- 1Department of Molecular Imaging, Carilion Clinic, Roanoke, VA, United States
- 2Lucerno Dynamics LLC, Cary, NC, United States
Nuclear medicine extravasations and prolonged venous stasis may cause poor quality and quantification errors that can affect image interpretation and patient management. Radiopharmaceutical remaining near the administration site means that some portion of the radioactivity is not circulating as required for the prescribed uptake period. This case describes how detection of excess presence of 99mTc-MDP near the injection site enabled the technologist to apply mitigation tactics early in the uptake process. It also suggests that detecting an extravasation or stasis early in the injection process can be important for image interpretation and minimizing radiation dose to tissue.
Introduction
Bone scanning is a widely accepted diagnostic tool in nuclear medicine for many disease processes including metastatic screening. Bone scanning is typically a qualitative exercise, but as healthcare is rapidly moving toward precision medicine, quantification of molecular imaging techniques, including bone scans, is beginning to play a larger role. Quantification allows for more accurate and objective scan interpretation (1), and is becoming more important for bone scans when using new-generation treatments (2).
Extravasations, the leakage of the radiopharmaceutical into the tissue surrounding the administration site, frequently occur in bone scans. In nine centers, during two studies, 450 bone scan images were retrospectively reviewed for extravasations. The average extravasation rate was found to be 17.5% (3). Other nuclear medicine procedures are also frequently extravasated. A literature review revealed seven studies in 3 centers (2,989 patients) with an average extravasation rate of 15%1 (4–9) and that significant extravasations can affect the qualitative and quantitative assessment of nuclear medicine studies (1, 10–14). The experience at our center confirms the negative effects of significant extravasations. We previously published how an extravasation during an FDG PET administration resulted in understating the SUVmax of a solitary lung tumor by 80% (15). In the same case, we saw the effects of an extravasation to the quality of the image when we reimaged the patient 3 day later and discovered metastatic disease previously not evident in the image with extravasated injection. Using the extravasated image would have resulted in an improper treatment plan (15). In another publication, we highlighted the effects of significant extravasations on the quality and quantification of additional FDG PET studies and the quality of an MDP study by reimaging these patients several days after their extravasated studies. These cases also had patient care implications (16).
Prolonged venous stasis, a condition of slow blood flow in the vein (17), is another common problem in nuclear medicine. Accurate quantification requires a bolus injection of the entire intended dose. In the case of a prolonged venous stasis, the dose is not being delivered as a bolus; this delays the actual delivery of the radiopharmaceutical. Additionally, there is no information of how much of the dose may have not been delivered. Those two factors can confound the quantification of the scan.
Since extravasations and stasis can cause quantification errors, they should be considered when interpreting patient images. However, in many nuclear medicine studies the administration site is outside the imaging field of view (FOV) (4) and/or is not imaged for several hours after the injection, so physicians may not know that an extravasation or stasis occurred.
Significant extravasations can also potentially lead to high absorbed radiation doses to underlying tissue and skin (18). While bone scans and other routine diagnostic studies are often viewed as very low risk to patients, recent research indicates that significant extravasations of routinely used diagnostic radiopharmaceuticals like 99mTc-MDP can result in high doses to the tissue and adverse tissue reactions (16, 18).
Detecting the excess presence of radiotracer immediately after administration can be important. Technologists, alerted that the injection quality may be compromised, can ensure the injection site is included in the field of view at time of imaging and can apply mitigation techniques early in the process. Early mitigation efforts for an extravasation or prolonged venous stasis can improve the image, minimize the absorbed dose, and decrease the latent effects of ionizing radiation to healthy tissue.
Case Presentation
A 63-year-old female patient had a history of breast cancer and MRI showed an indeterminate lesion at L1. A SPECT/CT bone scan was ordered to rule out bone metastases.
In our center, we monitor radiopharmaceutical injection quality using the Lara® System (Lucerno Dynamics, Cary, NC), consisting of two external scintillating detectors, a reader to collect and store data, a docking station, software to transfer data, and a web application to display and analyze data (Figure 1). The scintillating detectors are placed proximal to the injection site and mirrored on the other arm. The Lara® System provides a real time display of counts from each sensor and a time-activity curve (TAC) with a classifier score (19) once the administration is complete. Use of the device adds 20 additional seconds to the patient's imaging experience and <1 min/administration to the technologist's workload. A Certified Nuclear Medicine Technologist gained access to the right antecubital fossa using an IV cannula. The patient was manually injected with 27.2 mCi of 99mTc-MDP and the IV was flushed with 10 mL of saline. Per protocol, the IV was then removed and the injection site then covered by a 2 × 2 gauze pad, which the patient held in place with firm finger pressure. Following a brief delay to ensure the site would not bleed, the gauze was wrapped with Coban to hold the gauze in place. The injection and IV removal were both unremarkable and presented no complications.
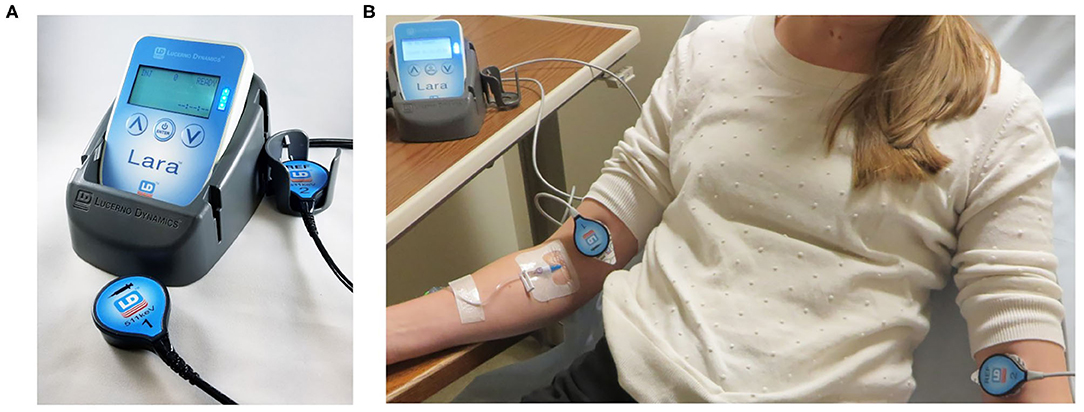
Figure 1. Lara device consists of 2 scintillation sensors, a reader, and a docking station (A). Sensors placed on injection arm and reference arm (B).
While observing the patient, the technologist checked the Lara reader display. Counts from the sensor on the injection arm, as compared to counts from the reference sensor, indicated a significant presence of excess 99mTc-MDP near the injection site (Figure 2). The technologist asked the patient to move her injection arm and to leave it raised above her head for several minutes. The technologist observed the real-time counts on the Lara display begin to decrease from the injection sensor, indicating the clearance of the MDP from the injection site. Over the next 8 min the counts from the injection sensor equilibrated with the counts from the reference sensor. The uptake period resumed with no further issue and the patient was imaged according to our standard protocol.
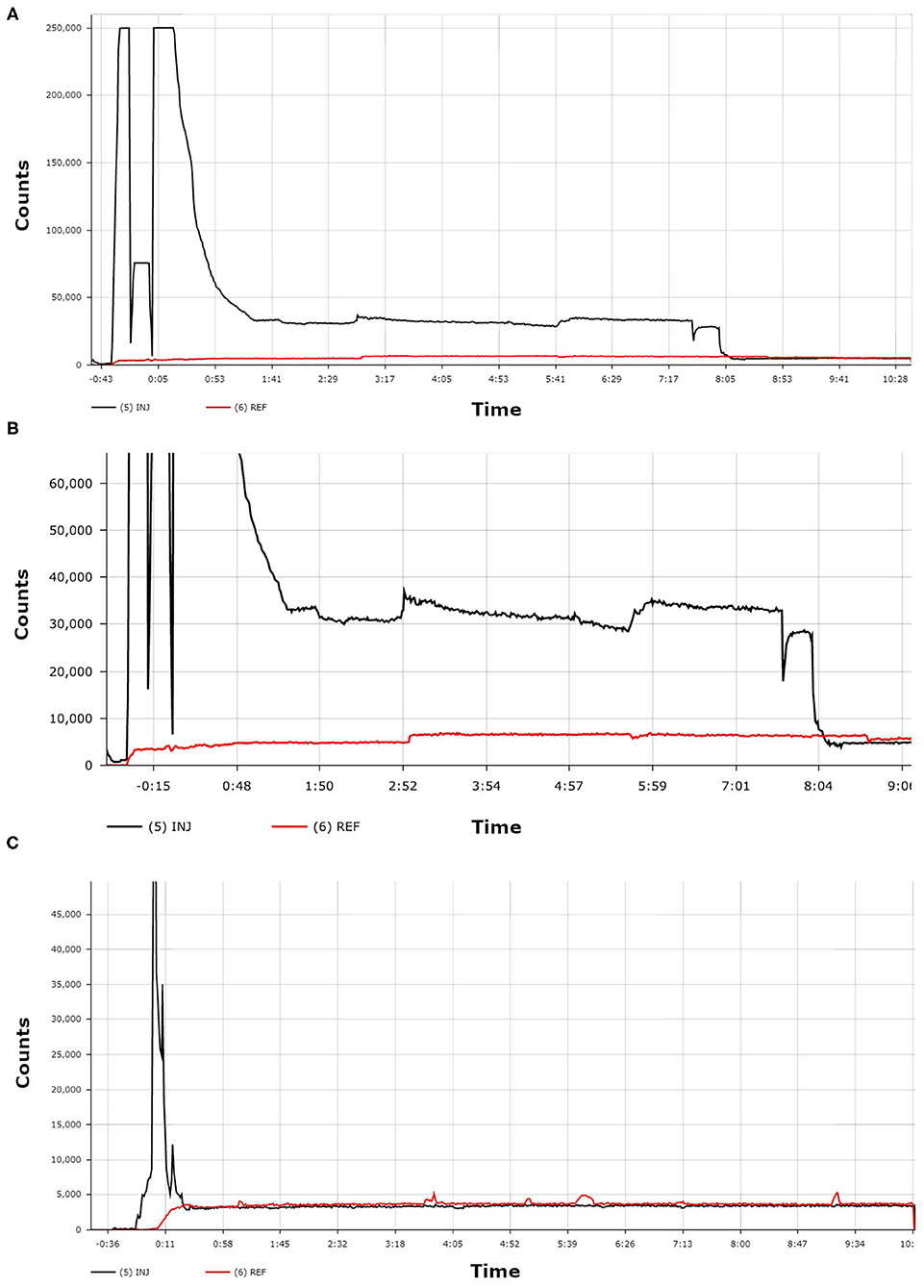
Figure 2. The first TAC (A) shows the patient's administration with 99mTc-MDP. A zoomed-in view of this same TAC (B) more clearly shows the dramatic difference between the counts from the injection arm sensor curve (black line) and the counts from the reference arm sensor curve (red line). The third TAC (C) was acquired from a different patient and shows an ideal administration where the injection arm sensor curve (black line) and the reference arm sensor curve (red line) reached equilibrium within seconds.
Bone SPECT images were acquired with a Siemens Intevo Bold (Siemens Medical Solutions USA, Inc.) after standard injected activity of 27mCi 99mTc-MDP using a LEHR collimator and matrix size of 256 x 256 with a zoom of 1. SPECT data were acquired using 30 views in a non-circular (body contour) orbit with an acquisition time of ~20 s per view. For CT Acquisition, data were acquired using 130 kVp with CareDose 4D and pitch of 1.5 and detector settings of 16 × 1.2 mm with 3 mm slice thickness.
Discussion
Without monitoring the quality of the administration, extravasation and stasis are difficult to detect during and after radiopharmaceutical administration for several reasons. First, nuclear medicine scans usually use small injection volumes of non-vesicant radiopharmaceuticals that do not cause immediate, visible changes to the overlying skin near the injection site, nor immediate pain to the patient. Second, during clinician interpretation of the images, the injection sites are often outside of the imaging FOV. Even if the injection site is in the imaging FOV, prolonged venous stasis is especially confounding since at the time of imaging there may be no evidence at the injection site that there had been a venous issue during the administration of the radiopharmaceutical (20).
In the case when an extravasation is visible, many centers use lead shielding to prevent artifacts. While this beneficial practice prevents artifacts from confounding image quality, it does not address quality issues associated with a reduced number of counts in the imaging area of interest. This unknown impact to the quality of the image is more pernicious because there is a lack of knowledge regarding how much activity remains near injection site.
Application of external scintillation detectors to monitor activity at the injection site provides dynamic insight into the quality of radiopharmaceutical administration without having to image the injection site and can assist the interpreting physician to determine if repeat imaging is needed. In a case of extravasation, monitoring with external scintillation detectors also provides patient-specific biological clearance information to perform more accurate dosimetry (18).
Prospective monitoring also enables early mitigation efforts. Mitigation steps can include one or more of the following: local application of warmth, elevation or movement of arm laterally, massaging, and flushing with saline (17, 21, 22). The purpose of these interventions is to either resolve a stasis or minimize the effects of an extravasation. Mitigation efforts increase blood flow and lymphatic clearance and disperse radioactivity. While some references suggest applying ice to a radiopharmaceutical extravasation (23), this will constrict blood flow and possibly retain radioactivity locally. Additionally, certain vascular access techniques prevent some mitigation tactics; it is impossible to flush saline if straight sticks have been used for vascular access.
This case report has limitations. At our center bone scan uptake periods often last for 3 h and we do not require that patients remain still. This prolonged stasis may have resolved itself sometime during the patient's 3-h uptake period without monitoring and then applying mitigation steps.
Results
During the standard imaging protocol for the study, additional bilateral antecubital static images in both anterior and posterior views were also acquired (Figure 3). The images revealed no evidence of remaining radioactivity in the right antecubital fossa, leading us to the conclusion that by having the patient raise the right arm above their head, the radiopharmaceutical was able to completely infuse into circulation resulting in an uncompromised study. The SPECT-CT bone scan showed that the patient had benign hemangioma with no uptake of radiotracer or any other signs of metastatic skeletal disease. The patient has had no further imaging evaluation since then regarding her cancer except for routine mammography.
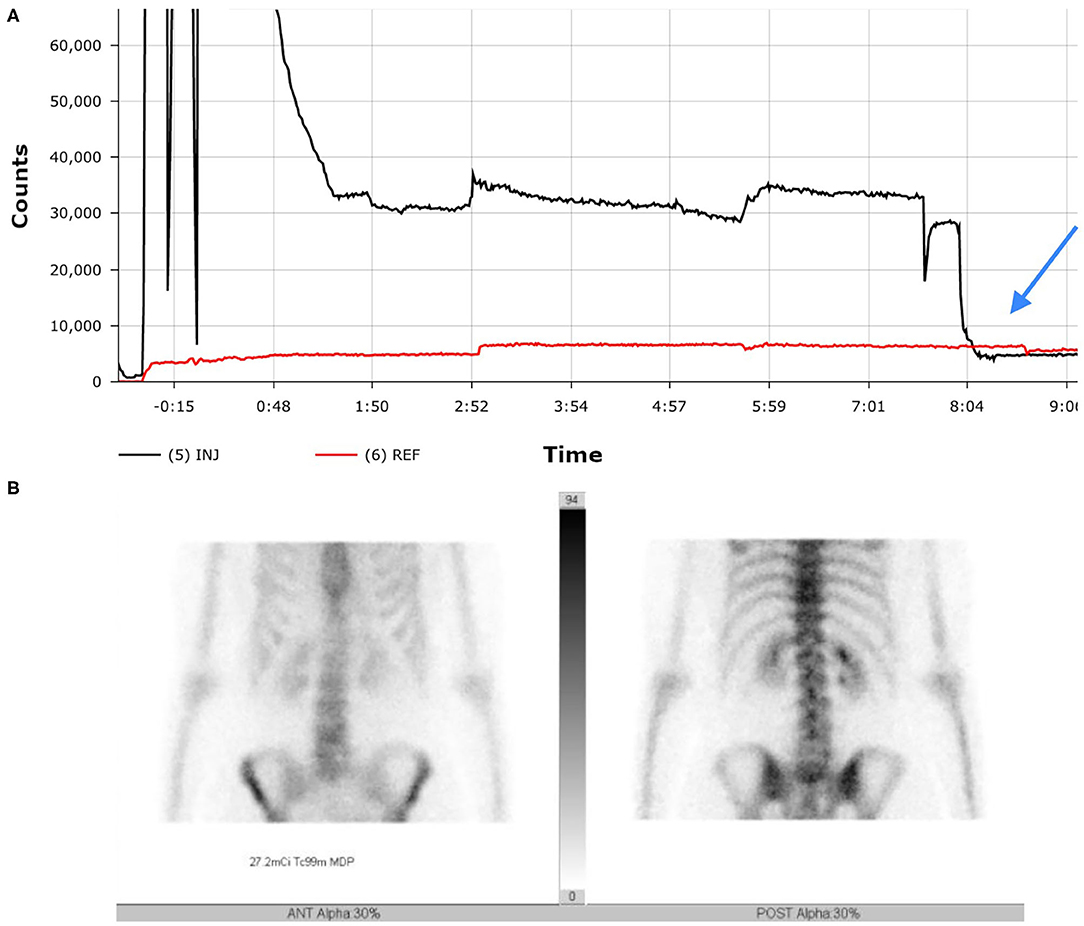
Figure 3. While some excessive level of radioactivity is indicated in the first few minutes of uptake on the TAC (A), the mitigation efforts at time 8 minutes cause rapid equilibrium (see blue arrow). This equilibrium is reflected in image (B) no remaining radioactivity evident at injection site.
Concluding Remarks
This case describes how an early detection of excess presence of 99mTc-MDP near injection site enables technologist to apply mitigation tactics early in the process. It also suggests that detecting an extravasation or stasis early in the injection process can be important for image interpretation and minimizing radiation dose to tissue.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JC contributed by obtaining patient data and preparation of the manuscript. IB and DG contributed to preparation of the manuscript. JK contributed by interpreting patient data and preparation of the manuscript. All authors contributed to review, editing, read, and approved the final manuscript.
Conflict of Interest
IB and DG are employed by the company Lucerno Dynamics, the manufacturer of the Lara System described in this manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
99mTc-MDP, Technetium 99m-methyl diphosphonate; mCi, Millicurie; SPECT/CT, Single Photon Emission Computed Tomography/Computed Tomography; TAC, Time-activity curve; FOV, Field of view; IV, Intravenous; mL, Milliliter.
Footnotes
1. ^Meyer A, Higgins Z, Packard A, Johnson D. Importance of Injection Site Image in DaTscans. Mayo Clinic, Department of Radiology, Rochester, Minnesota.
References
1. Sullivan DC. Precision prospectus: limiting variability in PET interpretation. In: In Practice. Leesburg, VA: ARRS (2020).
2. Swami U, Yap JT, Agrawal N. Radioisotope imaging and therapy for bone metastasis in men with castration-resistant prostate cancer. JAMA Oncol. (2020) 6:225–6. doi: 10.1001/jamaoncol.2019.4635
3. McIntosh C, Abele J. Frequency of interstitial radiotracer injection for patients undergoing bone scan. In: The Canadian Association of Radiologists. Montreal, QC (2016). Available online at: https://car.ca/
4. Osman MM, Muzaffar R, Altinyay ME, Teymouri C. FDG dose extravasations in PET/CT: frequency and impact on SUV measurements. Front Oncol. (2011) 1:41. doi: 10.3389/fonc.2011.00041
5. Hall N, Zhang J, Reid R, Hurley D, Knopp M. Impact of FDG extravasation on SUV measurements in clinical PET/CT. Should we routinely scan the injection site? J Nucl Med. (2006) 47(Suppl. 1):115P.
6. Bains A, Botkin C, Oliver D, Nguyen N, Osman M. Contamination in 18F-FDG PET/CT: an initial experience. J Nucl Med. (2009) 50(Suppl. 2):2222.
7. Krumrey S, Frye R, Tran I, Yost P, Nguyen N, Osman M. FDG manual injection verses infusion system: a comparison of dose precision and extravasation. J Nucl Med. (2009) 50(Suppl. 2):2031.
8. Silva-Rodriguez J, Aguiar P, Sanchez M, Mosquera J, Luna-Vega V, Cortes J, et al. Correction for FDG PET dose extravasations: Monte Carlo validation and quantitative evaluation of patient studies. Med Phys. (2014) 41:052502. doi: 10.1118/1.4870979
9. Muzaffar R, Frye SA, McMunn A, Ryan K, Lattanze R, Osman MM. Novel method to detect and characterize (18)F-FDG infiltration at the injection site: a single-institution experience. J Nucl Med Technol. (2017) 45:267–71. doi: 10.2967/jnmt.117.198408
10. Schaefferkoetter JD, Osman M, Townsend DW. The importance of quality control for clinical PET imaging. J Nucl Med Technol. (2017) 45:265–6. doi: 10.2967/jnmt.117.198465
11. Bogsrud TV, Lowe VJ. Normal variants and pitfalls in whole-body PET imaging with 18F FDG. Appl Radiol. (2006) 35:16–30.
12. Sonoda LI, Ghosh-Ray S, Sanghera B, Dickson J, Wong WL. FDG injection site extravasation: potential pitfall of misinterpretation and missing metastases. Clin Nucl Med. (2012) 37:1115–6. doi: 10.1097/RLU.0b013e318266cbdb
13. Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. Am J Roentgenol. (2010) 195:310–20. doi: 10.2214/AJR.10.4923
14. Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and roi definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. (2004) 45:1519–27.
15. Kiser JW, Crowley JR, Wyatt DA, Lattanze RK. Impact of an 18F-FDG PET/CT radiotracer injection infiltration on patient management - a case report. Front Med. (2018) 5:143. doi: 10.3389/fmed.2018.00143
16. Osborne D, Lattanze RK, Bryant TE, Barvi I, Fu Y, Kiser JW. The scientific and clinical case for reviewing diagnostic radiopharmaceutical extravasation long-standing assumptions. Front Med. (2021) 8:4157. doi: 10.3389/fmed.2021.684157
17. Taylor AT. Radionuclides in nephrourology, part 1: radiopharmaceuticals, quality control, and quantitative indices. J Nucl Med. (2014) 55:608–15. doi: 10.2967/jnumed.113.133447
18. Osborne D, Kiser JW, Knowland J, Townsend D, Fisher DR. Patient-specific extravasation dosimetry using uptake probe measurements. Health Phys. (2021) 20:339–43. doi: 10.1097/HP.0000000000001375
19. Andersson M, Johansson L, Eckerman K, Mattsson S. IDAC-Dose 2.1, an internal dosimetry program for diagnostic nuclear medicine based on the ICRP adult reference voxel phantoms. EJNMMI Res. (2017) 7:88. doi: 10.1186/s13550-017-0339-3
20. Lattanze RK, Osman M, Ryan KA, Frye SA, Townsend DW. Usefulness of topically applied sensors to assess the quality of 18F-FDG injections and validation against dynamic positron emission tomography (PET) images. Front Med. (2018) 5:303. doi: 10.3389/fmed.2018.00303
21. Hastings-Tolsma MT, Yucha CB, Tompkins J, Robson L, Szeverenyi N. Effect of warm and cold applications on the resolution of i.v. infiltrations. Res Nurs Health. (1993) 16:171–8. doi: 10.1002/nur.4770160304
22. Yucha CB, Hastings-Tolsma M, Szeverenyi NM. Effect of elevation on intravenous extravasations. J Intraven Nurs. (1994) 17:231–4.
23. Patient Preparation for Nuclear Medicine Procedures Involving Injections. Society of Nuclear Medicine and Molecular Imaging (2020). Available online at: http://www.snmmi.org/factsheets (accessed January 28, 2021).
Keywords: extravasation, infiltration, 99mTc-MDP, injection quality, bone scans
Citation: Crowley JR, Barvi I, Greulich D and Kiser JW (2021) Detection of Excess Presence of 99mTc-MDP Near Injection Site—A Case Report. Front. Med. 8:728542. doi: 10.3389/fmed.2021.728542
Received: 21 June 2021; Accepted: 20 August 2021;
Published: 17 September 2021.
Edited by:
Domenico Albano, University of Brescia, ItalyReviewed by:
Natale Quartuccio, ARNAS Ospedali Civico Di Cristina Benfratelli, ItalyAlberto Miceli, Università di Genova, Italy
Copyright © 2021 Crowley, Barvi, Greulich and Kiser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jackson W. Kiser, andraXNlckBjYXJpbGlvbmNsaW5pYy5vcmc=
 James R. Crowley
James R. Crowley Iryna Barvi
Iryna Barvi Debbie Greulich2
Debbie Greulich2 Jackson W. Kiser
Jackson W. Kiser