- 1Invasive Fungi Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
- 2Department of Medical Mycology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 3Center for Discovery and Innovation, Hackensack Meridian Health, Nutley, NJ, United States
- 4Antimicrobial Resistance Research Center/Department of Infectious Diseases, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 5Department of Biostatistics, Faculty of Health, Mazandaran University of Medical Sciences, Sari, Iran
- 6Medical Mycology, Shanghai Changzheng Hospital, Second Military Medical University, Shanghai, China
- 7Department of Medicine, University of Pittsburgh, Pittsburgh, PA, United States
- 8Clinical and Translational Fungal-Working Group, University of California, San Diego, La Jolla, CA, United States
- 9Section of Infectious Diseases and Tropical Medicine, Department of Internal Medicine, Medical University of Graz, Graz, Austria
- 10Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California, San Diego, San Diego, CA, United States
Background: Recent studies from multiple countries have shown a high prevalence of coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) among severely ill patients. Despite providing valuable insight into the clinical management of CAPA, large-scale prospective studies are limited. Here, we report on one of the largest multicenter epidemiological studies to explore the clinical features and prevalence of COVID-19-associated pulmonary mold infections (CAPMIs) among mechanically ventilated patients.
Methods: Bronchoalveolar lavage (BAL) and serum samples were collected for culture, galactomannan (GM), and β-D-glucan (BDG) testing. Patients were classified as probable CAPMI based on the presence of host factors, radiological findings, and mycological criteria.
Results: During the study period, 302 COVID-19 patients were admitted to intensive care units (ICUs), among whom 105 were mechanically ventilated for ≥4 days. Probable CAPMI was observed among 38% of patients (40/105), among whom BAL culture of 29 patients turned positive for molds, while galactomannan testing on BAL (GM index ≥1) and serum (GM index >0.5) samples were positive for 60% (24/40) and 37.5% (15/39) of patients, respectively. Aspergillus (22/29; 75.8%) and Fusarium (6/29; 20.6%) constituted 96.5% of the molds isolated. Diaporthe foeniculina was isolated from a COVID-19 patient. None of the patients who presented with CAPMI were treated with antifungal drugs.
Conclusion: Despite being prevalent, the absence of appropriate antifungal treatment highlights that CAPMI is a neglected complication among mechanically ventilated COVID-19 patients admitted to ICUs. CAPMI can be caused by species other than Aspergillus.
Introduction
Coronavirus disease 2019 (COVID-19) has hit almost every country in the world (1). The global mortality rate caused by COVID-19 has been ~3%. Numerous studies have shown that patients with underlying conditions, such as hypertension, obesity, diabetes, and higher age, are predisposed to develop more severe disease including acute respiratory distress syndrome (ARDS), which carries a higher mortality rate (2–6). Apart from these underlying conditions and the multiple organ damage exerted by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the presence of secondary infections, including bacterial and fungal infections, may contribute to a higher mortality (4). Therefore, rapid diagnosis and prompt initiation of antibiotic and/or antifungal therapies in the form of targeted treatment are of paramount importance to improve outcome. Fulfilling these goals, however, requires conducting prospective and large-scale epidemiological studies to establish appropriate therapeutic regimens as well as identify the most sensitive and specific diagnostic tools.
Invasive pulmonary aspergillosis (IPA), most commonly caused by Aspergillus fumigatus, is a recognized complication of severe influenza, especially among those patients with ARDS receiving corticosteroid therapy (7, 8). The mortality rate associated with influenza-associated IPA is high, even among patients with no known immunosuppression (7). IPA also has recently been linked to patients with severe COVID-19 requiring care in intensive care units (ICUs) (6, 9). There was evidence in some studies that antifungal therapy in these patients might improve outcome (6, 10). Prospective, large-scale studies, however, involving a large number of severely ill mechanically ventilated COVID-19 patients are limited to a recent study from the United Kingdom (10).
Iran has been ranked as the sixth country in terms of COVID-19 cases with a mortality rate two times higher than that reported globally (https://covid19.who.int/). Northern regions of Iran were among the initial epicenters of COVID-19. The upward and persisting trend of COVID-19 in this region has caused ~50,000 deaths since the beginning of the epidemic. Prospective multicenter studies assessing the prevalence of COVID-19-associated pulmonary mold infections (CAPMIs) from a large number of COVID-19 patients, especially from epicenters, are lacking in Iran. Herein, we explored the clinical features of CAPMI cases among 105 severely ill, mechanically ventilated COVID-19 cases in Iran.
Materials and Methods
Patients and Sample Collection
This cross-sectional study started on May 1 and ended on September 30, 2020. COVID-19 was diagnosed using real-time PCR and chest tomography (CT) images. Mechanically ventilated and PCR-confirmed COVID-19 patients with ARDS admitted to ICUs of three educational hospitals of Mazandaran University of Medical Sciences located in two cities of Mazandaran Province were recruited in our study. These centers are among the main centers for admission of severely ill COVID-19 cases from across the Mazandaran province.
In the study protocol that was approved by the institutional review board (IRB), bronchoalveolar lavage (BAL) sampling was set to occur 3–4 days after the start of mechanical ventilation in COVID-19 patients. Moreover, blood samples (5 ml) were collected from patients recruited in our study, and serum samples were obtained and stored at −80°C for future use. This study was approved by the ethics committee of the Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.1399.7697).
Sample Processing and Mold Isolate Identification
BAL samples were centrifuged for 10 min at 3,000 rpm. The supernatant was stored at −80°C for future uses. The sediment was inoculated on Sabouraud dextrose agar (QUELAB, Montreal, Quebec, Canada) supplemented with chloramphenicol (Sigma) (SC) and incubated at 27°C for 5–7 days and examined on a daily basis for fungal growth.
Mold colonies were subcultured onto SC and identified by morphological characteristics. Species-level identification involved sequencing beta-tubulin and ITS loci as described previously (11). The partial DNA sequence data from both genes were used as the BLAST query against three Web-accessible databases, those of the Centraalbureau voor Schimmelcultures (CBS-KNAW) Fungal Biodiversity Center, Utrecht, Netherlands (http://www.cbs.knaw.nl); the National Center for Biotechnology Information, Bethesda, MD (http://www.ncbi.nlm.nih.gov); and Fuasrium- ID (http://isolate.fusariumdb.org/blast.php).
Galactomannan and β-D-Glucan Assay
Serum and BAL galactomannan (GM) antigen was measured using Dynamiker Aspergillus Galactomannan Assay DNK-1402-1 (Dynamiker Biotechnology, Tianjin, China) following manufacturer's instructions for quantitative detection. Samples were tested in duplicate, and the mean value was used for interpretation. Serum GM index >0.5 and BAL GM index ≥1.0 were considered positive results.
Serum β-D-glucan (BDG) was detected using the Dynamiker® Fungus (1–3)-β-D-Glucan assay (Dynamiker Biotechnology, Tianjin, China) following manufacturer's instructions, with a positivity threshold >95 pg/ml. Samples were tested in duplicate, and the mean value was used for interpretation.
COVID-19-Associated Pulmonary Mold Infection Definition
To date, the case definition of CAPMI has not been standardized. In this current study, we used a combination of the definition proposed by Koehler et al. (12) and Verweij et al. (13) for diagnosis of COVID-19-associated pulmonary aspergillosis (CAPA). Patients were classified as probable CAPMI based on the presence of host factors (requiring ICU admission for respiratory distress with a positive SARS-CoV-2 PCR temporally related to ICU admission), radiological factors [pulmonary infiltrate, preferably documented by chest CT or cavitating infiltrate (not attributed to another cause)], and mycological criteria. The mycological criteria were defined as the presence of at least one of the following: serum GM index >0.5 or BAL GM index ≥1.0 or positive respiratory specimen culture for mold pathogen. Positive BDG in serum was considered a supplementary test for diagnosis of CAPMI. If respiratory culture grows Aspergillus spp. or Fusarium spp., patients fulfilling the above criteria will be classified as CAPA or CAPfusariosis, respectively.
Antifungal Susceptibility Testing
Isolates of Aspergillus and Fusarium species were subcultured on potato dextrose agar (PDA) for 7 days at 30°C. In vitro susceptibility testing was performed according to the Clinical & Laboratory Standards Institute (CLSI) M38-A2 guidelines (14). Antifungal agents included were as follows: amphotericin B (AMB; Bristol-Myers Squibb Co., Princeton, NJ, USA); itraconazole (ITR; Janssen Pharmaceutica N.V., Beerse, Belgium); voriconazole (VOR; Pfizer, Sandwich, United Kingdom); ravuconazole (RAV; Sigma-Aldrich, Steinheim, Germany); posaconazole (POS; Merck Sharp & Dohme BV, Haarlem, Netherlands); isavuconazole (ISA; Basilea Pharmaceuticals, Basel, Switzerland); and anidulafungin (ANF; Pfizer, Sandwich, United Kingdom).
Typically, final concentrations of the antifungal agents ranged from 0.016 to 16 μg/ml for AMB, VOR, ITR, RAV, and POS; and from 0.008 to 8 μg/ml for the echinocandins. Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were also used as quality control strains. The minimum inhibitory concentrations (MICs) were recorded visually with complete inhibition of growth following 48-h incubation at 37°C, while minimum effective concentrations (MECs) were read for echinocandins microscopically following 48–72-h incubation at 37°C.
Statistical Analysis
The demographic and clinical characteristics of patients were described using mean and median frequencies and percentage. Mann–Whitney or Student's t-test and chi-square test or Fisher's exact test were used to compare factors between the two groups (CAPMI and non-CAPMI). The analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 25.0. P < 0.05 was considered a statistically significant difference.
Results
Patients' Characteristics
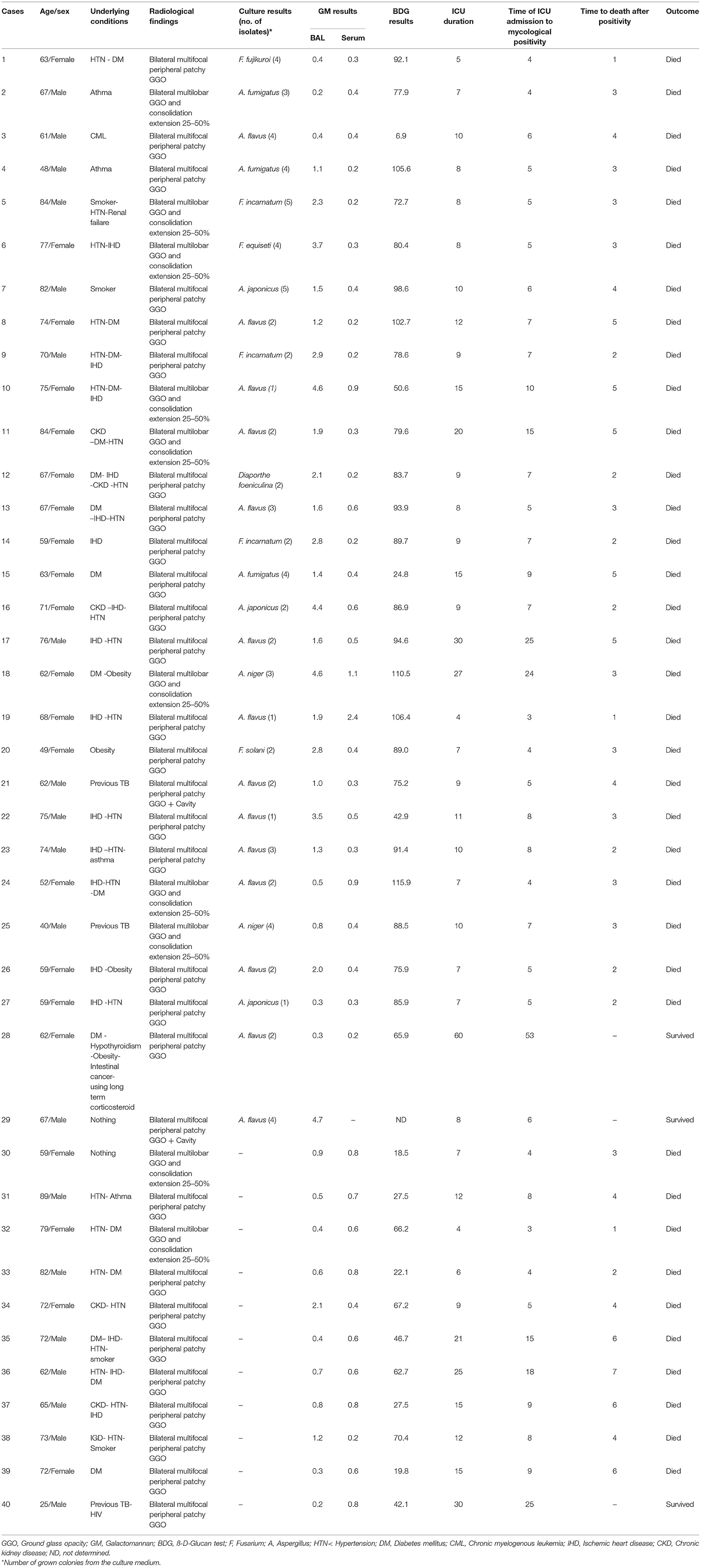
Over the study period, 302 patients were admitted to ICUs, of whom 185 required mechanical ventilation. Eighty patients were extubated within 4 days and were excluded from the analysis. The remaining 105 patients who required mechanical ventilation for ≥4 days formed the basis for this study (Figure 1). Of 105 patients, 58 (55.2%) were male. The demographic and clinical characteristics are presented in Table 1. The patients' ages ranged from 25 to 95 years with a mean of 65.2 years. Of 105 patients, 14 did not have any underlying conditions, while underlying conditions were noted in 91 cases, among which hypertension (48/105; 45.7%), ischemic heart disease (41/105; 39%), and diabetes mellitus (40/105; 38%) were the most common.
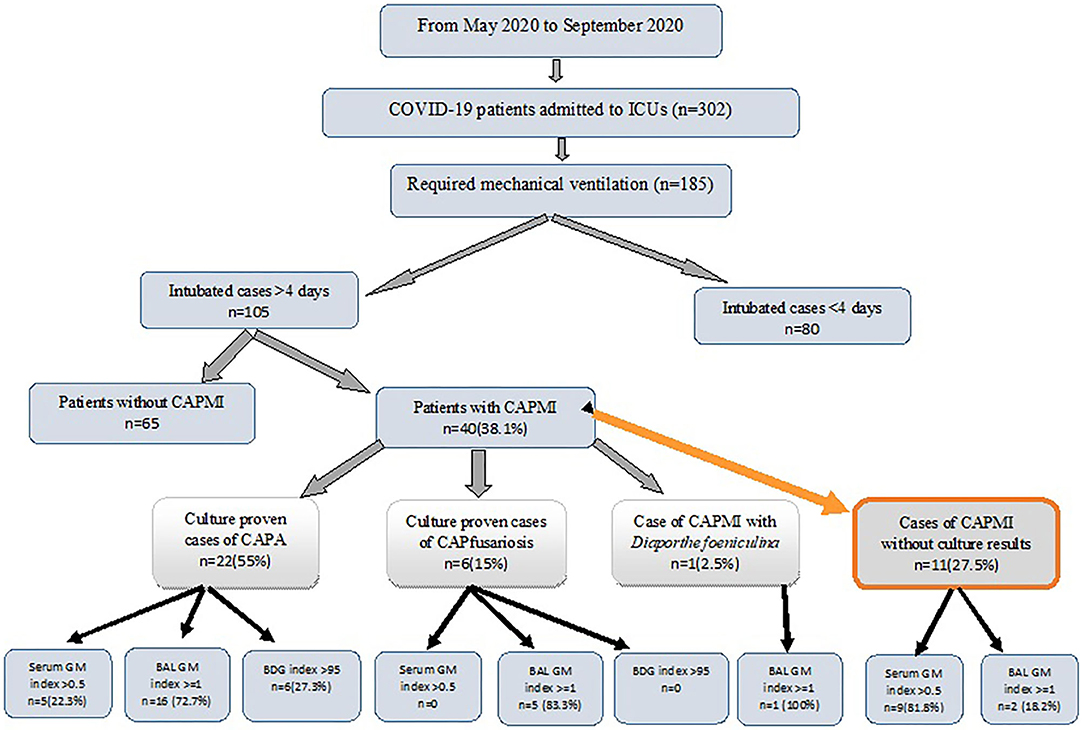
Figure 1. The diagnostic breakdown of the study population. ICU, intensive care unit; BAL, bronchoalveolar lavage; GM, Galactomannan; BDG, β-D-glucan test; CAPMI, COVID-19 associated pulmonary mold infections; CAPA, COVID-19 associated pulmonary aspergillosis; CAPfusariosis, COVID-19 associated pulmonary fusariosis.
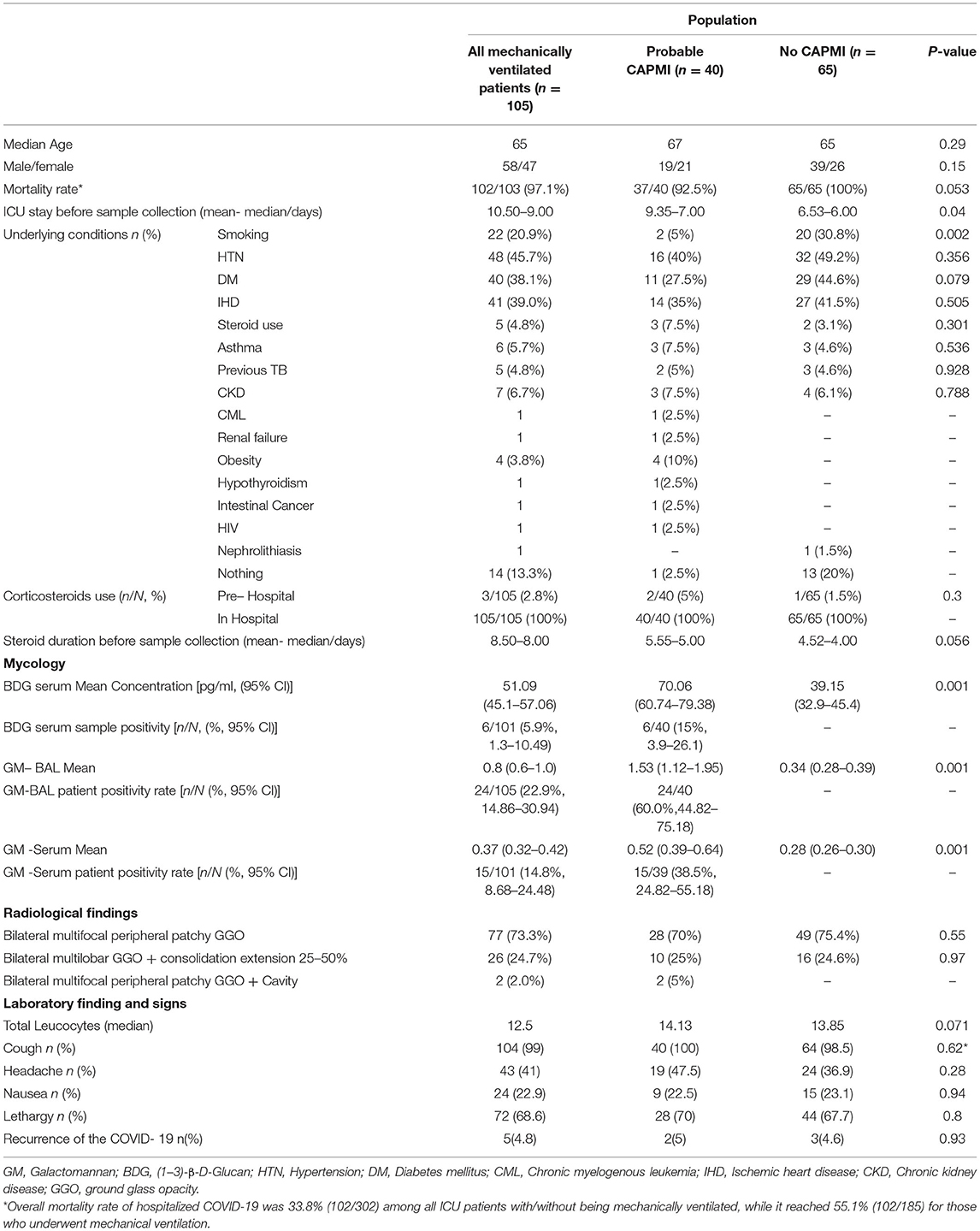
Table 1. Demographic, underlying conditions and clinical characteristics of critically ill COVID-19 patients with and without COVID-19 associated pulmonary mold infection.
As per institutional standard of care at the time the study was conducted, all 105 COVID-19 patients received antimicrobial therapy comprising of ceftriaxone (1 g IV once a day or in equally divided doses twice a day) and azithromycin (500 mg once per day for 5 days). Upon ICU admission, therapy was empirically broadened to vancomycin (1 g q12 h IV; total daily dose = 2.0 g) and imipenem (IPM; 500 mg q6 h IV; total daily dose = 2.0 g) due to high local rates of methicillin-resistant Staphylococcus aureus (MRSA) and multiresistant Gram-negative pathogens.
Three patients were receiving corticosteroid (prednisone: tablet, 5 mg/day) prior to hospitalization for control of asthma (Table 1). As per institutional standard of care (criteria for steroid use), all 105 patients also received dexamethasone (8 mg PO/IV qDay) after hospitalization and also during ICU stay. The mean and median duration of ICU stay was 10.5 and 9 days, respectively. The overall mortality rate of hospitalized COVID-19 cases in our study was 33.8% (102/302) among all ICU patients with/without being mechanically ventilated, while it reached 55.1% (102/185) for those who underwent mechanical ventilation.
Mycological Findings in COVID-19-Associated Pulmonary Mold Infection Patients
Forty patients (38.1%) fulfilled our definition of probable CAPMI. None had proven CAPMI. Twenty-nine (27.6%) had a positive BAL culture. CAPA was identified in 22 (75.9%) patients, of which Aspergillus flavus (14/22, 63.6%) was the most common agent, followed by A. fumigatus and Aspergillus japonicus (3/22 and 13.6% each) and Aspergillus niger (2/22, 9.1%). In patients with CAPfusariosis (6/29, 20.7%), Fusarium incarnatum (50%) was the most common followed by Fusarium fujikuroi (16.6%) and Fusarium equiseti and Fusarium solani (16.6% each). In one case of CAPMI, Diaporthe foeniculina was isolated (Table 2).
Of 40 patients with CAPMI, 17 (42.5%) had only one while 23 (57.5%) had more than one positive mycological findings. The patients with CAPMI were positive for GM BAL (GM index ≥1) and serum (GM index >0.5) in 24/40 (60%) and 15/39 (38.5%) cases, respectively. Serum BDG yielded positive results among 15.4% (6/39) of the CAPMI patients (Table 1). Of 29 patients with BAL-positive culture for mold, 22 (75.9%) had positive BAL GM index ≥1, including 16 patients with Aspergillus spp. growth, five with Fusarium species growth, and one with D. foeniculina growth. Of note, BAL and serum samples were positive in two and 10 patients for whom the BAL cultures were negative, respectively. Moreover, BDG testing was positive in six patients (>95 pg/ml), among whom five had positive GM on BAL, and three positive GM on serum (Table 2). More details are available in Figure 1.
Clinical Characteristics of COVID-19-Associated Pulmonary Mold Infection Patients
At the time of ICU admission, all 105 included patients underwent a CT scan, and bilateral multifocal peripheral patchy ground-glass opacity (GGO) was observed in 77 (73.3%), bilateral multilobar GGO + consolidation extension 25–50% was observed in 26 (24.7%), and bilateral multifocal peripheral patchy GGO + cavity was observed in two (2.0%) patients (Table 1).
In the CT scan of 40 patients with CAPMI, bilateral multifocal peripheral patchy GGO was reported in 28 (70%), bilateral multilobar GGO + consolidation extension 25–50% was reported in 10 (25%), and bilateral multifocal peripheral patchy GGO + cavity was reported in two (5%) cases. There was no significant difference between the two groups of COVID-19 patients with CAPMI and those without CAPMI with respect to radiological findings (Table 1).
It was identified that the ICU stay duration was significantly higher among those with CAPMI (p-value: 0.04), while no difference was noted with regard to underlying conditions and mortality rate (Table 1). This difference was driven by a significant difference in ICU stay before sample collection was performed (defined as the time of having mycological test results) in CAPMI patients [mean = 9.35 (CI: 6.53–12.16) and median = 7.00 (IQR: 5.66–8.33) days] vs. in others [mean = 6.53 (CI: 6.05–7.01) and median = 6.0 (IQR: 5.47–6.52) days (p-value: 0.04)], as well as there was a significant difference in ICU stay after sample collection was performed [mean = 3.52 (CI: 3.01–4.03) and median = 3.0 (IQR: 2.44–3.55) days vs. mean = 2.47 (CI: 2.10–2.85) and median = 2.0 (IQR: 1.56–2.43) days (p-value: 0.004)]. The mean and median duration of steroid in included patients was 8.5 and 8.0, respectively. The mean and median duration of steroid from hospitalization before sample collection in CAPMI patients was 5.55 (CI: 4.45–6.64) and 5.0 (IQR: 3.45–6.54) days and those without mycological findings was 4.52 (CI: 3.94–5.10) and 4.0 (IQR: 3.28–4.71) days (p-value: 0.056), respectively.
Of three patients who were receiving prednisone prior to hospitalization for control of asthma, two showed CAPMI.
Antifungal Susceptibility Testing
Table 3 represents the MICs of azole agents for the 29 mold strains. POS (MIC range 0.001–0.016 μg/ml, GM MIC 0.01 μg/ml) and AMB (MIC range 0.125–0.5 μg/ml, GM MIC 0.29 μg/ml) exhibited the lowest and the highest MICs against A. flavus isolates, respectively. The lowest and highest MIC50/MIC90 of A. flavus was observed against POS (0.016/0.016) and AMB (0.25/0.5). None of the Aspergillus species isolates showed MIC above epidemiologic cutoff value (ECV) against all tested antifungals. F. equiseti showed the highest MICs against VOR (16 μg/ml), ITR, POS, and ISA (8 μg/ml each). F. solani had MIC 8 μg/ml against VOR.
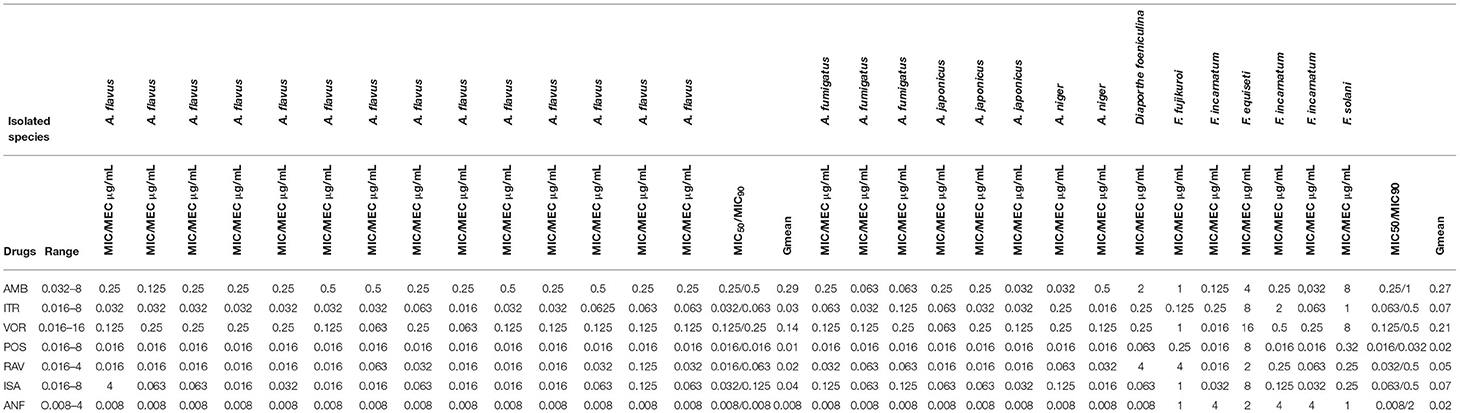
Table 3. In-vitro antifungal susceptibility profile of mold species isolated from COVID-19 patients against different antifungal agents.
Discussion
This study presents a unique picture of CAPA and CAPMI in Iran when compared to other studies exploring the burden of CAPA among severely ill COVID-19 patients. Firstly, the species distribution was different from previously published cases, and we found A. flavus as the most prevalent mold species. Furthermore, for the first time, we observed species belonging to Fusarium and an environmental mold complicating COVID-19 in mechanically ventilated patients. It is worth noting that A. flavus was reported as the main contaminant species of Aspergillus in clinical and indoor hospital samples from Iran (15, 16). This may be due to climatic conditions or ongoing construction work in some of the participating hospitals. We showed that radiology was not beneficial to diagnose CAPA/CAPMI, as in our study, there was no significant radiologic difference between COVID-19 patients with CAPMI and those without CAPMI, while both culture and GM testing on BAL samples provided the most specific and sensitive tools.
In this study, we found that ~28% of the mechanically ventilated patients had positive culture for mold species, among whom almost 76% had positive GM on BAL, while BDG and GM using serum samples showed much lower sensitivity. These findings are consistent with those identified in other studies (17–21), in which GM and culture of BAL samples yielded the most accurate results. It is important to note that neither culture nor GM in BAL is a definitive method to diagnose pulmonary angio-invasive disease caused by fungi including Aspergillus species, which may also be positive in airway invasive disease and colonization. While antifungal treatment has been associated with improved survival in CAPA patients, some patients improved without antifungals, which argued against true invasive disease in these individuals (20). While histologically confirmed CAPA is increasingly reported, Flikweert et al. (22) did not find postmortem histological evidence of CAPA in six patients diagnosed with probable CAPA, although given the unreliable radiologic signs of CAPA and overlap with severe COVID-19, their method of ultrasound and CT-guided postmortem needle biopsies may have very well-randomly missed focal Aspergillus infection. Indeed, the most recent consensus statements of the European Confederation of Medical Mycology (ECMM) and International Society for Human & Animal Mycology (ISHAM) also have advocated the use of BAL and lower respiratory tract sampling while advocating against the utility of serum to be tested for both GM and BDG (12). Although tissue biopsy and the observation of invasion by septate hyphae are the gold standard and required for proven infection, tissue biopsies are rarely obtained in vivo due to the high contagious nature of SARS-CoV-2 (12). In our study, radiological imaging did not differ in those with CAPMI and those without, as suggested previously (12). Collectively, our experience suggests that BAL culture and BAL GM represent the most useful diagnostic tools, while testing serum using GM and BDG and radiological findings provide a lower level of assistance in the diagnosis of CAPA/CAPMI.
One of the main challenges in fungal infection diagnosis in ICU patients including COVID-19 is the lack of consensus on a diagnostic algorithm. Critically ill COVID-19 patients are usually without classical host factors (neutropenia, receipt of an allogeneic stem cell transplant) and clinical criteria (halo signs) for CAPMI, as defined by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG), and will therefore not fulfill these criteria (23). Blot et al. (24) suggested a clinical algorithm to differentiate Aspergillus colonization from IPA in ICU patients without classical risk factors (AspICU algorithm) based on mycological criteria including detection of Aspergillus from respiratory specimens including BAL. More specifically for those with underlying acute viral diseases, Koehler et al. (12) and Verweij et al. (13) have recently proposed a diagnostic algorithm for CAPA and influenza-associated pulmonary aspergillosis, respectively. In these proposed criteria, patients with COVID-19 were divided into three groups of proven, probable, and possible invasive aspergillosis. Due to occupational safety and protection against virus transmission, classifying COVID-19 patients in proven IPA category is rarely possible in many institutions around the world, as local restrictions preclude biopsies and autopsies in COVID-19 patients. Using different algorithms for classification of fungal infections in COVID-19 patients may lead to different incidence rates of CAPMI. In the present study, two (cases 3 and 28) patients met the EORTC/MSG criteria (23), and the incidence rate of CAPMI was 27.6 and 38.1% according to the AspICU algorithm (24) and the combination definition proposed by Koehler et al. (12) and Verweij et al. (13), respectively. The prevalence of CAPA found in our study (22/105; 20.9%) is closer to that found in studies from Wales (14.1%) (10), Netherlands (19.4%) (19), and Pakistan (21.7%) (25) but lower than reported from Germany (26.3%) (21) and France (33.3%) (20). As for the species causing CAPMI, A. flavus constituted 63% of the Aspergillus isolates, which is in line with a recent systematic review on invasive aspergillosis in Iran, where A. flavus represented the most prevalent agent of IPA (26). This picture is entirely different from that of many other countries, where A. fumigatus has been shown to be the most prevalent agent of IPA and CAPA (10, 19–21). Importantly, we found that ~21% of the CAPMI cases were due to various species within the Fusarium genus, while to the best of our knowledge, there is only one previous case report of invasive pulmonary fusariosis due to Fusarium proliferatum in an immunocompetent critically ill patient with severe COVID-19 (27). Of note, five out of six fusariosis cases were identified via BAL GM testing; the cross-reactivity of GM with Fusarium spp. has been noted previously (28). Moreover, Fusarium incarnatum was the most common species among fusariosis cases (3/6), which is unlike previous studies that reported F. solani complex (comprising ~40–60% of infections), followed by F. oxysporum as the most common species in immunocompromised patients other than COVID-19 (29). Interestingly, we also found D. foeniculina in the BAL sample of one of the CAPMI cases, which is known for being a plant pathogen. This species has been rarely reported to cause infection in humans, and a few studies have shown a wide range of infections among transplant patients (30–32). Of note, the fact that our BAL sampling was restricted to a single sample obtained 3–4 days after initiation of mechanical ventilation may have resulted in underestimation of CAPMI.
Of particular concern is the lack of antifungal treatment among CAPA/CAPMI cases observed in this study, while it has been shown that CAPA cases receiving antifungal treatment had significantly lower mortality compared to those not treated (10). Moreover, the joint ECMM/ISHAM consensus statement (12) recommends using either VOR or ISA to treat CAPA. The underestimation of fungal infections, both superficial and invasive, is a well-known phenomenon in developing countries (33, 34), and increased awareness is needed.
In our study, the mean duration of steroid use from hospitalization to positive mycological tests in patients with CAPMI compared to those without was higher (5.55 vs. 4.52 days); however, the statistical significance was borderline (p-value: 0.056). These results may imply that the long stay in ICU and consequently the longer duration of steroids received by CAPMI patients predisposed them to opportunistic fungal infections. In addition, the widespread administration of different antibiotics among the patients recruited in this study and the ongoing construction work in some centers may have had an impact on the incidence of CAPMI in our study. However, the occurrence of CAPMI and construction requires more study to see if there is a link between the two.
The overall mortality rate of hospitalized COVID-19 cases in our study was 33.8% among all ICU patients with/without being mechanically ventilated, while it reached 55.1% for those who underwent mechanical ventilation. Since all patients recruited in our study were mechanically ventilated, no conclusions can be drawn from our data whether CAPMI is associated with higher overall mortality. On the other hand, the absence of a significant difference between CAPMI and non-CAPMI groups with respect to mortality may indicate fungal colonization rather than true invasive mold infection as has been suggested by Flikweert et al. (22).
In our study, only mechanically ventilated COVID-19 patients with ARDS were evaluated for fungal infections. Due to the special conditions of COVID-19 patients and the possibility of transmission, we were able to collect respiratory and blood samples only at one time point. Our study protocol also did not allow for obtaining biopsy or autopsy tissue samples from probable CAPMI cases for verification of definitive invasion of fungal agents in tissue, reflecting the current standard of care in Iran. These were among the main limitations of our study.
Conclusion
Although the current guidelines are in favor of appropriate administration of antifungal treatment in CAPMI cases, the absence of appropriate antifungal treatment highlights that CAPMI is a neglected complication among mechanically ventilated COVID-19 patients admitted to ICUs in our study. Finally, CAPMI can be caused by species other than Aspergillus.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the ethics committee of the Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.1399.7697). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MTH, LD, DSP, MH, WP, and AA were involved in the concept and design of the study. MG, MM, MA, IH, RM, SM, JC, WF, WL, and MHN were involved in the acquisition, analysis, and/or interpretation of the data. All authors participated in drafting the manuscript, its critical revisions for important intellectual content, and approved the final submitted article.
Funding
This study was supported by a research fund (No. 7697) from the Invasive Fungi Research Center of Mazandaran University of Medical Sciences, Sari, Iran.
Conflict of Interest
DSP received grant and contract funds from Merck, Regeneron, and Pfizer for COVID-19-related research programs. MHN received CDC-sponsored grant, CAPA. MH received research funding from Agile AS and Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The participating patients are thanked for their kind cooperation, which was essential for the completion of the study. The authors wish to thank Dr. Roudbari, Salari, and Divsalar at Zareh University Hospital for their kind support.
References
1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. (2020) 323:709–10. doi: 10.1001/jama.2020.1097
2. Zhou P, Liu Z, Chen Y, Xiao Y, Huang X, Fan XG. Bacterial and fungal infections in COVID-19 patients: a matter of concern. Infect Control Hosp Epidemiol. (2020) 41:1124–5. doi: 10.1017/ice.2020.156
3. Hoenigl M. Invasive fungal disease complicating COVID-19: when it rains it pours. Clin Infect Dis. (2020) 5:ciaa1342. doi: 10.1093/cid/ciaa1342
4. Bassetti M, Kollef MH, Timsit JF. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. (2020) 9:1–4. doi: 10.1007/s00134-020-06219-8
5. Arastehfar A, Carvalho A, Nguyen MH, Hedayati MT, Netea M. G., Perlin DS, et al. COVID-19-Associated Candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi. (2020) 6:E211. doi: 10.3390/jof6040211
6. Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi. (2020) 6:91. doi: 10.3390/jof6020091
7. Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Tienen CA, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. (2018) 6:782–92. doi: 10.1016/S2213-2600(18)30274-1
8. Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. (2012) 38:1761–8. doi: 10.1007/s00134-012-2673-2
9. Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. (2020) 28:ciaa1065. doi: 10.1093/cid/ciaa1065
10. White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. (2020) 29:ciaa1298. doi: 10.1093/cid/ciaa1298
11. Balajee SA, Borman AM, Brandt ME, Cano J, Cuenca-Estrella M, Dannaoui E, et al. Sequence based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J Clin Microbiol. (2009) 47:877–84. doi: 10.1128/JCM.01685-08
12. Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19 associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. (2020) 14:S1473–3099(20)30847–1. doi: 10.1016/S1473-3099(20)30847-1
13. Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. (2020) 46:1524–35. doi: 10.1007/s00134-020-06091-6
14. CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd edition. Wayne, PA: CLSI (2017).
15. Hedayati MT, Tavakoli M, Maleki M, Heidari S, Mortezaee V, Gheisari M, et al. Fungal epidemiology in cystic fibrosis patients with a special focus on Scedosporium species complex. Microb Pathog. (2019) 129:168–75. doi: 10.1016/j.micpath.2019.02.009
16. Zaini F, Hedayati MT. Study of airborne fungi in the wards of 3 Tehran hospitals. J Iran Med Council. (1995) 13:208–15.
17. Lamoth F, Glampedakis E, Boillat-Blanco N, Oddo M, Pagani JL. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect. (2020) 10:S1198–743X(20)30412–2. doi: 10.1016/j.cmi.2020.07.010
18. Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, Mertes H, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. (2020) 10:1–4. doi: 10.1186/s13613-020-00686-4
19. van Arkel AL, Rijpstra TA, Belderbos HN, Van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19–associated pulmonary aspergillosis. Am J Respir Crit Care Med. (2020) 202:132–5. doi: 10.1164/rccm.202004-1038LE
20. Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. (2020) 8:e48–9. doi: 10.1016/S2213-2600(20)30237-X
21. Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. (2020) 63:528–34. doi: 10.1111/myc.13096
22. Flikweert AW, Grootenboers MJ, Yick DC, Du Mée AW, van der Meerd NJM, Rettigd CD, et al. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. (2020) 59:149–55. doi: 10.1016/j.jcrc.2020.07.002
23. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
24. Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. (2012) 186:56–64. doi: 10.1164/rccm.201111-1978OC
25. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. (2020) 63:766–70. doi: 10.1111/myc.13135
26. Tavakoli M, Yazdani Charati J, Hedayati MT, Moosazadeh M, Badiee P, Seyedmousavi S, et al. National trends in incidence, prevalence and disability-adjusted life years of invasive aspergillosis in Iran: a systematic review and meta-analysis. Expert Rev Respir Med. (2019) 13:1121–34. doi: 10.1080/17476348.2019.1657835
27. Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin Microbiol Infect. (2020) 30:S1198–743X(20)30372–4. doi: 10.1016/j.cmi.2020.06.026
28. Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. (2007) 20:695–704. doi: 10.1128/CMR.00014-07
29. Batista BG, Chaves MA, Reginatto P, Saraiva OJ, Fuentefria AM. Human fusariosis: an emerging infection that is difficult to treat. Rev Soc Bras Med Trop. (2020) 53:e20200013. doi: 10.1590/0037-8682-0013-2020
30. Howard JC, Chen K, Werno A, Metcalf S. Soft tissue infection with diaporthe phaseolorum in heart transplant recipient with end-stage renal failure. Emerg Infect Dis. (2019) 25:1748–9. doi: 10.3201/eid2509.190768
31. Rakita RM, O'Brien KD, Bourassa L. Diaporthe soft tissue infection in a heart transplant patient. Transpl Infect Dis. (2017) 19:e12680. doi: 10.1111/tid.12680
32. Mattei AS, Severo CB, Guazzelli LS, Oliveira FM, Gené J, Guarro J, et al. Cutaneous infection by Diaporthe phaseolorum in Brazil. Med Mycol Case Rep. (2013) 2:85–7. doi: 10.1016/j.mmcr.2013.03.001
33. Arastehfar A, Wickes BL, Ilkit M, Pincus DH, Daneshnia F, Pan W, et al. Identification of Mycoses in developing countries. J Fungi. (2019) 5:90. doi: 10.3390/jof5040090
Keywords: COVID-19-associated pulmonary mold infections, mechanically ventilation, galactomannan, Aspergillus, Fusarium
Citation: Ghazanfari M, Arastehfar A, Davoodi L, Yazdani Charati J, Moazeni M, Abastabar M, Haghani I, Mirzakhani R, Mayahi S, Fang W, Liao W, Nguyen MH, Perlin DS, Hoenigl M, Pan W and Hedayati MT (2021) Pervasive but Neglected: A Perspective on COVID-19-Associated Pulmonary Mold Infections Among Mechanically Ventilated COVID-19 Patients. Front. Med. 8:649675. doi: 10.3389/fmed.2021.649675
Received: 13 January 2021; Accepted: 03 May 2021;
Published: 14 June 2021.
Edited by:
Piero Valentini, Università Cattolica del Sacro Cuore, ItalyReviewed by:
Archana Bhaskaran, University of Minnesota Twin Cities, United StatesRita Murri, The Catholic University of America, Rome Campus, Italy
Copyright © 2021 Ghazanfari, Arastehfar, Davoodi, Yazdani Charati, Moazeni, Abastabar, Haghani, Mirzakhani, Mayahi, Fang, Liao, Nguyen, Perlin, Hoenigl, Pan and Hedayati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad T. Hedayati, aGVkYXlhdGltdEBnbWFpbC5jb20=; Lotfollah Davoodi, bG90ZmRhdm9vZGlAeWFob28uY29t; Weihua Pan, cGFud2VpaHVhOUBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mona Ghazanfari
Mona Ghazanfari Amir Arastehfar
Amir Arastehfar Lotfollah Davoodi4*
Lotfollah Davoodi4* Maryam Moazeni
Maryam Moazeni Mahdi Abastabar
Mahdi Abastabar Iman Haghani
Iman Haghani Sabah Mayahi
Sabah Mayahi Wenjie Fang
Wenjie Fang David S. Perlin
David S. Perlin Weihua Pan
Weihua Pan Mohammad T. Hedayati
Mohammad T. Hedayati