- 1Duke-National University of Singapore (NUS) Medical School, Singapore, Singapore
- 2National Heart Centre Singapore, Singapore, Singapore
- 3Department of Geriatric Medicine, Institute of Geriatrics and Active Ageing, Tan Tock Seng Hospital, Singapore, Singapore
- 4Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
Cardiac alterations in structure and function, namely, the left ventricle, have been intensely studied for decades, in association with aging. In recent times, there has been keen interest in describing myocardial changes that accompany skeletal muscle changes in older adults. Initially described as a cardio-sarcopenia syndrome where alterations in myocardial structure were observed particularly among older adults with skeletal muscle sarcopenia, investigations into this syndrome have spurred a fresh level of interest in the cardiac-skeletal muscle axis. The purpose of this perspective is to summarize the background for this “syndrome of concern,” review the body of work generated by various human aging cohorts, and to explore future directions and opportunities for understanding this syndrome.
Introduction
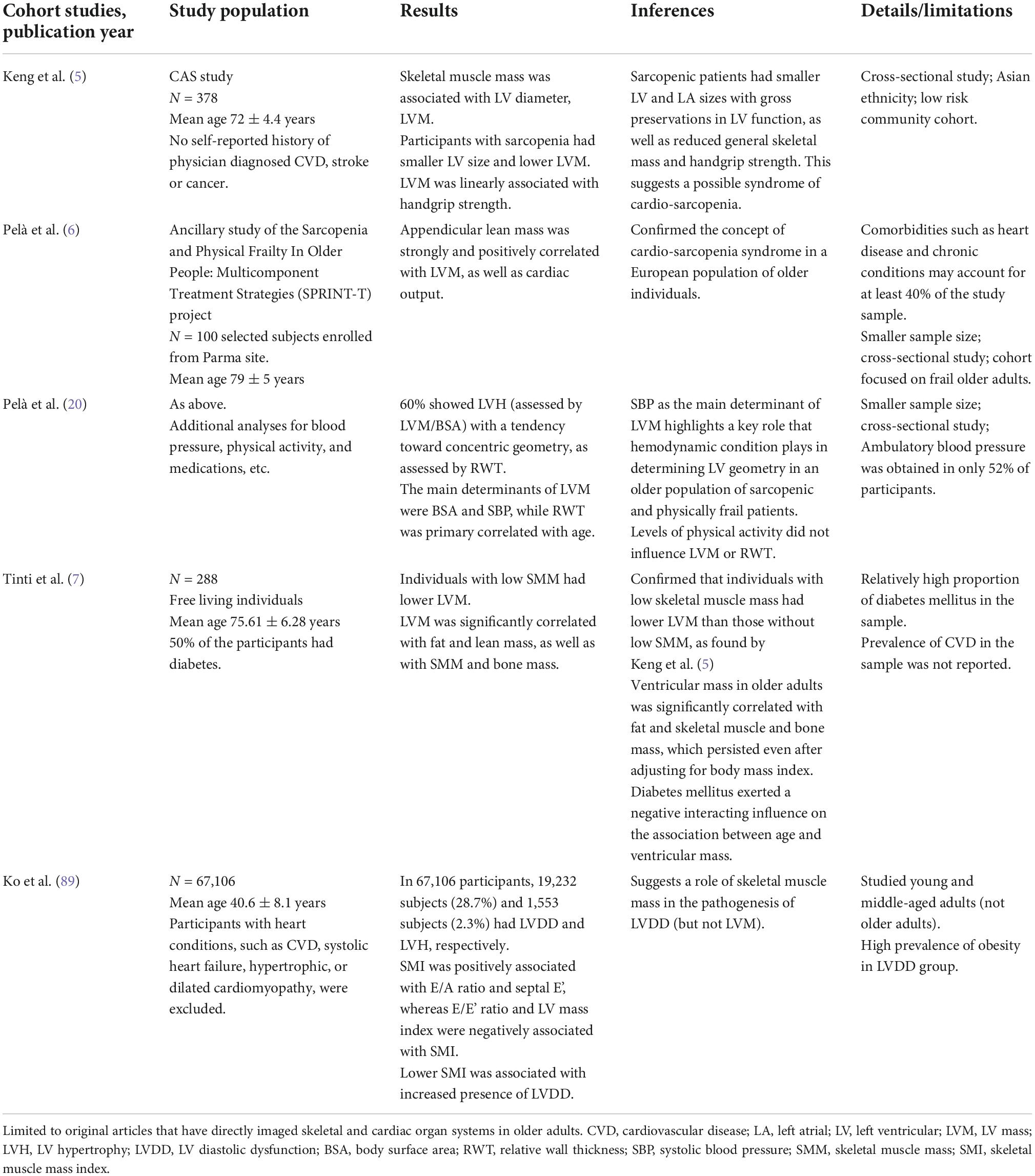
The traditional view of cardiovascular aging is that of age-related adaptations in the heart characterized by increased left ventricular (LV) mass (LVM) and LV hypertrophy (LVH), which are often secondary to increased systolic blood pressure mainly mediated by arterial stiffening (1, 2). These changes accumulate throughout the lifetime of an individual, increasing the risk of developing cardiovascular disease (CVD), such as heart failure (HF) and coronary artery disease (2). The incidence of CVD increases with age, rising from ∼78% among adults aged 60–79 years to ∼90% in those aged above 80 years (3). CVD is the leading cause of disease burden in the world, with global prevalence doubling from 271 million to 523 million between 1990 and 2019 (4). Incident CVD mortality increased from 12.1 million to 18.6 million in the same period (4), and accounted for 32% of all deaths. With rapidly aging national populations, these numbers are expected to increase. Despite the known association between cardiovascular aging and CVD, this knowledge has not translated into actionable changes that can specifically target aging-related CVD (2). This underscores the urgent need for in-depth study into the pathophysiology of cardiovascular aging and its prevention, and also highlights the unmet need for specific markers of cardiovascular aging that is “modifiable.” Emerging interests surrounding and research into a novel entity of “cardio-sarcopenia” have provided an added dimension to mainstream understanding of cardiovascular aging, and by extension, opened up new avenues for interventional strategies. This term was coined based on observations of associations between LVM and skeletal muscle mass and function that were independent of systemic risk factors such as hypertension and diabetes mellitus, as well as smaller left heart sizes in older adults with skeletal muscle sarcopenia (5). Observed among community older adults without clinical CVD, these distinctive patterns are hypothesis-generating for a possible syndrome of cardiac aging (5). Data from other cohorts have emerged that support relationships between markers of LV and skeletal muscle structure and function across the sarcopenia spectrum (6, 7).
Cardio-sarcopenia: A possible upstream marker of the heart failure spectrum
Skeletal muscle sarcopenia occurs with aging but may be accelerated in heart failure states (8). In advanced stages of heart failure, skeletal muscle wasting accompanied by severe exercise intolerance have long been observed in various cohorts (9–11). Several systemic and humoral mechanisms have been invoked as biological interactions (i.e., cross-talk) between the skeletal muscle system and the heart (12–15).
To date, observations pertaining to the cardiac muscle-skeletal muscle axis among non-heart failure cohorts have provided useful insights (Table 1). In a population-based cohort of older Asian subjects without clinical cardiovascular disease, skeletal muscle mass was associated with left ventricular mass, independent of age, diabetes mellitus status, and body size (5). In a selected cohort of frail sarcopenic older European subjects without severe cardiovascular disease (some had mild cardiovascular disease), appendicular lean mass was strongly associated with LVM and cardiac output (6). Although advanced age was associated with loss of skeletal muscle mass, the relationship between LVM and skeletal muscle mass appears to be independent of age (5, 7). Among 228 community adults aged 65–91 years, individuals with low skeletal muscle mass had lower LVM than those without low skeletal mass, without significant interaction between age and LVM (7). These observations are hypothesis-generating for possible age-related yet age-independent processes that mediate the cardiac and skeletal muscle systems in older persons.
Cardiac muscle: So, is it big or not big with aging?
The observations seem to run counter to the dogma of aging-associated LVH, especially in the context of hypertension which dominates aging. Traditionally, cardiac aging has been associated with increased LV wall thickness, with or without myocyte hypertrophy (16, 17). High LVM, and not low LVM, has been deemed to be clinically unfavorable (18). Among 3,220 subjects enrolled in the Framingham Heart Study who were 40 years of age or older and free of CVD who were followed up over 4 years, increments in LVM (corrected for height) predicted higher incidence of clinical events, including death, attributable to CVD (18). Interestingly, an earlier report from Framingham had also observed associations between subscapular skinfold thickness (and body mass index, among other variables) and LVM, in multivariable analyses, which may suggest that markers of lean body mass are indeed correlated with LVM (19). These historical considerations appropriately place most of the clinical emphasis on the significance of a large LVM.
In contrast, data from sarcopenic subjects suggest that the spotlight on “LVM” could be widened to include the “lower” end of the LVM spectrum. The observed correlates between LVM and specific measurements of skeletal muscle mass/function, emphasize a need to consider the skeletal muscle system as a possible variable in the evaluation of LVM, in addition to body surface area which is routinely calculated in indexed LVM measurements. This may be particularly important in clinical studies where older adults are the focus. Among older adults, there may be interactions between skeletal muscle sarcopenia and LVM. Aging appears to be associated with lower LVM among sarcopenic subjects (5, 7), suggesting that low LVM may be a phenotype of concern in older adults. Future longitudinal studies are needed to observe how subjects with low LVM evolve over time. However, concentric remodeling and LVH appear to occur when higher levels of physical frailty and sarcopenia are reached, albeit cross-sectionally (20). More understanding of events that occur between low LVM at lower levels of sarcopenia and LVH at higher levels of sarcopenia, would be critical. It is plausible that in the presence of sarcopenia, physiological adaptative responses are exceeded such that the development of LVH becomes pathologically significant. This hypothesis would have implications for the intensity and urgency of skeletal muscle management, in addition to routine hypertension management, in sarcopenic older adults.
The mechanism through which low LVM occurs in the setting of sarcopenia is unknown but potentially intriguing. Studies from autopsy specimens reveal that aging is associated with progressive attrition of myocyte numbers, in addition to hypertrophy of the remaining myocytes (21). While much of the developed literature have focused on myocyte hypertrophy, there is far less understanding about myocyte attrition, as a possible upstream phenomenon that occurs prior to adaptive hypertrophy. Cardiac muscle atrophy can occur in response to chronically reduced cardiac workload or to inflammatory disease states such as cancer (22). Interestingly, the failing heart can shrink and yet become stronger (23). In the studied cohorts of sarcopenic older patients with alterations in LVM, this phenomenon could be interpreted as pathologically adaptive with initial preservation of ejection fraction. Hence, there is value in targeting cardio-sarcopenia as an upstream phenotypic syndrome of concern in aging.
Skeletal muscle sarcopenia and cardiac aging: Which one came first, or does it matter?
Recent literature has shown that patients with HF have worse muscle function and atrophy, because of pathological alterations including altered metabolism, energetics, and decreased oxidative capacity (24–27). Possible metabolic pathways linking physical activity, cardiovascular health, and musculoskeletal function with aging have also been described and summarized using insights from metabolomics (28). Of note, genes involved in fatty acid oxidation and glucose metabolism are upregulated by physical activity, whereas these changes are absent or reversed in HF (29). These differences in metabolic gene expression demonstrate that maladaptive cardiac hypertrophy elicited by pathological stimuli should be differentiated from adaptive exercise-induced hypertrophy.
Most of the literature that are involved with dysregulated skeletal muscles in clinical HF have provided us with some understanding of possibly shared pathways. It is therefore reasonable to suggest that a cross-talk may in fact pre-exist even before clinical CVD. Conjecturally, the cardio-sarcopenia syndrome likely results from a framework of partially shared pathways leading to the loss of functional mass involving more than one organ system, with a predilection for HF development. As there is a dearth of longitudinal studies tracking skeletal muscle mass and cardiac function prospectively, any suggestion of a causal link between the two remains speculative. Nevertheless, the co-occurrence of skeletal muscle sarcopenia and myocardial perturbations in older adults without and with co-morbidities observed by us and others may be consistent with common upstream pathological pathway (or pathways) associated with aging.
Recently, a chronic low-grade inflammatory state known as “inflammaging” has been described in older adults (30) that is characterized by elevations in blood inflammatory markers related to aging-related immune dysregulation, high circulating levels of proinflammatory markers such as interleukins, C-reactive protein (CRP), transforming growth factor-beta, tumor necrosis factor (TNF) and TNF receptors, including in those free of active disease (31–33). Observed in association with multi-systemic geriatric conditions, it has been postulated as a mechanism contributing to conditions ranging from sarcopenia to frailty and CVD (34–37). Interleukin (IL)-6 is a promising translational frailty biomarker in humans and mice (38). In a prospective, population-based study of 986 old men and women, high levels of serum IL-6 and CRP were associated with risks of muscle mass and strength loss (39). In another prospective cohort study of 620 old women, high serum IL-6 levels were associated with accelerated declines in muscle strength and physical function (40). IL-6 is a powerful independent predictor of HF (41, 42) and has been associated with impaired coronary flow and cardiac function, and worsening HF (43). Upregulated IL-6 activates gp130/STAT3 signaling, induces reactive oxygen species (ROS) production, leading to mitochondrial dysfunction and increased expression of mitophagy-related proteins, which results in cardiac hypertrophy in HF (44). Excessive ROS appears to aggravate ongoing inflammation, feeding a proinflammatory microenvironmental vicious cycle that exacerbates maladaptive myocardial remodeling and consequent HF manifestation (45, 46).
Insulin resistance features heavily in the biochemical cross-talk between the musculoskeletal and cardiovascular systems. Decreased mitochondrial function and increased inflammatory and oxidative stresses that are observed with skeletal muscle aging induce muscle atrophy and insulin resistance (47). The latter is central to an entity known as sarcopenic obesity (48), which is interestingly also associated with concentric LV remodeling independent of age-adjusted indexed body mass (49). From the metabolic perspective, it is plausible that insulin resistance mediates the cardiac remodeling associated with sarcopenic obesity, along with contributions by fat and inflammation. Further research chronicling the development of insulin resistance, sarcopenia, and cardiac remodeling (and HF) in aging human subjects is urgently needed to confirm this mechanistic link. If true, age-associated sarcopenia might be a pre-disease state that is amenable to upstream preventive strategies, e.g., exercise training, to avert clinical CVD.
Given the increased incidence of HF among older adults with increased risk of sarcopenia, these additional insights would support early preventative and/or therapeutic strategies that target specific aspects of skeletal muscles, potentially benefitting frail older adults who have yet to develop heart disease. For one, it highlights the potential of using screening tools such as SARC-F to identify older population with sarcopenia as an approach to detect early cardiac dysfunction in the community (50). The cardio-sarcopenic phenotype could also be targeted as a modifiable risk factor that may be ameliorated by interventions.
Current detection tools
When considering the potential for scalability for case detection of older adults with cardio-sarcopenia syndrome in the geriatric population, detection tools should be easily accessible, safe with minimal radiation, non-invasive and robust. Currently, muscle mass can be estimated using bioelectrical impedance analysis (BIA) or dual-energy X-ray absorptiometry (DEXA) (5, 51), while myocardial structure and function can be assessed using echocardiography or cardiovascular magnetic resonance imaging, with newer variants including handheld echocardiography and the combined use of artificial intelligence tools (52, 53).
Importantly, some of these tools such as DEXA for body composition can be tagged onto existing imaging procedures for osteoporosis assessment of bone mineral density. Where available, the use of validated multi-frequency BIA facilitates access to measurement of muscle mass in the community setting, Lastly, as per the diagnostic algorithms of recent consensus statements for sarcopenia, the assessment of handgrip strength using a handheld dynamometer allows detection of “possible sarcopenia” defined by low muscle strength, circumventing the need for muscle mass measurement (54). Taken together, this allows clinicians to make more comprehensive risk assessment which combines inputs about the skeletal muscle in conjunction with more objective measurements of patients’ cardiac state (e.g., LVM).
Potential to improve human health
Changing the focus from disease management to prevention is a paradigm shift that calls attention to the need for a systems approach to tackling cardiac aging and its complexities. While current HF guidelines recognizes the intimate link between sarcopenia and heart failure, more granular upstream preventative actions before the onset of clinical heart disease can potentially avert joint deteriorations in both organ systems. It is therefore timely to advocate stronger population-based preventative efforts for improving the health of older adults.
Exercise is important for maintaining and promoting skeletal and cardiac muscle health. Resistance training is effective for inducing skeletal muscle growth in older adults (55–57). In addition, there is growing evidence that aerobic exercise favorably affects various mechanisms that collectively stimulate skeletal muscle hypertrophy, and should therefore be considered as a viable exercise prescription in populations prone to muscle loss (58).
Dietary protein constitutes a primary nutrient for maintenance and growth of skeletal muscles (59). To combat sarcopenia, dietary guidelines recommend high protein intake for older adults, who are prone to low energy intake and muscle loss (60, 61). Individual nutrient effects on age-related skeletal muscle preservation (and cardioprotection) are less clear. To date, many studies on dietary fat and diverse micronutrients like whey, casein, Vitamin D, and antioxidants have yielded few definitive conclusions (62, 63).
Angiotensin-converting enzyme inhibitors (ACE-I) are routinely prescribed to patients with hypertension, HF and diabetes due to their beneficial effects on the vasculature and cardiovascular outcomes. At the molecular level, ACE-I promotes glucose uptake (64) and suppresses proinflammatory cytokines (especially IL-6) in skeletal muscles (65). In older adults without HF, ACE-I therapy has been shown to retard loss in muscle strength (66) and increase both muscle strength (67) and exercise capacity (68). Therefore, ACE-I or related pathways constitute promising therapeutic targets for sarcopenia.
On the other hand, sodium-glucose cotransporter-2 (SGLT2) inhibitors have recently been shown to confer renal and cardiometabolic benefits in diabetic and non-diabetic subjects (69–73) but may be associated with myopathy (74) and sarcopenia (75, 76) which will be of concern to aged adults with low baseline pre-treatment skeletal muscle mass. There is potential for SGLT2 inhibitors to be studied as a candidate drug targeting aging-related CVD, although the totality of clinical effectiveness for this class of therapeutics may depend on the needs of specific patient cohorts.
Cardio-sarcopenia—Next steps
Firstly, there is a need for mechanistic understanding of the cross-talk between the various components of body composition and the cardiac muscles. Earlier research has revolved around obesity and its association with cardiovascular structure and function (77, 78). With emerging interest in skeletal muscles, sarcopenia enters the equation for the consideration of LVM. Notwithstanding the relative degree of involvement by adipose tissues or skeletal muscles, there is now greater awareness and scientific acknowledgment that there are close relationships between body composition and heart structure and function. This is in line with the opinion that the observed degenerative bodily changes in older adults is the result of the complex interplay between body composition, the cardiovascular system and aging process (79), far more granular than the body mass index metric (78).
In this regard, several studies have already demonstrated increased Framingham score or CVD risk in sarcopenic and/or obese older adults (80–82). In a large epidemiological study among the Korean population, it was observed that visceral obesity and low muscle mass may be patho-physiologically related, possibly through insulin resistance and inflammation, leading to subclinical LV changes independently and synergistically (83). Further elucidation of biological mechanisms that underlie these cardiometabolic alterations may require animal models.
Secondly, the cohort of older sarcopenic population should be followed up longitudinally with clear documentation of the changes in their skeletal and cardiac muscles as well as their cardiovascular status. This would provide clarity about the development of the cardio-sarcopenia syndrome in relation to the cardiac structure and function over time, which cannot be accomplished by cross-sectional studies that can only make associations at a single point in time. Concurrent biomarker annotation would provide additional mechanistic insights into the development of cardiac aging (84), useful for future clinical translation.
Thirdly, appropriate clinical trials should be conducted to evaluate the amount of change that is necessary to impact clinical outcomes. For example, in patients with clinically stable chronic HF (predominantly New York Heart Association class II or III), resistance exercise training can improve muscle strength (85, 86). More variability such as intensity and type of exercise should be trialed to determine the optimal level and type of physical exercise intervention for these patients. In addition to monitoring changes in the skeletal muscles, the cardiac structure and function can be concurrently assessed for any improvement over the course of the intervention. These features are not only therapeutic targets, but also markers requiring periodic surveillance, like blood pressure and low-density lipoprotein. The conduct of these interventional studies should additionally evaluate the impact of multi-component interventions such as nutrition, psychosocial support, and strategies personalized to individual needs (87).
Conclusion
In conclusion, the concept of cardio-sarcopenia has evolved from a syndrome which describes the coexistence of alterations in myocardial structure with skeletal muscle sarcopenia in older adults to portend pathophysiologic derangements in the cardiac-skeletal muscle axis. Emerging evidence implicating the involvement of adipose tissue, such as sarcopenic obesity (88), highlights the need to examine deeper into the muscle-, fat- and myocardium triad. From the preventative standpoint, the cardio-sarcopenic phenotype constitutes a potentially modifiable risk factor in older persons that may be amenable to early multi-modal intervention involving physical activity, resistance exercise, and nutrition. This raises the clarion call for greater inter-disciplinary collaboration between cardiologists, geriatricians, bio-scientists, exercise therapists, and nutrition specialists to push the frontiers in both research and clinical practice against this syndrome of concern in aging.
Author contributions
DRL, WSL, and ASK contributed to the conception and design of the manuscript. RST, DRL, WSL, and ASK performed literature critiques and contributed to the writing of the manuscript. All authors critically reviewed previous drafts and approved the final draft for submission.
Funding
ASK received funding support from the National Medical Research Council of Singapore (MOH-000153, HLCA21Jan-0052), Hong Leong Foundation, Duke-NUS Medical School, Estate of Tan Sri Khoo Teck Puat, and Singhealth Foundation. WSL received funding support from the National Medical Research Council of Singapore (MOH/NIC/HAIG03/2017), Lee Foundation, and the National Health Group. The funders had no role in the preparation, review, or approval of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lakatta EG. So! what’s aging? Is cardiovascular aging a disease? J Mol Cell Cardiol. (2015) 83:1–13. doi: 10.1016/j.yjmcc.2015.04.005
2. Paneni F, Cañestro CD, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: Understanding it at the cellular and clinical levels. J Am Coll Cardiol. (2017) 69:1952–67. doi: 10.1016/J.JACC.2017.01.064
3. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. (2019) 139:e56–528. doi: 10.1161/CIR.0000000000000659
4. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the Gbd 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
5. Keng BMH, Gao F, Teo LLY, Lim WS, Tan RS, Ruan W, et al. Associations between skeletal muscle and myocardium in aging: A syndrome of “cardio-sarcopenia”? J Am Geriatr Soc. (2019) 67:2568–73. doi: 10.1111/jgs.16132
6. Pelà G, Tagliaferri S, Perrino F, Bussolati G, Longobucco Y, Zerbinati L, et al. Interaction of skeletal and left ventricular mass in older adults with low muscle performance. J Am Geriatr Soc. (2021) 69:148–54. doi: 10.1111/jgs.16812
7. Tinti MG, Scillitani A, Guglielmi G, Nieddu L, Carnevale V. Left ventricular mass and parameters of body composition in older adults. Mayo Clin Proc. (2022) 97:626–8. doi: 10.1016/j.mayocp.2022.01.001
8. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 Esc guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (Esc) with the special contribution of the heart failure association (Hfa) of the Esc. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
9. Adams V, Linke A, Winzer E. Skeletal muscle alterations in Hfref Vs. Hfpef. Curr Heart Fail Rep. (2017) 14:489–97. doi: 10.1007/s11897-017-0361-9
10. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (Sica-Hf). Eur Heart J. (2013) 34:512–9. doi: 10.1093/eurheartj/ehs381
11. Saitoh M, Ishida J, Doehner W, Haehling Sv, Anker MS, Coats AJS, et al. Sarcopenia, cachexia, and muscle performance in heart failure: Review update 2016. Int J Cardiol. (2017) 238:5–11. doi: 10.1016/j.ijcard.2017.03.155
12. Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, et al. Sarcopenia and heart failure. Nutrients. (2020) 12:211. doi: 10.3390/nu12010211
13. Furihata T, Kinugawa S, Fukushima A, Takada S, Homma T, Masaki Y, et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int J Cardiol. (2016) 220:483–7. doi: 10.1016/j.ijcard.2016.06.231
14. Loncar G, Fülster S, Haehling Sv, Popovic V. Metabolism and the heart: An overview of muscle, fat, and bone metabolism in heart failure. Int J Cardiol. (2013) 162:77–85. doi: 10.1016/j.ijcard.2011.09.079
15. Seiler M, Bowen TS, Rolim N, Dieterlen M-T, Werner S, Hoshi T, et al. Skeletal muscle alterations are exacerbated in heart failure with reduced compared with preserved ejection fraction. Circulation. (2016) 9:e003027. doi: 10.1161/CIRCHEARTFAILURE.116.003027
16. Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. (1993) 73:413–67. doi: 10.1152/physrev.1993.73.2.413
17. Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises. Circulation. (2003) 107:139–46. doi: 10.1161/01.CIR.0000048892.83521.58
18. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. (1990) 322:1561–6. doi: 10.1056/NEJM199005313222203
19. Savage DD, Levy D, Dannenberg AL, Garrison RJ, Castelli WP. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study). Am J Cardiol. (1990) 65:371–6. doi: 10.1016/0002-9149(90)90304-J
20. Pelà G, Tagliaferri S, Perrino F, Righelli I, Montanari R, Longobucco Y, et al. Determinants of cardiac structure in frail and sarcopenic elderly adults. Exp Gerontol. (2021) 150:111351. doi: 10.1016/j.exger.2021.111351
21. Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation Res. (1991) 68:1560–8. doi: 10.1161/01.RES.68.6.1560
22. Harvey PA, Leinwand LA. Cardiac atrophy and remodeling. In: Willis MS, Homeister JW, Stone JR editor. Cellular and Molecular Pathobiology of Cardiovascular Disease. Cambridge, MA: Academic Press (2014). p. 37–50. doi: 10.1016/B978-0-12-405206-2.00003-X
23. Baskin KK, Taegtmeyer H. Taking pressure off the heart: The ins and outs of atrophic remodelling. Cardiovasc Res. (2011) 90:243–50. doi: 10.1093/cvr/cvr060
24. Bekfani T, Elsaied MB, Derlien S, Nisser J, Westermann M, Nietzsche S, et al. Skeletal muscle function, structure, and metabolism in patients with heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. Circulation. (2020) 13:e007198. doi: 10.1161/CIRCHEARTFAILURE.120.007198
25. Anderson M, Parrott CF, Haykowsky MJ, Brubaker PH, Ye F, Upadhya B. Skeletal muscle abnormalities in heart failure with preserved ejection fraction. Heart Fail Rev. (2022). doi: 10.1007/s10741-022-10219-9
26. Saw EL, Ramachandran S, Valero-Muñoz M, Sam F. Skeletal muscle (Dys)function in heart failure with preserved ejection fraction. Curr Opin Cardiol. (2021) 36:219–26. doi: 10.1097/HCO.0000000000000824
27. Zamani P, Proto E, Wilson N, Fazelinia H, Ding H, Spruce L, et al. Multimodality assessment of heart failure with preserved ejection fraction skeletal muscle reveals differences in the machinery of energy fuel metabolism. ESC Heart Fail. (2021) 8:2698–712. doi: 10.1002/ehf2.13329
28. Lim RMH, Koh AS. Cardiovascular aging and physical activity: Insights from metabolomics. Front Cardiovasc Med. (2021) 8:728228. doi: 10.3389/fcvm.2021.728228
29. Strøm CC, Aplin M, Ploug T, Christoffersen TEH, Langfort J, Viese M, et al. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. (2005) 272:2684–95. doi: 10.1111/j.1742-4658.2005.04684.x
30. Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
31. Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, et al. Proinflammatory State, hepcidin, and anemia in older persons. Blood. (2010) 115:3810–6. doi: 10.1182/blood-2009-02-201087
32. Cohen HJ, Pieper CF, Harris T, Rao KMK, Currie MS. The association of plasma Il-6 levels with functional disability in community-dwelling elderly. J Gerontol Ser A Biol Sci Med Sci. (1997) 52A:M201–8. doi: 10.1093/gerona/52A.4.M201
33. Gerli R, Monti D, Bistoni O, Mazzone AM, Peri G, Cossarizza A, et al. Chemokines, Stnf-Rs and Scd30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. (2001) 121:37–46. doi: 10.1016/S0047-6374(00)00195-0
34. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. (2017) 8:1045. doi: 10.3389/fphys.2017.01045
35. Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis. J Am Coll Cardiol. (2009) 54:2129–38. doi: 10.1016/j.jacc.2009.09.009
36. Forman DE, Maurer MS, Boyd C, Brindis R, Salive ME, Horne FM, et al. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. (2018) 71:2149–61. doi: 10.1016/j.jacc.2018.03.022
37. Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. (2009) 13:3103–9. doi: 10.1111/j.1582-4934.2009.00733.x
38. Heinze-Milne SD, Banga S, Howlett SE. Frailty and cytokines in preclinical models: Comparisons with humans. Mech Ageing Dev. (2022) 206:111706. doi: 10.1016/j.mad.2022.111706
39. Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (Sarcopenia) and strength. Am J Med. (2006) 119:.e9–17. doi: 10.1016/j.amjmed.2005.10.049
40. Ferrucci L, Penninx BWJH, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. (2002) 50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x
41. Orús J, Roig E, Perez-Villa F, Paré C, Azqueta M, Filella X, et al. Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant. (2000) 19:419–25. doi: 10.1016/S1053-2498(00)00083-8
42. Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. (2000) 102:3060–7. doi: 10.1161/01.CIR.102.25.3060
43. Su J-H, Luo M-Y, Liang N, Gong S-X, Chen W, Huang W-Q, et al. Interleukin-6: A novel target for cardio-cerebrovascular diseases. Front Pharmacol. (2021) 12:745061. doi: 10.3389/fphar.2021.745061
44. Huo S, Shi W, Ma H, Yan D, Luo P, Guo J, et al. Alleviation of inflammation and oxidative stress in pressure overload-induced cardiac remodeling and heart failure Via Il-6/Stat3 inhibition by raloxifene. Oxid Med Cell Longev. (2021) 2021:1–15. doi: 10.1155/2021/6699054
45. van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: Past, Present and Future. Eur J Heart Fail. (2019) 21:425–35. doi: 10.1002/ejhf.1320
46. Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, et al. Targeting the vicious inflammation–oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. (2010) 13:1033–49. doi: 10.1089/ars.2009.2930
47. Shou J, Chen P-J, Xiao W-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol Metab Syndr. (2020) 12:14. doi: 10.1186/s13098-020-0523-x
48. Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J Endocrinol. (2016) 229:R67–81. doi: 10.1530/JOE-15-0533
49. Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR, Wang L, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox. J Am Coll Cardiol. (2013) 61:1698–706. doi: 10.1016/j.jacc.2013.01.053
50. Yang X, Woo J, Lui LT, Li Q, Cheng KF, Fan Y, et al. Cardiac manifestations of sarcopenia. J Nutr Health I Aging. (2020) 24:478–84. doi: 10.1007/s12603-020-1358-8
51. Völgyi E, Tylavsky FA, Lyytikäinen A, Suominen H, Alén M, Cheng S. Assessing body composition with dxa and bioimpedance: Effects of obesity, physical activity, and age. Obesity. (2008) 16:700–5. doi: 10.1038/oby.2007.94
52. Chamsi-Pasha MA, Sengupta PP, Zoghbi WA. Handheld echocardiography. Circulation. (2017) 136:2178–88. doi: 10.1161/CIRCULATIONAHA.117.026622
54. Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Medl Direct Assoc. (2020) 21:300.e–7.e. doi: 10.1016/j.jamda.2019.12.012
55. Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. (2003) 95:1717–27. doi: 10.1152/japplphysiol.00347.2003
56. Fiatarone MA. High-intensity strength training in nonagenarians: Effects on skeletal muscle. JAMA. (1990) 263:3029. doi: 10.1001/jama.1990.03440220053029
57. Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥76 Yr old. Am J Physiol Endocrinol Metab. (1999) 277:E118–25. doi: 10.1152/ajpendo.1999.277.1.E118
58. Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. (2014) 42:53–61. doi: 10.1249/JES.0000000000000007
59. Carbone JW, Pasiakos SM. Dietary protein and muscle mass: Translating science to application and health benefit. Nutrients. (2019) 11:1136. doi: 10.3390/nu11051136
60. Deutz NEP, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the espen expert group. Clin Nutr. (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
61. Phillips SM, Chevalier S, Leidy HJ. Protein “requirements” beyond the Rda: Implications for optimizing health. Appl Physiol Nutr Metab. (2016) 41:565–72. doi: 10.1139/apnm-2015-0550
62. Casas R, Castro-Barquero S, Estruch R, Sacanella E. Nutrition and cardiovascular health. IJMS. (2018) 19:3988. doi: 10.3390/ijms19123988
63. Welch AA. Nutritional influences on age-related skeletal muscle loss. Proc Nutr Soc. (2014) 73:16–33. doi: 10.1017/S0029665113003698
64. Dietze GJ, Henriksen EJ. Review: Angiotensin-converting enzyme in skeletal muscle: Sentinel of blood pressure control and glucose homeostasis. J Renin Angiotensin Aldosterone Syst. (2008) 9:75–88. doi: 10.3317/jraas.2008.011
65. Gullestad L, Aukrust P, Ueland T, Espevik T, Yee G, Vagelos R, et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J Am Coll Cardiol. (1999) 34:2061–7. doi: 10.1016/S0735-1097(99)00495-7
66. Onder G, Penninx BWJH, Balkrishnan R, Fried LP, Chaves PHM, Williamson J, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: An observational study. Lancet. (2002) 359:926–30. doi: 10.1016/S0140-6736(02)08024-8
67. Di Bari M, Van De Rong Loh Poll-Franse LV, Onder G, Kritchevsky SB, Newman A, Harris TB, et al. Antihypertensive medications and differences in muscle mass in older persons: The health, aging and body composition study: Antihypertensive drugs and muscle mass. J Am Geriatr Soc. (2004) 52:961–6. doi: 10.1111/j.1532-5415.2004.52265.x
68. Sumukadas D, Witham MD, Struthers AD, McMurdo MET. Effect of perindopril on physical function in elderly people with functional impairment: A randomized controlled trial. Can Med Assoc J. (2007) 177:867–74. doi: 10.1503/cmaj.061339
69. Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol. (2015) 309:F889–900. doi: 10.1152/ajprenal.00267.2015
70. Gallo LA, Wright EM, Vallon V. Probing Sglt2 as a therapeutic target for diabetes: Basic physiology and consequences. Diab Vasc Dis Res. (2015) 12:78–89. doi: 10.1177/1479164114561992
71. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387:1089–98. doi: 10.1056/NEJMoa2206286
72. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (Sglt2) inhibitors. JACC. (2020) 5:632–44. doi: 10.1016/j.jacbts.2020.02.004
73. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors. J Am Coll Cardiol. (2020) 75:422–34. doi: 10.1016/j.jacc.2019.11.031
74. Gupta R, Alcantara R, Popli T, Mahajan S, Tariq U, Dusaj RS, et al. Myopathy associated with statins and Sglt2 – a review of literature. Curr Probl Cardiol. (2021) 46:100765. doi: 10.1016/j.cpcardiol.2020.100765
75. Sargeant JA, Henson J, King JA, Yates T, Khunti K, Davies MJA. Review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab. (2019) 34:247. doi: 10.3803/EnM.2019.34.3.247
76. Sasaki T. Sarcopenia, frailty circle and treatment with sodium-glucose cotransporter 2 inhibitors. J Diabetes Investig. (2019) 10:193–5. doi: 10.1111/jdi.12966
77. Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, et al. Longitudinal tracking of left ventricular mass over the adult life course. Circulation. (2009) 119:3085–92. doi: 10.1161/CIRCULATIONAHA.108.824243
78. Tan YH, Lim JP, Lim WS, Gao F, Teo LLY, Ewe SH, et al. Obesity in older adults and associations with cardiovascular structure and function. Obes Facts. (2022) 15:336–43. doi: 10.1159/000521729
79. Chumlea WC, Schubert CM, Towne B, Siervogel RM, Sun SS, Chumlea WC, et al. Left ventricular mass, abdominal circumference and age: The fels longitudinal study. J Nutr Health Aging. (2009) 13:821–5. doi: 10.1007/s12603-009-0219-2
80. Byeon CH, Kang KY, Kang SH, Bae EJ. Sarcopenia is associated with framingham risk score in the Korean population: Korean national health and nutrition examination survey (Knhanes) 2010-2011. J Geriatr Cardiol. (2015) 12:366–72. doi: 10.11909/j.issn.1671-5411.2015.04.007
81. Chen Y-Y, Chen W-L, Peng T-C, Liaw F-Y, Chao Y-P, Kao T-W. Relationship between sarcopenia and cardiovascular disease risk among taiwanese older adults. Public Health Nutr. (2022) 1–21. doi: 10.1017/S1368980022000684
82. Tyrovolas S, Panagiotakos D, Georgousopoulou E, Chrysohoou C, Tousoulis D, Haro JM, et al. Skeletal muscle mass in relation to 10 year cardiovascular disease incidence among middle aged and older adults: The Attica study. J Epidemiol Community Health. (2020) 74:26. doi: 10.1136/jech-2019-212268
83. Park J, Kim NH, Kim SH, Kim J-S, Kim YH, Lim HE, et al. Visceral adiposity and skeletal muscle mass are independently and synergistically associated with left ventricular structure and function: The Korean genome and epidemiology study. Int J Cardiol. (2014) 176:951–5. doi: 10.1016/j.ijcard.2014.08.108
84. Koh AS, Kovalik J-P. Metabolomics and cardiovascular imaging: A combined approach for cardiovascular ageing. ESC Heart Fail. (2021) 8:1738–50. doi: 10.1002/ehf2.13274
85. Savage PA, Shaw AO, Miller MS, Vanburen P, Lewinter MM, Ades PA, et al. Effect of resistance training on physical disability in chronic heart failure. Med Sci Sports Exerc. (2011) 43:1379–86. doi: 10.1249/MSS.0b013e31820eeea1
86. Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD, et al. Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. J Cardiac Fail. (2004) 10:21–30. doi: 10.1016/S1071-9164(03)00583-9
87. Loh DR, Yeo SY, Tan RS, Gao F, Koh AS. Explainable machine learning predictions to support personalized cardiology strategies. Eur Heart J Digit Health. (2022) 3:49–55. doi: 10.1093/ehjdh/ztab096
88. Khor EQ-E, Lim JP, Tay L, Yeo A, Yew S, Ding YY, et al. Obesity definitions in sarcopenic obesity: Differences in prevalence, agreement and association with muscle function. J Frailty Aging. (2020) 9:37–43. doi: 10.14283/jfa.2019.28
Keywords: aging, cardiovascular disease, myocardium, sarcopenia, frailty, prevention
Citation: Loh DR, Tan R-S, Lim WS and Koh AS (2022) Cardio-sarcopenia: A syndrome of concern in aging. Front. Med. 9:1027466. doi: 10.3389/fmed.2022.1027466
Received: 25 August 2022; Accepted: 11 October 2022;
Published: 25 October 2022.
Edited by:
Wook Song, Seoul National University, South KoreaReviewed by:
Luca Soraci, Unit of Geriatric Medicine, IRCCS INRCA, ItalyHyo-Bum Kwak, Inha University, South Korea
Copyright © 2022 Loh, Tan, Lim and Koh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela S. Koh, YW5nZWxhLmtvaC5zLm1Ac2luZ2hlYWx0aC5jb20uc2c=
 De Rong Loh
De Rong Loh Ru-San Tan
Ru-San Tan Wee Shiong Lim
Wee Shiong Lim Angela S. Koh
Angela S. Koh