- 1Department of Medical Education, Chung Shan Medical University Hospital, Taichung, Taiwan
- 2Institute of Medicine, College of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 3Department of Urology, Chung Shan Medical University Hospital, Taichung, Taiwan
- 4Department of Medical Research, Center for Health Data Science, Chung Shan Medical University Hospital, Taichung, Taiwan
- 5Department of Allergy, Immunology and Rheumatology, Chung Shan Medical University Hospital, Taichung, Taiwan
- 6Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
Objective: The association between autoimmune diseases (ADs) and interstitial cystitis/bladder pain syndrome (IC/BPS) has long been investigated. However, the lack of comprehensive descriptions of patients in the literature has made comparison and evaluation impossible. We aim to investigate the risk of systemic ADs in patients with IC/BPS in Taiwan using a population-based administrative database.
Methods: This study evaluated 1,095 patients newly diagnosed with IC/BPS between 2000 and 2013, using data from Taiwan's National Health Insurance Research Database. These patients were randomly matched by demographic characteristics with a comparison cohort of individuals without IC/BPS at a ratio of 1:20. Cox proportional hazards regression analysis was used to analyze the risk of ADs, adjusting for age, sex, urbanization, length of hospital stay, and comorbidities adjustment. Sensitivity analysis by propensity score was used to adjust for confounding factors.
Results: The adjusted Hazard Ratio (aHR) of ADs for IC/BPS patients was 1.409 (95% CI 1.152–1.725). The subgroup analysis indicated that female or 45–60 years of age had a greater risk of ADs. Furthermore, the subgroup analysis of primary outcomes indicated that IC/BPS had greater incidence with Hashimoto's thyroiditis (aHR = 2.767, 95% CI 1.039–7.368), ankylosing spondylitis (aHR = 2.429, 95% CI 1.264–4.67), rheumatoid arthritis (aHR = 1.516, 95% CI 1.001–2.296), and Sjogren's syndrome (aHR = 1.962, 95% CI 1.37–2.809).
Conclusion: IC/BPS was associated with the development of ADs in our study population, especially Hashimoto's thyroiditis, ankylosing spondylitis, rheumatoid arthritis, and Sjogren's syndrome. Clinicians are recommended to be alert to the increased likelihood of developing ADs, particularly for middle-aged women.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic inflammatory disease characterized by recurrent pain, discomfort, or tenderness in the urinary bladder and pelvic region and can be accompanied by various urinary symptoms, such as urinary frequency, persistent urge to void, and nocturia. The overall prevalence of IC/BPS is roughly 300 cases per 105 patients, with a five times higher incidence in women than men (1). IC/BPS has multiple possible etiologies that include (1) infection, (2) neuronal function change, (3) autoimmune reactivity, (4) hypersensitivity response with mast cell release, and (5) defects in the urothelial permeability barrier (2). Other confusable diseases with presentations similar to IC/BPS must be ruled out through proper examination before diagnosis (2).
Autoimmune diseases (ADs), although considered to occur infrequently, substantially influence mortality and morbidity of patients. The association between autoimmune reactivity and IC/BPS has long been investigated. Some studies have reported on autoantibodies in patients with IC/BPS (3–5). Kujala et al. proposed a possible link between ADs and IC/BPS in patients with certain immunological factors or genetic predispositions (6). Numerous studies on autoantibodies against nuclear or bladder epithelium antigens in patients with IC/BPS appear in the literature (7, 8). Other studies have also proposed activation of complement components (9). Although the precise identities of relevant autoantibodies have yet to be determined, some speculate that the increased prevalence of ADs among patients with IC/BPS may involve the presence of shared underlying autoimmune disturbances.
In previous population-based studies, patients with IC/BPS had an increased prevalence of several ADs, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren syndrome (SS), ankylosing spondylitis (AS), and inflammatory bowel syndrome (10–13). The correlation between IC/BPS and other ADs has also been described in several retrospective articles (7, 14, 15). However, the literature is limited due to the use of varying definitions of IC/BPS and approaches to cross-sectional study design. Furthermore, to date, no comprehensive studies have investigated systemic ADs in patients with IC/BPS. These limitations have made comparison and evaluation impossible. To overcome these limitations, we designed a cohort study using a nationwide population database to investigate the risk of systemic ADs in patients with IC/BPS. Furthermore, we performed subgroup analysis and sensitivity testing for further evaluation of the relationship between these two diseases.
Methods
Data Source
This population-based retrospective cohort study was constructed using Taiwan's National Health Insurance (NHI) Research Database (NHIRD), which was established in 1995. More than 99% of the population in Taiwan is covered under the NHI program. All medical claims for insured services reimbursement are contained in the NHIRD. Original claims data include inpatient stays, outpatient visits, ambulatory and emergent care, hospitalization records, and medicine prescriptions. The Longitudinal Health Insurance Database, a subset of the NHIRD comprising 1,000,000 systematically and randomly selected beneficiaries from the NHIRD, was used in this study. The diagnostic codes were defined according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. All identification data were encrypted to protect patient privacy. This study was approved by the Institutional Review Board of Chung Shan Medical University (IRB CS15134).
Participants
For the period 1997 to 2013, we identified patients with new onset of IC/BPS by using ICD-9-CM code 595.1, who had at least two outpatient or one inpatient diagnosis of IC/BPS. The date of first outpatient visit or admission with an ICD-9-CM code for IC/BPS was defined as the index date for the cohort study. To increase the diagnostic validity of IC/BPS, each patient in the study group achieved the prerequisite to use sodium hyaluronate (Cystistat), which works as an intravesical instillation therapy of IC/BPS. According to the NHI regulations, a strict pre-review of medical records was required to prescribe Cystistat for patients with IC/BPS. To date, numerous published studies have been conducted to assure the enrollment of IC/BPS (16–18). Initially, 1443 patients diagnosed with IC/BPS were identified. In order to enroll patients with new-onset IC/BPS to analyze the risk of ADs, we excluded those patients who had been diagnosed with IC/BPS before January 1, 2000 (n = 137). Additionally, diagnoses of ADs before the index date were excluded (n = 211). Individuals without IC/BPS were individually paired at a 1:20 ratio with IC/BPS patients based on age and sex at the index date. In total, 1,095 patients with IC/BPS and 21,900 persons without IC/BPS were selected for analysis (Figure 1). All individuals enrolled in the study were tracked from the index date until the development of ADs, withdrawal from the NHI program, death, or the end of the study (December 31, 2013).
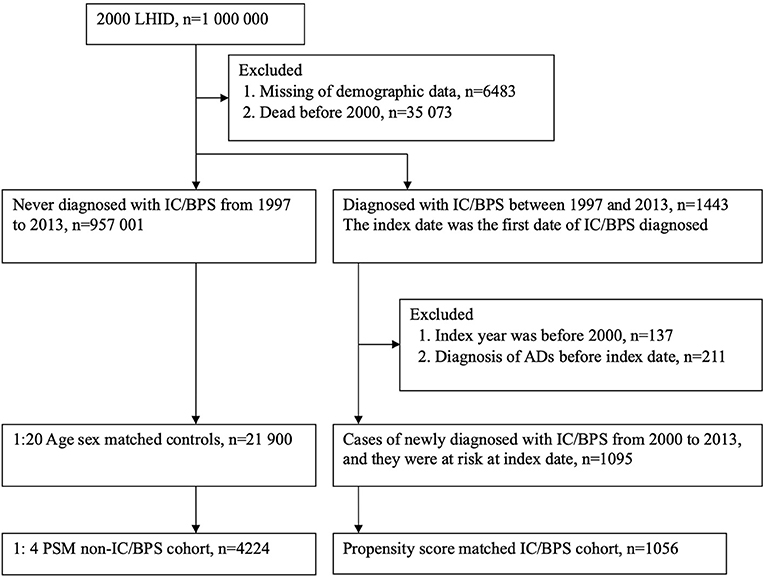
Figure 1. Study flow chart for population selection. LHID, Longitudinal Health Insurance Datasets; IC/BPS, interstitial cystitis/bladder pain syndrome; ADs, autoimmune diseases; PSM, propensity score matching.
Study Outcomes
Occurrence of ADs was defined as the primary outcome when subjects had at least 2 outpatient or 1 inpatient diagnosis of a condition listed below. These codes were validated and published in previous studies (19–21). The subcategories of ADs include Graves' disease (ICD-9-CM code 242.0), Crohn's disease (555), psoriasis and similar disorders (696), SLE (710.0), RA (714), AS (720.0), Guillain–Barré syndrome (357.0), SS (710.2), myasthenia gravis (358.0), vasculitis (446), uveitis (360.12, 363.0×, 363.1×, 363.20, 363.21, 363.22, 364.0×, 364.1×, 364.2×, 364.3), polymyalgia rheumatica (725), dermatomyositis (710.3), Hashimoto's thyroiditis (245.2), Behcet's disease (136.1), polymyositis (710.4), ulcerative colitis (556), autoimmune hemolytic anemia (283.0), multiple sclerosis (340), and systemic sclerosis (710.1). The diagnoses of ADs are highly trustworthy because systemic ADs such as SLE, SS, and RA are rigorously verified by two certified rheumatologists to review patients' medical records before issuing a catastrophic illness certificate, which is requested by the financial entity from the Bureau of NHI bearing the medical copayments of the patient (10, 12, 22).
Comorbidities
We considered comorbidities associated with ADs that should be included when adjusting for potential confounding factors. The following comorbidities were included as study covariates: hypertension (ICD-9-CM codes 401-405), type 2 diabetes mellitus (DM; 250), hyperlipidemia (272), coronary artery disease (CAD; 410-414), stroke (430-438), chronic kidney disease (CKD; 403.11, 403.91, 404.12, 404.13, 404.92, 404.93, 585, 586, 587, 274.1, 403.10, 403.90, 404.10, 404.11, 404.90, 404.91, 581, 582, 583, 590.0, 593.6, 593.9, 753.12, 753.13, 753.14, 250.4), chronic obstructive pulmonary disease (COPD; 490-492, 493-496), chronic liver disease (including cirrhosis and hepatic decompensation; 070.2, 070.3 070.41, 070.42, 070.44, 070.51, 070.52, 070.54, 070.6, 070.7, 070.9, 573.1, 273.4, 275.0, 275.1, 453.0, 571, 573, 576.1,456.0, 456.1, 456.2, 789.5, 789.59, 572.2, 567, 572.4), atopic dermatitis (691), allergic rhinitis (477), hepatitis B virus infection (070.2, 070.3, V02.61), hepatitis C virus infection (070.44, 070.51, 070.54, 070.7, V02.62), and depression (296, 300, 309, 311).
Statistical Analysis
Continuous variables (such as age and length of hospital stay) were transformed into ordinal variables. To evaluate the differences of study variables among IC/BPS and non-IC/BPS groups, the absolute standardized difference (ASD) was used. A small difference was defined as an ASD of <0.1. The incidence density (per 10,000 person-months) and its 95% confidence interval (95% CI) for AD was calculated by using Poisson distribution. The hazard ratio (HR) for AD with a 95% CI was estimated using the Cox proportional hazards regression model and then adjusted for age, sex, urbanization and comorbidities in a multivariable model. We used the propensity score matching to deal with the sensitivity analysis. The propensity score (predicted probability of IC/BPS exposure) was estimated for each patient using logistic regression, and the adjusted factors included age, sex, urbanization, hospital stay, and comorbidities. The paired patients with and without IC/BPS were randomly matched at a ratio of 1:4 where the difference of propensity score was closest. A significance level was set at two-tailed P < 0.05. Furthermore, we assessed the cumulative incidence curve using the Kaplan–Meier method and examined the difference by using the log-rank test.
Results
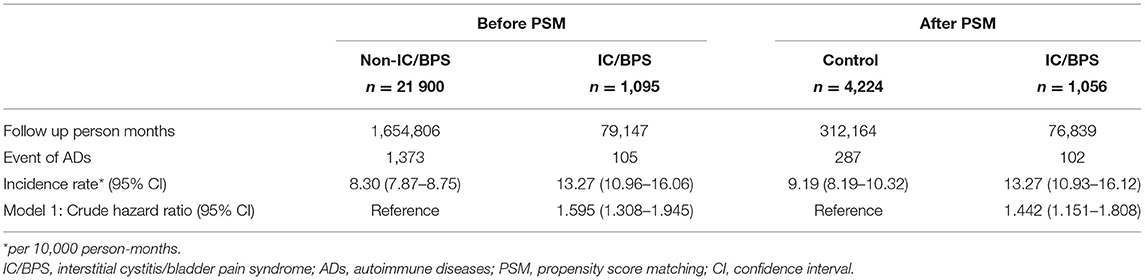
A total of 1,095 patients with IC/BPS and 21,900 1:20-matched non-IC/BPS controls were selected for this study. The median follow-up time was 66 months. Table 1 presents the information on the demographic variables and comorbidities of patients with IC/BPS and paired non-IC/BPS individuals. Of patients in the study groups, 80.18% were women. In IC/BPS groups, urban residence, longer hospital stay, and comorbidities, including hypertension, DM, hyperlipidemia, CAD, CKD, COPD, chronic liver diseases, allergic rhinitis, and depression, were more prevalent. The distributions of all observed baseline characteristics between the two groups were similar after propensity score matching.
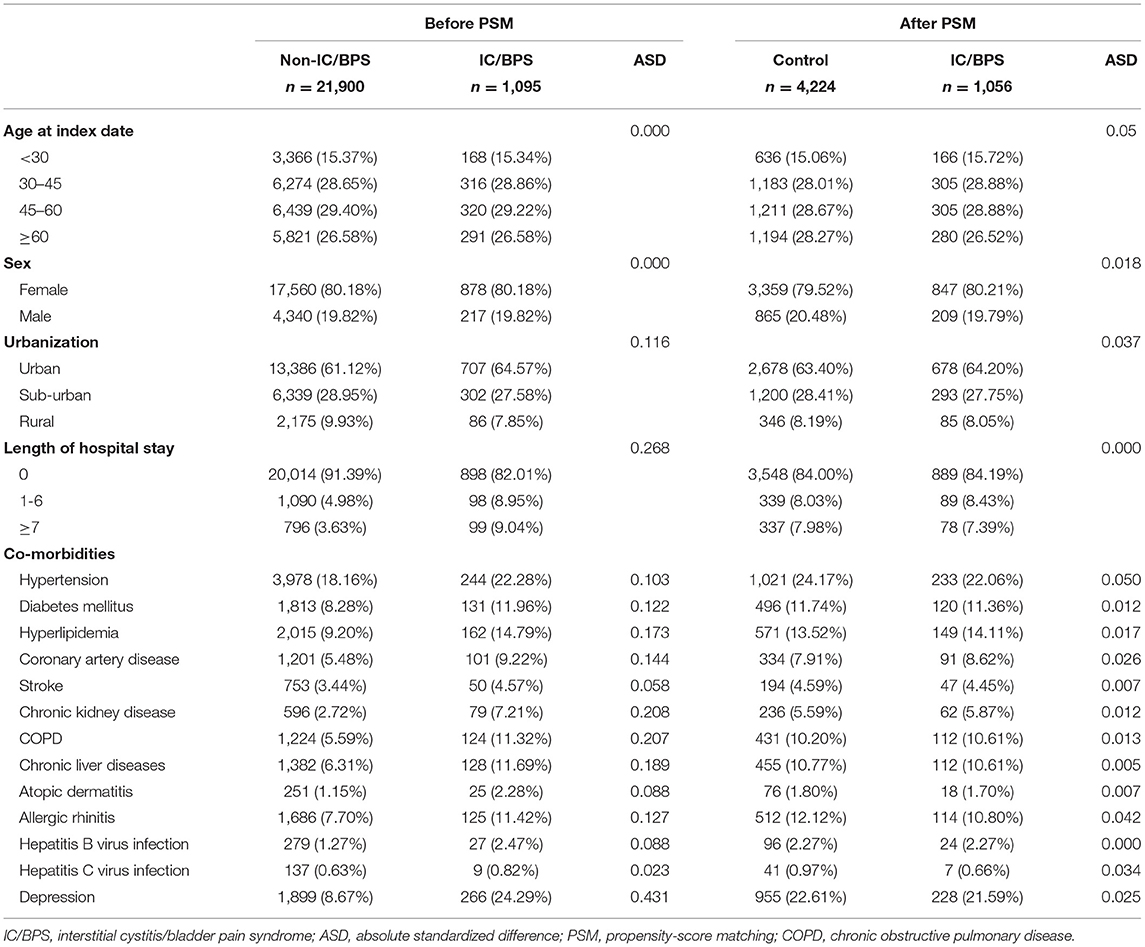
Table 1. Demographic variables and comorbidities of patients with IC/BPS and paired non-IC/BPS individuals.
The incidence rate of ADs was evaluated before and after propensity score matching. For patients with IC/BPS, the incidence rate was 13.27 (95% CI 10.96–16.06) per 10 000 person-months, and that for controls was 8.30 (95% CI 7.87–8.75) (Table 2) for the case cohort. The crude relative risk was 1.595(95% CI 1.308–1.945). Table 3 shows the HR of ADs calculated using Cox proportional hazards regression in age- and sex-matched populations. The aHR of ADs for patients with IC/BPS was 1.409 (95% CI 1.152–1.725; P < 0.01). Compared with patients aged under 30 years, patients aged 31–45, 46–60, and over 60 exhibited a gradually elevating aHRs of 1.35, 1.63, and 2.60, respectively. We also observed that CKD, COPD, and depression were significantly associated with higher risk of ADs. Figure 2 shows that the cumulative incidence of ADs in participants with IC/BPS was significantly higher than in those without IC/BPS (log-rank test, P = 0.0014).
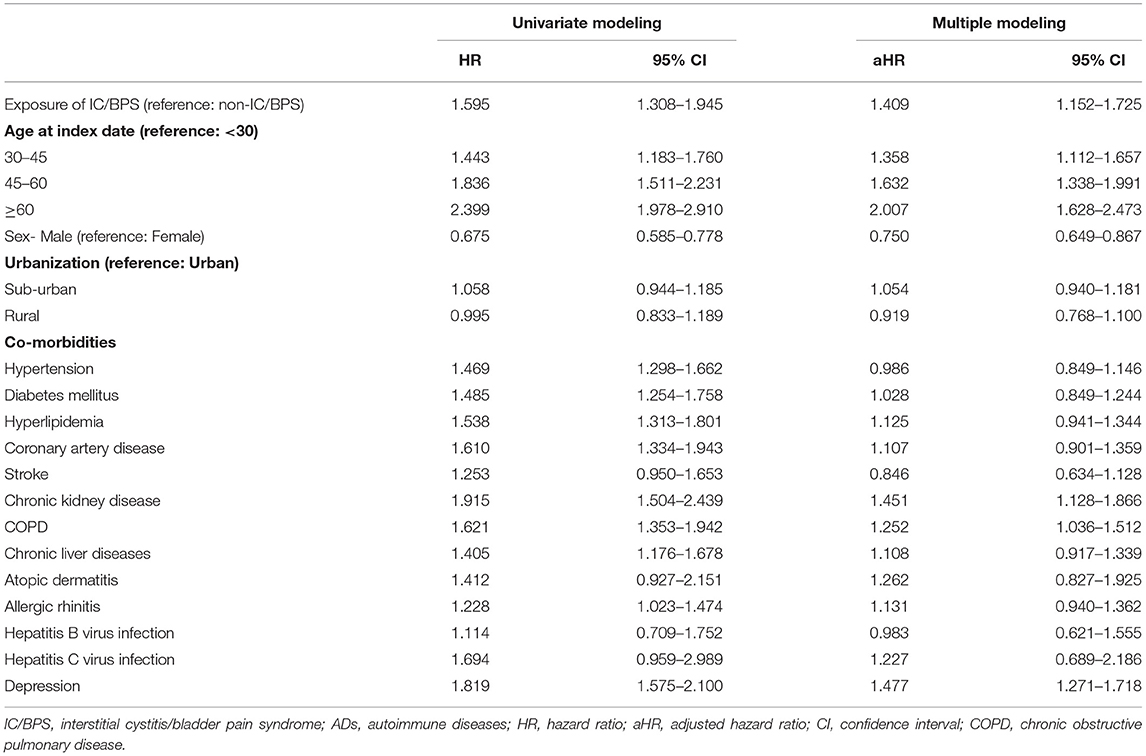
Table 3. Estimation of the hazard ratio of ADs by using Cox proportional hazard regression in age and sex matched population.
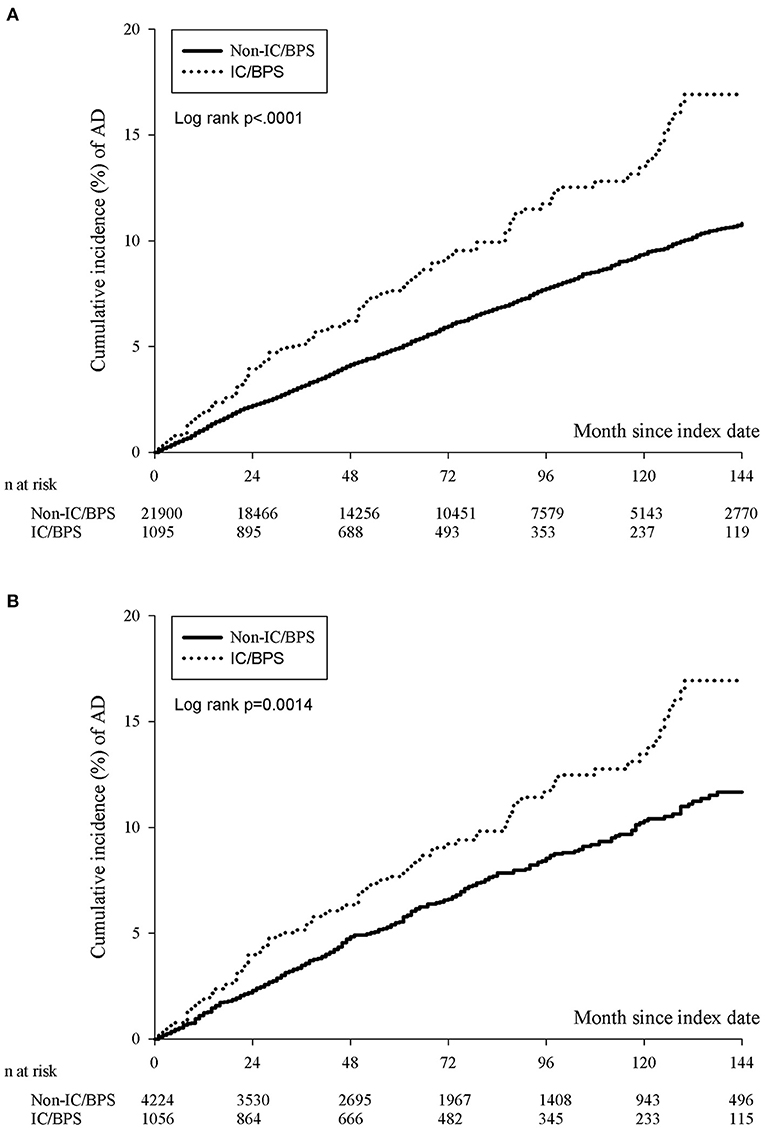
Figure 2. The cumulative incidence of ADs in participants with IC/BPS before (A) and after (B) PSM. The IC/BPS group was significantly higher than that in those without IC/BPS (log-rank test, p = 0.0014). IC/BPS, interstitial cystitis/bladder pain syndrome; ADs, autoimmune diseases; PSM, propensity score matching.
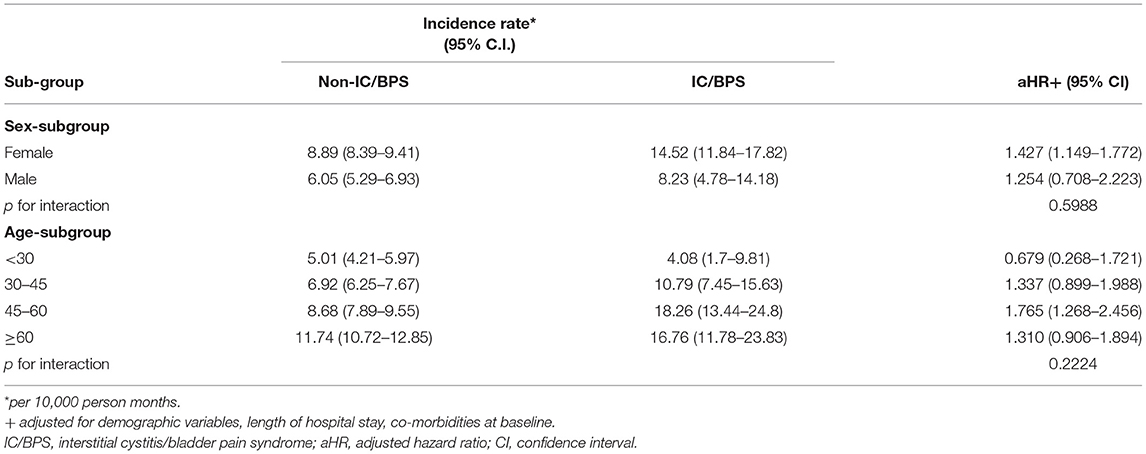
We present the risks of ADs in sex and age subgroups of patients with IC/BPS in Table 4. In the sex subgroup analysis, women with IC/BPS exhibited a higher risk of developing ADs (aHR = 1.427, 95% CI 1.149–1.772); for men with IC/BPS, the aHR was 1.254 (95% CI 0.708–2.223). However, the interaction between sex and IC/BPS was not significant (P = 0.5988). In the age subgroup analysis, those aged 45–60 exhibited a higher aHR of roughly 1.765 for developing ADs (95% CI 1.268–2.456), and no significant interaction between age and IC/BPS was noted (P = 0.2224).
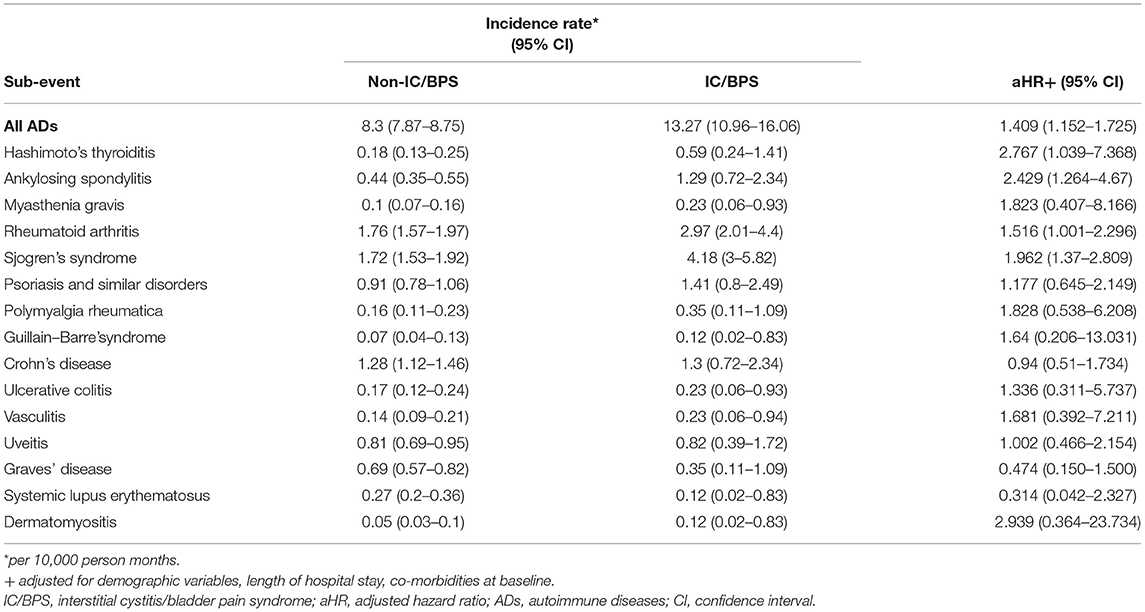
To clarify the causes of ADs, we analyzed the risk of each type of AD (Table 5). Owing to limited numbers of subjects with rarer ADs, we have not presented results for multiple sclerosis, systemic sclerosis, polymyositis, Behcet's disease, or autoimmune hemolytic anemia. Compared with the control group, the IC/BPS group had a 1.516-fold (95% CI 1.001–2.296) higher risk of RA, a 1.962-fold (95% CI 1.370–2.809) higher risk of SS, a 2.429-fold (95% CI 1.264–4.67) higher risk of AS, and a 2.767-fold (95% CI 1.039–7.368) higher risk of Hashimoto's thyroiditis. Sensitivity analysis by propensity score matching with balanced sex, age, and comorbidities yielded consistent results, namely HR = 1.442(95% CI 1.151–1.808).
Discussion
In the present study, we investigated the risk of concomitant ADs among individuals with IC/BPS using a nationwide database. The main results showed that patients with IC/BPS had an increased risk of developing ADs (aHR = 1.409, 95% CI 1.152–1.725) after adjusting for age, sex, and comorbidities. The risk was most prominent in Hashimoto's thyroiditis (aHR = 2.767, 95% CI 1.039–7.368), AS (aHR = 2.429, 95% CI 1.264–4.67), RA (aHR = 1.516, 95% CI 1.001–2.296), and SS (aHR = 1.962, 95% CI 1.37–2.809). Additionally, we independently stratified the subgroups of patients with IC/BPS into specific age and sex groups and identified a higher incidence rate in female patients (aHR = 1.427, 95% CI 1.149–1.772) and patients aged 45 to 60 years (aHR = 1.765, 95% CI 1.268–2.456) after adjusting for demographic variables, length of hospital stay, and comorbid conditions. However, the risks of SLE and inflammatory bowel disease (Crohn's disease and ulcerative colitis) were not increased in our study, a finding not in concordance with previous case-control studies (7, 14). These results indicated the possible causality underlying the association of IC/BPS and diverse ADs.
Some novel findings were made in our study. First, the risk of Hashimoto's thyroiditis was the highest in patients with IC/BPS in this study. However, there are currently no existing studies indicating a possible connection between IC/BPS and Hashimoto's thyroiditis. Hashimoto's thyroiditis is an autoimmune–endocrine disorder that mistakes the thyroid for an invader and attacks the thyroid tissue with a series of antibodies (23). In thyroid diseases, Chung et al. demonstrated that patients with IC/BPS had 2.16 times the odds ratio (OR) of patients without IC/BPS but with a previous diagnosis of hyperthyroidism (16). Another nationwide population-based study noted a higher prevalence of hypothyroidism with an adjusted OR of 2.3 times in patients with IC/BPS (24). These studies support our finding that IC/BPS may increase the risk of subsequent immune-related pathogenesis in the thyroid gland. Second, a positive relationship was shown between SLE and IC/BPS in the previous studies. A cross-sectional observational study, conducted by Keller et al., reported a higher prevalence of SLE (OR = 2.57) in patients with IC/BPS (24). Another cohort study demonstrated that patients with SLE had an increased risk for IC/BPS of 2.45 times (12). However, the aHR of SLE in our study was insignificant. We deduce that the sample size of IC/BPS patients with subsequent occurrence of SLE was still too small to provide enough statistical power to be statistically significant in our study.
In clinical practice, several major ADs such as SS, RA, and AS are pivotal and have drawn much attention for investigation by specialists. With regard to SS, patients showed a significantly increased risk of IC/BPS (HR = 2.34) in a large study involving 11,526 cases (10). As for RA, a case-control study showed that patients with IC/BPS and a previous diagnosis of RA had an OR of 1.66; however, men had a higher OR than women in their sex stratification (11), a finding contrasting with the female predominance seen in our results. AS has also been reported to be more prevalent in patients with IC/BPS (24). However, the specific mechanisms which underlie this relationship are still unknown. According to previous research, the pathogenesis between IC/BPS and ADs was induced by the release of specific cytokines evoked by autoantibodies transported to the lamina propria of the urinary bladder (10). Furthermore, relevant case reports have revealed abnormal laboratory tests with increased IgG, decreased C4, and antinuclear antibodies (ANA) in patients with SS presenting IC/BPS-related symptoms (25, 26). Merwe et al. determined that the antimuscarinic receptor IgG, an antagonist of the bladder and salivary and lachrymal glands, might play an important role in the early and late stages of the development of IC/BPS (27). Consequently, IC/BPS is likely to be a preceding local expression of origin participating in the immune responses of systemic ADs. As evidenced cited above, some studies have considered AD to be one of the causes of IC/BPS. However, further research is still required to verify whether IC/BPS triggers diverse ADs through any specific mechanism.
In our study, women and middle-aged patients had a higher risk of ADs. In previous research, the strong female preponderance has often been noted as a possible indicator of the autoimmune nature of IC/BPS (2). According to a study, the average age of onset of major ADs in women is as follows: SLE, age 15–55; RA, age 30–60; SS, age 40–60 (28). Therefore, middle-aged women are predisposed to present symptoms of IC/BPS and ADs due to their similar age of onset.
The strengths of our study are as follows. First, the use of a longitudinal and large-scale population database enables the results to be generalized up to a nationwide level. This not only minimizes the selection biases inherent in the investigation of the risks of ADs in patients with IC/BPS with various characteristics but also tracks a follow-up period of sufficient duration to allow for dysfunction to manifest in the urinary bladder. By contrast, previous studies have tended to analyze single AD and thus lack such a comprehensive review in their summaries. Second, this study examined the incidence risk of ADs in relation to chronic exposure to IC/BPS by utilizing various methods of matching analysis, including propensity score matching, and univariate- and multiple modeling to corroborate the results. Nevertheless, the present findings must be interpreted with caution due to several limitations. First, diagnoses from the NHIRD through ICD- 9-CM codes were concerned with miscoding or misclassification, and may thus be less accurate than standardized criteria. However, we further applied the same methods of the enrollment used in previous studies to improve the diagnostic validity. Second, the study design could not exclude the surveillance bias of patients with IC/BPS, especially middle-aged women, seeking health care services more often, thus resulting in a heightened tendency to receive medical examinations and be diagnosed with ADs. Third, although we have included many ADs to yield a generalized exploration in this study, some diseases with a potential relationship to autoimmunity might not yet be covered. Fourth, the confounding effects of comorbidities, such as health information and dietary habits and other environmental variables, still could not be completely avoided. However, we performed propensity score analysis to provide additional validity. Fifth, the NHIRD database was available until 2013 during the period of our IRB application, thus the trend in recent years was unable to be evaluated. However, our study has already included a 13-year cohort with adequate representation. Sixth, despite the large sample size in our study, the included population was still limited to a nation-base, which might influence the generalizability of the results to the worldwide population.
In conclusion, IC/BPS was associated with the development of ADs in patients in Taiwan's NHIRD research, especially in those with Hashimoto's thyroiditis, AS, RA, and SS. To date, no existing studies indicate a possible connection between IC/BPS and Hashimoto's thyroiditis. Clinicians are recommended to raise consensus on the patients with a history of IC/BPS and be alert to the increased likelihood of developing ADs, particularly for middle-aged women. Further studies are needed to confirm our findings and explore the underlying pathological mechanisms.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
This study was approved by the Institutional Review Board of Chung Shan Medical University (IRB, CS15134).
Author Contributions
H-ZY, M-HY, and J-YH were accountable for study conception and design and acquisition of data. J-YH performed the analyses and helped interpret the results. H-ZY and M-HY wrote the original draft of the manuscript. JW was involved in the supervision and revision of the manuscript. All authors have read, provided critical revision on intellectual content, and approved the final version of the manuscript.
Funding
The Longitudinal Health Insurance Database 2000 (LHID2000) was purchased from Taiwan National Health Insurance Administration by Chung Shan Medical University Hospital (Grant No. CSMU-INT-104-04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patnaik SS, Laganà AS, Vitale SG, Butticè S, Noventa M, Gizzo S, et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch Gynecol Obstet. (2017) 295:1341–59. doi: 10.1007/s00404-017-4364-2
2. Homma Y, Akiyama Y, Tomoe H, Furuta A, Ueda T, Maeda D, et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int J Urol. (2020) 27:578–89. doi: 10.1111/iju.14234
3. Silk MR. Bladder antibodies in interstitial cystitis. J Urol. (1970) 103:307–9. doi: 10.1016/S0022-5347(17)61948-7
4. Jokinen EJ, Alfthan OS, Oravisto KJ. Antitissue antibodies in interstitial cystitis. Clin Exp Immunol. (1972) 11:333–9.
5. Oravisto KJ. Interstitial cystitis as an autoimmune disease. A review. Eur Urol. (1980) 6:10–3. doi: 10.1159/000473278
6. Kujala MM, Tammela TL, Pöyhönen A, Forsell T, Pasanen S, Paananen I, et al. Prevalence of autoimmune disorders among bladder pain syndrome patients' relatives. Scand J Urol. (2020) 55:72–7. doi: 10.1080/21681805.2020.1821766
7. van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat Clin Pract Urol. (2007) 4:484–91. doi: 10.1038/ncpuro0874
8. Homma Y, Ueda T, Tomoe H, Lin AT, Kuo HC, Lee MH, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome: guidelines. Int J Urol. (2009) 16:597–615. doi: 10.1111/j.1442-2042.2009.02326.x
9. Mattila J, Linder E. Immunoglobulin deposits in bladder epithelium and vessels in interstitial cystitis: possible relationship to circulating anti-intermediate filament autoantibodies. Clin Immunol Immunopathol. (1984) 32:81–9. doi: 10.1016/0090-1229(84)90045-X
10. Lee CK, Tsai CP, Liao TL, Huang WN, Chen YH, Lin CH, et al. Overactive bladder and bladder pain syndrome/interstitial cystitis in primary Sjögren's syndrome patients: a nationwide population-based study. PLoS ONE. (2019) 14:1–10. doi: 10.1371/journal.pone.0225455
11. Keller JJ, Liu SP, Lin HC. A case-control study on the association between rheumatoid arthritis and bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. (2013) 32:980–5. doi: 10.1002/nau.22348
12. Wen JY, Lo TS, Chuang YC, Ho CH, Long CY, Law KS, et al. Risks of interstitial cystitis among patients with systemic lupus erythematosus: a population-based cohort study. Int J Urol. (2019) 26:897–902. doi: 10.1111/iju.14065
13. Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. (2010) 184:1358–63. doi: 10.1016/j.juro.2010.06.005
14. Peeker R, Atanasiu L, Logadottir Y. Intercurrent autoimmune conditions in classic and non-ulcer interstitial cystitis. Scand J Urol Nephrol. (2003) 37:60–3. doi: 10.1080/00365590310008721
15. Yamada T. Special contribution 2: significance of complications of allergic diseases in young patients with interstitial cystitis. Int J Urol. (2003) 10:56–8. doi: 10.1046/j.1442-2042.10.s1.12.x
16. Chung SD, Liu SP, Lin CC, Li HC, Lin HC. Bladder pain syndrome/interstitial cystitis is associated with hyperthyroidism. PLoS ONE. (2013) 8:8–12. doi: 10.1371/journal.pone.0072284
17. Wu MP, Luo HL, Weng SF, Ho CH, Chancellor MB, Chuang YC. Risk of urinary tract carcinoma among subjects with bladder pain syndrome/interstitial cystitis: a nationwide population-based study. Biomed Res Int. (2018) 2018:7495081. doi: 10.1155/2018/7495081
18. Keller J, Chiou HY, Lin HC. Increased risk of bladder cancer following diagnosis with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. (2013) 32:58–62. doi: 10.1002/nau.22283
19. Chu KA, Chen W, Hsu CY, Hung YM, Wei JCC. Association of scrub typhus with the risk of autoimmune diseases: a population-based cohort study. Am J Epidemiol. (2019) 188:1311–8. doi: 10.1093/aje/kwz074
20. Chen HH, Perng WT, Chiou JY, Wang YH, Huang JY, Wei JCC. Risk of dementia among patients with Sjogren's syndrome: a nationwide population-based cohort study in Taiwan. Semin Arthritis Rheum. (2019) 48:895–9. doi: 10.1016/j.semarthrit.2018.06.007
21. Zhu FX, Huang JY, Ye Z, Wen QQ, Wei JCC. Risk of systemic lupus erythematosus in patients with idiopathic thrombocytopenic purpura: a population-based cohort study. Ann Rheum Dis. (2020) 79:793–9. doi: 10.1136/annrheumdis-2020-217013
22. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Yang YHK, et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol. (2019) 11:349–58. doi: 10.2147/CLEP.S196293
23. Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, et al. Hashimotos' thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. (2019) 33:101367. doi: 10.1016/j.beem.2019.101367
24. Keller JJ, Chen YK, Lin HC. Comorbidities of bladder pain syndrome/interstitial cystitis: a population-based study. BJU Int. (2012) 110:E903–9. doi: 10.1111/j.1464-410X.2012.11539.x
25. Emmungil H, Kalfa M, Zihni FY, Karabulut G, Keser G, Sen S, et al. Interstitial cystitis: a rare manifestation of primary Sjögren's syndrome, successfully treated with low dose cyclosporine. Rheumatol Int. (2012) 32:1215–8. doi: 10.1007/s00296-010-1782-x
26. Liang D, Lu J, Guo A. Sjögren's syndrome accompanied with interstitial cystitis: a case report and review of the literature. Clin Rheumatol. (2014) 33:1189–93. doi: 10.1007/s10067-013-2480-3
27. Van De Merwe JP, Arendsen HJ. Interstitial cystitis: a review of immunological aspects of the aetiology and pathogenesis, with a hypothesis. BJU Int. (2000) 85:995–9. doi: 10.1046/j.1464-410x.2000.00646.x
Keywords: autoimmune diseases, interstitial cystitis, bladder pain syndrome, cohort study, nationwide population
Citation: Yueh H-Z, Yang M-H, Huang J-Y and Wei JC-C (2021) Risk of Autoimmune Diseases in Patients With Interstitial Cystitis/Bladder Pain Syndrome: A Nationwide Population-Based Study in Taiwan. Front. Med. 8:747098. doi: 10.3389/fmed.2021.747098
Received: 25 July 2021; Accepted: 27 August 2021;
Published: 20 September 2021.
Edited by:
Ying Ying Leung, Singapore General Hospital, SingaporeReviewed by:
Yoshiyuki Akiyama, The University of Tokyo, JapanEttore Silvagni, University of Ferrara, Italy
Copyright © 2021 Yueh, Yang, Huang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Cheng-Chung Wei, amNjd2VpQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Hann-Ziong Yueh
Hann-Ziong Yueh Min-Hsin Yang
Min-Hsin Yang Jing-Yang Huang
Jing-Yang Huang James Cheng-Chung Wei
James Cheng-Chung Wei