- 1Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 2Department of Nephrology, Zhongnan Hospital of Wuhan University, Wuhan, China
- 3Department of Emergency, Wuhan No.1 Hospital, Wuhan, China
- 4Department of Integrated Traditional Chinese and Western Medicine, The Central Hospital of Wuhan, Wuhan, China
Immune checkpoint inhibitors (ICIs), which can enhance antitumor immunity and inhibit cancer growth, have revolutionized the treatment of multiple cancers and dramatically decreased mortality. However, treatment with ICIs is directly associated with immune-related adverse events (irAEs) because of inflammation in off-target organs and autoimmunity resulting from non-specific immune activation. These irAEs can cause rheumatic diseases and manifestations such as inflammatory arthritis, polymyalgia rheumatica, myositis, vasculitis, Sicca and Sjogen's syndrome, and systemic lupus erythematosus. Early diagnosis and treatment of these adverse events will improve outcomes and quality of life for cancer patients. The treatment of rheumatic diseases induced by ICIs requires multidisciplinary cooperation among physicians. Furthermore, the underlying mechanisms are not fully understood and it is difficult to predict and evaluate these side effects precisely. In this review, we summarize available studies and findings about rheumatic irAEs, focusing mainly on the clinical manifestations, epidemiology, possible mechanisms, and guiding principles for treating these irAEs.
Introduction
Cancer, one of the most serious diseases affecting human health, has become the second leading cause of death worldwide—accounting for nearly a quarter of deaths—and the number of patients and deaths is growing rapidly every year (1). Immune-checkpoint inhibitor (ICI) therapy has provided a promising treatment strategy for patients (2). ICIs can overcome self-tolerance and enhance antitumor immunity through several approaches, mainly via the action of anti-cytotoxic T lymphocytes-associated antigen 4 (CTLA-4), anti-programmed death (PD)-1, and PD-ligand (L)-1 antibodies, according to the checkpoint inhibition target (3). ICIs therapy also can cause a unique and distinct immune-driven toxicity or autoimmune side effects termed “immune-related adverse events” (irAEs), which might be due to immune stimulation or loss of self-tolerance (4). IrAEs can affect multiple organs; this varies from patient to patient, and different patients present with diverse clinical manifestations ranging from mild (grade 1), to moderate (grade 2), severe, and generally requiring hospitalization (grade 3), life-threatening (grade 4), and death caused by adverse drug reactions (grade 5) according to the Common Terminology Criteria for Adverse Events (CTCAE) system (5, 6).
In some cases, irAEs are similar to various rheumatic diseases, including Sicca and Sjogren's syndrome (SS), myositis, arthritis, vasculitis, polymyalgia rheumatica (PMR), and systemic lupus erythematosus (SLE) (7). However, some of these inflammatory symptoms may be overlooked and confused with the related symptoms during the cancer treatment by physicians. In this review, we summarize the latest studies and findings about the clinical manifestations, unresolved questions, and underlying immune mechanisms, and propose approaches to the management of rheumatic diseases resulting from the use of ICIs.
Underlying Mechanisms and Potential Biomarkers
Most studies report clinical observations and the possible mechanisms are not fully understood (8). The incidence of rheumatic irAEs is associated with several factors, such as type of ICIs, dosage, the combination of ICIs used, and the accumulated dosage. Patients treated with an anti-CTLA-4 antibody are more vulnerable to irAEs compared with those treated using a PD-1/PD-L1 antibody (9). Different degrees of rheumatic disease have been observed in CTLA4- or PD-1-deficiency animal models (10). Autoimmune manifestations are likely to be associated with dysfunction or downregulation of CTLA4 (11). The deletion of CTLA-4 in adult mice showed an increase of T follicular helper and T follicular regulatory cells, resulted in antigen-specific expansion of antibody responses (12). ICIs target early stages of T-cell development, leading to increase in autoreactive T-cells, and autoimmune rheumatologic manifestations. When blocking PD-1, the autocrine binding of PD-1 to PD-1 ligands is inhibited, preventing apoptosis of B cells and improving the possibility for these cells to survive longer to produce specific antibodies.
In some anti-cyclic citrullinated peptide antibodies (ACPA)-positive patients with rheumatoid arthritis (RA), ICI therapy make them vulnerable to acute onset disease, especially those treated with anti-PD-1 therapeutics (13). In addition, T cell exhaustion could be correlated with disease activity in anti-nuclear cytoplasmic antibody (ANCA)-associated vasculitis and SLE (14). ICIs could induce epitope spreading, a diversification of epitope specificity of intra-tumoral T cells away from an initial focused, dominant epitope-specific immune response to a tumor (15). The increased levels of inflammatory cytokines, such as IL-17, TNF-α, are also associated with rheumatic irAEs, and targeted inhibitors can treat these adverse reactions (16, 17).
Unfortunately, it is difficult to predict the type and severity of rheumatic irAE because of the lack of no putative biomarker, classic HLA associations, perturbations of peripheral B cells, cytokines, and signature autoantibodies might provide crude estimates of the risk of irAEs (15, 18, 19).
Autoimmune IrAEs
Recent studies report that irAEs have occurred in ~90% of patients treated with ICIs, typically in the endocrine system, gastrointestinal tract, skin, heart, and lungs; the precise site varies from patient to patient (8). The incidence rates of rheumatic irAEs are evaluated based on numerous case series and retrospective clinical trials, many of which are phenotypically similar to classic rheumatic diseases (20). Most categories of rheumatic irAEs have been reported in ICIs trials; the most common event is arthritis (21).
Inflammatory Arthritis
Approximately 5% of the patients show clinical syndromes of arthritis or arthralgia after ICI treatment with anti-CTLA-4 (3–9%), anti-PD-1/PD-L1 (7–11%), and combination therapy (11%) (22, 23). A single-center prospective study revealed that 6.6% of patients (35/524) receiving ICIs developed rheumatic diseases (24). However, even this figure might be an underestimate because arthritis symptoms sometimes are ignored. The time between the onset of symptoms and the time of diagnosis in patients receiving ICI therapy varies. In a cohort study of 30 arthritis patients treated with ICIs (25), the median time of onset was 3 (1.3–12) months after ICI initiation, and knee arthritis was referred to a rheumatologist within one to two months, and arthritis in the small joints within 1 year. To date, three subtypes of inflammatory arthritis are characterized, including polyarthritis, which similar to RA; seronegative spondyloarthritis; and true reactive arthritis (26). Patients generally have joint pain and morning stiffness but no objective joint swelling, sometimes with accompanying tenosynovitis, enthesitis, or psoriasis (27, 28). Initially, arthritis has an additive pattern manifested as oligoarthritis, which subsequently proceeds to inflammatory polyarthritis. About two-thirds of arthritis affects the metacarpophalangeal joints, wrists, and larger joints, similar to RA (25, 29). Although joint fluid is found to show inflammation, laboratory tests do not show markers or specific findings. In the subtype of spondyloarthritis with large-joint phenotype, patients are HLA-B27 negative (30). A small number of patients are seropositive for RF and/or ACPA.
Arthritis can be detected by ultrasound, X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). Studies suggest that PET-CT and CT can be helpful in detecting synovitis with a high sensitivity and specificity in patients with ICI-induced arthritis (30, 31). In MRI and musculoskeletal ultrasonography tests, imaging always shows tenosynovitis, Doppler-positive synovitis, erosive disease, and joint effusions (26, 32). In a retrospective study of MRI in ICI-induced inflammatory arthritis (33), it is common to find tenosynovitis and synovitis in hands and wrists, while osseous erosions were found only in a few patients, which might indicate a worse prognosis. In other cases, joint pain and swelling also can result from tumor progression rather than ICIs (34). The severity of ICI-induced joint symptoms can range from mild to severe, but most are usually mild or moderate, and ICI therapy can be continued in these cases (35). Patients with mild syndromes only required NSAIDs or low-dose prednisone, with severe joint pain associated with stiffness or non-traumatic joint swelling should be referred to a rheumatologist.
PMR
A prospective study reported an estimated prevalence of 2.1% in 524 patients with ICI therapy developed a new-onset PMR-like syndrome (24). PMR syndrome is characterized by acute predominant joint pain and morning stiffness (shoulders, neck, hips, thighs) (24, 36–39). It commonly occurs about 3 months after starting ICI treatment and accompanied by other rheumatic irAEs (40). Given proximal limb involvement in ICI-associated PMR, it is important to consider myositis in the differential diagnosis. A multicenter and systematic review (40) evaluated 49 patients with ICI-induced PMR, and observed atypical features, such as the involvement of other joints (mostly knees and hands), the absence of high inflammatory indicators. In addition, it is important to seek symptoms suggesting temporal arteritis, such as headache and visual impairment, due to the same spectrum of disorders and frequently co-existence between PMR and temporal arteritis. Patients occasionally have a positive RF or ACPA. Most cases of ICI-induced PMR respond well to moderate doses of steroids, but the clinician should be aware that atypical cases with normal acute phase reactants exist and more aggressive therapy is sometimes required.
Myositis
Brahmer et al. reported an incidence of myalgia of 2% in patients receiving nivolumab treatment (41), while a prospective study showed the incidence to be up to 18.2% (42). However, prospective studies have only reported small-scale cases of myositis induced by ICIs, indicating a possible low prevalence. Myositis is characterized by muscle weakness in the proximal limbs and muscle inflammation as observed via neurophysiological and histopathologic detection, with or without myalgia (43). ICI-induced myositis can occur within 1 month of ICI initiation, with asymptomatic plasma creatine kinase (CK) and diffuse weakness (44). In some cases, CK can be highly elevated to 70 times the normal value, and accompanied by rhabdomyolysis (45). Moreover, ICI-induced myositis is strongly associated with myocarditis (11.3%) and myasthenia (11.9%), resulting in increased mortality (46, 47). Several studies have reported the appearance of myositis-like syndromes, myasthenia, and cardiac manifestations after a few infusions of ICI therapy (45, 48, 49). In addition, paraneoplastic myositis, such as dermatomyositis, induced by cancer but not ICI treatment, is difficult to distinguish from ICI-induced myositis (50). The time of onset of myositis symptoms might aid differentiation within 1–2 months of ICI treatment.
Muscle pathology in ICI myositis showed infiltration of cytotoxic T cells, multifocal necrotic myofibers, and endomysium inflammation, consisting mainly of CD68+ PD-L1+ cells and CD8+ PD-1+ cells (44, 51). An autopsy study in ICI myositis found CD81+ T-cell in the tumor, heart, and skeletal muscle, indicating the possible association between muscle injury and cross-reactive T cells in tumor and muscles (52).
CK levels do not perfectly reflect disease severity in patients: it is preferable to evaluate myositis via the detection of the severity of muscle weakness, CK levels, and extra-skeletal muscle organ complications (53). ICI treatment should not be discontinued unless severe pain, functional impairment, true muscle weakness, and/or myasthenia features, or cardiac involvement occurs. For severe or refractory patients, hospitalization and treatment are required, including corticoid pulse therapy, intravenous immunoglobulin, biological agents, plasmapheresis, and immunosuppressants.
Vasculitis
ICI-induced vasculitis is rare, the most frequent malignancy with vasculitis in ICI treatment is melanoma (54). Many different symptoms are observed in ICI-induced vasculitis, such as weight loss, fatigue, stomach ache, arthralgias or arthritis, fever, palpable purpura, and myalgias. In a study of anti-PD-1/PD-L1/CTLA-4 administration in cancer patients, 37.74% of them developed vasculitis, including patterns of large vasculitis (isolated aortitis, giant cell arteritis) and vasculitis of the nervous system (isolated vasculitis of the peripheral nervous system, primary angiitis of the central nervous system) (55). Isolated vasculitis can affect the retina and testicles (56, 57). Cutaneous granulomatous (58) and leukocytoclastic vasculitides (59) have also been shown in several studies. In addition, small-vessel vasculitis was also detected in patients receiving ICIs, such as eosinophilic granulomatosis with polyangiitis (60) and cryoglobulinemic vasculitis (61). Serologies rarely are positive, and the diagnosis is lack of autoantibodies in vasculitis. In a recently case with NSCLC, the patient developed acral vasculitis after receiving anti-PD-L1 mAb therapy (62). The laboratory test results revealed a positive perinuclear-ANCA, and negative anti-myeloperoxidase (MPO) and anti-proteinase 3 (PR3) antibodies. ANAs and cold agglutinins were not detected. Antiphospholipid body antibodies to IgG and IgM were negative. The detection of vasculitis only depends on imaging methods in a few cases; biopsy is required to confirm a diagnosis in some patients. Considering systemic vasculitides may result in severe organ damage, treatment of ICI may be discontinued, with the remedy therapy of hydroxychloroquine, glucocorticoid, and plasma exchange (63, 64). It should be noted that acral vasculitis with digital ischemia could be concurrent with cancer (65, 66); discontinuation of ICIs and steroid therapy might distinguish ICI-induced vasculitis and others.
SLE
SLE is not uncommon after ICI treatment (67), and likely involves different underlying mechanisms to other autoimmune diseases. In a study of 4,870 irAE cases after ICI, the median time for the development of SLE among 18 patients was 196 days after initiation of ICI, and the mean age was 61 years (68). SLE often manifests with lupus nephritis, immunoglobin (IgG and IgM) and complement complexes (C3 and C1q) in the mesangium with hypertrophy of podocytes detected in a melanoma patient treated with ipilimumab (69). The patient had increased titers of anti-double-stranded DNA antibodies and normal level of serum complement. Withholding ipilimumab and the administration of prednisolone relieved symptoms of ICI-induced lupus nephritis.
SS
A recent study reported that in 17 cases of SS following ICI treatment, the median age was 63 years, with median time of onset of SS after ICI initiation being 3.8 months (70). Although these percentages are not precise, the incidence of SS in ICI-treated patients has been reported to range from 1.2 to 24.2% in published studies (40, 71). ICIs-induced SS is mainly manifested as xerostomia and parotid enlargement (72). Antinuclear antibodies, anti-SSA/B antibodies, RF, and extractable nuclear antigens are absent in the majority of patients (73, 74). Salivary gland biopsies are helpful to make a diagnosis, with the characteristics of obvious lymphocyte infiltration (CD3+ and CD4+ T cells), epithelial injury, and a significant absence of B cells. Treatment of ICI-induced SS consists of saliva and tear substitutes in most cases; severe symptoms require steroids and cessation of ICI.
ICIs in Patients With Preexisting Rheumatic Disease
Most trials of ICIs exclude tumor patients with autoimmune diseases; therefore, whether the use of ICIs will affect the progression of rheumatic diseases is not completely understood. However, the non-specific upregulation of T cell activation and the inhibition of Treg activity due to ICI treatment could promote inflammation and autoimmunity in patients with preexisting rheumatic diseases (75). In a system review of 123 patients with malignant tumors and rheumatic diseases, it was reported that 92 cases (75%) developed irAEs and/or progression and recurrence of autoimmune disease (76). Several studies have reported that ICI therapy is associated with exacerbation of underlying rheumatic disease such as RA, SLE, psoriatic arthritis, SS, Crohn's disease, polymyalgia rheumatic, and Graves' disease, in 6–43% of patients with preexisting confirmed autoimmune disease; furthermore, ~75% of rheumatic disease patients developed irAEs after the therapy of ICIs (77–80). The exacerbation of rheumatic disease is related to the type of malignant tumors and previous treatment. At present, although preexisting rheumatic diseases are not considered be an absolute contraindication to ICIs, they represent a major obstacle to immunotherapy for patients with malignant tumors. In addition, the progression of rheumatic diseases has been reported frequently in patients with active disease symptoms rather than remission during ICI administration. Therefore, it is important to perform careful assessment before the initiation of ICI therapy because of the potential risk. Even if the risk of disease progression is higher in these patients, there is no reason to exclude them from receiving ICI treatment, especially considering rheumatic disease itself is not life-threatening (28).
Management and Remedy Strategies for Rheumatic irAEs
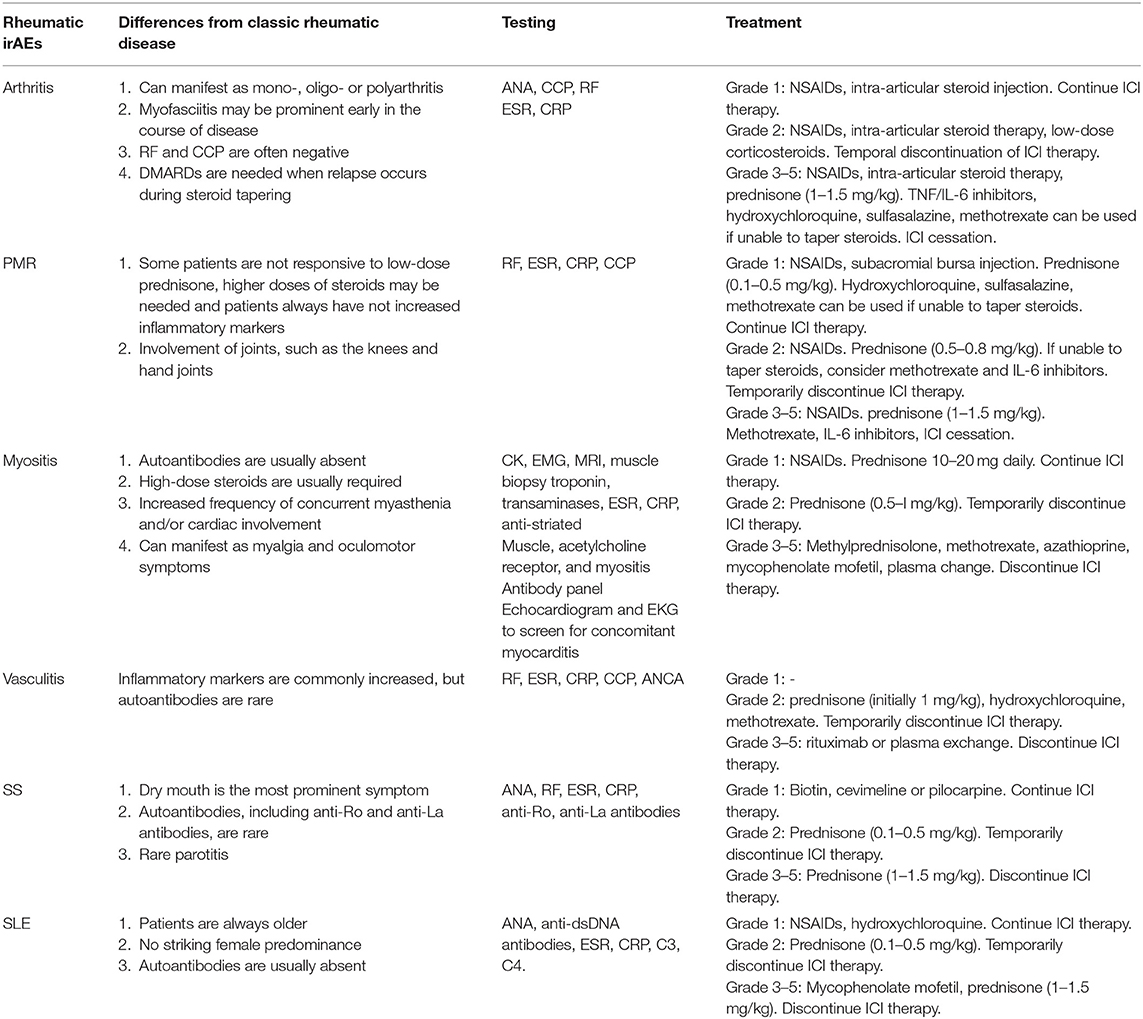
No definitive prospective trials and official guidelines have been conducted and treatment recommendations for rheumatic irAEs are thus lacking. In general, treatment approaches for rheumatic irAEs should be selected after discussion between experts from oncologists and rheumatologists, for optimal therapy (81). It is of the utmost importance to facilitate early diagnosis and treatment in the specific management for rheumatic irAEs in order to prevent permanent damage (82, 83). The general treatment principle suggests managing autoimmune irAEs depending on the severity of symptoms according to the CTCAE system (84) (Table 1). For grade 1, analgesics (such as NSAIDs) or local injections with steroids could be used to alleviate symptoms; systemic corticosteroids are generally not required and treatment with ICIs can continue with close monitoring. In case of grade 2 symptoms, ICI therapy may be temporarily withheld until symptoms are resolved or improve to grade 1 or less. Corticosteroids are generally needed; some of the disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, hydroxychloroquine, and sulfasalazine could also be used to manage irAEs in order to avoid side effects related to corticosteroids. In patients with grade 3 or higher events, ICI therapy should be discontinued, and high doses of steroids could be used with a gradual tapering course when symptoms alleviate to grade 1 or less (85). When steroids are not effective, or patients are unable to taper steroids, biological agents (TNF or IL-6 inhibitors) and DMARDS (such as hydroxychloroquine, methotrexate, and sulfasalazine) could be used as second-line therapy recommendations (86, 87). The early administration of these agents is associated with faster resolution of symptoms and a decreased infection risk compared with high-dose steroids alone (88–90). A retrospective study has suggested patients treated with ipilimumab can safely switch to PD-1 inhibitors if they have serious adverse reactions, and only 3% of people have serious adverse reactions again. This may indicate that a specific pathway, rather than systemic immunity, is involved in the occurrence of immunotoxicity (91). In future studies, rheumatic irAEs could be integrated into the grading system of non-irAEs induced by ICIs to improve management and facilitate decisions regarding decreasing ICIs and discontinuing therapy. Investigation of potential long-term benefits to patients requires more multi-center, large-scale prospective studies (92).
Future Directions
The effects of genetics, epigenetics, pre-existing autoimmune conditions, and immune status (Figure 1) could play an important role in mechanistic understanding and predictive biomarkers. HLA class I homozygosity is related with reduced survival in patients with melanoma, possibly due to a T cell antigenic repertoire and reduced anti-tumor cytotoxicity (93). Early changes in B-cells could identify patients who are at increased risk of irAEs and indicated that strategies targeting B-cells might limit toxicities (19). IL-6 and chemokine CXCL-9 are important for the growth and development of tumors, but it is unclear whether baseline cytokine and chemokine levels are associated with overall cancer survival and the development of irAEs.
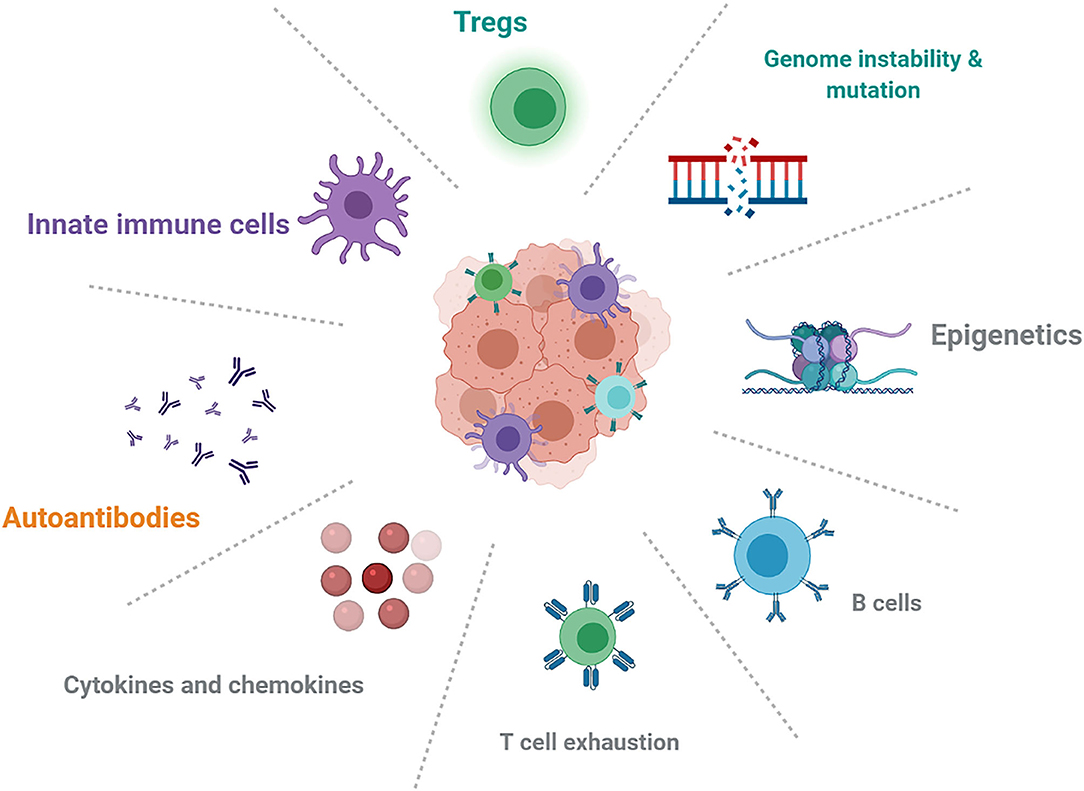
Figure 1. Future directions about the mechanistic understanding and possible biomarkers in autoimmune irAEs induced by ICIs.
Conclusion
While not among the most frequently encountered irAEs, rheumatic complications or diseases induced by ICIs are relatively common and are increasingly recognized, with musculoskeletal manifestations (including arthralgia, arthritis, myalgia, myositis) being the most common, and vasculitis, SS and SLE also detected in several cases. These complications can be clinically severe; therefore, early diagnosis and multidisciplinary management is of critical significance to attenuate pain and functional impairment, maintain immunotherapy, and preserve the quality of life of patients. Prospective large-scale studies will determine the clinical prevalence and characteristics of ICI-induced syndromes, find more predictive biomarkers, increase knowledge regarding diagnosis and management for physicians, and develop relevant therapeutic guidelines.
Author Contributions
PS wrote the first draft of the manuscript. All authors participated in manuscript revision and have approved the submitted version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Figure 1 was created with BioRender.com.
References
1. Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: a review. Scand J Public Health. (2018) 46:27–36. doi: 10.1177/1403494817715400
2. Singh S, Hassan D, Aldawsari HM, Molugulu N, Shukla R, Kesharwani P. Immune checkpoint inhibitors: a promising anticancer therapy. Drug Discov Today. (2020) 25:223–9. doi: 10.1016/j.drudis.2019.11.003
3. Wilson RAM, Evans TRJ, Fraser AR, Nibbs RJB. Immune checkpoint inhibitors: new strategies to checkmate cancer. Clin Exp Immunol. (2018) 191:133–48. doi: 10.1111/cei.13081
4. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
5. Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. (2016) 24:3669–76. doi: 10.1007/s00520-016-3297-9
6. Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. (2018) 17:610–6. doi: 10.1016/j.autrev.2018.01.010
7. Ghosh N, Bass AR. Rheumatic complications of immune checkpoint inhibitors. Med Clin North Am. (2021) 105:227–45. doi: 10.1016/j.mcna.2020.09.009
8. Chan KK, Bass AR. Autoimmune complications of immunotherapy: pathophysiology and management. BMJ. (2020) 369:m736. doi: 10.1136/bmj.m736
9. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. doi: 10.1056/NEJMoa1504030
10. Olde Nordkamp MJ, Koeleman BP, Meyaard L. Do inhibitory immune receptors play a role in the etiology of autoimmune disease? Clin Immunol. (2014) 150:31–42. doi: 10.1016/j.clim.2013.11.007
11. Lo B, Fritz JM, Su HC, Uzel G, Jordan MB, Lenardo MJ. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood. (2016) 128:1037–42. doi: 10.1182/blood-2016-04-712612
12. Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. (2014) 41:1026–39. doi: 10.1016/j.immuni.2014.12.005
13. Belkhir R, Burel S L, Dunogeant L, Marabelle A, Hollebecque A, Besse B, et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis. (2017) 76:1747–50. doi: 10.1136/annrheumdis-2017-211216
14. McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. (2015) 523:612–6. doi: 10.1038/nature14468
15. June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med. (2017) 23:540–7. doi: 10.1038/nm.4321
16. Manson G, Norwood J, Marabelle A, Kohrt H, Houot R. Biomarkers associated with checkpoint inhibitors. Ann Oncol. (2016) 27:1199–206. doi: 10.1093/annonc/mdw181
17. Esfahani K, Miller WH Jr. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med. (2017) 376:1989–91. doi: 10.1056/NEJMc1703047
18. Cappelli LC, Gutierrez AK, Bingham III CO, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res (Hoboken). (2017) 69:1751–63. doi: 10.1002/acr.23177
19. Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest. (2018) 128:715–20. doi: 10.1172/JCI96798
20. Chatzidionysiou K, Liapi M, Tsakonas G, Gunnarsson I, Catrina A. Treatment of rheumatic immune-related adverse events due to cancer immunotherapy with immune checkpoint inhibitors-is it time for a paradigm shift? Clin Rheumatol. (2021) 40:1687–95. doi: 10.1007/s10067-020-05420-w
21. Cappelli LC, Shah AA, Bingham III CO. Immune-related adverse effects of cancer immunotherapy- implications for rheumatology. Rheum Dis Clin North Am. (2017) 43:65–78. doi: 10.1016/j.rdc.2016.09.007
22. Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. (2011) 197:W992–1000. doi: 10.2214/AJR.10.6198
23. Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. (2019) 145:639–48. doi: 10.1002/ijc.32132
24. Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. (2018) 77:393–8. doi: 10.1136/annrheumdis-2017-212257
25. Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum. (2018) 48:553–7. doi: 10.1016/j.semarthrit.2018.02.011
26. Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. (2017) 76:43–50. doi: 10.1136/annrheumdis-2016-209595
27. Ruiz-Bañobre J, Pérez-Pampín E, García-González J, Gómez-Caamaño A, Barón-Duarte FJ, López-López R, et al. Development of psoriatic arthritis during nivolumab therapy for metastatic non-small cell lung cancer, clinical outcome analysis and review of the literature. Lung Cancer. (2017) 108:217–21. doi: 10.1016/j.lungcan.2017.04.007
28. Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis. (2021) 80:36–48. doi: 10.1136/annrheumdis-2020-217139
29. Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheumatol. (2019) 71:468–75. doi: 10.1002/art.40745
30. Leipe J, Christ LA, Arnoldi AP, Mille E, Berger F, Heppt M, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open. (2018) 4:e000714. doi: 10.1136/rmdopen-2018-000714
31. Narayan N, Owen DR, Taylor PC. Advances in positron emission tomography for the imaging of rheumatoid arthritis. Rheumatology (Oxford). (2017) 56:1837–46. doi: 10.1093/rheumatology/kew484
32. Chan MM, Kefford RF, Carlino M, Clements A, Manolios N. Arthritis and tenosynovitis associated with the anti-PD1 antibody pembrolizumab in metastatic melanoma. J Immunother. (2015) 38:37–9. doi: 10.1097/CJI.0000000000000060
33. Subedi A, Williams SG, Yao L, Maharjan S, Strauss J, Sharon E, et al. Use of magnetic resonance imaging to identify immune checkpoint inhibitor-induced inflammatory arthritis. JAMA Netw Open. (2020) 3:e200032. doi: 10.1001/jamanetworkopen.2020.0032
34. Albayda J, Bingham III CO, Shah AA, Kelly RJ, Cappelli L. Metastatic joint involvement or inflammatory arthritis? a conundrum with immune checkpoint inhibitor-related adverse events. Rheumatology (Oxford). (2018) 57:760–2. doi: 10.1093/rheumatology/kex470
35. Pundole X, Abdel-Wahab N, Suarez-Almazor ME. Arthritis risk with immune checkpoint inhibitor therapy for cancer. Curr Opin Rheumatol. (2019) 31:293–9. doi: 10.1097/BOR.0000000000000601
36. Bernier M, Guillaume C, Leon N, Alexandre J, Hamel-Senecal L, Chretien B, et al. Nivolumab causing a polymyalgia rheumatica in a patient with a squamous non-small cell lung cancer. J Immunother. (2017) 40:129–31. doi: 10.1097/CJI.0000000000000163
37. Garel B, Kramkimel N, Trouvin AP, Frantz C, Dupin N. Pembrolizumab-induced polymyalgia rheumatica in two patients with metastatic melanoma. Joint Bone Spine. (2017) 84:233–4. doi: 10.1016/j.jbspin.2016.01.007
38. Gauci ML, Baroudjian B, Laly P, Madelaine I, Da Meda L, Vercellino L, et al. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome induced by nivolumab. Semin Arthritis Rheum. (2017) 47:281–7. doi: 10.1016/j.semarthrit.2017.03.003
39. Mooradian MJ, Nasrallah M, Gainor JF, Reynolds KL, Cohen JV, Lawrence DP, et al. Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: a single center experience. Semin Arthritis Rheum. (2019) 48:1127–32. doi: 10.1016/j.semarthrit.2018.10.012
40. Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open. (2019) 5:e000906. doi: 10.1136/rmdopen-2019-000906
41. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
42. Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. (2015) 21:712–20. doi: 10.1158/1078-0432.CCR-14-2468
43. Wong VT, So H, Lam TT, Yip RM. Myositis-specific autoantibodies and their clinical associations in idiopathic inflammatory myopathies. Acta Neurol Scand. (2021) 143:131–9. doi: 10.1111/ane.13331
44. Seki M, Uruha A, Ohnuki Y, Kamada S, Noda T, Onda A, et al. Inflammatory myopathy associated with PD-1 inhibitors. J Autoimmun. (2019) 100:105–13. doi: 10.1016/j.jaut.2019.03.005
45. Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin Arthritis Rheum. (2019) 48:736–40. doi: 10.1016/j.semarthrit.2018.05.006
46. Suzuki S, Ishikawa N, Konoeda F, Seki N, Fukushima S, Takahashi K, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. (2017) 89:1127–34. doi: 10.1212/WNL.0000000000004359
47. Yanase T, Moritoki Y, Kondo H, Ueyama D, Akita H, Yasui T. Myocarditis and myasthenia gravis by combined nivolumab and ipilimumab immunotherapy for renal cell carcinoma: a case report of successful management. Urol Case Rep. (2021) 34:101508. doi: 10.1016/j.eucr.2020.101508
48. Matas-García A, Milisenda JC, Selva-O'Callaghan A, Prieto-González S, Padrosa J, Cabrera C, et al. Emerging PD-1 and PD-1L inhibitors-associated myopathy with a characteristic histopathological pattern. Autoimmun Rev. (2020) 19:102455. doi: 10.1016/j.autrev.2019.102455
49. Ma R, Wang Q, Meng D, Li K, Zhang Y. Immune checkpoint inhibitors-related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems. BMC Cancer. (2021) 21:38. doi: 10.1186/s12885-020-07741-0
50. Shibata C, Kato J, Toda N, Imai M, Fukumura Y, Arai J, et al. Paraneoplastic dermatomyositis appearing after nivolumab therapy for gastric cancer: a case report. J Med Case Rep. (2019) 13:168. doi: 10.1186/s13256-019-2105-9
51. Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Auré K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. (2018) 91:e985–94. doi: 10.1212/WNL.0000000000006124
52. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214
53. Stelmachowska-Banaś M, Czajka-Oraniec I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: an updated review. Endocr Connect. (2020) 9:R207–28. doi: 10.1530/EC-20-0342
54. Melissaropoulos K, Klavdianou K, Filippopoulou A, Kalofonou F, Kalofonos H, Daoussis D. Rheumatic manifestations in patients treated with immune checkpoint inhibitors. Int J Mol Sci. (2020) 21:3389. doi: 10.3390/ijms21093389
55. Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. (2018) 37:2579–84. doi: 10.1007/s10067-018-4177-0
56. Tsui E, Gonzales JA. Retinal vasculitis associated with ipilimumab. Ocul Immunol Inflamm. (2020) 28:868–70. doi: 10.1080/09273948.2019.1610460
57. Weiner R, Hanson B, Rehman J, Sun B. Isolated testicular vasculitis due to immune checkpoint inhibitor. Eur J Rheumatol. (2020) 7:35–6. doi: 10.5152/eurjrheum.2019.19061
58. Singh P, Wolfe SP, Alloo A, Gottesman SP. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. (2020) 47:65–9. doi: 10.1111/cup.13562
59. Tomelleri A, Campochiaro C, De Luca G, Cavalli G, Dagna L. Anti-PD1 therapy-associated cutaneous leucocytoclastic vasculitis: a case series. Eur J Intern Med. (2018) 57:e11–12. doi: 10.1016/j.ejim.2018.07.023
60. Roger A, Groh M, Lorillon G, Le Pendu C, Maillet J, Arangalage D, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) induced by immune checkpoint inhibitors. Ann Rheum Dis. (2019) 78:e82. doi: 10.1136/annrheumdis-2018-213857
61. Le Burel S, Champiat S, Routier E, Aspeslagh S, Albiges L, Szwebel TA, et al. Onset of connective tissue disease following anti-PD1/PD-L1 cancer immunotherapy. Ann Rheum Dis. (2018) 77:468–70. doi: 10.1136/annrheumdis-2016-210820
62. Gupta S, Xu D, Hadfield J, Prentice D. Durvalumab-associated vasculitis presenting as 'the blue toe syndrome'. BMJ Case Rep. (2020) 13:e235886. doi: 10.1136/bcr-2020-235886
63. Kang A, Yuen M, Lee DJ. Nivolumab-induced systemic vasculitis. JAAD Case Rep. (2018) 4:606–8. doi: 10.1016/j.jdcr.2018.03.013
64. Bui AN, Hirner J, Singer SB, Eberly-Puleo A, Larocca C, Lian CG, et al. De novo subacute cutaneous lupus erythematosus-like eruptions in the setting of programmed death-1 or programmed death ligand-1 inhibitor therapy: clinicopathological correlation. Clin Exp Dermatol. (2021) 46:328–37. doi: 10.1111/ced.14449
65. Gambichler T, Strutzmann S, Tannapfel A, Susok L. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. (2017) 17:327. doi: 10.1186/s12885-017-3313-6
66. Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. (2019) 19:449. doi: 10.1186/s12885-019-5661-x
67. Arnaud L, Lebrun-Vignes B, Salem JE. Checkpoint inhibitor-associated immune arthritis. Ann Rheum Dis. (2019) 78:e68. doi: 10.1136/annrheumdis-2018-213470
68. Raschi E, Antonazzo IC, Poluzzi E, De Ponti F. Drug-induced systemic lupus erythematosus: should immune checkpoint inhibitors be added to the evolving list? Ann Rheum Dis. (2021) 80:e120. doi: 10.1136/annrheumdis-2019-215819
69. Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. (2009) 361:211–2. doi: 10.1056/NEJMc0904283
70. Abdel-Wahab N, Suarez-Almazor ME. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology (Oxford). (2019) 58:vii40–8. doi: 10.1093/rheumatology/kez297
71. Abdel-Rahman O, Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. (2017) 17:387–94. doi: 10.1080/14737140.2017.1296765
72. Narváez J, Juarez-López P JLL, Narváez JA, Palmero R, García Del Muro X, et al. Rheumatic immune-related adverse events in patients on anti-PD-1 inhibitors: fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev. (2018) 17:1040–5. doi: 10.1016/j.autrev.2018.05.002
73. Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist. (2019) 24:1259–69. doi: 10.1634/theoncologist.2018-0823
74. Ortiz Brugués A, Sibaud V, Herbault-Barrés B, Betrian S, Korakis I, De Bataille C, et al. Sicca syndrome induced by immune checkpoint inhibitor therapy: optimal management still pending. Oncologist. (2020) 25:e391–5. doi: 10.1634/theoncologist.2019-0467
75. Ohnuma K, Hatano R, Dang NH, Morimoto C. Rheumatic diseases associated with immune checkpoint inhibitors in cancer immunotherapy. Mod Rheumatol. (2019) 29:721–32. doi: 10.1080/14397595.2018.1532559
76. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann Intern Med. (2018) 169:133–4. doi: 10.7326/L18-0209
77. Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. (2016) 2:234–40. doi: 10.1001/jamaoncol.2015.4368
78. Gutzmer R, Koop A, Meier F, Hassel JC, Terheyden P, Zimmer L, et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer. (2017) 75:24–32. doi: 10.1016/j.ejca.2016.12.038
79. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. (2017) 28:368–76. doi: 10.1093/annonc/mdw443
80. Richter MD, Pinkston O, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Brief report: cancer immunotherapy in patients with preexisting rheumatic disease: the mayo clinic experience. Arthritis Rheumatol. (2018) 70:356–60. doi: 10.1002/art.40397
81. Gediz F, Kobak S. Immune checkpoint inhibitors-related rheumatic diseases: what rheumatologist should know? Curr Rheumatol Rev. (2019) 15:201–8. doi: 10.2174/1573397115666190119094736
82. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–42. doi: 10.1093/annonc/mdx225
83. Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. (2018) 14:569–79. doi: 10.1038/s41584-018-0074-9
84. Puzanov I, Diab A, Abdallah K, Bingham III CO Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. (2017) 5:95. doi: 10.1186/s40425-017-0300-z
85. Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. (2021) 53:339–54. doi: 10.4143/crt.2020.790
86. Abu Samra K, Valdes-Navarro M, Lee S, Swan R, Foster CS, Anesi SD. A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur J Ophthalmol. (2016) 26:e46–8. doi: 10.5301/ejo.5000724
87. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) Induced by immune checkpoint inhibitor therapy. Front Pharmacol. (2017) 8:49. doi: 10.3389/fphar.2017.00049
88. Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett Jr RL, Abdel-Wahab N, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. (2018) 6:103. doi: 10.1186/s40425-018-0412-0
89. Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. (2019) 7:93. doi: 10.1186/s40425-019-0577-1
90. Leipe J, Mariette X. Management of rheumatic complications of ICI therapy: a rheumatology viewpoint. Rheumatology (Oxford). (2019) 58:vii49–58. doi: 10.1093/rheumatology/kez360
91. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
92. Londoño MC, Reig M. Multidisciplinary clinical approach to cancer patients with immune-related adverse events induced by checkpoint inhibitors. Cancers (Basel). (2020) 12:3446. doi: 10.3390/cancers12113446
Keywords: rheumatic diseases, immune-related adverse events, immune checkpoint inhibitors, arthritis, myositis, vasculitis
Citation: Shen P, Deng X, Hu Z, Chen Z, Huang Y, Wang K, Qin K, Huang Y, Ba X, Yan J, Han L and Tu S (2021) Rheumatic Manifestations and Diseases From Immune Checkpoint Inhibitors in Cancer Immunotherapy. Front. Med. 8:762247. doi: 10.3389/fmed.2021.762247
Received: 10 September 2021; Accepted: 13 October 2021;
Published: 04 November 2021.
Edited by:
Chris Wincup, University College London, United KingdomReviewed by:
Polona Žigon, University Medical Centre Ljubljana, SloveniaChengappa G. Kavadichanda, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), India
Chun Li, Peking University People‘s Hospital, China
Copyright © 2021 Shen, Deng, Hu, Chen, Huang, Wang, Qin, Huang, Ba, Yan, Han and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenghao Tu, c2h0dUB0amgudGptdS5lZHUuY24=
†These authors have contributed equally to this work
 Pan Shen
Pan Shen Xuan Deng2†
Xuan Deng2† Zhishuo Hu
Zhishuo Hu Zhe Chen
Zhe Chen Yao Huang
Yao Huang Kai Qin
Kai Qin Ying Huang
Ying Huang Shenghao Tu
Shenghao Tu