- 1Virus Reference Department, UK Health Security Agency, London, United Kingdom
- 2National Centre for Human Retrovirology, Imperial College Healthcare NHS Trust, London, United Kingdom
- 3Imperial College London, London, United Kingdom
HIV pre-exposure prophylaxis (HIV-PrEP) is effective in reducing the likelihood of HIV acquisition in HIV-negative people at high risk of exposure. Guidelines recommend testing for sexually transmitted infections (STIs) before starting, and periodically on PrEP, including bacterial infections, HIV, hepatitis C virus, and, for those who are non-immune, hepatitis B virus. Diagnosed infections can be promptly treated to reduce onward transmission. HTLV-1 is not mentioned; however, it is predominantly sexually transmitted, causes adult T-cell leukaemia/lymphoma (ATL) or myelopathy in 10% of those infected, and is associated with an increased risk of death in those without any classically HTLV-associated condition. The 2021 WHO Technical Report on HTLV-1 called for the strengthening of global public health measures against its spread. In this scoping review, we, therefore, (1) discuss the epidemiological context of HIV-PrEP and HTLV-1 transmission; (2) present current knowledge of antiretrovirals in relation to HTLV-1 transmission prevention, including nucleos(t)ide reverse transcriptase inhibitors (NRTIs) and integrase strand transfer inhibitors (INSTIs); and (3) identify knowledge gaps where data are urgently required to inform global public health measures to protect HIV-PrEP users from HTLV-1 acquisition. We suggest that systematic seroprevalence studies among PrEP-using groups, including men who have sex with men (MSM), people who inject drugs (PWIDs), and female sex workers (FSWs), are needed. Further data are required to evaluate antiretroviral efficacy in preventing HTLV-1 transmission from in vitro studies, animal models, and clinical cohorts. PrEP delivery programmes should consider prioritizing the long-acting injectable INSTI, cabotegravir, in HTLV-1 endemic settings.
Background
HIV pre-exposure prophylaxis (PrEP) reduces the likelihood of HIV acquisition and is recommended for high-risk groups. Indications include being HIV-negative and reporting anal or vaginal sex plus any of (1) HIV-positive partners with unknown or detectable viral load, (2) a bacterial sexually transmitted infection (STI), and (3) inconsistent condom use; or injecting drugs, and sharing injecting equipment or injecting with HIV-positive partners (1).
Guidelines recommend STI testing before starting and, periodically on PrEP (1) given the increased risk for STI acquisition. This includes testing for bacterial infections, HIV, hepatitis C virus (HCV), and for non-immune patients, hepatitis B virus (HBV). Diagnosed infections are promptly treated to reduce onward transmission. HTLV testing is not mentioned (1, 2), although HTLV-1 is predominantly sexually transmitted and causes adult T-cell leukaemia/lymphoma (ATL) or HTLV-1 associated myelopathy (HAM) in ~10% of those infected, and is associated with an increased risk of death (Relative Risk (RR) 1.57, 95%CI 1.37–1.80) in those without any classically HTLV-associated condition (3). HTLV-1 is endemic in South America, the Caribbean, Sub-Saharan Africa, Romania, Iran, Japan, and Melanesia. Populations with high seroprevalence are also described within many non-endemic countries. However, data for >2/3 of the global population are lacking (4, 5).
In 2021, the WHO HTLV-1 Technical Report called for the strengthening of public health measures against its spread (6). In this review, we (1) discuss the epidemiological context of HIV-PrEP and HTLV-1 transmission; (2) present current knowledge of antiretrovirals in relation to HTLV-1 transmission prevention; and (3) identify knowledge gaps where data are required to inform the global public health measures to protect HIV-PrEP users from HTLV-1.
HIV Pre-Exposure Prophylaxis
Efficacy
The current US Food and Drug Administration-approved HIV-PrEP antiretrovirals are an oral combination of nucleos(t)ide reverse transcriptase inhibitors (NRTI), emtricitabine (FTC) plus either tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF), taken daily or episodically around a potential exposure, or intramuscular, twice-monthly injections of the long-acting integrase strand transfer inhibitor (INSTI), cabotegravir. Randomized trials have shown the efficacy of TDF/FTC (7, 8) and TAF/FTC (9) PrEP in preventing sexual transmission of HIV-1 (86–97%), with most PrEP failures arising from reduced adherence to oral regimens. The efficacy of injectable cabotegravir is superior (10, 11).
Characteristics of Groups Using, or Eligible For, HIV-PrEP
Men who have sex with men (MSM) are the major PrEP-user group in industrialized countries. In the UK, 96% of 24,255 users were cis-gender MSM (12). Approximately 1.14 million U.S. adults have indications for PrEP, namely, 71% MSM, 23% heterosexual, and 6% people who inject drugs (PWIDs); notably, 69% were black or Latino (13). In non-industrialized settings, PrEP-user demographics differ. In Kenya, Lesotho, and Tanzania, 77% of 47,352 PrEP-users were women; 50% overall were female sex workers (FSWs) (14).
The MSM and others at risk of HIV acquisition in HTLV endemic countries are increasingly interested in, or already using, PrEP, including Brazil (15), Peru (16), Jamaica (17), and Nigeria (18). In a Brazilian PrEP delivery study, 450/738 (61%) of MSM and transgender women accepted an offer of PrEP (15). Across Brazil, the country with the greatest number of HTLV cases at almost 1 million (19), PrEP demand amongst eligible MSM was estimated at 66,000–98,000 (20). In Japan, modeling suggests that PrEP roll-out would reduce HIV transmission and will likely expand, although prescriptions are not yet widely accessible (21). HIV incidence in indigenous Australians is increasing, a group with one of the highest HTLV seroprevalences in the world (37%, 213/578) (22), and targeted PrEP programmes are expanding (23).
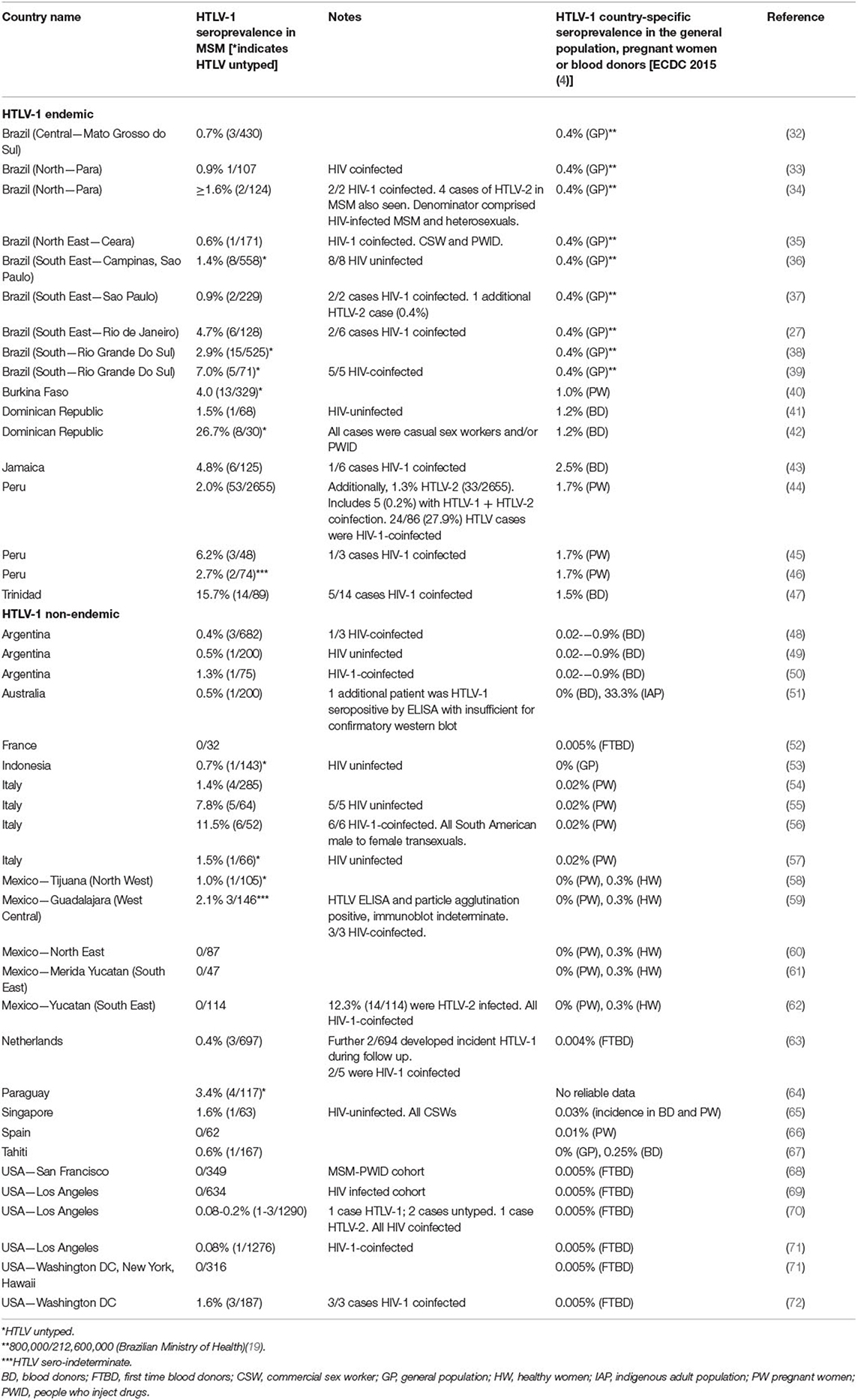
HTLV-1 Seroprevalence Amongst Groups Eligible for HIV-PrEP
Amongst STI clinic attendees in Jamaica, multiple partners, condomless sex, and a history of STIs or anogenital ulcers were associated with increased HTLV risk (24). HSV-2 seropositivity was associated with increased HTLV-1 seroprevalence amongst UK STI clinic attendees (25).
The HTLV-1 seroprevalence may be increased in MSM in endemic and non-endemic settings (Table 1). In Barbados, 0.7% (1/134) of a predominantly MSM cohort tested HTLV-1 positive at PrEP baseline (26). HTLV risk factors in MSM include HIV (27), multiple partners, condomless receptive anal intercourse (CRAI), syphilis, and HSV-2 (28), a profile that overlaps with that of PrEP-users (29, 30). HTLV-1 seroprevalence in HIV-positive individuals, including MSM, has been reviewed previously and is generally increased compared with that of the general population, indicating either greater exposure or susceptibility (31).
Increased HTLV-1 prevalence is reported amongst FSW in endemic settings. A systematic review in Latin America demonstrated greater prevalence than in blood donors or pregnant women, up to 21.8, 0.9, and 1.7%, respectively, in Peru (4, 73). A systematic review in Africa, mostly of endemic countries, identified increased HTLV-1 seroprevalence in FSWs although the difference could have been due to chance (74). In non-endemic settings, HTLV seroprevalence in FSW varies, for example, 0.3% in Spain (HTLV-1) (75) and 6.7% (HTLV-1/2) in the USA (76).
A high HTLV seroprevalence has been reported in PWIDs. HTLV-1 and HTLV-2 seroprevalence amongst HIV-1 positive PWID in Sao Paulo was 15.3 and 11.1%, respectively (37). HTLV-2 seroprevalence amongst U.S. STI clinic attendees was 7.6% vs. 0.7% for PWIDs vs. non-PWIDs, respectively (77). In other non-endemic countries, no HTLV cases among PWID were found, as in Germany (78).
HTLV-1 as an STI
The rising HTLV seroprevalence observed with increasing age in Japanese and Brazilian cohorts suggests that the major mode of transmission is sexual, with >80% of infections acquired in adulthood (79, 80). Seminal fluid contains T-lymphocytes and macrophages, cells permissive to HTLV infection, and enhances HTLV-1 replication by transactivation of the long terminal repeat promoter (81). Proviral DNA was detected in the cervical fluid of 68% HTLV-1 infected women (82). Phylogenetics has demonstrated sexual transmission within serodiscordant couples (83) and sexual acquisition has been described in other case reports, including subsequent rapidly progressive HAM (84, 85).
Male-to-female transmission is more efficient than vice versa, with a four times greater relative transmission rate (86). Sexual transmission risk per annum is ~0.6–4.9% (86–89). Data are limited on risk for male-to-male contact, for insertive and receptive anal sex. Female-to-female transmission has not been reported.
Reasons for increased efficiency of male-to-female transmission may include enhancing co-factors or greater lymphocyte content within a seminal fluid, inhibitory factors in cervical fluid, or the greater area of susceptible mucosa in the female genital tract. Most seroprevalence studies found that HTLV-1 seroprevalence increases after menopause, suggesting the mucosa becomes more susceptible after this event. Amongst MSM, the association of CRAI with transmission could be explained by the enhancing presence of seminal fluid, or a greater area of susceptible anorectal vs. penile mucosa.
The Anti-HTLV Activity of Antiretrovirals
Nucleos(t)ide Reverse Transcriptase Inhibitors
Zidovudine
In vitro
Zidovudine (AZT) reduced HTLV-1 proviral DNA production following co-culture of infected cell lines with lymphocytes when added at time zero of infection (90–94) but with no impact when added to cells with established infection (91). IC50 was reported as 0.11 μM (94). Enzymatic susceptibility of HTLV-1 and HIV-1 reverse transcriptase (RT) to AZT was equivalent (95).
In vivo
In an ATL model, where rabbits were inoculated intraperitoneally with an HTLV-1 transformed cell line, AZT at time zero prevented infection (96). In knockout mice inoculated with human PBMCs and a chronically infected cell line (MT-2), AZT at time zero blocked HTLV-1 infection, but not if given 1 week later (97). In baboons naturally infected with simian T-lymphotropic virus type 1 (STLV-1), treatment with the histone deacetylase inhibitor, valproate, which induces viral expression, followed by AZT, persistently reduced the proviral load (PVL), with a rebound on treatment discontinuation (98). PVL decline was associated with an increase in STLV-1-specific cytotoxic T-lymphocytes. However, AZT did not impact PVL in patients with HAM (99).
Zidovudine/Interferon-Alpha (IFN-Alpha)
The combination AZT/interferon (IFN)-alpha improves survival and is the first-line treatment for certain ATL types (100, 101) although the mechanism is uncertain. Although treatment is usually administered indefinitely, cases are described by ATL remission following discontinuation (102). One report demonstrated AZT/IFN-induced inhibition of HTLV-1 RT in responding but not resistant patients with ATL (103), consistent with direct antiviral effects. As RT-mediated viral replication does not occur in malignant cells, this suggests antiviral activity in the ATL microenvironment, either within infected cells or in preventing de novo T-cell infection, as such cells are critical for the survival of the malignant clone (104).
Lamivudine
In vitro
Lamivudine (3TC) protected lymphocytes from HTLV-1 infection in vitro, although with reduced potency vs. AZT (105), in one study, 200 times lower (94). However, a methionine-to-valine substitution in the conserved motif of HTLV-1 RT, tyrosine (Y)-methionine (M)-aspartic acid (D)-aspartic acid (D) (YMDD), conferred high-level lamivudine resistance. This motif is homologous with that of HIV-1 RT, where the M184V substitution confers lamivudine resistance, indicating HTLV-1 RT as the target of inhibition (94).
Others reported that lamivudine did not block infection (106) and high-level resistance was observed in an RT enzymatic assay (95). By homology with the reduced susceptibility to 3TC conferred by V118I in association with other HIV-1 RT mutations, HTLV-1 RT resistance to 3TC may be due to the naturally occurring presence of isoleucine at this codon (107).
In vivo
Lamivudine therapy in five people with HAM initially reduced the HTLV-1 proviral DNA load by 10-fold, with the nadir reached over a variable period of 1–6 months. However, the effect was not sustained (99).
Lamivudine/Zidovudine
AZT-3TC therapy vs. placebo over 48 weeks in 16 patients with HAM had no effect on PVL or clinical response (108) with lack of effect not due to the development of phenotypic NRTI resistance. AZT consistently inhibited HTLV-1 from primary isolates; however, sensitivity was reduced compared to HTLV-1 from MT-2 cells and varied amongst patients. HTLV-1 RT from both primary isolates and MT-2 cells was 3TC-insensitive (109).
Tenofovir and Adefovir (Acyclic Nucleoside Phosphonates)
In vitro
Inhibition of HTLV-1 cell-to-cell transmission by tenofovir was twenty times more potent in one study than AZT (94). Others reported a similar activity of tenofovir and AZT (110). In cell-free assays, tenofovir inhibited HTLV-1 RT enzymatic activity (93). Others reported the activity of the prodrugs, TDF and TAF, but not of tenofovir, in blocking HTLV-1 cell-to-cell transmission (106). Adefovir and its prodrug, adefovir dipivoxil, approved for anti-HBV therapy, were more active against HTLV-1 than AZT (106). Other groups also observed the blocking of HTLV-1 infection of cells by TDF (111).
In vivo
Limited data are available on in vivo activity of tenofovir. In a knock-out mouse model, TDF at time zero of inoculation prevented infection, with no effect if administered 1 week later (97). TDF administered for a mean of 9 months in six patients with HTLV-1 had no benefit on PVL or clinical status (109). In the analyses of primary isolates of HTLV-1 RT from patients' peripheral blood mononuclear cells (PBMCs), variable sensitivity to TDF was noted prior to and not altered by AZT/3TC exposure in vivo, in contrast with consistent sensitivity to AZT (109).
Other NRTIs
Abacavir blocked HTLV-1 infection of cells in vitro although less potently than tenofovir (94). The historic NRTIs, didanosine, zalcitabine, and stavudine, were partially active (94, 95, 112). An experimental NRTI class, phosphonated carbocyclic 2′-oxa-3′aza nucleosides (PCOANs), prevented HTLV-1 infection of PBMCs in vitro more potently than tenofovir and inhibited the growth of infected cells (109, 110).
Integrase Strand Transfer Inhibitors
In vitro
INSTI, including raltegravir, inhibit HTLV-1 integrase and blocks PBMC infection (113, 114). Barski et al. described INSTI effects against HTLV-1 integrase enzymatic activity and cell-to-cell infection. The EC50 for blocking infection was 0.3, 6.4, and 9.6 nM for bictegravir, raltegravir, and elvitegravir, respectively, and 17.8 nM for TDF. Antiviral activities were comparable with HIV-1 and HTLV-1 integrase (111). Dolutegravir exerted anti-enzymatic activity but potency in blocking infection was not evaluated (111).
In vivo
Raltegravir for 12 months reduced the PVL in patients with HAM (n = 2) for the first 6 months followed by a rebound, with no impact on the asymptomatic infection (n = 3) (115). Genetic substitutions were not observed post-therapy, consistent with a lack of drug selection pressure. Amongst patients with HAM (n = 16) receiving 6 months of raltegravir, PVL in PBMC and/or cerebrospinal fluid (CSF) declined in some individuals but with no impact on the overall cohort. Spontaneous lymphoproliferation, a measure of ex vivo T-cell activation, was significantly reduced overall. Clinical measurements remained stable (116).
Other Antiretroviral Classes
Non-nucleoside Reverse Transcriptase Inhibitors
The non-nucleoside reverse transcriptase inhibitor (NNRTI), dapivirine intravaginal ring, has been approved by the European Medicines Agency for use as HIV-PrEP in high prevalence settings (117, 118). However, NNRTI is ineffective against HIV-2, a closer relative of HIV-1 than HTLV-1, and nevirapine exerted no activity on HTLV-1 RT in vitro (95, 119). This may reflect the greater sequence diversity in RT at the NNRTI-binding pocket than at the substrate-binding site, and the dependence of NNRTI binding on RT structure (120, 121).
Protease Inhibitors
Ritonavir inhibits ATL cell growth ex vivo, probably due to the anti-nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-kB) activity rather than HTLV-1 protease inhibition (122). The historic HIV-1 protease inhibitor, indinavir, did not inhibit HTLV-1 (123). In vitro, darunavir weakly inhibits HTLV-1 protease; novel darunavir analogs have increased activity (124).
Entry Inhibitors
The anti-HTLV-1 activity of other antiretrovirals is unknown, including the C-C Motif Chemokine Receptor 5 (CCR5) co-receptor antagonist, maraviroc; fusion inhibitor, enfuvirtide; attachment inhibitor, fostemsavir; or the post-attachment inhibitor, ibalizumab-uiyk. However, a lack of anti-HIV-2 activity of enfuvirtide and fostemsavir suggests inactivity against HTLV-1. A lack of use of a cluster of differentiation 4 (CD4) receptors or CCR5 co-receptors for HTLV-1 attachment predicts the inactivity of ibalizumab and maraviroc, respectively.
Antiretrovirals: Summary
Data on HTLV-1 susceptibility to NRTIs are conflicting across in vitro studies reflecting (1) different assays, particularly comparing cell-to-cell transmission vs. enzyme inhibition, (2) cell-type-dependent drug uptake and intracellular phosphorylation, (3) RT sequence variation, and (4) intracellular RT activity, which may vary with the cellular environment. In vivo, most studies have yielded disappointing results for NRTI in established HTLV-1 infection (106). Amongst INSTI, the second-generation agent, bictegravir, showed greater potency in blocking HTLV-1 transmission in vitro than TDF. In vivo, the antiviral effects of raltegravir in established infection have been disappointing.
The lack of antiretroviral activity in patients probably reflects the greater contribution of HTLV-1-driven cellular proliferation to PVL than viral replication. Extrapolating from HIV-1, a predominance of HTLV-1 transmission through cell-to-cell contacts over virus entry from plasma could reduce RT inhibitor sensitivity, by producing a greater number of infection events per target cell and, therefore, reducing the likelihood of all transmitted viruses being exposed to drugs (106, 125).
NRTI and/or INSTI may be insufficiently potent to treat established HTLV-1 infection. However, the signal for preventing cell-to-cell transmission, mostly marked for AZT, TDF, and bictegravir, suggests a potential role as HTLV-1 PrEP or post-exposure prophylaxis (PEP).
Current Knowledge of HTLV-PrEP and HTLV-PEP
It has been speculated that following exposure, HTLV-1 proliferation is first through viral replication and infectious transmission, and then predominantly although not exclusively by the mitotic expansion of infected clones (126). However, early infection is asymptomatic and dynamics are poorly understood. The effect of HIV-PrEP is likely through early inhibition of HIV replication, before the infection is established, and similar pre-exposure or post-exposure antiretrovirals with anti-HTLV activity could plausibly prevent HTLV-1 transmission. However, no studies have evaluated HTLV-PrEP, and information on HTLV-PEP is limited to case reports.
HTLV-PEP
Organ Transplantation
In iatrogenically immunosuppressed patients infected with HTLV-1 through organ transplantation, any initial phase of infectious spread lasted no more than a few weeks (127). For three organ recipients of one infected donor, AZT/raltegravir was administered from days 17–26 to 43–80 post-transplantation, following the detection of low-level PVL. Peak proviral doubling time was achieved on days 38–45, followed by a steady state. HTLV-1 antibodies were detected on days 16–39. Findings suggested rapid virus dissemination despite antivirals, with both the early infectious spread and mitotic expansion. No rapidly progressive HAM was observed (127).
Elsewhere, AZT/3TC/raltegravir was initiated 24 h post-renal transplantation from an infected donor for 1 month. At PEP cessation, both PVL and HTLV antibodies were undetected. Two months later, high-level PVL and HTLV-1 antibodies were detected, followed by HAM. Antivirals were re-initiated but HAM rapidly progressed (128). The authors speculated that early PEP might delay but not prevent viral propagation. AZT/3TC/raltegravir initiated within 1 week of renal transplantation from another infected donor, and continued for >18 months, did not prevent HAM development in the recipient at 8 months. A second kidney recipient, who received AZT/3TC/raltegravir in the first 2-months post-transplant, and whose allograft was removed following rejection, developed asymptomatic infection over 3 years of follow-up (129).
Consensus, opinion-based guidelines recommend 6 weeks of AZT/raltegravir PEP initiated within 48 h of transplantation from an HTLV-1 seropositive donor; or as pre-emptive therapy, where the donor's HTLV-1 seropositivity is detected >48 h post-transplant and the recipient's PVL is undetected (130). Some clinicians also recommend raltegravir post-stem cell transplant for ATL as this may prevent infection of the donor's lymphocytes; however, data are required to evaluate this approach.
Neonatal
Although most mother-to-child HTLV-1 transmission (MTCT) occurs via breastfeeding, intrauterine and perinatal infection is also reported. Transmission rates are 7-32% for long term (>6 months) breastfeeding vs. 2.5–5.7% for exclusive formula-feeding (131). There are insufficient data on the use of antivirals to prevent MTCT. In UK patients with ATL, MTCT occurred in one case where no antivirals were administered; no MTCT occurred in two cases where maternal AZT/raltegravir plus neonatal AZT were provided (132).
Occupational
Although occupational exposure, such as in healthcare workers, is a recognized transmission route for HTLV-1 and HTLV-2 (133, 134), data on PEP efficacy are lacking. Some recommend 6 weeks of AZT/3TC/raltegravir after cellular fluid exposure from an HTLV-1 source, especially with a known high PVL (135).
Sexual
There are no data on antiviral PEP after sexual exposure to HTLV-1.
Future Directions
HIV-PrEP roll-out has critical implications for HTLV transmission. First, sexually transmitted HTLV outbreaks could occur, as described for HCV (136). Data are urgently required to ascertain the risk and to inform public health interventions. Seroprevalence studies amongst HIV-PrEP-using groups, including MSM, PWID, and FSW, should be performed. In other populations, HTLV seroprevalence may vary considerably even within one country, probably due to founder effects and high transmission rates. Epidemiological findings should inform guidelines indicating country-specific HTLV testing algorithms within PrEP programmes.
Second, PrEP programmes should evaluate antiretroviral efficacy in preventing HTLV-1 transmission. Unlike HIV, antiviral therapy to treat established HTLV is lacking, placing greater emphasis on transmission prevention. Observational cohort studies should measure HTLV-1 incidence in PrEP-users and matched non-PrEP user controls. Cohorts should include MSM and PWID, as most HTLV incidence data are from heterosexual couple studies.
Third, further data on antiretroviral activity against HTLV-1 transmission are required. This should include cabotegravir in vitro and in animal models, including in combination with NRTI. Antiretrovirals under development and clinical trials should also be evaluated, particularly, long-acting agents such as the HIV-1 capsid inhibitor, lenacapavir. Data could also be obtained from HBV MTCT prevention studies of TDF, where individuals are HTLV-1-coinfected and compared to non-TDF-receiving controls; however, such studies might only assess the effect of transplacental TDF to prevent intrapartum transmission. In the interim, PrEP programmes should prioritize injectable cabotegravir for roll-out in HTLV-1 endemic settings, both for its predicted greater efficacy in blocking HTLV-1 transmission, and superior efficacy in preventing HIV-1 transmission vs. oral NRTI PrEP.
Finally, country-specific data are required to understand HTLV-1 awareness amongst PrEP users, clinicians, and community groups, including risk factors, clinical consequences, treatments for HTLV-related conditions, and measures to prevent onward transmission. Findings should encourage campaigns to improve HTLV-1 knowledge and clinical care.
Author Contributions
DB and GT devised the manuscript. DB wrote the first draft and GT revised it. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
3TC, lamivudine; ATL, adult T-cell leukaemia/lymphoma; AZT, zidovudine; CCR5, C-C Motif Chemokine Receptor 5; CD4, cluster of differentiation 4; CRAI, condomless receptive anal intercourse; CSF, cerebrospinal fluid; FSW, female sex worker; FTC, emtricitabine; HAM, HTLV-1 associated myelopathy; IFN, interferon; INSTI, integrase strand transfer inhibitor; MSM, men who have sex with men; MTCT, mother to child transmission; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PBMC, peripheral blood mononuclear cell; PCOAN, phosphonated carbocyclic 2′-oxa-3′aza nucleosides; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; PVL, proviral load; PWID, people who inject drugs; RT, reverse transcriptase; STI, sexually transmitted infection; STLV-1, simian T-lymphotropic virus type 1; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
References
1. Preexposure prophylaxis for the prevention of HIV infection in the United States-−2021 update: a clinical practice guideline 2021. Available from: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
2. Brady M, Rodger A, Asboe D, Cambiano V, Clutterbuck D, Desai M, et al. BHIVA/BASHH guidelines on the use of HIV pre-exposure prophylaxis (PrEP) 2018. HIV Med. (2019) 20:s2–s80. doi: 10.1111/hiv.12718
3. Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis. (2020) 20:133–43. doi: 10.1016/S1473-3099(19)30402-5
4. Geographical distribution of areas with a high prevalence of HTLV-1 infection 2015. Available from: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/geographical-distribution-areas-high-prevalence-HTLV1.pdf
5. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. (2012) 3:388. doi: 10.3389/fmicb.2012.00388
6. Human T-lymphotropic virus type 1: technical report 2020. Available from: https://www.who.int/news/item/03-03-2021-new-who-report-on-human-t-lymphotropic-virus-type-1-indicates-the-future-path-for-its-prevention-and-control
7. McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. (2016) 387:53–60. doi: 10.1016/S0140-6736(15)00056-2
8. Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. (2015) 373:2237–46. doi: 10.1056/NEJMoa1506273
9. Mayer KH, Molina JM, Thompson MA, Anderson PL, Mounzer KC, De Wet JJ, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. (2020) 396:239–54. doi: 10.1016/S0140-6736(20)31065-5
10. Landovitz RJ, Donnell D, Clement ME, Hanscom B, Cottle L, Coelho L, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. (2021) 385:595–608. doi: 10.1056/NEJMoa2101016
11. HPTN 084 study demonstrates superiority of CAB LA to Oral TDF/FTC for the prevention of HIV 2020. Available from: https://www.hptn.org/news-and-events/press-releases/hptn-084-study-demonstrates-superiority-of-cab-la-to-oral-tdfftc-for
12. Sullivan AK. The HIV pre-exposure prophylaxis (PrEP) IMPACT trial: baseline demographics, coverage and first regimen choice. Fifth Joint Conference of the British HIV Association (BHIVA) and the British Association for Sexual Health and HIV (BASHH) (2021).
13. Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. (2018) 28:850–7 e9. doi: 10.1016/j.annepidem.2018.05.003
14. Reed JB, Shrestha P, Were D, Chakare T, Mutegi J, Wakhutu B, et al. HIV PrEP is more than ART-lite: longitudinal study of real-world PrEP services data identifies missing measures meaningful to HIV prevention programming. J Int AIDS Soc. (2021) 24:e25827. doi: 10.1002/jia2.25827
15. Hoagland B, Moreira RI, De Boni RB, Kallas EG, Madruga JV, Vasconcelos R, et al. High pre-exposure prophylaxis uptake and early adherence among men who have sex with men and transgender women at risk for HIV Infection: the PrEP Brasil demonstration project. J Int AIDS Soc. (2017) 20:21472. doi: 10.7448/IAS.20.1.21472
16. Torres TS, Konda KA, Vega-Ramirez EH, Elorreaga OA, Diaz-Sosa D, Hoagland B, et al. Factors associated with willingness to use pre-exposure prophylaxis in Brazil, Mexico, and Peru: web-based survey among men who have sex with men. JMIR Public Health Surveill. (2019) 5:e13771. doi: 10.2196/13771
17. Global AIDS Monitoring Report: Jamaica: United Nations Programme on HIV/AIDS (2020). Available from: https://www.unaids.org/sites/default/files/country/documents/JAM_2020_countryreport.pdf
18. Ogunbajo A, Iwuagwu S, Williams R, Biello K, Mimiaga MJ. Awareness, willingness to use, and history of HIV PrEP use among gay, bisexual, and other men who have sex with men in Nigeria. PLoS ONE. (2019) 14:e0226384. doi: 10.1371/journal.pone.0226384
19. Prevalência da infecção por HTLV-1/2 no Brasil: Coordenação-Geral de Vigilância das Infecções Sexualmente Transmissíveis do Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis (CGIST/DCCI/SVS) (2020). Available from: https://www.gov.br/saude/pt-br/media/pdf/2020/dezembro/11/boletim_epidemiologico_svs_48.pdf
20. Luz PM, Benzaken A, Alencar TM, Pimenta C, Veloso VG, Grinsztejn B. PrEP adopted by the brazilian national health system: What is the size of the demand? Medicine (Baltimore). (2018) 97:S75–S7. doi: 10.1097/MD.0000000000010602
21. Gilmour S, Peng L, Li J, Oka S, Tanuma J. New strategies for prevention of HIV among Japanese men who have sex with men: a mathematical model. Sci Rep. (2020) 10:18187. doi: 10.1038/s41598-020-75182-7
22. Einsiedel L, Pham H, Talukder MR, Taylor K, Wilson K, Kaldor J, et al. Very high prevalence of infection with the human T cell leukaemia virus type 1c in remote Australian Aboriginal communities: results of a large cross-sectional community survey. PLoS Negl Trop Dis. (2021) 15:e0009915. doi: 10.1371/journal.pntd.0009915
23. Ward J, McManus H, McGregor S, Hawke K, Giele C, Su JY, et al. HIV incidence in Indigenous and non-Indigenous populations in Australia: a population-level observational study. Lancet HIV. (2018) 5:e506–e14. doi: 10.1016/S2352-3018(18)30135-8
24. Murphy EL, Figueroa JP, Gibbs WN, Brathwaite A, Holding-Cobham M, Waters D, et al. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann Intern Med. (1989) 111:555–60. doi: 10.7326/0003-4819-111-7-555
25. Turner CG, Cohen CE, Sabin CA, Tosswill JH, Best JM, Taylor GP, et al. The seroepidemiology of HTLV-I amongst genitourinary medicine (GUM) attendees in South East London. J Clin Virol. (2008) 43:253–4. doi: 10.1016/j.jcv.2008.06.011
26. Best A, Rambarran N. The demographic features and outcome indicators of the Barbados HIV Pre-exposure Prophylaxis Program, 2018-2019. Rev Panam Salud Publica. (2021) 45:e51. doi: 10.26633/RPSP.2021.51
27. Cortes E, Detels R, Aboulafia D, Li XL, Moudgil T, Alam M, et al. HIV-1, HIV-2, and HTLV-I infection in high-risk groups in Brazil. N Engl J Med. (1989) 320:953–8. doi: 10.1056/NEJM198904133201501
28. Zunt JR, La Rosa AM, Peinado J, Lama JR, Suarez L, Pun M, et al. Risk factors for HTLV-II infection in Peruvian men who have sex with men. Am J Trop Med Hyg. (2006) 74:922–5. doi: 10.4269/ajtmh.2006.74.922
29. Traeger MW, Schroeder SE, Wright EJ, Hellard ME, Cornelisse VJ, Doyle JS, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. (2018) 67:676–86. doi: 10.1093/cid/ciy182
30. Ong JJ, Baggaley RC Wi TE, Tucker JD, Fu H, Smith MK, et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis. JAMA Netw Open. (2019) 2:e1917134. doi: 10.1001/jamanetworkopen.2019.17134
31. Dhasmana D, Taylor GP. Human T-lymphotropic virus/HIV co-infection: a clinical review. Curr Opin Infect Dis. (2014) 27:16–28. doi: 10.1097/QCO.0000000000000027
32. Castro LS, Rezende GR, Fernandes FRP, Bandeira LM, Puga MAM, Tanaka TSO, et al. Human T cell lymphotropic virus type 1 infection among men who have sex with men in Central Brazil. Braz J Infect Dis. (2018) 22:472–6. doi: 10.1016/j.bjid.2018.11.003
33. Alencar SP, Souza MC, Fonseca RRS, Menezes CR, Azevedo VN, Ribeiro ALR, et al. Prevalence and molecular epidemiology of human T-lymphotropic virus (HTLV) infection in people living with HIV/AIDS in the Para State, Amazon Region of Brazil. Front Microbiol. (2020) 11:572381. doi: 10.3389/fmicb.2020.572381
34. Vallinoto AC, Azevedo VN, Santos DE, Caniceiro S, Mesquita FC, Hall WW, et al. Serological evidence of HTLV-I and HTLV-II coinfections in HIV-1 positive patients in Belem, state of Para, Brazil. Mem Inst Oswaldo Cruz. (1998) 93:407–9. doi: 10.1590/S0074-02761998000300026
35. Broutet N, de Queiroz Sousa A, Basilio FP, Sa HL, Simon F, Dabis F. Prevalence of HIV-1, HIV-2 and HTLV antibody, in Fortaleza, Ceara, Brazil, 1993-1994. Int J STD AIDS. (1996) 7:365–9. doi: 10.1258/0956462961918103
36. Soares CC, Georg I, Lampe E, Lewis L, Morgado MG, Nicol AF, et al. HIV-1, HBV, HCV, HTLV, HPV-16/18, and Treponema pallidum infections in a sample of Brazilian men who have sex with men. PLoS ONE. (2014) 9:e102676. doi: 10.1371/journal.pone.0102676
37. de Araujo AC, Casseb JS, Neitzert E, de Souza ML, Mammano F, Del Mistro A, et al. HTLV-I and HTLV-II infections among HIV-1 seropositive patients in Sáo Paulo, Brazil. Eur J Epidemiol. (1994) 10:165–71. doi: 10.1007/BF01730366
38. Barcellos NT, Fuchs SC, Mondini LG, Murphy EL. Human T lymphotropic virus type I/II infection: prevalence and risk factors in individuals testing for HIV in counseling centers from Southern Brazil. Sex Transm Dis. (2006) 33:302–6. doi: 10.1097/01.olq.0000194598.47821.b6
39. Galetto LR, Lunge VR, Beria JU, Tietzmann DC, Stein AT, Simon D. Short communication: Prevalence and risk factors for human T cell lymphotropic virus infection in Southern Brazilian HIV-positive patients. AIDS Res Hum Retroviruses. (2014) 30:907–11. doi: 10.1089/aid.2013.0210
40. Ouedraogo HG, Kouanda S, Grosso A, Compaore R, Camara M, Dabire C, et al. Hepatitis B, C, and D virus and human T-cell leukemia virus types 1 and 2 infections and correlates among men who have sex with men in Ouagadougou, Burkina Faso. Virol J. (2018) 15:194. doi: 10.1186/s12985-018-1110-8
41. Rodriguez EM, de Moya EA, Guerrero E, Monterroso ER, Quinn TC, Puello E, et al. HIV-1 and HTLV-I in sexually transmitted disease clinics in the Dominican Republic. J Acquir Immune Defic Syndr (1988). (1993) 6:313–8.
42. Paulino-Ramirez R, Tapia L, Ruiz-Matuk C, Charow R, Budhwani H, Routy JP. Human T-cell lymphotropic virus 1/2 and human immunodeficiency virus antibodies identification among transactional sex workers and drug users in the Dominican Republic. Trans R Soc Trop Med Hyg. (2019) 113:293–7. doi: 10.1093/trstmh/trz012
43. Murphy EL, Gibbs WN, Figueroa JP, Bain B, LaGrenade L, Cranston B, et al. Human immunodeficiency virus and human T-lymphotropic virus type I infection among homosexual men in Kingston, Jamaica. J Acquir Immune Defic Syndr (1988). (1988) 1:143–9.
44. La Rosa AM, Zunt JR, Peinado J, Lama JR, Ton TG, Suarez L, et al. Retroviral infection in Peruvian men who have sex with men. Clin Infect Dis. (2009) 49:112–7. doi: 10.1086/599609
45. Zurita S, Costa C, Watts D, Indacochea S, Campos P, Sanchez J, et al. Prevalence of human retroviral infection in Quillabamba and Cuzco, Peru: a new endemic area for human T cell lymphotropic virus type 1. Am J Trop Med Hyg. (1997) 56:561–5. doi: 10.4269/ajtmh.1997.56.561
46. Juscamaita Z, Torrealva M, Cairampoma R, Gotuzzo E. Seroprevalencia del virus linfotropo T humano tipo 1 (HTLV-1) en gestantes y grupos de elevada prevalencia para enfermedades de transmisión sexual de Ayacucho, Perú. Revista Peruana de Medicina Experimental y Salud Publica. (2004) 21.
47. Bartholomew C, Saxinger WC, Clark JW, Gail M, Dudgeon A, Mahabir B, et al. Transmission of HTLV-I and HIV among homosexual men in Trinidad. JAMA. (1987) 257:2604–8. doi: 10.1001/jama.1987.03390190082024
48. Berini CA, Pando MA, Bautista CT, Eirin ME, Martinez-Peralta L, Weissenbacher M, et al. HTLV-1/2 among high-risk groups in Argentina: molecular diagnosis and prevalence of different sexual transmitted infections. J Med Virol. (2007) 79:1914–20. doi: 10.1002/jmv.21036
49. Pampuro SE, Rabinovich RD, Martinez Peralta L, Gallo D, Hanson C, Libonatti O. Presence of human T-cell lymphotropic virus types I and II and coinfection with human immunodeficiency virus in different groups at risk in Argentina. J Acquir Immune Defic Syndr (1988). (1993) 6:851–2.
50. Gastaldello R, Hall WW, Gallego S. Seroepidemiology of HTLV-I/II in Argentina: an overview. J Acquir Immune Defic Syndr. (2004) 35:301–8. doi: 10.1097/00126334-200403010-00012
51. Nicholson SR, Efandis T, Dimitrakakis M, Karopoulos A, Lee H, Gust ID, et al. infection in selected populations in Australia and the western Pacific region. Med J Aust. (1992) 156:878–80. doi: 10.5694/j.1326-5377.1992.tb137167.x
52. Janier M, Agbalika F, Lassau F, Pezin P, Ferchal F, Timsit F, et al. (Prevalence of HIV-1, HIV-2 and HTLV-1 infections. Experience in a Parisian center for sexually transmitted diseases. Presse Med. (1990) 19:1747–50.
53. Prasetyo AA, Ariapramuda R, Kindi EA, Dirgahayu P, Sari Y, Dharmawan R, et al. Men having sex with men in Surakarta, Indonesia: demographics, behavioral characteristics and prevalence of blood borne pathogens. Southeast Asian J Trop Med Public Health. (2014) 45:1032–47.
54. Giuliani M, Rezza G, Lepri AC, Di Carlo A, Maini A, Crescimbeni E, et al. Risk factors for HTLV-I and II in individuals attending a clinic for sexually transmitted diseases. Sex Transm Dis. (2000) 27:87–92. doi: 10.1097/00007435-200002000-00006
55. Manca N, Caruso A, Bonfanti C, Savoldi E, Li Vigni R, Pirali F, et al. HTLV-1 seroprevalence in AIDS patients and in HIV-1 seropositive and seronegative subjects at risk for AIDS in northern Italy. Eur J Epidemiol. (1989) 5:37–41. doi: 10.1007/BF00145042
56. Zehender G, Colasante C, De Maddalena C, Bernini F, Savasi V, Persico T, et al. High prevalence of human T-lymphotropic virus type 1 (HTLV-1) in immigrant male-to-female transsexual sex workers with HIV-1 infection. J Med Virol. (2004) 74:207–15. doi: 10.1002/jmv.20165
57. Chironna M, Calabro ML, Quarto M, Germinario C, Fiore JR, Favero A, et al. HTLV-I and HTLV-II infections in subjects at risk for HIV-I infection from southeastern Italy (Apulia region). Int J Cancer. (1996) 65:746-50. doi: 10.1002/(SICI)1097-0215(19960315)65:6<746::AID-IJC6>3.0.CO;2-Z
58. Guerena-Burgueno F, Benenson AS, Sepulveda-Amor J, Ascher MS, Vugia DJ, Gallo D. Prevalence of human T cell lymphotropic virus types 1 and 2 (HTLV-1/2) in selected Tijuana subpopulations. Am J Trop Med Hyg. (1992) 47:127–32. doi: 10.4269/ajtmh.1992.47.127
59. Vazquez-Valls E, Campos-Lopez PI, Torres-Mendoza BM, Alvarez-Maya I, Gonzalez-Mendoza A. Prevalence of anti-HTLV-I antibodies in HIV-seropositive individuals in Guadalajara, Mexico. J Acquir Immune Defic Syndr (1988). (1993) 6:965–6.
60. Zapata-Benavides P, Lara-Rodriguez MA, Alcocer-Gonzalez JM, Rodriguez-Padilla C, Tamez-Guerra R, Trejo-Avila LM. Seroprevalence of HTLV-I/II in different groups at risk in northeast Mexico. Vox Sang. (1996) 70:181–2. doi: 10.1111/j.1423-0410.1996.tb01321.x
61. Gongora-Biachi RA, Gonzalez-Martinez P, Puerto FI, Sosa-Munoz J, Duarte-Zapata L, Bastarrachea-Ortiz J, et al. A low prevalence of HTLV-I/-II infection among eight population groups from Merida Yucatan, Mexico. J Acquir Immune Defic Syndr (1988). (1992) 5:104–6.
62. Castro-Sansores CJ, Santos-Rivero A, Gonzalez-Martinez P, Lara-Perera DM, Alonso-Salomon G, Gongora-Biachi RA. Co-infection by the human T-cell lymphotropic virus type II in patients infected by the human immunodeficiency virus in Yucatan, Mexico. Arch Med Res. (2006) 37:365–9. doi: 10.1016/j.arcmed.2005.06.013
63. Goudsmit J, de Wolf F, van de Wiel B, Smit L, Bakker M., Albrecht-van Lent N, et al. Spread of human T-cell leukemia virus (HTLV-I) in the Dutch homosexual community. J Med Virol. (1987) 23:115–21. doi: 10.1002/jmv.1890230204
64. Zoulek G, Schatzl H, Kawabata M, de Cabral MB, Cabello A, Freutsmiedl K, et al. A seroepidemiological survey of antibodies to HTLV-I/HTLV-II in selected population groups in Paraguay. Scand J Infect Dis. (1992) 24:397–8. doi: 10.3109/00365549209061351
65. Sng EH, Thirumoorthy T, Levin A, Alexander S, Sng I, Blattner W. Evidence of HTLV-I infection in Singapore prostitutes. Int J STD AIDS. (1991) 2:172–5. doi: 10.1177/095646249100200304
66. Soriano V, Tor J, Ribera A, Clotet B, Muga R, Pauplana M, et al. Evidence of HTLV-1 infection in different groups at risk in Barcelona. Med Clin (Barc). (1990) 94:5–8.
67. Chungue E, Burucoa C, Cartel JL, Roux J, Gras C. Serological survey of human T-cell leukaemia virus type I in homosexual men in Tahiti. Med J Aust. (1989) 150:349. doi: 10.5694/j.1326-5377.1989.tb136511.x
68. Feigal E, Murphy E, Vranizan K, Bacchetti P, Chaisson R, Drummond JE, et al. Human T cell lymphotropic virus types I and II in intravenous drug users in San Francisco: risk factors associated with seropositivity. J Infect Dis. (1991) 164:36–42. doi: 10.1093/infdis/164.1.36
69. Aboulafia DM, Mitsuyasu RT, Slamon DJ. Human T-cell leukemia virus infection in non-intravenous drug using HIV seropositive men in Los Angeles. AIDS Res Hum Retroviruses. (1991) 7:333–6. doi: 10.1089/aid.1991.7.333
70. Meyer RD, Moudgil T, Detels R, Phair JP, Hirsch MS, Ho DD. Seroprevalence of human T cell leukemia viruses in selected populations of homosexual men. J Infect Dis. (1990) 162:1370–2. doi: 10.1093/infdis/162.6.1370
71. Manns A, Obrams I, Detels R, Diwan A, Ginzburg HM, Goedert JJ, et al. Seroprevalence of human T-cell lymphotropic virus type 1 among homosexual men in the United States. N Engl J Med. (1988) 319:516–7. doi: 10.1056/NEJM198808253190811
72. Chang KS, Wang LC, Gao CL, Alexander S, Ting RC, Bodner A., et al. Concomitant infection of HTLV-I and HIV-1: prevalence of IgG and IgM antibodies in Washington, DC area. Eur J Epidemiol. (1988) 4:426–34. doi: 10.1007/BF00146393
73. Eusebio-Ponce E, Candel FJ, Anguita E. Human T-cell lymphotropic virus type 1 and associated diseases in Latin America. Trop Med Int Health. (2019) 24:934–53. doi: 10.1111/tmi.13278
74. Fox JM, Mutalima N, Molyneux E, Carpenter LM, Taylor GP, Bland M, et al. Seroprevalence of HTLV-1 and HTLV-2 amongst mothers and children in Malawi within the context of a systematic review and meta-analysis of HTLV seroprevalence in Africa. Trop Med Int Health. (2016) 21:312–24. doi: 10.1111/tmi.12659
75. Belza MJ, Spanish Group for the Unlinked Anonymous Survey of HIVSiSTDP. Prevalence of HIV, HTLV-I and HTLV-II among female sex workers in Spain, 2000-2001. Eur J Epidemiol. (2004) 19:279–82. doi: 10.1023/B:EJEP.0000020443.29480.d0
76. Khabbaz RF, Darrow WW, Hartley TM, Witte J, Cohen JB, French J, et al. Seroprevalence and risk factors for HTLV-I/II infection among female prostitutes in the United States. JAMA. (1990) 263:60–4. doi: 10.1001/jama.1990.03440010058030
77. Khabbaz RF, Onorato IM, Cannon RO, Hartley TM, Roberts B, Hosein B, et al. Seroprevalence of HTLV-1 and HTLV-2 among intravenous drug users and persons in clinics for sexually transmitted diseases. N Engl J Med. (1992) 326:375–80. doi: 10.1056/NEJM199202063260604
78. Hohn O, Norley S, Kucherer C, Bazarbachi A, El Hajj H, Marcus U, et al. No significant HTLV seroprevalence in German people who inject drugs. PLoS ONE. (2017) 12:e0183496. doi: 10.1371/journal.pone.0183496
79. Satake M, Iwanaga M, Sagara Y, Watanabe T, Okuma K, Hamaguchi I. Incidence of human T-lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: a nationwide retrospective cohort analysis. Lancet Infect Dis. (2016) 16:1246–54. doi: 10.1016/S1473-3099(16)30252-3
80. Nunes D, Boa-Sorte N, Grassi MF, Taylor GP, Teixeira MG, Barreto ML, et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS ONE. (2017) 12:e0171303. doi: 10.1371/journal.pone.0171303
81. Moriuchi M, Moriuchi H. Seminal fluid enhances replication of human T-cell leukemia virus type 1: implications for sexual transmission. J Virol. (2004) 78:12709–11. doi: 10.1128/JVI.78.22.12709-12711.2004
82. Zunt JR, Dezzutti CS, Montano SM, Thomas KK, Alarcon JO, Quijano E, et al. Cervical shedding of human T cell lymphotropic virus type I is associated with cervicitis. J Infect Dis. (2002) 186:1669–72. doi: 10.1086/345364
83. Iga M, Okayama A, Stuver S, Matsuoka M, Mueller N, Aoki M, et al. Genetic evidence of transmission of human T cell lymphotropic virus type 1 between spouses. J Infect Dis. (2002) 185:691–5. doi: 10.1086/339002
84. Caswell RJ, Nall P, Boothby M, Taylor GP. Rapid onset and progression of myelopathy following an STI: a case for screening? Sex Transm Infect. (2019) 95:244–5. doi: 10.1136/sextrans-2019-053978
85. Gout O, Baulac M, Gessain A, Semah F, Saal F, Peries J, et al. Rapid development of myelopathy after HTLV-I infection acquired by transfusion during cardiac transplantation. N Engl J Med. (1990) 322:383–8. doi: 10.1056/NEJM199002083220607
86. Stuver SO, Tachibana N, Okayama A, Shioiri S, Tsunetoshi Y, Tsuda K, et al. Heterosexual transmission of human T cell leukemia/lymphoma virus type I among married couples in southwestern Japan: an initial report from the Miyazaki Cohort Study. J Infect Dis. (1993) 167:57–65. doi: 10.1093/infdis/167.1.57
87. Roucoux DF, Wang B, Smith D, Nass CC, Smith J, Hutching ST, et al. A prospective study of sexual transmission of human T lymphotropic virus (HTLV)-I and HTLV-II. J Infect Dis. (2005) 191:1490–7. doi: 10.1086/429410
88. Stuver SO, Mueller NE. Re: “Sexual transmission of human T-lymphotropic virus type I among female prostitutes and among patients with sexually transmitted diseases in Fukuoka, Kyushu, Japan”. Am J Epidemiol. (1995) 142:1247–8. doi: 10.1093/oxfordjournals.aje.a117586
89. Figueroa JP, Ward E, Morris J, Brathwaite AR, Peruga A, Blattner W, et al. Incidence of HIV and HTLV-1 infection among sexually transmitted disease clinic attenders in Jamaica. J Acquir Immune Defic Syndr Hum Retrovirol. (1997) 15:232–7. doi: 10.1097/00042560-199707010-00007
90. Matsushita S, Mitsuya H, Reitz MS, Broder S. Pharmacological inhibition of in vitro infectivity of human T lymphotropic virus type I. J Clin Invest. (1987) 80:394–400. doi: 10.1172/JCI113085
91. Macchi B, Faraoni I, Zhang J, Grelli S, Favalli C, Mastino A, et al. AZT inhibits the transmission of human T cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J Gen Virol. (1997) 78:1007–16. doi: 10.1099/0022-1317-78-5-1007
92. Zhang J, Balestrieri E, Grelli S, Matteucci C, Pagnini V, D'Agostini C, et al. Efficacy of 3'-azido 3'deoxythymidine (AZT) in preventing HTLV-1 transmission to human cord blood mononuclear cells. Virus Res. (2001) 78:67–78. doi: 10.1016/S0168-1702(01)00285-4
93. Balestrieri E, Sciortino MT, Mastino A, Macchi B. Protective effect of the acyclic nucleoside phosphonate tenofovir toward human T-cell leukemia/lymphotropic virus type 1 infection of human peripheral blood mononuclear cells in vitro. Antiviral Res. (2005) 68:154–62. doi: 10.1016/j.antiviral.2005.09.001
94. Hill SA, Lloyd PA, McDonald S, Wykoff J, Derse D. Susceptibility of human T cell leukemia virus type I to nucleoside reverse transcriptase inhibitors. J Infect Dis. (2003) 188:424–7. doi: 10.1086/376531
95. Garcia-Lerma JG, Nidtha S, Heneine W. Susceptibility of human T cell leukemia virus type 1 to reverse-transcriptase inhibitors: evidence for resistance to lamivudine. J Infect Dis. (2001) 184:507–10. doi: 10.1086/322785
96. Isono T, Ogawa K, Seto A. Antiviral effect of zidovudine in the experimental model of adult T cell leukemia in rabbits. Leuk Res. (1990) 14:841–7. doi: 10.1016/0145-2126(90)90172-6
97. Miyazato P, Yasunaga J, Taniguchi Y, Koyanagi Y, Mitsuya H, Matsuoka M, et al. novo human T-cell leukemia virus type 1 infection of human lymphocytes in NOD-SCID, common gamma-chain knockout mice. J Virol. (2006) 80:10683–91. doi: 10.1128/JVI.01009-06
98. Afonso PV, Mekaouche M, Mortreux F, Toulza F, Moriceau A, Wattel E, et al. Highly active antiretroviral treatment against STLV-1 infection combining reverse transcriptase and HDAC inhibitors. Blood. (2010) 116:3802–8. doi: 10.1182/blood-2010-02-270751
99. Taylor GP, Hall SE, Navarrete S, Michie CA, Davis R, Witkover AD, et al. Effect of lamivudine on human T-cell leukemia virus type 1 (HTLV-1) DNA copy number, T-cell phenotype, and anti-tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J Virol. (1999) 73:10289–95. doi: 10.1128/JVI.73.12.10289-10295.1999
100. Gill PS, Harrington W. Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. (1995) 332:1744–8. doi: 10.1056/NEJM199506293322603
101. Cook LB, Fuji S, Hermine O, Bazarbachi A, Ramos JC, Ratner L, et al. Revised adult T-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol. (2019) 37:677–87. doi: 10.1200/JCO.18.00501
102. Cook LB, Rowan AG, Demontis MA, Sagawe S, Gillet NA, Melamed A, et al. Long-term clinical remission maintained after cessation of zidovudine and interferon-alpha therapy in chronic adult T-cell leukemia/lymphoma. Int J Hematol. (2018) 107:378–82. doi: 10.1007/s12185-017-2361-7
103. Macchi B, Balestrieri E, Frezza C, Grelli S, Valletta E, Marcais A, et al. Quantification of HTLV-1 reverse transcriptase activity in ATL patients treated with zidovudine and interferon-alpha. Blood Adv. (2017) 1:748–52. doi: 10.1182/bloodadvances.2016001370
104. Tsukasaki K, Marcais A, Nasr R, Kato K, Fukuda T, Hermine O, et al. Diagnostic approaches and established treatments for adult T cell leukemia lymphoma. Front Microbiol. (2020) 11:1207. doi: 10.3389/fmicb.2020.01207
105. Balestrieri E, Forte G, Matteucci C, Mastino A, Macchi B. Effect of lamivudine on transmission of human T-cell lymphotropic virus type 1 to adult peripheral blood mononuclear cells in vitro. Antimicrob Agents Chemother. (2002) 46:3080–3. doi: 10.1128/AAC.46.9.3080-3083.2002
106. Pasquier A, Alais S, Roux L, Thoulouze MI, Alvarez K, Journo C, et al. How to control HTLV-1-associated diseases: preventing de novo cellular infection using antiviral therapy. Front Microbiol. (2018) 9:278. doi: 10.3389/fmicb.2018.00278
107. Toro C, Rodes B, Mendoza C, Soriano V. Lamivudine resistance in human T-cell leukemia virus type 1 may be due to a polymorphism at codon 118 (V–>I) of the reverse transcriptase. Antimicrob Agents Chemother. (2003) 47:1774. doi: 10.1128/AAC.47.5.1774-1775.2003
108. Taylor GP, Goon P, Furukawa Y, Green H, Barfield A, Mosley A, et al. Zidovudine plus lamivudine in Human T-Lymphotropic Virus type-I-associated myelopathy: a randomised trial. Retrovirology. (2006) 3:63. doi: 10.1186/1742-4690-3-63
109. Macchi B, Balestrieri E, Ascolani A, Hilburn S, Martin F, Mastino A, et al. Susceptibility of primary HTLV-1 isolates from patients with HTLV-1-associated myelopathy to reverse transcriptase inhibitors. Viruses. (2011) 3:469–83. doi: 10.3390/v3050469
110. Balestrieri E, Matteucci C, Ascolani A, Piperno A, Romeo R, Romeo G, et al. Effect of phosphonated carbocyclic 2'-oxa-3'-aza-nucleoside on human T-cell leukemia virus type 1 infection in vitro. Antimicrob Agents Chemother. (2008) 52:54–64. doi: 10.1128/AAC.00470-07
111. Barski MS, Minnell JJ, Maertens GN. Inhibition of HTLV-1 infection by HIV-1 first- and second-generation integrase strand transfer inhibitors. Front Microbiol. (2019) 10:1877. doi: 10.3389/fmicb.2019.01877
112. Anantharaman VV, Moen LK. Effects of nucleoside analogs on native and site-directed mutants of HTLV Type 1 reverse transcriptase. Bioorg Chem. (2000) 28:293–305. doi: 10.1006/bioo.2000.1181
113. Rabaaoui S, Zouhiri F, Lancon A, Leh H. d'Angelo J, Wattel E. Inhibitors of strand transfer that prevent integration and inhibit human T-cell leukemia virus type 1 early replication. Antimicrob Agents Chemother. (2008) 52:3532–41. doi: 10.1128/AAC.01361-07
114. Seegulam ME, Ratner L. Integrase inhibitors effective against human T-cell leukemia virus type 1. Antimicrob Agents Chemother. (2011) 55:2011–7. doi: 10.1128/AAC.01413-10
115. Trevino A, Parra P, Bar-Magen T, Garrido C, de Mendoza C, Soriano V. Antiviral effect of raltegravir on HTLV-1 carriers. J Antimicrob Chemother. (2012) 67:218–21. doi: 10.1093/jac/dkr404
116. Enose-Akahata Y, Billioux BJ, Azodi S, Dwyer J, Vellucci A, Ngouth N, et al. Clinical trial of raltegravir, an integrase inhibitor, in HAM/TSP. Ann Clin Transl Neurol. (2021) 8:1970–85. doi: 10.1002/acn3.51437
117. Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. (2016) 375:2133–43. doi: 10.1056/NEJMoa1602046
118. Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. (2016) 375:2121–32. doi: 10.1056/NEJMoa1506110
119. Marino-Merlo F, Balestrieri E, Matteucci C, Mastino A, Grelli S, Macchi B. Antiretroviral therapy in HTLV-1 infection: an updated overview. Pathogens. (2020) 9:342. doi: 10.3390/pathogens9050342
120. Soltani A, Hashemy SI, Zahedi Avval F, Soleimani A, Rafatpanah H, Rezaee SA, et al. Molecular targeting for treatment of human T-lymphotropic virus type 1 infection. Biomed Pharmacother. (2019) 109:770–8. doi: 10.1016/j.biopha.2018.10.139
121. Smerdon SJ, Jager J, Wang J, Kohlstaedt LA, Chirino AJ, Friedman JM, et al. Structure of the binding site for nonnucleoside inhibitors of the reverse transcriptase of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. (1994) 91:3911–5. doi: 10.1073/pnas.91.9.3911
122. Dewan MZ, Uchihara JN, Terashima K, Honda M, Sata T, Ito M, et al. Efficient intervention of growth and infiltration of primary adult T-cell leukemia cells by an HIV protease inhibitor, ritonavir. Blood. (2006) 107:716–24. doi: 10.1182/blood-2005-02-0735
123. Sohraby F, Aryapour H. Comparative analysis of the unbinding pathways of antiviral drug Indinavir from HIV and HTLV1 proteases by supervised molecular dynamics simulation. PLoS ONE. (2021) 16:e0257916. doi: 10.1371/journal.pone.0257916
124. Lockbaum GJ, Henes M, Talledge N, Rusere LN, Kosovrasti K, Nalivaika EA, et al. Inhibiting HTLV-1 Protease: A Viable Antiviral Target. ACS Chem Biol. (2021) 16:529–38. doi: 10.1021/acschembio.0c00975
125. Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. (2011) 477:95–8. doi: 10.1038/nature10347
126. Laydon DJ, Sunkara V, Boelen L, Bangham CRM, Asquith B. The relative contributions of infectious and mitotic spread to HTLV-1 persistence. PLoS Comput Biol. (2020) 16:e1007470. doi: 10.1371/journal.pcbi.1007470
127. Cook LB, Melamed A, Demontis MA, Laydon DJ, Fox JM, Tosswill JH, et al. Rapid dissemination of human T-lymphotropic virus type 1 during primary infection in transplant recipients. Retrovirology. (2016) 13:3. doi: 10.1186/s12977-015-0236-7
128. Moreno-Ajona D, Yuste JR, Martin P, Gallego Perez-Larraya J. HTLV-1 myelopathy after renal transplant and antiviral prophylaxis: the need for screening. J Neurovirol. (2018) 24:523–5. doi: 10.1007/s13365-018-0627-3
129. Roc L, de Mendoza C, Fernandez-Alonso M, Reina G, Soriano V, Spanish HN. Rapid subacute myelopathy following kidney transplantation from HTLV-1 donors: role of immunosuppresors and failure of antiretrovirals. Ther Adv Infect Dis. (2019) 6:2049936119868028. doi: 10.1177/2049936119868028
130. Armstrong MJ, Corbett C, Rowe IA, Taylor GP, Neuberger JM. HTLV-1 in solid-organ transplantation: current challenges and future management strategies. Transplantation. (2012) 94:1075–84. doi: 10.1097/TP.0b013e318263ad7a
131. Rosadas C, Taylor GP. Mother-to-Child HTLV-1 Transmission: Unmet Research Needs. Front Microbiol. (2019) 10:999. doi: 10.3389/fmicb.2019.00999
132. Cook L, Haddow J, Olavarria E, Ayto R, Wilkins A, Drysdale S editors. Managing human T-lymphotropic virus (HTLV) associated adult T-cell leukaemia/lymphoma in pregnancy and prevention of transmission to the neonate: the UK experience. 35th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID). (2017) Madrid.
133. Menna-Barreto M, HTLV-II. transmission to a health care worker. Am J Infect Control. (2006) 34:158–60. doi: 10.1016/j.ajic.2005.12.002
134. Goubau P, Carton H, Cornet P, Vercauteren G, Van Gompel A, De Vooght H, et al. Human T-cell lymphotropic virus type 1 infection and tropical spastic paraparesis in Belgian expatriates. J Med Virol. (1992) 36:13–5. doi: 10.1002/jmv.1890360103
135. Guidance: Human T-cell lymphotropic virus (HTLV) types 1 2: UK Health Security Agency. (2020). Available from: https://www.gov.uk/guidance/human-t-cell-lymphotropic-virus-htlv-types-1-and-2
Keywords: HTLV-1, PrEP (pre-exposure prophylaxis), antiretroviral (ARV), transmission prevention, integrase inhibitors, nucleoside reverse transcriptase inhibitor (NRTI), PEP (post-exposure prophylaxis)
Citation: Bradshaw D and Taylor GP (2022) HTLV-1 Transmission and HIV Pre-exposure Prophylaxis: A Scoping Review. Front. Med. 9:881547. doi: 10.3389/fmed.2022.881547
Received: 22 February 2022; Accepted: 22 March 2022;
Published: 29 April 2022.
Edited by:
Juarez Antonio Simões Quaresma, Universidade do Estado do Pará, BrazilReviewed by:
Fred Luciano Neves Santos, Gonçalo Moniz Institute (IGM), BrazilCopyright © 2022 Bradshaw and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Bradshaw, ZGFuaWVsLmJyYWRzaGF3MkBuaHMubmV0
 Daniel Bradshaw
Daniel Bradshaw Graham Philip Taylor
Graham Philip Taylor