- 1Department of Emergency Medicine, Cheng Hsin General Hospital, Taipei, Taiwan
- 2Department of Hematology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, China
- 3Enze Medical Research Center, Affiliated Taizhou Hospital of Wenzhou Medical College, Taizhou, China
Background: Coronavirus disease-2019 (COVID-19), a worldwide disaster, has already affected lots of people. Effective care and therapy are currently being evaluated in full swing.
Purpose: Our purpose was to investigate the effects of tocilizumab, an interleukin-6 receptor inhibitor, on treatment of adult patients with COVID-19 pneumonia.
Data Sources, Study Selection, and Data Extraction: We conducted a meta-analysis and searched for relevant studies on Pubmed, Embase, and the Cochrane Library without restrictions on language from inception until February 1, 2021. Fifteen studies were included for this meta-analysis. Two authors independently selected and screened these studies, assessed the quality of included studies, and extracted related information.
Results: Fifteen studies were included in this meta-analysis. The main studies showed that tocilizumab was associated with lower mortality (risk ratio = 0.62, 95% confidence interval = 0.46–0.83; and hazard ratio = 0.61, 95% confidence interval = 0.51–0.72). Using tocilizumab might also affect biochemistry indicators (lowered C-reactive protein and ferritin, increased lymphocyte count).
Conclusion: These current bodies of evidence could indicate that early use of tocilizumab was associated with lower mortality in adult patients with COVID-19. Early use of tocilizumab could reduce the mortality rate of adult patients with COVID-19 without obvious fatal side effects, which may be a treatment option in patients with COVID-19 pneumonia.
Systematic Review Registration: The study protocol was registered on PROSPERO (ID:242811).
Introduction
An outbreak of pneumonia caused by severe acute respiratory syndrome coronavirus 2 [coronavirus disease 2019 (COVID-19)] emerged in Wuhan, Hubei province, China in December 2019. COVID-19 represents lots of clinical manifestations that typically include fever, cough, and fatigue (1). There is no definite, quite effective treatment for patients with COVID-19 infection until February 2021. Compared to disease caused by previously known human CoVs, COVID-19 showed less severe pathogenesis but higher transmission competence. Since there are no definite antiviral drugs existing to fully treat or prevent COVID-19, potential therapeutic strategies arecurrently being evaluated in full swing (2). For example, systemic steroids have been reported to benefit severely ill mechanically ventilated patients with COVID-19 (3). Severe dysregulated systemic inflammation is the putative mechanism. Thus, early administration of prolonged, low-dose methylprednisolone treatment was associated with lower mortality and decreased ventilator dependence (4).
Tocilizumab (trade name Actemra), also known as atlizumab, is an immunosuppressive drug mainly used for treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. This interleukin-6 receptor inhibitor showed possible efficacy and safety in patients with COVID-19. After some review of related literature, we could speculate that using the IL-6 inhibitor tocilizumab might lower the mortality of patients suffering from COVID-19 pneumonia (5–7). However, more substantial and integrative results are required. Because of highly infectious COVID-19 pneumonia, finding out a definite therapy was the top priority. Critically ill patients with COVID-19 might add heavy pressure on the medical system (3). The use of the IL-6 inhibitor in treating COVID-19 is still controversial. The object of this meta-analysis was to assess the curative effect of tocilizumab on COVID-19, regardless of race (including Europeans, Americans, and Asians) and severity of the disease.
Methods
We performed a meta-analysis of observational studies to examine the effects of tocilizumab on COVD-19. The study protocol was registered on PROSPERO.
Searching for Literature
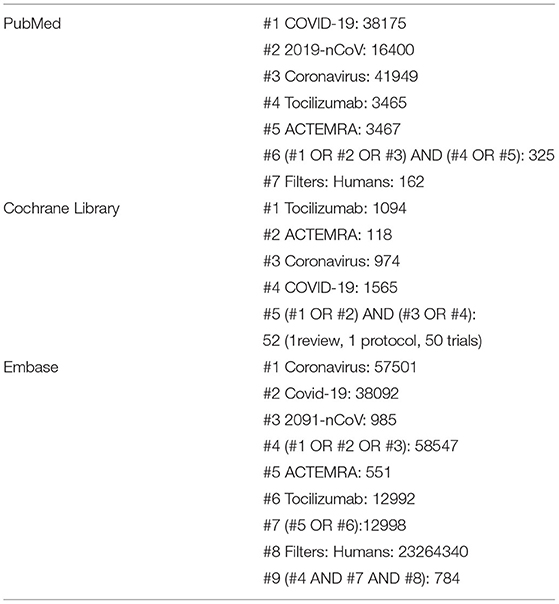
We followed MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines to conduct and report this study (8). We searched PubMed, Embase, and the Cochrane Library for relevant studies with no language limitation from inception to February 1, 2021. The search strategy is illustrated in Table 1.
Study Selection
We included observational studies that examined the effects of tocilizumab on COVID-19. Two authors (Chi-Chung Chen and Hsien-Lung Tsai) independently scanned the search results, reviewed the full text of potential studies, and decided whether these studies met our selection criteria. The third author (Tao-Hsin Tung) was consulted if there was any disagreement.
Data Extraction and Risk of Bias Assessment
The following data were extracted from the included studies: first author, year of publication, country, study subjects, and outcomes. Outcomes included mortality rates between tocilizumab and control groups. At the end of 2020, treatment of COVID-19 was various. Classes of drugs used are antiviral agents (e.g., lopinavir/ritonavir and remdesivir), inflammation inhibitors/antirheumatic drugs (e.g., tocilizumab, antimalarials, and methylprednisolone), low molecular-weight heparin, plasma, and hyperimmune immunoglobulins (3, 9). Definite therapy was still under development and research. Two authors (Chi-Chung Chen and Hsien-Lung Tsai) independently utilized the Newcastle-Ottawa Scale (NOS) to assess the quality of included non-randomized studies. The NOS applies three domains (selection of study groups, comparability, and outcome assessment) to evaluate the quality of these studies (10). A study could be awarded up to one star for each item in the selection and outcome domains and up to two stars for comparability. We considered a study of high quality if seven or more stars were awarded. Any disagreement was adjudicated by the third author (Tao-Hsin Tung).
Statistical Analysis
Review Manager 5.4.1 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2020) was used to conduct the meta-analysis. We presented the risk of outcome as risk ratio (RR), hazard ratio (HR), and 95% confidence interval (CI), and assessed heterogeneity using I2 statistic, which evaluated the degree of variation across studies that was due to heterogeneity rather than chance alone. An I2 value of ≥50% represented substantial heterogeneity. We conducted a random-effects model meta-analysis because we expected considerable clinical heterogeneity.
Results
Characteristics of Included Studies
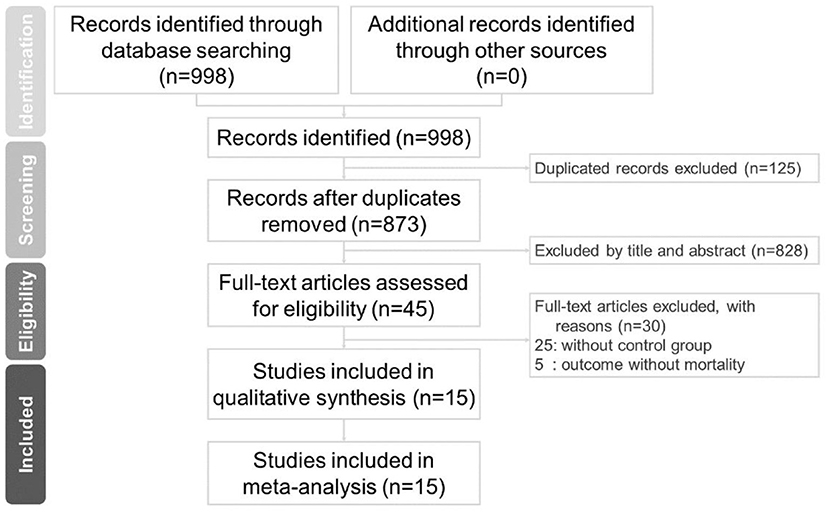
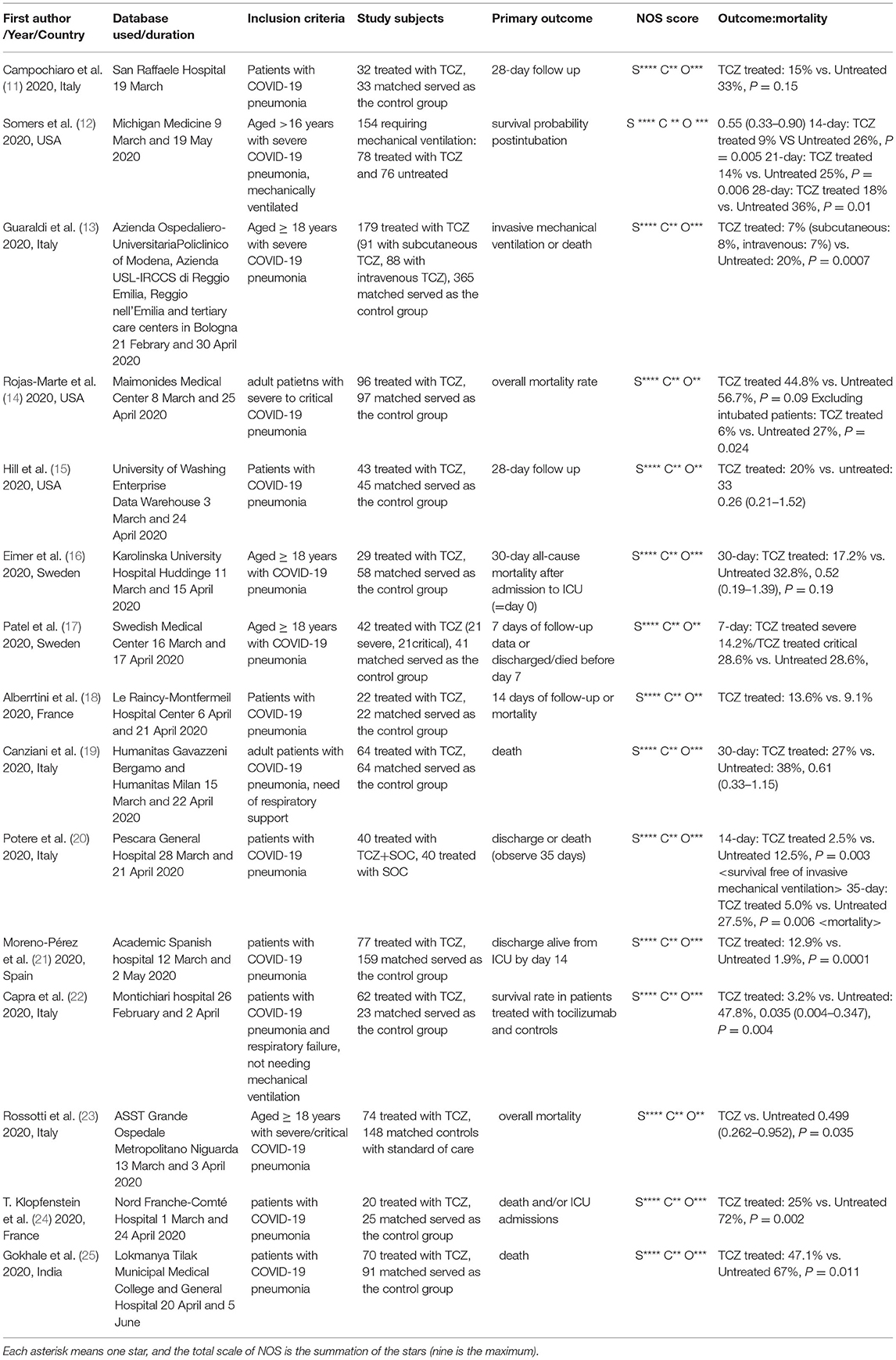
There were 784 studies found on Embase, 142 on PubMed. and 52 studies on the Cochrane Library. After removing duplicates, 853 articles were identified. A total of 15 studies meet the inclusion criteria, as shown in Figure 1. A total of 1,993 study subjects were involved in these 15 studies. Table 2 illustrates the characteristics of these included studies. Most of the included studies were conducted in Europe and the United States. All the included studies were focused on the effects of tocilizumab on the severity of COVID-19, and all the studies contained experiment groups (patients treated with tocilizumab) and control groups (patients not treated with tocilizumab).
Drugs used in control groups were various, such as hydroxychloroquine, azithromycin, remdesivir, and methylprednisolone (12, 14, 23). It is worth acknowledging that using adjunctive corticosteroid in patient with COVID-19 with acute respiratory distress syndrome could be a choice (12, 26).
The risk of bias assessment for included studies is also illustrated in Table 2. We used The Newcastle-Ottawa Scale (NOS) to assess the quality of these studies (10). Despite the short research time, there were two studies considered to not have a relatively long study period (<2 weeks) (8, 9). However, these two studies still had some valuable statistical information.
Benefits of Using Tocilizumab in Patients With COVID-19
Figure 2 indicates the results of mortality in patients with COVID-19 receiving tocilizumab. Fifteen studies with a total of 854 COVID-19 patients receiving tocilizumab and 1139 patients as controlled group were collected. Our study found substantial heterogeneity (I2 = 65%) among the 15 studies. Overall decreased mortality was found (pooled RR:0.62, 95% CI = 0.46–0.83). Subgroup heterogeneity was significant (I2 = 65%).
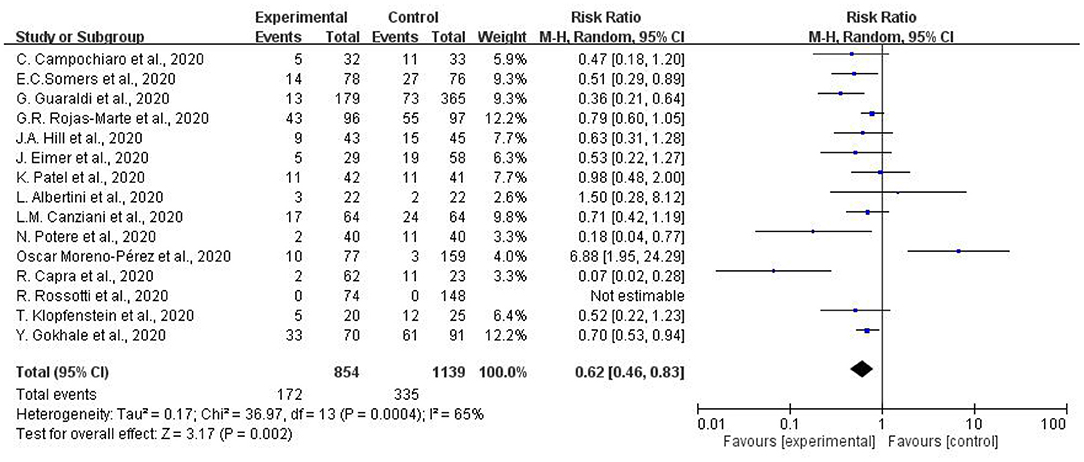
Figure 2. Meta-analysis of effects of tocilizumab on coronavirus disease-2019 (COVID-19) (risk ratio).
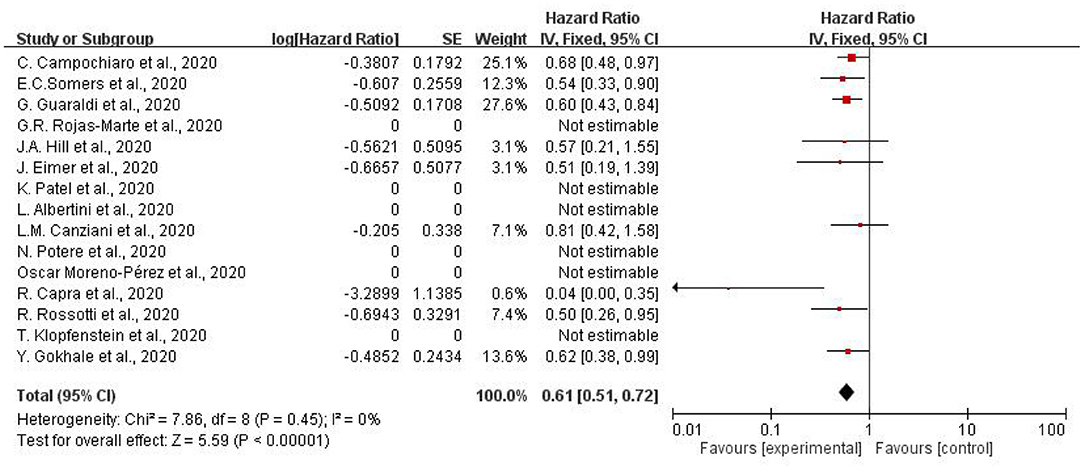
Figure 3 illustrates the hazard ratio of using tocilizumab to treat patients with COVID-19. Overall decreased mortality was also found (pooled HR.61, 95% CI = 0.51–0.72). I2 of subgroup heterogeneity was 0%. In summary, adult patients suffering from COVID-19 showed lower mortality compared to the controlled-group, which was treated with other therapy.
Good Effect of Using Tocilizumab
Among our studies, one of them showed a good effect of using tocilizumab on treatment of patients with COVID-19. Patients receiving tocilizumab showed significantly better survival rate than control groups (HR for death 0.035; 95% CI =0.004–0.347; p = 0.004) (22). After detailed analysis, the included subjects were all diagnosed as having COVID-19 but without mechanical ventilation. Maybe the relatively less severity of COVID-19 infection could explain the good effect of using tocilizumab.
Disadvantages of Using Tocilizumab in Patients With COVID-19
Tocilizumab was associated with improved survival; however, it increased the proportion of patients with superinfections (54 vs. 26%; p < 0.001). Despite higher superinfection occurrence, there was no difference in 28-day case fatality rate (12).
Possibly because of the result of several biochemical, respiratory, and infectious complications, using tocilizumab in patients with COVID-19 was associated with longer hospital stay (HR 1.658, 95% CI = 1.088–2.524, p = 0.019) (23). By the way, a study in India showed longer days of hospitalization in the tocilizumab group (14 days) than in the control group (6 days) (p = 0.001) (25).
On the contrary, another study conducted in Sweden showed different results. Median length of stay in hospital was 20.5 (days) in the tocilizumab group and 30 (days) in the control group (p = 0.04) (16).
Variety of Biochemistry Indicators After Using Tocilizumab in Patients With COVID-19
Treating COVID-19 with tocilizumab showed some variations in biochemistry indicators. After treatment, C-reactive protein (CRP) showed a clear reduction (P < 0.001). Meanwhile, D-dimer and lymphocyte both showed a significant increase (p < 0.001) (23). Despite short period, tocilizumab still showed positive influence on lowering CRP level (from 23.7 to 0.9 mg/dl) and ferritin level (from 1,522 to 940 ng/ml) and increasing lymphocyte count (from 800 to 1,700 cell/mmc) (17). Although these acute phase reactants are unreliable biomarkers for disease improvement, changes in biochemistry indicators could help us know about patients' current physical condition.
Corticosteroid in the Study
In addition to tocilizumab, a corticosteroid was used in eight of the fifteen studies (among both experiment and control groups). Since steroids had been reported to benefit patients with COVID-19 with acute respiratory distress syndrome (26), use of steroids had no influence on results of the studies (12, 14, 16, 19).
Publication Bias of the Study
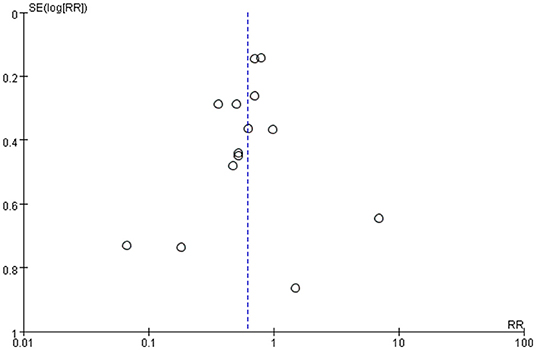
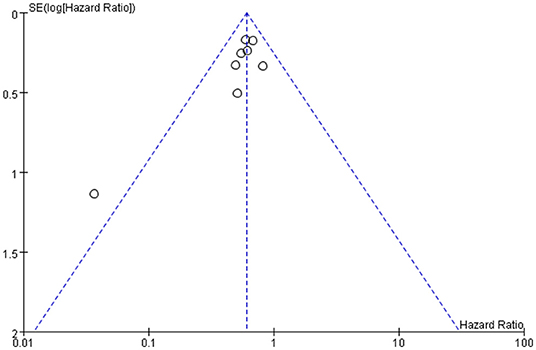
Publication bias was defined as publication or non-publication of studies depending on the direction and statistical significance of the results. Figures 4, 5 show that the funnel plots are not absolutely symmetry, which indicates some inevitable publication bias in this study.
Discussion
Clinical Implications
In this meta-analysis of fifteen studies, as of February 2021, we observed that tocilizumab reduced the mortality of adult patients with COVID-19 pneumonia infection. The results indicated that early use of tocilizumab was associated with lower mortality in adults with COVID-19 pneumonia. Perhaps the relative less severity of COVID-19 infection could explain the good effect of using toxilizumab in treatment of patients with COVID-19. Lots of patients with COVID-19 suffered from severe complications, such as respiratory distress, intensive care unit (ICU) stay and even death. Current studies suggest that increased alveolar exudates caused by host immune response and inflammatory cytokine storm might contribute to mortality (27).
As observed in respiratory distress syndrome, interleukin-6 (IL-6) played a key role in inducing acute inflammation. The recombinant human monoclonal antibody tocilizumab, directed against the IL-6 receptor, mediated an inhibition strategy, preventing IL-6 binding to its receptor and, therefore, IL-6 biological activity (6).
The tocilizumab treatment showed inspiring clinical results (27). Tocilizumab is a potential recombinant monoclonal antibody against IL-6. Currently, tocilizumab is under investigation for management of patients with COVID-19 infection (28).
Previous studies have assessed the efficacy of tocilizumab in treatment of COVID-19 and showed that all-cause mortality of COVID-19 between tocilizumab and control groups was 16.3 and 24.1%, respectively. However, the difference did not have statistical significance (95% CI = 0.31–1.22). Besides, a similar risk of ICU admission was noted between the tocilizumab and control groups (35.1 vs. 15.8%, 95% CI = 0.33–6.78). From a clinical viewpoint, no clear evidence showed that tocilizumab could provide definite benefit to patients with COVID-19 infection (29).
From an evidence-based medicine viewpoint, ordinary meta-analyses on the efficacy of interventions obtain confident quality evidence from randomized controlled trials only (30–32). However, randomized trials are often not the best source of clinical evidence on harm, as the period of study is often too short to detect long-term or rare adverse outcomes (33, 34). Including observational studies in our analysis was a strength, as these studies could detect the effect of tocilizumab, which might be important in treatment of patients with COVID-19 pneumonia.
Methodological Considerations
Another strength of our study was that we included most case-control studies regarding the possible therapeutic effects of tocilizumab on treatment of patients with COVID-19 pneumonia.
Several limitations should be considered when interpreting the results of this meta-analysis. First, the main limitation of the study was inclusion of only observational studies. There were not enough double-blind randomized controlled trials until February 2021. Second, research time was not long enough. Due to this urgent global crisis, nearly all studies to date have a relatively short research time. The longest research time is only more than a few months. Third, the main research subjects included in our studies were adults. Effect of the treatment on minors was still unknown. Lastly, the patients enrolled in the study were treated with different drugs and started treatment with tocilizumab in different stages of infection. Disease severity was inconsistent.
Heterogeneity of Meta-Analysis
In this meta-analysis, heterogeneity may exist if sample estimates for the population risk were of different magnitudes. For existence of heterogeneity, it is crucial to assess heterogeneity in the meta-analysis. We aggregate studies that have different methodologies; however, heterogeneity in the results is still inevitable.
Conclusion
This meta-analysis included 15 publications with more than 1,993 individuals to analyze the correlation of tocilizumab with clinical mortality among adult patients with COVID-19 pneumonia. Although the patients in our study were treated with tocilizumab in different stages of disease, our study showed that early use of tocilizumab was associated with lower mortality in adults with COVID-19 pneumonia. Despite some possible increased risks of infection and prolonged hospital stay, the use of tocilizumab could reduce mortality without obvious fatal side effects, which might be a treatment option for adult patients with COVID-19 pneumonia if it is used early. Besides, relatively less severe symptoms of COVID-19 pneumonia might explain the good effect of using tocilizumab.
Further studies should have a more in-depth insight into the effect of tocilizumab on treatment of patients with COVID-19 pneumonia.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
Author Contributions
C-CC and H-LT conducted the study and drafted the manuscript. T-HT and Y-PY participated in the design and data collection of the study, conceived the study, and participated in its design and coordination. C-CC and T-HT calculated the results of this study. All the authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the Sunflower Statistical Consulting Company, Kaohsiung, Taiwan for the statistical advice.
References
1. Shi Y, Wang G, Cai XP, Deng JW, Zheng L, Zhu HH, et al. An overview of COVID-19. J Zhejiang Univ Sci B. (2020) 21:343–60. doi: 10.1631/jzus.B2000083
2. Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. (2020) 33:e00028–20. doi: 10.1128/CMR.00028-20
3. Rahman MU, Nair SC, Din MU, Dar MR, Masood M, Al Menhali ARS, et al. Methylprednisolone treatment versus standard supportive care for adult COVID-19 mechanically ventilated, acute respiratory distress syndrome patients. SN Compr Clin Med. (2022) 4:11. doi: 10.1007/s42399-021-01084-y
4. Salton F, Confalonieri P, Meduri GU, Santus P, Harari S, Scala R, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. (2020)7:ofaa421. doi: 10.1093/ofid/ofaa421
5. Cortegiani A, Ippolito M, Greco M, Granone V, Protti A, Gregoretti C, et al. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. (2021) 27:52–66. doi: 10.1016/j.pulmoe.2020.07.003
6. Belladonna ML, Orabona C. Potential benefits of tryptophan metabolism to the efficacy of tocilizumab in COVID-19. Front Pharmacol. (2020) 11:959. doi: 10.3389/fphar.2020.00959
7. Lakatos B, Gopcsa L, Gondos E, Riczu A, Várnai Z, Nagy É, et al. [Anti-cytokine therapy in novel coronavirus disease (COVID-19) – the first administration of tocilizumab in Hungary at a department of infectology]. Orv Hetil. (2020) 161:1070–7. doi: 10.1556/650.2020.31899
8. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
9. Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID-19: an overview. Eur J Pharmacol. (2020) 889:173644. doi: 10.1016/j.ejphar.2020.173644
10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
11. Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. (2020) 76:43–9. doi: 10.1016/j.ejim.2020.05.021
12. Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. (2021) 73:e445–54. doi: 10.1093/cid/ciaa954
13. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. (2020) 2:e474–84. doi: 10.1016/S2665-9913(20)30173-9
14. Rojas-Marte G, Khalid M, Mukhtar O, Hashmi AT, Waheed MA, Ehrlich S, et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM. (2020) 113:546–50. doi: 10.1093/qjmed/hcaa206
15. Hill JA, Menon MP, Dhanireddy S, Wurfel MM, Green M, Jain R, et al. Tocilizumab in hospitalized patients with COVID-19: clinical outcomes, inflammatory marker kinetics, and safety. J Med Virol. (2021) 93:2270–80. doi: 10.1002/jmv.26674
16. Eimer J, Vesterbacka J, Svensson AK, Stojanovic B, Wagrell C, Sönnerborg A, et al. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J Intern Med. (2021) 289:434–6. doi: 10.1111/joim.13162
17. Patel K, Gooley TA, Bailey N, Bailey M, Hegerova L, Batchelder A, et al. Use of the IL-6R antagonist tocilizumab in hospitalized COVID-19 patients. J Intern Med. (2021) 289:430–3. doi: 10.1111/joim.13163
18. Albertini L, Soletchnik M, Razurel A, Cohen J, Bidegain F, Fauvelle F, et al. Observational study on off-label use of tocilizumab in patients with severe COVID-19. Eur J Hosp Pharm. (2021) 28:22–7. doi: 10.1136/ejhpharm-2020-002414
19. Canziani LM, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J Autoimmun. (2020) 114:102511. doi: 10.1016/j.jaut.2020.102511
20. Potere N, Di Nisio M, Cibelli D, Scurti R, Frattari A, Porreca E, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study. Ann Rheum Dis. (2021) 80:1–2. doi: 10.1136/annrheumdis-2020-218243
21. Moreno-Pérez O, Andres M, Leon-Ramirez JM, Sánchez-Payá J, Rodríguez JC, Sánchez R, et al. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: a retrospective cohort study. J Autoimmun. (2020) 114:102523. doi: 10.1016/j.jaut.2020.102523
22. Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. (2020) 76:31–5. doi: 10.1016/j.ejim.2020.05.009
23. Rossotti R, Travi G, Ughi N, Corradin M, Baiguera C, Fumagalli R, et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J Infect. (2020) 81:e11–7. doi: 10.1016/j.jinf.2020.07.008
24. Klopfenstein T, Zayet S, Lohse A, Balblanc JC, Badie J, Royer PY, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. (2020) 50:397–400. doi: 10.1016/j.medmal.2020.05.001
25. Gokhale Y, Mehta R, Kulkarni U, Karnik N, Gokhale S, Sundar U, et al. Tocilizumab improves survival in severe COVID-19 pneumonia with persistent hypoxia: a retrospective cohort study with follow-up from Mumbai, India. BMC Infect Dis. (2021) 21:241. doi: 10.1186/s12879-021-05912-3
26. Meduri GU, Annane D, Confalonieri M, Chrousos GP, Rochwerg B, Busby A, et al. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med. (2020) 46:2284–96. doi: 10.1007/s00134-020-06289-8
27. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. (2020) 18:164. doi: 10.1186/s12967-020-02339-3
28. Khiali S, Khani E, Entezari-Maleki T. A comprehensive review of tocilizumab in COVID-19 acute respiratory distress syndrome. J Clin Pharmacol. (2020) 60:1131–46. doi: 10.1002/jcph.1693
29. Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. (2020) 56:106103. doi: 10.1016/j.ijantimicag.2020.106103
30. Chi C-C. Evidence-based dermatology. Dermatologica Sinica. (2013) 31:2–6. doi: 10.1016/j.dsi.2012.06.002
31. Chen YJ, Chen YY, Wu CY, Chi CC. Oral Chinese herbal medicine in combination with phototherapy for vitiligo: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2016) 26:21–7. doi: 10.1016/j.ctim.2016.02.009
32. Chen TH, Tung TH, Chen PS, Wang SH, Chao CM, Hsiung NH, et al. The clinical effects of aromatherapy massage on reducing pain for the cancer patients: meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2016) 2016:9147974. doi: 10.1155/2016/9147974
33. Chi CC, Wang SH, Wojnarowska F, Kirtschig G, Davies E, Bennett C. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. (2015) 2015:Cd007346. doi: 10.1002/14651858.CD007346.pub3
Keywords: COVID-19, tocilizumab, meta-analysis, mortality, side effects, treatment
Citation: Chen C-C, Yang Y-P, Tsai H-L and Tung T-H (2022) Effects of Tocilizumab on Adults With COVID-19 Pneumonia: A Meta-Analysis. Front. Med. 9:838904. doi: 10.3389/fmed.2022.838904
Received: 18 December 2021; Accepted: 14 February 2022;
Published: 30 March 2022.
Edited by:
Giovanni Damiani, University of Milan, ItalyReviewed by:
Nicola Luigi Bragazzi, York University, CanadaBarbara Ruaro, University of Trieste, Italy
Copyright © 2022 Chen, Yang, Tsai and Tung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao-Hsin Tung, Y2gyODc2QHllYWgubmV0; Hsien-Lung Tsai, dWZzaGFubm9AbXMxNS5oaW5ldC5uZXQ=
 Chi-Chung Chen
Chi-Chung Chen Yu-Pei Yang2
Yu-Pei Yang2 Tao-Hsin Tung
Tao-Hsin Tung