- Division of Critical Care, Department of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Introduction: Data on the characteristics and outcomes of patients hospitalized for Coronavirus Disease 2019 (COVID-19) in Thailand are limited.
Objective: To determine characteristics and outcomes and identify risk factors for hospital mortality for hospitalized patients with COVID-19.
Methods: We retrospectively reviewed the medical records of patients who had COVID-19 infection and were admitted to the cohort ward or ICUs at Siriraj Hospital between January 2020 and December 2021.
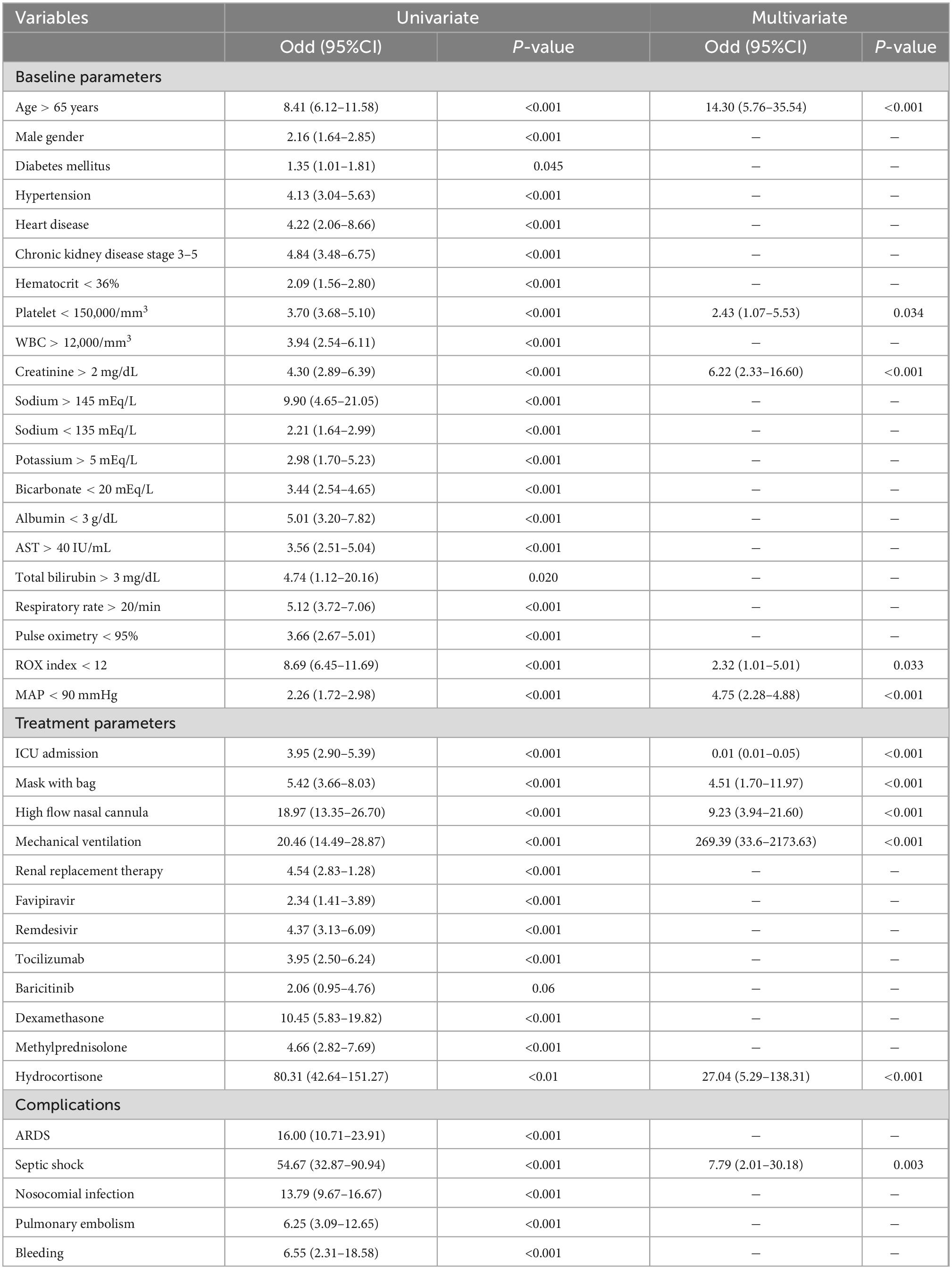
Results: Of the 2,430 patients included in this study, 229 (9.4%) died; the mean age was 54 years, 40% were men, 81% had at least one comorbidity, and 13% required intensive care unit (ICU). Favipiravir (86%) was the main antiviral treatment. Corticosteroids and rescue anti-inflammatory therapy were used in 74 and 6%, respectively. Admission to the ICU was the only factor associated with reduced mortality [odds ratio (OR) 0.01, 95% confidence interval (CI) 0.01–0.05, P < 0.001], whereas older age (OR 14.3, 95%CI 5.76–35.54, P < 0.001), high flow nasal cannula (HFNC; OR 9.2, 95% CI 3.9–21.6, P < 0.001), mechanical ventilation (OR 269.39, 95%CI 3.6–2173.63, P < 0.001), septic shock (OR 7.79, 95%CI, 2.01–30.18, P = 0.003), and hydrocortisone treatment (OR 27.01, 95%CI 5.29–138.31, P < 0.001) were factors associated with in-hospital mortality.
Conclusion: The overall mortality of hospitalized patients with COVID-19 was 9%. The only factor associated with reduced mortality was admission to the ICU. Therefore, appropriate selection of patients for admission to the ICU, strategies to limit disease progression and prevent intubation, and early detection and prompt treatment of nosocomial infection can improve survival in these patients.
Introduction
COVID-19 (Corona virus disease 2019) is an emerging disease declared by the WHO (World Health Organization) as a Public Health Emergency of International Concern in January 2020. Since the first case reported from China in December 2019, more than 500 million people have been infected worldwide, with an overall mortality rate of 1.14% (1), a mortality rate 17–28% for hospitalized patients, and 49–60% for mechanically ventilated patients (2–10). Risk factors for increased mortality from previous studies included older age, pre-existing medical illnesses, high Sequential Organ Failure Assessment (SOFA) score, receipt of IMV, acute respiratory distress syndrome (ARDS), and elevated D-dimer (2, 6, 9, 11, 12). However, most data were reported from China and developed countries, while the epidemiological data for COVID-19 diseases from low- and middle-income countries are lacking. The limitation of healthcare resources and treatment capacity may impact patient outcomes. This study aimed to determine the characteristics and outcomes and identify risk factors for in-hospital mortality for hospitalized COVID-19 patients in Thailand.
Materials and methods
Study design and population
This is a retrospective cohort study conducted at Siriraj Hospital, a tertiary care academic hospital in Bangkok, Thailand. The study protocol was approved by the Human Research Protection Unit of Siriraj Hospital Faculty of Medicine, Mahidol University. The requirement for written informed consent was waived.
In Thailand, the COVID-19 pandemic has occurred since January 2020. All COVID-19 patients who require admission to our hospital are treated in cohort wards and intensive care units (ICU) with modified airborne infection isolation rooms (AIIR). The patients requiring vasopressors, high flow nasal cannula (HFNC), non-invasive ventilation (NIV), or invasive mechanical ventilation (IMV) were admitted to the ICUs when beds were available. Guideline for critical care management of severe COVID-19 patients in our hospital has been previously published (13–15). Moreover, the recommendation for antiviral and anti-inflammatory therapies for COVID-19 patients in Thailand have been changed regularly according to the updated published data (16, 17). Since the publication of the preliminary report of the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial (18), dexamethasone has been recommended for all patients with a resting oxygen saturation of < 96% or a reduction in oxygen saturation of ≥ 3% after exercise-induced desaturation or those requiring respiratory support. However, before the publication of the RECOVERY study, some critically ill patients who had rapidly worsening respiratory symptoms with evidence of hyperinflammation and were less likely to have superimposed bacterial infections received corticosteroid therapy as previously described (13).
In this study, we investigated all adult patients aged ≥ 18 years diagnosed with COVID-19 and admitted to the cohort wards and ICUs at Siriraj Hospital from January 2020 to December 2021. The diagnosis of COVID-19 disease was confirmed by detecting severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) from any respiratory specimens by reverse transcription-polymerase chain reaction (RT-PCR). Patients who were readmitted due to diagnoses other than reinfection of COVID-19 were excluded.
Data collection
We initially obtained data from electronic medical records (EMRs) in collaboration with Siriraj Informatics and Data Innovation Center (SiData +) and then manually reviewed the EMRs of all patients to verify the diagnosis of COVID-19 infections and recorded the data that could not be collected electronically.
The data collected included demographic data of the patient, comorbidities, vital signs, and laboratory results at admission, antiviral and anti-inflammatory medications used for the treatment of COVID-19 disease, admission to the ICU, the requirement for renal replacement therapy (RRT) and respiratory support, including oxygen therapy, HFNC, NIV, IMV. The respiratory rate oxygenation index (ROX), the ratio of oxygen saturation measured by pulse oximetry (SpO2)/FiO2 to respiratory rate, was also calculated in all patients (19).
Patient outcomes included survival at hospital discharge, ICU and hospital length of stay, complications during admission, including ARDS according to the Berlin Definition (20), nosocomial infections, septic shock, venous thromboembolism, and bleeding complications.
Statistical analysis
Categorical variables were presented as numbers and column percentages, while continuous variables were presented as mean with standard deviation (SD), or median with 25th and 75th quartiles, as appropriate. Categorical variables were compared using Chi-square or Fisher exact tests, while continuous variables were compared using the t-test or Mann-Whitney U-test, as appropriate. A multivariate logistic regression model with a stepwise forward method was used to identify independent clinical risk factors associated with in-hospital mortality. We report risk factors with odds ratios (OR) and 95% confidence intervals (CI). All p-values < 0.05 were considered statistical significance. All statistical analyses were performed using IBM SPSS Statistics version 18 (SPSS, Inc., Chicago, IL, USA) (21).
Results
Study patients
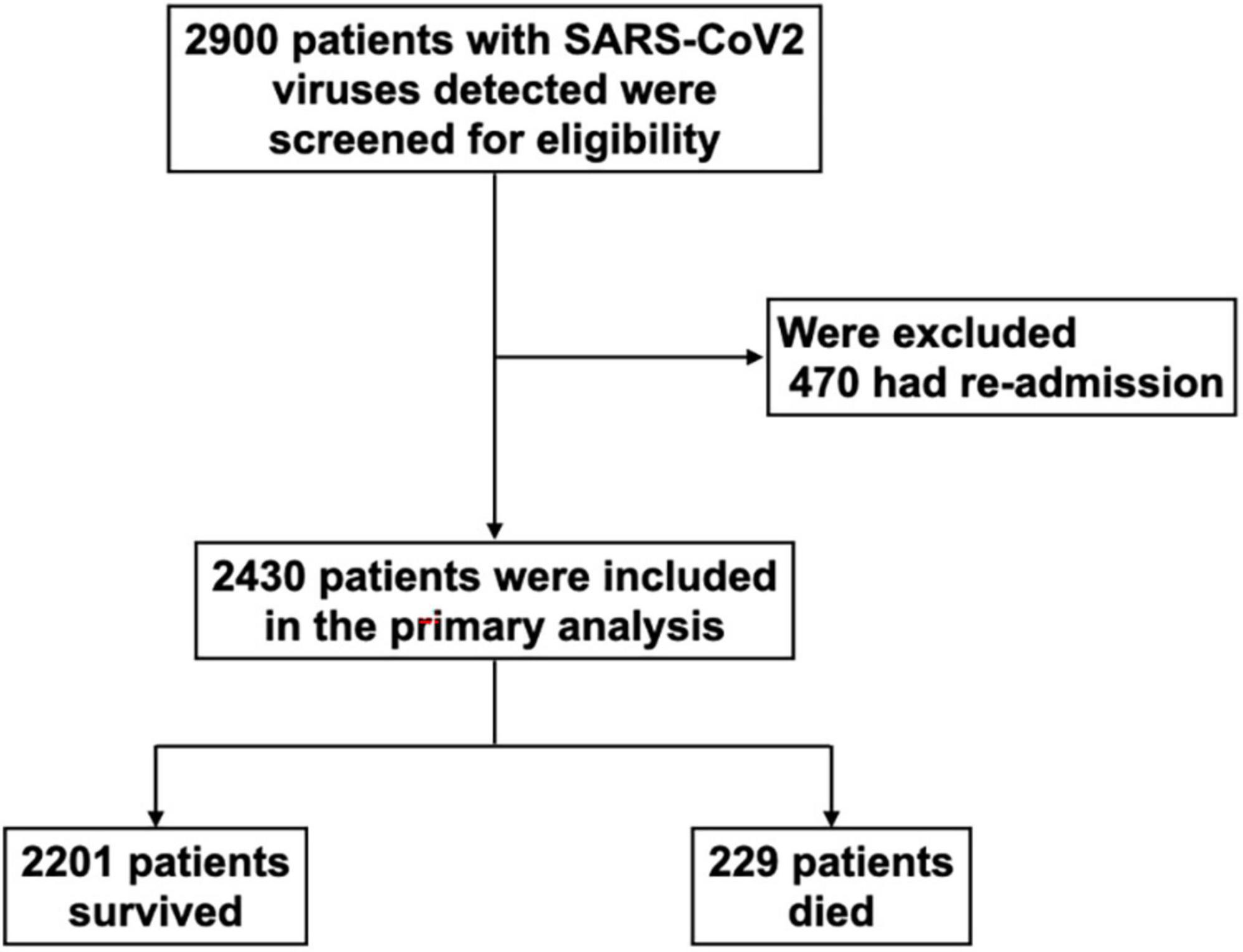
From January 2020 to December 2021, 2,900 patients with SARS-CoV2 viruses detected by RT-PCR were admitted to the cohort wards and ICUs of Siriraj hospital. Of these patients, 470 were excluded due to readmission for diagnoses other than COVID-19 reinfections; the remaining 2,430 patients were included in the final analysis (Figure 1); 2,201 (91%) survived and 229 (9%) died. In Thailand, the first case of the delta variant was reported in December 2020, and the first case of the omicron variant was found in December 2021. In our cohort, most patients (n = 2,329) were admitted from December 2020 to December 2021. The mortality rates changed over time; the highest mortality (14%) occurred at the same time as the highest admission rate (1,204 hospitalized patients) in July–September 2021 (Figure 2). Only 101 patients were admitted between January and April 2020; no mortality was observed in that period, and no COVID-19 patient was hospitalized from May to November 2020.

Figure 2. Trends in mortality from December 2020 to December 2021. Most cases (n = 2,329) were admitted from December 2020 to December 2021. The mortality rates changed over time; the highest mortality (14%) occurred at the same time as the highest admission rate (1,204 hospitalized patients) in July–September 2021. Only 101 patients were admitted between January and April 2020; no mortality was observed in that period, and no COVID-19 patient was hospitalized from May to November 2020.
Baseline characteristics and laboratory results
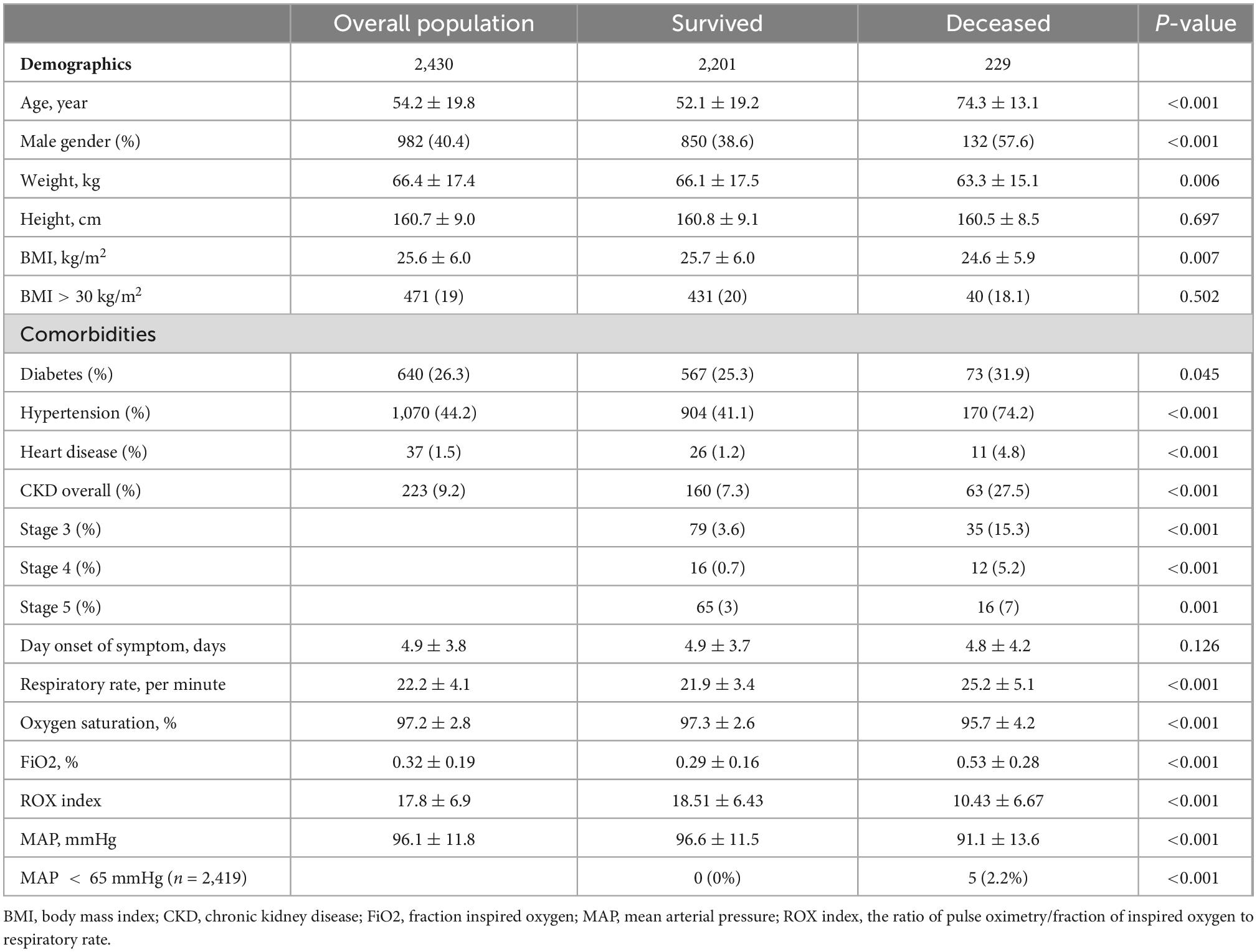
The mean age of the hospitalized patients was 54 years, 40% were men and 19% had a body mass index (BMI) > 30 kg/m2. Most of the patients (81%) had at least one comorbid disease; 44% had hypertension, 26% had diabetes mellitus, 9% had chronic kidney disease, and 2% had chronic heart disease. The mean duration from the onset of symptoms to hospital admission was 5 days, and only 2% of the patients had hypotension [mean arterial pressure (MAP) < 65 mmHg] at admission. For patients receiving HFNC, the mean ROX index was 17.8. In the univariate analysis, non-survived patients were older [74 (13) vs. 52 (19) years, P < 0.001], and were more often men (58% vs. 37%, P < 0.001) and had hypertension (74% vs. 41%, P < 0.001), diabetes mellitus (32% vs. 25%, P = 0.045), chronic kidney disease (28% vs. 7%, P < 0.001), and chronic heart disease (5% vs. 1%, P < 0.001), required a higher FiO2 [0.5 (0.3) vs. 0.3 (0.2), P < 0.001], and had a lower ROX index [10.4 (6.7) vs. 18.5 (6.4), P < 0.001] compared to patients who were discharged alive. The BMI and duration from the onset of the symptoms to admission were not significantly different between the survived and non-survived patients (Table 1).
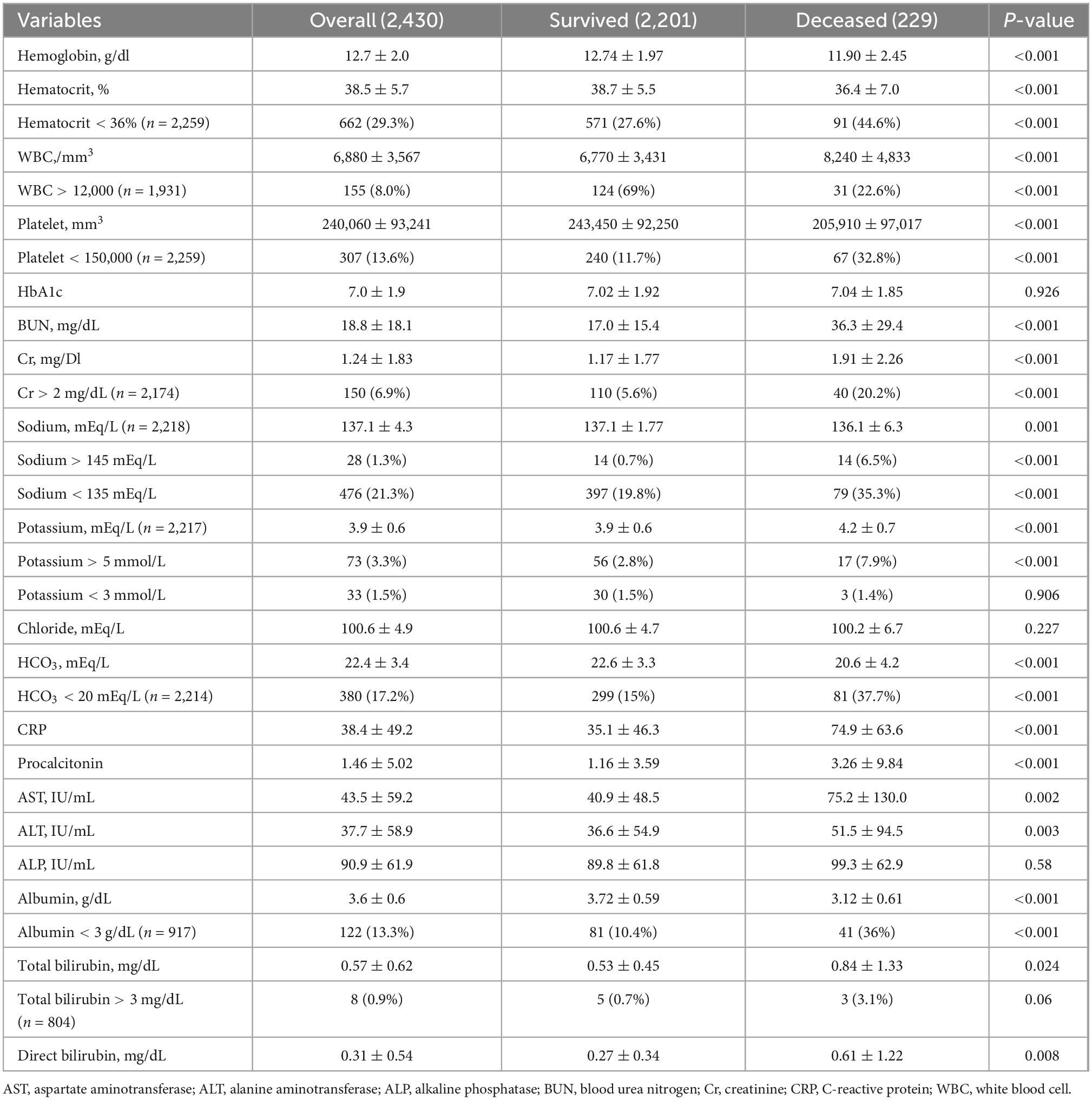
For baseline laboratory results, patients who did not survive had slightly lower hemoglobin [12 (2) vs. 13 (2) g/dl, P < 0.001], platelet counts [206 (97) vs. 243 (92) × 103/mm3, P < 0.001], and serum sodium [136 (6) vs. 137 (2) mEq/L, P < 0.001], bicarbonate [21 (4) vs. 22 (3) mEq/L, P < 0.001] and albumin level [3.1 (0.6) vs. 3.7 (0.6) g/dl, P < 0.001], but higher white blood cells [8.2 (4.8) vs. 6.8 (3.4) × 103 cells/mm3, P < 0.001], serum creatinine [Cr; 1.9 (2.3) vs. 1.2 (1.8) mg/dl, P < 0.001], potassium [4.2 (0.7) vs. 3.9 (0.6) mEq/L, P < 0.001], total bilirubin [0.8 (1.3) vs. 0.5 (0.5) mg/dl, P = 0.024], aspartate aminotransferase [AST; 75 (130) vs. 41 (49) U/L, P = 0.002], alanine aminotransferase [ALT; 52 (95) vs. 37 (55) U/L, P = 0.003], C-reactive protein [CRP; 75 (64) vs. 35 (46) mg/L, P < 0.001], and procalcitonin level [3.3 (9.8) vs. 1.2 (3.6) mg/mL, P < 0.001]. There were no significant differences in hemoglobin A1c, serum chloride, and alkaline phosphatase levels (Table 2).
Treatments and outcomes
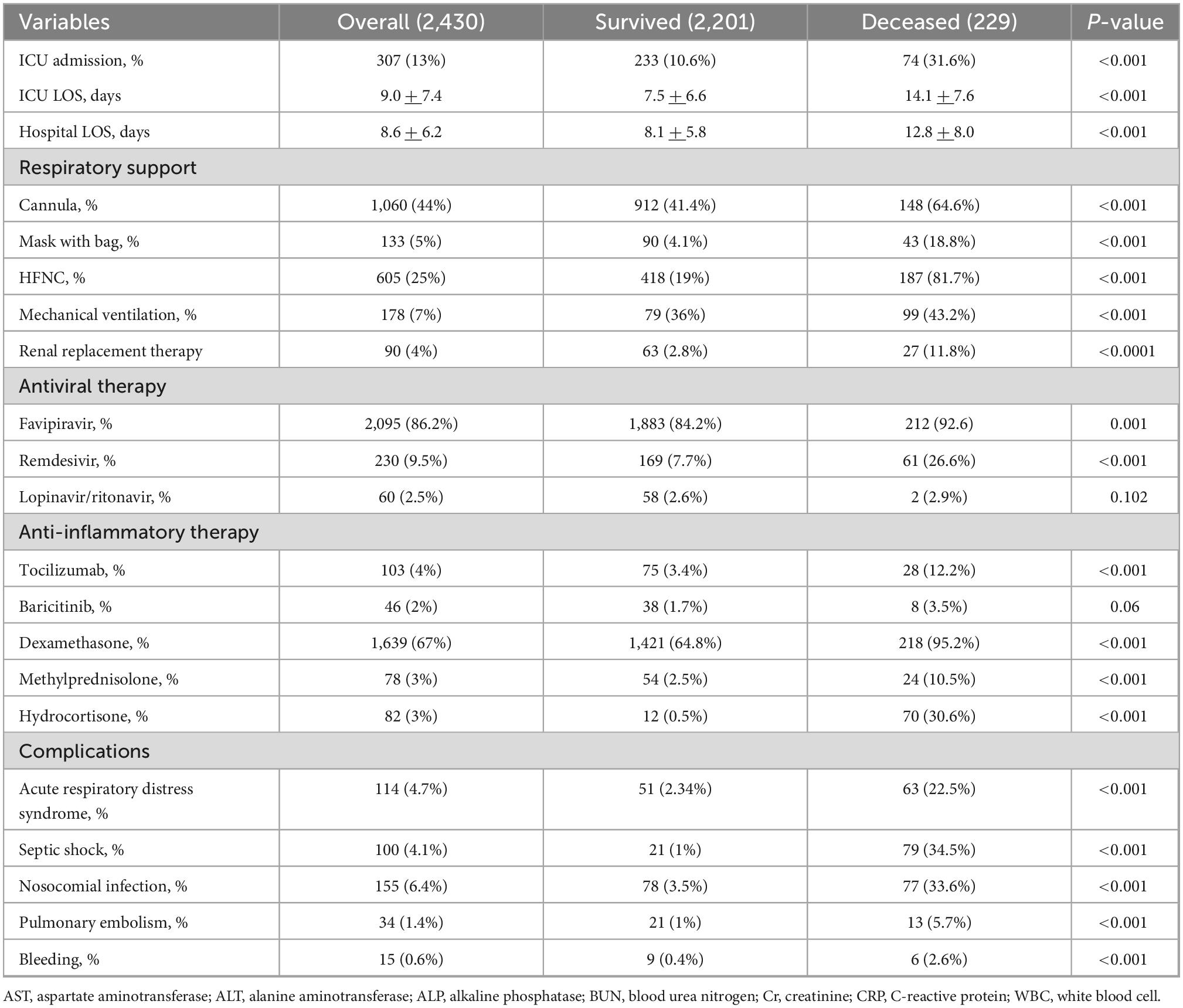
Eighty-one percent of patients received respiratory support; 49% required low flow oxygen via nasal cannula or non-rebreathing mask with a reservoir bag, 25% required HFNC or NIV, and 7% required IMV. However, admission to the ICU was available for only 307 patients (13%). RRT was used in 90 patients (4%). Favipiravir (86%) was the most common antiviral medication, followed by remdesivir (9%) and lopinavir boosted with ritonavir (2%). Corticosteroids were administered to 1,799 patients (74%); dexamethasone (67%) was the most common type of corticosteroids used in patients with COVID-19, followed by methylprednisolone (3%) and hydrocortisone (3%). Rescue anti-inflammatory therapies with tocilizumab (4%) or baricitinib (2%) were required in 149 patients (6%). Of these patients, 113 survived (76%), and 36 died (24%; Table 3).
Overall in-hospital mortality was 9%; the mortality rate increased to 31% (187 of 605) in patients requiring HFNC or NIV, and 56% (99 of 178) in patients requiring IMV. The mortality rate for patients admitted to the ICU was 24% (74 of 307). Deceased patients were admitted more frequently to the ICU and received antiviral and anti-inflammatory medications, respiratory support, and RRT than survived patients (Table 3). Nosocomial infections (6.4%) were the most common hospital complications, followed by ARDS (4.7%), septic shock (4.1%), pulmonary embolism (1.4%), and bleeding complications (0.6%). All complications occurred more frequently in patients who did not survive than in those who survived.
Factors associated with hospital mortality
In a multivariate logistic regression analysis, the only factor related to mortality reduction in mortality was admission to the ICU (OR 0.01, 95%CI 0.01–0.05, P < 0.001). Factors associated with increased mortality included age > 65 years (OR 14.3, 95%CI 5.8–35.5, P < 0.001), thrombocytopenia (platelet < 150 × 103/mm3; OR 2.4, 95%CI 1.1–5.5, P = 0.034), renal dysfunction (Cr > 2 mg/dL; OR 6.2, 95%CI 2.3–16.6, P < 0.001), ROX index < 12 (OR 2.32, 95%CI 1.0–5.0, P = 0.033), MAP < 90 mmHg (OR 4.8, 95%CI 2.3–4.9, P < 0.001), the use of respiratory support with low flow O2 through a non-rebreathing mask with a reservoir bag (OR 4.5, 95%CI 1.7–12.0, P < 0.001), non-invasive respiratory support (HFNC or NIV, OR 9.2, 95%CI 3.9–21.6, P < 0.001), and IMV (OR 269.4, 95%CI 33.6–2173.6, P < 0.001), receiving hydrocortisone (OR 27.0, 95%CI 5.3–138.3, P < 0.001) and occurrence of septic shock (OR 7.8, 95%CI 2.0–30.2, P < 0.001; Table 4).
A predictive model including only baseline parameters was shown in Supplementary Table 1. Similar baseline parameters including age > 65 years, platelet < 150 × 103/mm3, Cr > 2 mg/dL, ROX index < 12, and MAP < 90 mmHg were persistently associated with increased mortality. Moreover, abnormal liver test namely serum albumin < 3 g/dL, AST > 40 IU/mL, and total bilirubin > 3 mg/dL were independently associated with greater mortality.
Discussion
In this large cohort of patients hospitalized for COVID-19 infections in Thailand, the overall mortality rate was 9%; the rate was higher in patients requiring admission to the ICU (24%) and IMV (56%). In multivariate analysis, admission to the ICU was the only factor associated with a reduction in mortality. Patients who were elderly, required high-level respiratory support, especially those with a lower ROX index, had renal dysfunction and thrombocytopenia at admission, were complicated by septic shock, and received hydrocortisone were at high risk of mortality in hospital.
The overall mortality of 9% in our cohort was lower than previously reported during the early stage of the pandemic (2, 3), but the mortality in patients who required admission to the ICU (24%) and IMV (56%) was comparable (3–5, 7–9). In previous studies, the mortality rate of hospitalized patients with COVID-19 ranged from 17 to 28% (2–4, 6, 18), and the mortality for ICU patients was 24–62% and for mechanically ventilated patients was 49–62% (3–5, 7–11).
A better understanding of the natural history of the disease and the availability of antiviral and anti-inflammatory therapies can lead to a reduction in mortality over time. Prior studies were carried out during the early stage of the pandemic, January–April 2020, in which information on the natural course and effective treatment strategies for emerging diseases was limited (3, 4, 6), while our study period was longer (January 2020–December 2021) and included the later stage of the pandemic when more scientific knowledge about COVID-19 was available (18, 22–27). The difference in mortality of hypoxemic COVID-19 who did not require mechanical ventilation at admission was also found in two randomized controlled trials conducted in different periods. The RECOVERY trial revealed the effectiveness of dexamethasone in COVID-19 and was carried out between March and June 2020. That trial reported that the mortality at 28 days was 23% in the dexamethasone group and 26% in the usual care group (18). The Adaptive COVID-19 Treatment Trial 4 (ACTT-4) compared baricitinib and dexamethasone in combination with remdesivir for the treatment of hospitalized patients with COVID-19 was carried out from December 2020 to April 2021. ACTT-4 reported that mortality at 60 days was only 7 and 8% in the baricitinib and dexamethasone groups, respectively (28).
The different variants of the virus and the availability of COVID-19 vaccines may be the other important reasons for the decreased mortality in our cohort (29). A prior study also reported a lower fatality of COVID-19 patients during the outbreak of the omicron variant compared to that of the delta and beta variants (30). At the end of 2021, 63% of the Thai population had already received at least two doses of vaccines (31), and all types of COVID-19 vaccines are proven to reduce the risk of developing severe disease and death (32–36). Unfortunately, information on COVID-19 vaccination status for our individual patients was not available, so the influence of the vaccination status on hospital mortality could not be determined.
This study also revealed the survival benefit of admission to the ICU for patients with severe COVID-19 (Table 4). Of the 307 patients admitted to the ICU, 76% were discharged alive. According to our results, patients who needed high-level respiratory support, HFNC or IMV, and who had septic shock and renal failure were at the highest risk of death; these patients should be the main priority for admission to the ICU. However, we also demonstrated the shortage of cohort ICU beds in our hospital, since almost one-third of our patients received HFNC or IMV, but only 13% of all patients were treated in the ICU. Therefore, increasing the capacity of the ICU with AIIR is an essential plan to prepare for the next pandemic.
Previous studies have reported an association between the degree of hypoxemia and increased mortality; our results supported this finding (4, 9, 11, 37). The lower ROX index and use of respiratory supports represented more severe hypoxemia in our patients. Since the risk of death was much higher in patients requiring IMV than in those requiring HFNC and low-flow O2 therapy (Table 4), strategies to limit disease progression and prevent intubation, including early antiviral treatment for patients at high risk of disease progression, and rescue anti-inflammatory therapy with tocilizumab or baricitinib for those who had rapid worsening despite corticosteroid treatment, appear reasonable (25–27, 38).
Hydrocortisone is the only type of corticosteroid found to be associated with mortality in this study (Table 4). In contrast to dexamethasone, no prior research has demonstrated the benefit of hydrocortisone in patients with COVID-19 (39, 40). We found that septic shock was associated with high mortality in the multivariate model. This association between hydrocortisone and mortality could be explained by the fact that in our hospital hydrocortisone was used primarily in patients with septic shock who received high-dose vasopressor treatment. An observational study in Argentina also found that septic shock and refractory hypoxemia were the major causes of death in patients with COVID-19 requiring IMV, and the use of vasopressors on admission was an independent predictor of hospital mortality (10), the rate of nosocomial infection in our cohort was 6% (155 of 2,430) and 65% (100 of 155) of these patients developed septic shock (Table 3). Therefore, early detection and treatment of superimposed bacterial infections are essential to improve patient outcomes.
The associations between increased mortality and older age, thrombocytopenia, and renal dysfunction on admission in patients with COVID-19 have been reported in previous studies (2, 4, 8, 11, 41–43), but older age is not modifiable, and thrombocytopenia and renal dysfunction appear to be attributable to the severity of the disease (44–47). Thrombocytopenia, defined as platelet counts < 150 × 103/mm3, is common in COVID-19 disease. The proposed pathophysiological mechanisms include reducing platelet synthesis from the bone marrow due to direct viral infection or cytokine storm, increasing platelet destruction by the immune system, and increased platelet consumption resulting from aggregation and formation of microthrombi in damaged lung parenchyma and pulmonary endothelial cells (48). The possible pathophysiology of acute kidney injury (AKI) in COVID-19 consists of direct effects of the virus that causes endothelial and tubular epithelial damage and indirect impact from volume depletion, nephrotoxic drugs, sepsis-associated AKI from superimposed bacterial infection, increased renal venous pressure complicated from mechanical ventilation with high intrathoracic pressure, organ crosstalk from lung injury or cardiorenal syndrome, and rhabdomyolysis (46, 47). Patients with any of these factors need close observation for severe disease.
The strengths of our study are that we reported the characteristics and outcomes of a relatively large cohort of hospitalized patients with COVID-19 and performed a multivariate analysis to determine the independent factors associated with in-hospital mortality. We also performed manual searches on the EMR to verify the diagnosis of COVID-19 infection in all patients and to obtain the data that could not be collected electronically. We also acknowledge several limitations of this study. First, this is a single-center study, which may limit the generalizability of our findings. Second, we did not have data on underlying chronic lung disease, SOFA score, and D-dimer level, which increased mortality risks in previous studies. Third, concurrent life-threatening medical or surgical diseases necessitating urgent treatment were not recorded; these conditions may impact patient outcomes. Fourth, we were unable to obtain clinical outcomes after hospital discharge. Finally, similar to other observational and retrospective studies, unmeasured confounders may affect the results.
Conclusion
The overall mortality of hospitalized COVID-19 patients in Thailand was 9%. Admission to the ICU was the only protective factor. Patients who received invasive or non-invasive respiratory support and were complicated by septic shock had the highest risk of death. Therefore, appropriate patient selection for admission to the ICU, strategies to limit disease progression and prevent intubation, and early detection and prompt treatment of nosocomial infection may improve survival. Although unmodifiable, elderly patients and those with thrombocytopenia and renal dysfunction need close observation for the development of severe disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Human Research Protection Unit of Siriraj Hospital Faculty of Medicine, Mahidol University (Certificate of Approval no. Si 335/2020). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
TN had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, designed the study, performed manual searches for additional data collection and interpretation, drafted and revised the manuscript. TV designed the study, reviewed, interpreted the data, drafted, and revised the manuscript. ST designed the study, performed the statistical analysis, drafted, and revised the manuscript. AD, TP, RW, RR, PP, PT, AP, and CP assisted in data collection, data interpretation, and critically reviewed the manuscript. All authors have read and approved the final manuscript and agreed to be responsible for all aspects of the work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1061955/full#supplementary-material
Abbreviations
COVID-19, corona virus disease 2019; SOFA, sequential organ failure assessment; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; AIIR, airborne infection isolation rooms; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; RT-PCR, reverse transcription-polymerase chain reaction; EMR, electronic medical records; SiData+, Siriraj Informatics and Data Innovation Center; RRT, renal replacement therapy; HFNC, high flow nasal cannula; NIV, non-invasive ventilation; IMV, invasive mechanical ventilation; ROX, respiratory rate oxygenation index; SpO2/FiO2, pulse oxygen saturation/fractional inspired oxygen; SD, standard deviation; OR, odds ratios; CI, confidence intervals; BMI, body mass index; MAP, mean arterial pressure; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CRP, C-reactive protein; AKI, acute kidney injury.
References
2. Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. (2021) 21:855. doi: 10.1186/s12879-021-06536-3
3. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
4. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. doi: 10.1136/bmj.m1966
5. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
6. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
8. Xu J, Yang X, Yang L, Zou X, Wang Y, Wu Y, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. (2020) 24:394. doi: 10.1186/s13054-020-03098-9
9. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
10. Estenssoro E, Loudet CI, Ríos FG, Kanoore Edul VS, Plotnikow G, Andrian M, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. (2021) 9:989–98. doi: 10.1016/S2213-2600(21)00229-0
11. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. (2020) 180:1345–55. doi: 10.1001/jamainternmed.2020.3539
12. Kim L, Garg S, O’Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. (2021) 72:e206–14. doi: 10.1093/cid/ciaa1012
13. Ratanarat R, Sivakorn C, Viarasilpa T, Schultz MJ. Critical Care Management of Patients with COVID-19: early Experience in Thailand. Am J Trop Med Hyg. (2020) 103:48–54. doi: 10.4269/ajtmh.20-0442
14. Bhurayanontachai R. Mechanical ventilator support and prone positioning in COVID-19 related pneumonia. Clin Crit Care. (2021) 29:e0004.
15. Tongyoo S, Kongsayreepong S. Extracorporeal membrane oxygenation (ECMO) for COVID-19 patients. Clin Crit Care. (2021) 29:e0005.
16. Department of Medical Services. Thai Clinical Practice Guidelines for COVID-19 Patients. (2022).
17. Theerawit P. Anti-inflammatory agents other than corticosteroid in SARs-CoV-2 pneumonia. Clin Crit Care. (2021) 29:e0001.
18. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. (2021) 384:693–704. doi: 10.1056/NEJMoa2021436
19. Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. (2019) 199:1368–76. doi: 10.1164/rccm.201803-0589OC
20. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
22. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a Review. JAMA. (2020) 324:782–93. doi: 10.1001/jama.2020.12839
23. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. (2020) 383:2451–60. doi: 10.1056/NEJMcp2009575
24. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 – Final Report. N Engl J Med. (2020) 383:1813–26. doi: 10.1056/NEJMoa2007764
25. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: a Meta-analysis. JAMA. (2021) 326:499–518. doi: 10.1001/jama.2021.11330
26. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. (2021) 384:795–807. doi: 10.1056/NEJMoa2031994
27. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda, MA: National Institutes of Health (2022).
28. Wolfe CR, Tomashek KM, Patterson TF, Gomez CA, Marconi VC, Jain MK, et al. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial. Lancet Respir Med. (2022) 10:888–99. doi: 10.1016/S2213-2600(22)00088-1
29. Myrstad M, Rønningen PS, Tveita A, Rønning EJ, Ernø PE, Andersen EL, et al. Three waves of COVID-19 in a Norwegian local hospital. Tidsskr Nor Laegeforen. (2021) 141. doi: 10.4045/tidsskr.21.0750
30. Jassat W, Abdool Karim SS, Mudara C, Welch R, Ozougwu L, Groome MJ, et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study. Lancet Glob Health. (2022) 10:e961–9. doi: 10.1016/S2214-109X(22)00114-0
32. Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. (2021) 385:1761–73. doi: 10.1056/NEJMoa2110345
33. El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N Engl J Med. (2021) 385:1774–85. doi: 10.1056/NEJMoa2113017
34. Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. (2021) 373:n1088. doi: 10.1136/bmj.n1088
35. Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. (2021) 385:2348–60. doi: 10.1056/NEJMoa2105290
36. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. (2021) 385:875–84. doi: 10.1056/NEJMoa2107715
37. Cordova E, Mykietiuk A, Sued O, De Vedia L, Pacifico N, Garcia Hernandez MH, et al. Clinical characteristics and outcomes of hospitalized patients with SARS-CoV-2 infection in a Latin American country: results from the ECCOVID multicenter prospective study. PLoS One. (2021) 16:e0258260. doi: 10.1371/journal.pone.0258260
38. Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. (2022) 386:305–15. doi: 10.1056/NEJMoa2116846
39. Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. (2020) 324:1298–306. doi: 10.1001/jama.2020.16761
40. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: the REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. (2020) 324:1317–29. doi: 10.1001/jama.2020.17022
41. Maquet J, Lafaurie M, Sommet A, Moulis G. Thrombocytopenia is independently associated with poor outcome in patients hospitalized for COVID-19. Br J Haematol. (2020) 190:e276–9. doi: 10.1111/bjh.16950
42. Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. (2020) 31:490–6. doi: 10.1080/09537104.2020.1754383
43. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
44. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
45. Jiang SQ, Huang QF, Xie WM, Lv C, Quan XQ. The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br J Haematol. (2020) 190:e29–33. doi: 10.1111/bjh.16817
46. Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. (2020) 16:747–64. doi: 10.1038/s41581-020-00356-5
47. Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. (2020) 8:738–42. doi: 10.1016/S2213-2600(20)30229-0
Keywords: mortality, coronavirus, COVID-19, SARS-CoV-2, risk factor, Thailand, developing country
Citation: Naorungroj T, Viarasilpa T, Tongyoo S, Detkaew A, Pinpak T, Wimolwattanaphan R, Ratanarat R, Promsin P, Thamrongpiroj P, Phumpichet A and Permpikul C (2023) Characteristics, outcomes, and risk factors for in-hospital mortality of COVID-19 patients: A retrospective study in Thailand. Front. Med. 9:1061955. doi: 10.3389/fmed.2022.1061955
Received: 05 October 2022; Accepted: 05 December 2022;
Published: 04 January 2023.
Edited by:
Giuliana Scarpati, University of Salerno, ItalyReviewed by:
Artem N. Kuzovlev, Research Institute General Resuscitation im.V.A.Negovskogo, RussiaHåkon Ihle-Hansen, Vestre Viken Hospital Trust, Norway
Copyright © 2023 Naorungroj, Viarasilpa, Tongyoo, Detkaew, Pinpak, Wimolwattanaphan, Ratanarat, Promsin, Thamrongpiroj, Phumpichet and Permpikul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tanuwong Viarasilpa, ✉ dGFudXdvbmcudmlhQG1haGlkb2wuYWMudGg=
 Thummaporn Naorungroj
Thummaporn Naorungroj Tanuwong Viarasilpa
Tanuwong Viarasilpa Surat Tongyoo
Surat Tongyoo Aeckapholpholladet Detkaew
Aeckapholpholladet Detkaew Chairat Permpikul
Chairat Permpikul