- Department of Anesthesia, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Background: Shivering is a common complication after subarachnoid administration of local anesthetics. Intravenous ketamine and tramadol are widely available anti-shivering drugs, especially in developing settings. This meta-analysis aimed to compare the effects of intravenous ketamine vs. tramadol for post-spinal anesthesia shivering.
Materials and methods: PubMed/MEDLINE, Web of Science, Cochrane Library, Embase, and Google Scholar databases were used to search for relevant articles for this study. Mean difference (MD) with 95% confidence interval (CI) was used to analyze continuous outcomes, and risk ratio (RR) with 95% CI to analyze categorical results. The heterogeneity of the included studies was assessed using the I2 test. We utilized Review Manager 5.4.1 to perform statistical analysis.
Results: Thirteen studies involving 1,532 patients were included in this meta-analysis. Ketamine had comparable effects in preventing post-spinal anesthetics shivering [RR = 1.06; 95% CI (0.94, 1.20), P = 0.33, I2 = 77], and onset of shivering [MD = −0.10; 95%CI (– 2.68, 2.48), P = 0.94, I2 = 0%], lower incidences of nausea and vomiting [RR = 0.51; 95%CI (0.26, 0.99), P = 0.05, I2 = 67%], and lower incidences of bradycardia [RR = 0.16; 95%CI (0.05, 0.47), P = 0.001, I2 = 33%], higher incidence of hallucinations [RR = 12; 95%CI (1.58, 91.40), P = 0.02, I2 = 0%], and comparable effects regarding the incidences of hypotension [RR = 0.60; 95%CI (0.30, 1.21), P = 0.15, I2 = 54%] as compared to tramadol.
Conclusions: Intravenous ketamine and tramadol are comparable in the prevention of post-spinal anesthetic shivering. Ketamine had a better outcome with less occurrences of nausea, vomiting, and bradycardia. However, ketamine was associated with higher incidences of hallucinations than tramadol.
Introduction
Shivering is defined as an involuntary, repetitive activity of skeletal muscles to raise the core body temperature (1–5). Spinal anesthesia is known to decrease the shivering threshold, preceded by core hypothermia and vasoconstriction above the level of the block (6). The review of 21 studies reported that the median incidence of shivering related to neuraxial anesthesia was 55% in ranges of 40% to 64% (7).
Shivering may have beneficial thermoregulatory effects; however, it is a distressing experience and causes several undesirable detrimental effects (8). It leads to an increase in oxygen consumption and carbon dioxide production, intraocular and intracranial pressure (9–11). It may also lead to an increase in sympathetic tone that enhances the chances of myocardial ischemia (12, 13), pain (14), and bleeding (15). Shivering may impede monitoring techniques (non-invasive blood pressure, electrocardiogram, and pulse oximetry) (16–19).
A variety of pharmacologic and non-pharmacologic techniques for the prevention and treatment of shivering have been used; however, there is no globally accepted preferred technique for the treatment or prevention of post-spinal anesthetic shivering (7). Ketamine acts as a competitive N-methyl-D-aspartic acid receptor antagonist and can control post-spinal anesthetic shivering; In addition, it may decrease core-to-peripheral redistribution of heat by direct central sympathetic stimulation and by blocking inhibition of norepinephrine uptake into postganglionic sympathetic nerve endings, and it has a κ-opioid agonist property (17, 20–23). Tramadol has a μ-opioid agonist effect with minimum effect at kappa and delta receptors. Tramadol inhibits the re-uptake of serotonin and norepinephrine at the spinal cord level, which increases 5-hydroxytryptamine production. These actions of the drug make it effective in preventing and controlling post-spinal anesthetic shivering (24–27).
Intravenous tramadol and ketamine are widely available and cheap drugs, especially in the low resource settings. However, there is no high-quality data (meta-analysis) or large-sized randomized controlled trials on the relative efficacy and safety (anti-shivering agent with lesser side effects) of intravenous ketamine vs. tramadol. Hence, this meta-analysis aimed to compare the effects of intravenous tramadol vs. ketamine in preventing shivering after spinal anesthesia and associated side effects.
Materials and methods
This study is reported as per Preferred Reporting Items for Systematic and Meta-analysis. Thirteen randomized controlled trials with a total of 1,532 patients were included. This meta-analysis was registered in Prospero with registration number CRD42022342030 on July 5, 2022.
Search strategy
PubMed/MEDLINE, Web of Science, Cochrane Library, Embase, and Google Scholar databases were used for searching relevant articles. The terms used for searching were “Ketamine,” “Tramadol,” “Spinal Anesthesia,” and “Shivering” through June 2022.
Inclusion criteria
Patients undergoing surgery under spinal anesthesia; studies that compare intravenous ketamine with intravenous tramadol on shivering; the incidence of side effects reported in both tramadol and ketamine groups; and randomized controlled trials were included.
Data extraction
The titles and abstracts of all articles were reviewed by two authors. Studies that are deemed to fall outside the inclusion criteria were excluded. Full paper copies of the remaining studies were reviewed by two authors (EF and DT) independently, and decisions made regarding selection/rejection. Any disagreements arising were resolved by a third reviewer (TT). The authors' name, publication year, characteristics of study participants, sample size, type of surgery, the dose and type of drug used for spinal anesthesia, the anti-shivering dose of intravenous ketamine and intravenous tramadol, and the outcomes of each included study were extracted.
Evaluation of the risk of bias (quality) assessment
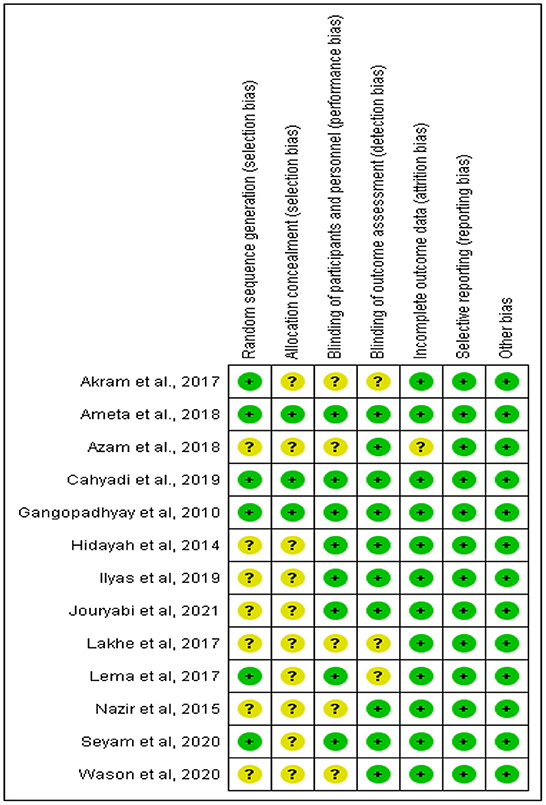
The risk of bias was assessed using the Cochrane risk of bias tool and graded as low, unclear, or high risk of bias by two researchers independently. The included articles were rated according to random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. The disagreements between the researchers arising were resolved by a third reviewer.
Statistical analysis
We performed a meta-analysis of the effects of intravenous tramadol vs. ketamine in preventing post-spinal anesthetic shivering.
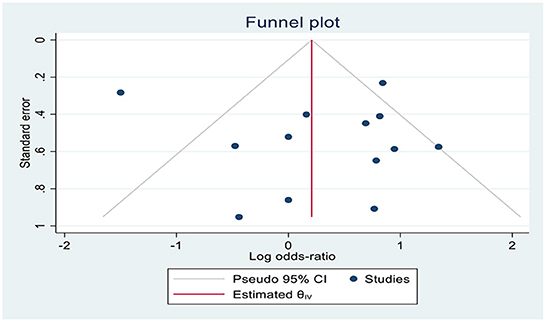
The Review Manager 5.4.1 (Cochrane Library, Oxford, UK) was used for this meta-analysis (Figure 1). The effective rate of shivering, the incidence rate of nausea and vomiting, hypotension, bradycardia, and hallucination were expressed in risk ratio (RR) with a 95% confidence interval (CI); and the onset of shivering in minutes was expressed in mean difference (MD) with 95% confidence interval (CI). If the I2 was >50% or <50%, a fixed-effect model and a random-effect model, respectively, were utilized. The symmetry of the funnel plot showed that there was no publication bias.
Results
Characteristics of the included studies
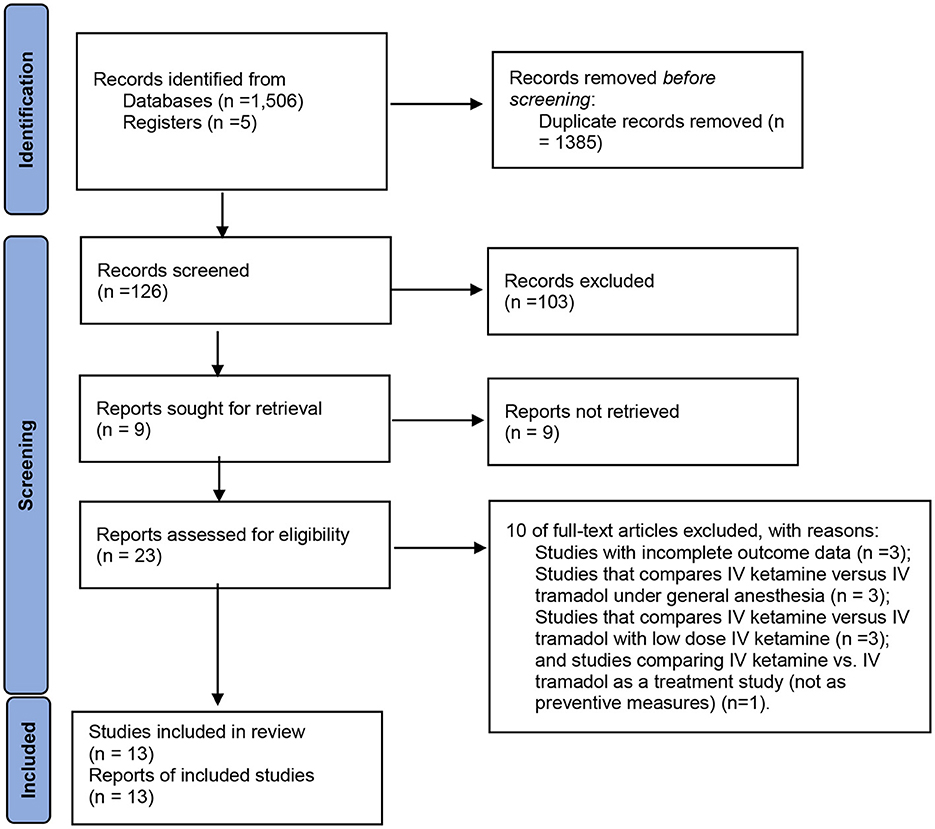
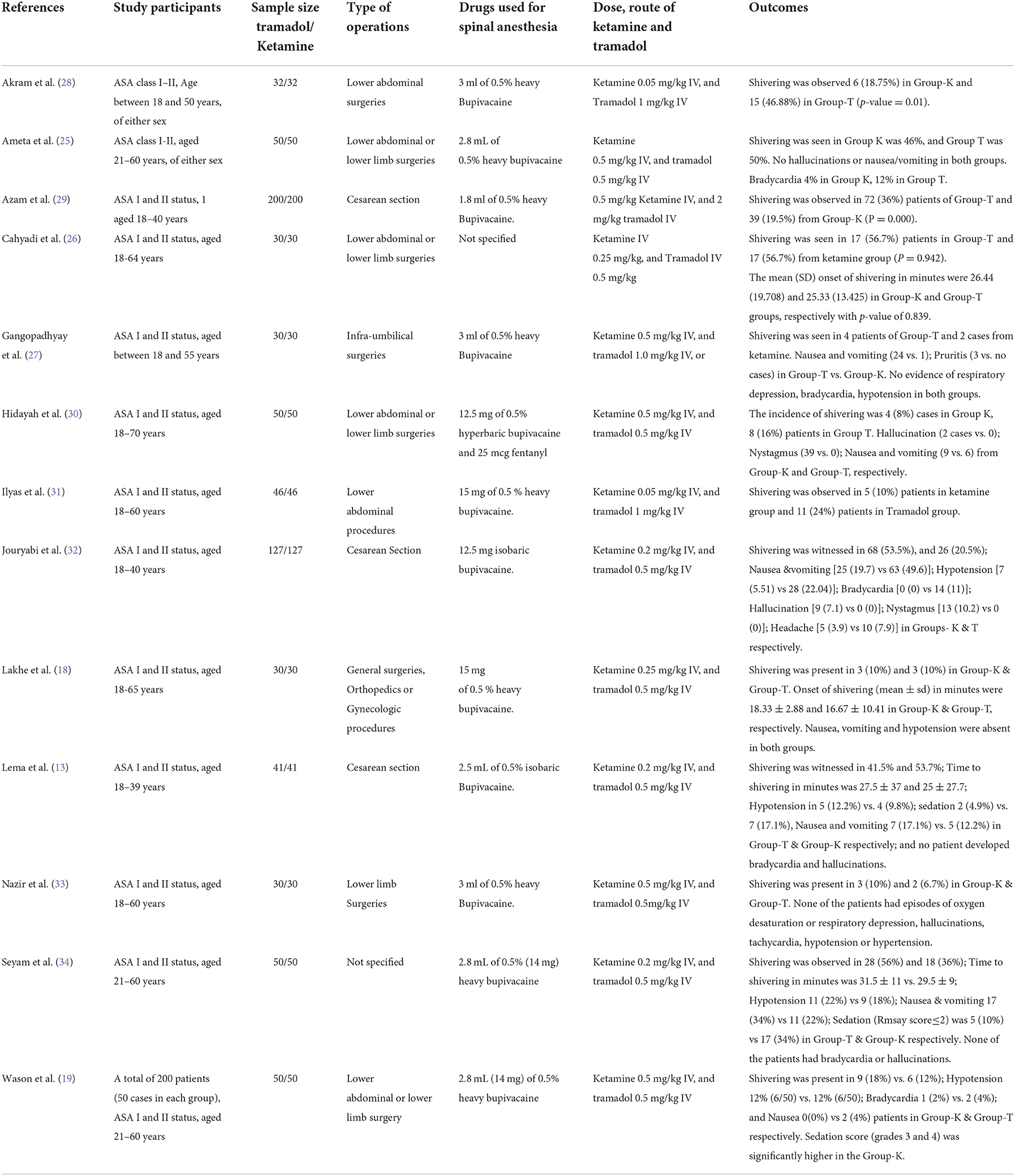
Figure 2 demonstrates the flow chart of this meta-analysis. Thirteen RCTs (13, 18, 19, 25–34) were included in this meta-analysis, having 1,532 patients (Table 1). Eight trials (13, 26, 28–31, 33, 34) compared ketamine with tramadol; three trials (18, 32) compared ketamine with tramadol and ondansetron, clonidine (19), pethidine (27), or dexmedetomidine (25).
In six RCTs (18, 19, 25–27, 30), patients underwent lower abdominal and lower limb surgeries; patients in 2 trials (28, 31) underwent lower abdominal surgeries; patients in 3 trials (13, 29, 32) underwent cesarean section, and patients in a single trial (33) underwent lower limb surgery. However, a single trial (34) did not report the specific type of surgery.
Regarding the dose of bupivacaine used for spinal anesthesia, five trials (18, 27, 28, 31, 33) administered 15 mg of heavy bupivacaine, three studies (19, 25, 34) used 14 mg of heavy bupivacaine, and three studies (13, 30, 32) administered 12.5 mg of heavy bupivacaine, and a single trial (29) administered 9 mg of heavy bupivacaine. However, one trial (26) did not report the dose of local anesthetics used for spinal anesthesia.
The effect of ketamine vs. tramadol on the prevention of shivering
Thirteen RCTs (13, 18, 19, 25–34) reported the effective rate of shivering control. The random effects model was utilized because the value of I2 was >50%. The effective rate of shivering control was comparable between groups (RR =1.06; 95% CI [0.94, 1.20], P = 0.33, I2 = 77%) (Figure 3). Sensitivity analysis was executed for the effective rate of shivering control by excluding a single study consecutively but with no source of heterogeneity detected and publication bias detected (Figure 4).
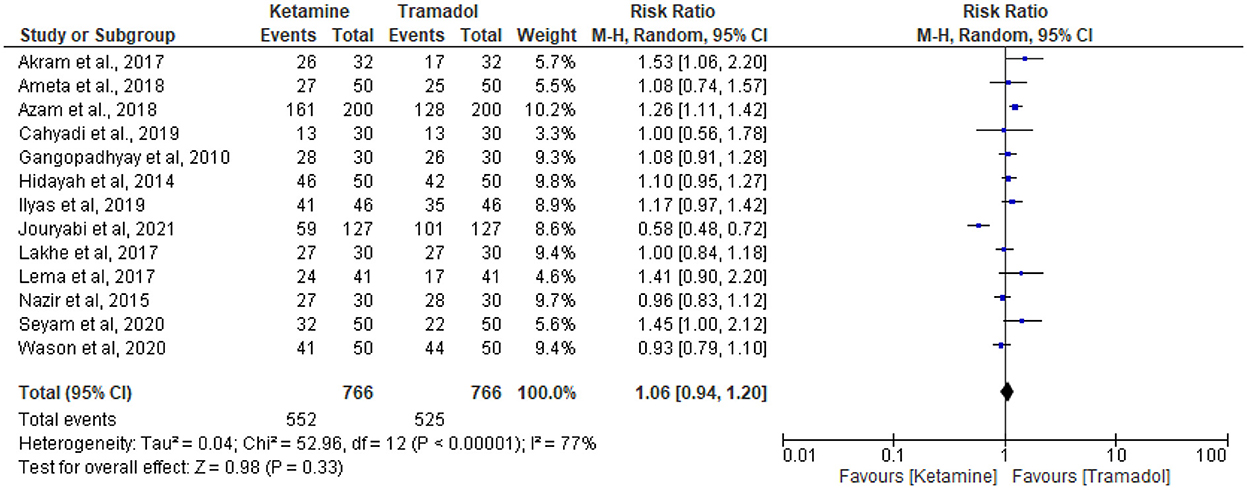
Figure 3. The effect of ketamine vs. tramadol on the prevention of shivering following spinal anesthesia.
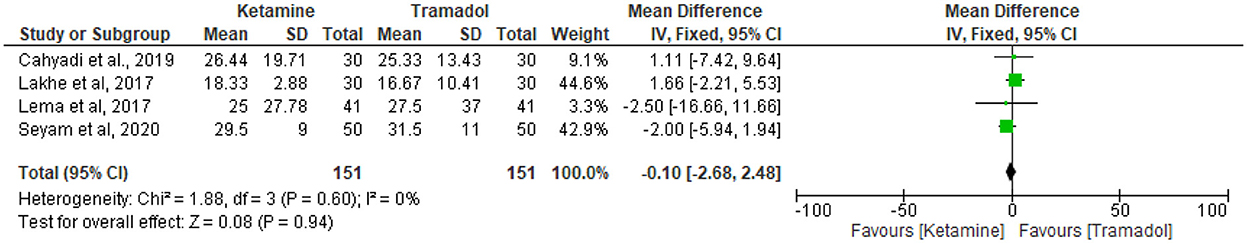
The effect of tramadol vs. ketamine on onset of shivering
Four RCTs (13, 18, 26, 34) compared time to onset of shivering of ketamine vs. tramadol. Since there was no heterogeneity detected (I2 = 0%), the fixed effect model was utilized. The result showed that there was no significant differences regarding time to the onset of shivering time in minutes (MD = −0.10; 95%CI [– 2.68, 2.48], P = 0.94, I2 = 0%) (Figure 5).
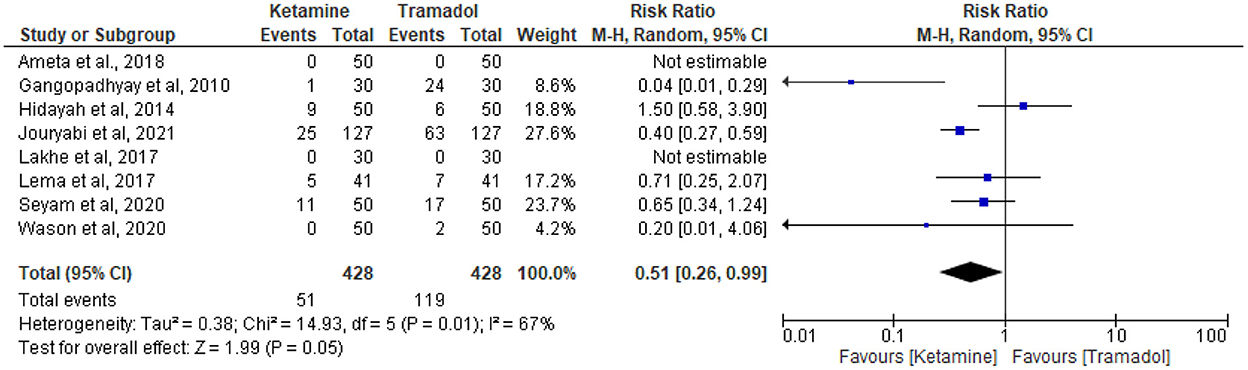
The effect of tramadol vs. ketamine on the incidence of nausea and vomiting
Eight articles reported the incidence of nausea and vomiting (13, 18, 19, 25, 27, 30, 32, 34). Fifty-one patients receiving intravenous ketamine and 119 patients receiving intravenous tramadol experienced nausea and vomiting out of 428 patients in each group. Ketamine had lower incidences of nausea and vomiting than tramadol (RR = 0.51; 95%CI [0.26, 0.99], P = 0.05, I2 = 67%) (Figure 6).
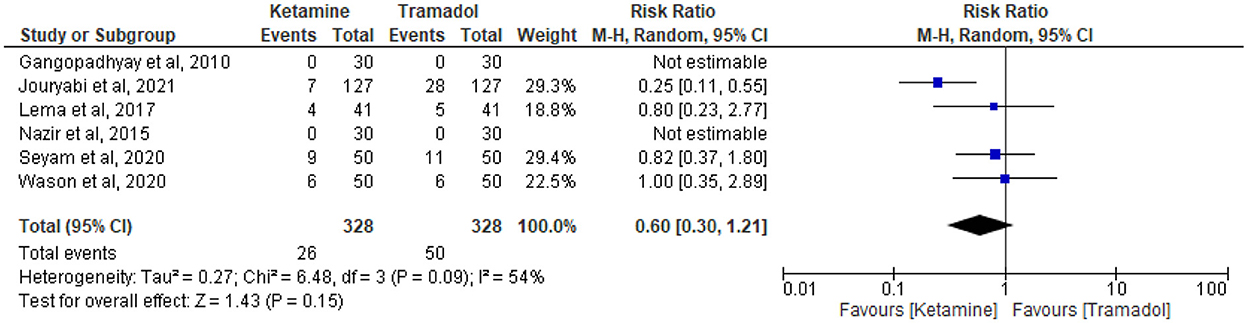
The effect of tramadol vs. ketamine on the incidence of hypotension
The incidence of hypotension was reported in six trials (13, 19, 27, 32–34). Twenty-six patients receiving ketamine and 50 patients receiving tramadol experienced hypotension out of 328 patients in each group. Tramadol had comparable results with ketamine regarding the incidence of hypotension (RR = 0.60; 95%CI [0.30, 1.21], P = 0.15, I2 = 54%) (Figure 7).
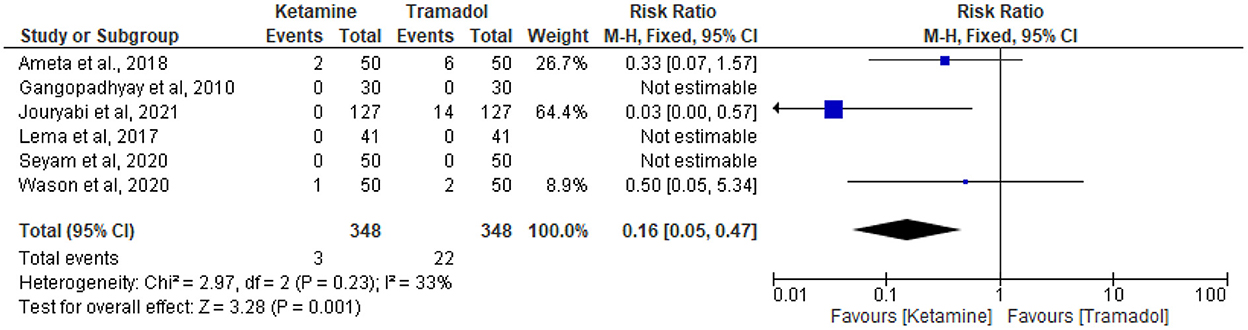
The effect of tramadol vs. ketamine on the incidence of bradycardia
The incidence of bradycardia was reported in six trials (13, 19, 25, 27, 32, 34). Three patients receiving ketamine and 22 patients receiving tramadol experienced bradycardia out of 348 patients in each group. Tramadol was associated with higher incidence of bradycardia (RR = 0.16; 95%CI [0.05, 0.47], P = 0.001, I2 = 33%) (Figure 8).
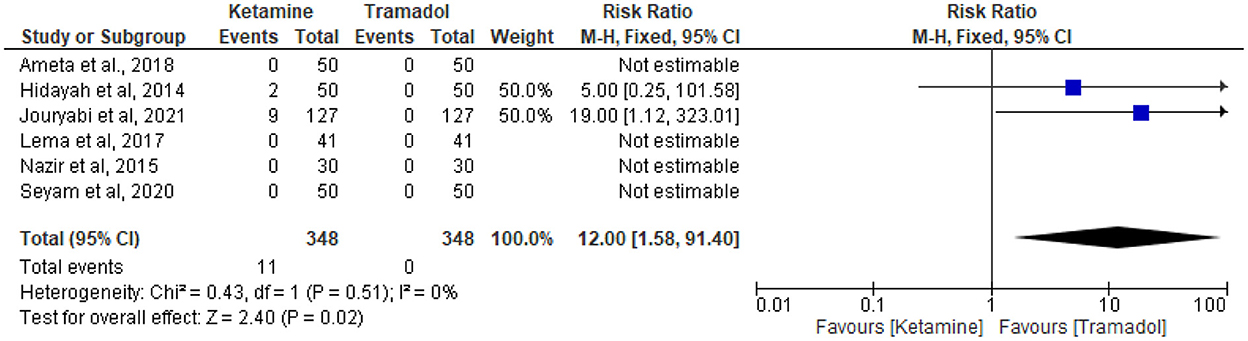
The effect of tramadol vs. ketamine on the incidence of hallucinations
The incidence of hallucination was reported in six RCTs (13, 25, 30, 32–34). Eleven patients in the ketamine group and no patient in the tramadol group experienced hallucination in a total of 348 patients in each group. Ketamine was associated with higher incidence of hallucinations (RR = 12; 95%CI [1.58, 91.40], P = 0.02, I2 = 0%) (Figure 9).
Discussion
Post-spinal anesthetic shivering is a common complication following subarachnoid administration of local anesthetics which results in repression of thermoregulatory mechanism for hypothermia (14, 34). In this meta-analysis, we compared the effect of intravenous ketamine and tramadol on the prevention of post-spinal anesthetics shivering.
In this meta-analysis, ketamine had comparable effects to tramadol in preventing post-spinal anesthetic shivering with a P-value of 0.51; and there were no significant differences regarding the onset of shivering with a P-value of 0.94. Tramadol inhibits the reuptake of serotonin and noradrenaline in the spinal cord and also has an effect on alpha-2 adrenergic and opioid receptors that might have anti-shivering effects (35–37). Ketamine is a competitive NMDA (N-Methyl D-Aspartate) receptor antagonist that inhibits noradrenergic and serotonergic neurons that might result in anti-shivering effects. Intravenous administration of ketamine and tramadol can be used for preventing post-spinal anesthetic shivering (19, 33, 34, 38).
The current study included a pooled analysis of the incidences of adverse events (nausea and vomiting, bradycardia, hypotension, and hallucinations) after the administration of anti-shivering agents. Ketamine showed a better outcome with less occurrences of nausea and vomiting (P = 0.03) and bradycardia (P = 0.001). Ketamine can cause a dose-dependent direct stimulation of the central nervous system that leads to an activation of the sympathetic nervous system and sustains heart rate (39). Ketamine was associated with a higher incidence of hallucinations (P = 0.02), and this might be due to its effect on glutamatergic signaling in psychosis that results in hallucination (40, 41). Intravenous ketamine had a comparable incidence of hypotension with intravenous tramadol with a P-value of 0.15. Research included in this meta-analysis (13, 19, 34) reported that ketamine was related with higher sedation scores than tramadol, despite the fact that we have not performed a pooled analysis due to variances in sedation scales employed in the included studies. Ketamine may be a more effective anti-shivering medicine in this context than tramadol because of the higher sedation scores in the ketamine group, which may be crucial in maintaining optimal surgical circumstances and decreasing patient pain following spinal anesthesia. However, because there are variances in the types and dosages of local anesthetics used for spinal anesthesia as well as the types and durations of the procedures carried out in the included RCTs, the findings may be highly heterogeneous.
The main limitation of this meta-analysis might be the relatively inadequate sample size to make generalizations, and therefore further studies should be conducted. The other limitation of this meta-analysis could be the heterogeneity of the scales used for shivering to run a pooled analysis.
Conclusions
Intravenous ketamine and tramadol are comparable in the prevention of post-spinal anesthetic shivering. Ketamine could be a better anti-shivering agent with less occurrences of nausea, vomiting, and bradycardia. Ketamine had comparable effects regarding the incidence of hypotension. However, ketamine was associated with higher incidences of hallucinations in comparison to tramadol.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EF, SK, MH, TT, and DT conceived the data, participated in the study design, conducted the statistical analysis, and drafted the manuscript. EF, TT, MH, and DT were involved in collecting the data, performing data analysis, and drafting the manuscript. YF and SS have also participated in the study design, data analysis, and revising of the manuscript. All authors have read and approved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MD, Mean difference; RR, Relative risk; CI, Confidence interval; RCTs, Randomized controlled trials.
References
1. Ahmed ZU, Aziz MA, Khan SN, Zia M. Comparative study of tramadol versus ketamine for postoperative shivering. Pak J Med Health Sci. (2018) 12:1348–50.
2. Askri MR, Maqbool S, Imran-ul-Hassan S, Ashraf A, Awan K, Liaqat M. Prophylactic use of ketamine and tramodol for the prevention of intra-operative shivering in lower limb surgeries done in spinal anesthesia. Med Forum. (2020) 31:100–3.
3. Baig MS, Naz A, Kumar V, Khan SS, Javed S. Addition of low dose ketamine to tramadol for prevention of post anesthetic shivering: a comparative study. Anaesth Pain Inten Care. (2021) 25:596–601. doi: 10.35975/apic.v25i5.1626
4. Koroglu A, Yilmaz H, Kazak Bengisun Z, Suer AH, Tuzuner F. Meperidine, ketamine, and tramadol's effects on postanesthesia shivering and pain in patients under general anaesthesia. Anesth Analg. (2016) 123:595–6. doi: 10.1213/01.ane.0000492856.02973.cc
5. Kumar RA, Ammu S. Comparing the efficacy of tramadol, ketamine and dexmedetomidine in the prevention of intraoperative shivering in patients undergoing surgery under subarachnoid blockade. Indian J Clin Anaesth. (2021) 8:446–51. doi: 10.18231/j.ijca.2021.085
6. Kabade SD, Venkatesh Y, Karthik S, Kumar V. Comparative study of granisetron versus pethidine for the prevention of perioperative shivering under spinal anesthesia. Karnataka Anaesth J. (2016) 2:14–8. doi: 10.4103/2394-6954.190769
7. Crowley LJ, Buggy DJ. Shivering and neuraxial anesthesia. Reg Anesth Pain Med. (2008) 33:241–52. doi: 10.1016/j.rapm.2007.11.006
8. Mahmood MA, Zweifler RM. Progress in shivering control. J Neurol Sci. (2007) 261:47–54. doi: 10.1016/j.jns.2007.04.038
9. Gargari RM, Anvari HMP. Effect of different doses of granisetron on preventing postoperative shivering in patients undergoing septorhinoplasty under general anesthesia. Adv Biosci Clin Med. (2017) 5:21–6. doi: 10.7575/aiac.abcmed.17.05.02.04
10. Gomathi M, Sudhakar R. Magnesium sulphate infusion prevents shivering during spinal anaesthesia: a randomised double blinded controlled study. J Evol Med Dent Sci. (2016) 5:4614–9. doi: 10.14260/jemds/2016/1052
11. Biswa D, Sharma A, Gogna R. Comparative assessment of clonidine and tramadol on post-spinal anaesthesia shivering among patients with lower abdominal and lower limb surgeries. IJMA. (2021) 4:130–2. doi: 10.33545/26643766.2021.v4.i2b.245
12. Kiekkas P, Poulopoulou M, Papahatzi A, Souleles P. Effects of hypothermia and shivering on standard PACU monitoring of patients. AANA J. (2005) 73:47.
13. Lema GF, Gebremedhn EG, Gebregzi AH, Desta YT, Kassa AA. Efficacy of intravenous tramadol and low-dose ketamine in the prevention of post-spinal anesthesia shivering following cesarean section: a double-blinded, randomized control trial. Int J Women's Health. (2017) 9:681–88. doi: 10.2147/IJWH.S139655
14. Lopez MB. Postanaesthetic shivering–from pathophysiology to prevention. Rom J Anaesth Inten Care. (2018) 25:73. doi: 10.21454/rjaic.7518.251.xum
15. Lee MJ, Lee KC, Kim HY, Lee WS, Seo WJ, Lee C. Comparison of ramosetron plus dexamethasone with ramosetron alone on postoperative nausea, vomiting, shivering and pain after thyroid surgery. Korean J Pain. (2015) 28:39–44. doi: 10.3344/kjp.2015.28.1.39
16. Omar H, Aboella WA, Hassan MM, Hassan A, Hassan P, Elshall A, et al. Comparative study between intrathecal dexmedetomidine and intrathecal magnesium sulfate for the prevention of post-spinal anaesthesia shivering in uroscopic surgery) (RCT). BMC Anesthesiol. (2019) 19:1–10. doi: 10.1186/s12871-019-0853-0
17. Sagir O, Gulhas N, Toprak H, Yucel A, Begec Z, Ersoy O. Control of shivering during regional anaesthesia: prophylactic ketamine and granisetron. Acta Anaesthesiol Scand. (2007) 51:44–9. doi: 10.1111/j.1399-6576.2006.01196.x
18. Lakhe G, Adhikari KM, Khatri K, Maharjan A, Bajracharya A, Khanal H. Prevention of shivering during spinal anesthesia: comparison between tramadol, ketamine, and ondansetron. JNMA. (2017) 56:395–400. doi: 10.31729/jnma.3377
19. Wason R, Jain N, Gupta P, Gogia AR. Randomized double-blind comparison of prophylactic ketamine, clonidine and tramadol for the control of shivering under neuraxial anaesthesia. Indian J Anaesth. (2012) 56:370–75. doi: 10.4103/0019-5049.100821
20. Kose E, Honca M, Dal D, Akinci S, Aypar U. Prophylactic ketamine to prevent shivering in parturients undergoing Cesarean delivery during spinal anesthesia. J Clin Anesth. (2013) 25:275–80. doi: 10.1016/j.jclinane.2012.11.014
21. Gecaj-Gashi A, Hashimi M, Sada F, Salihu S, Terziqi H. Prophylactic ketamine reduces incidence of postanaesthetic shivering. Nig J Med. (2010) 19:60181. doi: 10.4314/njm.v19i3.60181
22. Zhou Y, Mannan A, Han Y, Liu H, Guan H-L, Gao X, et al. Efficacy and safety of prophylactic use of ketamine for prevention of postanesthetic shivering: a systematic review and meta analysis. BMC Anesthesiol. (2019) 19:1–11. doi: 10.1186/s12871-019-0910-8
23. Shakya B, Chaturvedi A, Sah B. Prophylactic low dose ketamine and ondansetron for prevention of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. (2010) 26:465. doi: 10.4103/0970-9185.74586
24. Mohta M, Kumari N, Tyagi A, Sethi A, Agarwal D, Singh M. Tramadol for prevention of postanaesthetic shivering: a randomised double-blind comparison with pethidine. Anaesthesia. (2009) 64:141–6. doi: 10.1111/j.1365-2044.2008.05711.x
25. Ameta N, Jacob M, Hasnain S, Ramesh G. Comparison of prophylactic use of ketamine, tramadol, and dexmedetomidine for prevention of shivering after spinal anesthesia. J Anaesthesiol Clin Pharmacol. (2018) 34:352–6. doi: 10.4103/joacp.JOACP_211_16
26. Cahyadi RAD, Nasution AH, Lubis A. Comparison of Intravenous Ketamine 0.25 Mg/KgBW and intravenous tramadol 0.5 Mg/KgBW for prevention of shivering post spinal anesthesia. Int J Innovat Sci Res Technol. (2019) 4:262–70.
27. Gangopadhyay S, Gupta K, Acharjee S, Nayak SK, Dawn S, Piplai G. Ketamine, tramadol and pethidine in prophylaxis of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. (2010) 26:59–63. doi: 10.4103/0970-9185.75111
28. Akram M, Raza H, Imam SM. Efficacy of prophylactic low dose ketamine and tramadol for prevention of shivering during spinal anaesthesia in patients undergoing lower abdominal surgeries. Pak J Med Health Sci. (2017) 11:378–80.
29. Azam M, Asad N, Butt TA, Ahmad W. Efficacy of prophylactic intravenous ketamine vs tramadol for prevention of intraoperative shivering in spinal anesthesia for patient undergoing cesarean section. Pak J Med Health Sci. (2018) 12:455–58.
30. Hidayah MN, Liu CY, Joanna OS. Ketamine and tramadol for the prevention of shivering during spinal anaesthesia. Clin Terap. (2014) 165:193–8. doi: 10.7417/CT.2014.1732
31. Ilyas M, Naz A, Wadood F, Halimi N, Jawaid Z, Khan P. Randomized control trial of tramadol versus ketamine in the prevention of shivering during spinal anesthesia. Pak J Surg. (2019) 35:311–15.
32. Jouryabi AM, Sharami SH, Ghanaie MM, Sedighinejad A, Imantalab V, Sorouri ZR, et al. Comparing the effects of low dose of ketamine, tramadol, and ondansetron in prevention of post spinal anesthesia shivering in cesarean section. Anesthesiol Pain Med. (2021) 11:6429. doi: 10.5812/aapm.116429
33. Nazir A, Dar FA, Javed T. Comparative study prophylactive intravenous ketamine and tramodol in preventing intraoperative shivering in patients undergoing elective lower limb surgery under spinal anaesthesia. Am J Adv Drug Delivery. (2015) 3:011–7.
34. Seyam SH. Prevention of post-spinal anesthesia shivering: Low dose ketamine vs. tramadol. Al-Azhar Int Med J. (2020) 1:108–15. doi: 10.21608/aimj.2020.22925.1102
35. Nain P, Kundra S, Singh T, Singh MR, Kapoor R, Singh A. Comparative evaluation of oral tramadol and gabapentin for prophylaxis of post-spinal shivering. Indian J Anaesth. (2021) 65:S5. doi: 10.4103/ija.IJA_979_20
36. Arora N. Prophylactic tramadol versus dexmedetomidine for prevention of shivering during spinal anaesthesia. Int J Scien Study. (2014) 2:17–20.
37. Guha S, Nath PK, Halder R, Bandyopadhyay U. Prophylactic use of intravenous clonidine compared to tramadol in prevention of intraoperative shivering under regional anesthesia. Anesth Essays Res. (2017) 11:477. doi: 10.4103/aer.AER_6_17
38. Abdelrahman RS. Prevention of shivering during regional anaesthesia: comparison of midazolam, midazolam plus ketamine, tramadol, and tramadol plus ketamine. Life Sci J. (2012) 9:132–9.
39. Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T. Pharmacological aspects and potential new clinical applications of ketamine: reevaluation of an old drug. J Clin Pharmacol. (2009) 49:957–64. doi: 10.1177/0091270009337941
40. Powers III AR, Gancsos MG, Finn ES, Morgan PT, Corlett PR. Ketamine-induced hallucinations. Psychopathology. (2015) 48:376–85. doi: 10.1159/000438675
Keywords: ketamine, tramadol, spinal, anesthesia, shivering
Citation: Fenta E, Kibret S, Hunie M, Tamire T, Fentie Y, Seid S and Teshome D (2022) The effects of intravenous tramadol vs. intravenous ketamine in the prevention of shivering during spinal anesthesia: A meta-analysis of randomized controlled trials. Front. Med. 9:1011953. doi: 10.3389/fmed.2022.1011953
Received: 04 August 2022; Accepted: 21 November 2022;
Published: 05 December 2022.
Edited by:
Shun Ming Chan, Tri-Service General Hospital, TaiwanReviewed by:
Chian Yong Liu, National University of Malaysia, MalaysiaIvana Budic, University of Niš, Serbia
Copyright © 2022 Fenta, Kibret, Hunie, Tamire, Fentie, Seid and Teshome. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Efrem Fenta, ZXBoZmVuMjAwN0BnbWFpbC5jb20=
 Efrem Fenta
Efrem Fenta Simegnew Kibret
Simegnew Kibret Metages Hunie
Metages Hunie Tadese Tamire
Tadese Tamire Yewlsew Fentie
Yewlsew Fentie Shimelis Seid
Shimelis Seid Diriba Teshome
Diriba Teshome