- 1Department of Microbiology and Virology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 2Molecular and Cell Biology Research Center (MCBRC), Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran
- 3Gastrointestinal Cancer Research Center, Non-communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
- 4Faculty of Medicine, University of Medical Sciences, Sari, Iran
- 5Medical Sciences Technologies, Molecular and Cell Biology Research Center (MCBRC), Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
Objectives: Infections in the male genitourinary system with bacterial and viral agents may play a significant role in male infertility. These agents usually infect the urethra, seminal vesicles, prostate, epididymis, vas deferens, and testes retrograde through the reproductive system. A meta-analysis review study was performed to evaluate the presence of bacterial and viral agents in the semen of infertile men and its correlation with infertility.
Methods: Relevant cross-sectional and/or case-control studies were found by an online review of national and international databases (Web of Science, PubMed, Scopus, Science Direct, and Google scholar), and suitable studies were selected. A checklist determined the qualities of all studies. Heterogeneity assay among the primary studies was evaluated by Cochran’s Q test and I2 index (significance level 50%). A statistical analysis was conducted using the Comprehensive Stata ver. 14 package (StataCorp, College Station, TX, United States).
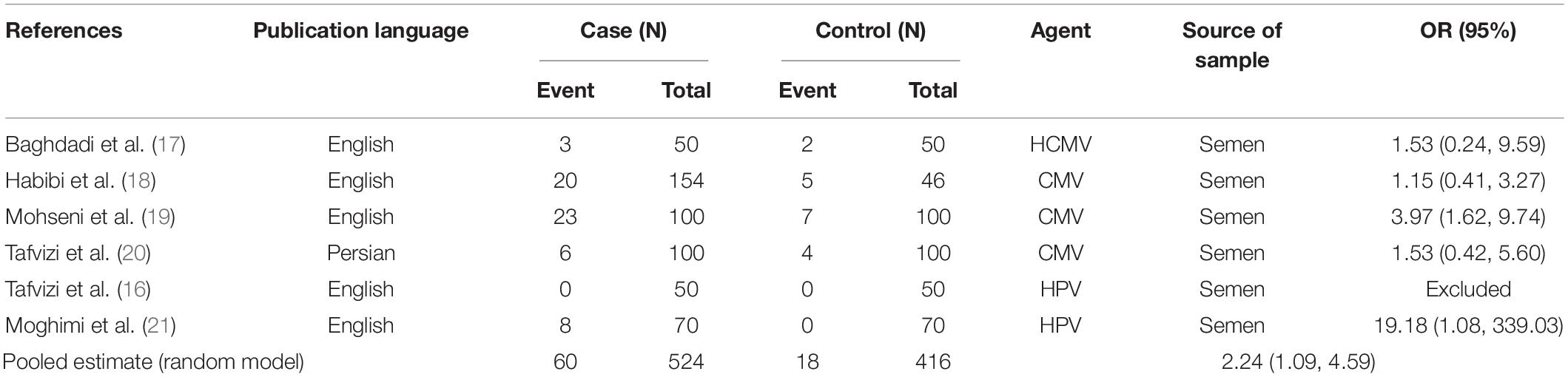
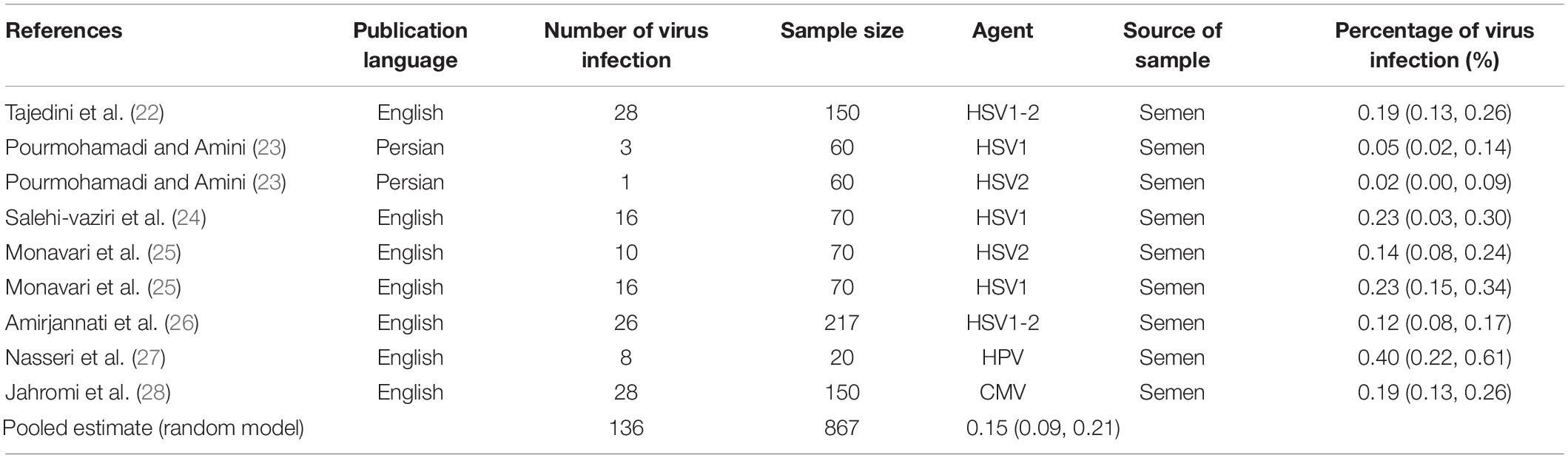
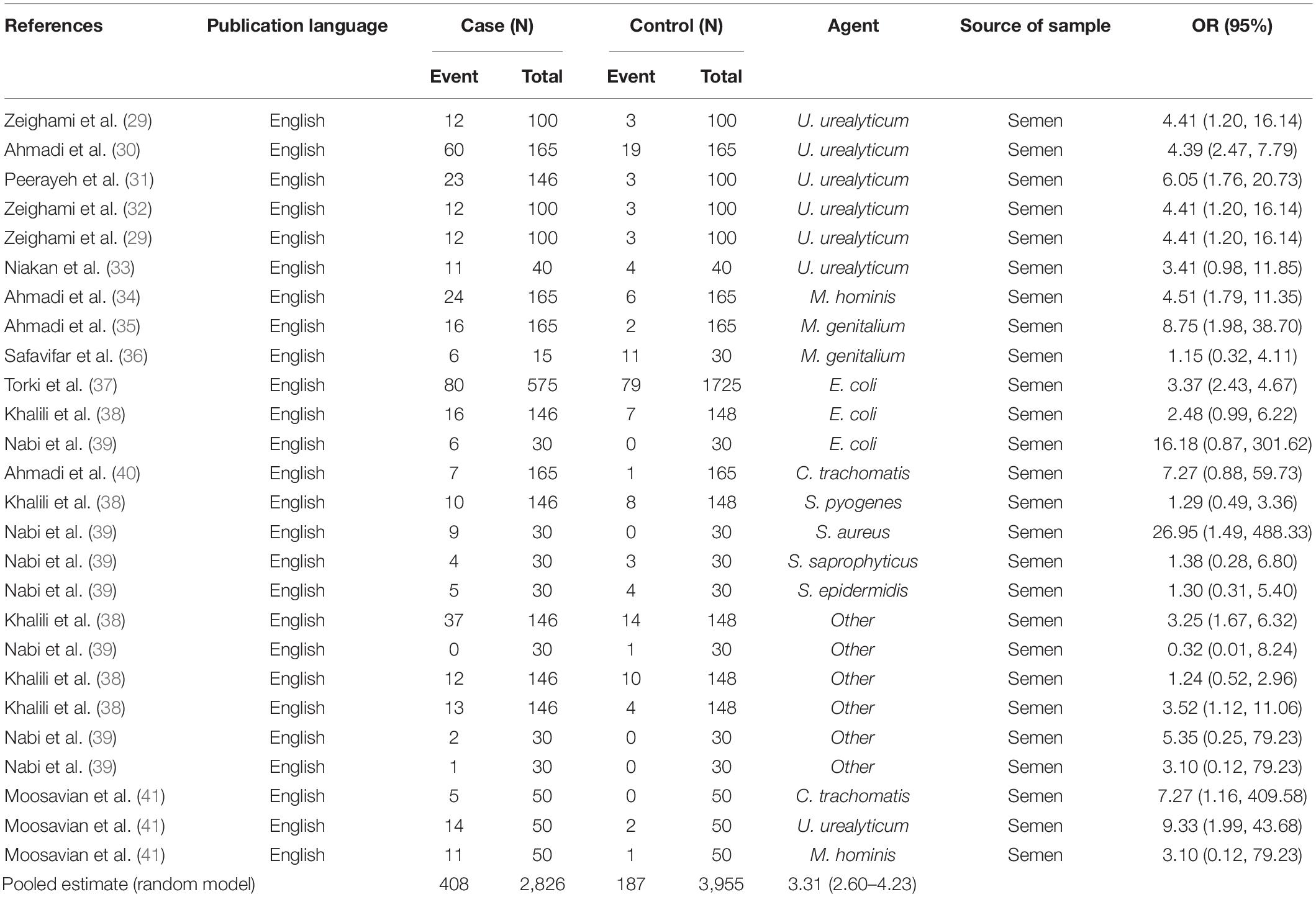
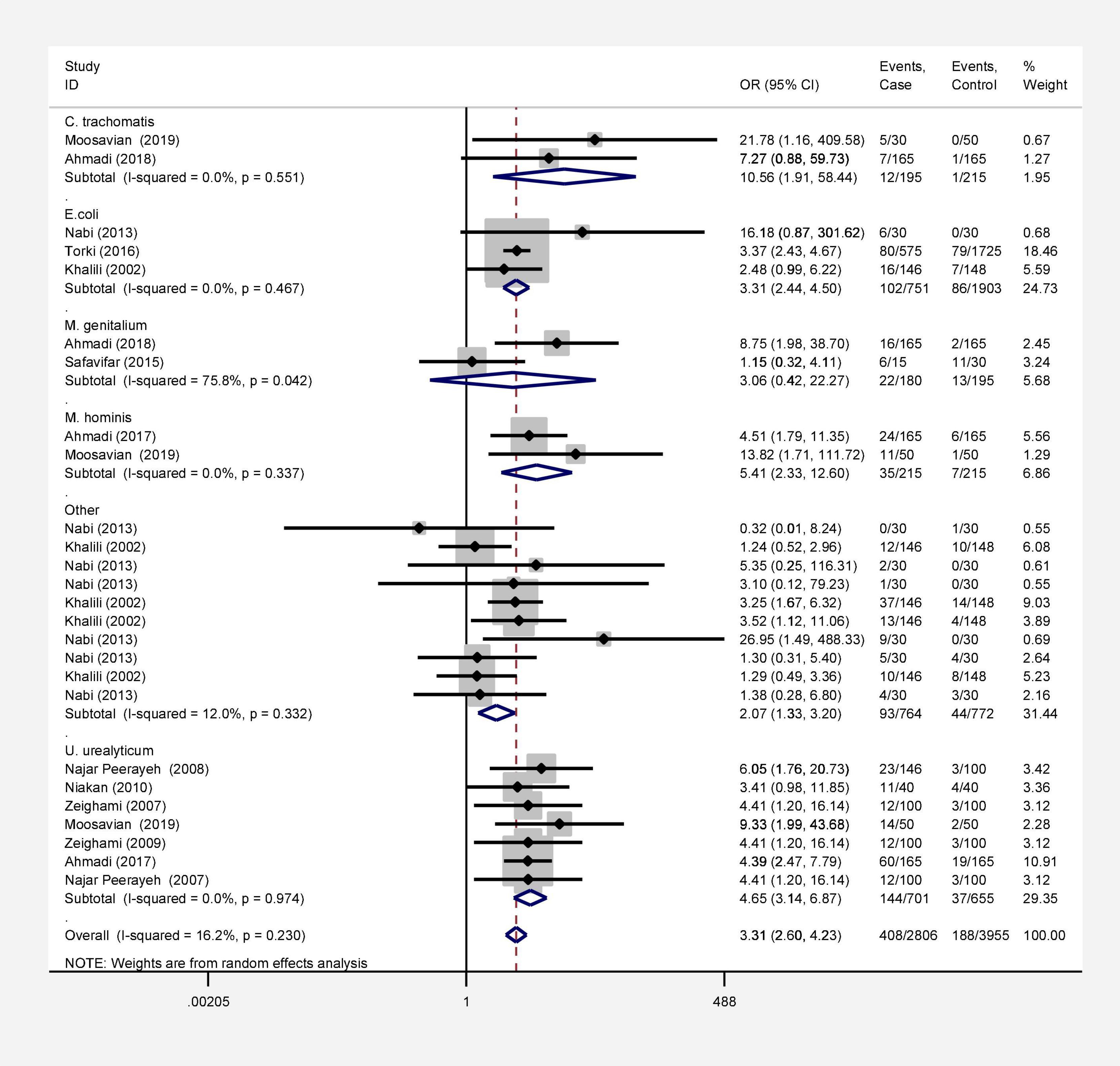
Results: Seventy-two studies were included in this meta-analysis. Publication bias was compared with Egger’s test, and the impact of each research on overall estimate was evaluated by sensitivity analysis. In 56 studies, the rate of bacterial infections in the semen of infertile men was 12% [95% confidence interval (CI): 10–13]. Also, in 26 case-control studies, the association of infertility in men with bacterial infections was evaluated. The results show that the odds ratio of infertility in men exposed to bacterial infections is 3.31 times higher than that in non-infected men (95% CI: 2.60–4.23). Besides, in 9 studies that examined the prevalence of human papillomavirus (HPV), herpes simplex virus 1 (HSV1), herpes simplex virus 2 (HSV2), and herpes simplex virus 1-2 (HSV1-2) in infertile men, the frequency of these viruses was 15% (95% CI: 9–21). In 6 case-control studies, the association between human cytomegalovirus (HCMV), Cytomegalovirus (CMV), and HPV and male infertility was evaluated. The chance of male infertility due to exposure to these viruses was 2.24 times higher than those without exposure to these viruses (CI 95%: 1.9–4.52). The results show that the chance of infertility in men exposed to bacteria was significantly higher than that in the uninfected population.
Conclusion: This meta-analysis showed that viral and bacterial infections are a risk factor and could impair male fertility potential. Moreover, our study supports the hypothesis that bacterial and viral infections of the genital tract correlate positively with impairment of sperm quality in the male population.
Introduction
Infertility refers to the inability to have a baby and pregnancy after at least 1 year of marriage and attempts of pregnancy without contraceptives. Causes of infertility can refer to women, men, or both (1). According to WHO studies, about 50–60% of cases are of men. In 10%, the cause of infertility is unclear, that is, a couple’s examination does not indicate a pathological problem, but the cause of infertility is unclear (2). Major causes in men include genital injuries, semen infections, testis problems, genital tracts, genital glands, varicocele, genital tract obstruction, endocrine, and metabolic diseases (3). One of the essential reasons for male infertility is semen and genital tract infections. Male urinary tract infection is one of the most important causes of male infertility. As many as 8–35% of infertility cases worldwide are due to genitourinary tract infections (4). Although the mechanism and physiopathology of infections in infertility are not fully understood, viral and bacterial infections appear to cause semen or sperm abnormalities and morphological changes in sperm, and reduce motility directly, thus reducing fertility (5). It can also indirectly cause infection, testicular damage, and inflammation and, subsequently, stimulate the immune system against intrinsic antigens associated with leukocytospermia, all of which can cause male infertility (6). The presence of leukocytes in the semen (pivotal sperm) can play an essential role in reducing the qualitative parameters of the ejaculatory fluid and, on the other hand, is a good sign in determining genital infection. Some studies suggest that leukocytes in the semen indicate infection and that those with urethritis have more leukocytes in their semen (7).
According to WHO criteria, a concentration of more than 10,00,000 leukocytes per ml of semen is called leukocytopenia. Meanwhile, polymorphonuclear leukocytes make up 50–60% and macrophages 20–30% of semen-positive peroxidase leukocytes. Leukocytes are one of the most important sources of reactive oxygen species in semen (8). How these cells enter the seminiferous tubules is not well understood. Still, studies have shown that, as a result of infection, tight junctions between Sertoli cells are destroyed, or their resistance is reduced, and leukocytes invade the seminiferous tubules (9). Since the cytoplasm of a mature sperm is low, and the concentration of ROS-destroying antioxidants is low in sperm cells, sperm cells are more susceptible to oxidative stress than any other cell (10). Also, because the sperm membrane contains large amounts of unsaturated fatty acids, it is highly vulnerable to oxidative stress.
Furthermore, due to the specific form of sperm, intracellular antioxidant enzymes cannot protect the plasma membrane surrounding the acrosome and tail (11). Among the common bacterial species, male genitourinary tract infections are caused by Streptococcus pyogenes, enterococci, Escherichia coli, coagulase positive, and negative staphylococci bacteria (12). If the infection destroys the blood-testicular barrier, it results in the formation of anti-sperm antibody exposure levels that are detectable in the serum and semen (13). However, it is not yet known whether serum antibodies significantly affect patients’ fertility. However, anti-sperm antibodies in semen can impair sperm function. Some bacteria also cause sperm cells to immobilize by adhesion or agglutination, which depends on the density of bacteria in the semen. Escherichia coli and Chlamydia trachomatis cause sperm agglutination, and, on the other hand, the attachment of bacteria to the sperm cell membrane results in reduced sperm attachment to the ovum (14). Some leading causes of chronic sperm viral infections are human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). In particular, the role of HIV infection in chronic lower genital inflammation, sperm infection, and fertility decline is significant. Recent studies show that the presence of HBV or HCV in semen adversely affects sperm parameters and, in particular, reduces sperm motility. Also, seminal viral infection is associated with increased frequency of sperm abnormalities and DNA damage (4). According to importance of genital infections in male infertility, therefore, this study will be designed to survey viral and bacterial agents in semen of infertile men. Therefore, this study will be designed to survey viral and bacterial agents in semen of infertile men. This is a systematic review and meta-analysis of various bodies of literature in this field, so it may be possible to obtain a single result from different studies and clarify the role of viruses and bacteria in male infertility.
Methods
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) described the present meta-analysis statement and the guidelines of the Meta-analysis of Observational Studies in Epidemiology.
Search Strategy
A systematic search for studies was performed using online international databases, namely, Web of Science,
Science Direct, PubMed, Scopus, and Google scholar, to determine suitable studies published from 2000 to 2021. The references of these studies were examined to improve search sensitivity. The following search terms were used in combination for search strategies: “Semen,” “Infertility men,” “Iran,” “Virus,” and “Bacteria,” which were combined with and/or not.
Study Selection
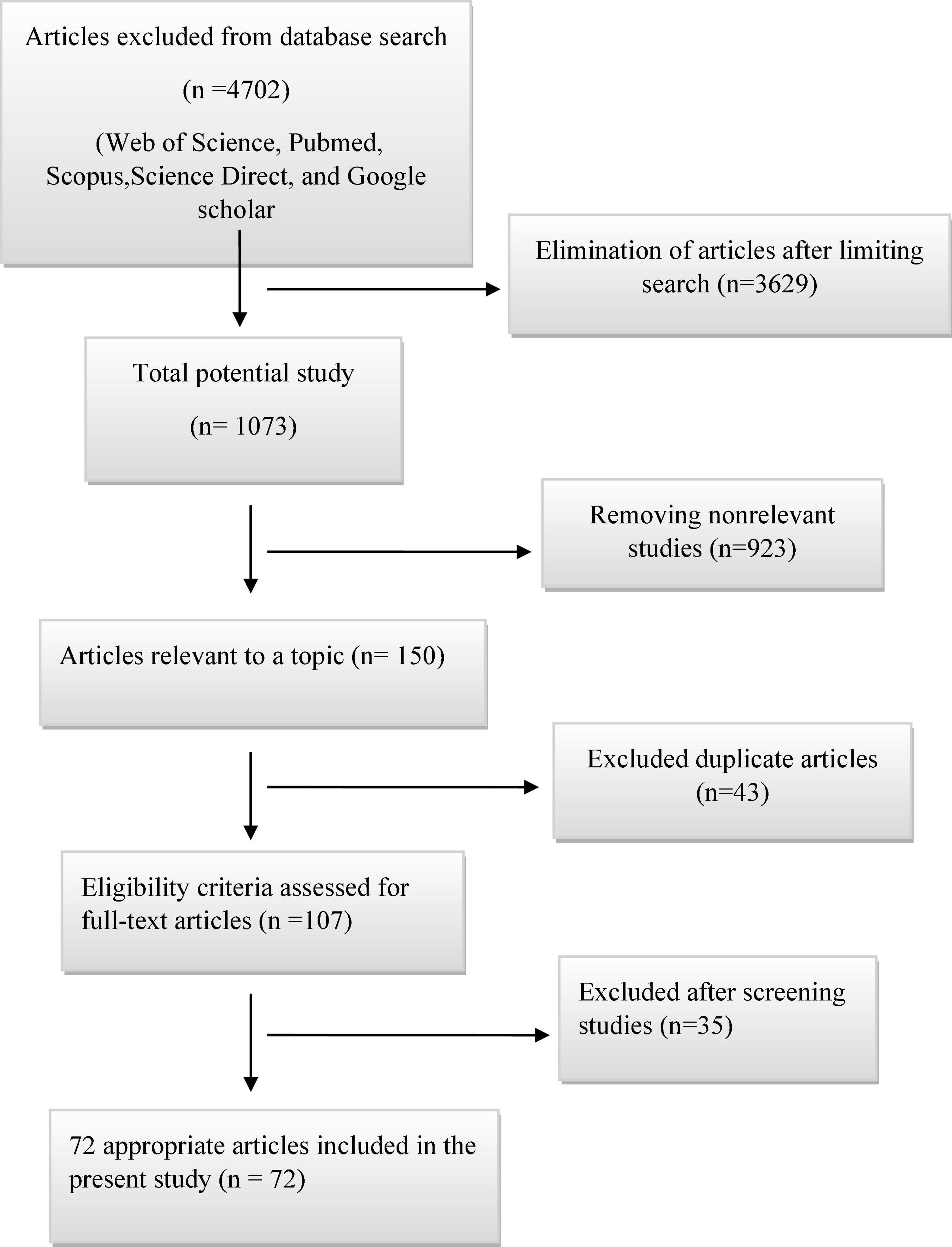
All full texts or abstracts of the literature were excluded from the database search and reviewed on the advanced search. First, after limiting the search, non-relevant and duplicate studies were excluded. Then, studies were screened after checking the titles, abstracts, and full texts. Later, appropriate studies were included in our study.
This study aimed to estimate the prevalence of viral and bacterial agents in the semen of infertile men and to analyze the relationship between bacterial and viral agents and infertility in men; the inclusion and exclusion criteria are as follows.
Inclusion Criteria
In this study, (PICOS) search strategy for descriptive studies aimed to determine infertile men (P) and the prevalence of viral and bacterial agents in descriptive studies (S). For analytical studies, PICOS was used to identify infertile men (P), viral and bacterial agents (E), fertile men (C), odds ratio of viral and bacterial agents in the semen of infertile men to fertile (O), and case-control studies (S).
All studies that passed the above assessment phases for high-quality scores were selected if they met the following conditions: (1) case-control studies on associations between bacterial infections and infertility, (2) case-control studies on associations between viral infections and infertility, (3) cross-sectional studies based on the prevalence of bacterial infections in the semen of infertile men, (4) cross-sectional studies based on the prevalence of viral infections in the semen of infertile men, and (5) both English and Persian studies.
Exclusion Criteria
The following types of studies were excluded: (1) case reports or case series articles, (2) articles with no complete access to full text, (3) duplicate studies, (4) conference abstracts without full-text letters or review studies, (5) studies with low quality, and (6) studies published in languages other than English and Persian.
Quality Assessment
The quality of this meta-analysis was determined following the Newcastle-Ottawa Scale (NOS) statement. The NOS checklist covers methodology, comparability, and outcome. Depending on the quality of the analysis, the studies were divided into three groups: good quality, fair quality, and poor quality.
Data Extraction
After selection of appropriate literature, the following data were extracted: authors, publication year, geographical regions, publication language, type of study, number of infertile men infected with viral/or bacterial agents, sample size, agent, and source of the sample. Data were extracted and entered into a Microsoft Excel spreadsheet.
Statistical Analysis
In this study, Stata ver. 14 package (StataCorp, College Station, TX, United States) was used for data analysis. A contingency table was formed for each case-control study for the case and control groups. Data were weighted and combined using the inverse variance method. The heterogeneity index (I2) between studies was determined by Cochran (Q) and I2 tests. Also, according to Higgins and Thompson (15), an I2 value of less than 25% indicates low heterogeneity, 25–75% indicates moderate heterogeneity, and over 75% indicates high heterogeneity. A random-effects model was used to estimate the odds ratio of male infertility in the case group and the control group and to estimate the frequency of viruses or bacteria in the semen of infertile men. Odds ratios were calculated with 95% CIs on forest plots. In this diagram, the square size shows the weight of each study, and related lines on either side represent a 95% CI. A meta-regression test was performed to evaluate the effect of microorganisms on heterogeneity. An Egger’s test was conducted and a funnel plot chart with a significant level of less than 0.1 was used to evaluate publication bias. In addition, a sensitivity analysis was performed to investigate the effect of each early study on the overall pooled odds ratio.
Results
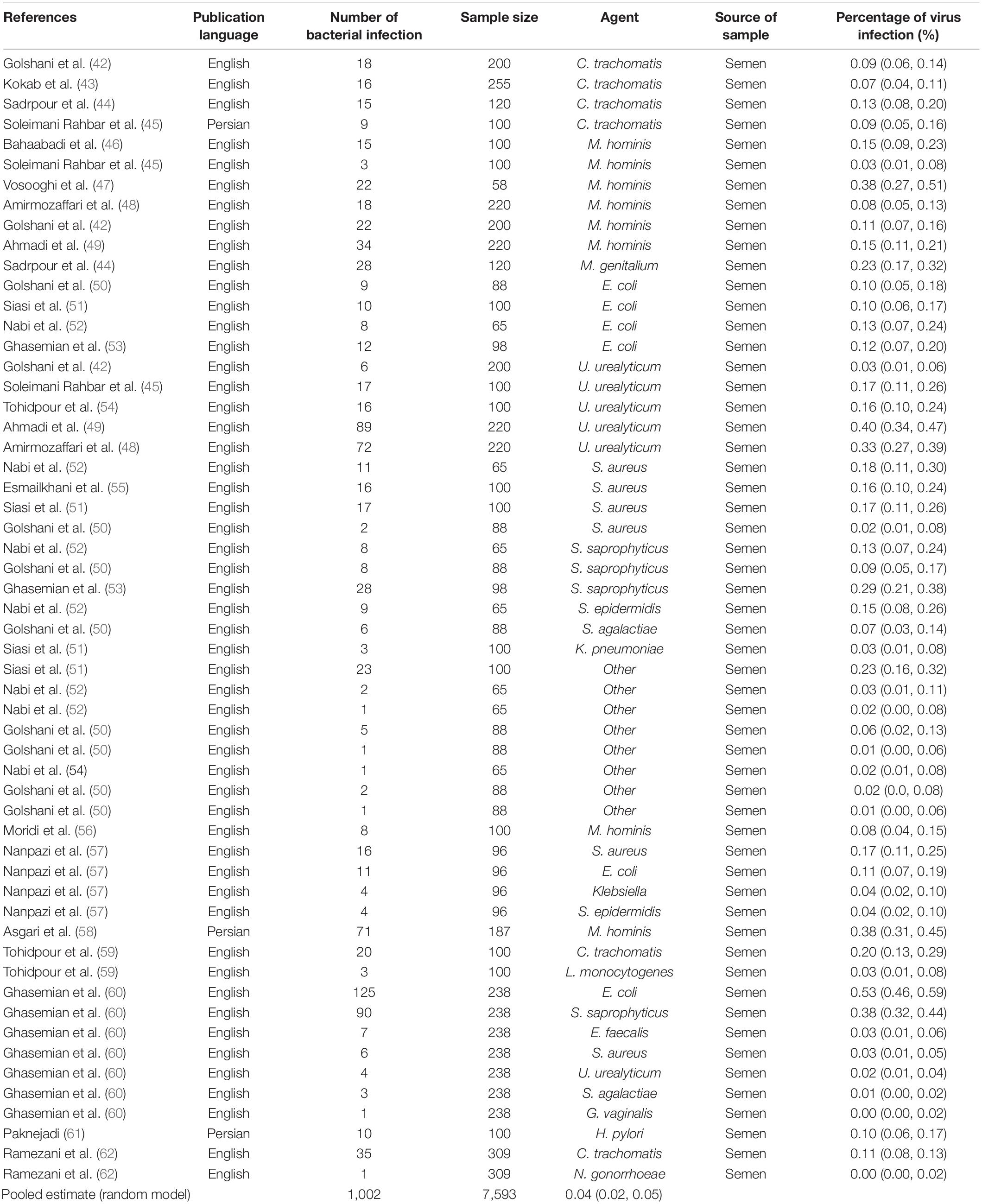
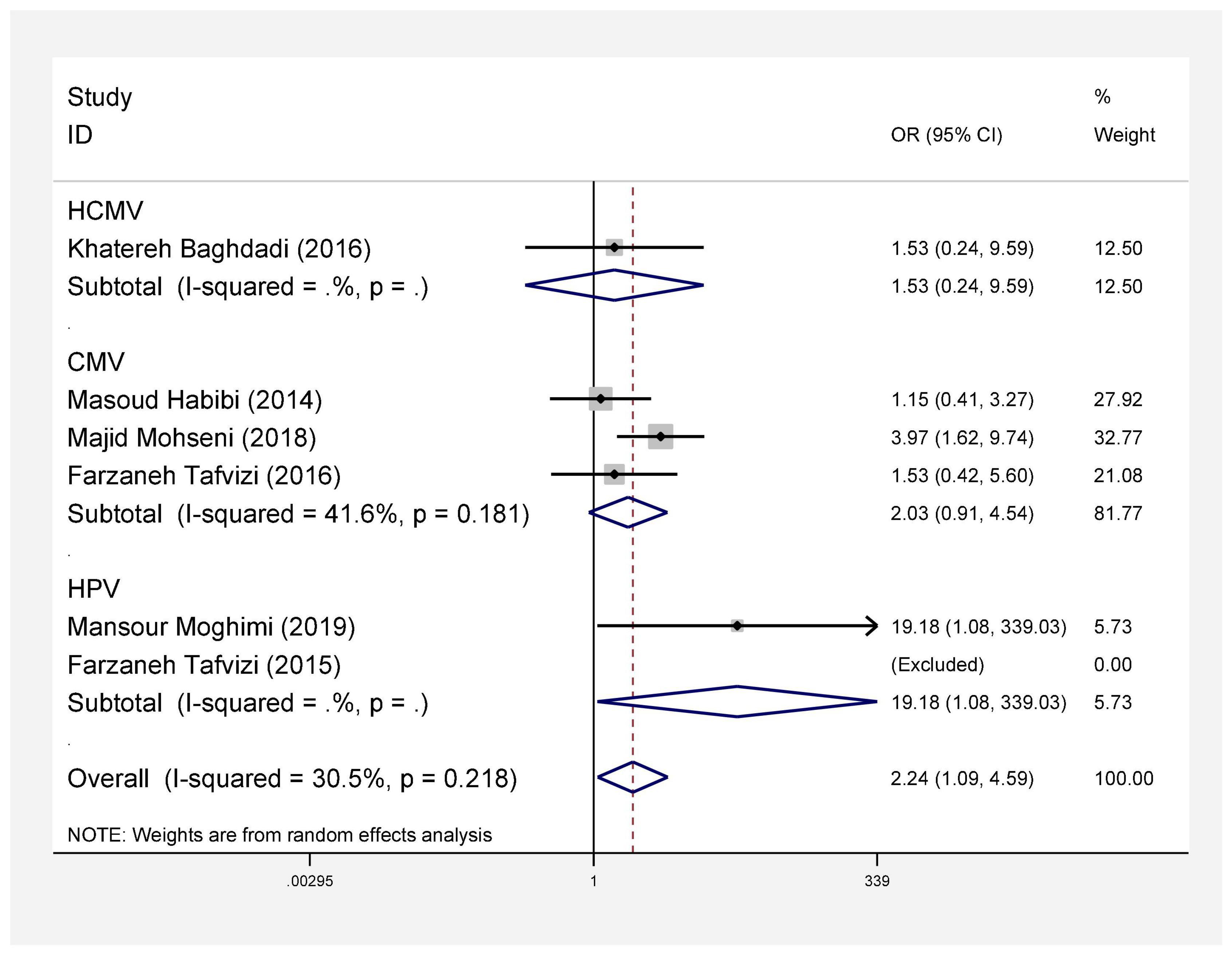
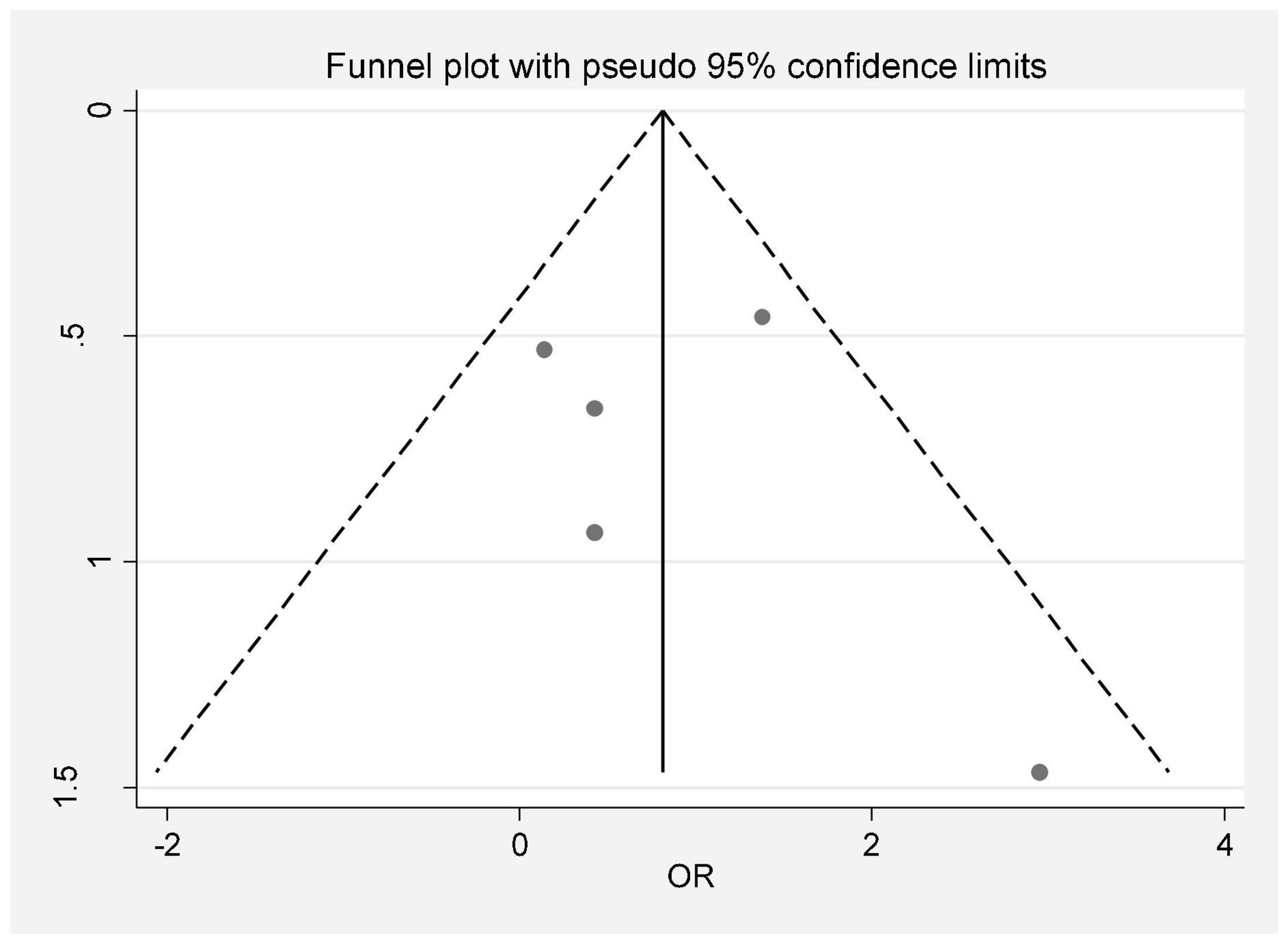
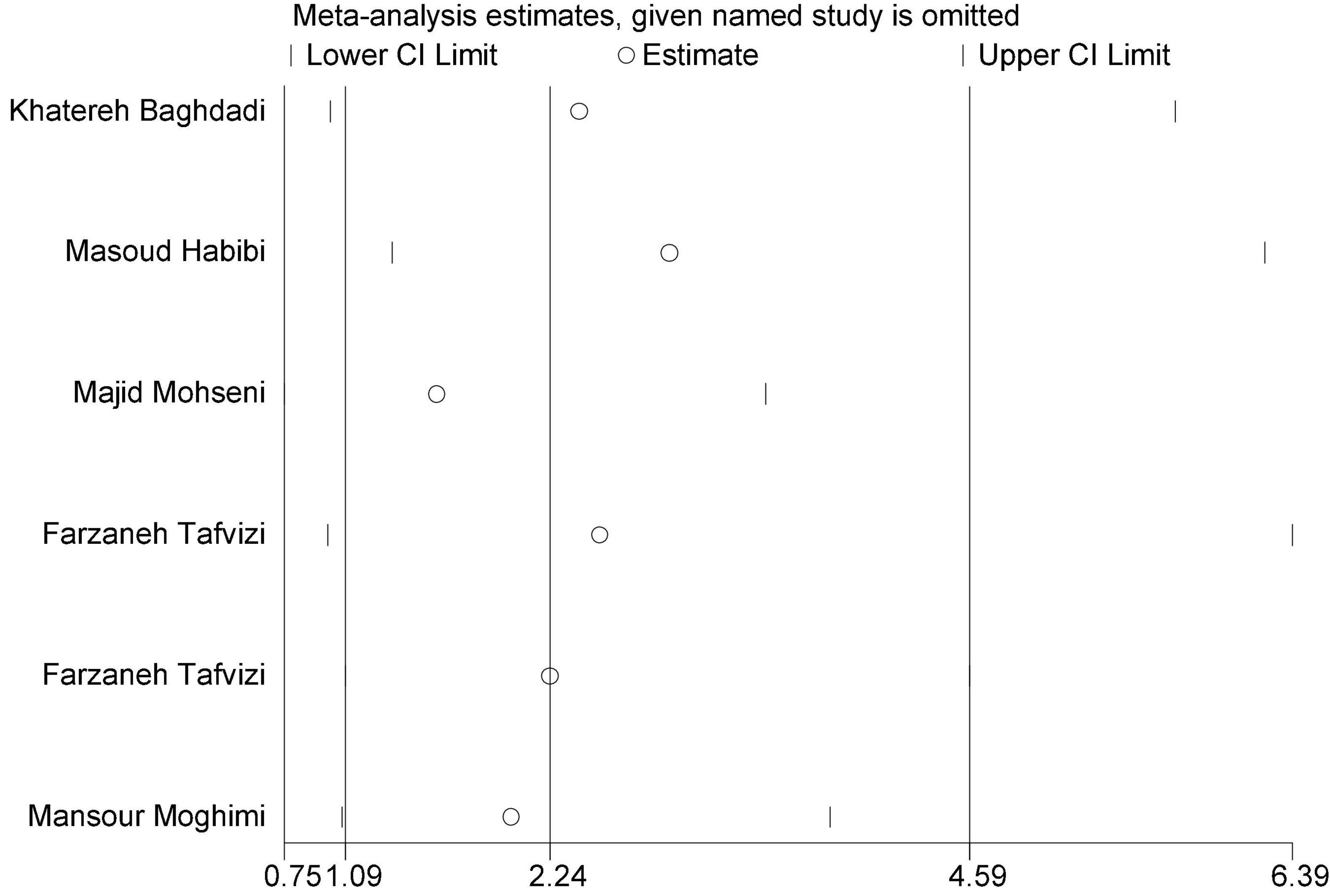
In this review, a total of 4,702 articles were found from the online databases (Web of Science, PubMed, Scopus, Science Direct, and Google scholar). After limiting the search strategy, the studies were restricted to 1,073. A total of 923 non-relevant studies were then excluded. After removing 43 duplicate studies, 107 articles were assessed for eligibility criteria. Finally, 72 appropriate studies were included in this meta-analysis (Figure 1 and Tables 1–4). The relationship between human cytomegalovirus (HCMV), cytomegalovirus (CMV), and human papillomavirus (HPV) and male infertility was investigated in six case-control studies. The heterogeneity among the primary studies was not significant (I2: 30.5%, Q: 5.8, P: 0.218). By combining the results of primary studies based on the random effect model, the odds ratio of male infertility in men exposed to HCMV, CMV, and HPV was higher than that in men without exposure to these viruses 2.24 (1.09–4.59) 95%. This difference was statistically significant. It should be noted that, in Tafvizi’s (16) study, the frequency of HPV was zero in the case and control groups (Figure 2). A funnel plot is used to investigate publication bias to estimate the odds ratio of male infertility due to exposure to CMV, HCMV, and HPV. According to the funnel plot and Egger test, there is no publication bias (β = 0.74, P = 0.698). Also, the results of the meta-regression test show that the virus type did not affect the heterogeneity and diversity of the results (β = 0.96, P = 0.351 (Figure 3). The sensitivity analysis results showed that the effect of each primary study on the overall estimation of the odds ratio of male infertility due to exposure to CMV, HCMV, and HPV was not significant (Figure 4). The heterogeneity of 9 studies in estimating the frequency of the HPV, herpes simplex virus 1 (HSV1), herpes simplex virus 2 (HSV2), and herpes simplex virus 1-2 (HSV1-2) is high (I2: 87.86%, Q: 65.9, P < 0.001). By combining the results of eight primary studies, the overall incidence of these viruses in infertile men was 15% (95% CI: 9–21 (Figure 5). A total of 26 case-control studies examined the association between Ureaplasma urealyticum, Mycoplasma hominis, Mycoplasma genitalium, Escherichia coli, Chlamydia trachomatis, Streptococcus pyogenes, Staphylococcus aureus, Staphylococcus saprophyticus, Staphylococcus epidermidis, and other bacterial exposure and male infertility. The heterogeneity among the primary studies was not significant (I2: 10.2%, Q: 29.85, P = 0.23). The statistical significance of the results shows that the odds ratio of male infertility in men exposed to the bacteria was higher than that in men without exposure to these bacteria (3.15; 95% CI: 2.60–4.23) (Figure 6). A funnel plot was used to determine publication bias in estimating the odds ratio of male infertility due to exposure to different bacteria. According to the funnel plot and Egger test, there is no publication bias (β = 0.31, P = 0.431) (Figure 7). The sensitivity analysis showed that the effect of each primary study on the overall estimation of the odds ratio of male infertility due to exposure to different bacteria was not significant (Figure 8). The result of the study for estimating the frequency of bacteria showed significant heterogeneity among the results of 56 primary studies (I2: 95.3%, Q: 1,166.1, P < 0.001), and the overall incidence of bacteria in infertile men was 12% (95% CI: 10–13) (Figure 9).
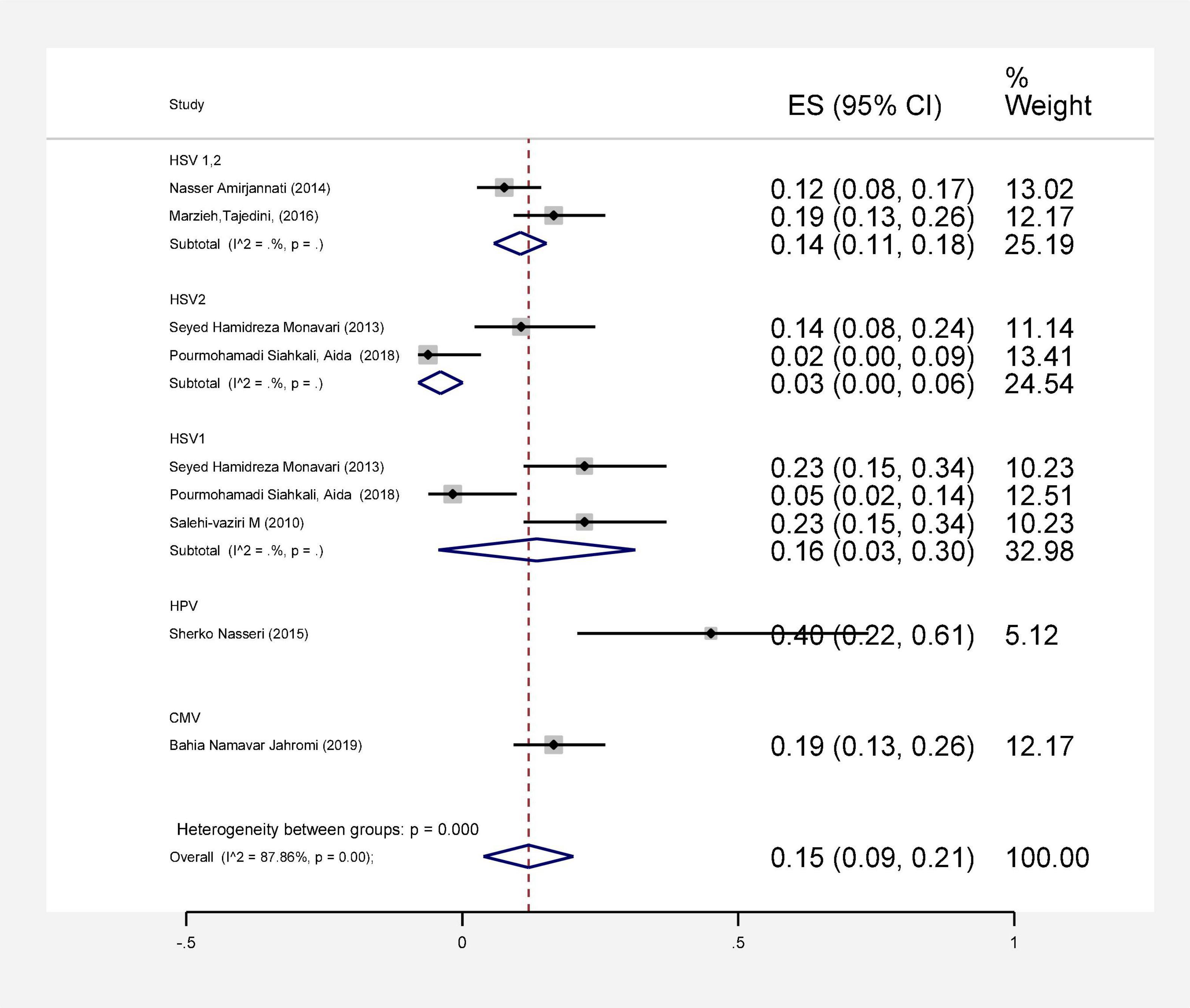
Figure 5. Estimating the prevalence of viral agents in each of the initial studies and pooled estimate with a 95% confidence interval.
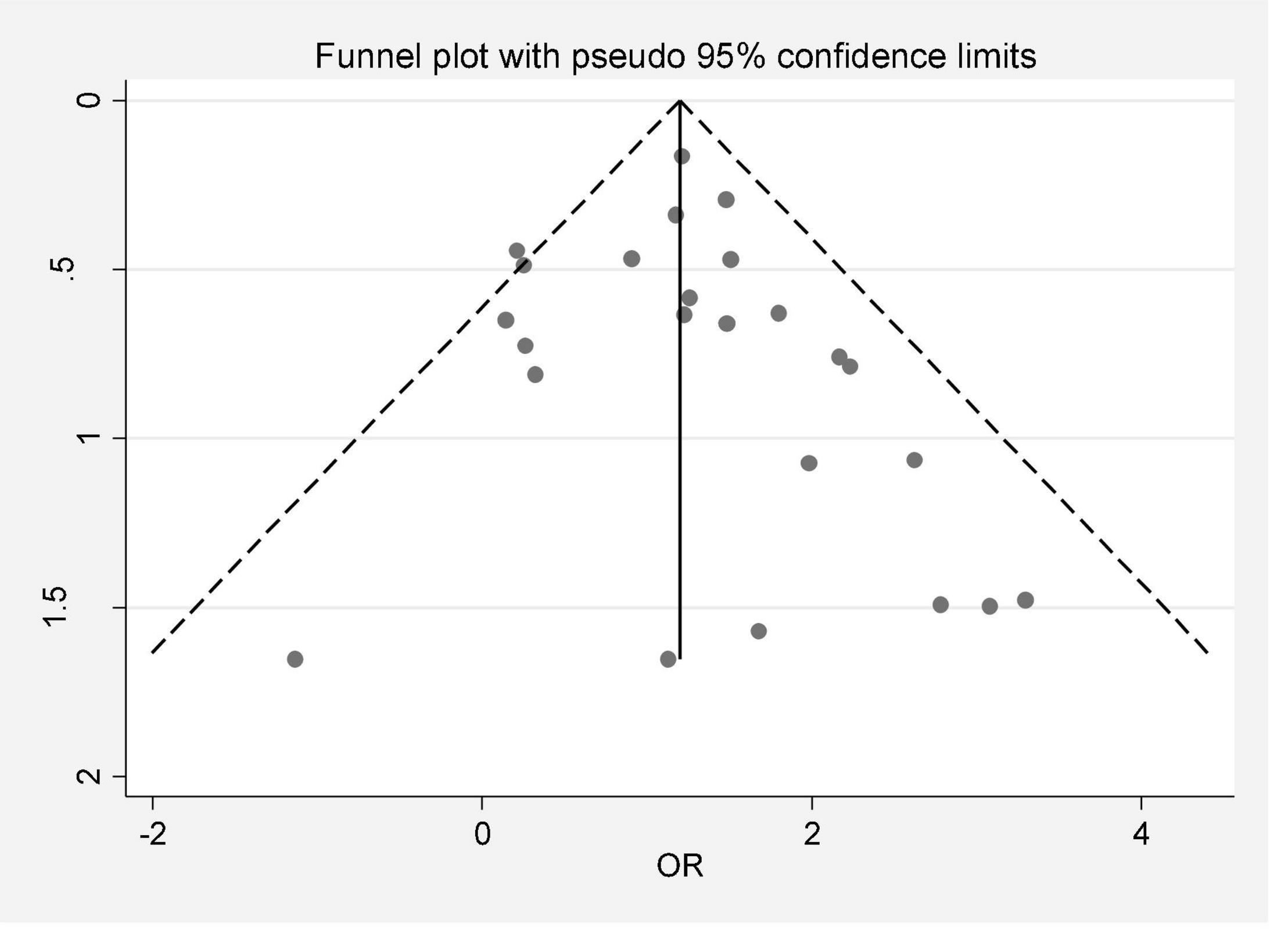
Figure 7. Publication bias (odds ratio of male infertility due to exposure to different bacteria based on the funnel plot).
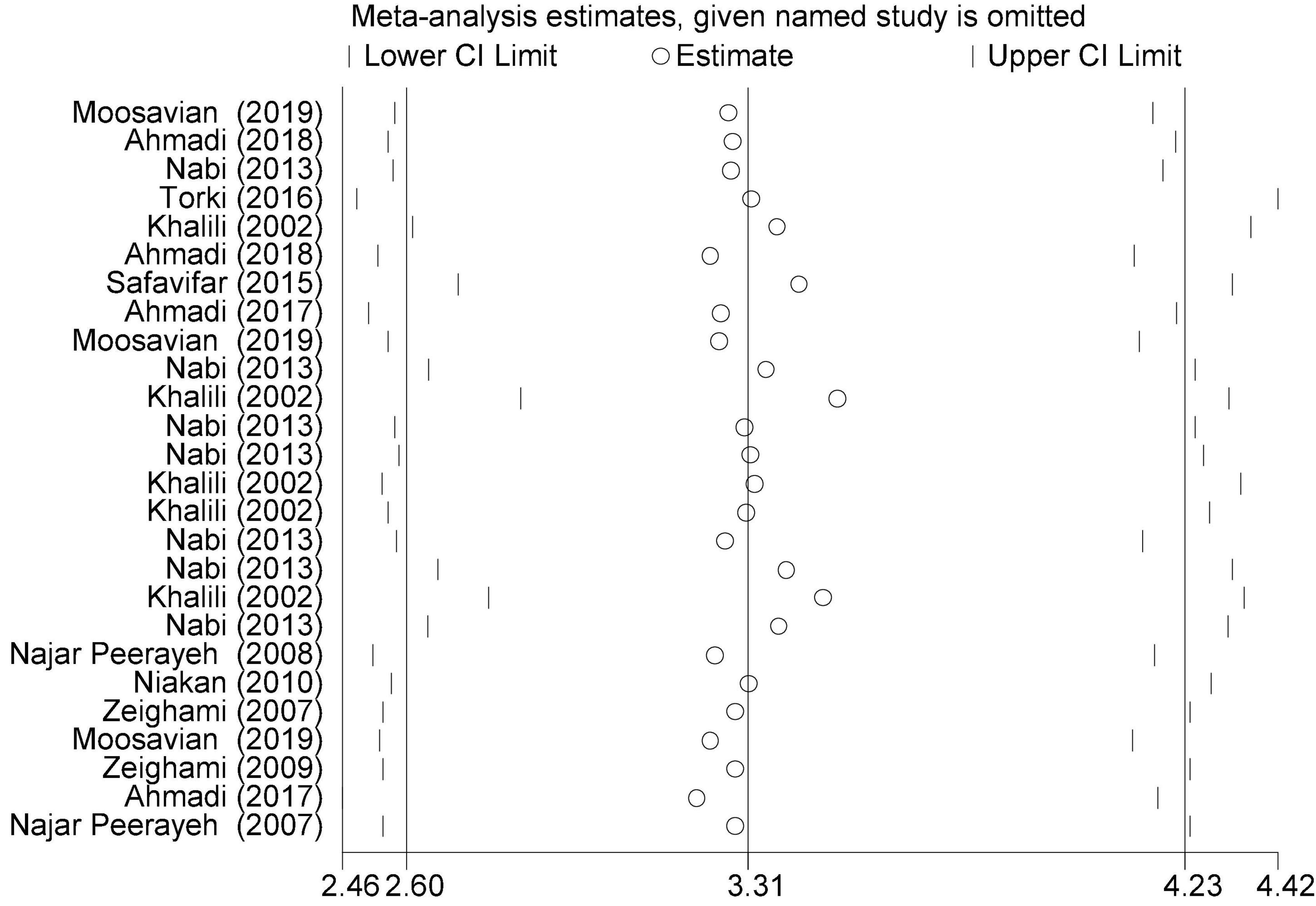
Figure 8. Sensitivity analysis (overall estimation of the odds ratio of male infertility due to exposure to different bacteria).
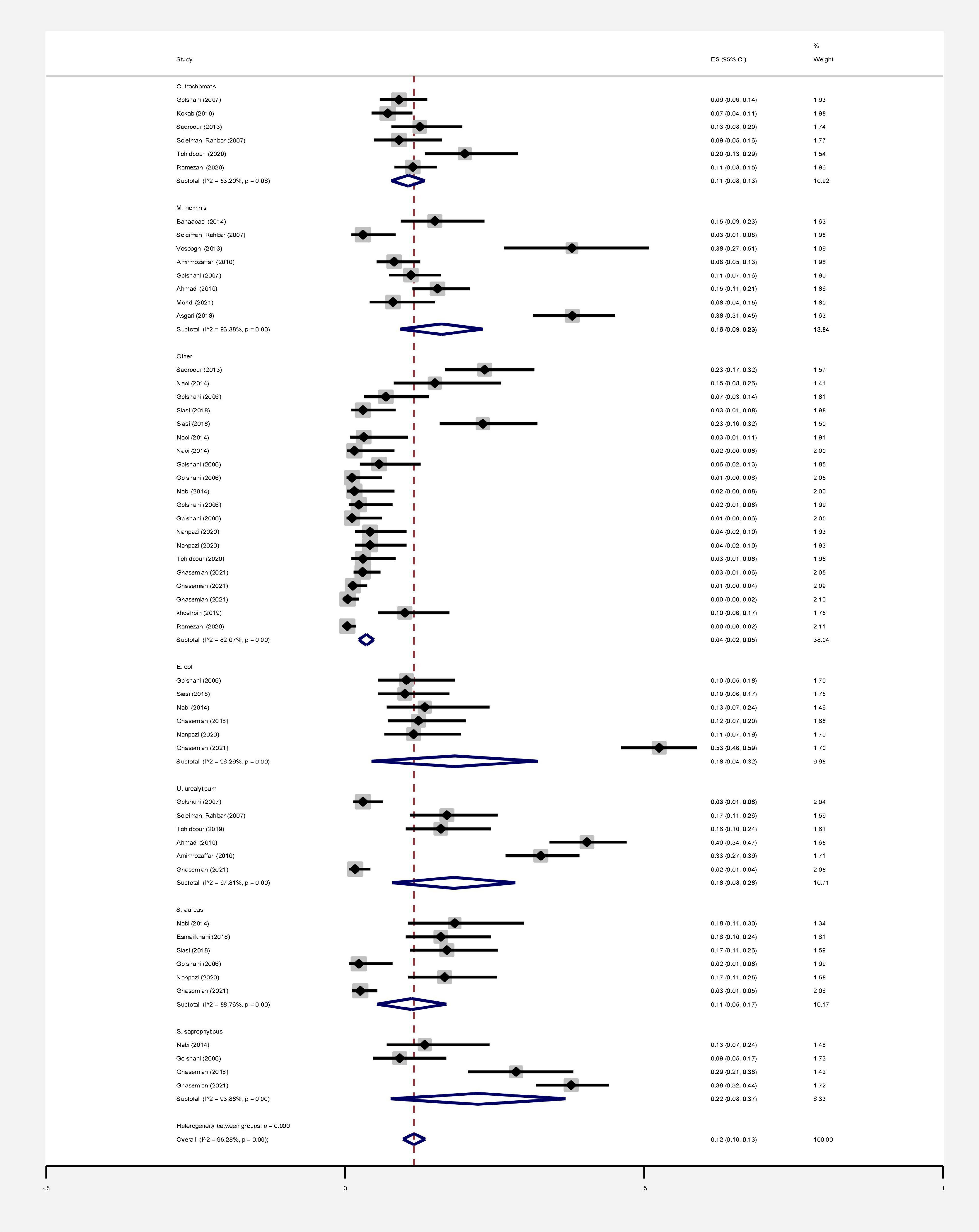
Figure 9. Estimating the prevalence of bacterial agents in each of the initial studies and pooled estimate with a 95% confidence interval.
Discussion
Infertility affects about 15% of couples globally and about half of these cases are because of male factors. Major etiological variants include microbial infectious and inflammatory disorders in the reproductive system. Male infertility can be caused by various microorganisms, although the direct effects of bacterial and viral infections on male infertility are still debated (63, 64). To date, there has been no systematic review and meta-analysis study on the frequency and role of viral and bacterial infections simultaneously in infertile men in Iran. Based on our results, all 9 studies investigating the prevalence of HPV, HSV1, HSV2, and HSV1-2 in infertile men have a frequency of 15% (CI 95%: 9–21). The association between HCMV, CMV, and HPV and male infertility was evaluated in 6 case-control studies. The ratio of male infertility due to these viruses (1.09–4.59) was 2.24 times higher than those without viruses. The association between HPV infection and male infertility has also been recently explored, and the available data are controversial (65, 66). In a cross-sectional clinical study, Foresta et al. (67) have also shown that sperm motility in patients positive for HPV was considerably reduced (67). Perino et al. indicated that HPV infection was associated with infertility in couples. These presented a diminished pregnancy rate and an elevated abortion frequency in couples infected with HPV than those not infected (68). Male infertility and aberrant sperm parameters are linked to HSV infection, which disrupts the male accessory genital system (69, 70). HSV infections in sperm vary greatly among investigations (71). Kurscheidt et al. have previously reported that HSV infections in male partners of infertile couples could cause alterations in spermatozoa and seminal fluid, affecting fertility (72). This analysis is consistent with a previous report by Bezold et al. (69). They found that HSV DNA-positive semen specimens had meaningfully decreased sperm count and motility. Based on available data, the occurrence of HCMV DNA in the semen of fertile and infertile men with seropositive HCMV is between 8 and 65% (73). However, HCMV-caused male infertility is commonly unusual (74, 75). Pallier et al. have previously reported that HCMV particles in the semen are not correlated with sperm motility (76). Most of the evaluated articles in our study were about viruses that were possibly associated with male infertility. In line with studies conducted on viral infection, numerous efforts have been directed toward identifying the role of bacterial infections in male infertility. Bacterial infection was considered a significant element of infertility in the semen of asymptomatic infertile men (35). In a recent study, among a total of 50 infertile male semen samples, 45 (90%) were infected with at least one type of bacterial strain. In comparison, in five samples of infertile semen, microorganisms were not detected. The most common bacterial genus was Enterococcus (32%), followed by Klebsiella (22%) (77). In another study, Domes et al. reported 15% bacteriospermia in a male factor infertility population (1,200/7,852), including 22 bacterial species. Among positive cultures, Enterococcus faecalis was the most frequent, with an occurrence of 56% (15/60) (78). In 56 studies that entered our meta-analysis, the pooled prevalence of bacterial infections in the semen of infertile men was 12% (95% CI 10–13%). The relationship between infertility in men and bacteria was evaluated in 26 case-control studies. The results show that the ratio of chance of infertility in men exposed to bacteria with a 95% confidence interval is equal to 2.60–4.23, which is 3.31 times that of people without bacteria. Moreover, Zeyad et al. showed that bacterial infections have significant negative effects on sperm parameters. Sperm concentration, motility, and progression, and chromatin condensation were significantly lower in infected patients (79).
Limitation
There are some limitations in this meta-analysis:
• High heterogeneity among the results of preliminary studies, which included study population, methodology of studies, sampling, and semen analysis method in infertile males.
• Lack of socioeconomic and demographic information of infertile men may have led to the heterogeneity.
• Different criteria for the screening and recruitment of infertile men in the included studies.
Conclusion
The presence of viral and bacterial infections is a risk factor and could impair male fertility potential by decreasing sperm quality. The current systematic review and meta-analysis provides the overall bacterial and viral infection frequency in infertile men and data on etiologic agents of viruses and bacteria in Iran. Also, this meta-analysis supports the hypothesis that co-infection could play a key role in impairing sperm quality, motility, and mobility in the male population.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TM and MG conceived and designed the study, performed the literature search, and collected the data. HJ and MH performed the literature review. MM performed the statistical analysis. All authors read and approved the final version of the manuscript.
Funding
We would like to thank Mazandaran University of Medical Sciences for financial support (IR.MAZUMS.REC.1401.11569).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.835254/full#supplementary-material
References
1. Schuppe H-C, Pilatz A, Hossain H, Diemer T, Wagenlehner F, Weidner W. Urogenital infection as a risk factor for male infertility. Deutsch Ärzteblatt Int. (2017) 114:339. doi: 10.3238/arztebl.2017.0339
2. Fijak M, Pilatz A, Hedger MP, Nicolas N, Bhushan S, Michel V, et al. Infectious, inflammatory and ‘autoimmune’male factor infertility: how do rodent models inform clinical practice? Hum Reprod Update. (2018) 24:416–41. doi: 10.1093/humupd/dmy009
3. Rusz A, Pilatz A, Wagenlehner F, Linn T, Diemer T, Schuppe H, et al. Influence of urogenital infections and inflammation on semen quality and male fertility. World J Urol. (2012) 30:23–30. doi: 10.1007/s00345-011-0726-8
4. Bukharin O, Kuz’min M, Ivanov I. The role of the microbial factor in the pathogenesis of male infertility. Zh Mikrobiol Epidemiol Immunobiol. (2000). 2:106–10.
5. Weidner W, Pilatz A, Diemer T, Schuppe H, Rusz A, Wagenlehner F. Male urogenital infections: impact of infection and inflammation on ejaculate parameters. World J Urol. (2013) 31:717–23. doi: 10.1007/s00345-013-1082-7
6. Fraczek M, Kurpisz M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: potential inflammatory markers in semen. Folia Histochem Cytobiol. (2015) 53:201–17. doi: 10.5603/fhc.a2015.0019
7. Brunner RJ, Demeter JH, Sindhwani P. Review of guidelines for the evaluation and treatment of leukocytospermia in male infertility. World J Men Health. (2019) 37:128–37. doi: 10.5534/wjmh.180078
8. Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. (2017) 32:18–31.
9. Kim SJ, Paik D-J, Lee JS, Lee HS, Seo JT, Jeong MS, et al. Effects of infections with five sexually transmitted pathogens on sperm quality. Clin Exp Reprod Med. (2017) 44:207. doi: 10.5653/cerm.2017.44.4.207
10. Vilvanathan S, Kandasamy B, Jayachandran AL, Sathiyanarayanan S, Tanjore Singaravelu V, Krishnamurthy V, et al. Bacteriospermia and its impact on basic semen parameters among infertile men. Interdiscip Perspect Infect Dis. (2016) 2016:2614692. doi: 10.1155/2016/2614692
11. Xiong Y-Q, Chen Y-X, Cheng M-J, He W-Q, Chen Q. The risk of human papillomavirus infection for male fertility abnormality: a meta-analysis. Asian J Androl. (2018) 20:493. doi: 10.4103/aja.aja_77_17
12. Fode M, Fusco F, Lipshultz L, Weidner W. Sexually transmitted disease and male infertility: a systematic review. Eur Urol Focus. (2016) 2:383–93. doi: 10.1016/j.euf.2016.08.002
13. Pourmasumi S, Sabeti P, Rahiminia T, Mangoli E, Tabibnejad N, Talebi AR. The etiologies of DNA abnormalities in male infertility: an assessment and review. Int J Reprod Biomed. (2017) 15:331. doi: 10.1093/humupd/dmg027
14. Diemer T, Huwe P, Ludwig M, Hauck E, Weidner W. Urogenital infection and sperm motility. Andrologia. (2003) 35:283–7. doi: 10.1111/j.1439-0272.2003.tb00858.x
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
16. Tafvizi F, Fathi MH, Tahmasbi FZ. Lack of association between HPV and sperm parameters as a risk factor in infertile men admitted to an infertility clinic. Pars Jahrom Univ Med Sci. (2015) 13:37–45. doi: 10.29252/jmj.13.3.37
17. Baghdadi K, Tafvizi F, Hayati Roodbari N. Molecular detection of HCMV and investigation of Its relationship with quality of sperm parameters in male infertility. Electron J Biol. (2016) 12:73–6.
18. Habibi M, Bahrami A, Morteza A, Sadighi Gilani MA, Hassanzadeh G, Ghadami M, et al. Study of cytomegalovirus infection in idiopathic infertility men referred to Shariati hospital Tehran, Iran. Iran J Reprod Med. (2014) 12:151–4.
19. Mohseni M, Mollaei HR, Arabzadeh SA, Mirshekari TR, Ghorbani P. Frequency of cytomegalovirus in fertile and infertile men, referring to Afzalipour Hospital IVF research center Kerman, IRAN: a case-control study. Int J Reprod Biomed. (2018) 16:443. doi: 10.29252/ijrm.16.7.443
20. Tafvizi F, Baghdadi K, Roodbari H. Lack of relatedness between Human cytomegalovirus in semen and male infertility. Iran J Med Microbiol. (2016) 10:39–46. doi: 10.1007/978-1-4612-1848-7_3
21. Moghimi M, Zabihi-Mahmoodabadi S, Kheirkhah-Vakilabad A, Kargar Z. Significant correlation between high-risk HPV DNA in semen and impairment of sperm quality in infertile men. Int J Fertil Steril. (2019) 12:306–9. doi: 10.22074/ijfs.2019.5421
22. Tajedini M, Sadighi Gilani MA, Heydari T, Choobineh K, Choobineh H. Detection of herpes simplex virus 1 and 2 in semen, blood and urine of idiopathic infertile men and its association with sperm count and motility. J Payavard Salamat. (2017) 10:523–9.
23. Pourmohamadi SA, Amini K. Identification of herpes simplex virus, Chlamydia trachomatis, and Mycoplasma genitalium in infertile seminal fluid samples using multiplex-PCR in Kerman Province, Iran (2016). Iran J Obstet Gynecol Infertil. (2018) 21:69–74.
24. Salehi-vaziri M, Monavari S, Khalili M, Shamsi-Shahrabadi M, Keyvani H, Mollaei H, et al. Detection of HSV-1 DNA in the semen of infertile men and evaluation of its correlation with semen parameters in Iran. Iran J Virol. (2010) 4:1–6.
25. Monavari SH, Vaziri MS, Khalili M, Shamsi-Shahrabadi M, Keyvani H, Mollaei H, et al. Asymptomatic seminal infection of herpes simplex virus: impact on male infertility. J Biomed Res. (2013) 27:56. doi: 10.7555/JBR.27.20110139
26. Amirjannati N, Yaghmaei F, Akhondi MM, Nasiri M, Heidari-Vala H, Sehhat Z. Molecular and serologic diagnostic approaches; the prevalence of herpes simplex in idiopathic men infertile. Iran J Reprod Med. (2014) 12:327.
27. Nasseri S, Monavari SH, Keyvani H, Nikkhoo B, Roudsari RV, Khazeni M. The prevalence of human papilloma virus (HPV) infection in the oligospermic and azoospermic men. Med J Islam Republ Iran. (2015) 29:272.
28. Jahromi BN, Yaghobi R, Matlub N, Fazelzadeh A, Ramzi A, Anvar Z, et al. Prevalence of cytomegalovirus in semen of male partners of infertile couples and the virus impact on sperm parameters. J Reprod Infertil. (2020) 21:124.
29. Zeighami H, Peerayeh SN, Safarlu M. Detection of Ureaplasma urealyticum in semen of infertile men by PCR. Pak J Biol Sci. (2007) 10:3960–3. doi: 10.3923/pjbs.2007.3960.3963
30. Ahmadi MH, Mirsalehian A, Gilani MA, Bahador A, Talebi M, Yazdi RS. Antibiotic treatment of asymptomatic Ureaplasma infection improves semen parameters in infertile men. J Appl Biomed. (2017) 15:139–45. doi: 10.1016/j.jab.2016.11.004
31. Peerayeh SN, Yazdi RS, Zeighami H. Association of Ureaplasma urealyticum infection with varicocele-related infertility. J Infect Dev Ctries. (2008) 2:116–9. doi: 10.3855/t2.2.116
32. Zeighami H, Peerayeh SN, Yazdi RS, Sorouri R. Prevalence of Ureaplasma urealyticum and Ureaplasma parvum in semen of infertile and healthy men. Int J STD AIDS. (2009) 20:387–90. doi: 10.1258/ijsa.2008.008334
33. Niakan M, Moradi B, Ragheb S. The prevalence of Ureaplasma urealyticumin semen of infertile men Niakan M*, Moradi B, Ragheb SH. Iran J Med Microbiol. (2009) 3:31–5.
34. Ahmadi MH, Mirsalehian A, Gilani MA, Bahador A, Talebi M. Asymptomatic infection with Mycoplasma hominis negatively affects semen parameters and leads to male infertility as confirmed by improved semen parameters after antibiotic treatment. Urology. (2017) 100:97–102. doi: 10.1016/j.urology.2016.11.018
35. Ahmadi MH, Mirsalehian A, Gilani MA, Bahador A, Talebi M. Improvement of semen parameters after antibiotic therapy in asymptomatic infertile men infected with Mycoplasma genitalium. Infection. (2018) 46:31–8. doi: 10.1007/s15010-017-1075-3
36. Safavifar F, Bandehpour M, Hosseiny SJ, Khorramizadeh MR, Shahverdi A, Kazemi B. Mycoplasma infection in Pyospermic infertile and healthy fertile men. Novel Biomed. (2015) 3:25–9.
37. Torki A, Khalili MB, Ghasemzadeh J. Association of E.coli in the semen with male infertility in infertility research center of Shahid Sadoughi University of Medical Sciences in Yazd. Res Med. (2016) 40:12–6.
38. Khalili MB, Khalili MA. Correlation between asymptomatic urethritis with bacteriospermia in seminal plasma of fertile and infertile men. J Reprod Infertil. (2002) 3:21–8.
39. Nabi A, Khalili MA, Halvaei I, Ghasemzadeh J, Zare E. Seminal bacterial contaminations: probable factor in unexplained recurrent pregnancy loss. Iran J Reprod Med. (2013) 11:925.
40. Ahmadi MH, Mirsalehian A, Sadighi Gilani MA, Bahador A, Afraz K. Association of asymptomatic Chlamydia trachomatis infection with male infertility and the effect of antibiotic therapy in improvement of semen quality in infected infertile men. Andrologia. (2018) 50:e12944. doi: 10.1111/and.12944
41. Moosavian M, Ghadiri A, Amirzadeh S, Rashno M, Afzali M, Ahmadi K. Investigating Chlamydia trachomatis and genital Mycoplasma prevalence and apoptosis markers in infertile and fertile couples. Jundishapur J Microbiol. (2019) 12:e84954.
42. Golshani M, Eslami G, Ghobadloo SM, Fallah F, Goudarzi H, Rahbar AS, et al. Detection of Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum by multiplex PCR in semen sample of infertile men. Iran J Public Health. (2017) 36:50–7.
43. Kokab A, Akhondi MM, Sadeghi MR, Modarresi MH, Aarabi M, Jennings R, et al. Raised inflammatory markers in semen from men with asymptomatic chlamydial infection. J Androl. (2010) 31:114–20. doi: 10.2164/jandrol.109.008300
44. Sadrpour P, Bahador A, Asgari S, Bagheri R, Chamani-Tabriz L. Detection of Chlamydia trachomatis and Mycoplasma genitalium in semen samples of infertile men using multiplex PCR. Tehran Univ Med J. (2013) 70:623–9.
45. Soleimani Rahbar AA, Golshani M, Fayyaz F, Rafiee Tabatabaei S, Moradi A. Identification of Mycoplasma genome in sperm of infertile men by PCR. Iran J Med Microbiol. (2007) 1:47–53.
46. Bahaabadi SJ, Moghadam NM, Kheirkhah B, Farsinejad A, Habibzadeh V. Isolation and molecular identification of Mycoplasma hominis in infertile female and male reproductive system. Nephrourol Mon. (2014) 6:e22390. doi: 10.5812/numonthly.22390
47. Vosooghi S, Kheirkhah B, Mir-shekari TR, Karimi Nik A, Hamidavi Mohammadpour S, Mohseni Moghadam N. Molecular detection of Mycoplasma hominis from genital secretions of infertile men referred to the Kerman infertility center. J Microb World. (2013) 6:14–22.
48. Amirmozaffari N, Ahmadi M, Sedighi Gilani M, Kazemi B, Masjedian Jazi F. Detection of Mycoplasma hominis and Ureaplasma urealyticum from semen samples of infertile men referred to royan institute in 2008. RJMS. (2010) 17:14–26.
49. Ahmadi MH, Mozafari NA, Kazemi B, Sedighi-Gilani MA, Masjedian F. Use of pcr to detect Mycoplasma hominis and Ureaplasma urealyticum from semen samples of infertile men who referred to royan institute in 2009. Cell J. (2010) 12:371–80.
50. Golshani M, Taheri S, Eslami G, Suleimani Rahbar AA, Fallah F, Goudarzi H. Genital tract infection in asymptomatic infertile men and its effect on semen quality. Iran J Public Health. (2006) 35:81–4.
51. Siasi E, Sohrabvand F, Gohari N. Relation of fim H, pap and afa genes in urinary infection E.coli with male infertility. NCMBJ. (2018) 8:110–23.
52. Nabi A, Khalili MA, Halvaei I. An investigation of bacterial infection of seminal fluid in men with infertility with unknown etiology. Qom Univ Med Sci J. (2014) 8:48–53.
53. Ghasemian F, Esmaeilnezhad S, Mehdipoor MM. The comparison of bacterial infection effects on semen parameters and assisted reproductive outcomes in infertile men. J Dev Biol. (2018) 10:59–68.
54. Tohidpour M, Shahhosseiny M, Mehrabian S, Saremi A. Detection of Ureaplasma urealyticum in clinical semen samples in infertile men using molecular method. J Mazandaran Univ Med Sci. (2019) 28:10–21.
55. Esmailkhani A, Akhi MT, Sadeghi J, Niknafs B, Bialvaei AZ, Farzadi L, et al. Assessing the prevalence of Staphylococcus aureus in infertile male patients in Tabriz, northwest Iran. Int J Reprod Biomed. (2018) 16:469. doi: 10.29252/ijrm.16.7.469
56. Moridi K, Ghazvini K, Hemmati M, Hoseiniun H, Torkaman M. Prevalence determination of M. hominis and M. genitalium in the semen samples in the northeast of Iran using culture and multiplex polymerase chain reaction. Arch Razi Inst. (2021) 76:41. doi: 10.22092/ari.2019.125966.1338
57. Nanpazi E, Fozouni L, Dadgar T. In-vitro antimicrobial effect of Silybum marianum extract on bacterial isolates from the semen of infertile men in Iran. Int J Infect. (2020) 7:e104297.
58. Asgari A, Nazari R, Razavian SMH. Investigation of frequency of mycoplasma hominis and biological parameters in semen sample of men referred to Qom Jihad Daneshgahi infertility treatment center in 2016 (Iran). Qom Univ Med Sci J. (2018) 12:81–8. doi: 10.29252/qums.12.4.81
59. Tohidpour M, Shahhosseiny M, Mehrabian S, Saremi A. Prevalence of Chlamydia trachomatis and Listeria monocytogenes in infertile men and the effect on semen parameters. Jundishapur J Microbiol. (2020) 13:e97780.
60. Ghasemian F, Esmaeilnezhad S, Moghaddam MJ. Staphylococcus saprophyticus and Escherichia coli: tracking from sperm fertility potential to assisted reproductive outcomes. Clin Exp Reprod Med. (2021) 48:142. doi: 10.5653/cerm.2020.04203
61. Paknejadi M. Molecular diagnosis of Helicobacter pylori in semen of infertile men. N Cell Mol Biotechnol J. (2019) 9:67–76.
62. Ramezani M, Hakimi H, Zainodini N, Rahnama A, Sayadi A. Survey on the prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections and their possible effects on seminal quality in infertile men. Int J Infect. (2019) 6:e101243.
63. Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. (2018) 50:e13140. doi: 10.1111/and.13140
64. Pai MO, Venkatesh S, Gupta P. The role of infections in infertility: a review. Int J Acad Med. (2020) 6:189.
65. Rintala MA, Greénman SE, Pöllänen PP, Suominen JJ, Syrjänen SM. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int J STD AIDS. (2004) 15:740–3. doi: 10.1258/0956462042395122
66. Lai YM, Lee JF, Huang HY, Soong YK, Yang FP, Pao CC. The effect of human papillomavirus infection on sperm cell motility. Fertil Steril. (1997) 67:1152–5. doi: 10.1016/s0015-0282(97)81454-9
67. Foresta C, Garolla A, Zuccarello D, Pizzol D, Moretti A, Barzon L, et al. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil Steril. (2010) 93:802–6. doi: 10.1016/j.fertnstert.2008.10.050
68. Perino A, Giovannelli L, Schillaci R, Ruvolo G, Fiorentino FP, Alimondi P, et al. Human papillomavirus infection in couples undergoing in vitro fertilization procedures: impact on reproductive outcomes. Fertil Steril. (2011) 95:1845–8. doi: 10.1016/j.fertnstert.2010.11.047
69. Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. (2007) 87:1087–97. doi: 10.1016/j.fertnstert.2006.08.109
70. Gimenes F, Souza RP, Bento JC, Teixeira JJ, Maria-Engler SS, Bonini MG, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. (2014) 11:672–87. doi: 10.1038/nrurol.2014.285
71. Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigiannakis A. Prevalence of human herpes virus types 1–7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil Steril. (2009) 91:2487–94. doi: 10.1016/j.fertnstert.2008.03.074
72. Kurscheidt FA, Damke E, Bento JC, Balani VA, Takeda KI, Piva S, et al. Effects of herpes simplex virus infections on seminal parameters in male partners of infertile couples. Urology. (2018) 113:52–8. doi: 10.1016/j.urology.2017.11.050
73. Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol. (2013) 100:20–9. doi: 10.1016/j.jri.2013.03.004
74. Naumenko VA, Tyulenev YA, Yakovenko SA, Lubov’F K, Shileyko LV, Segal AS, et al. Detection of human cytomegalovirus in motile spermatozoa and spermatogenic cells in testis organotypic culture. Herpesviridae. (2011) 2:1–8. doi: 10.1186/2042-4280-2-7
75. Eggert-Kruse W, Reuland M, Johannsen W, Strowitzki T, Schlehofer JR. Cytomegalovirus (CMV) infection—related to male and/or female infertility factors? Fertil Steril. (2009) 91:67–82. doi: 10.1016/j.fertnstert.2007.11.014
76. Pallier C, Tebourbi L, Chopineau-Proust S, Schoevaert D, Nordmann P, Testart J, et al. Herpesvirus, cytomegalovirus, human sperm and assisted fertilization. Hum Reprod. (2002) 17:1281–7. doi: 10.1093/humrep/17.5.1281
77. Al-Saadi BQ, Abd AS. The effect of bacterial infection on male infertility. Iraqi J Biotechnol. (2019) 18:72–84.
78. Domes T, Lo KC, Grober ED, Mullen JB, Mazzulli T, Jarvi K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril. (2012) 97:1050–5. doi: 10.1016/j.fertnstert.2012.01.124
Keywords: bacteria, virus, infection, semen, infertility, Iran
Citation: Gholami M, Moosazadeh M, Haghshenash MR, Jafarpour H and Mousavi T (2022) Evaluation of the Presence of Bacterial and Viral Agents in the Semen of Infertile Men: A Systematic and Meta-Analysis Review Study. Front. Med. 9:835254. doi: 10.3389/fmed.2022.835254
Received: 14 December 2021; Accepted: 05 April 2022;
Published: 04 May 2022.
Edited by:
Remco P. H. Peters, University of Pretoria, South AfricaReviewed by:
Andrea Garolla, University of Padua, ItalyMassimo Menegazzo, University of Padua, Italy
Copyright © 2022 Gholami, Moosazadeh, Haghshenash, Jafarpour and Mousavi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tahoora Mousavi, c3RtLmptdW1zQGdtYWlsLmNvbQ==
 Mehrdad Gholami
Mehrdad Gholami Mahmood Moosazadeh3
Mahmood Moosazadeh3 Tahoora Mousavi
Tahoora Mousavi