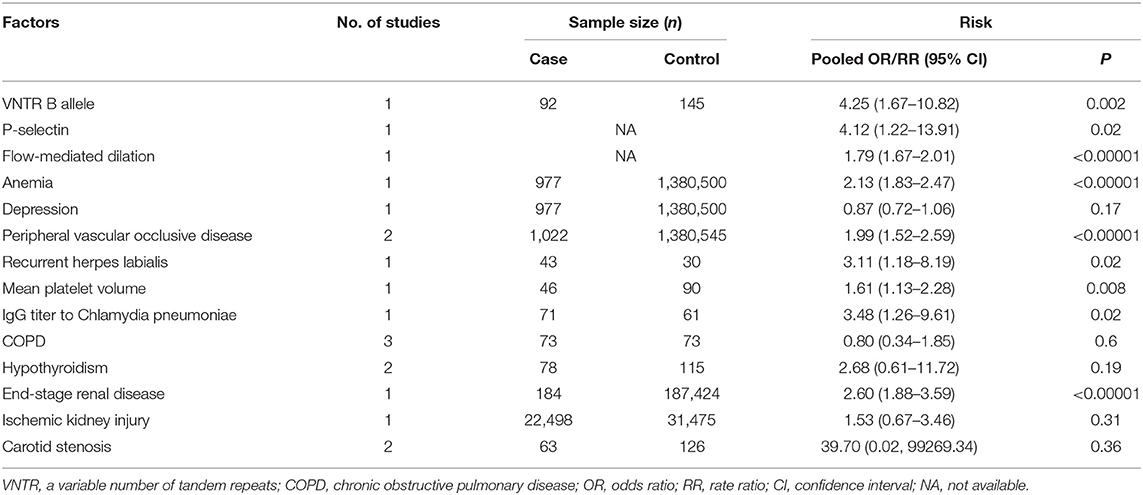
Table 3 provides 14 factors researched in <3 studies. Positive B allele of the glycoprotein Ibα variable number of tandem repeats (48), increased P-selectin (19), decreased flow-mediated dilation (28), anemia (9), peripheral vascular occlusive disease (9, 58), recurrent herpes labialis (55), increased mean platelet volume (24), elevated IgG titer to Chlamydia pneumonia (50), and end-stage renal disease (31), all indicated a significant association with NAION, while depression (9), COPD (10, 54), hypothyroidism (11, 56), ischemic kidney injury (38), and carotid stenosis (23, 57) had no such relationship.
Discussion
In our systematic review and meta-analysis, we included articles studying a variety of risk factors: age, gender, ethnicity, systematic diseases, ocular factors, genotypes, cardiovascular drugs, and so on. We finally concluded the following risk factors to be significantly associated with NAION: male gender, hypertension, hyperlipidemia, DM, CHD, sleep apnea, medication history of cardiovascular drugs, and factor V Leiden heterozygous. Some other systematic and ocular diseases were researched in <3 studies and did not seem to be significant risk factors. In the subgroup analyses based on ethnicities, we found that the influences of gender in Asians and CHD and sleep apnea in Europeans were not significant. Therefore, we should take notice when applying our results to different populations.
In our meta-analysis, the cardiovascular factors were highly associated with NAION and studied in most literature, including hypertension, hyperlipidemia, and DM. NAION probably results from topical and/or systematic hypoperfusion (2). Although this is not a thrombotic event, many predisposing factors related to thrombogenesis or hypercoagulative state can disturb the systematic blood circulation via different pathways (41). For example, hyperlipidemia is harmful to endothelial cells and accelerates the formation of atherosclerotic plaques, further leading to hypertension and CHD (27). Some biochemical markers (such as hyperhomocysteinemia) and genetic polymorphisms (such as factor V Leiden heterozygous) indicating hypercoagulative state were also significant risk factors in our meta-analysis. Therefore, the above factors are co-factors of NAION with similar mechanisms. Drugs treating cardiovascular diseases were proved to be significantly associated with NAION, too, such as antithrombotics, β-blockers, and statins. These drugs could not be considered as independent risk factors because they were only applied to treat the diseases.
Although the above major factors were reported to induce NAION, we summarized and reconfirmed these conclusions. In addition, we included the hypercoagulative biomarkers and possible risky genetic polymorphisms from the published literature. We first performed the meta-analyses on these factors, demonstrating that increased homocysteinemia, fibrinogen, lipoprotein(a), and factor V Leiden heterozygous were risk factors of NAION. Several diseases and biomarkers were identified in <3 studies, which are listed in Table 3. They might be the potential risk factors if more original studies were carried out. Specifically, we conducted the subgroup analyses based on ethnicities in seven risk factors. The cases were divided into Asians, Europeans, and mixed ethnicities from original publications. The ethnicity did not affect the association between smoking, hypertension, hyperlipidemia, DM, and NAION. Nevertheless, the incidence of NAION had no disparity in different gender among the Asians. CHD and sleep apnea were not significant risk factors in Europeans. This subgroup analysis expanded the application of our results to different peoples.
Diabetes and sleep apnea were proved to be important risk factors in the previously published meta-analyses (7, 14). We reconducted meta-analyses on both factors with 11 new studies included on diabetes and three on sleep apnea. Apart from the verification of their conclusions with larger sample sizes, we also performed the subgroup analyses according to ethnicities as stated above. For DM, its influence on NAION was not related to ethnicity in our pooled results, similar to the conclusions of Chen et al. (14). Furthermore, our conclusions were more powerful with three extra cohort studies compared with the previous meta-analysis (14), in which the included studies were all case-controlled because the cohort studies had a higher level of evidence. Sleep apnea was not a significant risk factor of NAION in the Europeans after our subgroup analysis, although a published study found that the Europeans were more likely to have NAION (14). However, only three studies were included in this subgroup, and we achieved marginal data (P = 0.06). We expected more valid original studies on different ethnicities, in case, a high-quality meta-analysis could be conducted.
We found no apparent association between the occurrence of NAION and 1-month use of PDE5-Is, which was published by us in 2018 (8). Although PDE5-Is mainly cause vasodilation and systematic hypotension (32), their influences on the NAION pathogenesis remain controversial. Because of the relatively low incidence of NAION and difficulties in diagnosis, it was hard to include adequate samples in the published literature, and confounders were not adjusted in several case-control studies. More clinical studies are necessarily needed to provide strong evidence on this point.
A crowded optic disc was often observed in NAION because hypoperfusion or ischemia of the optic nerve head is apparent in a tight optic disc structure (26). An optical coherence tomography (OCT) is a useful tool to measure the CDR. Several studies showed the association between the CDR and NAION. For example, González Martín-Moro et al. reported a smaller CDR to be a risk factor and poor prognostic marker of NAION based on the measurements by OCT (60). OCT angiography can detect the perfusion and vessel density of optic disc in patients with NAION, further providing more information of blood supply around optic nerve head. Fard et al. and Gandhi et al. found that the patients with NAION lost some vascular networks and perfusion in the optic disc areas (62, 63). However, most of the studies compared the CDR between NAION and controls, but did not explain the definition of “small optic disc.” Pooled ORs or RRs of small CDR on NAION could not be extracted from these studies, so they did not meet our inclusion criteria. A meta-analysis studying this topic can be conducted in our future work, specifically collecting the continuous variables from these original articles.
Some other factors were researched in <3 studies. Johnson and colleagues (55) researched the impact of recurrent herpes labialis on NAION and reached a significant relationship. Weger et al. observed an elevated IgG titer to Chlamydia pneumonia in NAION cases compared with normal controls (50). Both pathogens disturb the function of endothelial cells, activate the secretion of inflammatory cytokines, and thus induce or promote atherosclerosis. In the cohort study by Chang et al., end-stage renal disease was proved to increase the risk of NAION (31), probably because these patients had received repeated hemodialysis, which resulted in systematic hypotension. Zhu et al. (23) and Fry et al. (57) achieved different results on the relationship between carotid stenosis and NAION. Although the carotid arterial abnormities can possibly cause ocular arterial disturbance by potential embolism and decreased perfusion (64), no consistent conclusions were reached due to the lack of well-planned studies with large sample sizes. All the above research were carried out in a small number of studies, so the studies with larger samples size are necessary to confirm these findings.
Although our meta-analysis was conducted comprehensively and included multiple potential risk factors of NAION, it still has several limitations. First, all the included original articles were case-control or retrospective cohort studies. No RCTs or prospective cohort studies have been published yet. Recalled data might be incomplete and inaccurate, bringing biases to these studies. Second, most NAION possible factors have similar mechanisms and act as confounders. Independence of these factors may probably be examined by RCTs; however, no RCTs can be conducted on studying the risk factors. Third, the time span of the included studies lasts from 1991 to 2019, during which the changes in lifestyles and medical techniques influence the type of risk factors. Finally, some risk factors were examined in <3 studies and it was hard to confirm their association with NAION. The above limitations reduced the quality of our meta-analysis partly and restricted our results to be applied.
Consequently, our study concluded that the following risk factors were associated with NAION: male gender, hypertension, hyperlipidemia, DM, CHD, sleep apnea, medication history of cardiovascular drugs, and factor V Leiden heterozygous. Better understanding of these risk factors in NAION can direct future research and therapeutic approaches.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
BL, TD, and DX: conceptualization and project administration. BL and YY: literature search and screening, formal analysis, data curation, and writing—original draft preparation. BL and TD: methodology and software. BL, YY, and TD: validation. WL, TD, and DX: writing—review and editing. TD and DX: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the Natural Science Foundation of Guangdong Province, China (No. 2020A1515110113) and the Science and Technology Planning Project of Guangzhou, China (No. 202102020113).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye. (2015) 29:65–79. doi: 10.1038/eye.2014.144
2. Berry S, Lin WV, Sadaka A, Lee AG. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain. (2017) 27:23–28. doi: 10.2147/EB.S125311
3. Barros AE, Amram AL, Derham AM, Smith SV, Lee AG. Management of ischemic optic neuropathies. Expert Rev Ophthalmol. (2017) 12:99–109. doi: 10.1080/17469899.2017.1291341
4. Fraser JA, Rueløkke LL, Malmqvist L, Hamann S. Prevalence of optic disc drusen in young patients with nonarteritic anterior ischemic optic neuropathy: a 10-year retrospective study. J Neuroophthalmol. (2021) 41:200–5. doi: 10.1097/WNO.0000000000000974
5. Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol. (2008) 246:1029–46. doi: 10.1007/s00417-008-0805-8
6. Kaderli B, Avci R, Yucel A, Guler K, Gelisken O. Intravitreal triamcinolone improves recovery of visual acuity in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. (2007) 27:164–8. doi: 10.1097/WNO.0b013e31814a5a9a
7. Wu Y, Zhou LM, Lou H, Cheng JW, Wei RL. The association between obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Curr Eye Res. (2016) 41:987–92. doi: 10.3109/02713683.2015.1075221
8. Liu B, Zhu L, Zhong J, Zeng G, Deng T. The association between phosphodiesterase type 5 inhibitor use and risk of non-arteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Sex Med. (2018) 6:185–92. doi: 10.1016/j.esxm.2018.03.001
9. Cestari DM, Gaier ED, Bouzika P, Blachley TS, De Lott LB, Rizzo JF, et al. Demographic, systemic, and ocular factors associated with nonarteritic anterior ischemic optic neuropathy. Ophthalmology. (2016) 123:2446–55. doi: 10.1016/j.ophtha.2016.08.017
10. Li J, McGwin G Jr, Vaphiades MS, Owsley C. Non-arteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ). Br J Ophthalmol. (2007) 91:1524–7. doi: 10.1136/bjo.2006.113803
11. Deramo VA, Sergott RC, Augsburger JJ, Foroozan R, Savino PJ, Leone A. Ischemic optic neuropathy as the first manifestation of elevated cholesterol levels in young patients. Ophthalmology. (2003) 110:1041–6. doi: 10.1016/S0161-6420(03)00079-4
12. Moghimi S, Afzali M, Akbari M, Ebrahimi KB, Khodabande A, Yazdani-Abyaneh AR, et al. Crowded optic nerve head evaluation with optical coherence tomography in anterior ischemic optic neuropathy. Eye. (2017) 31:1191–8. doi: 10.1038/eye.2017.56
13. Sakai T, Shikishima K, Matsushima M, Tsuneoka H. Genetic polymorphisms associated with endothelial function in nonarteritic anterior ischemic optic neuropathy. Mol Vis. (2013) 19:213–9.
14. Chen T, Song D, Shan G, Wang K, Wang Y, Ma J, et al. The association between DM and nonarteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. PLoS ONE. (2013) 8:e76653. doi: 10.1371/journal.pone.0076653
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Higgins J, Green S (editors). Cochrane handbook for systematic reviews of interventions. In: The Cochrane Collaboration,. A John Wiley & Sons, Ltd., Publication (2011). Available online at: https://handbook-5–1.cochrane.org/ (accessed July 21, 2018).
17. Chen YY, Chou P, Huang YF, Chien HJ, Wu YC, Lee CC, et al. Repeated intravitreal injections of antivascular endothelial growth factor in patients with neovascular age-related macular degeneration may increase the risk of ischemic optic neuropathy. BMC Ophthalmol. (2019) 19:268. doi: 10.1186/s12886-019-1284-x
18. Yang HK, Park SJ, Byun SJ, Park KH, Hwang JM. Risk of nonarteritic anterior ischemic optic neuropathy after cataract surgery. Am J Ophthalmol. (2019) 207:343–50. doi: 10.1016/j.ajo.2019.08.001
19. Nagy V, Kolozsvari B, Balogh Z, Csutak A, Kasza M, Nagy B Jr, et al. Increased level of platelet P-selectin in nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. (2013) 251:917–22. doi: 10.1007/s00417-012-2196-0
20. Lee JY, Park KA, Oh SY. Risk of ischemic kidney injury in patients with nonarteritic anterior ischemic optic neuropathy: a nationwide population-based study. Am J Ophthalmol. (2018) 187:153–7. doi: 10.1016/j.ajo.2017.12.025
21. Sun MH, Lee CY, Liao YJ, Sun CC. Nonarteritic anterior ischaemic optic neuropathy and its association with obstructive sleep apnoea a health insurance database study. Acta Ophthalmol. (2019) 97:e64–70. doi: 10.1111/aos.13832
22. Chen T, Ma J, Shan G, Zhong Y. The polymorphisms of ATOH 7, ET-1 and ACE in non-arteritic anterior ischemic optic neuropathy. Exp Eye Res. (2018) 174:147–51. doi: 10.1016/j.exer.2018.05.021
23. Zhu WH, Chen TT, Jin L, Wang HY, Yao FJ, Wang CY, et al. Carotid artery intimal medial thickness and carotid artery plaques in hypertensive patients with non-arteritic anterior ischaemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. (2017) 255:2037–43. doi: 10.1007/s00417-017-3744-4
24. Kuhli-Hattenbach C, Hellstern P, Kohnen T, Hattenbach LO. Platelet activation by ADP is increased in selected patients with anterior ischemic optic neuropathy or retinal vein occlusion. Platelets. (2017) 28:720–3. doi: 10.1080/09537104.2016.1276548
25. Flahavan EM, Li H, Gupte-Singh K, Rizk RT, Ruff DD, Francis JL, et al. Prospective case-crossover study investigating the possible association between nonarteritic anterior ischemic optic neuropathy and phosphodiesterase type 5 inhibitor exposure. Urology. (2017) 105:76–84. doi: 10.1016/j.urology.2017.02.044
26. Kim DH, Shin GR, Choi YJ. Risk factors for non-arteritic anterior ischaemic optic neuropathy in a Korean population. Neuroophthalmology. (2017) 41:68–75. doi: 10.1080/01658107.2016.1267771
27. Zotz RB, Finger C, Scharf RE, Unsöld R. Associations between thrombophilic risk factors and determinants of atherosclerosis and inflammation in patients with non-arteritic anterior ischaemic optic neuropathy. Hamostaseologie. (2016) 36:46–54. doi: 10.5482/HAMO-15-10-0028
28. Yao F, Wan P, Su Y, Liao R, Zhu W. Impaired systemic vascular endothelial function in patients with non-arteritic anterior ischaemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. (2016) 254:977–81. doi: 10.1007/s00417-015-3212-y
29. Sahin M, Sahin A, Elbey B, Yüksel H, Türkcü FM, Cingü AK. Mean platelet volume in patients with nonarteritic anterior ischemic optic neuropathy. J Ophthalmol. (2016) 2016:1051572. doi: 10.1155/2016/1051572
30. Lee YC, Wang JH, Huang TL, Tsai RK. Increased risk of stroke in patients with nonarteritic anterior ischemic optic neuropathy: a nationwide retrospective cohort study. Am J Ophthalmol. (2016) 170:183–9. doi: 10.1016/j.ajo.2016.08.006
31. Chang YS, Weng SF, Chang C, Wang JJ, Su SB, Huang CC, et al. Risk of nonarteritic anterior ischemic optic neuropathy following end-stage renal disease. Medicine. (2016) 95:e3174. doi: 10.1097/MD.0000000000003174
32. Nathoo NA, Etminan M, Mikelberg FS. Association between phosphodiesterase-5 inhibitors and nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. (2015) 35:12–5. doi: 10.1097/WNO.0000000000000186
33. Campbell UB, Walker AM, Gaffney M, Petronis KR, Creanga D, Quinn S, et al. Acute nonarteritic anterior ischemic optic neuropathy and exposure to phosphodiesterase type 5 inhibitors. J Sex Med. (2015) 12:139–51. doi: 10.1111/jsm.12726
34. Bilgin G, Koban Y, Arnold AC. Nonarteritic anterior ischemic optic neuropathy and obstructive sleep apnea. J Neuroophthalmol. (2013) 33:232–4. doi: 10.1097/WNO.0b013e31828eecbd
35. Arda H, Birer S, Aksu M, Ismailogullari S, Karakucuk S, Mirza E, et al. Obstructive sleep apnoea prevalence in non-arteritic anterior ischaemic optic neuropathy. Br J Opthalmol. (2013) 97:206–9. doi: 10.1136/bjophthalmol-2012-302598
36. Stein JD, Kim DS, Mundy KM, Talwar N, Nan B, Chervin RD, et al. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. (2011) 152:989–98. doi: 10.1016/j.ajo.2011.04.030
37. Markoula S, Giannopoulos S, Asproudis I, Kostoulas C, Nikas A, Bagli E, et al. Renin-angiotensin-aldosterone system genes and nonarteritic anterior ischemic optic neuropathy. Mol Vis. (2011) 17:1254–60.
38. Lee MS, Grossman D, Arnold AC, Sloan FA. Incidence of nonarteritic anterior ischemic optic neuropathy: increased risk among diabetic patients. Ophthalmology. (2011) 118:959–63. doi: 10.1016/j.ophtha.2011.01.054
39. Felekis T, Kolaitis NI, Kitsos G, Vartholomatos G, Bourantas KL, Asproudis I. Thrombophilic risk factors in the pathogenesis of non-arteritic anterior ischemic optic neuropathy patients. Graefes Arch Clin Exp Ophthalmol. (2010) 248:877–84. doi: 10.1007/s00417-010-1308-y
40. Kuhli-Hattenbach C, Scharrer I, Lüchtenberg M, Hattenbach LO. Selective thrombophilia screening of patients with nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. (2009) 247:485–90. doi: 10.1007/s00417-008-0987-0
41. Giambene B, Sodi A, Sofi F, Marcucci R, Fedi S, Abbate R, et al. Evaluation of traditional and emerging cardiovascular risk factors in patients with non-arteritic anterior ischemic optic neuropathy: a case-control study. Graefes Arch Clin Exp Ophthalmol. (2009) 247:693–7. doi: 10.1007/s00417-008-0981-6
42. Kesler A, Irge D, Rogowski O, Bornstein N, Berliner S, Shapira I, et al. High-sensitivity C-reactive protein measurements in patients with non-arteritic anterior ischaemic optic neuropathy: a clue to the presence of a microinflammatory response. Acta Opthalmol. (2009) 87:216–21. doi: 10.1111/j.1755-3768.2008.01199.x
43. Pinna A, Solinas G, Masia C, Zinellu A, Carru C, Carta A. Glucose-6-phosphate dehydrogenase (G6PD) deficiency in nonarteritic anterior ischemic optic neuropathy in a Sardinian Population, Italy. Invest Ophthalmol Vis Sci. (2008) 49:1328–32. doi: 10.1167/iovs.07-1115
44. French DD, Margo CE. Post-marketing surveillance of ischaemic optic neuropathy in male veterans co-prescribed phosphodiesterase-5 inhibitors with organic nitrates or alpha-blockers. Drug Saf. (2008) 31:241–7. doi: 10.2165/00002018-200831030-00005
45. Margo CE, French DD. Ischemic optic neuropathy in male veterans prescribed phosphodiesterase-5 inhibitors. Am J Ophthalmol. (2007) 143:538–9. doi: 10.1016/j.ajo.2006.10.006
46. Palombi K, Renard E, Levy P, Chiquet C, Deschaux Ch, Romanet JP, et al. Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol. (2006) 90:879–82. doi: 10.1136/bjo.2005.087452
47. Nagy V, Steiber Z, Takacs L, Vereb G, Berta A, Bereczky Z, et al. Trombophilic screening for nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. (2006) 244:3–8. doi: 10.1007/s00417-005-1154-5
48. Salomon O, Rosenberg N, Steinberg DM, Huna-Baron R, Moisseiev J, Dardik R, et al. Nonarteritic anterior ischemic optic neuropathy is associated with a specific platelet polymorphism located on the glycoprotein Ibα gene. Ophthalmology. (2004) 111:184–8. doi: 10.1016/j.ophtha.2003.05.006
49. Glueck CJ, Wang P, Bell H, Rangaraj V, Goldenberg N. Nonarteritic anterior ischemic optic neuropathy: associations with homozygosity for the C677T methylenetetrahydrofolate reductase mutation. J Lab Clin Med. (2004) 143:184–92. doi: 10.1016/j.lab.2003.10.015
50. Weger M, Haas A, Stanger O, El-Shabrawi Y, Temmel W, Maier R, et al. Chlamydia pneumoniae seropositivity and the risk of nonarteritic ischemic optic neuropathy. Ophthalmology. (2002) 109:749–52. doi: 10.1016/S0161-6420(01)01031-4
51. Mojon DS, Hedges TR III, Ehrenberg B, Karam EZ, Goldblum D, Abou-Chebl A, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. (2002) 120:601–5. doi: 10.1001/archopht.120.5.601
52. Salomon O, Dardik R, Steinberg DM, Kurtz S, Rosenberg N, Moisseiev J, et al. The role of angiotensin converting enzyme and angiotensin II type 1 receptor gene polymorphisms in patients with nonarteritic anterior ischemic optic neuropathy. Ophthalmology. (2000) 107:1717–20. doi: 10.1016/S0161-6420(00)00220-7
53. Pianka P, Almog Y, Man O, Goldstein M, Sela BA, Loewenstein A. Hyperhomocystinemia in patients with nonarteritic anterior ischemic optic neuropathy, central retinal artery occlusion, and central retinal vein occlusion. Ophthalmology. (2000) 107:1588–92. doi: 10.1016/S0161-6420(00)00181-0
54. Jacobson DM, Vierkant RA, Belongia EA. Nonarteritic anterior ischemic optic neuropathy a case-control study of potential risk factors. Arch Ophthalmol. (1997) 115:1403–7. doi: 10.1001/archopht.1997.01100160573008
55. Johnson LN, Krohel GB, Allen SD, Mozayeni R. Recurrent herpes labialis as a potential risk factor for nonarteritic anterior ischemic optic neuropathy. J Natl Med Assoc. (1996) 88:369–73.
56. Talks SJ, Chong NHV, Gibson M, Dodson PM. Fibrinogen, cholesterol and smoking as risk factors for non-arteritic anterior ischaemic optic neuropathy. Eye. (1995) 9:85–8. doi: 10.1038/eye.1995.13
57. Fry CL, Carter JE, Kanter MC, Tegeler CH, Tuley MR. Anterior ischemic optic neuropathy is not associated with carotid artery atherosclerosis. Stroke. (1993) 24:539–42. doi: 10.1161/01.STR.24.4.539
58. Kalenak JW, Kosmorsky GS, Rockwood EJ. Nonarteritic anterior ischemic optic neuropathy and intraocular pressure. Arch Ophthalmol. (1991) 109:660–1. doi: 10.1001/archopht.1991.01080050074032
59. Salomon O, Huna-Baron R, Kurtz S, Steinberg DM, Moisseiev J, Rosenberg N, et al. Analysis of prothrombotic and vascular risk factors in patients with nonarteritic anterior ischemic optic neuropathy. Ophthalmology. (1999) 106:739–42. doi: 10.1016/S0161-6420(99)90159-8
60. González Martín-Moro J, Contreras I, Gutierrez-Ortiz C, Gómez-Sanz F, Castro-Rebollo M, Fernández-Hortelano A, et al. Disc Configuration as a risk and prognostic factor in NAION: the impact of cup to disc ratio, disc diameter, and crowding index. Semin Ophthalmol. (2019) 34:177–81. doi: 10.1080/08820538.2019.1620792
61. Yang HK, Park SJ, Byun SJ, Park KH, Kim JW, Hwang JM. Obstructive sleep apnoea and increased risk of nonarteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. (2019) 103:1123–8. doi: 10.1136/bjophthalmol-2018-312910
62. Fard MA, Yadegari S, Ghahvechian H, Moghimi S, Soltani-Moghaddam, Subramanian PS. Optical coherence tomography angiography of a pale optic disc in demyelinating optic neuritis and ischemic optic neuropathy. J Neuroophthalmol. (2019) 39:339–44. doi: 10.1097/WNO.0000000000000775
63. Gandhi U, Chhablani J, Badakere A, Kekunnaya R, Rasheed MA, Goud A, et al. Optical coherence tomography angiography in acute unilateral nonarteritic anterior ischemic optic neuropathy: a comparison with the fellow eye and with eyes with papilledema. Indian J Opthalmol. (2018) 66:1144–8. doi: 10.4103/ijo.IJO_179_18
Keywords: risk factor, non-arteritic anterior ischemic optic neuropathy, large scale, meta-analysis, systematic review
Citation: Liu B, Yu Y, Liu W, Deng T and Xiang D (2021) Risk Factors for Non-arteritic Anterior Ischemic Optic Neuropathy: A Large Scale Meta-Analysis. Front. Med. 8:618353. doi: 10.3389/fmed.2021.618353
Received: 29 October 2020; Accepted: 02 September 2021;
Published: 04 October 2021.
Edited by:
Michael S. Vaphiades, University of Alabama at Birmingham, United StatesReviewed by:
Masoud Aghsaei Fard, Farabi Eye Hospital, IranHoward Pomeranz, Northwell Health, United States
Copyright © 2021 Liu, Yu, Liu, Deng and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daoman Xiang, eGlhbmdkbTM1QDE2My5jb20=; Tuo Deng, Z3pneV9kZW5ndHVvQHNpbmEuY29t
†These authors have contributed equally to this work
 Bing Liu
Bing Liu Ying Yu1†
Ying Yu1† Tuo Deng
Tuo Deng Daoman Xiang
Daoman Xiang