- 1Chronic Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 2Osteoporosis Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 3Elderly Health Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 4The Persian Gulf Marine Biotechnology Research Center, Bushehr University of Medical Sciences, Bushehr, Iran
- 5Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Background: Sarcopenia is characterized by low skeletal muscle mass and function, which is associated with cardiovascular risk factors and may even be related to adverse cardiovascular events and mortality. This study aimed to evaluate whether sarcopenia is related to electrocardiographic (ECG) abnormalities in a large sample of older adults.
Methods: We performed a cross-sectional study based on the data collected during the Bushehr Elderly Health (BEH) cohort study. Body composition was measured by dual X-ray absorptiometry (DXA) and muscle strength was measured using a digital dynamometer for each hand of every participant. A person who had low muscle strength, as well as low muscle mass was identified as having sarcopenia. The subjects were classified into three groups according to the Minnesota Code (MC) as major, minor ECG abnormalities and participants with no abnormalities ECG.
Results: Of the 2,426 participants, 354 (14.6%) had major ECG abnormalities and 193 (8%) had minor ECG abnormalities. Sarcopenia was associated with an increased risk of major ECG abnormality in all models. After adjustment for confounders of CHD in full model, the OR for major ECG abnormality was 1.47 (95% CI 1.11–1.95) in those with sarcopenia. Low muscle strength and low muscle performance were both with an increased risk of major ECG abnormality in all models. Sarcopenia and low muscle strength increased 28% and 62% risk of any ECG abnormality in the full models [sarcopenia: 1.28(1.01–1.63), low muscle strength: 1.62(1.30–2.03)], respectively.
Conclusions: This study showed that sarcopenia and its components are associated with ECG abnormalities in Iranian older people. Although some older adults have higher cardiovascular risk factors, these data showed that further factors such as sarcopenia may be identified as a particular risk factor for future cardiovascular events. Therefore, sarcopenia could be added to the screening of the older population to reduce the risk of cardiovascular events.
Introduction
Aging is related to changes in body composition, including decreases in muscle mass and bone mass and an increase in adipose tissue, which can lead to cardiovascular diseases and metabolic disorders (1).
Sarcopenia, an age-related muscle disorder characterized by a decline in muscle mass and function (2), is a major risk factor of falling, disability and death in old adults. Also, sarcopenia is associated with cardiometabolic risk factors such as glucose intolerance and metabolic syndrome and other diseases such as, cardiovascular diseases (CVD) and respiratory diseases (3).
Among all CVDs, Coronary Heart disease (CHD) is one of the most common and important causes of mortality, morbidity, and disability in older people (4).
Some studies determined cardiometabolic risk factors and comorbidities that associated with CHD (5, 6). The non-modifiable factors such age, sex, race, family history, and modifiable risk factors include obesity, physical inactivity, smoking, hyperlipidemia, hypertension, and diabetes are traditional cardiovascular disease risk factors (5). Also, electrocardiograms (ECG) abnormalities can predict of all- cause, CHD mortality independent of other cardiomeytabolic risk factors (7–11).
Few studies have shown that the association between low muscle mass or sarcopenia and various heart disease in older people (12–14). Some mechanisms including changes in anabolic androgenic hormones, insulin resistance, protein intake, physical activity and muscle structure can be common between CVDs and sarcopenia and can also impact the outcomes of patients with these diseases (15–17).
Although the prevalence and association of ECG abnormalities with chronic diseases such as diabetes and metabolic syndrome and its components have been studied (18, 19), this association has not been investigated in persons with sarcopenia. If ECG abnormalities are common and have associated with sarcopenia, these might help to identify of sarcopenic persons who are at especially high risk for the CVD events. Therefore, to identify subjects with increased risk of CVDs and study on the association between age-related diseases such as sarcopenia with CHD can improve public health problems in elderly.
Given the high prevalence of CHD and also sarcopenia in the Iranian elderly, in the present study, we investigated the association of sarcopenia and its components with ECG abnormalities in older people.
Materials and Methods
The Study Population
This cross-sectional study was based on the Bushehr Elderly Health (BEH) program. The BEH study was a prospective population-based cohort study performed on a sample of older people ≥60 years in the urban population of Bushehr city, in the south of Iran. Overall 3,000 persons were recruited using a multistage, stratified cluster sampling method. BEH study aimed to assess non-communicable diseases in older people. The methodology and protocol of the BEH program were previously described elsewhere (20, 21).
The Research Ethics Committee of Bushehr and Tehran University of Medical Sciences approved the protocol of the BEH program (ID:IR.TUMS.EMRI.REC.1394.0036) and written informed consent was signed by all participants.
Data Collection
A comprehensive questionnaire including sociodemographic characteristics, medical history, smoking and lifestyle data was completed for each person through a personal interview. Anthropometric measurements were carried out using standard protocols. Height and weight were measured with a fixed stadiometer and a digital scale respectively. The body mass index (BMI) was calculated as weight in kg divided by the square of height in meters.
The measurement of Blood pressure (BP) was performed using a standardized mercury sphygmomanometeron the right arm after 15 min of rest in the seated position.
Standard 12-lead electrocardiograms (ECGs) were recorded at baseline in the resting supine position according to the standard procedures. Two qualified physicians coded the ECGs in parallel according to the Minnesota codes using a measuring loop, specially manufactured by the University of Minnesota (22). Any discordant results were resolved by a third qualified physician who was a cardiologist. The physical activity was evaluated by a validated questionnaire based on metabolic equivalent task (MET) (23, 24). A 24-h dietary recall was used for dietary assessment.
A trained operator measured the body composition using dual x-ray absorptiometry (DXA, Discovery WI, HologicInc, USA). The skeletal muscle mass index (SMI) was defined as the sum of the muscle masses of the four limbs as appendicular skeletal muscle mass divided by squared height. Maximum handgrip strength was measured in both hands by a digital grip strength dynamometer, 3 times, and the highest value was used as muscle strength. Walking speed over 4.57 m was used for estimating physical performance.
The biochemical parameters were measured by laboratory testing in a fasting condition, according to standard protocols. Serum lipid profiles and fasting plasma glucose (FPG) were measured by an enzymatic colorimetric technique using a commercial kit (Pars Azmun, Karaj, Iran).
Definition of Variables
According to the European Working Group on Sarcopenia in Older People 2 (EWGSOP- 2), sarcopenia was defined as low muscle strength plus reduced skeletal muscle mass (3). Also, the EWGSOP and Asia Working Group for Sarcopenia (AWGS) (25) recommend the use of reference data of the same population to determine cut- off points for muscle mass. Therefore, we used reference data from a normative Iranian population that was available for detecting sarcopenia for our study. Based on these data, the cut-off values for low skeletal muscle mass index (SMI) were 7.0 kg/m2 and <5.4 kg/m2 among men and women, respectively (26). The low muscle strength was handgrip strength <26 kg for men and <18 kg for women; while the cut-off value for low physical performance was a usual walking speed <0.8 m/s for both genders (25). According to these cut-off points, we identified sarcopenic persons (27). Current smoking was defined as smoking cigarettes or water pipes, at the time of study.
Diabetes mellitus was defined as fasting plasma glucose (FPG) ≥ 126 mg/dl or HbA1C ≥ 6.5 or current use of pharmacological medication. Hypertension (HTN) was defined as systolic blood pressure 140 mmHg and/or diastolic blood pressure 90 mmHg or current use of antihypertensive medication. Subjects with total cholesterol ≥200 mg/dl were named as hypercholesterolemia. High Fat Mass was outlined as total body percent fat >30 for men and >40 for women (28).
The Minnesota coding system and Whitehall criteria was used to classify ECG findings as having a major or minor abnormality (22, 29).
Criteria for major ECG abnormalities were any of the following: Q-QS wave abnormalities (MC 1-1 to 1-2-8); left ventricular hypertrophy (MC 3-1); Wolff–Parkinson–White syndrome (MC 6- 4-1 or 6-4-2); complete bundle branch block or intraventricular block (MC 7-1-1, 7-2-1, 7-4, or 7-8); atrial fibrillation or atrial flutter (MC 8-3); or major ST-T changes (MC 4-1, 4-2, 5-1, and 5-2).
Criteria for minor ECG abnormalities were minor ST-T changes (MC 4-3, 4-4, 5-3, and 5-4).
Participants with only minor ECG abnormalities were classified as having “minor abnormalities,” and participants with major ECG abnormalities with or without coexisting minor ECG abnormalities were classified as having “major ECG abnormalities.” Participants with both major and minor abnormalities were classified as having major abnormalities. Participants without minor or major ECG abnormalities were classified as having no abnormalities and their ECG was considered normal (22, 30).
Statistical Analysis
Normal distribution of continuous variables was assessed using the Shapiro-Wilks test and visual inspection of the group histograms. Continuous data that followed a normal distribution was described with means ± standard deviation (SD). Categorical variables are expressed as percentages. Comparisons between ECG abnormality categories were made using the t-test for continuous variables and the chi-squares tests for percentages. We used the best subset method with the Akaike Information Criterion (AIC), to select the final model from all possible subsets. The multinominal regression analyses were used to investigate the associations of sarcopenia and its components with ECG abnormality category. Results were presented as odds ratios and 95% confidence intervals. Data were analyzed using the Stata 14 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) and P ≤ 0.05 was considered as statistically significant in all tests.
Results
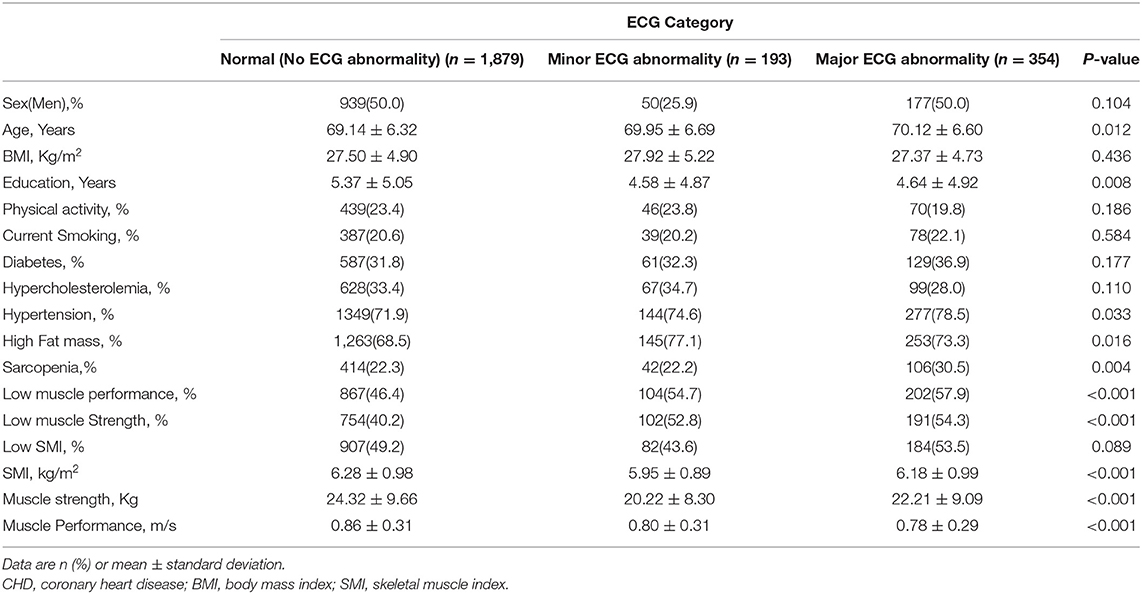
General characteristics of the study group are shown in Table 1. Among 2,426 participants, 193 (8%) had minor ECG abnormalities and 354 (14.6%) had major ECG abnormalities. Subjects in the group with minor or major ECG abnormalities were older and had fewer years of education and also the prevalence of HTN and obesity was higher among these subjects (P < 0.05). Participants with minor or major ECG abnormalities walked more slowly and had lower means of SMI and muscle strength (P < 0.001).
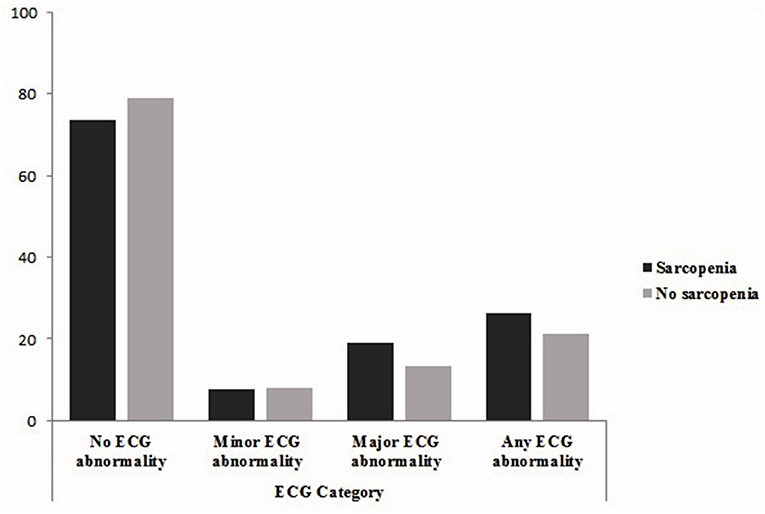
Figure 1 shows that the prevalence major or any ECG abnormalities was higher in sarcopenic patients (major ECG abnormalities: 18.9 vs. 13.2%, any ECG abnormalities: 26.3 vs. 21.2%).
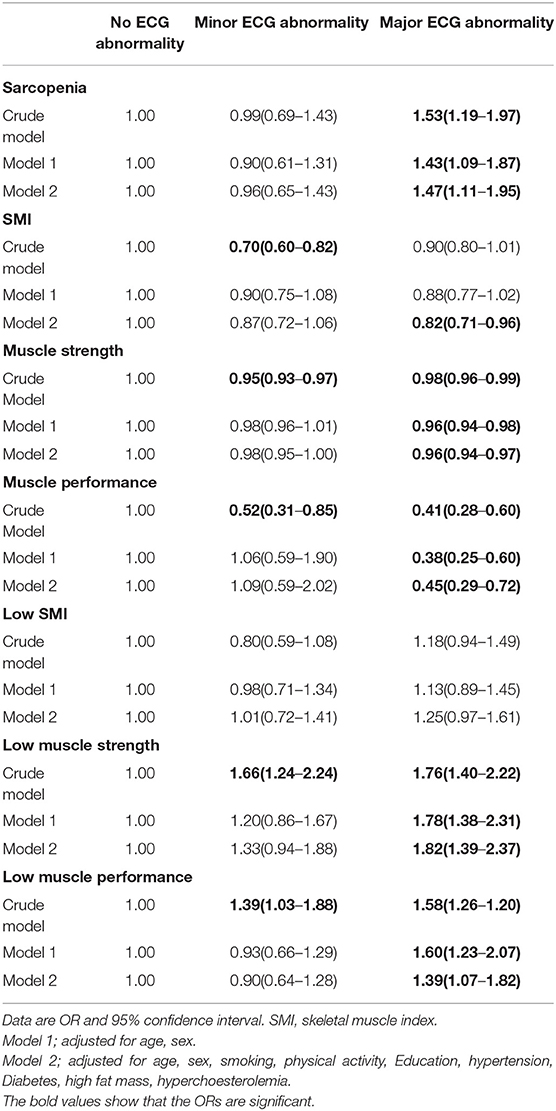
Table 2 presents the results of the multinomial logistic regression models to define the association sarcopenia and its components with minor and major ECG abnormalities. Sarcopenia was associated with an increased risk of major ECG abnormality in all models. After adjustment for confounders of CHD in full model, the OR for major ECG abnormality was 1.47 (95% CI 1.11–1.95) in those with sarcopenia. The associations between sarcopenia and minor ECG abnormality were not statistically significant in all models.
Low muscle strength and low muscle performance were both with an increased risk of major ECG abnormality in all models. Also, the results show that muscle strength and muscle performance decrease risk of major ECG abnormality in all models.
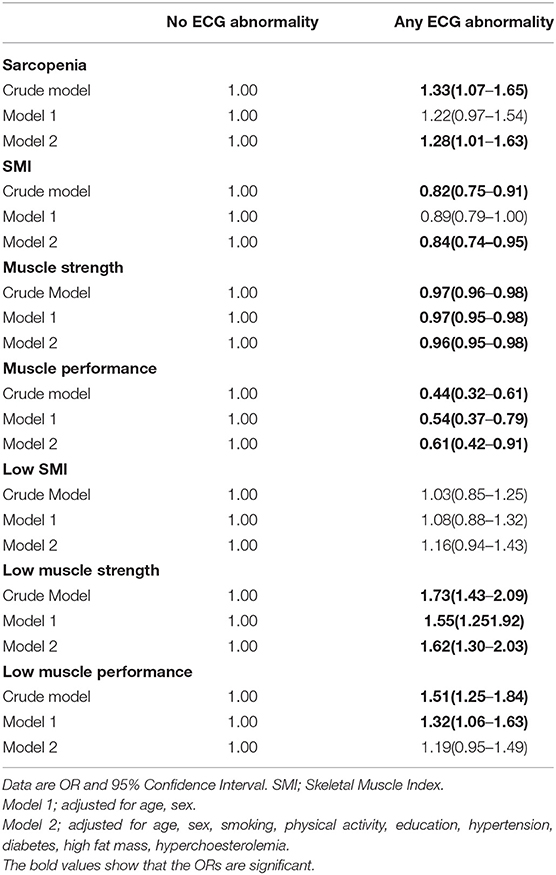
Table 3 demonstrates the association of sarcopenia and muscle components with any ECG abnormality. Sarcopenia and low muscle strength increased 28 and 62% risk of any ECG abnormality in the full models [sarcopenia: 1.28(1.01–1.63), low muscle strength: 1.62(1.30–2.03)], respectively. The relationship between low muscle performance and any ECG abnormality was significant only in crude and age, sex adjusted model. The associations between low SMI and any ECG abnormality were not statistically significant in all models.
Also, we observed statistically significant associations of SMI, muscle strength and muscle performance with any ECG abnormality in all models especially in full models.
Discussion
This cross-sectional study aimed to assay the association of sarcopenia and components of muscle with major and minor ECG abnormalities. The results of the present study demonstrated sarcopenia to be independently and strongly associated with major ECG abnormalities in older people. Furthermore, participants with low muscle strength or low muscle performance had higher risk of major ECG abnormality than those with normal ECG.
Studies explained that skeletal muscle, as the most tissue in the human body, is involved in some metabolic functions including energy expenditure, protein metabolism and insulin sensitivity (31, 32). Also, these muscles have endocrine actions with releasing some myokines and play in regulation inflammation and immune function. Therefore, decline of muscle mass and muscle function, as named sarcopenia can lead to several age-related metabolic disorders and some diseases such as CHD, hypertension and heart failure (33–35).
CHD is a major cause of death and disability in the world. Understanding the risk factors and comorbidities is essential for prevention, early diagnosis, reduction of mortality and to evaluate management effectiveness (4). Among comorbidities, sarcopenia with common mechanisms can associate with CHD. Previous reports have shown that the associations of sarcopenia and its parameters with carotid atherosclerosis, myocardial infarction (MI), chronic heart failure and other heart diseases (14, 33). A recent study found that sarcopenia was associated with MI and atrial fibrillation. However, in the mention study, sarcopenia was defined based on the skeletal muscle mass and muscle strength did not assay in their population (14). In another study, researchers showed that sarcopenia was as prognostic predictor in older people with acute MI (12).
ECG is an available, low cost and useful tool for risk prediction of asymptomatic subjects with CHD, especially in older people given their higher prevalence of CVD events (36, 37). In some studies, CHD was defined as a symptom of angina pectoris based on the Rose Angina Questionnaire, a positive history of CHD, or a positive ECG for CHD. There are also studies that use only one definition of CHD separately. Therefore, CHD could be defined based on ECG abnormalities (29, 38, 39).
In this study, we revealed the relationship between sarcopenia and major ECG abnormalities. The model analysis indicated that the risk of major ECG abnormality was increased in sarcopenic participants (OR = 1.47, 95% CI: 1.11–1.95) even after adjustment for several risk factors. Also, among components of sarcopenia, we found the independent association between muscle strength and muscle performance with ECG abnormalities. Considerable evidence supports muscle strength, an important component of sarcopenia, has an independent role in the prevention of cardiovascular events and mortality (40–42). A systematic review of 23 selected publications showed that muscle strength was inversely and independently associated with all cause and cardiovascular mortality. Furthermore, a strong and inverse association of muscle strength with mortality had been confirmed in patients with chronic diseases such as CHD, cancer, and peripheral artery disease (43).
Our findings also supported that low muscle mass alone may not completely reveal muscle performance and that muscle strength should be considered an important parameter in defining sarcopenia. This issue has also been considered by the EWGSOP and in the recent definition of sarcopenia, the importance of muscle strength over muscle mass has been emphasized (3). Furthermore, the present study demonstrated the important of muscle performance as a risk factor for CHD. It implied that the muscle function of the lower extremities may be important in cardiovascular outcomes. Consistent with our findings, recent studies revealed gait speed was associated with an increased HRs for death and cardiovascular mortality (44, 45).
Both muscle strength and muscle performance are used to assess the function of skeletal muscle and these tests are simple and rapid and can be performed in older people. On the other hand, due to the relevance of these parameters with health outcomes, these are useful for predicting CVD and all- cause mortality. This may, at least in part, explain our observations that muscle strength and muscle performance are independently and significantly associated with health outcomes regardless of muscle mass (45, 46).
The principal mechanism for the relationships between sarcopenia and its components, especially muscle strength or muscle performance, with CHD has not been clearly explored. Muscle mitochondrial dysfunction, which is involved in the pathogenesis of sarcopenia, may also play a role to develop of cardiovascular disease by increasing oxidative stress production and damage of vascular endothelium (47). In addition, insulin resistance, chronic inflammation, and abnormalities in anabolic hormones such as low testosterone levels are other common mechanisms involve in sarcopenia especially low muscle function and atherosclerosis diseases (48–50). Physical activity and activities of daily living may be reduced after developing CVDs and also muscle atrophy occurs by reducing blood flow in skeletal muscle and infiltration of adipocytes into muscle fibers (50). The above mechanisms suggest that sarcopenia and its parameters associate with CVDs and prevention and treatment of sarcopenia would decrease the risk of atherosclerosis and death in older people.
In this study, some limitations should be considered. The cross-sectional design limited the possibilities of determining the causal inferences and further longitudinal designs are needed to clarify any causal relationship. However, ECG is an easy screening test for CHD, but not the gold standard method such as coronary angiography. Therefore, this issue might be impacted on our results. Despite these limitations, the strength of this study is that our findings were based on a great sample size from a population-based study provided data on musculoskeletal disorders such as sarcopenia among older people in Iran. Also, skeletal muscle mass was directly measured with DXA as a gold standard for diagnosing low muscle mass.
Conclusions
This study highlighted that sarcopenia was associated with ECG abnormalities independent of the well-known cardiovascular risk factors in Iranian older people. Among parameters of sarcopenia, muscle strength and muscle performance were the most important factors to associate with ECG abnormalities.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Bushehr University of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the personnel of the Bushehr Elderly Health program and all the individuals who took part in the study.
References
1. Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. (2011) 27:337–9. doi: 10.1016/j.cger.2011.03.003
2. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age and Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
3. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
4. Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. (2016) 4:256. doi: 10.21037/atm.2016.06.33
5. Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. (2003) 290:898–904. doi: 10.1001/jama.290.7.898
6. Sarrafzadegan N, Mohammmadifard N. Cardiovascular Disease in Iran in the Last 40 Years: Prevalence, Mortality, Morbidity, Challenges and Strategies for Cardiovascular Prevention. Arch Iran Med. (2019) 22:204–10.
7. Auer R, Bauer DC, Marques-Vidal P, Butler J, Min LJ, Cornuz J, et al. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. (2012) 307:1497–505. doi: 10.1001/jama.2012.434
8. Liao YL, Liu KA, Dyer A, Schoenberger JA, Shekelle RB, Colette P, et al. Major and minor electrocardiographic abnormalities and risk of death from coronary heart disease, cardiovascular diseases and all causes in men and women. J Am Coll Cardiol. (1988) 12:1494–500. doi: 10.1016/s0735-1097(88)80016-0
9. Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women's Health Initiative. Circulation. (2006) 113:473–80. doi: 10.1161/CIRCULATIONAHA.104.496091
10. Scheidt-Nave C, Barrett-Connor E, Wingard DL. Resting electrocardiographic abnormalities suggestive of asymptomatic ischemic heart disease associated with non-insulin-dependent diabetes mellitus in a defined population. Circulation. (1990) 81:899–906. doi: 10.1161/01.cir.81.3.899
11. Shafiee G, Sharifi F, Alizadeh M, Arzaghi SM, Maleki A, Esfahani MA, et al. Electrocardiogram abnormalities and risk of cardiovascular mortality and all-cause mortality in old age: the Kahrizak Elderly Study (KES). Arch Gerontol Geriatr. (2014) 59:69–73. doi: 10.1016/j.archger.2014.03.011
12. Santana NM, Mendes RML, Silva NFD, Pinho CPS. Sarcopenia and sarcopenic obesity as prognostic predictors in hospitalized elderly patients with acute myocardial infarction. Einstein. (2019) 17:eAO4632. doi: 10.31744/einstein_journal/2019AO4632
13. Requena Calleja MA, Arenas Miquelez A, Diez-Manglano J, Gullon A, Pose A, Formiga F, et al. Sarcopenia, frailty, cognitive impairment and mortality in elderly patients with non-valvular atrial fibrillation. Rev Clin Esp. (2019) 219:424–32. doi: 10.1016/j.rce.2019.04.001
14. Xia MF, Chen LY, Wu L, Ma H, Li XM, Li Q, et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: a cross-sectional study. Clin Nutr. (2020) 40:571–80. doi: 10.1016/j.clnu.2020.06.003
15. Yamada S, Kamiya K, Kono Y. Frailty may be a risk marker for adverse outcome in patients with congestive heart failure. ESC Heart Fail. (2015) 2:168–70. doi: 10.1002/ehf2.12052
16. Doehner W, Frenneaux M, Anker SD. Metabolic impairment in heart failure: the myocardial and systemic perspective. J Am Coll Cardiol. (2014) 64:1388–400. doi: 10.1016/j.jacc.2014.04.083
17. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) 16:21. doi: 10.1186/s40200-017-0302-x
18. Gupta S, Gupta RK, Kulshrestha M, Chaudhary RR. Evaluation of ECG Abnormalities in Patients with Asymptomatic Type 2 Diabetes Mellitus. J Clin Diagn Res. (2017) 11:OC39-OC41. doi: 10.7860/JCDR/2017/24882.9740
19. Ebong IA, Bertoni AG, Soliman EZ, Guo M, Sibley CT, Chen YD, et al. Electrocardiographic abnormalities associated with the metabolic syndrome and its components: the multi-ethnic study of atherosclerosis. Metab Syndr Relat Disord. (2012) 10:92–7. doi: 10.1089/met.2011.0090
20. Ostovar A, Nabipour I, Larijani B, Heshmat R, Darabi H, Vahdat K, et al. Bushehr Elderly Health (BEH) Programme, phase I (cardiovascular system). BMJ Open. (2015) 5:e009597. doi: 10.1136/bmjopen-2015-009597
21. Shafiee G, Ostovar A, Heshmat R, Darabi H, Sharifi F, Raeisi A, et al. Bushehr Elderly Health (BEH) programme: study protocol and design of musculoskeletal system and cognitive function (stage II). BMJ Open. (2017) 7:e013606. doi: 10.1136/bmjopen-2016-013606
22. Prineas RJ, Harland WR, Janzon L, Kannel W. Recommendations for use of non-invasive methods to detect atherosclerotic peripheral arterial disease–in population studies. American Heart Association Council on Epidemiology. Circulation. (1982) 65:1561A-6A.
23. Aadahl M, Jorgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. (2003) 35:1196–202. doi: 10.1249/01.MSS.0000074446.02192.14
24. Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. (2007) 14:422–8. doi: 10.1097/HJR.0b013e3280128d00
25. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
26. Shafiee G, Ostovar A, Heshmat R, Keshtkar AA, Sharifi F, Shadman Z, et al. Appendicular Skeletal Muscle Mass Reference Values and the Peak Muscle Mass to Identify Sarcopenia among Iranian Healthy Population. Int J Prev Med. (2018) 9:25. doi: 10.4103/ijpvm.IJPVM_295_17
27. Shafiee G, Heshmat R, Ostovar A, Khatami F, Fahimfar N, Arzaghi SM, et al. Comparison of EWGSOP-1and EWGSOP-2 diagnostic criteria on prevalence of and risk factors for sarcopenia among Iranian older people: the Bushehr Elderly Health (BEH) program. J Diabetes Metab Disord. (2020) 19:727–34. doi: 10.1007/s40200-020-00553-w
28. Hill KD, Farrier K, Russell M, Burton E. Dysmobility syndrome: current perspectives. Clin Interv Aging. (2017) 12:145–52. doi: 10.2147/CIA.S102961
29. Ford ES, Giles WH, Croft JB. Prevalence of nonfatal coronary heart disease among American adults. Am Heart J. (2000) 139:371–7. doi: 10.1016/s0002-8703(00)90076-0
30. Ashley EA, Raxwal V, Froelicher V. An evidence-based review of the resting electrocardiogram as a screening technique for heart disease. Prog Cardiovasc Dis. (2001) 44:55–67. doi: 10.1053/pcad.2001.24683
31. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. (2014) 33:737–48. doi: 10.1016/j.clnu.2014.03.007
32. Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol. (2017) 34:49–55. doi: 10.1016/j.coph.2017.05.005
33. Li CW, Yu K, Shyh-Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle. (2019) 10:586–600. doi: 10.1002/jcsm.12417
34. Dogan MH, Karadag B, Ozyigit T, Kayaoglu S, Ozturk AO, Altuntas Y. Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta Clin Belg. (2012) 67:328–32. doi: 10.2143/ACB.67.5.2062685
35. Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. (2009) 73:13–8. doi: 10.1253/circj.cj-08-0961
36. Chou R, Arora B, Dana T, Fu R, Walker M, Humphrey L. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. (2011) 155:375–85. doi: 10.7326/0003-4819-155-6-201109200-00006
37. Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and minor ECG abnormalities in asymptomatic women and risk of cardiovascular events and mortality. JAMA. (2007) 297:978–85. doi: 10.1001/jama.297.9.978
38. Hadaegh F, Hatami M, Mohebi R, Hasheminia M, Bozorgmanesh M, Sheikholeslami F, et al. Electrocardiography-defined silent CHD and risk of cardiovascular events among diabetic patients in a Middle Eastern population. Eur J Prev Cardiol. (2012) 19:1227–33. doi: 10.1177/1741826711428065
39. Nilsson U, Blomberg A, Johansson B, Backman H, Eriksson B, Lindberg A. Ischemic ECG abnormalities are associated with an increased risk for death among subjects with COPD, also among those without known heart disease. Int J Chron Obstruct Pulmon Dis. (2017) 12:2507–14. doi: 10.2147/COPD.S136404
40. Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, et al. Associations of Muscle Mass and Strength with All-Cause Mortality among US Older Adults. Med Sci Sports Exerc. (2018) 50:458–67. doi: 10.1249/MSS.0000000000001448
41. Artero EG, Lee DC, Lavie CJ, Espana-Romero V, Sui X, Church TS, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. (2012) 32:351–8. doi: 10.1097/HCR.0b013e3182642688
42. Lee MR, Jung SM, Kim HS, Kim YB. Association of muscle strength with cardiovascular risk in Korean adults: Findings from the Korea National Health and Nutrition Examination Survey (KNHANES) VI to VII (2014–2016). Medicine. (2018) 97:e13240. doi: 10.1097/MD.0000000000013240
43. Volaklis KA, Halle M, Meisinger C. Muscular strength as a strong predictor of mortality: a narrative review. Eur J Intern Med. (2015) 26:303–10. doi: 10.1016/j.ejim.2015.04.013
44. Lee YH, Kim JS, Jung SW, Hwang HS, Moon JY, Jeong KH, et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. (2020) 21:166. doi: 10.1186/s12882-020-01831-8
45. Veronese N, Stubbs B, Volpato S, Zuliani G, Maggi S, Cesari M, et al. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc. (2018) 19:981–8. e7. doi: 10.1016/j.jamda.2018.06.007
46. Menant JC, Weber F, Lo J, Sturnieks DL, Close JC, Sachdev PS, et al. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people: time to abandon the term sarcopenia? Osteoporos Int. (2017) 28:59–70. doi: 10.1007/s00198-016-3691-7
47. Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. (2017) 8:349–69. doi: 10.1002/jcsm.12178
48. Srinivas-Shankar U, Wu F. Frailty and muscle function: role for testosterone? Front Horm Res. (2009) 37:133–49. doi: 10.1159/000176050
49. Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, Blackman MR, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab. (2006) 290:E234–42. doi: 10.1152/ajpendo.00059.2005
Keywords: sarcopenia, ECG abnormalities, older people, risk, components of sarcopenia
Citation: Heshmat R, Shafiee G, Ostovar A, Fahimfar N, Maleki Birjandi S, Jabbari M, Sharifi F, Nabipour I and Larijani B (2021) Relationship Between Sarcopenia and Electrocardiographic Abnormalities in Older People: The Bushehr Elderly Health Program. Front. Med. 8:656181. doi: 10.3389/fmed.2021.656181
Received: 20 January 2021; Accepted: 12 April 2021;
Published: 07 May 2021.
Edited by:
Emanuele Marzetti, Catholic University of the Sacred Heart, ItalyReviewed by:
Natalia Sharashkina, Pirogov Russian National Research Medical University, RussiaMasahide Hamaguchi, Kyoto Prefectural University of Medicine, Japan
Copyright © 2021 Heshmat, Shafiee, Ostovar, Fahimfar, Maleki Birjandi, Jabbari, Sharifi, Nabipour and Larijani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gita Shafiee, Z3NoYWZpZWUuZW5kb2NyaW5lQGdtYWlsLmNvbQ==
 Ramin Heshmat
Ramin Heshmat Gita Shafiee
Gita Shafiee Afshin Ostovar
Afshin Ostovar Noushin Fahimfar2
Noushin Fahimfar2 Bagher Larijani
Bagher Larijani