- 1Scientific Consultant, Chattanooga, TN, United States
- 2Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3Department of Emergency Medicine, University of Tennessee, Chattanooga, TN, United States
- 4Vapotherm, Inc., Exeter, NH, United States
High flow nasal oxygen is a relatively new option for treating patients with respiratory failure, which decreases work of breathing, improves tidal volume, and modestly increases positive end expiratory pressure. Despite well-described physiologic benefits, the clinical impact of high flow nasal oxygen is still under investigation. In this article, we review the most recent findings on the clinical efficacy of high flow nasal oxygen in Type I, II, III, and IV respiratory failure within adult and pediatric patients. Additionally, we discuss studies across clinical settings, including emergency departments, intensive care units, outpatient, and procedural settings.
Introduction
High flow nasal oxygen (HFNO) is a relatively new modality for treating patients with respiratory failure. Historically, the term 'high-flow' referred to an increased bore size of the nasal cannula with associated gas flow. Technological advancements have significantly augmented this concept creating a class of devices called “high flow nasal oxygen.” While there are a variety of HFNO machines available, they can broadly be divided into two groups. Classic HFNO utilizes a high-flow nasal cannula providing heated, humidified air at flow rates up to 60 Liters/min with a corresponding fraction of inspired oxygen from 21 to 100%. The second category is high-velocity nasal insufflation (HVNI), which utilizes small-bore nasal cannulas to flush large airways, reducing anatomic dead space and increasing oxygen. Flow levels are limited to 40 L/min, but the air has greater kinetic energy resulting in a larger flush at equivalent flow rates (1). This flush difference led to different FDA classifications (DEN170001). Whether these engineering differences have a measurable clinical impact remains a subject of study.
The physiological benefits of HFNO are well-described, including decreased work of breathing, improved tidal volumes, modest increases in positive end-expiratory pressure, enhanced mucociliary clearance of secretions, and accurate delivery of FiO2 (2–5). Mechanistically, HFNO has many beneficial characteristics, but clinical efficacy is debated. For this review, an electronic literature search was conducted using PubMed and Google Scholar to summarize recent findings within key adult and pediatric patient populations.
Clinical efficacy of high flow nasal oxygenation
Type I respiratory failure
Adult patients
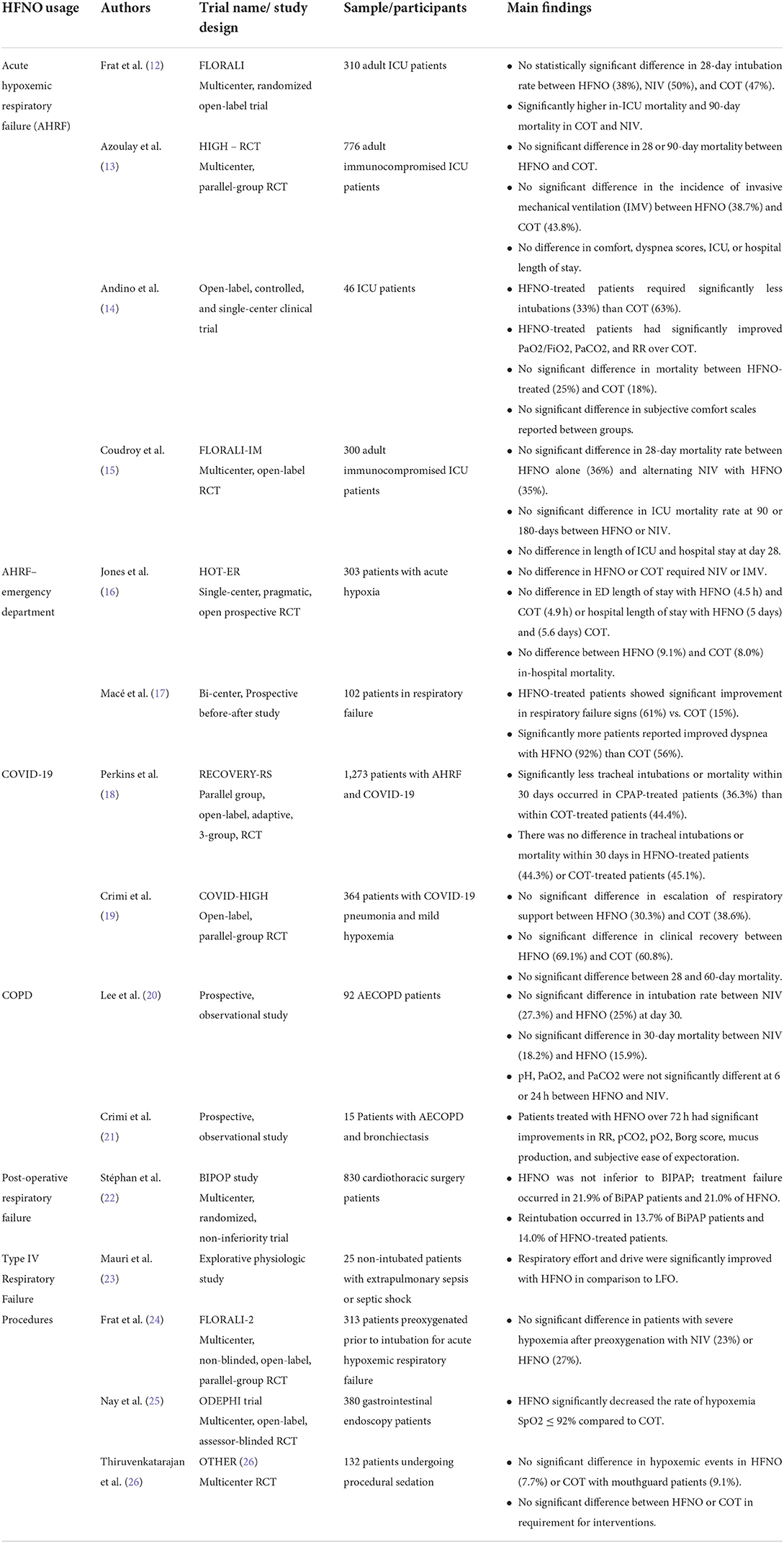
Acute hypoxemic respiratory failure (AHRF) describes patients with inadequate tissue oxygenation associated with partial pressures of oxygen < 60 mmHG. Non-invasive positive pressure ventilation (NIPPV) is associated with improved oxygenation but may cause lung damage through overdistention (6). HFNO increases alveolar recruitment relative to conventional oxygen therapy (COT) without negatively affecting tidal volume (6) and improves oxygenation, inspiratory effort, respiratory rate (RR), lung volume, and other metrics compared to face masks (7). These benefits led to recommendations for HFNO over NIPPV for treatment of AHRF by the American College of Physicians (8) and a strong recommendation with moderate certainty using GRADE guidelines for usage over COT during hypoxemic respiratory failure by a joint panel of experts within the European Society of Intensive Care Medicine (9). Moreover, HFNO may provide some advantages in terms of patients' comfort compared to NIPPV, and it is generally considered a well-tolerated device despite few studies having specifically addressed this aspect (10). However, it has been shown that higher temperature may negatively impact comfort regardless of flow rate and in more severe patients, higher flow rates may improve comfort (11). Therefore, HFNO settings that produce both optimal comfort and therapeutic effect vary with individual patients.
Adult critically ill patients in the intensive care unit
One of the significant recent studies was the FLORALI trial which compared intubation rates in 310 adult ICU patients with AHRF (Table 1). The authors reported no difference in intubation rates at 28 days with HFNO usage. However, there were significantly higher in-ICU mortality and 90-day mortality rates with COT and NIPPV compared to HFNO (12). In contrast, HFNO-treated AHRF ICU patients had significantly lower intubation rates than COT and significant improvements in PaCO2, PaO2/FiO2, and RR (14). Unlike the FLORALI study, there was no significant difference in mortality.
The data are less clear in critically ill, immunocompromised patients. In a study of 776 such patients with AHRF, HFNO was not superior to COT in reducing 28-day mortality (13). Other studies in this population found no difference between HFNO and venturi mask in escalation to intubation or NIPPV (27). Given sample size differences, the studies may have been underpowered to detect a difference in efficacy between HFNO and COT. In a retrospective study, immunocompromised patients treated with HFNO or NIPPV showed significantly lower intubation rates and mortality with HFNO (28). However, the authors noted significant differences at baseline, patients with more severe illnesses were treated with NIPPV and not evenly distributed between interventions (28). Therefore, these results may be an outlier among more extensive studies. The recent FLORALI-IM study compared mortality rates in severely immunocompromised patients treated with alternating NIPPV and HFNO or HFNO alone. Consistent with prior studies, the authors found no significant difference in mortality, intubation rates, length of stay (LOS) in the ICU and hospital, and ventilator-free days (15, 29).
Adult patients in the emergency department
Mace et al., 2019 compared oxygen treatments in 102 ED patients with acute hypoxemia. 61% of HFNO patients showed improvement in respiratory failure symptoms after 1 h compared to 15% improvement in COT-treated (17). However, there was no significant difference in LOS within the ED or intubation rates (17). Similarly, the HOT-ER study (n = 303) found no difference in the need for mechanical ventilation in the ED, LOS in the ED or hospital, and no difference in mortality in patients with respiratory distress (16).
In 204 ED patients, HVNI was non-inferior to NIPPV for all-cause respiratory distress in patients without a need for emergency intubation (30). In adult patients with AHRF, HVNI produces similar intubation rates and clinical outcomes. These studies also demonstrated the safety of HFNO and HVNI usage outside the context of the ICU.
Neonates/pediatric patients
Respiratory support for preterm infants and young children in respiratory failure can be provided through non-invasive methods prior to endotracheal intubation. Recent studies have sought to evaluate the efficacy of HFNO compared to non-invasive options to ensure clinical outcomes are not worse than standard practices. Unfortunately, many studies provide contradictory evidence, which may be due to variability of methods, flow rates, and patient populations. Recent meta-analyses suggest HFNO has a higher risk of treatment failure in infants (31–33). In a subgroup of infants (<2 yo) by diagnosis, the significant increase in HFNO treatment failure risk was specific to patients diagnosed with acute viral bronchiolitis (31).
Individual studies have found HFNO non-inferior to nCPAP or BiPAP (12), with no significant differences in treatment failure or intubation rates in preterm or pediatric patients (34–36). Non-invasive methods (nCPAP) are associated with nasal injury, particularly in infants (37). Preterm or low-weight newborns are at higher risk of skin breakdown and injury from nCPAP, resulting in higher pain scores and salivary cortisol concentrations than HFNO (38). Therefore, given the protective benefits of HFNO, more RCTs are needed to identify specific pediatric patient populations that receive the most clinical benefit.
COVID-19 respiratory failure
COVID-19 infections resulted in critically-ill patients with AHRF worldwide (39). In many cases, invasive mechanical ventilation was required due to the clinical severity. Initially, concerns about the risk of HFNO spreading COVID-19 through aerosolization were expressed. However, recent evidence confirmed that HFNO poses a low risk of spread, and delivery with a surgical mask prevents aerosol dispersal (40–43).
Recent studies have evaluated whether HFNO has a specific benefit over COT during COVID-19. HFNO-treated adults with COVID-19 had significantly reduced need for intubation (44, 45) and reduced time to recovery (44). Despite reduced intubation risk, HFNO was not associated with decreased LOS in the hospital or ICU, and mortality rates were unchanged (44, 45). HFNO for COVID-19-associated hypoxemic respiratory failure may reduce the likelihood of intubation, but the effect on recovery time is unclear. The RECOVERY-RS trial compared CPAP or HFNO to COT in patients with COVID-19-related respiratory failure (n = 1,273). CPAP reduced intubation risk and mortality over COT, but HFNO did not (18). This trial stopped early due to waning COVID-19 numbers; therefore, the HFNO group might have been underpowered to detect the same benefit. In COVID-19-pneumonia patients with mild hypoxemia (n = 364) there was no significant difference in escalation to respiratory support (30.3% HFNO, 38.6% COT) or clinical recovery (69.9% HFNO, 60.8% COT) (19). The authors report that this trial may have been underpowered to detect the hypothesized difference between the groups due to a lower event rate than anticipated (19).
Type II respiratory failure
Acute hypercapnic respiratory failure in adult patients
Acute respiratory failure with hypercapnia is common in chronic obstructive pulmonary disease (COPD) patients, and NIV is the current gold standard for care (46). Given the physiological benefits, ease of use, and comfort, it has been increasingly studied in COPD patients and those with hypercapnic respiratory failure (47). Hypercapnic respiratory failure treatment success with HFNO suggests that patients have some assistance with ventilation and oxygenation.
In patients with severe COPD and ventilatory limitations during exercise, HFNO improved endurance time and dyspnea rates over COT (48). Additionally, HFNO enhanced ventilation efficiency in COPD patients (49, 50). In 19 COPD patients, HFNO improved pCO2 despite reduced calculated minute ventilation, suggesting a reduction in dead space ventilation (51). One hundred two severe COPD patients with hypercapnia had no difference in pCO2, quality of life, or dyspnea symptoms after 6 weeks of HFNO or NIPPV usage (52). Additionally, increased mean airway pressures have been reported in COPD patients (53). In 14 stable, severe COPD patients on HFNO, COT, or NIPPV, HFNO patients showed reduced inspiratory effort, RR, and pCO2 compared to COT (54). For severe COPD patients with intrinsic PEEP, vulnerable to dynamic hyperinflation, the smaller amount of positive pressure generated by HFNO compared to NIPPV could be beneficial.
In the acute care setting, NIPPV has significant benefits and remains strongly recommended for acute exacerbation of COPD (AECOPD) (55, 56). However, NIPPV remains underutilized and, when utilized, fails in 10–25% of patients (57–59). Evidence for using HFNO in outpatient settings for stable COPD is promising, but its role during AECOPD is still unknown. Given its ease of use and tolerability, HFNO utilization is increasing in all acute care settings for all causes of respiratory failure. In 15 patients with AECOPD and bronchiectasis, HFNO usage improved dyspnea, gas exchange, mucus production, and decreased RR without any significant safety concerns (21). A study of 92 patients with AECOPD showed no difference in intubation rate or mortality with HFNO compared to NIPPV (20). Several ED studies comparing HFNO to NIPPV or COT for mixed or undifferentiated respiratory failure showed no difference in outcomes between the different modalities, with one showing HFNO to be non-inferior to NIPPV (16, 30, 60, 61). In a recent case study, an elderly male with severe bronchiectasis was prescribed HFNO for long-term home usage and displayed reduced mucus buildup after 6 months (62). More studies are needed to delineate the benefits of HFNO home usage.
HVNI was shown to have comparable efficacy to NIPPV in hypercapnic respiratory failure, with no significant difference in treatment failure or intubation rate (63). Multiple studies confirm that HFNO produces similar treatment failure, intubation, and mortality rates (20, 63–65) and provides similar RR, PaCO2, and PaO2/FiO2 ratios (64, 66). Evidence for HFNO usage during COPD with hypercapnia is growing, but more studies are needed.
Hypercapnia in pediatric patients
Hypercapnia is relatively rare in children and is typically associated with advanced lung diseases, such as cystic fibrosis or neuromuscular diseases (67–69). Affected children may require NIV to offset the effects of nocturnal hypercapnia to reduce the risk of alveolar hypoventilation during sleep (67, 69). Unfortunately, complications from NIPPV-induced pressure can lead to gastric distention, gastroesophageal reflux, pulmonary aspiration, and other adverse effects (67, 70). Few studies have analyzed the effectiveness of HFNO compared to other methods in children with hypercapnia. However, no studies have confirmed the effectiveness of NIV in children with acute cystic fibrosis exacerbations, and no validated criteria currently exist to determine when to initiate long-term NIV in pediatric cases (67, 69). One crossover study of hospitalized adults with cystic fibrosis found that HFNO significantly reduced RR and minute ventilation compared to NIV (71). Multiple trials are underway, which may provide critical information needed for evidence-based clinical recommendations.
Type III respiratory failure
Post-operative respiratory failure is associated with morbidity and mortality in surgical patients. HFNO was given a conditional recommendation with moderate certainty using GRADE guidelines by a joint panel of experts within the European Society of Intensive Care Medicine for usage post-operatively in high-risk and obese patients after cardiac and thoracic surgery (9, 72). The greater portability of HFNO makes it an attractive option for patients that benefit from early mobilization.
In a multicenter, non-inferiority trial of patients after cardiothoracic surgery, HFNO was non-inferior to BiPAP with similar levels of treatment failure (21.9% BiPAP, 21.0% HFNO), reintubation rates (13.7% BiPAP, 14.0% HFNO) and ICU mortality (5.5% BiPAP, 6.8% HFNO) (22). Additionally, when diaphragm thickening fractions were analyzed as a measure of respiratory workload, BiPAP and HFNO significantly reduced respiratory workload compared to COT (73). A recent meta-analysis found when HFNO is used within 24 h post-operatively, there is a moderate likelihood that HFNO reduces reintubation rates and lessens escalation of respiratory support frequency compared to COT (72). However, the data was criticized for excluding a study of patients who underwent major abdominal surgery where a benefit from HFNO was not seen (74, 75). Recent data contradicts prior studies that found similar reintubation rates in HFNO-treated patients compared to COT (76) and no significant difference in post-operative complications (77).
Patients are also at risk for type 3 respiratory failure in the immediate post-extubation period. HFNO significantly reduced reintubation rates and post-extubation respiratory failure incidence compared to COT and performed similarly to NIPPV (78, 79). Presently, there is insufficient evidence to support HFNO as routine prophylactic post-operative care, but some patient populations may benefit and further research is needed to clarify this issue.
Type IV respiratory failure
Type 4 respiratory failure occurs due to failure of respiratory muscles resulting from hypoperfusion in shock. The physiologic concept behind using HFNO in this setting would provide supplemental oxygen and reduce work of breathing, allowing for lower cardiac output requirements to support respiration. This may enhance the ability of the patient to resolve metabolic acidosis through typical respiratory compensation methods. Treatment focuses on supporting respiration while identifying and correcting the source of shock. Few studies examined the clinical role of HFNO during shock-induced respiratory failure. Mauri et al. (23) found that HFNO significantly reduced respiratory effort, drive, and rate in septic and septic shock patients compared to COT (23).
The authors measured respiratory effort by esophageal pressure and correlated it with plasma lactate levels and dynamic lung compliance. Both factors independently increased respiratory effort when plasma lactate levels increase, or dynamic lung compliance worsens (23). Further studies are needed to determine what, if any, impact this might have on clinical outcomes.
Use of high flow nasal oxygen in procedures
Many patients benefit from preoxygenation prior to endotracheal intubation (80). Obese ICU patients preoxygenated with HFNO had a significantly reduced risk of severe hypoxemia compared to patients managed with a non-rebreather mask (81). In contrast, the FLORALI-2 study of 313 adult ICU patients with AHRF and preoxygenated with either NIPPV or HFNO prior to intubation found no significant difference in severe hypoxemia incidence between patients treated with NIPPV (23%) or HFNO (27%) (24).
In a recent meta-analysis, patients preoxygenated with HFNO prior to endotracheal intubation had significantly shortened ICU LOS (mean = 1.8 days). Subgroup analysis demonstrated that HFNO significantly reduced severe hypoxemia incidence during endotracheal intubation in patients with mild hypoxemia (PaO2/FiO2 > 200 mmHg), with a number needed to treat (NNT) = 16.7. The authors concluded that there was no apparent benefit to HFNO use compared to standard care for non-hypoxic patients (82).
HFNO usage during apnea has recently been studied using the newly coined “Transnasal Humidified Rapid Insufflation Ventilatory Exchange” (THRIVE) technique, where HFNO maintains oxygenation during intubation and extends apnea time (83, 84). HFNO administration reported an average apneic time of 17 min for difficult airways in 25 adult patients (85).
The THRIVE technique (HFNO with jaw support) was studied in 48 healthy children (0–10 yo) undergoing general anesthesia; results showed significantly longer apnea without desaturation times during intubation compared to jaw support alone (86). Recently, the SHINE study compared HVNI to standard care for preoxygenation of neonates undergoing endotracheal intubation. Here, 50% of first-attempt intubations were successful with HVNI compared to 31.5% with standard of care (87). Desaturation in HVNI-treated neonates occurred at a lower percentage with a longer mean time to desaturation (44.3 and 35.5 s, respectively); NNT =6 (87). These results suggest HVNI improves intubation success with lowered risk of adverse events and these data suggest that neonates, infants, and children likely benefit from preoxygenation with HFNO before intubation. Preoxygenation with HFNO is likely to benefit some patient groups and is non-inferior to NIPPV for patients with obesity (80, 82, 88).
Gastrointestinal (GI) endoscopy procedures may have complications stemming from sedation, such as respiratory depression, airway obstruction, and decreased chest wall compliance, which may induce hypoxia (89). Recent RCTs have explored using HFNO during GI procedures compared to standard methods. In the ODEPHI trial, HFNO usage during GI endoscopy reduced desaturation frequency compared to COT and significantly reduced the need for maneuvers to maintain the upper airways (25).
Evidence from other GI procedures produced similar results; patients undergoing advanced esophagogastroduodenoscopy with HFNO had an absolute risk reduction of 11.9% of hypoxic events compared to patients provided oxygen with low flow nasal cannulas (LFNC); NNT = 8.4 (90). Similarly, in a trial comparing LFNC to HFNO in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), HFNO patients had no hypoxemic events. The lowest mean SpO2 was higher than LFNC, suggesting that HFNO provided superior oxygenation support during ERCP (91). In a large trial of adult outpatients undergoing elective gastroscopy with propofol sedation, there was significantly lower incidence of adverse events and subclinical respiratory depression in HFNO patients. Additionally, results showed a significant reduction in mild and severe hypoxia compared to patients given LFNC (89). These data indicate that undifferentiated patients undergoing GI procedures may benefit from HFNO.
However, high-risk patient studies failed to observe any benefits. Morbidly obese (BMI >40 kg/m2) patients undergoing elective colonoscopy with propofol sedation were supported with HFNO or LFNO with no significant differences in desaturation incidence (92). Additionally, the OTHER trial found no significant difference in hypoxemic events in high-risk adults during ECRP (26). More high-quality studies are needed to evaluate patient populations with the highest clinical benefit.
Conclusion
HFNO is a valuable addition to the options for managing respiratory distress. HFNO is more often portable than NIPPV, allowing greater freedom of movement for the patient and the ability to eat and speak with healthcare providers and loved ones. Additionally, HFNO patients may be managed in a range of hospital bedding areas due to mechanical constraints of NIPPV machines. Overall, more studies are needed in pediatrics, peri-operative patients, during medical procedures, type 4 respiratory distress, COVID-19, and unique patient populations.
Author contributions
KW, NG, and JW participated in the conception, development, and writing of this manuscript. All authors agree to be accountable for the content of the work. All authors contributed to the article and approved the submitted version.
Conflict of interest
Author JW is VP of Clinical Research for Vapotherm, Inc–a manufacturer of high flow oxygen systems. Author KW has been employed within the past 12 months as a scientific consultant.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miller T, Saberi B, Saberi S. Computational fluid dynamics modeling of extrathoracic airway flush: evaluation of high flow nasal cannula design elements. J Pulm Respir Med. (2016) 6:376. doi: 10.4172/2161-105X.1000376
2. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. (2009) 103:1400–5. doi: 10.1016/j.rmed.2009.04.007
3. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. (2018) 15:145–55. doi: 10.1513/AnnalsATS.201707-548FR
4. Lodeserto FJ, Lettich TM, Rezaie SR. High-flow nasal cannula: mechanisms of action and adult and pediatric indications. Cureus. (2018) 10:e3639. doi: 10.7759/cureus.3639
5. Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care. (2019) 64:735–42. doi: 10.4187/respcare.06718
6. Artaud-Macari E, Bubenheim M, Le Bouar G, Carpentier D, Grangé S, Boyer D, et al. High-flow oxygen therapy versus noninvasive ventilation: a randomised physiological crossover study of alveolar recruitment in acute respiratory failure. ERJ Open Res. (2021) 7:00373-2021. doi: 10.1183/23120541.00373-2021
7. Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. (2017) 195:1207–15. doi: 10.1164/rccm.201605-0916OC
8. Qaseem A, Etxeandia-Ikobaltzeta I, Fitterman N, Williams JW Jr, Kansagara D. Appropriate use of high-flow nasal oxygen in hospitalized patients for initial or postextubation management of acute respiratory failure: a clinical guideline from the American college of physicians. Ann Intern Med. (2021) 174:977–84. doi: 10.7326/M20-7533
9. Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. (2020) 46:2226–37. doi: 10.1007/s00134-020-06312-y
10. Cortegiani A, Crimi C, Noto A, Helviz Y, Giarratano A, Gregoretti C, et al. Effect of high-flow nasal therapy on dyspnea, comfort, and respiratory rate. Crit Care. (2019) 23:201. doi: 10.1186/s13054-019-2473-y
11. Mauri T, Galazzi A, Binda F, Masciopinto L, Corcione N, Carlesso E, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit Care. (2018) 22:120. doi: 10.1186/s13054-018-2039-4
12. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. (2015) 372:2185–96. doi: 10.1056/NEJMoa1503326
13. Azoulay E, Lemiale V, Mokart D, Nseir S, Argaud L, Pene F, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. (2018) 320:2099–107. doi: 10.1001/jama.2018.14282
14. Andino R, Vega G, Pacheco SK, Arevalillo N, Leal A, Fernández L, et al. High-flow nasal oxygen reduces endotracheal intubation: a randomized clinical trial. Ther Adv Respir Dis. (2020) 14:1–14. doi: 10.1177/1753466620956459
15. Coudroy R, Frat J-P, Ehrmann S, Pène F, Decavèle M, Terzi N, et al. High-flow nasal oxygen alone or alternating with non-invasive ventilation in critically ill immunocompromised patients with acute respiratory failure: a randomised controlled trial. Lancet Respir Med. (2022). 10:641–9. doi: 10.1016/S2213-2600(22)00096-0
16. Jones PG, Kamona S, Doran O, Sawtell F, Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respir Care. (2016) 61:291–9. doi: 10.4187/respcare.04252
17. Macé J, Marjanovic N, Faranpour F, Mimoz O, Frerebeau M, Violeau M, et al. Early high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure in the ED: a before-after study. Am J Emerg Med. (2019) 37:2091–6. doi: 10.1016/j.ajem.2019.03.004
18. Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. (2022) 327:546–58. doi: 10.1001/jama.2022.0028
19. Crimi C, Noto A, Madotto F, Ippolito M, Nolasco S, Campisi R, et al. High-flow nasal oxygen versus conventional oxygen therapy in patients with COVID-19 pneumonia and mild hypoxaemia: a randomised controlled trial. Thorax. (2022) 1–8. doi: 10.1136/thoraxjnl-2022-218806
20. Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim S-H, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. (2018) 12:2046–56. doi: 10.1111/crj.12772
21. Crimi C, Noto A, Cortegiani A, Campisi R, Heffler E, Gregoretti C, et al. High flow nasal therapy use in patients with acute exacerbation of COPD and bronchiectasis: a feasibility study. COPD. (2020) 17:184–90. doi: 10.1080/15412555.2020.1728736
22. Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery. JAMA. (2015) 313:2331. doi: 10.1001/jama.2015.5213
23. Mauri T, Spinelli E, Pavlovsky B, Grieco DL, Ottaviani I, Basile MC, et al. Respiratory drive in patients with sepsis and septic shock: modulation by high-flow nasal cannula. Anesthesiology. (2021) 135:1066–75. doi: 10.1097/ALN.0000000000004010
24. Frat J-P, Ricard J-D, Quenot J-P, Pichon N, Demoule A, Forel J-M, et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Resp Med. (2019) 7:303–12. doi: 10.1016/S2213-2600(19)30048-7
25. Nay M-A, Fromont L, Eugene A, Marcueyz J-L, Mfam W-S, Baert O, et al. High-flow nasal oxygenation or standard oxygenation for gastrointestinal endoscopy with sedation in patients at risk of hypoxaemia: a multicentre randomised controlled trial (ODEPHI trial). Br J Anaesth. (2021) 127:133–42. doi: 10.1016/j.bja.2021.03.020
26. Thiruvenkatarajan V, Dharmalingam A, Arenas G, Wahba M, Liu WM, Zaw Y, et al. Effect of high-flow vs. low-flow nasal plus mouthguard oxygen therapy on hypoxaemia during sedation: a multicentre randomised controlled trial. Anaesthesia. (2021) 77:46–53. doi: 10.1111/anae.15527
27. Lemiale V, Mokart D, Mayaux J, Lambert J, Rabbat A, Demoule A, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care. (2015) 19:380. doi: 10.1186/s13054-015-1097-0
28. Coudroy R, Jamet A, Petua P, Robert R, Frat J-P, Thille AW. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care. (2016) 6:45. doi: 10.1186/s13613-016-0151-7
29. Lemiale V, Mokart D, Resche-Rigon M, Pène F, Mayaux J, Faucher E, et al. Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure. JAMA. (2015) 314:1711. doi: 10.1001/jama.2015.12402
30. Doshi P, Whittle JS, Bublewicz M, Kearney J, Ashe T, Graham R, et al. High-velocity nasal insufflation in the treatment of respiratory failure: a randomized clinical trial. Ann Emerg Med. (2018) 72:73–83.e5. doi: 10.1016/j.annemergmed.2017.12.006
31. Zhao X, Qin Q, Zhang X. Outcomes of high-flow nasal cannula vs. nasal continuous positive airway pressure in young children with respiratory distress: a systematic review and meta-analysis. Front Pediatr. (2021) 9:759297. doi: 10.3389/fped.2021.759297
32. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. (2019) 104:564–76. doi: 10.1136/archdischild-2018-315846
33. Roberts CT, Owen LS, Manley BJ, Frøisland DH, Donath SM, Dalziel KM, et al. Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med. (2016) 375:1142–51. doi: 10.1056/NEJMoa1603694
34. Lavizzari A, Colnaghi M, Ciuffini F, Veneroni C, Musumeci S, Cortinovis I, et al. Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity. JAMA Pediatr. (2016). doi: 10.1001/jamapediatrics.2016.1243. [Epub ahead of print].
35. Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D, et al. randomized pilot study comparing heated humidified high-flow nasal cannulae with NIPPV for RDS. Pediatr Pulmonol. (2015) 50:576–83. doi: 10.1002/ppul.23022
36. Sarkar M, Sinha R, Roychowdhoury S, Mukhopadhyay S, Ghosh P, Dutta K, et al. Comparative study between noninvasive continuous positive airway pressure and hot humidified high-flow nasal cannulae as a mode of respiratory support in infants with acute bronchiolitis in pediatric intensive care unit of a tertiary care hospital. Indian J Crit Care Med. (2018) 22:85–90. doi: 10.4103/ijccm.IJCCM_274_17
37. Zivanovic S, Scrivens A, Panza R, Reynolds P, Laforgia N, Ives KN, et al. Nasal high-flow therapy as primary respiratory support for preterm infants without the need for rescue with nasal continuous positive airway pressure. Neonatology. (2019) 115:175–81. doi: 10.1159/000492930
38. Osman M, Elsharkawy A, Abdel-Hady H. Assessment of pain during application of nasal-continuous positive airway pressure and heated, humidified high-flow nasal cannulae in preterm infants. J Perinatol. (2015) 35:263–7. doi: 10.1038/jp.2014.206
39. Crimi C, Pierucci P, Renda T, Pisani L, Carlucci A. High-flow nasal cannula and COVID-19: a clinical review. Respir Care. (2022) 67:227–40. doi: 10.4187/respcare.09056
40. Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. (2020) 55:2000892. doi: 10.1183/13993003.00892-2020
41. Leonard S, Atwood CW, Walsh BK, Debellis RJ, Dungan GC, Strasser W, et al. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask. Chest. (2020) 158:1046–9. doi: 10.1016/j.chest.2020.03.043
42. Hamada S, Tanabe N, Inoue H, Hirai T. Wearing of medical mask over the high-flow nasal cannula for safer oxygen therapy in the COVID-19 era. Pulmonology. (2021) 27:171–3. doi: 10.1016/j.pulmoe.2020.10.009
43. Roca O, Pacheco A, Rodon J, Antón A, Vergara-Alert J, Armadans L, et al. Nasal high-flow oxygen therapy in COVID-19 patients does not cause environmental surface contamination. J Hosp Infect. (2021) 116:103–5. doi: 10.1016/j.jhin.2021.04.034
44. Ospina-Tascón GA, Calderón-Tapia LE, García AF, Zarama V, Gómez-Álvarez F, Álvarez-Saa T, et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19. JAMA. (2021) 326:2161. doi: 10.1001/jama.2021.20714
45. Bonnet N, Martin O, Boubaya M, Levy V, Ebstein N, Karoubi P, et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care. (2021) 11:37. doi: 10.1186/s13613-021-00825-5
46. Macrea M, Oczkowski S, Rochwerg B, Branson RD, Celli B, Coleman JM, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American thoracic society clinical practice Guideline. Am J Respir Crit Care Med. (2020) 202:e74–87. doi: 10.1164/rccm.202006-2382ST
47. Chatila W, Nugent T, Vance G, Gaughan J, Criner GJ. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. (2004) 126:1108–15. doi: 10.1378/chest.126.4.1108
48. Cirio S, Piran M, Vitacca M, Piaggi G, Ceriana P, Prazzoli M, et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med. (2016) 118:128–32. doi: 10.1016/j.rmed.2016.08.004
49. Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. (2016) 71:759–61. doi: 10.1136/thoraxjnl-2015-207962
50. McKinstry S, Pilcher J, Bardsley G, Berry J, Van de Hei S, Braithwaite I, et al. Nasal high flow therapy and PtCO2 in stable COPD: a randomized controlled cross-over trial. Respirology. (2018) 23:378–84. doi: 10.1111/resp.13185
51. Braunlich J, Kohler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. (2016) 11:1077–85. doi: 10.2147/COPD.S104616
52. Braunlich J, Dellweg D, Bastian A, Budweiser S, Randerath W, Triche D, et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD. Int J Chron Obstruct Pulmon Dis. (2019) 14:1411–21. doi: 10.2147/COPD.S206111
53. Braunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth HJ, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. (2013) 85:319–25. doi: 10.1159/000342027
54. Pisani L, Fasano L, Corcione N, Comellini V, Musti MA, Brandao M, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. (2017) 72:373–5. doi: 10.1136/thoraxjnl-2016-209673
55. Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. (2017) 50:1602426. doi: 10.1183/13993003.02426-2016
56. Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. (1995) 333:817–22. doi: 10.1056/NEJM199509283331301
57. Sweet DD, Naismith A, Keenan SP, Sinuff T, Dodek PM. Missed opportunities for noninvasive positive pressure ventilation: a utilization review. J Crit Care. (2008) 23:111–7. doi: 10.1016/j.jcrc.2007.04.002
59. Fisher KA, Mazor KM, Goff S, Stefan MS, Pekow PS, Williams LA, et al. Successful use of noninvasive ventilation in chronic obstructive pulmonary disease. How do high-performing hospitals do it? Ann Am Thorac Soc. (2017) 14:1674–81. doi: 10.1513/AnnalsATS.201612-1005OC
60. Rittayamai N, Tscheikuna J, Praphruetkit N, Kijpinyochai S. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care. (2015) 60:1377–82. doi: 10.4187/respcare.03837
61. Bell N, Hutchinson CL, Green TC, Rogan E, Bein KJ, Dinh MM. Randomised control trial of humidified high flow nasal cannulae versus standard oxygen in the emergency department. Emerg Med Australas. (2015) 27:537–41. doi: 10.1111/1742-6723.12490
62. Impellizzeri P, Nolasco S, Campisi R, Cipolla A, Borgese A, Alia S, et al. Acute and long-term management of severe bronchiectasis with high flow nasal therapy: a case report. Monaldi Arch Chest Dis. (2022). doi: 10.4081/monaldi.2022.2333. [Epub ahead of print].
63. Doshi PB, Whittle JS, Dungan G II, Volakis LI, Bublewicz M, Kearney J, et al. The ventilatory effect of high velocity nasal insufflation compared to non-invasive positive-pressure ventilation in the treatment of hypercapneic respiratory failure: a subgroup analysis. Heart Lung. (2020) 49:610–5. doi: 10.1016/j.hrtlng.2020.03.008
64. Sun J, Li Y, Ling B, Zhu Q, Hu Y, Tan D, et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. Int J Chron Obstruct Pulmon Dis. (2019) 14:1229–37. doi: 10.2147/COPD.S206567
65. Tan D, Walline JH, Ling B, Xu Y, Sun J, Wang B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial. Crit Care. (2020) 24:489. doi: 10.1186/s13054-020-03214-9
66. Cortegiani A, Longhini F, Madotto F, Groff P, Scala R, Crimi C, et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. (2020) 24:692. doi: 10.1186/s13054-020-03409-0
67. Atag E, Krivec U, Ersu R. Non-invasive ventilation for children with chronic lung disease. Front Pediatr. (2020) 8:561639. doi: 10.3389/fped.2020.561639
68. Bersanini C, Khirani S, Ramirez A, Lofaso F, Aubertin G, Beydon N, et al. Nocturnal hypoxaemia and hypercapnia in children with neuromuscular disorders. Eur Respir J. (2012) 39:1206–12. doi: 10.1183/09031936.00087511
69. Fauroux B, Khirani S, Griffon L, Teng T, Lanzeray A, Amaddeo A. Non-invasive ventilation in children with neuromuscular disease. Front Pediatr. (2020) 8:482. doi: 10.3389/fped.2020.00482
70. Praud J-P. Long-term non-invasive ventilation in children: current use, indications, and contraindications. Front Pediatr. (2020) 8:584334. doi: 10.3389/fped.2020.584334
71. Sklar MC, Dres M, Rittayamai N, West B, Grieco DL, Telias I, et al. High-flow nasal oxygen versus noninvasive ventilation in adult patients with cystic fibrosis: a randomized crossover physiological study. Ann Intensive Care. (2018) 8:85. doi: 10.1186/s13613-018-0432-4
72. Chaudhuri D, Granton D, Wang DX, Burns KEA, Helviz Y, Einav S, et al. High-flow nasal cannula in the immediate postoperative period. Chest. (2020) 158:1934–46. doi: 10.1016/j.chest.2020.06.038
73. Laverdure F, Genty T, Rezaiguia-Delclaux S, Herve P, Stephan F. Ultrasound assessment of respiratory workload with high-flow nasal oxygen versus other noninvasive methods after chest surgery. J Cardiothorac Vasc Anesth. (2019) 33:3042–7. doi: 10.1053/j.jvca.2019.05.020
74. Thille AW, Coudroy R, Futier E. Does prophylactic use of high-flow nasal cannula in the immediate postoperative period actually decrease the risk of intubation? Chest. (2021) 159:2113–4. doi: 10.1016/j.chest.2020.11.066
75. Futier E, Paugam-Burtz C, Godet T, Khoy-Ear L, Rozencwajg S, Delay J-M, et al. Effect of early postextubation high-flow nasal cannula vs conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: a French multicentre randomised controlled trial (OPERA). Intensive Care Med. (2016) 42:1888–98. doi: 10.1007/s00134-016-4594-y
76. Huang H-W, Sun X-M, Shi Z-H, Chen G-Q, Chen L, Friedrich JO, et al. Effect of high-flow nasal cannula oxygen therapy versus conventional oxygen therapy and noninvasive ventilation on reintubation rate in adult patients after extubation: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care Med. (2018) 33:609–23. doi: 10.1177/0885066617705118
77. Lu Z, Chang W, Meng S-S, Zhang X, Xie J, Xu J-Y, et al. Effect of high-flow nasal cannula oxygen therapy compared with conventional oxygen therapy in postoperative patients: a systematic review and meta-analysis. BMJ Open. (2019) 9:e027523. doi: 10.1136/bmjopen-2018-027523
78. Granton D, Chaudhuri D, Wang D, Einav S, Helviz Y, Mauri T, et al. High-flow nasal cannula compared with conventional oxygen therapy or noninvasive ventilation immediately postextubation: a systematic review and meta-analysis. Crit Care Med. (2020) 48:e1129–36. doi: 10.1097/CCM.0000000000004576
79. Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients. JAMA. (2016) 316:1565. doi: 10.1001/jama.2016.14194
80. Rodriguez M, Ragot S, Coudroy R, Quenot J-P, Vignon P, Forel J-M, et al. Noninvasive ventilation vs. high-flow nasal cannula oxygen for preoxygenation before intubation in patients with obesity: a post hoc analysis of a randomized controlled trial. Ann Intensive Care. (2021). 11:114. doi: 10.1186/s13613-021-00892-8
81. Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. (2015) 43:574–83. doi: 10.1097/CCM.0000000000000743
82. Jhou H-J, Chen P-H, Lin C, Yang L-Y, Lee C-H, Peng C-K. High-flow nasal cannula therapy as apneic oxygenation during endotracheal intubation in critically ill patients in the intensive care unit: a systematic review and meta-analysis. Sci Rep. (2020) 10:3541. doi: 10.1038/s41598-020-60636-9
83. Hodgson KA, Owen LS, Kamlin CO, Roberts CT, Donath SM, Davis PG, et al. A multicentre, randomised trial of stabilisation with nasal high flow during neonatal endotracheal intubation (the SHINE trial): a study protocol. BMJ Open. (2020) 10:e039230. doi: 10.1136/bmjopen-2020-039230
84. Sud A, Patel A. THRIVE five years on and into the COVID-19 era. Br J Anaesth. (2021) 126:768–73. doi: 10.1016/j.bja.2020.12.030
85. Patel A, Nouraei SAR. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. (2015) 70:323–9. doi: 10.1111/anae.12923
86. Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. (2017) 118:232–8. doi: 10.1093/bja/aew401
87. Hodgson KA, Owen LS, Kamlin COF, Roberts CT, Newman SE, Francis KL, et al. Nasal high-flow therapy during neonatal endotracheal intubation. N Engl J Med. (2022) 386:1627–37. doi: 10.1056/NEJMoa2116735
88. Frat JP, Coudroy R, Thille AW. Non-invasive ventilation or high-flow oxygen therapy: when to choose one over the other? Respirology. (2019) 24:724–31. doi: 10.1111/resp.13435
89. Lin Y, Zhang X, Li L, Wei M, Zhao B, Wang X, et al. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: a randomized multicenter clinical trial. Gastrointest Endosc. (2019) 90:591–601. doi: 10.1016/j.gie.2019.06.033
90. Mazzeffi MA, Petrick KM, Magder L, Greenwald BD, Darwin P, Goldberg EM, et al. high-flow nasal cannula oxygen in patients having anesthesia for advanced esophagogastroduodenoscopy: HIFLOW-ENDO, a randomized clinical trial. Anesth Analg. (2021) 132:743–51. doi: 10.1213/ANE.0000000000004837
91. Kim SH, Bang S, Lee K-Y, Park SW, Park JY, Lee HS, et al. Comparison of high flow nasal oxygen and conventional nasal cannula during gastrointestinal endoscopic sedation in the prone position: a randomized trial. Can J Anaesth. (2021) 68:460–6. doi: 10.1007/s12630-020-01883-2
Keywords: high-velocity therapy, high-flow oxygen, respiratory failure, non-invasive ventilation, high flow nasal cannula, respiratory distress
Citation: Wyatt KD, Goel NN and Whittle JS (2022) Recent advances in the use of high flow nasal oxygen therapies. Front. Med. 9:1017965. doi: 10.3389/fmed.2022.1017965
Received: 12 August 2022; Accepted: 26 September 2022;
Published: 10 October 2022.
Edited by:
Murali Shyamsundar, Queen's University Belfast, United KingdomReviewed by:
Claudia Crimi, Gaspare Rodolico Hospital, ItalyBairbre AIne Mcnicholas, Saolta University Health Care Group, Ireland
Alison Bell, Saolta Hospital Group, Ireland, in collaboration with reviewer BM
Copyright © 2022 Wyatt, Goel and Whittle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica S. Whittle, d2hpdHRsZS5qZXNzaWNhQGdtYWlsLmNvbQ==
 Kara D. Wyatt
Kara D. Wyatt Neha N. Goel2
Neha N. Goel2