- 1Department of Tropical Pathology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
- 2Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
- 3Information Technology Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
- 4Division of Hematology, Department of Medicine, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok, Thailand
- 5Department of Medical Technology and Sciences, International University of Health and Welfare, Ohkawa, Japan
Particulate matter 2.5 (PM2.5) in the air enters the human body by diffusion into the blood. Therefore, hematological abnormalities might occur because of these toxic particles, but few studies on this issue have been reported. According to Cochrane guidance, we performed a systematic review on the relationship between exposure to PM2.5 and the risk of hematological disorders. Ten articles were included in this review. Anemia was found among children and elderly populations with 2- to 5-year PM2.5 exposure. Young children from mothers exposed to air pollution during pregnancy had a higher incidence of leukemia similar to the elderly. Supporting these data, outdoor workers also showed abnormal epigenetic modifications after exposure to very high PM2.5 levels. Adults living in high PM2.5 areas for 2 years were more likely to develop thrombocytosis. Finally, elderly populations with 7- to 8-year PM2.5 exposure showed increased risks of venous thromboembolism. In conclusion, the associations between PM2.5 and hematological aberrations among high-risk people with long-term exposure were reported.
Introduction
Air pollution is one of the most important environmental and health problems worldwide (1, 2). Thailand and other countries have been encountering excessive amounts of particulate matter (PM) primarily from combustion of fuels, coal, and natural gases. PMs are complex mixtures of airborne particles with differences in chemical compositions, sizes, and origin (3). They can be classified into coarse (PM10; diameter range 2.5–10 μm), fine (PM2.5; size <2.5 μm), and ultrafine particulate (UFP; size <100 nm) matter (4–6). Both PM10 and PM2.5 concentrations are widely measured in the environment. After human exposure to PM, PM10 remains in the nasal cavities and upper respiratory tracts, but PM2.5 can reach the alveoli and then penetrate into the blood (3, 7–10). Fine PM can directly cause health problems such as respiratory (reactive airway, lung cancer, and chronic obstructive pulmonary diseases), cardiovascular (heart failure and myocardial infarction), and allergic diseases (rhinitis and eczema) (9, 11). In 2013, the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) reported that outdoor air pollution is toxic to humans (12). The annual mean PM2.5 level over 10 μg/m3 or 24-h mean over 25 μg/m3 was defined as deleterious to the health of the population (13). Children and women are high-risk groups that are prone to develop cardiovascular diseases (18–27%) and pulmonary diseases (8–20%) from PM exposure (14). As PM2.5 can be absorbed from the respiratory tract into the blood, they may interact with blood cells and plasma components (7, 10). PM2.5 also affects red blood cells, white blood cells, or platelet functions, and therefore, some patients may acquire hematological diseases. Apart from exposure to PM2.5, other risk factors also play synergistic roles in causing the diseases. Therefore, high-risk groups for developing hematologic diseases need to be identified. In this review, we aim to study the relationship between PM2.5 exposure and risks for abnormal hematological parameters.
Methods
This systematic review followed the Cochrane guidelines. The search used PubMed®, Science Direct, Mendeley, Google Scholar, and EBSCOhost database. The keywords were separated into three categories. Searching group1 used “air pollution” or “particulate matter” or “PM2.5” and “anemia.” In group2, the terms “air pollution” or “particulate matter” or “PM2.5” and “leukemia” were searched. In group3, “air pollution” or “particulate matter” or “PM2.5” and “thrombosis” or “coagulation” were used. In group4, “air pollution” or “particulate matter” or “PM2.5” and “lymph nodes” were used. In group5, “air pollution” or “particulate matter” or “PM2.5” and “spleen” were added. The inclusion criteria were studies on PM2.5 in relation to hematological diseases, such as anemia, leukemia, and thrombosis in humans, and publications between 2014 and 2021. Duplicated articles, irrelevant titles, the non-English languages, and unavailable full papers were excluded. Afterwards, eligible articles were archived to the EndNote X7.7.1 for Windows (Thomson Reuters, USA). Interesting information from matched articles of the PM2.5 related to hematologic diseases were extracted to identify the risk factors and assessed by three independent hematologists. The search algorithms are shown in Figure 1.
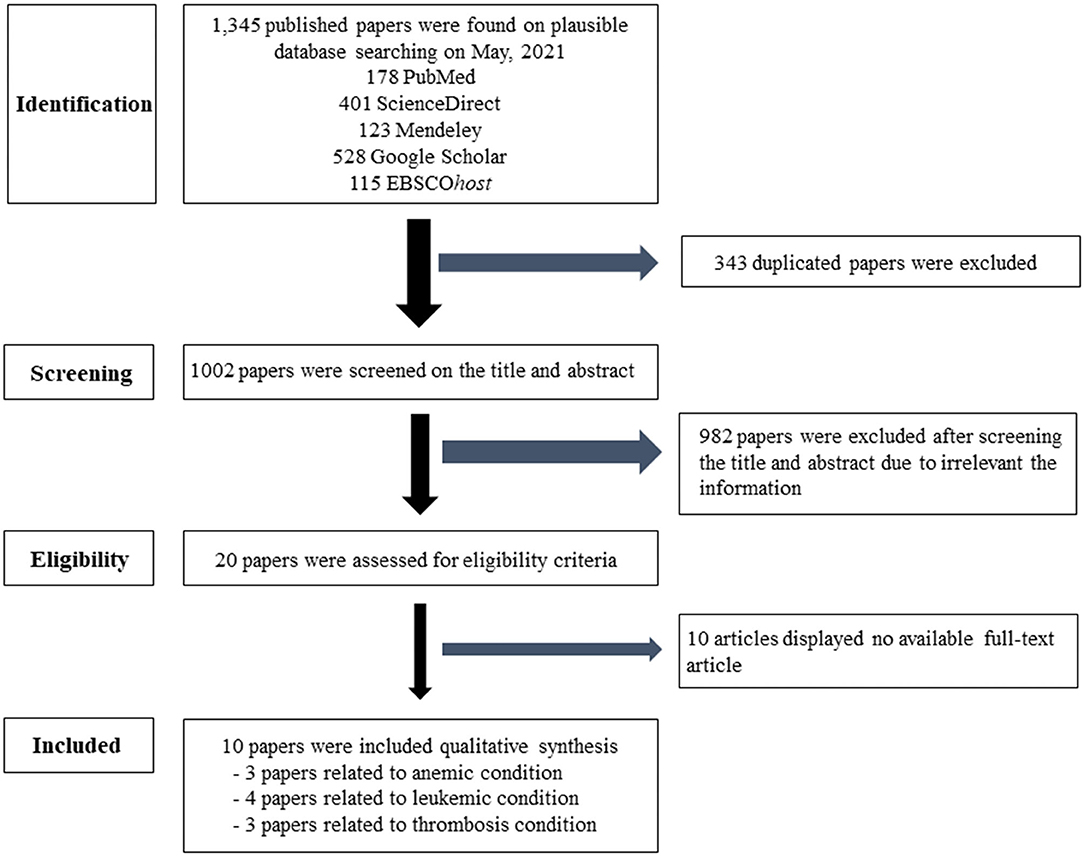
Figure 1. Flow chart of the selection criteria. Ten eligible articles were included and extracted data for this article.
Results
Study Characteristics
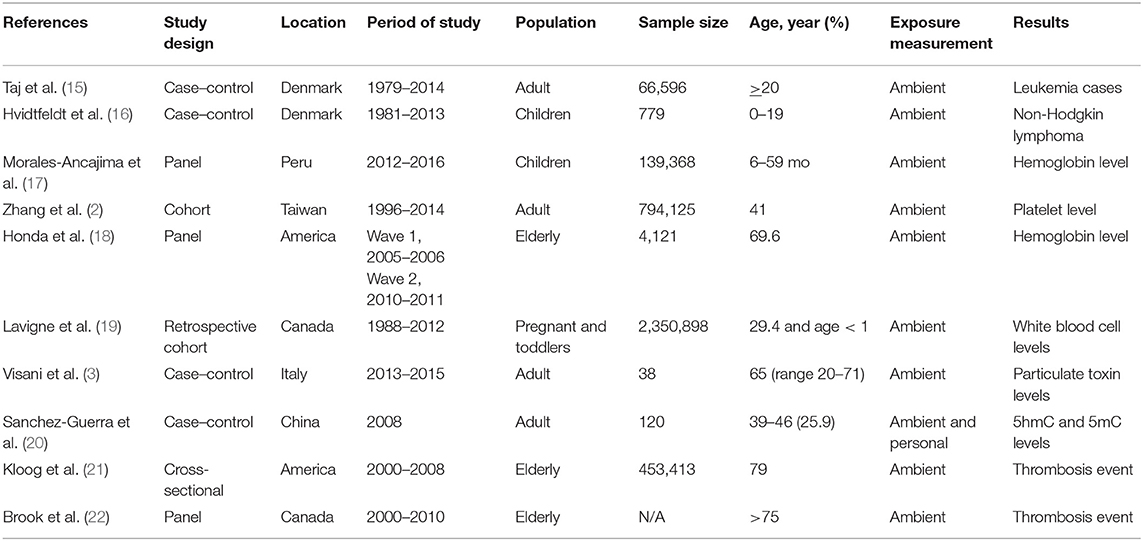
Table 1 shows the characteristics of the 10 available studies included in this systematic review. Of these, there were three studies using the panel design, four case–control studies, two cohort studies, and one cross-sectional study. Sample sizes of the population per study ranged from 4,121 to 139,368 for the panel design and 453.413 for the cross-sectional study. Most studies included subjects over 35 years of age. The studies were located in Peru, Denmark, the United States, Italy, Canada, China, and Taiwan. Air pollutant data were obtained from fixed sites or personal systems or both. Three studies reported the results related to erythrocyte disorders, four studies related to white blood cells, and three studies demonstrated results associated with platelets or thrombotic conditions.
Hematologic Disorders (Anemia, Leukemia, and Thrombosis)
Is PM2.5 a Risk Factor for Anemia?
A few studies linked PM2.5 exposure to erythrocyte disorders, especially anemia. According to the WHO criteria, anemia was defined as a hemoglobin (Hb) level lower than 13.0 g/dl in men, 12.0 g/dl in women, and 11.0 g/dl in children (23, 24). A study by Morales-Ancajima et al. (17) was performed in 139,368 children (age 6–35 months) to evaluate the association between Hb and air pollution in their residential areas. Hb levels decreased among children who were exposed to PM2.5 (range 24.97–28.84 μg/m3) during the 4-year study. Mild to moderate anemia in children was related to areas with high 24-h PM2.5 concentrations with the means between 25 (25) and 50 μg/m3 (26). Moderate to severe anemia in children were associated with very high 24-h mean PM2.5 of over 50 μg/m3 [odds ratio (OR) = 2.83, 95% confidence interval (CI) 1.39–5.75] (27). Anemic children were detectable in 30.8% of the population, while moderate to severe anemia was observed in 8.8% of the population (17). Similarly, Honda et al. (18) showed that 4,121 elderly people with anemia (34.9%) lived in areas with high annual mean PM2.5 levels (>11.1 ± 2.8 μg/m3). Interestingly, elderly subjects with 2- to 5-year exposure to PM2.5 pollution showed declines in Hb levels of approximately 0.81 ± 0.06 g/dl. Honda et al. (18) explained the relationship between air pollution and anemia in the elderly population with elevated C-reactive protein (CRP) levels that indicated chronic inflammatory responses to PM2.5. In an animal model, the increased harmful effects of PM2.5 exposure to young rather than adolescent mice were mediated by the impairment of bone marrow microenvironment (27, 28). The mechanism of PM2.5 action is probably a reactive oxygen species formation which increases inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the cells. Inflammation may inhibit differentiation and proliferation of erythroid precursor cells and enhance an erythropoietin (Epo) resistant state (27–29). Another potential explanation is that inflammatory cytokines can upregulate hepcidin synthesis causing breakdown of ferroportin, thus reducing iron absorption in the gastrointestinal tract (30, 31). In another mouse model, an association between particulate matters (PM2.5 and PM0.1) and the deformation of murine erythrocytes was found (6). The mice exposed to PM2.5 (100 s for 8 days) increased erythrocyte distortion, which finally led to hemolytic anemia (6). Anemia observed in mice exposed to air pollution showed significant dose and time dependency (27).
How Is PM2.5 Related to Leukemia?
It has been known since 1997 that gasoline pollution is one of the factors in gene–toxin–environmental interactions promoting leukemogenesis (32, 33). Leukemia is a malignant clonal disease that results in abnormal proliferation and impaired cellular differentiation of hematopoietic stem cells. Multiple myelotoxicity substances commonly injure hematopoietic cells (3). Exposure to environment toxins might be one of the etiologies of childhood leukemia (34). Acute leukemia represents approximately 30% of pediatric and 25% of adult malignancies (3, 35, 36). A study by Visani et al. (3) measured the particulate toxin in blood samples of adult patients (age range 20–71 years) with acute myeloid leukemia (AML). The levels of PM2.5 were higher among AML patients compared to healthy controls (3). Similarly, Taj et al. (15) demonstrated that PM2.5 components were more positively associated with AML [OR = 1.14; interquartile range (IQR) = 1.00–1.29] than chronic myeloid leukemia and chronic lymphocytic leukemia. The effects of PM2.5 and other components are related not only to adult leukemia but also to childhood hematological malignancy as reported in studies by Lavigne et al. (19) and Hvidtfeldt et al. (16). Consistently, Lavigne et al. (19) demonstrated the relationship between traffic air pollution exposure in approximately a million pregnant women and the incidence of childhood acute lymphoblastic leukemia (ALL) in their offspring. They found a high hazard ratio in exposure during the first trimester of pregnancy [hazard ratio (HR) = 1.20, 95% CI = 1.02–1.41] (19). This study design showed the strongest relationship between fetal exposure to air pollution during pregnancy and leukemia in the first year of age. A study by Hvidtfeldt et al. (16) showed a high OR ratio of 2.05 (IQR = 1.10–3.38) in childhood non-Hodgkin lymphoma with exposure to PM2.5. Molecular epidemiology studies suggested that DNA methylation of the leukemic gene was positively correlated with exposure to environmental toxins (37, 38). DNA methylation incorporates a methyl group to the position of the fifth carbon of cytosine to produce 5-methylcytosine (5mC). Subsequently, 5mC is oxidized into 5-hydroxymethylcytosine (5hmC) which suppresses gene expression. Both 5mC and 5hmC are markers for DNA methylation in malignancy cells (39, 40). A study by Sanchez-Guerra et al. (20) demonstrated the increase in methylated genomic contents (5hmC and 5mC) among subjects who were exposed to PM10 and PM2.5 for 4–7 work-days. Blood samples of 60 outdoor workers exposed to PM10 showed significantly higher 5hmC levels (p = 0.001 at 4 work-days, p = 0.005 at 7 work-days, and p < 0.001 at 14 work-days) and increasing 5mC levels in people exposed to PM2.5 (p = 0.005). This study is limited by the exposure times to PM2.5. In addition, high 5hmC and 5mC levels, which indicated epigenetic modification, were detectable among people in Beijing (20). The mechanism of PM2.5 in AML development could be explained by specific proteins. Protein-forming nanoparticles, termed corona proteins, that cover PM2.5 particles can alter epigenetics and tumor suppressor gene expression (3, 15, 41, 42). DNA methylation is highly sensitive to environmental PM2.5 exposures (16, 20). Therefore, this modification can inhibit gene expression, impeding cellular differentiation as one of the steps of leukemic development. Long-term exposure to PM2.5 might alter both gene-coding and non-coding DNA methylation (20). An in vitro study by Jin et al. (43) found that the progression of leukemic cells was induced by prolonged exposure to PM2.5. The proposed mechanism is the reactive oxygen species-mediated pathway (43). Moreover, the PM2.5 component had been classified as a carcinogenic class I agent since 2013 (3). Apart from PM2.5 particles, its components, i.e., aluminum, black carbon, sulfur, lead, titanium, and silicon, may be involved in the pathogenesis of leukemia (20). In conclusion, a positive relationship between PM2.5 and leukemic cells is suggested by epidemiological evidence, and the molecular mechanisms are used to explain the pathophysiology of diseases.
Can Thrombosis and Coagulation Be Promoted by PM2.5?
Evidence from related studies links particulate matters to platelet function (2, 4, 22, 44–46). The American Heart Association-AHA (2004) proposed that thrombotic mechanisms may be explained by daily exposure to PM2.5 among patients with atherosclerotic cardiovascular disorders (22). Thrombosis is the most common pathology in patients with acute cardiac ischemia and stroke on top of atherosclerosis (4, 47, 48). Brook et al. (22) found that deep venous thrombosis (DVT) and hypercoagulability were also the results of long-term (almost 8-year) exposure to PM2.5. Similarly, the Zhang et al. (2) study was performed with 175,959 men (with 396,248 observations) and 186,437 women (with 397,877 observations) correlating with 2-year average PM2.5 concentrations. They found a relationship between thrombocytosis (men, 0.42% and women, 0.49%) and increment in PM2.5 (every 10 μg/m3 increase of PM2.5) (2). In the United States, a study by Kloog et al. (21) found a slight increment in DVT (0.63%, 95% CI = 0.03–1.25) but no significant increase in pulmonary embolism (PE), after short-term exposure to PM2.5. Interestingly, there were increased risks of DVT (6.98%; 95% CI = 5.65–8.33) and PE (2.67%; 95% CI = 5.65–8.33) events after long-term exposure to PM2.5 (21). The mechanism of thrombosis by PM2.5 may be from increased inflammatory cytokine (IL-6) levels, oxidative stress, platelet activation, stimulated coagulation pathway, and reduced fibrinolysis (3, 46, 49, 50). These conditions are known to promote thrombotic phenomena in humans (51). In animal models, there is a study on the effects of PM2.5 that can cause disseminated intravascular coagulation (DIC) through coagulation activation in rats (46). Liang et al. (46) found that rat exposure to PM2.5 increasingly expressed inflammatory cytokines, IL-6, IL-1β, and CRP in plasma. Moreover, tissue factor-dependent extrinsic pathways coagulation systems, as well as expression of adhesion molecules, such as VCAM-1 and ICAM-1, were upregulated after moderate to high doses of PM2.5 (average 35 μg/m3 for 30 exposure days). Finally, a shift of hemostatic balance to a pro-thrombotic/pro-coagulation state is induced by exposure to PM2.5 (4).
Conclusions
High PM2.5 exposure is one of the most important avoidable hazards to human health. The effects on hematological systems have been less well-studied. There were fewer than 20 available entries in the web search according to the Cochrane recommendation as the damages are not easily analyzed and are mostly unnoticed. All available studies found harmful effects of long-term exposure to PM2.5 to the hematopoietic system. Hematological parameter changes in people exposed to medium to high PM2.5 concentrations for more than a year are summarized in Table 2. The pathophysiology of PM2.5 pollution may be from increased inflammatory responses affecting each hematological component. For the public, avoidance of exposure and reduction in PM2.5 pollution should be encouraged. Interventions should be focused on the high-risk groups including the elderly with cardiovascular diseases, young children, and pregnant women. Personal facemasks can minimize inhaled small particles and reduce pollution exposure time. Air purifiers also lessen indoor pollution concentrations. Improving air quality has to be addressed for the general population. Decreasing PM2.5 pollution is essential to prevent hematological adverse events. Further studies are needed to determine whether reducing PM2.5 exposure can decrease these disorders.
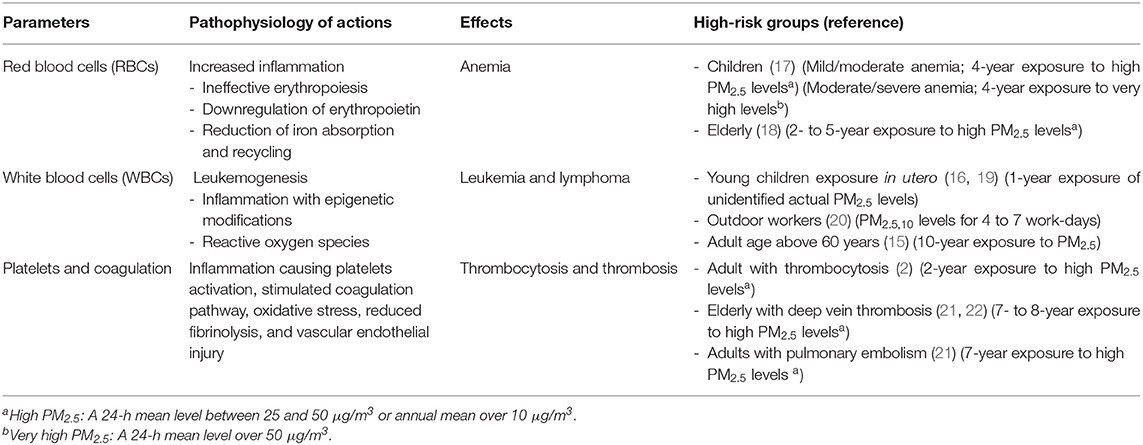
Table 2. Pathophysiology of PM2.5 effects on individual hematologic parameters and the high-risk groups.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
KF and SC: conception of the article and updating of the article as per the suggestions. KF, SC, VD, VT, and DS: writing-first draft preparation. KF, SC, VD, VT, DS, PR, and TU: writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Multicenter Research Grant from the Thai Society of Hematology (grant year 2019–2021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ms. Boongong Noochan for organization of files.
References
1. Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. (2015) 525:367–71. doi: 10.1038/nature15371
2. Zhang Z, Chan TC, Guo C, Chang LY, Lin C, Chuang YC, et al. Long-term exposure to ambient particulate matter (PM2.5) is associated with platelet counts in adults. Environ Pollut. (2018) 240:432–9. doi: 10.1016/j.envpol.2018.04.123
3. Visani G, Manti A, Valentini L, Canonico B, Loscocco F, Isidori A, et al. Environmental nanoparticles are significantly over-expressed in acute myeloid leukemia. Leuk Res. (2016) 50:50–6. doi: 10.1016/j.leukres.2016.09.004
4. Robertson S, Miller MR. Ambient air pollution and thrombosis. Part Fibre Toxicol. (2018) 15:1. doi: 10.1186/s12989-017-0237-x
5. Us Environmental Protection Agency. National Standards Air Quality Criteria for Particulate Matter (2019). Available online at: https://www.epa.gov/criteria-air-pollutants (accessed April 2, 2021).
6. Wardoyo A, Juswono U, Noor J. How exposure to ultrafine and fine particles of car smoke can alter erythrocyte forms of male mice. Polish J Environ Stud. (2019) 28:2901–10. doi: 10.15244/pjoes/94047
7. Mukae H, Hogg JC, English D, Vincent R, Van Eeden SF. Phagocytosis of particulate air pollutants by human alveolar macrophages stimulates the bone marrow. Am J Physiol Lung Cell Mol Physiol. (2000) 279:L924–31. doi: 10.1152/ajplung.2000.279.5.L924
8. Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol. (2006) 34:949–57. doi: 10.1080/01926230601080502
9. Mannucci PM, Harari S, Martinelli I, Franchini M. Effects on health of air pollution: a narrative review. Intern Emerg Med. (2015) 10:657–62. doi: 10.1007/s11739-015-1276-7
10. Accinelli RA, Leon-Abarca JA. Solid fuel use is associated with anemia in children. Environ Res. (2017) 158:431–5. doi: 10.1016/j.envres.2017.06.032
11. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. (2017) 389:1907–18. doi: 10.1016/S0140-6736(17)30505-6
12. World Health Organization. Ambient (Outdoor) Air Pollution (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed April 2, 2021).
13. World Health Organization. Global Health Observatory (GHO) Data; Exposure to Ambient Air Pollution (2019). Available online at: https://www.who.int/gho/phe/outdoor_air_pollution/exposure/en/ (accessed April 2, 2021).
14. World Health Organization. Pollution Affects All of Us (2012). Available online at: https://www.who.int/gho/phe/outdoor_air_pollution/exposure/en/ (accessed April 2, 2021).
15. Taj T, Poulsen AH, Ketzel M, Geels C, Brandt J, Christensen JH, et al. Exposure to PM2.5 constituents and risk of adult leukemia in Denmark: a population-based case-control study. Environ Res. (2021) 196:110418. doi: 10.1016/j.envres.2020.110418
16. Hvidtfeldt UA, Erdmann F, Urhoj SK, Brandt J, Geels C, Ketzel M, et al. Residential exposure to PM2.5 components and risk of childhood non-hodgkin lymphoma in Denmark: a nationwide register-based case-control Study. Int J Environ Res Public Health. (2020) 17:8949. doi: 10.3390/ijerph17238949
17. Morales-Ancajima VC, Tapia V, Vu BN, Liu Y, Alarcon-Yaquetto DE, Gonzales GF. Increased outdoor PM2.5 concentration is associated with moderate/severe anemia in children aged 6-59 months in Lima, Peru. J Environ Public Health. (2019) 2019:6127845. doi: 10.1155/2019/6127845
18. Honda T, Pun VC, Manjourides J, Suh H. Anemia prevalence and hemoglobin levels are associated with long-term exposure to air pollution in an older population. Environ Int. (2017) 101:125–32. doi: 10.1016/j.envint.2017.01.017
19. Lavigne E, Belair MA, Do MT, Stieb DM, Hystad P, Van Donkelaar A, et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: a population-based study in Ontario, Canada. Environ Int. (2017) 100:139–47. doi: 10.1016/j.envint.2017.01.004
20. Sanchez-Guerra M, Zheng Y, Osorio-Yanez C, Zhong J, Chervona Y, Wang S, et al. Effects of particulate matter exposure on blood 5-hydroxymethylation: results from the Beijing truck driver air pollution study. Epigenetics. (2015) 10:633–42. doi: 10.1080/15592294.2015.1050174
21. Kloog I, Zanobetti A, Nordio F, Coull BA, Baccarelli AA, Schwartz J. Effects of airborne fine particles (PM2.5) on deep vein thrombosis admissions in the northeastern United States. J Thromb Haemost. (2015) 13:768–74. doi: 10.1111/jth.12873
22. Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. (2010) 121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1
23. Wouters H, Van Der Klauw MM, De Witte T, Stauder R, Swinkels DW, Wolffenbuttel BHR, et al. Association of anemia with health-related quality of life and survival: a large population-based cohort study. Haematologica. (2019) 104:468–76. doi: 10.3324/haematol.2018.195552
24. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity (2020). Available online at: https://www.who.int/health-topics/anaemia#tab=tab_1 (accessed April 2, 2021).
25. World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease (2016). Available online at: https://www.who.int/airpollution/publications/en/ (accessed April 2, 2021).
26. Ministry of Environment of Peru-Minam. Climate Change Adaptation (UNDP) (2020). Available online at: https://www.adaptation-undp.org/partners/ministry-environment-peru-minam (accessed April 4, 2021).
27. Abu-Elmagd M, Alghamdi MA, Shamy M, Khoder MI, Costa M, Assidi M, et al. Evaluation of the effects of airborne particulate matter on bone marrow-mesenchymal stem cells (BM-MSCs): cellular, molecular and systems biological approaches. Int J Environ Res Public Health. (2017) 14:440. doi: 10.3390/ijerph14040440
28. Cui Y, Xie X, Jia F, He J, Li Z, Fu M, et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell Physiol Biochem. (2015) 35:353–63. doi: 10.1159/000369701
29. Bhattarai G, Lee JB, Kim MH, Ham S, So HS, Oh S, et al. Maternal exposure to fine particulate matter during pregnancy induces progressive senescence of hematopoietic stem cells under preferential impairment of the bone marrow microenvironment and aids development of myeloproliferative disease. Leukemia. (2020) 34:1481–4. doi: 10.1038/s41375-019-0665-8
30. Ferrucci L, Balducci L. Anemia of aging: the role of chronic inflammation and cancer. Semin Hematol. (2008) 45:242–9. doi: 10.1053/j.seminhematol.2008.06.001
31. Kido T, Tamagawa E, Bai N, Suda K, Yang HH, Li Y, et al. Particulate matter induces translocation of IL-6 from the lung to the systemic circulation. Am J Respir Cell Mol Biol. (2011) 44:197–204. doi: 10.1165/rcmb.2009-0427OC
32. Lynge E, Andersen A, Nilsson R, Barlow L, Pukkala E, Nordlinder R, et al. Risk of cancer and exposure to gasoline vapors. Am J Epidemiol. (1997) 145:449–58. doi: 10.1093/oxfordjournals.aje.a009127
33. Wu J, Houston D, Lurmann F, Ong P, Winer A. Exposure of PM2.5 and EC from diesel and gasoline vehicles in communities near the Ports of Los Angeles and Long Beach, California. Atmos Environ. (2009) 43:1962–71. doi: 10.1016/j.atmosenv.2009.01.009
34. Whitehead TP, Metayer C, Wiemels JL, Singer AW, Miller MD. Childhood leukemia and primary prevention. Curr Probl Pediatr Adolesc Health Care. (2016) 46:317–52. doi: 10.1016/j.cppeds.2016.08.004
35. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. (2015) 373:1136–52. doi: 10.1056/NEJMra1406184
36. Chen T, Zhang J, Zeng H, Zhang Y, Zhang Y, Zhou X, et al. The impact of inflammation and cytokine expression of PM2.5 in AML. Oncol Lett. (2018) 16:2732–40. doi: 10.3892/ol.2018.8965
37. Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, et al. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. (2012) 131:1565–89. doi: 10.1007/s00439-012-1189-8
38. Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. Int J Epidemiol. (2012) 41:79–105. doi: 10.1093/ije/dyr154
39. Madakashira BP, Sadler KC. DNA methylation, nuclear organization, and cancer. Front Genet. (2017) 8:76. doi: 10.3389/fgene.2017.00076
40. Koch A, Joosten SC, Feng Z, De Ruijter TC, Draht MX, Melotte V, et al. Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol. (2018) 15:459–66. doi: 10.1038/s41571-018-0004-4
41. Lundqvist M, Sethson I, Jonsson BH. Protein adsorption onto silica nanoparticles: conformational changes depend on the particles' curvature and the protein stability. Langmuir. (2004) 20:10639–47. doi: 10.1021/la0484725
42. Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. (2009) 20:455101. doi: 10.1088/0957-4484/20/45/455101
43. Jin XT, Chen ML, Li RJ, An Q, Song L, Zhao Y, et al. Progression and inflammation of human myeloid leukemia induced by ambient PM2.5 exposure. Arch Toxicol. (2016) 90:1929–38. doi: 10.1007/s00204-015-1610-x
44. Spiezia L, Campello E, Bon M, Maggiolo S, Pelizzaro E, Simioni P. Short-term exposure to high levels of air pollution as a risk factor for acute isolated pulmonary embolism. Thromb Res. (2014) 134:259–63. doi: 10.1016/j.thromres.2014.05.011
45. Franchini M, Mengoli C, Cruciani M, Bonfanti C, Mannucci PM. Association between particulate air pollution and venous thromboembolism: a systematic literature review. Eur J Intern Med. (2016) 27:10–3. doi: 10.1016/j.ejim.2015.11.012
46. Liang S, Zhao T, Hu H, Shi Y, Xu Q, Miller MR, et al. Repeat dose exposure of PM2.5 triggers the disseminated intravascular coagulation (DIC) in SD rats. Sci Total Environ. (2019) 663:245–53. doi: 10.1016/j.scitotenv.2019.01.346
47. Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. (2005) 36:2549–53. doi: 10.1161/01.STR.0000189687.78760.47
48. The Lancet Haematology. Thromboembolism: an under appreciated cause of death. Lancet Haematol. (2015) 2:e393. doi: 10.1016/S2352-3026(15)00202-1
49. Ruckerl R, Hampel R, Breitner S, Cyrys J, Kraus U, Carter J, et al. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ Int. (2014) 70:32–49. doi: 10.1016/j.envint.2014.05.013
50. Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, et al. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the multi-ethnic study of atherosclerosis (MESA). Epidemiology. (2015) 26:310–20. doi: 10.1097/EDE.0000000000000267
Keywords: air pollution, particulate matter, anemia, leukemia, thrombosis, blood coagulation
Citation: Fongsodsri K, Chamnanchanunt S, Desakorn V, Thanachartwet V, Sahassananda D, Rojnuckarin P and Umemura T (2021) Particulate Matter 2.5 and Hematological Disorders From Dust to Diseases: A Systematic Review of Available Evidence. Front. Med. 8:692008. doi: 10.3389/fmed.2021.692008
Received: 13 April 2021; Accepted: 14 June 2021;
Published: 14 July 2021.
Edited by:
Uday Prakash Kulkarni, Christian Medical College & Hospital, IndiaReviewed by:
Ching-Ying Kuo, National Taiwan University, TaiwanHong Yue, Marshall University, United States
Copyright © 2021 Fongsodsri, Chamnanchanunt, Desakorn, Thanachartwet, Sahassananda, Rojnuckarin and Umemura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Supat Chamnanchanunt, c3VwYXQuY2hhQG1haGlkb2wuYWMudGg=
 Kamonpan Fongsodsri
Kamonpan Fongsodsri Supat Chamnanchanunt
Supat Chamnanchanunt Varunee Desakorn2
Varunee Desakorn2 Vipa Thanachartwet
Vipa Thanachartwet