- 1Department of Ultrasound, Sheng Jing Hospital of China Medical University, Shenyang, China
- 2Department of Hospice, Sheng Jing Hospital of China Medical University, Shenyang, China
Background: Endometriosis mainly affects female pelvic tissues and organs, and the presence of endometriosis in the kidney is extremely rare.
Case Presentation: We report a case of a 48-year-old woman who presented with intermittent hematuria. She was found to have a cystic mass on renal ultrasonography, and contrast-enhanced computed tomography (CT) showed slight enhancement of the cystic wall and septa. These findings were indicative of cystic renal tumor. The patient subsequently underwent partial right nephrectomy. Histopathology revealed endometriosis of the right renal parenchyma. The patient recovered well and had no evidence of a recurrent renal mass at the 3 months' follow up.
Conclusion: The possibility of renal endometriosis should be considered in a female patient with a cystic renal mass and clinical symptoms related to the menstrual cycle.
Introduction
Endometriosis is a common disease occurring in women of reproductive age. Most lesions occur in the reproductive system (1), and endometriosis of the kidneys is rare (2). Because of the insufficient knowledge of renal endometriosis, it can be easily misdiagnosed. Herein, we report our experience with renal endometriosis with the goal of increasing the scholarly awareness of this rare entity.
Case Presentation
A 48-year-old married woman presented to our hospital with intermittent gross hematuria for 3 months. She had regular menstrual cycle of 28 days without pain around her waist line or abdominal pain and no history of gynecologic surgery. She denied a family history of cancer, endometriosis, genetic and psychosocial diseases.
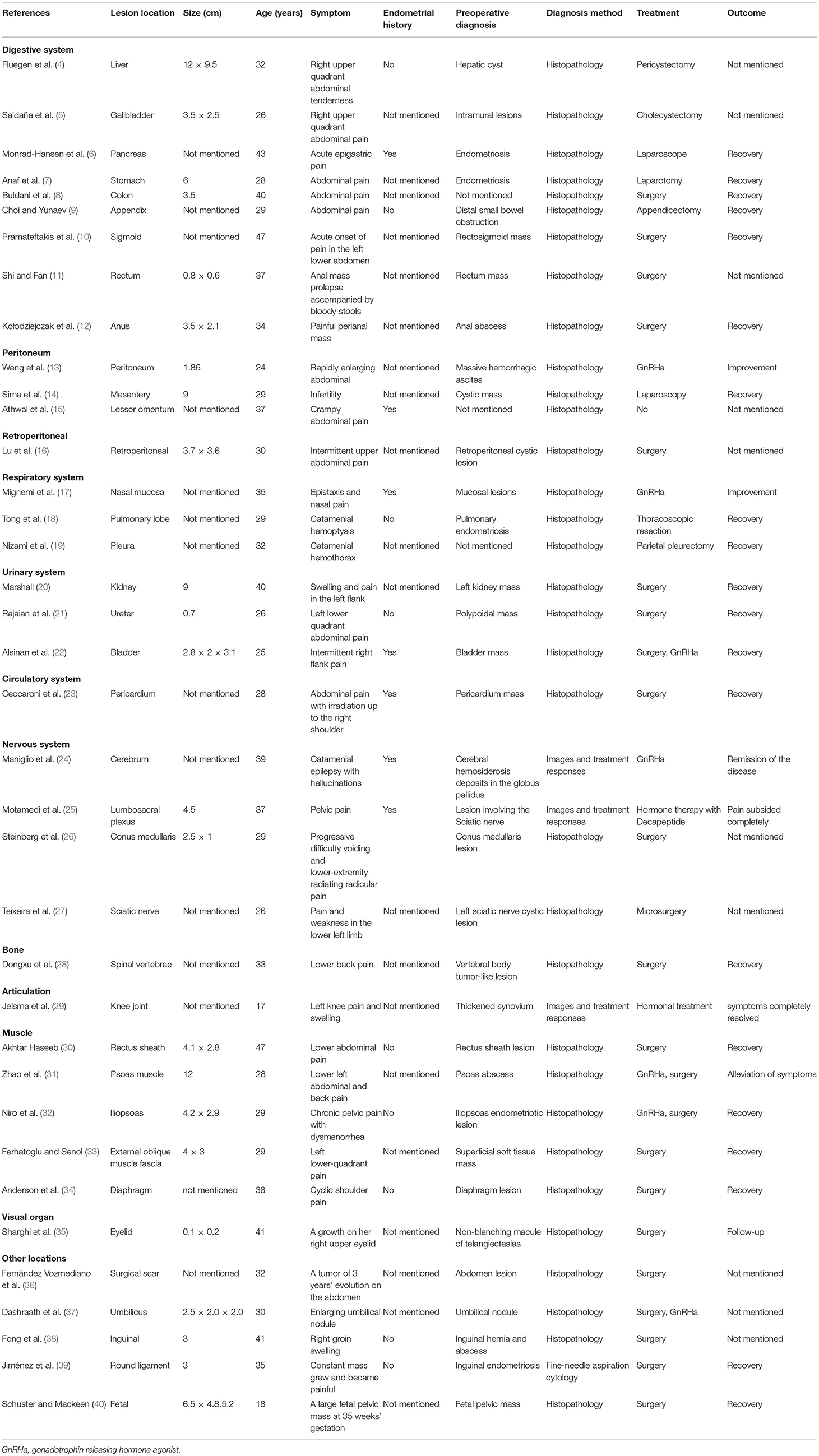
There was no obvious mass on the palpation of the bilateral kidney area, and no percussion pain. An ultrasound of the urinary system was recommended first. Conventional ultrasound examination was performed under standardized settings using Toshiba ultrasound systems (Aplio500, Tokyo, Japan) (ultrasound transducer: PVT-375BT, frequency 3.5 MHz). Ultrasonography showed a cystic mass in the lower pole of the right kidney with septa and well-circumscribed. The thickness of the cystic mass wall is about 4 mm. Doppler color flow imaging showed a small signal in the wall of the mass, but no blood flow was detected in the cystic part. Contrast-enhanced computed tomography (CT) of the kidney was then performed. It showed a heterogeneous hypodense mass in the lower pole of the right kidney, measuring ~1.8 × 1.5 × 1.4 cm (Figure 1). The mass was polycystic, with slight enhancement of the wall and septa and no enhancement of the cystic area. Contrast filling of the mass was not observed during the excretory phase. The right lower pole of the kidney was partially depressed, and there was some local thinning of the renal cortex. Given the slight enhancement of the mass, the possibility of cystic renal tumor could not be ruled out, and surgical resection was planned. Laparoscopic right renal mass resection was subsequently performed at our hospital on the 7th day after her menstruation. After successful anesthesia, the surgeon opened the lateral retroperitoneum under laparoscopy, pushed the colon away and opened the Gerota's fascia, then carefully dissociated the right kidney. The right lower pole of the kidney was partially irregular and contracted. A well-demarcated cystic tumor with the size of 2.5 × 2.0 × 2.0 cm could be seen at the lower pole of the right kidney after surgical incision, which involved the renal parenchyma but had clear boundaries, and the mass was easily peeled off. The mass composed of brown central area showed several branching cavities, with 5 mm wide homogenous light-yellow zone at its periphery. Perioperatively, the tumor was confined to the right kidney without extracapsular invasion, which did not encroach on the perirenal fat sac or surrounding organs. The mass was completely resected, with an ~0.5 cm margin of normal renal tissue. The resected mass had a diameter of ~1.5 cm. There were several capsular spaces containing brown fluid, and the cut surface of the mass was yellowish. Microscopic examination confirmed the diagnosis of renal endometriosis characterized by endometrial glands and embedded stromal cells (Figure 2). Immunohistochemical analysis revealed that the stromal cells and epithelial cells were positive for estrogen receptor (ER), progestin receptor (PR), and vimentin, which further supported the diagnosis of renal endometriosis. Histopathology was reviewed by two senior pathologists separately, without any atypia. The patient recovered well after the surgery, and there were no obvious space-occupying lesions by ultrasonography in the kidney at the 3 months follow-up. The patient is very satisfied with the treatment of this disease.
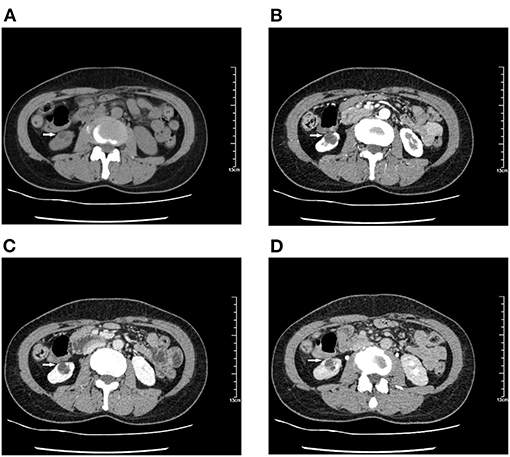
Figure 1. Computed tomography (CT) shows a hypodense mass of 1.8 × 1.5 × 1.4 cm in the lower pole of the right kidney. Contrast-enhanced CT scan shows a polycystic mass with slight enhancement of the wall and septa and no enhancement in the cystic part. The right lower pole of the kidney is partially depressed, and contrast-enhanced images show local thinning of the renal cortex during cortical enhancement. (A) Unenhanced images. (B–D) With contrast. (B) Renal cortical phase. (C) Renal corticomedullary phase. (D) Renal excretory phase.
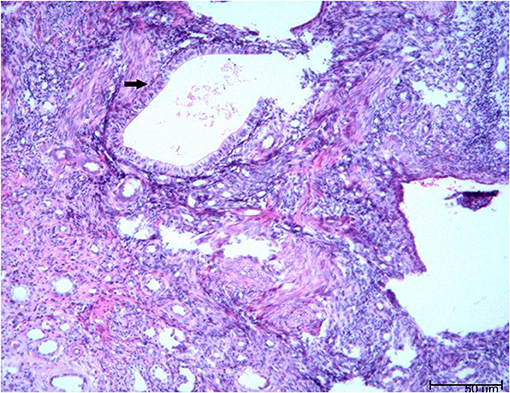
Figure 2. The microscopic pathology proved the diagnosis of renal endometriosis characterized by endometrial glands and embedded stromal cells [hematoxylin and eosin stain (H and E), × 200 magnification].
Discussion
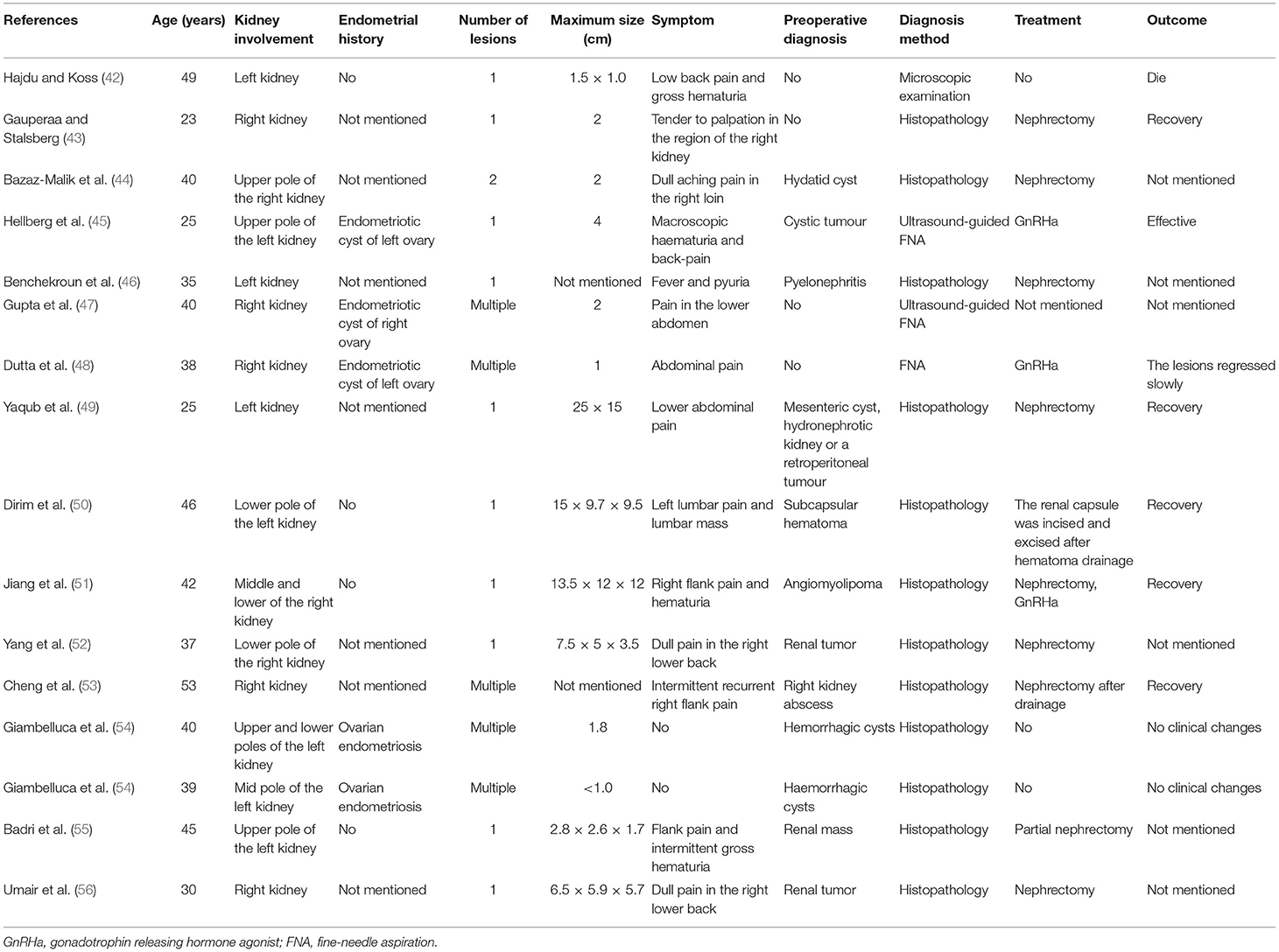
Endometriosis is a common gynecologic disease, occurring in up to 10% of women, mainly of reproductive age (1). The main pathologic changes are cyclic shedding of the ectopic endometrium and surrounding tissue fibrosis with the formation of heterotopic nodules. Endometriosis usually affects pelvic tissues and organs (3), but extragenital endometriosis can occur anywhere in the body (4–40), as shown in Table 1. Urinary tract involvement is uncommon and primarily manifests in the bladder, followed by the ureters and kidneys, at a ratio of 40:5:1 (41). Marshall first reported renal endometriosis in 1943 (20), and only 16 pathologically confirmed cases have been reported in the past 30 years (Table 2) (42–56). The median age was 37 years (range, 23–53 years).
The mechanism of extragenital endometriosis remains controversial. The main theories can be categorized as migratory, embryonic, and immunologic, these theories have been discussed in previous literature (43, 44, 46, 48, 50–54, 56, 57). Migratory theories propose that retrograde menstruation, lymphovascular metastasis, and direct extension allow the endothelial cells to transplant into ectopic sites (46, 48, 50–54, 56, 57). Embryonic theories suggest that endometriosis results from metaplastic changes of Wolffian, Mullerian, and occasionally peritoneal (celomic) structures (43, 44, 46, 48, 50, 51, 53, 54). Immunologic theories suggest that a suboptimal immune response may result in ectopic endometrial implantation (46, 50–54). In these literatures, Bazaz-Malik et al., Dutta et al., and Cheng et al. believe that the pathogenesis of their cases of renal endometriosis is rationally explained by embryonic theory (44, 48, 53).
The qualitative features of Bosniak III cystic masses: cystic masses with one or more thick or irregular enhancing walls or septa without nodular enhancement. The Bosniak Classification, version 2019 defines “thick” as 4 mm or thicker (58). In our case, the thickness of the cystic mass wall is 4 mm and with enhancement. It was classified as Bosniak III according to Bosniak Classification of Cystic Renal Masses, Version 2019 (58). Approximately 50% of Bosniak III masses are malignant (59). The imaging features of multilocular cystic renal neoplasm of low malignant potential are different from benign renal cystic masses. In general, necrosis of carcinoma tends to be central, with a thickened solid peripheral “rind” and a central ill-defined area of non-enhancement (60). Different imaging modalities may have some roles in the diagnosis of renal endometriosis. Shedding of the ectopic endometrium may result in a heterogeneous mass density on CT scan (52). The hyperplastic fibromuscular tissue surrounding the lesion is unevenly enhanced and may show protrusion into the central part (49). The continuous proliferation of muscle fibers around the lesion can distort the surrounding renal parenchyma and change the shape of the kidney. Unlike the CT scan, MRI can show renal pelvis compression or displacement. Mixed cystic-solid masses with fibromuscular hyperplasia and residual blood will produce heterogeneous MRI signals (49). The location, size, and composition (solid vs. cystic) of a renal mass can also be visualized using ultrasound. Ultrasound color Doppler flow imaging can demonstrate blood flow signals in the wall and septa. Ultrasound can also be used to facilitate tissue diagnosis. Ultrasound-guided fine needle aspiration (FNA) biopsy is an accurate and minimally invasive method that can greatly help in the diagnosis of endometriosis. To date, three cases of renal endometriosis have been confirmed by FNA (45, 47, 48). Ultrasound is very useful for detecting endometriosis in the uterus and ovary (61), and most importantly, it is cheap, especially suitable for developing countries.
Pain and hematuria are often intermittent and associated with the menstrual cycle in patients with renal endometriosis. Our patient had no abdominal pain, but her hematuria was cyclical and corresponded to her menstrual cycle. This important information was overlooked at the time of her initial presentation.
The histopathology of renal endometriosis is characterized by endometrial glands and stromal cells involving renal cortex and medulla (62). Immunohistochemical analysis shows stromal cell and epithelial cell positivity for CD10, ER, and PR (62). In our case, the stromal cells and epithelial cells were positive for ER, PR, and vimentin, which further supported the diagnosis of renal endometriosis.
As there are no treatment guidelines for renal endometriosis because of its rarity (51), the treatment should be based on the patient's clinical symptoms, characteristics of the lesion, and the patient's reproductive plans (41). Although renal endometriosis is a benign lesion, surgical treatment is usually considered due to its invasiveness (63). The feasibility of laparoscopic management is now widely proven and may reduce the length of hospital stay (64). Asymptomatic patients with multiple small lesions unchanged during subsequent imaging examinations generally do not require any definitive therapy for renal lesions (54). Hormone therapy, such as GnRH agonists and oral contraceptives, can be used for symptom management (45, 48, 51). Hormonal treatment reduces the pain in the short-term follow up and is the best treatment for patients of reproductive age (64).
Conclusion
We report a patient who presented with a single small cystic kidney mass and was found to have endometriosis. Although imaging can be helpful, the final diagnosis of endometriosis relies on histopathologic findings. The possibility of renal endometriosis should be considered in a female patient with a cystic renal mass and clinical symptoms related to the menstrual cycle.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the participant for the publication of this case report. Ethical approval was given by the Medical Ethics Committee of our hospital.
Author Contributions
YY and YH diagnosed the patient. All authors wrote and revised the manuscript.
Funding
This study was supported by grants from the Scientific Research Project of the Educational Department of Liaoning Province (ZF2019023) and 345 Talent Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the patient for agreeing and providing her case history. We would like to thank Editage (www.editage.cn) for English language editing.
References
1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. (1997) 24:235–58. doi: 10.1016/S0889-8545(05)70302-8
2. Eisenberg VH, Weil C, Chodick G, Shalev V. Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG. (2018) 125:55–62. doi: 10.1111/1471-0528.14711
3. Machairiotis N, Stylianaki A, Dryllis G, Zarogoulidis P, Kouroutou P, Tsiamis N, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. (2013) 8:194. doi: 10.1186/1746-1596-8-194
4. Fluegen G, Jankowiak F, Zacarias Foehrding L, Kroepil F, Knoefel WT, Topp SA. Intrahepatic endometriosis as differential diagnosis: case report and literature review. World J Gastroenterol. (2013) 9:4818–22. doi: 10.3748/wjg.v19.i29.4818
5. Saldaña DG, de Acosta DA, Alemán HP, Gebrehiwot D, Torres E. Gallbladder endometrioma associated with obstructive jaundice and a serous ovarian cystic adenoma. South Med J. (2010) 103:1250–2. doi: 10.1097/SMJ.0b013e3181faee63
6. Monrad-Hansen PW, Buanes T, Young VS, Langebrekke A, Qvigstad E. Endometriosis of the pancreas. J Minim Invasive Gynecol. (2012) 19:521–3. doi: 10.1016/j.jmig.2012.03.011
7. Anaf V, Buggenhout A, Franchimont D, Nöel JC. Gastric endometriosis associated with transverse colon endometriosis: a case report of a very rare event. Arch Gynecol Obstet. (2014) 290:1275–7. doi: 10.1007/s00404-014-3373-7
8. Buldanli MZ, Özemir IA, Yener O, Dölek Y. A rare case of acute mechanical intestinal obstruction: colonic endometriosis. Ulus Travma Acil Cerrahi Derg. (2020) 26:148–51. English.
9. Choi JDW, Yunaev M. Endometriosis of the appendix causing small bowel obstruction in a virgin abdomen. BMJ Case Rep. (2019) 12:e230496. doi: 10.1136/bcr-2019-230496
10. Pramateftakis MG, Psomas S, Kanellos D, Vrakas G, Roidos G, Makrantonakis A, et al. Large bowel obstruction due to endometriosis. Tech Coloproctol. (2010) 14(Suppl. 1):S87–9. doi: 10.1007/s10151-010-0616-x
11. Shi X, Fan C. Endometriosis in the rectum accompanied by hemorrhoids leading to diagnostic pitfalls: a rare case report. BMC Womens Health. (2018) 18:120. doi: 10.1186/s12905-018-0615-z
12. Kołodziejczak M, Sudoł-Szopińska I, Santoro GA, Bielecki K, Wiaczek A. Ultrasonographic evaluation of anal endometriosis: report of four cases. Tech Coloproctol. (2014) 18:1099–104. doi: 10.1007/s10151-014-1176-2
13. Wang X, Li Y, Tong J, Chang B, Zhang Y, Liu Y, et al. Endometriosis presenting with recurrent massive hemorrhagic ascites and diagnosed by core needle biopsy: a case report. Medicine (Baltimore). (2019) 98:e15477. doi: 10.1097/MD.0000000000015477
14. Sima RM, Radosa JC, Zamfir R, Ionescu CA, Carp D, Iordache II, et al. Novel diagnosis of mesenteric endometrioma: case report. Medicine (Baltimore). (2019) 98:e16432. doi: 10.1097/MD.0000000000016432
15. Athwal P, Patel K, Hassani C, Bahadori S, Nardi P. A case of multisystem endometriosis. J Radiol Case Rep. (2013) 7:1–6. doi: 10.3941/jrcr.v7i10.1483
16. Lu J, Zhao JY, Xiong XZ, Cheng NS. An unusual retroperitoneal endometriotic cyst in a young woman. Dig Liver Dis. (2019) 51:324. doi: 10.1016/j.dld.2018.09.002
17. Mignemi G, Facchini C, Raimondo D, Montanari G, Ferrini G, Seracchioli R. A case report of nasal endometriosis in a patient affected by Behcet's disease. J Minim Invasive Gynecol. (2012) 19:514–6. doi: 10.1016/j.jmig.2012.03.005
18. Tong SS, Yin XY, Hu SS, Cui Y, Li HT. Case report of pulmonary endometriosis and review of the literature. J Int Med Res. (2019) 47:1766–70. doi: 10.1177/0300060518800868
19. Nizami M, Mani A, Begum S. Catamenial hemothorax: a rare case of thoracic endometriosis. Ann Thorac Surg. (2019) 108:e127. doi: 10.1016/j.athoracsur.2018.12.062
20. Marshall VF. The occurrence of endometrial tissue in the kidney: case report and discussion. J Urol. (1943) 50:652–6.
21. Rajaian S, Pragatheeswarane M, Krishnamurthy K, Narasimhachar SC. Isolated intraluminal ureteral endometriosis mimicking tuberculosis. J Postgrad Med. (2019) 65:58–9. doi: 10.4103/jpgm.JPGM_458_18
22. Alsinan TA, AlDahleh LA, Alreefi HAA, Albiabi SA, Alsouss YO, Alshayeb FA, et al. Endometriosis of the urinary bladder causing a right hydronephrosis: a case report. Am J Case Rep. (2019) 20:1360–3. doi: 10.12659/AJCR.917445
23. Ceccaroni M, Clarizia R, Placci A. Pericardial, pleural, and diaphragmatic endometriosis. J Thorac Cardiovasc Surg. (2010) 140:1189–90. doi: 10.1016/j.jtcvs.2010.07.064
24. Maniglio P, Ricciardi E, Meli F, Tomao F, Peiretti M, Caserta D. Complete remission of cerebral endometriosis with dienogest: a case report. Gynecol Endocrinol. (2018) 34:837–9. doi: 10.1080/09513590.2018.1463362
25. Motamedi M, Mousavinia F, Naser Moghadasi A, Talebpoor M, Hajimirzabeigi A. Endometriosis of the lumbosacral plexus: report of a case with foot drop and chronic pelvic pain. Acta Neurol Belg. (2015) 115:851–2. doi: 10.1007/s13760-015-0445-9
26. Steinberg JA, Gonda DD, Muller K, Ciacci JD. Endometriosis of the conus medullaris causing cyclic radiculopathy. J Neurosurg Spine. (2014) 21:799–804. doi: 10.3171/2014.7.SPINE14117
27. Teixeira AB, Martins WA, d'Ávila R, Stochero L, Alberton L, Bezerra S, et al. Endometriosis of the sciatic nerve. Arq Neuropsiquiatr. (2011) 69:995–6. doi: 10.1590/S0004-282X2011000700032
28. Dongxu Z, Fei Y, Xing X, Bo-Yin Z, Qingsan Z. Low back pain tied to spinal endometriosis. Eur Spine J. (2014) 23(Suppl. 2):214–7. doi: 10.1007/s00586-013-2988-x
29. Jelsma J, Mayne A, Steffanie B. Monthly swelling of the knee—case report and review of the literature. Knee. (2016) 23:559–60. doi: 10.1016/j.knee.2016.01.010
30. Akhtar Haseeb Y. Mullerian tissue in the rectus abdominis muscle forming an endometrioma: a case report. Pan Afr Med J. (2019) 34:159. doi: 10.11604/pamj.2019.34.159.19193
31. Zhao L, Wang P, Zhao F, Wu W. A case of psoas muscle endometriosis: a distinct approach to diagnosis and management. J Minim Invasive Gynecol. (2018) 25:1305–8. doi: 10.1016/j.jmig.2018.03.036
32. Niro J, Fournier M, Oberlin C, Le Tohic A, Panel P. Endometriotic lesions of the lower troncular nerves. Gynecol Obstet Fertil. (2014) 42:702–5. doi: 10.1016/j.gyobfe.2014.08.003
33. Ferhatoglu MF, Senol K. Primary abdominal wall endometriosis: presentation of rarely seen two cases. G Chir. (2018) 39:107–10.
34. Anderson TL, Aguirre F, Ayuso A. Diaphragmatic endometriosis in a patient with cyclic shoulder pain. J Minim Invasive Gynecol. (2014) 21:23–4. doi: 10.1016/j.jmig.2013.05.012
35. Sharghi KG, Ramey NA, Rush PS, Grider DJ. Endometriosis of the eyelid, an extraordinary extra-abdominal location highlighting the spectrum of disease. Am J Dermatopathol. (2019) 41:593–5. doi: 10.1097/DAD.0000000000001386
36. Fernández Vozmediano JM, Armario Hita JC, Cuevas Santos J. Cutaneous endometriosis. Int J Dermatol. (2010) 49:1410–2. doi: 10.1111/j.1365-4632.2010.04585.x
37. Dashraath P, Kailun C, Li M. Umbilical endometriosis (Villar's nodule). Am J Obstet Gynecol. (2019) 221:653. doi: 10.1016/j.ajog.2019.04.032
38. Fong KNY, Lau TWS, Mak CCC, Lui KW. Inguinal endometriosis: a differential diagnosis of right groin swelling in women of reproductive age. BMJ Case Rep. (2019) 12:e229864. doi: 10.1136/bcr-2019-229864
39. Jiménez JS, Barbero P, Tejerizo A, Guillén C, Strate C. A laparoscopic approach to Nuck's duct endometriosis. Fertil Steril. (2011) 96:e103–5. doi: 10.1016/j.fertnstert.2011.05.083
40. Schuster M, Mackeen DA. Fetal endometriosis: a case report. Fertil Steril. (2015) 103:160–2. doi: 10.1016/j.fertnstert.2014.09.045
41. Maccagnano C, Pellucchi F, Rocchini L, Ghezzi M, Scattoni V, Montorsi F, et al. Ureteral endometriosis: proposal for a diagnostic and therapeutic algorithm with a review of the literature. Urol Int. (2013) 91:1–9. doi: 10.1159/000345140
42. Hajdu SI, Koss LG. Endometriosis of the kidney. Am J Obstet Gynecol. (1970) 106:314–5. doi: 10.1016/0002-9378(70)90284-X
43. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. (1977) 11:189–91. doi: 10.3109/00365597709179714
44. Bazaz-Malik G, Saraf V, Rana BS. Endometrioma of the kidney: case report. J Urol. (1980) 123:422–3. doi: 10.1016/S0022-5347(17)55965-0
45. Hellberg D, Fors B, Bergqvist C. Renal endometriosis treated with a gonadotrophin releasing hormone agonist. Case report. Br J Obstet Gynaecol. (1991) 98:406–7. doi: 10.1111/j.1471-0528.1991.tb13434.x
46. Benchekroun A, Nouini Y, Zennoud M, Iken A, Ould Jdoud C. Endométriose rénale. A propos d'un cas [Renal endometriosis. Case report]. Ann Urol (Paris). (2001) 35:266–9. French. doi: 10.1016/S0003-4401(01)00041-9
47. Gupta K, Rajwanshi A, Srinivasan R. Endometriosis of the kidney: diagnosis by fine-needle aspiration cytology. Diagn Cytopathol. (2005) 33:60–1. doi: 10.1002/dc.20228
48. Dutta P, Bhat MH, Bhansali A, Kumar V. A young woman with endometriosis of kidney. Saudi Med J. (2006) 27:244–6.
49. Yaqub U, Hassan SE, Yusaf Z, Yusuf AW. Endometriosis in the renal area. J Coll Physicians Surg Pak. (2008) 18:174–5.
50. Dirim A, Celikkaya S, Aygun C, Caylak B. Renal endometriosis presenting with a giant subcapsular hematoma: case report. Fertil Steril. (2009) 92:391.e5-7. doi: 10.1016/j.fertnstert.2009.04.013
51. Jiang YH, Kuo HC, Hsu YH. Renal endometriosis mimicking an angiomyolipoma. Urol Sci. (2013) 24:24–6. doi: 10.1016/j.urols.2013.01.001
52. Yang J, Song RJ, Xu C, Zhang SQ, Zhang W. Renal endometriosis tends to be misdiagnosed as renal tumor: a rare case report. Int Surg. (2015) 100:376–80. doi: 10.9738/INTSURG-D-13-00190.1
53. Cheng CH, Kuo HC, Su B. Endometriosis in a kidney with focal xanthogranulomatous pyelonephritis and a perinephric abscess. BMC Res Notes. (2015) 8:591. doi: 10.1186/s13104-015-1574-1
54. Giambelluca D, Albano D, Giambelluca E, Bruno A, Panzuto F, Agrusa A, et al. Renal endometriosis mimicking complicated cysts of kidney: report of two cases. G Chir. (2017) 38:250–5. doi: 10.11138/gchir/2017.38.5.250
55. Badri AV, Jennings R, Patel P, Eun DD. Renal endometriosis: the case of an endometrial implant mimicking a renal mass. J Endourol Case Rep. (2018) 4:176–8. doi: 10.1089/cren.2018.0070
56. Umair M, Nawaz M, Murtaza B, Ali A, Khan FB, Wahab AU. Renal endometriosis mimicking a renal tumor in a pregnant patient. Urol Case Rep. (2020) 33:101374. doi: 10.1016/j.eucr.2020.101374
57. Signorile PG, Baldi A. Endometriosis: new concepts in the pathogenesis. Int J Biochem Cell Biol. (2010) 42:778–80. doi: 10.1016/j.biocel.2010.03.008
58. Silverman SG, Pedrosa I, Ellis JH, Hindman NM, Schieda N, Smith AD, et al. Bosniak classification of cystic renal masses, version 2019: an update proposal and needs assessment. Radiology. (2019) 292:475–88. doi: 10.1148/radiol.2019182646
59. Smith AD, Carson JD, Sirous R, Sanyal R, Remer EM, Allen BC, et al. Active surveillance versus nephron-sparing surgery for a bosniak IIF or III renal cyst: a cost-effectiveness analysis. Am J Roentgenol. (2019) 212:830–8. doi: 10.2214/AJR.18.20415
60. Hindman NM. Cystic renal masses. Abdom Radiol (NY). (2016) 41:1020–34. doi: 10.1007/s00261-016-0761-4
61. Kumar S, Tiwari P, Sharma P, Goel A, Singh JP, Vijay MK, et al. Urinary tract endometriosis: review of 19 cases. Urol Ann. (2012) 4:6–12. doi: 10.4103/0974-7796.91613
62. Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol. (2007) 14:241–60. doi: 10.1097/PAP.0b013e3180ca7d7b
63. Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol. (2004) 18:349–71. doi: 10.1016/j.bpobgyn.2003.03.001
Keywords: endometriosis, renal endometriosis, computed tomography, cystic renal tumor, ultrasonography
Citation: Yang Y, Zhao X and Huang Y (2021) Renal Endometriosis Mimicking Cystic Renal Tumor: Case Report and Literature Review. Front. Med. 8:684474. doi: 10.3389/fmed.2021.684474
Received: 23 March 2021; Accepted: 27 May 2021;
Published: 21 June 2021.
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Salim Alfred Bassil, Al-Arz Hospital, LebanonWen Kong, Shanghai JiaoTong University, China
Copyright © 2021 Yang, Zhao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Huang, aHVhbmd5aW5nNzEyQDE2My5jb20=
 Ye Yang
Ye Yang Xinxin Zhao
Xinxin Zhao Ying Huang
Ying Huang