Abstract
Purpose: Over the last decade, there has been tremendous progress in the treatment of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). To understand whether this has resulted in better ophthalmic outcomes, we aimed to study the incidence of severe ocular complications (SOCs) in the acute and chronic stage among SJS/TEN patients, major causative medications, and therapeutic effect of medical and surgical treatment.
Methods: Using electronic medical records review of patients of Chang Gung Memorial Hospital Linkou Branch from 2010 to 2020, 119 patients (236 eyes) received ophthalmic consultation during the acute stage and were retrospectively studied. Sotozono's grading score systems for acute and chronic SJS/TEN were employed for accessing correlation between acute and chronic presentations, the therapeutic effect of systemic etanercept treatment, and outcome of early amniotic membrane transplantation (AMT) performed in patients with severe acute SOCs.
Results: There were 46 male and 73 female patients with a mean age of 45.6 ± 22.7 years old (2–90 years), and follow-up time of 408.3 ± 351.0 (116–1,336) days. The numbers of patients with SJS, overlap syndrome, and TEN were 87, 9, and 23, respectively. In total, 109 eyes (55 patients) had acute SOCs, which comprised 46.2% of patients who underwent ophthalmic examination. Antiepileptics were the most common category of culprit drugs causing SOCs in the acute stage. At the end of follow-up, there were 14 eyes (9 patients) with chronic SOCs (5.9%), and non-steroidal anti-inflammatory drugs and cold medicine were the most common causative medications that were associated with severe chronic sequela. The correlation between Sotozono's acute and chronic grading score showed a positive relationship [Spearman's rank correlation coefficient (r) = 0.52, p < 0.001]. The average chronic grading scores in patients receiving systemic corticosteroid combined with etanercept treatment were significantly lower than those receiving corticosteroid only, Finally, the average chronic grading scores in patients receiving AMT <7 days after onset were significantly lower than those performed beyond 7 days.
Conclusion: Our study implies that acute manifestation can be an indicator for chronic sequelae. Additional early etanercept treatment and early AMT showed beneficial effect in reducing chronic ocular sequela.
Introduction
Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is a potentially life-threatening and vision-impairing immune-mediated disease with an estimated annual incidence of 1–5 per 1,000,000 individuals globally (1). The incidence differs among countries. A study from Korea reported incidences of 3.96–5.03 for SJS and 0.94–1.45 for TEN per million individuals from 2010 to 2013 (2). An incidence rate of 5.76 SJS/TEN cases per million person-years were reported from the UK (database between 1995 and 2013) (3).
Two studies from the US reported similar incidences. The annual SJS spectrum incidence rate reported by White et al. was 12.35 per million people per year (database between 2010 and 2012) (1), and Hsu et al. reported that the mean estimated incidences of SJS, SJS/TEN, and TEN were 9.2, 1.6, and 1.9 per million adults per year, respectively (database between 2010 and 2012) (4).
In Taiwan, utilizing data from the National Health Insurance Research Database (NHIRD) of Taiwan from 2000 to 2008, Syu et al. reported that the overall SJS incidence rate was 3.6 per million people per year (study database between 2000 and 2008) (5). These data may potentially reveal a considerable number of patients affected by ocular complications. Severe ocular complications (SOCs) in chronic SJS/TEN include dry eye, chronic inflammation and neovascularization of the cornea, symblepharon and keratinization of the conjunctiva. If not properly handled, SOCs often result in permanent visual loss.
Because of severe dry eye, SOCs in SJS/TEN are more difficult to treat by surgery; therefore, prevention is the key to reduce the incidence of SOCs in SJS/TEN.
Although Taiwan was the first country to report an intense association of human leukocyte antigen (HLA)-B*15:02 and carbamazepine-induced SJS/TEN (6), and the first to implement HLA-B*15:02 screening for carbamazepine users (7), reports focusing on ocular manifestations of SJS/TEN from Taiwan are limited. In 2007, Chang et al. reported a total of 207 patients with 213 episodes/attacks of SJS/TEN. The most frequent causative drugs were carbamazepine and allopurinol. Dry eye was the most frequent ocular sequelae identified within 3 months after hospital discharge (17.2%) followed by symblepharon (4.7%) and corneal scarring (4.7%) (8).
Over the last decade, there has been considerable progress not only in diagnostic methods but also in treatment protocols, especially the early application of amniotic membrane transplantation (AMT) (9–12), and it has been shown that the long-term outcomes of AMT to be quite favorable (9, 13). In this article, we aim to study the incidence of SOCs among SJS/TEN patients, major causative medications, the final outcome of affected patients, therapeutic effect of systemic corticosteroids combined with etanercept treatment, and beneficial effect of early AMT.
Materials and Methods
This retrospective study to collect and analyze electronic medical records of SJS/TEN was approved by the Institutional Review Boards of Chang Gung Medical Foundation (IRB approval No. 202100092B0). All experimental procedures were conducted in accordance with the principles set forth in the Helsinki Declaration.
The list of SJS/TEN patients who were admitted to Chang Gung Memorial Hospital, Linkou Branch from January 2010 to July 2020 was retrieved from the database maintained by the Department of Dermatology. The electronic medical records were retrospectively reviewed, and the recorded data included information on gender, presenting age, causative medications/diseases, and systemic treatments. Regarding disease chronicity, Shanbhag et al. have defined the acute phase as the period between symptom onset of SJS/TEN up to 2 months later; the subacute phase was defined as 2–6 months after symptom onset; and the chronic phase was defined as more than 6 months after symptom onset (14). Ocular manifestations in the acute stage were retrieved from ophthalmic consultation records. To record the severity of ocular involvement at the first time of consultation in the acute stage (within 1 week from the first manifestation), the Sotozono acute stage ocular surface grading score (OSGS) was employed (15). OSGS ranges from grade 0 to 3, with grade 0 indicating no ocular surface involvement, grade 1 indicating mild involvement with conjunctival hyperemia, grade 2 indicating severe involvement with accompanying pseudomembrane formation or ocular surface epithelial defects, and grade 3 indicating very severe involvement with both pseudomembrane formation and ocular surface epithelial defects. The eyes with greater than grade 2 ocular involvement were considered to have acute severe ocular complications (SOCs). The initial VA and ophthalmic medical and surgical treatments were also reviewed. Visual acuity (VA) was evaluated in naked-eye with standard Snellen E chart. Regarding the causative medications, those with acute SOCs (grade 2 and grade 3) were analyzed and compared in percentage.
In the chronic stage (defined as at least 6 months from the first manifestation), ocular involvement was assessed according to the scoring system proposed by Sotozono et al. (16). Complications were broadly defined as corneal, conjunctival, and eyelid complications. There are a total of 13 categories of findings, such as superficial punctate keratopathy (SPK), hyperemia, trichiasis, and symblepharon. Each category scored from 0 to 3, and the maximal score was 39. Treatment in the chronic stage, Schirmer test values and vision were also recorded. Because there was a high portion of missing data for Schirmer test values, the diagnosis of dry eye was made if the patient's Schirmer's test I value (without topical anesthesia) was <10 mm and/or the cornea showed greater than grade 1 SPK. To judge the severity of the ocular manifestation in the chronic stage, the patient's last electronic medical records with a detailed eye examination was used as a reference (Table 1). Patients with Sotozono's chronic stage grading scores 0–5 were considered to have non- or minimal ocular involvement. Patients with scores 6–10 exhibited mild involvement, scores 11–15 indicate moderate involvement, and scores > 16 were considered to have severe involvement.
Table 1
| Demographics/characteristics | |
|---|---|
| Number of patients | 119 |
| Total eye number | 236 |
| Mean age ± SD (range) | 45.6 ± 22.7 (2–90) |
| Gender, n (%) | |
| Male | 46 (38.7) |
| Female | 73 (61.3) |
| Laterality, n (%) | |
| Bilateral | 117 (98.3) |
| Unilateral | 2 (1.7) |
| Diagnosis, n (%) | |
| SJS | 87 (73.1) |
| Overlap syndrome | 9 (7.6) |
| TEN | 23 (19.3) |
| Mean follow-up time (days) | 408.3 ± 351.0 (116–1,336) |
| Sotozono's acute stage grading scores, n=eyes (%) | |
| Grade 0 | 30 (12.7) |
| Grade 1 | 97 (41.1) |
| Grade 2 | 79 (33.5) |
| Grade 3 | 30 (12.7) |
| Mean OSGS in acute stage (range) | 1.46 ± 0.87 (0–3) |
| Sotozono's chronic stage grading scores, n=eyes (%) | |
| 0–5 | 195 (82.6) |
| 6–10 | 22 (9.3) |
| 11–15 | 5 (2.1) |
| >16 | 14 (5.9) |
| Mean OSGS in chronic stage (range) | 3.54 ± 5.50 (0–33) |
Demographics and grading of patients receiving ophthalmic examination in acute and chronic stage of SJS/TEN from 2010 to 2020.
SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; OSGS, ocular surface grading score.
Based on published therapeutic benefits of steroid therapy at disease onset in preventing ocular complications (17), systemic treatment with steroid pulse therapy (1–1.5 mg/kg/day prednisolone by intravenous injection until the skin lesions were healed), intravenous methylprednisolone or hydrocortisone has also been commonly administered to SJS/TEN patients admitted to dermatology wards (17, 18). Based on the conclusion from a randomized and controlled trial from our hospital (2009–2015) (19), additional subcutaneous etanercept (Enbrel; Pfizer) has been prescribed for recalcitrant SJS/TEN patients with progression of blistering or erythema even after methylprednisolone (>1 mg/kg/day) treatment for 3–5 days. To investigate the effect of additional systemic etanercept on long-term ocular outcome, we focused on patients whose medical records showed treatment with intravenous corticosteroids with or without additional subcutaneous etanercept. Demographics of the patients in each group such as age, gender, and distribution of top 5 culprit drugs were compared, and we found no significant difference in these parameters (Table 5). Thereafter, the acute and chronic stage severity grading scores were compared between these two groups.
In the acute stage, we used topical balanced salt solution (BSS, Alcon) for lubrication, levofloxain (Cravit, Santen) as a prophylactic antibiotic, Tobradex ointment (Alcon) as topical corticosteroid ointment for eyelid wounds, and preservative-free 0.1% betamethasone (Fusone, AIM Medicine, Taiwan) qid to q3h when conjunctival congestion was evident. Conjunctival pseudomembranes were removed every 2–4 days. If grade 3 and sometimes grade 2 ocular involvement was noted, amniotic membrane (AM) dressing using cryopreserved AM was performed to protect the entire ocular surface. Before 2019, suture-fixated AM dressing was performed (20–22). Since 2020, sutureless AM dressing using cyanoacrylate glue to fixate AM at the lid margin has been adopted (23). This novel technique significantly reduced the time and discomfort of the surgery. To study the influence of timing of AM transplantation (AMT; AM grafting or AM dressing) on the outcome, we compared the Sotozono's chronic grading score of patients who received AMT less than or more than 7 days after onset.
Statistics
All statistics were calculated using SPSS software version 23.0 for Windows (SPSS, Inc., Chicago, IL, USA). Continuous variables are presented as the mean and standard deviation (SD). Spearman's rank correlation was used to present the association between Sotozono's acute and chronic grading score. Correlation coefficients (ρ) were also calculated. Student's t-test was used to compare the difference in demographics, follow-up duration, acute and chronic grading scores between patients treated with or without additional etanercept, and chronic grading scores in patients receiving AMT before or beyond 7 days after onset. Chi-squared test was used to compare distribution of causative medications between patients treated with or without additional etanercept. A p < 0.05 was deemed to be statistically significant.
Results
From January 2010 until July 2020, a total of 294 patients were registered in the Department of Dermatology database. Among them, 119 patients (236 eyes) received ophthalmic consultation during the acute stage and were recruited in this study. There were 46 male and 73 female patients with a mean age of 45.6 +/– 22.7 years old (2–90 years). The numbers (percentages) of patients with SJS, overlap syndrome, and TEN were 87 (73.1%), 9 (7.6%), and 23 (19.3%), respectively. When the number of involved eyes was calculated, using Sotozono's acute OSGS, there were 30 eyes without ocular involvement (grade 0, 12.7%), 97 eyes with mild involvement (grade 1, 41.1%), 79 eyes with severe involvement (grade 2, 33.5%), and 30 eyes with the most severe involvement (grade 3, 12.7%). Given that patients with greater than grade 2 ocular involvement were considered to have SOCs, there were 109 eyes (55 patients) in total with acute SOCs, comprising 46.2% of patients who had ophthalmic examination, and 18.6% of total patients (Table 1). In the chronic stage, when the assessment was done at an averaged 408.3 ± 351.0 (116–1,336) days after onset, there were 195 eyes (82.6%) with Sotozono's chronic grading scores 0–5 (non- or minimal ocular involvement). Twenty-two eyes (9.3%) with scores 6–10 (mild involvement), 5 eyes (2.1%) with scores 11–15 (moderate involvement), and 14 eyes (5.9%) with scores >16 (severe involvement) (Table 1).
Among 67 identifiable drugs that were associated with acute SOCs, antiepileptics (carbamazepine, phenytoin, lamitrogine, etc.) was the category of drugs that were associated with most acute SOCs (n = 15, 22.4%) followed by antibiotics (n = 12, 17.9%), allopurinol (n = 8, 11.9%), non-steroidal anti-inflammatory drug (NSAID; n = 7, 10.4%), and sulfa drugs (n = 6, 9.0%) (Table 2). In terms of a single drug, allopurinol was the drug that was associated with most acute SOCs. In 14 eyes of the 9 patients with chronic SOCs, NSAID and cold medicine were the most common causative medications that were associated with chronic SOCs (44.4%, Table 2).
Table 2
| Name of causative drugs | No. of patients (%) |
|---|---|
| Acute SOCs | N = 67 |
| Antiepileptics | 15 (22.4) |
| Phenytoin | 2 (3.0) |
| Carbamazepine | 4 (6.0) |
| Oxcarbazepine | 5 (7.5) |
| Lamotrigine | 2 (3.0) |
| Zonisamide | 2 (3.0) |
| Antibiotics | 12 (17.9) |
| Cephalexin | 1 (1.5) |
| Cefuroxime | 1 (1.5) |
| Ceftazidime | 1 (1.5) |
| Ceftriaxone | 1 (1.5) |
| Norfloxacin | 1 (1.5) |
| Levofloxacin | 1 (1.5) |
| Moxifloxacin | 1 (1.5) |
| Amoxicillin | 3 (4.5) |
| Vancomycin | 2 (3.0) |
| Allopurinol | 8 (11.9) |
| NSAIDs | 7 (10.4) |
| Diclofenac | 3 (4.5) |
| Ibuprofen | 1 (1.5) |
| Mefenamic acid | 3 (4.5) |
| Sulfa drugs | 6 (9.0) |
| Sulfasalazine | 3 (4.5) |
| Sulfonamide | 3 (4.5) |
| Miscellaneous | 19 (28.4) |
| Chronic SOCs | N = 9 |
| NSAIDs | 4 (44.4) |
| Diclofenac | 2 (22.2) |
| Mefenamic acid | 1 (11.1) |
| Cold medicine | 19 (11.1) |
| Anti-epileptics | 2 (22.2) |
| Carbamazepine | 1 (11.1) |
| Lamotrigine | 1 (11.1) |
| Antibiotics | |
| Cefuroxime | 1 (11.1) |
| Sulfa drugs | |
| Sulfasalazine | 1 (11.1) |
| Tenofovir | 1 (11.1) |
Top 5 causative drug categories responsible for acute and chronic SOCs in SJS/TEN patients.
SOCs, Severe ocular complications.
NSAIDs, Non-steroidal anti-inflammatory drugs.
The average grading score for all patients in the acute stage was 1.46 ± 0.87. The value in the chronic stage (at the end of follow-up) was 3.54 ± 5.50. For patients whose grading score ranged from 0 to 3 during the acute stage, their corresponding chronic stage grading scores were 0.67 ± 0.80, 1.62 ± 1.99, 4.70 ± 6.38, and 9.60 ± 7.73, respectively. The correlation between Sotozono's acute and chronic grading score showed a moderately correlated relationship [Spearman's rank correlation coefficient (ρ) = 0.52, p < 0.001] (Figure 1). This finding implies that patients who presented with more severe acute ocular manifestations tended to suffer from sequelae in the chronic stage.
Figure 1
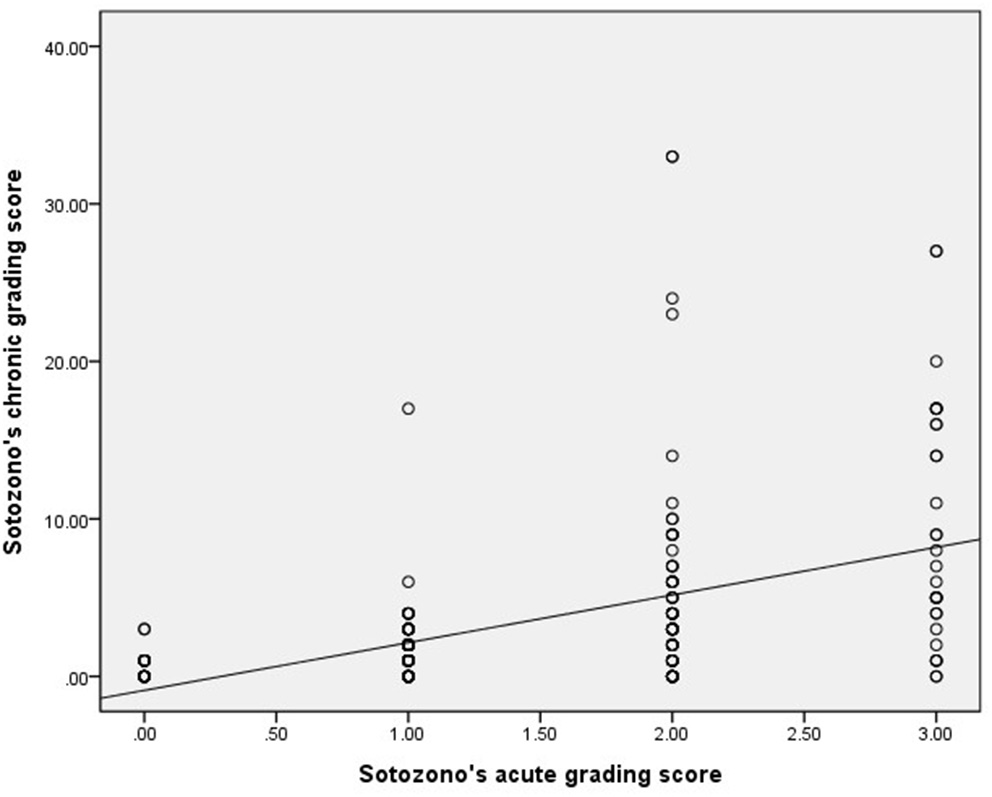
Correlations between Sotozono's acute (15) and chronic (16) stage grading score of SJS/TEN patients evaluated during the acute and chronic stage, respectively.
Judging from Sotozono's chronic stage ocular complication category, hyperemia was the most common chronic stage ocular complication, followed by SPK, meibomian gland dysfunction, mucocutaneous junction involvement, and trichiasis. Judged from the Schirmer test value and the presence of corneal staining, the incidence of dry eye in the chronic stage was estimated to be 49.2% (116 eyes) (Table 3).
Table 3
| Characteristic | Number of eye | Percentage (%) |
|---|---|---|
| Corneal complication | ||
| Superficial punctate keratitis | 117 | 49.58 |
| Epithelium defect | 29 | 12.29 |
| Loss of POV* | 9 | 3.81 |
| Conjunctivalization | 13 | 5.51 |
| Neovasculization | 22 | 9.32 |
| Opacification | 19 | 8.05 |
| Keratinization | 14 | 5.93 |
| Conjunctival complication | ||
| Hyperemia | 122 | 51.69 |
| Symblepharon | 8 | 3.39 |
| Eyelid complication | ||
| Tichiasis | 49 | 20.76 |
| MC* junction involvement | 62 | 26.27 |
| Meibomian gland involvement | 90 | 38.14 |
| Punctal damage | 7 | 2.97 |
Ocular manifestations in chronic stage of SJS/TEN patients.
POV, Palisades of Vogt; MC, Mucocutaneous.
When best corrected vision in the chronic stage was measured, data were available from 174 eyes. Among them, 112 eyes (64.4%) had vision better than 20/40, and 43 eyes (24.7%) had vision between 20/40 and 20/200. Nine eyes (5.2%) had vision between 20/200 and 20/2,000, and 10 eyes (5.7%) had vision worse than 20/2,000.
To study the effect of additional systemic etanercept on long-term ocular manifestations, there was no significant difference in gender, age, distribution of culprit drugs, and follow-up time between patients receiving corticosteroid treatment only and patients receiving corticosteroid plus etanercept treatment (Table 4). The average acute stage score for patients who received additional systemic etanercept was 1.32 ± 0.76 (0–3). The value for patients who received systemic corticosteroid only treatment was 1.49 ± 0.90 (0–3; p = 0.171). In the chronic stage, the average score for additional etanercept treated patients was 1.64 ± 2.47 (0–14), which was significantly lower than the score for corticosteroid only treated patients (3.95 ± 5.76; range 0–33, p < 0.001) (Table 4).
Table 4
| Corticosteroid + | Corticosteroid | p-value | |
|---|---|---|---|
| etanercept | only | ||
| Number (patient/eye) | 31/62 | 82/164 | |
| Gender (M/F) | 15/16 | 32/50 | 0.126 |
| Age (years old) | 48.00 ± 18.72 | 45.79 ± 24.06 | 0.601 |
| Mean follow up time (days) | 485.00 ± 502.42 | 360.82 ± 319.64 | 0.319 |
| Time to intervention (days) | 1.96 ± 1.49 | 0.78 ± 0.64 | 0.003 |
| Major causative drugs | |||
| Anti-epileptics | 24 (38.71%) | 48 (29.27%) | 0.264 |
| Antibiotics | 11 (17.74%) | 26 (15.58%) | |
| Allopurinol | 6 (9.68%) | 10 (6.10%) | |
| NSAID | 6 (9.68%) | 26 (15.58%) | |
| Sulfa drugs | 2 (3.23%) | 18 (10.98%) | |
| Others | 13 (20.97%) | 36 (21.95%) | |
| Sotozono's grading score | |||
| Acute stage | |||
| Mean | 1.323 | 1.488 | 0.171 |
| Std. deviation | 0.763 | 0.903 | |
| Std. Error Mean | 0.097 | 0.071 | |
| Chronic stage | |||
| Mean | 1.645 | 3.951 | <0.001 |
| Std. deviation | 2.470 | 5.765 | |
| Std. error mean | 0.314 | 0.450 | |
Comparison between corticosteroid with or without additional etanercept treatment on Sotozono's acute and chronic stage grading score.
Finally, for surgical treatment, during the acute stage (<2 months after onset), 7 patients (13 eyes) received AM grafting (AMG) and 7 patients (14 eyes) received AM dressing (AMD) at an averaged 8.15 ± 4.5 days and 4.93 ± 4.6 days, respectively, after onset. During the chronic stage, seven patients (12 eyes) received oral mucosal transplantation to correct lid margin keratinization, 4 patients (7 eyes) received punctal occlusion to treat dry eye, and 2 patients (4 eyes) received correction of entropion (Table 5). When we compare the Sotozono's chronic stage grading scores in patients receiving AM transplantation (AMG + AMD) less than or more than 7 days after onset, we found that in AMG group and combined AMG and AMD group, the scores were significantly lower when the operation was performed <7 days (2.17 ± 2.63 vs. 15.6 ± 12.94, p = 0.031; 6.29 ± 4.69 vs. 13.60 ± 11.22, p = 0.025; Chi-squared test). This implies that patients receiving earlier AM transplantation were associated with less severe chronic SOCs (Table 5).
Table 5A
| Type of | Patient | Eye | Time to |
|---|---|---|---|
| surgery | (%) | (%) | intervention (days) |
| Acute stage | |||
| AMG* | 7 (5.88) | 13 (5.51) | 8.15 ± 4.5 (3–15) |
| AMD* | 7 (5.88) | 14 (5.93) | 4.93 ± 4.6 (0–14) |
| Chronic stage | |||
| OMT* | 7 (5.88) | 12 (5.08) | |
| Punctum suture | 4 (3.36) | 7 (2.97) | |
| Correction of entropion | 2 (1.68) | 4 (1.69) | |
Surgical intervention in the acute and the chronic stage of SJS/TEN.
Table 5B
| Type of surgery | Onset to intervention | Chronic stage | p-value |
|---|---|---|---|
| (in days) | grading score | ||
| AMG | ≧7 day (n = 7) | 15.6 ± 12.94 (4~33) | 0.031 |
| <7 day (n = 6) | 2.17 ± 2.63 (0~6) | ||
| AMD | ≧7 day (n = 3) | 8.33 ± 2.08 (6~10) | 0.904 |
| <7 day (n = 11) | 8.55 ± 3.98 (5~16) | ||
| AMG + AMD | ≧7 day (n = 10) | 13.60 ± 11.22 (4~33) | 0.025 |
| <7 day (n = 17) | 6.29 ± 4.69 (0~16) |
Comparison of Sotozono's chronic stage grading score in patients receiving AM transplantation more than or < 7 days after onset.
*AMG, Amniotic membrane graft; *AMD, Amniotic membrane dressing; *OMT, Oral mucosal transplantation.
Discussion
In multiple-country epidemiological studies of medication risk related to SJS/TEN in Asian populations (1998–2017), Wang et al. reported that antiepileptic drugs/antipsychotics (53%) were the most common category of drugs that caused SJS/TEN followed by antibiotics/antiviral agents (20%), allopurinol (19%), and NSAIDs (4%) (24). In Taiwan, Chang et al. previously reported that carbamazepine was the most common medication that caused SJS/TEN followed by allopurinol and phenytoin (8). With the ground-breaking finding that the human leukocyte antigen HLA-B*15:02 was intensively associated with carbamazepine-induced SJS/TEN in Han Chinese individuals (6), subsequent government health insurance covering HLA-B*15:02 screening dramatically reduced the incidence of carbamazepine-induced SJS/TEN (7). When all registered patients within the last 10 years were reviewed, antiepileptics were still the most common category of drugs that caused acute SOCs followed by antibiotics and allopurinol. However, allopurinol was the most common single culprit drug that caused acute SOC followed by oxcarbazepine and carbamazepine. Recently, cold medicine (antipyretic or analgesic but not antibiotics a patient takes when catching cold) was thought to be the most common drug that induces SOCs in chronic SJS/TEN (25–29). We also had similar observations in our recent study based on patients from outpatient clinics, and identified HLA B*0207 to be associated with cold medicine-induced SJS/TEN with SOCs (30). In this study, NSAID and cold medicine were found to be the most common causative medications that were associated with chronic SOCs. A significant association was noted between HLA-B*15:02 and oxcarbazepine-induced SJS/TEN; however, the incidence and severity were lower than those of carbamazepine-induced STS/TEN (31). On the other hand, although a strong association of HLA-B*5801 with allopurinol-induced SJS/TEN has been reported (32, 33), the relatively lower incidence of allopurinol-induced SJS/TEN does not support the implementation of gene screening.
One may think that patients with more severe dermatological involvement also have more severe ocular involvement. However, Morales et al. reported that although ocular damage in the acute stage was more frequent in patients with epidermal detachment >10% of the total body surface area, the SCORTEN value did not correlate with the severity of eye involvement in the acute stage (34). Heng et al. reported that the grading of acute ocular disease severity does not correlate with systemic disease severity but is significantly associated with the time to resolution of ocular involvement in TEN (35). Yip et al. also stressed that the severity of acute ocular disease and abnormal laboratory tests were not found to be significant risk factors for late complications (36).
To identify predictors for the development of chronic ocular complications, Gueudry et al. retrospectively reviewed the records for demographics, cause of the condition, and severity of ocular involvement. They found that (1) Patients with TEN had more frequent but not more severe acute ocular involvement; (2) Dry eye syndrome was the most common late complication; (3) The severity of acute ocular disease was found to be the only significant risk factor for late complications. Although late complications are more frequent in patients with severe initial eye involvement, these complications may also develop in patients without initial ocular symptoms (37). In this study, we found a good correlation between the severity of acute ocular manifestations and chronic ocular sequelae; nevertheless, we also observed that some patients with only minimal ocular involvement in the beginning deteriorated over the years due to insults from chronic inflammation, dry eye, trichiasis and lid margin keratinization. Therefore, we agree with Shanbhag et al. that patients with any acute ocular involvement regardless of severity should be seen by an ophthalmologist for life given that severe and irreversible complications can occur at any time, even decades after acute disease (38).
There are several different grading systems to define the severity of ocular involvement in chronic SJS/TEN (16, 39, 40). We chose to use Sotozono's 2007 grading system because the system includes the most observation features, each with a score range, which facilitates quantitative comparison between different groups (16). We found hyperemia to be the most common chronic stage ocular complication followed by dry eye, SPK, and meibomian gland dysfunction. However, in our hospital, not all acute SJS/TEN patients (294 patients in the last decade) were seen by ophthalmologists. It was only when patients presented with red eye or other ophthalmic symptoms that dermatologists sent out a consultation request (119 patients, 236 eyes in total). This is the major drawback in the study as this might overestimate the SOC rate given that many non-consulted patients were in fact patients without any ocular involvement. For example, the percentages of patients with acute SOCs, chronic SOCs, and late severe vision impairment (< 20/200) were 46.2, 5.9, and 10.9%, respectively, among consulted patients, but the value could be as low as 18.7, 2.4, and 3.4%, respectively, if non-examined patients were all considered free of eye involvement. This projected value would be very similar to the report by Power et al. which indicates that 16% of patients with SJS-spectrum will experience severe ocular involvement (41), and the report by Shanbhag et al. which indicates that only 3% eyes had vision worse than 20/200 after aggressive treatment (14).
Previously, a high portion of SJS/TEN patients often suffered from chronic debilitating sequelae. In 2007, Sotozono et al. reported that greater than half of their patients had final vision worse than 20/200 (74 eyes, 53.6%) with an average chronic SJS grading score of 25.66 (16). With the awareness that early and aggressive intervention in the acute stage is key to prevent long-term complications, a specific protocol for acute ocular care in SJS/TEN, including aggressive use of AMT (22, 23), was instituted at Massachusetts Eye Infirmary in January 2008, which was highly successful in reducing corneal blindness and severe vision-threatening complications (14). In the report of Shanbhag et al. after adapting the protocol, only 3% (2/78) of eyes had vision worse than 20/200 in contrast to 50% (9/18) before the protocol (14). In this study, systemic corticosteroids were administered to all but 5 patients in the acute stage, and 27 of the 30 eyes with acute grade 3 involvement received either AMG or AMD. We believe that in the last decade, aggressive systemic immunosuppressives and early AM transplantation have already contributed to reduced long-term ocular complications. In this study, the average chronic grading scores in patients receiving AMT <7 days after onset were significantly lower than those performed beyond 7 days, suggesting the beneficial effect of early AMT in reducing chronic SOCs (9, 13).
In addition to systemic corticosteroids (17, 39), other immunomodulatory medications, such as intravenous immunoglobulin (IVIG) (39, 42) and cyclosporine A (43, 44), have been used to treat acute SJS/TEN. Although IVIG was found to be ineffective, the use of cyclosporine may offer a greater mortality benefit (45) in the treatment of SJS/TEN, and cyclosporine and glucocorticosteroids were shown to be the more promising systemic immunomodulating therapies (43). Recently, our dermatology colleagues reported the beneficial effect of systemic etanercept treatment in promoting epidermal regeneration and reducing the incidence of gastrointestinal hemorrhage and mortality in acute SJS/TEN in a randomized, controlled study (19). The use of subcutaneous etanercept is generally safe, but we need to pay attention to whether there is tuberculosis, hepatitis B, hepatitis C, and serious infections before the injection. In the present study, we also found that patients receiving additional systemic etanercept exhibited better final vision and significantly lower chronic SJS/TEN grading scores. This encourages us to conduct a study to trace the long-term ocular condition in the original cohort of patients (manuscript in preparation).
In summary, we found that patients with severe acute ocular manifestations in SJS/TEN tend to suffer from chronic ocular involvement. Antiepileptics were the category of drugs that caused most acute SOCs, whilst NSAIDs and cold medicines were associated with most chronic SOCs. With aggressive medical and surgical treatment, eyes suffering from chronic complications of SJS/TEN have decreased in the last decade. Finally, systemic etanercept given in the acute stage showed promise in reducing long-term ocular morbidity; however, the efficacy and long-term benefit still await further investigation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Boards of Chang Gung Medical Foundation. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
DM: writing of the manuscript, patient care and surgery. T-YT: review of electronic medical records, statistical analysis, and making of tables and figure. L-YP: review of electronic medical records, statistical analysis, and making of tables. S-YC: patient care and surgery. C-HH, H-YT, and L-KY: ophthalmic consultation. C-WL: dermatological care of patients. C-BC: dermatological care of patients and provides patient list. W-HC: dermatological care of patients and inventor of systemic etanercept treatment. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by research grants from the Ministry of Science and Technology, Taiwan (MOST) 106-2314-B-182A-043-; and Chang Gung Memorial Hospital, Taiwan (CMPRPG1J0011) to DM.
Acknowledgments
The authors are grateful to Biostatistical Section, Clinical Trial Center, Chang Gung Memorial Hospital (MOHW110-TDU-B-212-123005) for the statistical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor declared a past collaboration with several of the authors DM, W-HC, and S-YC.
References
1.
White KD Abe R Ardern-Jones M Beachkofsky T Bouchard C Carleton B et al . SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. (2018) 6:38–69. 10.1016/j.jaip.2017.11.023
2.
Yang MS Lee JY Kim J Kim GW Kim BK Kim JY et al . Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS ONE. (2016) 11:e0165933. 10.1371/journal.pone.0165933
3.
Frey N Jossi J Bodmer M Bircher A Jick SS Meier CR et al . The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. J Invest Dermatol. (2017) 137:1240–7. 10.1016/j.jid.2017.01.031
4.
Hsu DY Brieva J Silverberg NB Silverberg JI . Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. (2016) 136:1387–97. 10.1016/j.jid.2016.03.023
5.
Syu FK Pan HY Chuang PC Huang YS Cheng CY Cheng FJ . Incidence of stevens-johnson syndrome following combination drug use of allopurinol, carbamazepine and phenytoin in Taiwan: a case-control study. J Dermatol. (2018) 45:1080–7. 10.1111/1346-8138.14528
6.
Chung WH Hung SI Hong HS Hsih MS Yang LC Ho HC et al . Medical genetics: a marker for Stevens-Johnson syndrome. Nature. (2004) 428:486. 10.1038/428486a
7.
Chen P Lin JJ Lu CS Ong CT Hsieh PF Yang CC et al . Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. (2011) 364:1126–33. 10.1056/NEJMoa1009717
8.
Chang YS Huang FC Tseng SH Hsu CK Ho CL Sheu HM . Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea. (2007) 26:123–9. 10.1097/ICO.0b013e31802eb264
9.
Shanbhag SS Hall L Chodosh J Saeed HN . Long-term outcomes of amniotic membrane treatment in acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul Surf. (2020) 18:517–22. 10.1016/j.jtos.2020.03.004
10.
Sharma N Thenarasun SA Kaur M Pushker N Khanna N Agarwal T et al . Adjuvant role of amniotic membrane transplantation in acute ocular Stevens-Johnson Syndrome: a randomized control trial. Ophthalmology. (2016) 123:484–91. 10.1016/j.ophtha.2015.10.027
11.
Kohanim S Palioura S Saeed HN Akpek EK Amescua G Basu S et al . Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf. (2016) 14:168–88. 10.1016/j.jtos.2016.02.001
12.
Ciralsky JB Sippel KC Gregory DG . Current ophthalmologic treatment strategies for acute and chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Curr Opin Ophthalmol. (2013) 24:321–8. 10.1097/ICU.0b013e3283622718
13.
Yang Y Fung SSM Chew H Mireskandari K Ali A . Amniotic membrane transplantation for Stevens-Johnson syndrome/toxic epidermal necrolysis: the Toronto experience. Br J Ophthalmol. (2020). 10.1136/bjophthalmol-2020-316056. [Epub ahead of print].
14.
Shanbhag SS Rashad R Chodosh J Saeed HN . Long-term effect of a treatment protocol for acute ocular involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis. Am J Ophthalmol. (2019) 208:331–41. 10.1016/j.ajo.2019.07.006
15.
Sotozono C Ueta M Nakatani E Kitami A Watanabe H Sueki H et al . Japanese research committee on severe cutaneous adverse, predictive factors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. (2015) 160:228–37.e2. 10.1016/j.ajo.2015.05.002
16.
Sotozono C Ang LP Koizumi N Higashihara H Ueta M Inatomi T et al . New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. (2007) 114:1294–302. 10.1016/j.ophtha.2006.10.029
17.
Araki Y Sotozono C Inatomi T Ueta M Yokoi N Ueda E et al . Successful treatment of Stevens-Johnson syndrome with steroid pulse therapy at disease onset. Am J Ophthalmol. (2009) 147:1011.e1. 10.1016/j.ajo.2008.12.040
18.
Lin CY Wang CW Hui CR Chang YC Yang CH Cheng CY et al . Delayed-type hypersensitivity reactions induced by proton pump inhibitors: a clinical and in vitro T-cell reactivity study. Allergy. (2018) 73:221–9. 10.1111/all.13235
19.
Wang CW Yang LY Chen CB Ho HC Hung SI Yang CH et al . The Taiwan severe cutaneous adverse reaction, randomized, controlled trial of TNF-alpha antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. (2018) 128:985–96. 10.1172/JCI93349
20.
Kobayashi A Yoshita T Sugiyama K Miyashita K Niida Y Koizumi S et al . Amniotic membrane transplantation in acute phase of toxic epidermal necrolysis with severe corneal involvement. Ophthalmology. (2006) 113:126–32. 10.1016/j.ophtha.2005.09.001
21.
Shay E Kheirkhah A Liang L Sheha H Gregory DG Tseng SC . Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. (2009) 54:686–96. 10.1016/j.survophthal.2009.03.004
22.
Ma KN Thanos A Chodosh J Shah AS Mantagos IS . A novel technique for amniotic membrane transplantation in patients with acute Stevens-Johnson syndrome. Ocul Surf. (2016) 14:31–6. 10.1016/j.jtos.2015.07.002
23.
Shanbhag SS Chodosh J Saeed HN . Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul Surf. (2019) 17:560–4. 10.1016/j.jtos.2019.03.001
24.
Wang YH Chen CB Tassaneeyakul W Saito Y Aihara M Choon SE et al . Asian severe cutaneous adverse reaction, the medication risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in Asians: the major drug causality and comparison with the US FDA label. Clin Pharmacol Ther. (2019) 105:112–20. 10.1002/cpt.1071
25.
Ueta M Kannabiran C Wakamatsu TH Kim MK Yoon KC Seo KY et al . Trans-ethnic study confirmed independent associations of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe ocular surface complications. Sci Rep. (2014) 4:5981. 10.1038/srep05981
26.
Ueta M . Cold medicine-related Stevens-Johnson syndrome/toxic epidermal necrolysis with severe ocular complications-phenotypes and genetic predispositions. Taiwan J Ophthalmol. (2016) 6:108–18. 10.1016/j.tjo.2016.06.001
27.
Wakamatsu TH Ueta M Tokunaga K Okada Y Loureiro RR Costa KA et al . Human leukocyte antigen class I genes associated with Stevens-Johnson syndrome and severe ocular complications following use of cold medicine in a Brazilian population. JAMA Ophthalmol. (2017) 135:355–60. 10.1001/jamaophthalmol.2017.0074
28.
Jongkhajornpong P Lekhanont K Pisuchpen P Chantaren P Puangsricharern V Prabhasawat P et al . Association between HLA-B*44:03-HLA-C*07:01 haplotype and cold medicine-related Stevens-Johnson syndrome with severe ocular complications in Thailand. Br J Ophthalmol. (2018) 102:1303–7. 10.1136/bjophthalmol-2017-311823
29.
Tangamornsuksan W Chanprasert S Nadee P Rungruang S Meesilsat N Ueta M et al . HLA genotypes and cold medicine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis with severe ocular complications: a systematic review and meta-analysis. Sci Rep. (2020) 10:10589. 10.1038/s41598-020-67610-5
30.
Ma KS Chung WH Hsueh YJ Chen SY Tokunaga K Kinoshita S et al . Human leucocyte antigen association of patients with Stevens-Johnson syndrome/toxic epidermal necrolysis with severe ocular complications in Han Chinese. Br J Ophthalmol. (2021). 10.1136/bjophthalmol-2020-317105. [Epub ahead of print].
31.
Chen CB Hsiao YH Wu T Hsih MS Tassaneeyakul W Jorns TP et al . Taiwan severe cutaneous adverse reaction, risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology. (2017) 88:78–86. 10.1212/WNL.0000000000003453
32.
Chung WH Hung SI . Genetic markers and danger signals in stevens-johnson syndrome and toxic epidermal necrolysis. Allergol Int. (2010) 59:325–32. 10.2332/allergolint.10-RAI-0261
33.
Hung SI Chung WH Liou LB Chu CC Lin M Huang HP et al . HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA. (2005) 102:4134–9. 10.1073/pnas.0409500102
34.
Morales ME Purdue GF Verity SM Arnoldo BD Blomquist PH . Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthalmol. (2010) 150:505–10.e1. 10.1016/j.ajo.2010.04.026
35.
Heng JS Malik N Joshi N Hayes M Jones I Fuller LC et al . Severity of acute ocular involvement is independently associated with time to resolution of ocular disease in toxic epidermal necrolysis patients. Br J Ophthalmol. (2015) 99:251–4. 10.1136/bjophthalmol-2014-305506
36.
Yip LW Thong BY Lim J Tan AW Wong HB Handa S et al . Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy. (2007) 62:527–31. 10.1111/j.1398-9995.2006.01295.x
37.
Gueudry J Roujeau JC Binaghi M Soubrane G Muraine M . Risk factors for the development of ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Arch Dermatol. (2009) 145:157–62. 10.1001/archdermatol.2009.540
38.
Shanbhag SS Chodosh J Fathy C Goverman J Mitchell C Saeed HN . Multidisciplinary care in Stevens-Johnson syndrome. Ther Adv Chronic Dis. (2020) 11:2040622319894469. 10.1177/2040622319894469
39.
Kim DH Yoon KC Seo KY Lee HS Yoon SC Sotozono C et al . The role of systemic immunomodulatory treatment and prognostic factors on chronic ocular complications in Stevens-Johnson syndrome. Ophthalmology. (2015) 122:254–64. 10.1016/j.ophtha.2014.08.013
40.
Sharma N Venugopal R Maharana PK Chaniyara M Agarwal T Pushker N et al . Multistep grading system for evaluation of chronic ocular sequelae in patients with Stevens-Johnson syndrome. Am J Ophthalmol. (2019) 203:69–77. 10.1016/j.ajo.2019.01.028
41.
Power WJ Ghoraishi M Merayo-Lloves J Neves RA Foster CS . Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology. (1995) 102:1669–76. 10.1016/S0161-6420(95)30811-1
42.
Yip LW Thong BY Tan AW Khin LW Chng HH Heng WJ . High-dose intravenous immunoglobulin in the treatment of toxic epidermal necrolysis: a study of ocular benefits. Eye. (2005) 19:846–53. 10.1038/sj.eye.6701653
43.
Zimmermann S Sekula P Venhoff M Motschall E Knaus J Schumacher M et al . Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. (2017) 153:514–22. 10.1001/jamadermatol.2016.5668
44.
Hall LN Shanbhag SS Rashad R Chodosh J Saeed HN . The effects of systemic cyclosporine in acute Stevens-Johnson syndrome/toxic epidermal necrolysis on ocular disease. Ocul Surf. (2020) 19:128–32. 10.1016/j.jtos.2020.05.003
45.
Kirchhof MG Miliszewski MA Sikora S Papp A Dutz JP . Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. (2014) 71:941–7. 10.1016/j.jaad.2014.07.016
Summary
Keywords
Stevens-Johnson syndrome, toxic epidermal necrolysis, ocular complication, amniotic membrane transplantation, etanercept, Taiwan
Citation
Ma DH-K, Tsai T-Y, Pan L-Y, Chen S-Y, Hsiao C-H, Yeh L-K, Tan H-Y, Lu C-W, Chen C-B and Chung W-H (2021) Clinical Aspects of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis With Severe Ocular Complications in Taiwan. Front. Med. 8:661891. doi: 10.3389/fmed.2021.661891
Received
31 January 2021
Accepted
08 April 2021
Published
12 May 2021
Volume
8 - 2021
Edited by
Mayumi Ueta, Kyoto Prefectural University of Medicine, Japan
Reviewed by
Mee Kum Kim, Seoul National University, South Korea; Swapna Shanbhag, L. V. Prasad Eye Institute, India; Passara Jongkhajornpong, Decision Research, United States
Updates
Copyright
© 2021 Ma, Tsai, Pan, Chen, Hsiao, Yeh, Tan, Lu, Chen and Chung.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Hui-Kang Ma davidhkma@yahoo.com
This article was submitted to Ophthalmology, a section of the journal Frontiers in Medicine
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.