- 1Department of Immunology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- 2Department of Otorhinolaryngology, Head and Neck Surgery, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- 3Hospital Universiti Sains Malaysia, Kubang Kerian, Malaysia
Allergic rhinitis (AR) represents a global health concern where it affects approximately 400 million people worldwide. The prevalence of AR has increased over the years along with increased urbanization and environmental pollutants thought to be some of the leading causes of the disease. Understanding the pathophysiology of AR is crucial in the development of novel therapies to treat this incurable disease that often comorbids with other airway diseases. Hence in this mini review, we summarize the well-established yet vital aspects of AR. These include the epidemiology, clinical and laboratory diagnostic criteria, AR in pediatrics, pathophysiology of AR, Th2 responses in the disease, as well as pharmacological and immunomodulating therapies for AR patients.
Introduction
Atopic allergic sensitization is defined by the production of immunoglobulin E (IgE)-mediated immune response toward allergens. Allergic rhinitis (AR) is a common disorder that afflicts 400 million people worldwide and it represents a global concern as its prevalence has increased over the years (1). AR usually comorbids with other diseases such as asthma (2), leading to impaired quality of life, school or work performance, and significant financial impact. AR is shown to be caused by aberrantly high Th2 cytokines, and recent findings on the cause of AR are directed toward impairment of the nasal epithelial barrier integrity (3–8). In this review, we summarize the well-established yet important concepts of AR including the epidemiology, clinical and laboratory diagnostic criteria, pathophysiology of AR including allergens as well as Th2 responses in AR.
Epidemiology of Allergic Rhinitis
Allergic rhinitis prevalence has increased significantly since the 1990s (9–11). It is reported to affect approximately 25 and 40% of children and adult globally, respectively. Approximately 80% of AR symptoms develop before the age of 20 years (12) and peak at age 20–40 years before gradually declining (13). The incidence rate of AR in children over the first 5 years of life was reported to be 17.2%, with a peak age at diagnosis between 24 and 29 months (2.5%) (14). Meta-analysis studies have shown the sex-specific differences in the prevalence of AR with male predominance in childhood and a female predominance in adolescents (15, 16).
Prevalence of AR has increased with years due to several risk factors including global urbanization as shown by several studies comparing AR prevalence in urban settings with rural areas (17, 18). This is mainly caused by increased levels of pollutants [e.g., traffic-related pollutants and particulate matter 2.5 (PM2.5)] that can exacerbate pollen-sensitized AR (19–21). It has been reported that AR is more prevalent in urban areas compared with rural areas (18). Climate changes also prolong pollen season as reported in Europe over the last three decades along with more frequent seasonal allergies (22).
Smoking, however, did not show a significant association with the severity of nasal symptoms in AR but usually impacted patients with chronic rhinitis (23, 24). Conversely, maternal smoking conferred the greatest risk in pediatric AR (25). Novel tobacco products such as electronic cigarette and heated tobacco products increase the risk of AR in adolescents compared with traditional smoking (26).
The economic impact of AR is significant where the total annual cost of self-reported AR in Sweden is estimated at €1.3 billion (27) and up to $20.9 billion in United States (1). In addition, AR is a systemic inflammatory disease and often comorbids with other disease such as asthma, atopic disease, sinusitis, conjunctivitis, and otitis media (9), complicating the treatment and management of AR patients.
Clinical Signs and Symptoms of Allergic Rhinitis
AR is characterized by the presence of nasal and non-nasal symptoms. Nasal symptoms include anterior or posterior rhinorrhea, sneezing, nasal blockage and/or itching of the nose (9). These symptoms may persist for hours after allergic reaction upon the exposure of allergens that cause mucosal inflammation (13). In consequences, the mucosa is rendered more reactive to the triggering allergen as well as to other allergens and to non-allergenic stimuli (e.g., strong odors and other irritants). Non-nasal symptoms are characterized by ocular symptoms such as allergic rhinoconjunctivitis (i.e., itching and redness of the eyes and tearing) which frequently occurs in AR patients (10). Other symptoms include itching of the palate, postnasal drip and cough.
Hypersensitivity reactions can be observed in AR, bronchial asthma, allergic conjunctivitis, allergic dermatitis, food allergy and anaphylactic shock (28). Over 30% of AR patients suffer debilitating allergic symptoms that can lead to severe disability and life-threatening conditions such as anaphylaxis (29). In severe cases, intense bronchospasm, laryngeal edema, cyanosis, hypotension and shock may occur (28).
In terms of AR severity, it can be classified as mild and moderate/severe based on the AR and its Impact on Asthma (ARIA) guidelines (9). It is measured based on four aspects including sleep abnormality, impairment in daily activities, impairment in school or work performance, and troublesome symptoms. Patients without the aforementioned problems are considered as mild AR while patients suffering from one or more of the items are considered as moderate/severe AR. The ARIA guidelines also classify AR symptoms into intermittent and persistent based on the duration of symptoms present in AR patient. For intermittent symptoms, the symptoms occur in less than 4 days per week or less than 4 consecutive weeks while for persistent symptoms, they occur in more than 4 days per week and more than 4 consecutive weeks.
Laboratory Characteristics of Allergic Rhinitis
To determine the specific allergen that causes the production of IgE antibodies in AR, multiple tests can be conducted such as in vivo skin tests including skin prick test (SPT; percutaneous) and intradermal (intracutaneous) skin tests (IDST), and in vitro serum allergen-specific IgE (ssIgE) immunoassay. SPT and ssIgE immunoassay are the most common laboratory tests to determine the causative allergen(s) (30, 31). However, there is no “gold standard” laboratory test in diagnosing AR but SPT represents the first-line approach in the assessment of allergic sensitivities (9, 32). SPT is a quick and cost-effective methodology in diagnosing any allergic sensitization (33).
ssIgE immunoassay utilizes commercially available test panels which are more costly (33). It is also less sensitive for the diagnosis of allergy due to inhalant allergens compared with SPT. However, ssIgE immunoassay can be useful when skin testing is not available or cannot be performed due to patients have extensive skin disease, unable to discontinue antihistamines or other interfering medications, dermatographic, or other issues that complicate skin testing (34, 35). A novel non-invasive local diagnosis is through dried blood spot (DBS)-based diagnosis to detect IgE reactivity which can test up to more than 170 allergen molecules (36, 37).
Another uncommon diagnostic test for AR includes intradermal skin testing (IDST) in which tiny quantity of allergen is injected into the dermis with a hypodermic needle for the diagnosis of IgE-mediated allergic conditions (38). Other alternative or supporting tests include eosinophil cationic protein (ECP) and the percentage of eosinophils (39, 40), tryptase (marker of mast cell activation) (41), leukotriene B4 (40) and basophil activation test (through flow cytometry) to determine the causative allergen in local AR (42, 43). These tests are usually conducted in research settings and not routinely used for AR diagnosis.
Diagnostic Criteria of Allergic Rhinitis
Diagnostic criteria are generally broad and must reflect the different features of a disease (i.e., heterogeneity), with a view to accurately identify as many patients with the condition as possible (44). Due to the lack of gold standards in diagnosing AR, definitive diagnostic criteria have been challenging to establish. The choice of confirmatory test is a matter of clinical judgment and the results obtained must be considered together with additional risk factors, rather than definitive indicators of disease (45).
However, for patients to be diagnosed with AR, they must have clinical symptoms and possess laboratory characteristics as discussed in the previous section. AR patients must encounter two or more of the following clinical symptoms for more than 1 h on most days: (1) Watery rhinorrhea; (2) Sneezing, especially paroxysmal; (3) Nasal obstruction; (4) Nasal pruritis; (5) With or without conjunctivitis. When individuals present with clinical symptoms of AR, allergy laboratory tests are conducted for confirmation.
In skin tests, positive result is considered when the wheal-and-flare reaction occurs on the skin test site after 20 min of exposure to allergens. For SPT, positive result must demonstrate wheal (i.e., a red and itchy raised bump with surrounding inflammation that indicates the presence of allergic antibodies) size in diameter of ≥4 mm (46, 47). Figure 1 shows a positive SPT on house dust mite (HDM) allergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae and Blomia tropicalis) tested on an AR patient who attended the ORL-HNS clinic of Hospital Universiti Sains Malaysia, and each wheal size was greater than 4 mm. The SPT protocols were approved by the Human Research Ethics Committee of Universiti Sains Malaysia (JEPeM; approved ethics code: USM/JEPeM/18060273).
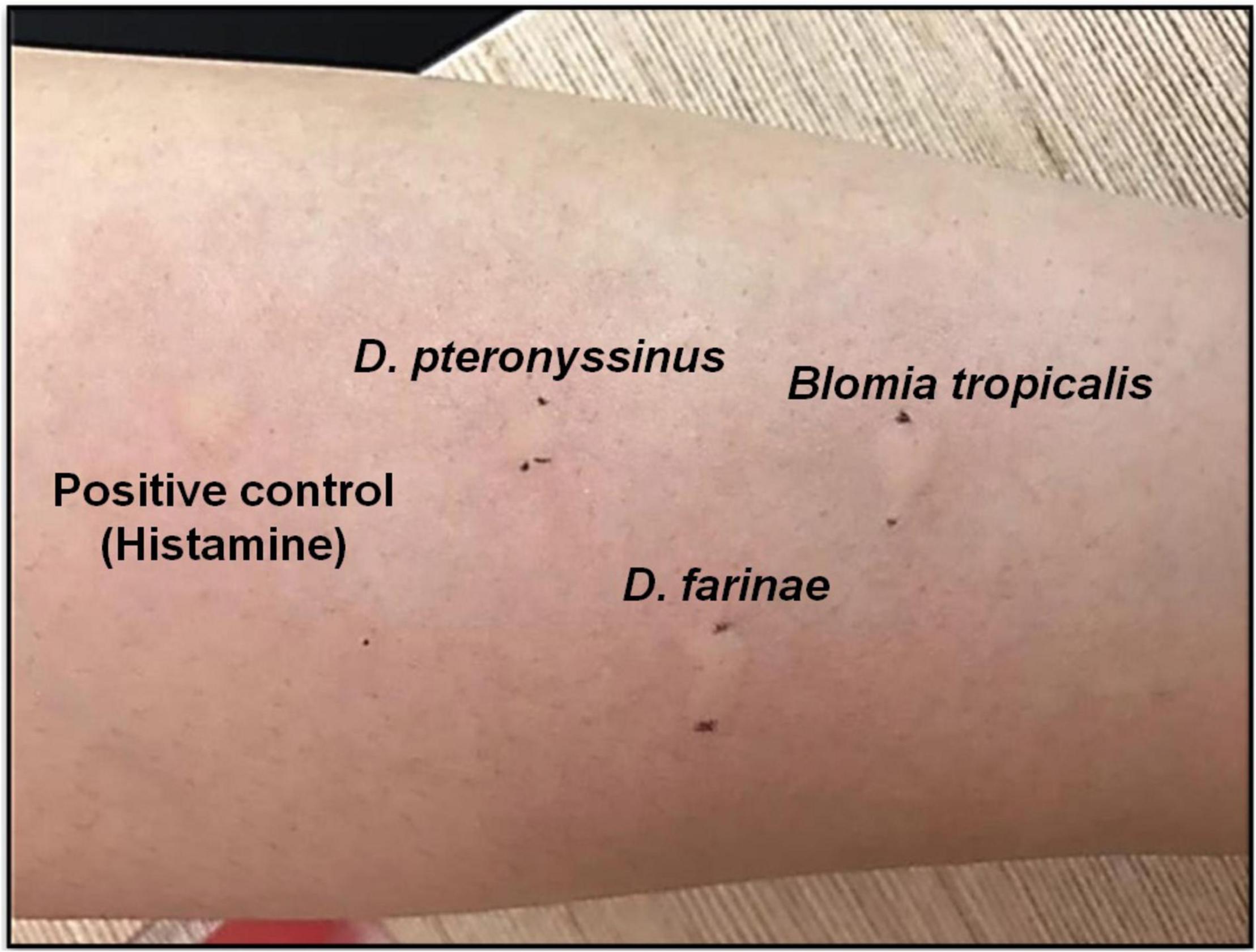
Figure 1. Positive SPT of HDM allergen Dermatophagoides pteronyssinus (D. pteronyssinus; wheal size 9 mm), Dermatophagoides farinae (D. farinae; wheal size 11 mm) and Blomia tropicalis (B. tropicalis; wheal size 10 mm) tested on an AR patient at ORL-HNS clinic, Hospital Universiti Sains Malaysia.
The performance of any diagnostic criteria is dependent on the prevalence of the disease in a given geographical area or clinical setting (e.g., community clinic vs. tertiary care facility). AR diagnostic criteria are summarized in Table 1.

Table 1. Diagnosis criteria of allergic rhinitis (AR) based on clinical symptoms and laboratory characteristics criteria (9).
Pediatric Allergic Rhinitis
Allergic rhinitis represents a common pediatric problem where approximately 40% of pediatric AR patients develop symptoms as early as age 6 years old and increase with age (48–50). Symptoms of pediatric AR are similar with adolescents; however, young children may frequently exhibit sniffing, snorting, throat-clearing, and coughing (51). Comorbidities in pediatric AR are often combination of several conditions which indicate AR is a disease manifestation that involves systemic inflammation including conjunctivitis, atopic dermatitis, asthma, rhinosinusitis, otitis media with effusion, or food allergies (51, 52). The MeDALL (Mechanisms of the Development of ALLergy) project that involved more than 12,000 children from 12 ongoing longitudinal cohort studies showed the co-existence of asthma, rhinitis and eczema prevalence in the same child (53). In addition, findings from the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) have shown higher prevalence of AR, asthma and aeroallergen sensitization in children exposed to urban environment in their early age compared with those exposed to rural environment (54).
Diagnosis of AR in pediatrics includes complete history taking, physical examinations (e.g., nose, oropharynx, tympanic membranes, and eyes) as well as differential diagnosis based on the clinical symptoms suggestive of AR but not evidence of systemic atopy (55). A very young child with persistent nasal symptoms should be considered as having other disorders that can mimic AR (56). For children under 2 years of age, other disorders include adenoidal hypertrophy, acute or chronic sinusitis, congenital abnormalities (choanal atresia), foreign bodies and nasal polyps will be taken into consideration during diagnosis. On the other hand, older children may present with other disorders including acute infectious rhinitis, chronic non-allergic rhinitis, chronic rhinosinusitis, rhinitis medicamentosa, rhinitis due to systemic medications, atrophic rhinitis, rhinitis associated with hormonal changes, unilateral rhinitis or nasal polyps and rhinitis with immunologic disorders. Descriptions on each of these specific disorders (56) and differential diagnosis based on age (57) have been discussed in the cited references.
Pathophysiology of Allergic Rhinitis
Early and Late Phase of Allergic Rhinitis
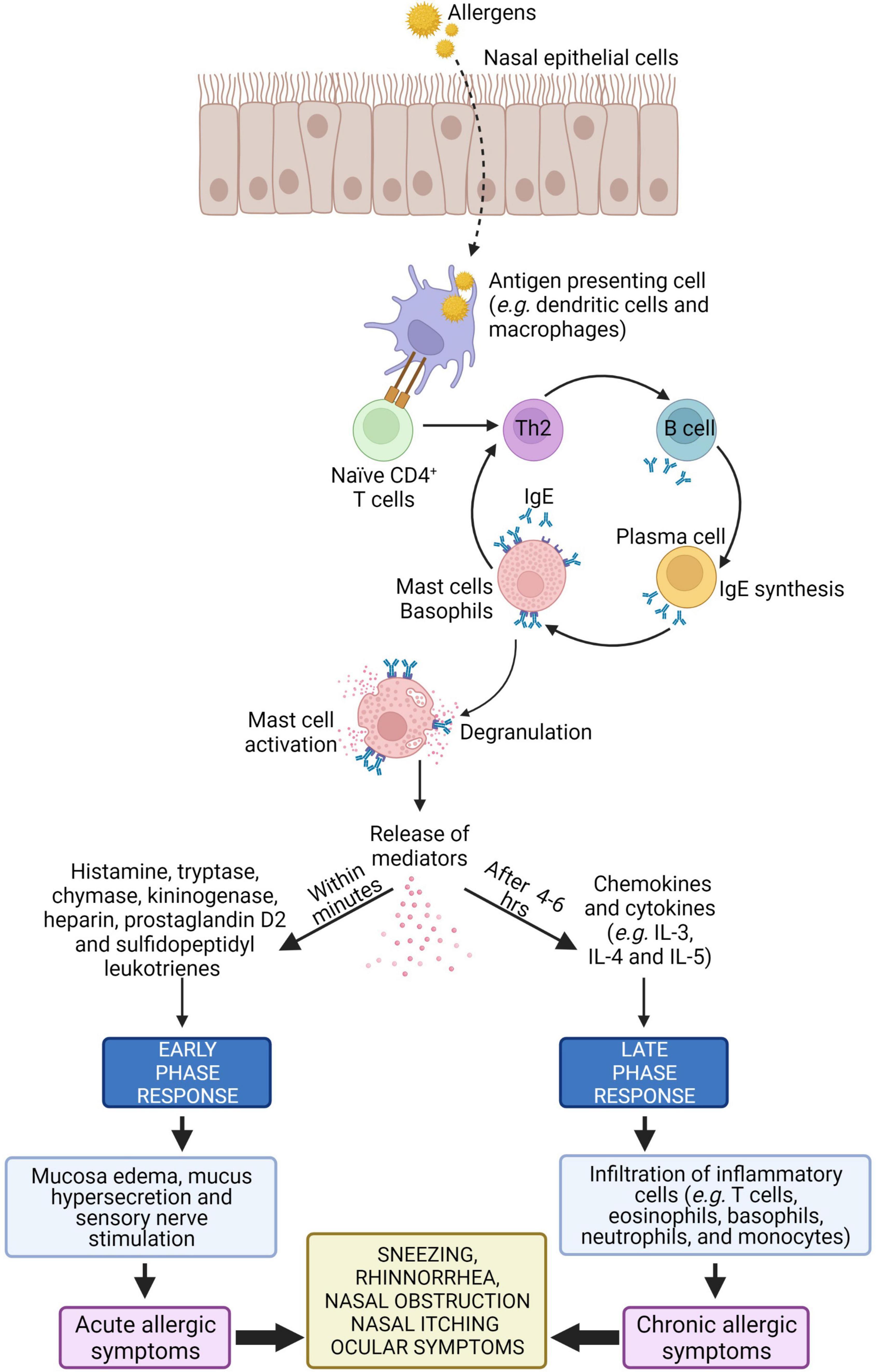
Type I hypersensitivity is an allergic reaction mediated by IgE antibody in response to allergens (13). Type I hypersensitivity reactions occur rapidly, usually within 20 min after allergen exposure, and it is characterized by activation of mast and inflammatory cells as well as their infiltration in tissues (58). The allergic response in AR can be divided into two phases i.e., the early and late phase.
The early phase starts within 20 min after exposure to harmful allergens. Antigen presenting cells such as dendritic cells in the mucosal surface uptake, process and present peptides from allergens on the major histocompatibility complex (MHC) class II molecule. The antigen complex and the MHC class II molecule serve as a ligand for T cell receptors on naïve CD4+ T cells, resulting in differentiation of naïve CD4+ T cells into allergen-specific Th2 cell. Cytokines such as IL-4 and IL-13 released from the activated Th2 cells interact with B cells to produce allergen-specific IgE. This allergen-specific IgE binds to high-affinity Fc receptor for IgE (FcεR) present on mast cells, leading to mast cell activation (6, 59, 60).
Cross-linking of the FcεR on mast cells causes release of allergic mediators consisting of histamine, proteases and lipid mediators such as leukotriene (LT) C4, and prostaglandin D2 (PGD2) that cause vascular leak, bronchoconstriction, inflammation, and intestinal hypermotility (28, 61–63). These mediators induce mucosal edema and watery rhinorrhea characteristic of AR by causing the blood vessels to leak. Histamine is the major mediator in AR where it activates H1 receptors on sensory nerve endings and causes sneezing, pruritus, and reflex secretory responses, and it also interacts with H1 and H2 receptors on mucosal blood vessels, leading to vascular engorgement (nasal congestion) and plasma leakage (64).
After 4–6 h of allergens exposure, the late phase of allergic response is initiated. In this phase, nasal mucosal inflammation occurs with the influx and activation of a variety of inflammatory cells such as T cells, eosinophils, basophils, neutrophils, and monocytes into the nasal mucosa (64). Recruitment of these inflammatory cells is triggered by cytokines such as IL-4 and IL-5. These cytokines upregulate the expression of adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells which facilitate inflammatory cellular influx (65). The activation of structural cells in the nasal mucosa, such as epithelial cells and fibroblasts, can promote the release of additional chemokines (e.g., eotaxin, RANTES, and TARC) that facilitate cellular influx from the peripheral blood (66). The schematic representation of pathophysiology of AR is illustrated in Figure 2.
Allergens in Allergic Rhinitis
Allergens are typically proteins with molecular weight ranging from 10 to 40 kDa that induce type I hypersensitivity by reacting with specific IgE antibodies (28, 67). Common types of allergens include food allergens (e.g., shrimp, soybean, crab, clam, wheat, peanut, yolk egg, and cow’s milk), pet allergens (e.g., cat and dog dander), and HDMs (68, 69).
Major indoor allergens (e.g., HDMs, cockroaches, cat, and dog dander) have been consistently demonstrated to be the strongest risk factor for AR (70). Lifestyle heavily impacts the diversity and composition of the airway and gut microbiotas. The hygiene hypothesis proposes the importance of symbiotic relationship with relevant microorganisms in boosting immune system maturation and shifting the immune system toward a more tolerogenic state, and it thus suggests to be the fundamental cause for allergy onset (71).
In terms of HDM, one of the most common causes of AR, the protease activity of HDM leads to excessive IgE production. In normal physiological state, the level of IgE production by B cells is controlled by a negative feedback mechanism that involves IgE binding to CD23 (i.e., the low-affinity receptor for IgE FceRII). Upon binding of IgE/allergen-complexes to CD23, IgE production by B cell is then downregulated (72). However in HDM-sensitized AR patients, Der p 1 (a HDM cysteine proteinase allergen) disrupts this IgE-feedback mechanism via selective cleavage of CD23, causing overproduction of IgE by B cells (73). Lastly, in allergic diseases, pulmonary surfactants [i.e., surface protein (SP)-A and SP-D)] are vital in the clearance of allergens (72). They bind the allergens and lessen allergic sensitization by allergen removal or interference with IgE-binding (74, 75). Der p 1 has been shown to cleave SP-A and SP-D, leading to decreased lung clearance of allergens (76). A summary of the effects resulting from proteolytic activity of HDM is presented in Figure 3.
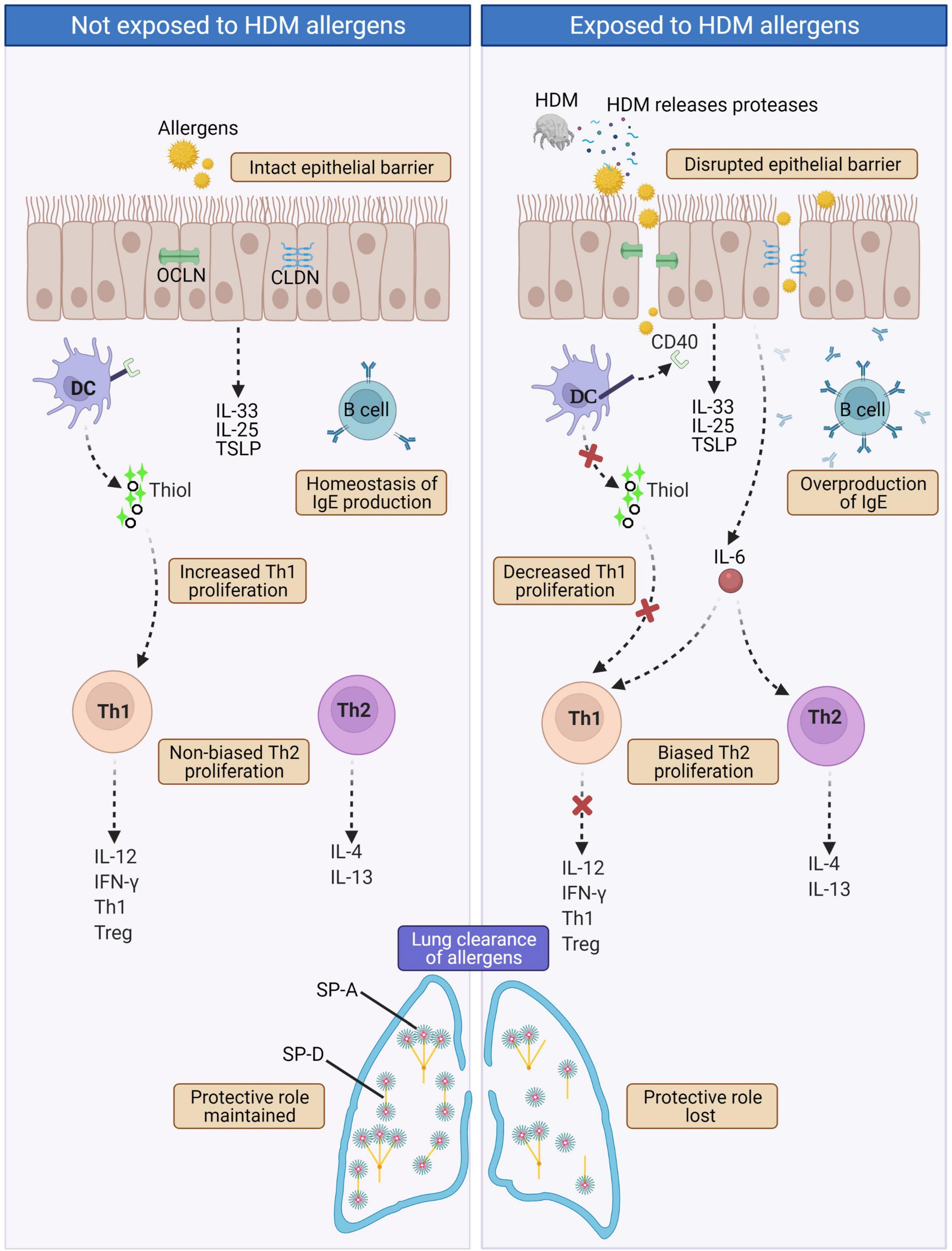
Figure 3. In normal physiological state (left panel), intact epithelial barrier prevents allergens infiltration and hence homeostasis of immune components and functions are maintained. In AR such as HDM-sensitized AR (right panel), proteases released by HDMs disrupt tight junctions leading to disrupted epithelial barrier that allows infiltration of allergens. This triggers a cascade of IgE overproduction by B cells, cleaved CD40 on the surface of DCs disrupts the production of thiols by DCs causing decreased Th1 proliferation and collectively with increased IL-6 secretion leads to biased Th2 proliferation. Th2 cells produce the hallmark AR cytokines IL-4 and IL-13. HDM proteases also cleave the pulmonary surfactants SP-A and SP-D, causing decreased lung clearance of allergens. CLDN, Claudin; DC, Dendritic cell; HDM, House dust mite; IL-4, Interleukin 4; IL-12, Interleukin 12; IL-13, Interleukin 13; IL-25, Interleukin 25; IL-33; Interleukin 33; IFNγ, Interferon gamma; OCLN, Occludin; SP-A, Surface protein A; SP-D, Surface protein D; Th1, T helper type 1; Th2, T helper type 2; Treg, Regulatory T cell; TSLP, Thymic stromal lymphopoietin.
Apart from HDMs, previous reports have demonstrated that pollen allergens also impair tight junction (TJ) barrier function of epithelial cells. Pollens conferred proteolytic activities by degrading the TJ occludin in monolayers of Calu-3 cells (lung cancer cells of epithelial origin), resulted in increased paracellular permeability of the cells (77). Reduced expression of another TJ protein, CLDN1, enhanced Calu-3 cell transepithelial permeability. This has also been reported for pollen allergens widespread in the Mediterranean area (e.g., olive tree, orchard grass, Italian cypress, and Scots pine) where their exposure increased Calu-3 transepithelial permeability by disrupting TJ proteins (78). Likewise in Asia, 32.4% of self-reported pollen-induced AR was reported in the northern grassland region of China, a region with frequent occurrence of seasonal pollen (79).
T Helper 2 Responses in Allergic Rhinitis
T Helper 2 (Th2) cells activate type 2 responses by stimulating B cells to proliferate and differentiate into plasma cells through the production of Th2 cytokines including IL-4, IL-5, IL-6, and IL-13 (80). Th2 cells are major contributors of IgE-producing B cells (81, 82), and Th2 cells play a predominant role in AR pathogenesis. Together with eosinophils and basophils, Th2 cells infiltrate the nasal mucosa tissue, resulting in late phase allergic response (83). IL-4 is a key cytokine in promoting Th2 differentiation from naïve CD4+ T cells (84). The mechanism is dependent on the activation of signal transducer and activator of transcription 6 (STAT6) signaling through IL-4 receptor complex.
Th2 cytokines not only enhance inflammatory cell activation but also may deregulate epithelial cell barrier integrity in allergic disease (e.g., AR, eosinophilic esophagitis, asthma, and chronic rhinosinusitis) (4, 5, 84–87). The cytokines may also be released within the sinonasal microenvironment including sinonasal epithelial cells, causing increased epithelial cell permeability (88, 89). This is thought to be due to regulation of transmembrane transcription involved in TJ remodeling where the “tight” barrier properties of TJ proteins are switched to “leaky” properties (89, 90). Th2 cytokines also hinder the epithelial barrier from resealing which may maintain the inflammation and exposure to inflammatory antigens (88).
Treatment of Allergic Rhinitis
Symptomatic Therapies in Allergic Rhinitis
Pharmaceutical management of AR rests on symptomatic treatments with antihistamines, nasal or oral glucocorticoids, nasal decongestants and leukotriene receptor antagonists that act as symptoms reliever in AR. Antihistamines is the most utilized first line medication to treat mild AR, however, first generation of antihistamines (e.g., diphenhydramine and hydroxyzine) are no longer recommended due to various adverse side effects impacting the central nervous system, anticholinergic side effects and cardiac toxicity (91–93). Newer generation of antihistamines (e.g., cetirizine, loratadine, desloratadine, fexofenadine, rupatadine, and bilastine) should be chosen as they demonstrate enhanced efficacy and safety profile (94). A newer type of intranasal antihistamine (e.g., olopatadine, levocabastine, and azelastine) ensures improved drug delivery to nasal mucosa exposed to release mediators during allergic inflammation in AR (95).
Besides, intranasal corticosteroid that acts as first-line pharmacotherapy by suppressing immune cells infiltration in AR is effective for both mild and moderate-severe AR in both children and adults (96). Currently approved intranasal corticosteroid for children are mometasone furoate (≥3 years old), fluticasone propionate (≥4 years old), triamcinolone acetonide (≥4 years old) and ciclesonide (≥6 years old) (97). A meta-analysis study was conducted to determine which combination therapies resulted in improved symptoms in AR patients. The meta-analysis demonstrated that intranasal H1 antihistamines and intranasal corticosteroids combination therapies were better compared with oral H1 antihistamines plus intranasal corticosteroid combination therapies (98).
Next, leukotriene receptor antagonists (e.g., montelukast, zafirlukast, and pranlukast) block the activity of cysteinyl leukotrienes, an important potent allergic mediator that causes allergic inflammation and various allergic symptoms such as nasal congestion and mucus production (94, 99). A meta-analysis study showed higher efficacy of leukotriene receptor antagonist compared with H1 antihistamines during nighttime symptoms but not in daytime symptoms (100). Other meta-analysis studies demonstrated that combination therapy of leukotriene receptor antagonist plus H1 antihistamines conferred increased efficacy in reducing daytime symptoms (101, 102).
Treatment using nasal decongestants reduces nasal congestion symptoms through their agonistic action at α1 and α2-adrenergic receptors on endothelial cells of nasal mucosa, leading to reduced mucosa swelling (103). Available nasal sprays in stores are oxymetazoline (Afrin), phenylephrine (Neo-synephrine) and pseudoephedrine (Sudafed). Overuse of nasal decongestants can cause rhinitis medicamentosa (i.e., a condition of rebound congestion upon withdrawal of nasal decongestants) (104) and this condition can be treated by administering intranasal corticosteroid (105, 106).
Immunomodulating Therapies in Allergic Rhinitis
Treatment of AR targeting immune modulation aims to modify the natural courses of AR rather than to cause a shift to an immunologically naive or unresponsive state. In this section, we focused on allergen immunotherapy (AIT) in AR. A fraction of AR patients do not respond toward treatment with conventional pharmacotherapy, thus disease-modifying therapeutic agents are adopted such as AIT. Administration of AIT can be delivered either through subcutaneous (SCIT) or sublingual (SLIT) route. AIT of HDM-SCIT can induce temporary increase in FcεRI expression on DCs, but not on basophils, demonstrating potential tolerogenic roles of IgE/FcεRI signaling in DCs in the setting of AIT (107).
Basophils are important mediators in initiating early phase responses in AR, thus targeting these biomarkers through AIT is promising. One year therapy with SLIT for Parietaria can reduce the threshold of basophil activation which highlights the importance of AIT in treating disease and halting the disease progression (108). High serum periostin levels were observed after HDM-SLIT, indicating that serum periostin appears to be a useful biomarker in AIT (109). Furthermore, multiple cytokines profiling was investigated in treated AR patients with SLIT where serum BAFF, IFNγ, IL10, and IL33 levels were strongly predictive of the efficacy of SLIT (110). These sets of biomarkers are closely associated with Th1 immune response and type 2 innate lymphoid cells downstream signaling pathway in AR.
Another independent study measuring SNOT-20 score after SLIT treatment in AR reported a reduction in AR symptoms and improvement in QOL of AR patients (111). AIT is proven to be a safe treatment for pediatrics AR. Adverse reactions related to SCIT in pediatric patients were reported to be infrequent, and grade 1 adverse reactions being the most frequently reported (112). In the recent large retrospective cohort study of AIT clinical trial REACT (real-world effectiveness in allergy immunotherapy) (113), it involved 46,024 AIT-treated subjects composed of AR patients with or without asthma vs. control subjects without AIT treatment. The AIT group exhibited greater reduction in AR and asthma prescriptions together with reduced severe asthma exacerbations. The REACT clinical trial demonstrated longer and sustained effectiveness of AIT in real-world settings.
Conclusion and Future Directions
Increased prevalence of AR and that it is an incurable disease pose major unmet needs to alleviate these issues. AR comorbidities also confer increased health and socioeconomic burden on AR patients. Moreover, diagnosing AR is more challenging in patients with dual AR (DAR), a recently defined AR phenotype in which the DAR patients display perennial and seasonal allergies-related nasal symptoms, and only allergic to seasonal allergies. This indicates the importance of measuring inflammation at local allergic sites which might be different from peripheral inflammation in DAR patients (114–116). Thus, future studies warrant a better understanding of allergen-mediated cellular mechanisms at local sites of AR patients, in conjunction with further research on its pathogenic mechanisms such as breakdown of nasal epithelial barrier integrity that may lead to the discovery of novel therapeutic agents for the disease.
Author Contributions
SMNH, HTT, NSMA, and KKW conceived the manuscript. SMNH and KKW designed the manuscript, performed literature search, wrote and revised the manuscript. SMNH prepared the figures and table. HTT, NSMA, and NMS revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the Universiti Sains Malaysia (USM) grants comprising of the Research University Grant (1001/PPSP/8012349) awarded to KKW and Research University Grant (1001.PPSP.8012285) awarded to NSMA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. (2014) 7:12. doi: 10.1186/1939-4551-7-12
2. Licari A, Manti S, Ciprandi G. What are the effects of rhinitis on patients with asthma? Expert Rev Respir Med. (2019) 13:503–5. doi: 10.1080/17476348.2019.1604227
3. Steelant B, Farre R, Wawrzyniak P, Belmans J, Dekimpe E, Vanheel H, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. (2016) 137:1043–53.e5. doi: 10.1016/j.jaci.2015.10.050
4. Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology. (2016) 54:195–205. doi: 10.4193/Rhin15.376
5. Steelant B, Seys SF, Van Gerven L, Van Woensel M, Farre R, Wawrzyniak P, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. (2018) 141:951–63.e8. doi: 10.1016/j.jaci.2017.08.039
6. Nur Husna SM, Tan H-TT, Md Shukri N, Mohd Ashari NS, Wong KK. Nasal epithelial barrier integrity and tight junctions disruption in allergic rhinitis: overview and pathogenic insights. Front Immunol. (2021) 12:663626. doi: 10.3389/fimmu.2021.663626
7. Nur Husna SM, Siti Sarah CO, Tan HT, Md Shukri N, Mohd Ashari NS, Wong KK. Reduced occludin and claudin-7 expression is associated with urban locations and exposure to second-hand smoke in allergic rhinitis patients. Sci Rep. (2021) 11:1245. doi: 10.1038/s41598-020-79208-y
8. Siti Sarah COS, Shukri NM, Ashari NSM, Wong KK. Zonula occludens and nasal epithelial barrier integrity in allergic rhinitis. PeerJ. (2020) 8:e9834. doi: 10.7717/peerj.9834
9. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. (2008) 63(Suppl. 86):S8–160. doi: 10.1111/j.1398-9995.2007.01620.x
10. Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. (2017) 140:950–8. doi: 10.1016/j.jaci.2017.03.050
11. Li J, Wang H, Chen Y, Zheng J, Wong GW, Zhong N. House dust mite sensitization is the main risk factor for the increase in prevalence of wheeze in 13- to 14-year-old school children in Guangzhou city, China. Clin Exp Allergy. (2013) 43:1171–9. doi: 10.1111/cea.12157
12. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. (2001) 108(Suppl. 1):S2–8. doi: 10.1067/mai.2001.115569
13. Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. (2015) 372:456–63. doi: 10.1056/NEJMcp1412282
14. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. (2016) 16:133. doi: 10.1186/s12887-016-0673-z
15. Frohlich M, Pinart M, Keller T, Reich A, Cabieses B, Hohmann C, et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood? A meta-analysis. Clin Transl Allergy. (2017) 7:44. doi: 10.1186/s13601-017-0176-5
16. Pinart M, Keller T, Reich A, Frohlich M, Cabieses B, Hohmann C, et al. Sex-related allergic rhinitis prevalence switch from childhood to adulthood: a systematic review and meta-analysis. Int Arch Allergy Immunol. (2017) 172:224–35. doi: 10.1159/000464324
17. Elholm G, Linneberg A, Husemoen LL, Omland O, Gronager PM, Sigsgaard T, et al. The Danish urban-rural gradient of allergic sensitization and disease in adults. Clin Exp Allergy. (2016) 46:103–11. doi: 10.1111/cea.12583
18. Li CW, Chen DD, Zhong JT, Lin ZB, Peng H, Lu HG, et al. Epidemiological characterization and risk factors of allergic rhinitis in the general population in Guangzhou city in China. PLoS One. (2014) 9:e114950. doi: 10.1371/journal.pone.0114950
19. Wang IJ, Tung TH, Tang CS, Zhao ZH. Allergens, air pollutants, and childhood allergic diseases. Int J Hyg Environ Health. (2016) 219:66–71. doi: 10.1016/j.ijheh.2015.09.001
20. Leung TF, Ko FW, Wong GW. Roles of pollution in the prevalence and exacerbations of allergic diseases in Asia. J Allergy Clin Immunol. (2012) 129:42–7. doi: 10.1016/j.jaci.2011.11.031
21. D’Amato G, Akdis C. Global warming, climate change, air pollution and allergies. Allergy. (2020) 75:2158–60. doi: 10.1111/all.14527
22. Bergmann KC, Buters J, Karatzas K, Tasioulis T, Werchan B, Werchan M, et al. The development of birch pollen seasons over 30 years in Munich, Germany—an EAACI task force report. Allergy. (2020) 75:3024–6. doi: 10.1111/all.14470
23. Bousquet PJ, Cropet C, Klossek JM, Allaf B, Neukirch F, Bousquet J. Effect of smoking on symptoms of allergic rhinitis. Ann Allergy Asthma Immunol. (2009) 103:195–200. doi: 10.1016/S1081-1206(10)60181-0
24. Hisinger-Molkanen H, Piirila P, Haahtela T, Sovijarvi A, Pallasaho P. Smoking, environmental tobacco smoke and occupational irritants increase the risk of chronic rhinitis. World Allergy Organ J. (2018) 11:6. doi: 10.1186/s40413-018-0184-5
25. Singh S, Sharma BB, Salvi S, Chhatwal J, Jain KC, Kumar L, et al. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. Clin Respir J. (2018) 12:547–56. doi: 10.1111/crj.12561
26. Chung SJ, Kim BK, Oh JH, Shim JS, Chang YS, Cho SH, et al. Novel tobacco products including electronic cigarette and heated tobacco products increase risk of allergic rhinitis and asthma in adolescents: analysis of Korean youth survey. Allergy. (2020) 75:1640–8. doi: 10.1111/all.14212
27. Cardell LO, Olsson P, Andersson M, Welin KO, Svensson J, Tennvall GR, et al. Totall: high cost of allergic rhinitis-a national Swedish population-based questionnaire study. NPJ Prim Care Respir Med. (2016) 26:15082. doi: 10.1038/npjpcrm.2015.82
28. Justiz Vaillant AA, Zito PM. Hypersensitivity Reactions, Immediate. Treasure Island, FL: Statpearls (2018).
29. Valenta R, Karaulov A, Niederberger V, Gattinger P, van Hage M, Flicker S, et al. Molecular aspects of allergens and allergy. Adv Immunol. (2018) 138:195–256. doi: 10.1016/bs.ai.2018.03.002
30. Wongpiyabovorn J, Suratannon N, Boonmee S, Chatchatee P. Comparison of specific Ige detection by immunoblotting and fluorescence enzyme assay with in vivo skin prick test. Asian Pac J Allergy Immunol. (2018) 36:159–65. doi: 10.12932/AP-270217-0035
31. Nam YH, Lee SK. Comparison between skin prick test and serum immunoglobulin E by cap system to inhalant allergens. Ann Allergy Asthma Immunol. (2017) 118:608–13. doi: 10.1016/j.anai.2017.03.005
32. Erel F, Sarioglu N, Kose M, Kaymakci M, Gokcen M, Kepekci AH, et al. Intradermal skin testing in allergic rhinitis and asthma with negative skin prick tests. Iran J Allergy Asthma Immunol. (2017) 16:193–7.
33. Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. (2012) 67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x
34. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. (2011) 127(1 Suppl.):S1–55. doi: 10.1016/j.jaci.2010.09.034
35. Mansfield L, Hutteman HR, Tyson S, Enriquez A. A multiallergen and miniscreen can change primary care provider diagnosis and treatment of rhinitis. Am J Rhinol Allergy. (2012) 26:218–21. doi: 10.2500/ajra.2012.26.3764
36. Castelli S, Arasi S, Tripodi S, Villalta D, Martelli P, Conte M, et al. Ige antibody repertoire in nasal secretions of children and adults with seasonal allergic rhinitis: a molecular analysis. Pediatr Allergy Immunol. (2020) 31:273–80. doi: 10.1111/pai.13148
37. Garib V, Rigler E, Gastager F, Campana R, Dorofeeva Y, Gattinger P, et al. Determination of Ige and Igg reactivity to more than 170 allergen molecules in paper-dried blood spots. J Allergy Clin Immunol. (2019) 143:437–40. doi: 10.1016/j.jaci.2018.08.047
38. Tanno LK, Calderon MA, Li J, Casale T, Demoly P, Joint Allergy A. Updating allergy and/or hypersensitivity diagnostic procedures in the WHO ICD-11 revision. J Allergy Clin Immunol Pract. (2016) 4:650–7. doi: 10.1016/j.jaip.2016.01.015
39. Li Y, Wu R, Tian Y, Bao T, Tian Z. The correlation of serum eosinophil cationic protein level with eosinophil count, and total Ige level in Korean adult allergic rhinitis patients. Asian Pac J Allergy Immunol. (2016) 34:33–7.
40. A G, Rasheed Z, Salama RH, Salem T, Ahmed AA, Zedan K, et al. Filaggrin, major basic protein and leukotriene B4: biomarkers for adult patients of bronchial asthma, atopic dermatitis and allergic rhinitis. Intract Rare Dis Res. (2018) 7:264–70. doi: 10.5582/irdr.2018.01111
41. Kim JH, Yoon MG, Seo DH, Kim BS, Ban GY, Ye YM, et al. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. (2016) 8:329–37. doi: 10.4168/aair.2016.8.4.329
42. Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. (2015) 70:1393–405. doi: 10.1111/all.12698
43. Campo P, Eguiluz-Gracia I, Bogas G, Salas M, Plaza Seron C, Perez N, et al. Local allergic rhinitis: implications for management. Clin Exp Allergy. (2019) 49:6–16. doi: 10.1111/cea.13192
44. Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken). (2015) 67:891–7. doi: 10.1002/acr.22583
45. Rich RR, Fleisher TA, Shearer WT, Schroeder HW Jr, Frew AJ, Weyand CM. Clinical Immunology E-Book: Principles and Practice. 5th ed. Amsterdam: Elsevier (2019).
46. Nur Husna SM, Shukri N, Tan HTT, Mohd Ashari NS, Wong KK. Higher wheal sizes of dermatophagoides farinae sensitization exhibit severe nasal symptoms in allergic rhinitis patients. Front Med. (2022) 9:461. doi: 10.3389/fmed.2022.843432
47. van der Valk JP, Gerth van Wijk R, Hoorn E, Groenendijk L, Groenendijk IM, de Jong NW. Measurement and interpretation of skin prick test results. Clin Transl Allergy. (2015) 6:8. doi: 10.1186/s13601-016-0092-0
48. Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the pediatric allergies in america survey. J Allergy Clin Immunol. (2009) 124:S43–70. doi: 10.1016/j.jaci.2009.05.013
49. Aït−Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the international study of asthma and allergies in childhood (ISAAC) phase three. Allergy. (2009) 64:123–48. doi: 10.1111/j.1398-9995.2008.01884.x
50. Nieto A, Mazon A, Martin−Mateos MA, Plaza AM, Garde J, Alonso E, et al. Pediatric allergy and immunology in Spain. Pediatr Allergy Immunol. (2011) 22:742–50.
51. Gentile D, Bartholow A, Valovirta E, Scadding G, Skoner D. Current and future directions in pediatric allergic rhinitis. J Allergy Clin Immunol Pract. (2013) 1:214–26; quiz 27. doi: 10.1016/j.jaip.2013.03.012
52. Mariño-Sánchez F, Valls-Mateus M, de Los Santos G, Plaza AM, Cobeta I, Mullol J. Multimorbidities of pediatric allergic rhinitis. Curr Allergy Asthma Rep. (2019) 19:1–11. doi: 10.1007/s11882-019-0843-9
53. Pinart M, Benet M, Annesi-Maesano I, von Berg A, Berdel D, Carlsen KC, et al. Comorbidity of eczema, rhinitis, and asthma in Ige-sensitised and non-Ige-sensitised children in medall: a population-based cohort study. Lancet Respir Med. (2014) 2:131–40. doi: 10.1016/S2213-2600(13)70277-7
54. Lehtimäki J, Thorsen J, Rasmussen MA, Hjelmsø M, Shah S, Mortensen MS, et al. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. J Allergy Clin Immunol. (2021) 148:234–43. doi: 10.1016/j.jaci.2020.12.621
56. DeShazo RD, Kemp SF, Corren J, Feldweg A. Allergic Rhinitis: Clinical Manifestations, Epidemiology, and Diagnosis. Up to Date. Tehran: MediLib (2018).
57. Zacharisen MC. Rhinitis in children, adolescents, the elderly, and pregnant women: special considerations. Immunol Allergy Clin North Am. (2000) 20:425–44.
58. Gangwar RS, Friedman S, Seaf M, Levi-Schaffer F. Mast cells and eosinophils in allergy: close friends or just neighbors. Eur J Pharmacol. (2016) 778:77–83. doi: 10.1016/j.ejphar.2015.10.036
59. Tan H-TT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. (2016) 16:70. doi: 10.1007/s11882-016-0650-5
60. Sani MM, Ashari NSM, Abdullah B, Wong KK, Musa KI, Mohamud R, et al. Reduced Cd4+ terminally differentiated effector memory T cells in moderate-severe house dust mites sensitized allergic rhinitis patients. Asian Pac J Allergy Immunol. (2019) 37:138–46. doi: 10.12932/AP-191217-0220
61. He JS, Narayanan S, Subramaniam S, Ho WQ, Lafaille JJ, Curotto de Lafaille MA. Biology of Ige production: Ige cell differentiation and the memory of Ige responses. Curr Top Microbiol Immunol. (2015) 388:1–19. doi: 10.1007/978-3-319-13725-4_1
62. Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. (2014) 5:569. doi: 10.3389/fimmu.2014.00569
63. Finkelman FD, Khodoun MV, Strait R. Human Ige-independent systemic anaphylaxis. J Allergy Clin Immunol. (2016) 137:1674–80.
64. Sin B, Togias A. Pathophysiology of allergic and nonallergic rhinitis. Proc Am Thorac Soc. (2011) 8:106–14. doi: 10.1513/pats.201008-057RN
65. Pawankar R, Yamagishi S, Yagi T. Revisiting the roles of mast cells in allergic rhinitis and its relation to local Ige synthesis. Am J Rhinol. (2000) 14:309–17. doi: 10.2500/105065800781329582
66. Plewako H, Holmberg K, Oancea I, Gotlib T, Samolinski B, Rak S. A follow-up study of immunotherapy-treated birch-allergic patients: effect on the expression of chemokines in the nasal mucosa. Clin Exp Allergy. (2008) 38:1124–31. doi: 10.1111/j.1365-2222.2008.03005.x
67. Baldacci S, Maio S, Cerrai S, Sarno G, Baiz N, Simoni M, et al. Allergy and asthma: effects of the exposure to particulate matter and biological allergens. Respir Med. (2015) 109:1089–104. doi: 10.1016/j.rmed.2015.05.017
68. Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. (2000) 106:228–38. doi: 10.1067/mai.2000.108434
69. Hosseini S, Shoormasti RS, Akramian R, Movahedi M, Gharagozlou M, Foroughi N, et al. Skin prick test reactivity to common aero and food allergens among children with allergy. Iran J Med Sci. (2014) 39:29–35.
70. Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J. (2015) 2:24642. doi: 10.3402/ecrj.v2.24642
71. Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. (2017) 18:1076–83. doi: 10.1038/ni.3829
72. Reithofer M, Jahn-Schmid B. Allergens with protease activity from house dust mites. Int J Mol Sci. (2017) 18:1368. doi: 10.3390/ijms18071368
73. Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving Cd23: innate protection by antiproteases. J Exp Med. (1995) 182:1537–44. doi: 10.1084/jem.182.5.1537
74. Wang JY, Kishore U, Lim BL, Strong P, Reid KB. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin Exp Immunol. (1996) 106:367–73. doi: 10.1046/j.1365-2249.1996.d01-838.x
75. Madan T, Kishore U, Singh M, Strong P, Clark H, Hussain EM, et al. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. (2001) 107:467–75. doi: 10.1172/JCI10124
76. Deb R, Shakib F, Reid K, Clark H. Major house dust mite allergens Dermatophagoides pteronyssinus 1 and Dermatophagoides farinae 1 degrade and inactivate lung surfactant proteins A and D. J Biol Chem. (2007) 282:36808–19. doi: 10.1074/jbc.M702336200
77. Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. (2007) 12:834–42. doi: 10.1111/j.1440-1843.2007.01175.x
78. Vinhas R, Cortes L, Cardoso I, Mendes VM, Manadas B, Todo−Bom A, et al. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy. (2011) 66:1088–98. doi: 10.1111/j.1398-9995.2011.02598.x
79. Ma T, Wang X, Zhuang Y, Shi H, Ning H, Lan T, et al. Prevalence and risk factors for allergic rhinitis in adults and children living in different grassland regions of Inner Mongolia. Allergy. (2020) 75:234–9. doi: 10.1111/all.13941
80. Brzustewicz E, Bryl E. The role of cytokines in the pathogenesis of rheumatoid arthritis–practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine. (2015) 76:527–36.
81. Guo L, Huang Y, Chen X, Hu-Li J, Urban JF Jr, Paul WE. Innate immunological function of Th2 cells in vivo. Nat Immunol. (2015) 16:1051–9. doi: 10.1038/ni.3244
82. Kubo M. T follicular helper and Th2 cells in allergic responses. Allergol Int. (2017) 66:377–81. doi: 10.1016/j.alit.2017.04.006
83. Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. (2016) 46:1139–51. doi: 10.1111/cea.12780
84. Heijink IH, Nawijn MC, Hackett TL. Airway epithelial barrier function regulates the pathogenesis of allergic asthma. Clin Exp Allergy. (2014) 44:620–30. doi: 10.1111/cea.12296
85. Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. (2007) 120:1233–44; quiz 45–6. doi: 10.1016/j.jaci.2007.10.025
86. Gruber R, Bornchen C, Rose K, Daubmann A, Volksdorf T, Wladykowski E, et al. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am J Pathol. (2015) 185:2777–89. doi: 10.1016/j.ajpath.2015.06.021
87. Travers J, Rochman M, Miracle CE, Cohen JP, Rothenberg ME. Linking impaired skin barrier function to esophageal allergic inflammation via Il-33. J Allergy Clin Immunol. (2016) 138:1381–3. doi: 10.1016/j.jaci.2016.09.001
88. London NR Jr, Tharakan A, Ramanathan M Jr. The role of innate immunity and aeroallergens in chronic rhinosinusitis. Adv Otorhinolaryngol. (2016) 79:69–77. doi: 10.1159/000445132
89. Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. (2009) 1788:864–71. doi: 10.1016/j.bbamem.2008.08.027
90. Nur Husna SM, Shukri N, Tuan Sharif SE, Tan HTT, Mohd Ashari NS, Wong KK. Il-4/Il-13 axis in allergic rhinitis: elevated serum cytokines levels and inverse association with tight junction molecules expression. Front Mol Biosci. (2022) 9:819772. doi: 10.3389/fmolb.2022.819772
91. Bousquet J, Khaltaev N, Cruz A, Denburg J, Fokkens W, Togias A, et al. Ga Len; Allergen. Allergic Rhinitis and Its Impact on Asthma (ARIA) 2008 Update (in Collaboration with the World Health Organization, Ga Len and Allergen). Geneva: World Health Organization (2008).
93. McKay MP, Groff L. 23 years of toxicology testing fatally injured pilots: implications for aviation and other modes of transportation. Accid Anal Prev. (2016) 90:108–17. doi: 10.1016/j.aap.2016.02.008
94. Hossenbaccus L, Linton S, Garvey S, Ellis AK. Towards definitive management of allergic rhinitis: best use of new and established therapies. Allergy Asthma Clin Immunol. (2020) 16:1–17. doi: 10.1186/s13223-020-00436-y
95. Bjermer L, Westman M, Holmstrom M, Wickman MC. The complex pathophysiology of allergic rhinitis: scientific rationale for the development of an alternative treatment option. Allergy Asthma Clin Immunol. (2019) 15:24. doi: 10.1186/s13223-018-0314-1
96. Zhang M, Ni JZ, Cheng L. Safety of intranasal corticosteroids for allergic rhinitis in children. Expert Opin Drug Saf. (2022) 1–8. **VOLQ, doi: 10.1080/14740338.2022.2046731
97. Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA editors. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. International forum of Allergy & Rhinology. New York, NY: Wiley Online Library (2018).
98. Du K, Qing H, Zheng M, Wang X, Zhang L. Intranasal antihistamine is superior to oral H1 antihistamine as an add-on therapy to intranasal corticosteroid for treating allergic rhinitis. Ann Allergy Asthma Immunol. (2020) 125:589–96.e3. doi: 10.1016/j.anai.2020.06.038
99. Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. (2006) 36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x
100. Feng Y, Meng Y-P, Dong Y-Y, Qiu C-Y, Cheng L. Management of allergic rhinitis with leukotriene receptor antagonists versus selective H1-antihistamines: a meta-analysis of current evidence. Allergy Asthma Clin Immunol. (2021) 17:1–12. doi: 10.1186/s13223-021-00564-z
101. Seresirikachorn K, Chitsuthipakorn W, Kanjanawasee D, Khattiyawittayakun L, Snidvongs K. Leukotriene receptor antagonist addition to H1-antihistamine is effective for treating allergic rhinitis: a systematic review and meta-analysis. Am J Rhinol Allergy. (2019) 33:591–600. doi: 10.1177/1945892419844459
102. Liu G, Zhou X, Chen J, Liu F. Oral antihistamines alone vs in combination with leukotriene receptor antagonists for allergic rhinitis: a meta-analysis. Otolaryngol Head Neck Surg. (2018) 158:450–8. doi: 10.1177/0194599817752624
103. Mandhane SN, Shah JH, Thennati R. Allergic rhinitis: an update on disease, present treatments and future prospects. Int Immunopharmacol. (2011) 11:1646–62. doi: 10.1016/j.intimp.2011.07.005
104. Ramey J, Bailen E, Lockey R. Rhinitis medicamentosa. J Investig Allergol Clin Immunol. (2006) 16:148.
105. Ferguson BJ, Paramaesvaran S, Rubinstein E. A study of the effect of nasal steroid sprays in perennial allergic rhinitis patients with rhinitis medicamentosa. Otolaryngol Head Neck Surg. (2001) 125:253–60. doi: 10.1067/mhn.2001.117717
106. Vaidyanathan S, Williamson P, Clearie K, Khan F, Lipworth B. Fluticasone reverses oxymetazoline-induced tachyphylaxis of response and rebound congestion. Am J Respir Crit Care Med. (2010) 182:19–24. doi: 10.1164/rccm.200911-1701OC
107. Berings M, Gevaert P, De Ruyck N, Derycke L, Holtappels G, Pilette C, et al. Fcepsilonri expression and ige binding by dendritic cells and basophils in allergic rhinitis and upon allergen immunotherapy. Clin Exp Allergy. (2018) 48:970–80. doi: 10.1111/cea.13157
108. Caruso M, Cibella F, Emma R, Campagna D, Tringali G, Amaradio MD, et al. Basophil biomarkers as useful predictors for sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. (2018) 60:50–8. doi: 10.1016/j.intimp.2018.04.034
109. Hoshino M, Akitsu K, Kubota K, Ohtawa J. Serum periostin as a biomarker for predicting clinical response to house dust mite sublingual immunotherapy in allergic rhinitis. J Allergy Clin Immunol Pract. (2021) 9:1864–70. doi: 10.1016/j.jaip.2020.11.046
110. Zhang Y, Zhu K, Xia C, Chen J, Yu C, Gao T, et al. Multiple-cytokine profiling: a novel method for early prediction of the efficacy of sublingual immunotherapy in allergic rhinitis patients. J Inflamm Res. (2022) 15:603–12. doi: 10.2147/JIR.S350003
111. Das D, Sit S, Khaowas AK, Das C, Mukherjee D. Outcome of sublingual immunotherapy in allergic rhinitis. Indian J Otolaryngol Head Neck Surg. (2021) 73:467–73. doi: 10.1007/s12070-021-02465-4
112. Pavon-Romero GF, Larenas-Linnemann DE, Xochipa Ruiz KE, Ramirez-Jimenez F, Teran LM. Subcutaneous allergen-specific immunotherapy is safe in pediatric patients with allergic rhinitis. Int Arch Allergy Immunol. (2021) 182:553–61. doi: 10.1159/000513158
113. Fritzsching B, Contoli M, Porsbjerg C, Buchs S, Larsen JR, Elliott L, et al. Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: results from the react study, a retrospective cohort study. Lancet Reg Health Eur. (2022) 13:100275. doi: 10.1016/j.lanepe.2021.100275
114. Kortekaas Krohn I, Seys SF, Lund G, Jonckheere AC, Dierckx de Casterlé I, Ceuppens JL, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy. (2020) 75:1155–64. doi: 10.1111/all.14132
115. Molfetta R, Lecce M, Quatrini L, Caracciolo G, Digiacomo L, Masuelli L, et al. Immune complexes exposed on mast cell-derived nanovesicles amplify allergic inflammation. Inflammation. (2020) 4:5. doi: 10.1111/all.14103
Keywords: allergic rhinitis, epidemiology, diagnostic criteria, pathophysiology, Th2 responses, immunotherapy
Citation: Nur Husna SM, Tan HTT, Md Shukri N, Mohd Ashari NS and Wong KK (2022) Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med. 9:874114. doi: 10.3389/fmed.2022.874114
Received: 11 February 2022; Accepted: 15 March 2022;
Published: 07 April 2022.
Edited by:
Sara Manti, University of Catania, ItalyReviewed by:
Amelia Licari, University of Pavia, ItalyCopyright © 2022 Nur Husna, Tan, Md Shukri, Mohd Ashari and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noor Suryani Mohd Ashari, c3VyeWFuaUB1c20ubXk=; Kah Keng Wong, a2Foa2VuZ0B1c20ubXk=, a2Foa2VuZzNAZ21haWwuY29t
 Siti Muhamad Nur Husna
Siti Muhamad Nur Husna Hern-Tze Tina Tan1
Hern-Tze Tina Tan1 Norasnieda Md Shukri
Norasnieda Md Shukri Noor Suryani Mohd Ashari
Noor Suryani Mohd Ashari Kah Keng Wong
Kah Keng Wong