- 1Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Obstetrics and Gynecology, Liuzhou Worker's Hospital, Liuzhou, China
- 3Department of Obstetrics and Gynecology, Panjin Central Hospital, Panjin, China
Background: Antimicrobial resistance to metronidazole has emerged after several decades of worldwide use of the drug. The purpose of this study was to evaluate the effectiveness, safety and population pharmacokinetics of morinidazole plus levofloxacin in adult women with pelvic inflammatory disease (PID).
Methods: Patients in 30 hospitals received a 14-day course of 500 mg intravenous morinidazole twice daily plus 500 mg of levofloxacin daily. A total of 474 patients were included in the safety analysis set (SS); 398 patients were included in the full analysis set (FAS); 377 patients were included in the per protocol set (PPS); 16 patients were included in the microbiologically valid (MBV) population.
Results: The clinical resolution rates in the FAS and PPS populations at the test of cure (TOC, primary effectiveness end point, 7–30 days post-therapy) visit were 81.91 and 82.49% (311/377), respectively. There were 332 patients who did not receive antibiotics before treatment, and the clinical cure rate was 82.83%. Among 66 patients who received antibiotics before treatment, 51 patients were clinically cured 7–30 days after treatment, with a clinical cure rate of 77.27%. The bacteriological success rate in the MBV population at the TOC visit was 87.5%. The minimum inhibitory concentration (MIC) values of morinidazole for use against these anaerobes ranged from 1 to 8 μg/mL. The rate of drug-related adverse events (AEs) was 27.43%, and no serious AEs or deaths occurred during the study.
Conclusions: The study showed that treatment with a 14-day course of intravenous morinidazole, 500 mg twice daily, plus levofloxacin 500 mg daily, was effective and safe. The results of this study were consistent with the results of a phase III clinical trial, which verified the effectiveness and safety of morinidazole.
Introduction
PID is a common acute inflammatory disease associated with the upper reproductive tract of women, and this condition includes endometritis, salpingitis, tubal and ovary abscess, and pelvic peritonitis (1). PID is mainly caused by the upward spread of lower genital tract infection or by a sexually transmitted infection; it is mainly observed in young sexually mature women, and the most frequent age of onset is 20~35 years old (2, 3). According to some epidemiological data in China, the incidence rate of PID among adult women who are sexually active is 2% or higher. Untreated or inadequately treated PID can have serious clinical consequences, including infertility, ectopic pregnancy, and chronic pelvic pain. Early diagnosis and broad-spectrum antibacterial therapy are important to reduce the risk of both short-term and long-term complications (4–6). In addition, the risks of PID also include a possible association with ovarian cancer (7).
Antibiotic therapy is the basis of PID therapy. The treatment regimens for PID should include empiric broad-spectrum therapy to cover a wide range of pathogens, but the optimal treatment regimens still have not been determined. Previous studies have confirmed that PID is caused by various microorganisms, including sexually transmitted organisms, such as Chlamydia trachomatis and Neisseria gonorrhoeae, as well as microorganisms that are present in the vaginal flora. Bacteroides fragilis is the most prevalent anaerobe in abdominal and pelvic infections. The significant regional changes in the antibiotic sensitivity that are associated with anaerobes directly lead to poor results. Some antibiotic regimens that were recommended in the 1970s−80s are no longer suitable for the empiric treatment of PID, such as clindamycin, cefoxitin, cefotetan, and fluoroquinolones (including moxifloxacin) (8–13). Metronidazole is included in the regimens that are recommended for targeting anaerobic bacteria. Antimicrobial resistance to metronidazole has emerged after several decades of worldwide use of the drug. In addition, systemic metronidazole treatment is associated with several adverse reactions, including gastrointestinal discomfort and adverse events associated with the nervous system (14–18). One goal of the use of the new nitroimidazoles is to minimize the side effects and improve patient adherence to treatment.
Morinidazole is a new generation of nitroimidazole drugs. The China Food and Drug Administration (CFDA) approved the use of morinidazole in February 2014. Morinidazole has the following characteristics: (1) Its antibacterial activity is higher in vitro than those of metronidazole, ornidazole, and other nitroimidazoles, (2) Its high water solubility prevents it from easily penetrating the blood–brain barrier, so the incidence of adverse reactions in the central nervous system is lower than those observed for metronidazole, ornidazole, and other nitroimidazoles, (3) The main metabolic pathway does not go through the CYP system, unlike the pathway involved in the metabolism of metronidazole, ornidazole, and other nitroimidazoles. Therefore, there is little influence of morinidazole use on liver function, and there is less drug interaction. The results of a phase III clinical trial showed that morinidazole had slightly better efficacy than that of ornidazole and significantly better efficacy than that of metronidazole for anaerobes isolated in clinical tests. The results of pharmacodynamic tests in vivo and in vitro indicated that the antimicrobial activity of morinidazole against isolated pathogenic anaerobes was stronger than or equal to those of metronidazole, tinidazole, and ornidazole (19).
This paper describes a multicenter, prospective, open-label phase IV trial (NCT03391440, clinical trial registered on ClinicalTrials.gov) evaluating the effectiveness, safety and population pharmacokinetics of morinidazole in the treatment of adult females with PID.
Methods
Design scheme
This was a multicenter, prospective, and open-label trial conducted in mainland China from February 2017 to July 2019 (September 2016 to December 2018 on ClinicalTrials.gov were estimated when registering). The trial was performed in strict accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines. Ethical approval was obtained from the Huazhong University of Science and Technology, Tongji Medical College Affiliated Tongji Hospital Medical Ethics Committee (2014S00156). We obtained full written informed consent from all of the participants before the trial was performed.
According to the results of the phase III trial of morinidazole, the sample size was estimated to be 375 based on an α-value of 2.5% (one-sided) and a power of 80%. Assuming an 80% validity rate, 469 women were planned to be included in the study.
Patients
The study enrolled women who were aged between 18 and 65 years old and who had PID (including endometritis, tubal phlogistic, oviduct ovarian abscess, pelvic peritonitis, etc.). The diagnosis of PID was based on the following symptoms: sexually active women or other patients with the risk of sexually transmitted infections; women with obvious lower abdominal tenderness, uterine tenderness, and/or adnexal and/or cervical motion tenderness on a biannual vaginal examination, accompanied by at least one of the following signs [from the China Guidelines for Diagnosis and Treatment of Pelvic Inflammatory Diseases (Revised Edition), 2014] (20): (A) pyrexia (axillary temperature ≥37.8°C); (B) mucopurulent cervical or vagina discharge; (C) an elevated white blood cell (WBC) count in vaginal discharge; (D) an elevated erythrocyte sedimentation rate; (E) an elevated C-reactive protein level; (F) cervical Chlamydia trachomatis infection confirmed by laboratory examination; and (G) a WBC count of ≥10×109/L. Patients with an allergy to nitroimidazole, patients who had antibiotic therapy for 3 days within the last week, patients with any condition likely to require surgery, patients with impaired liver or renal function, patients who received an abortion within the last month, or patients with severe systemic diseases likely to affect the therapy (e.g., cardiovascular abnormalities, severe neuropathy, or epilepsy) were excluded from this study.
Intervention
All participants received morinidazole (500 mg intravenous, twice daily for 14 days). Because of the diverse microflora involved in PID, levofloxacin was also administered to the participants. An intravenous infusion of 500 mg levofloxacin was administered once daily for the first week, followed by the oral administration of 500 mg levofloxacin tablets once daily for the second week.
The morinidazole and levofloxacin injections and levofloxacin tablets were provided free of charge by Shanghai Hansoh Biomedical Technology Co., Ltd. The morinidazole package was a 100 mL bottle containing 500 mg of morinidazole and 900 mg of sodium chloride. The batch No. used was NMPA Approval No. H20140022. The levofloxacin injection was produced by Jiangsu Hansoh Pharmaceutical Group Co., Ltd. The package was a 100 mL bottle containing 500 mg of levofloxacin and 900 mg of sodium chloride. The batch No. used was NMPA Approval No. H20041833. The levofloxacin tablets were produced by Jiangsu Hengrui Pharmaceutical Co., Ltd. The package contained 0.5 g/tablet. The batch No. used was NMPA Approval No. H20066387.
Patients attended the following study visits: a pre-treatment visit (3 days before the initiation of the study drug), an in-therapy visit (day 8), a first day post-therapy visit, and a TOC visit. The microbiology assessments were performed on cervical secretions that were taken from the pre-treatment visit, the first day post-therapy visit, and the TOC visit. The safety evaluations included patient compliance assessments, physical examinations and clinical laboratory assessments, which were performed at the pre-treatment visit, the in-therapy visit (day 8), the first day post-therapy visit and the TOC visit.
Population pharmacokinetic study
According to the results of an analysis of the classification of the study population, a total of 70 subjects were enrolled in pharmacokinetic analysis subsets (PKSS). Participation in the population pharmacokinetic study was voluntary after full informed consent was obtained, and the subjects were randomly assigned to five groups (using Central stochastic system IWRS).
To ensure the accuracy of the blood sampling time of the population pharmacokinetic subjects, the infusion time of morinidazole was 45 ± 5 min, the interval between the first dose and the second dose on the first day was 8 h ± 10 min, and the interval between the first dose on the first day and the first dose on the second day was 24 h ± 30 min. According to the population requirements for the pharmacokinetic study, 70 subjects were randomly divided into 5 groups. Four blood samples were collected from each subject (3 mL each). The blood sampling time points are shown in Table 1.
Analysis
The FAS group included all of the participants irrespective of the inclusion criteria. The PPS population was used for the effectiveness analysis and included all women who fulfilled the study inclusion criteria. The microbiologically valid (MBV) population (anaerobic cultures were performed in all subjects at the pre-treatment visit, but only subjects with positive anaerobic cultures were included in the MBV population) was included in the PPS population. The specimens from all of the patients were collected once during the screening period, the first day and 7–30 days after treatment for bacteriological examination. Each research center was responsible for the isolation, culture and identification of pathogenic bacteria.
The primary effectiveness indicator was the clinical response at the TOC visit in the PPS population. Clinical cure was defined as follows: an axillary temperature of ≤ 37.5°C, a WBC count of <10 × 109/L, and a modified McCormack Scale score reduction of >90% compared with baseline values (according to the diagnosis and treatment guidelines of PID, the minimum diagnostic criteria for PID is uterine and/or adnexal tenderness. Corresponding with the diagnostic criteria, the criteria for clinical cure of PID include a modified McCormack Scale score reduction of >90% compared with the baseline value. The McCormack scale was used in this study to assess the severity of PID based on the self-perception of the subjects. The modified scale scores were taken before treatment, on day 1 in-therapy, day 8 in-therapy, the first day and 7–30 days after treatment), and a significant improvement or the disappearance of cervical or vaginal purulent secretions. The secondary effectiveness endpoints were as follows: (1) the clinical response on the first day post-therapy and (2) the bacteriological response (at the TOC visit and the first day post-therapy). Microbiological cure was defined as a negative result on the second cervical secretion culture.
Statistical analysis
Statistical analysis was performed using SAS 9.4. The study mainly used descriptive statistics. The hypothesis test of the primary effectiveness indicator was one-sided and used an α = 0.025; all other statistical tests were two-sided and were performed at the 0.05 significance level.
Results
Patient and baseline characteristics
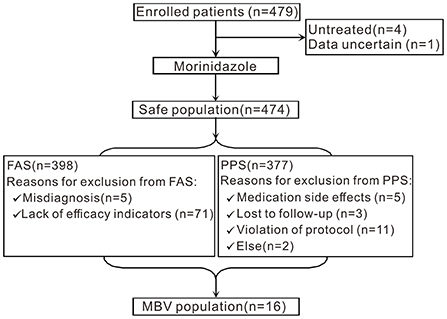
A flowchart of the study is shown in Figure 1. A total of 479 participants with PID from 30 centers were recruited for this study. Four patients were untreated, and 1 patient had ambiguous data. Therefore, 474 patients were included in the SS. A total of 398 patients were eligible for the FAS. Seventy-six patients were excluded from the FAS analyses (5 patients had a misdiagnosis, while the other 71 patients lacked effectiveness indicators). A total of 377 patients were eligible for the PPS analyses. Twenty-one patients were excluded from the PPS; among them, 5 patients developed medication side effects and 3 patients were lost to follow-up, and protocol violations occurred in 11 patients. Two patients were excluded for other reasons. Sixteen patients were included in the MBV population.
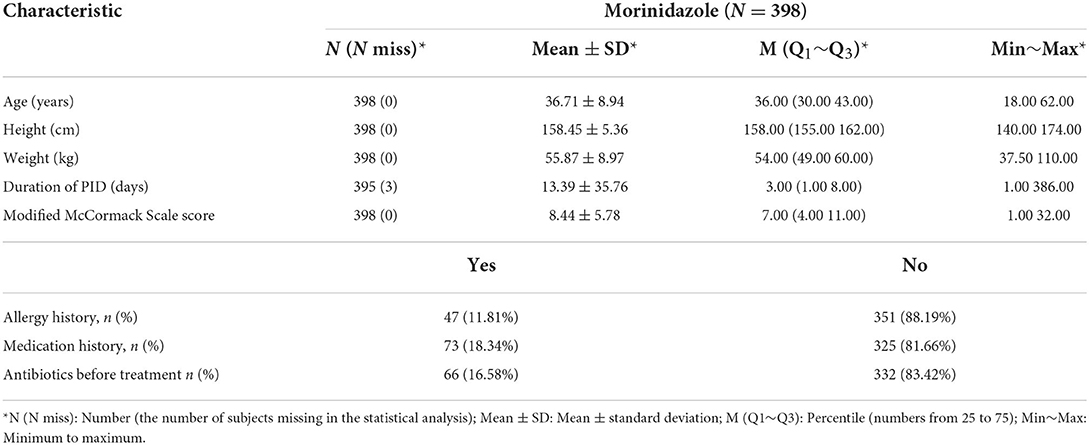
The demographic and baseline characteristics are shown in Table 2. The age of the subjects ranged from 18 to 62 years old, basically covering the whole range from youth to old, which is also the main demographic of PID patients. The duration from the onset of PID is as short as 1 day up to more than a year, representing acute, subacute, and chronic PID. As shown in Table 2, we observed that weight and height, which ranged from low to high, are good proxies for the different physical characteristics of the entire female population. The proportion of subjects with allergy history and drug history was 11.81 and 18.34%, respectively, and the proportion who had previously used antibiotics was 16.58%.
Effectiveness
Clinical response
Based on the clinical success criteria, the clinical resolution rate in the PPS population at the primary efficacy end point (TOC) was 82.49% (311/377). The clinical resolution rates for the FAS population were consistent with those for the PPS population. Among the 332 patients who did not receive antibiotics before treatment, 275 patients were clinically cured 7–30 days after treatment, and the clinical resolution rate was 82.83% (95% CI: 78.34–86.73%). Among the 66 patients who received antibiotics before treatment, 51 patients were clinically cured 7–30 days after treatment, with a clinical resolution rate of 77.27% (95% CI: 65.30–86.69%). According to the final results, the clinical resolution rate was less than 70% on the first day post-therapy, while the final clinical resolution rate reached 82.83%, which was considered to be related to the characteristics of the drug itself. Even in the patients who had previously taken other antibiotics with poor efficacy, the overall clinical effectiveness of morinidazole was satisfactory (Table 3).
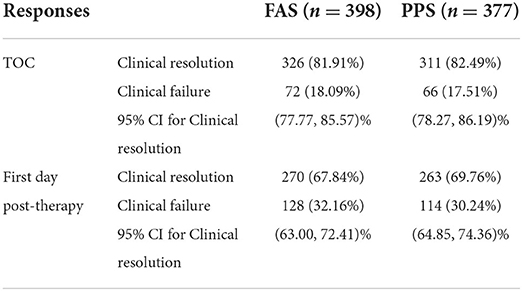
Table 3. Clinical responses of the PPS and FAS populations at the TOC visit and the first day post-therapy visit.
Bacteriological response
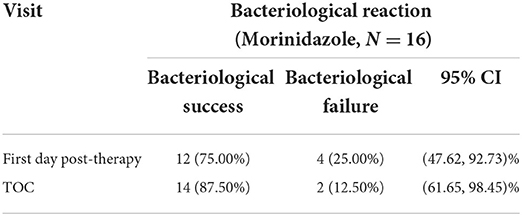
All patients had culture samples taken from the cervical canal. Due to the small number of strains extracted in a single trial, to improve the reliability of the analysis, we analyzed the anaerobic strains extracted from two clinical trials together. Eighty-one strains of anaerobes were isolated from 43 patients (including 27 patients from another trial of morinidazole in patients with appendicitis, NCT03380793, clinical trial registered on ClinicalTrials.gov). In the study of patients with appendicitis, samples were obtained from surgically removed appendix tissue or from abdominal pus. Samples from both studies were sent to the same testing unit for MIC testing. The purpose of bacteriological research was to evaluate whether clinical isolates were susceptible to morinidazole. The bacteriological success rate in the MBV population at the TOC visit was 87.5% (14/16) (Table 4). The most commonly identified anaerobes were Bacteroides fragilis (n = 19), Finegoldia magna (n = 5), Bacteroides thetaiotaomicron (n = 6), Prevotella bivia (n = 12), and Lactobacillus (n = 6) (Table 5). Most of the tested strains were sensitive to morinidazole. The minimum inhibitory concentration (MIC) values for morinidazole ranged from 1 to 8 μg/mL and were very close to those of ornidazole and metronidazole (Table 5).
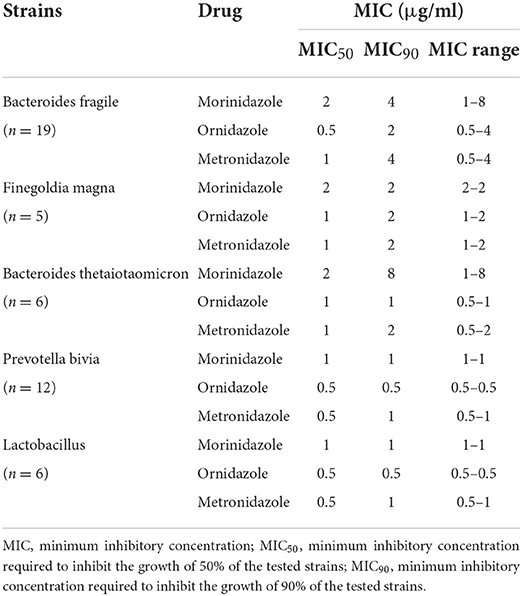
Table 5. The antibacterial activity (MIC) of morinidazole (compared with those of ornidazole and metronidazole) against anaerobes in vitro.
Improved McCormack scale score
In the FAS, the modified McCormack scale scores gradually decreased from day 8 in therapy to the first day after treatment and until 7–30 days after treatment, with reductions of 5.65 ± 4.43, 7.99 ± 5.86, and 8.08 ± 5.95, respectively. The modified McCormack scale scores at these three time points were compared with those obtained at baseline, and all of the differences were significant (P < 0.0001).
Population pharmacokinetic parameters
This analysis assesses the blood drug concentrations of the subjects based on PKSS. Descriptive statistics were calculated for the blood drug concentrations of the subjects at the planned blood collection time, and the average drug concentration-time curves (linear and semilogarithmic) were drawn. As stated in the report, approximately 0.75 h and 9.25 h after the start of the first infusion, the average blood concentration of morinidazole was approximately 13,000 ng/mL, where its concentration peaked, and the concentration observed after the second infusion was slightly higher than the first concentration. These data suggested that morinidazole has stable pharmacokinetics in the population (Figure 2).
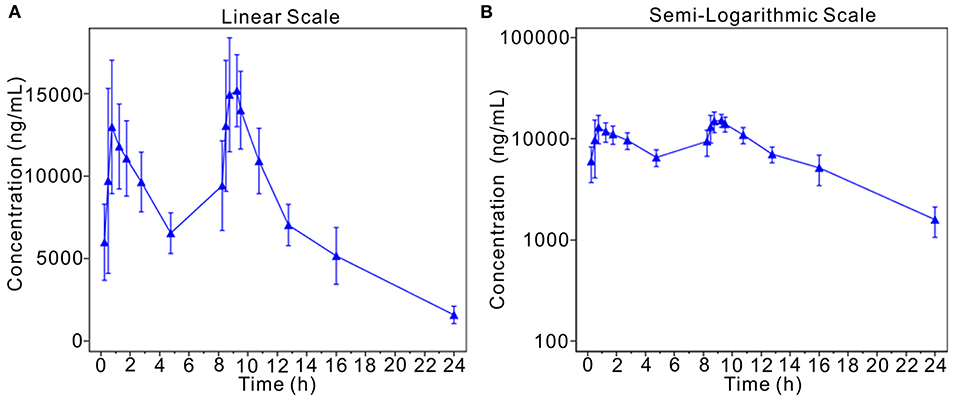
Figure 2. Mean plasma concentration-time curve (linear and semilogarithmic). The average drug concentration-time curves (A, linear; B, semilogarithmic).
Safety
In the FAS, the incidence of AEs was 44.73% (212/474), and the incidence of drug-related AEs was 27.43% (130/474). Most of the events were of mild to moderate severity. The most common drug-related AEs were nausea (5.91%), dizziness (3.80%), abdominal discomfort (2.32%), vomiting (2.11%), pruritus (1.90%), epigastric pain (1.69%), headache (1.69%), a decreased white blood cell count (1.69%), diarrhea (1.48%), flatulence (1.27%), and elevated blood creatinine levels (1.05%). Serious AEs occurred in 6 cases (1.27%), including somatization disorder, ovarian serous adenocarcinoma, adjustment disorder, gonorrhoeae-infected PID, endometrial polyp, and chronic gastroenteritis. Among them, 2 cases (somatization disorder and adjustment disorder) were judged to be probably unrelated to drugs, while the rest were unrelated. No serious drug-related AEs or deaths occurred during the study (Table 6).
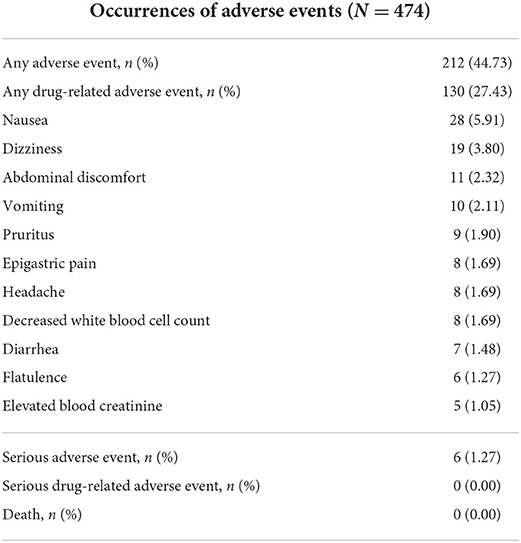
Table 6. Occurrences of adverse events, including the most common drug-related adverse events (>1%).
Discussion
PID is often a mixed infection. If there is an anaerobic infection and the patient is older, the patient is prone to multiple recurrences, usually accompanied by abscess formation, such that treatment is more problematic (20, 21). PID patients usually exhibit long-term sequelae, and some scholars believe that this is due to the formation of scars and adhesions during the healing of infected tissues. There are different views on the exact mechanism. For example, it is unknown whether it is related to the standard and rational use of antibiotics, or whether it is related to insufficient targeting of anaerobic bacteria by the antibiotics or resistance to antibiotics. However, the role of anaerobic bacteria in PID is still controversial.
Due to the difficulty associated with culturing anaerobic bacteria, the overall clinical detection rate was not high in previous studies. The rate of the detection of anaerobic bacteria in the genital tract is higher in patients with acute PID, especially for vaginal disease-related anaerobic bacteria (22–25). Even so, the isolation and cultivation of anaerobes is still difficult and requires a long time, and this process may not be able to guide clinical treatment within a reasonable amount of time, so anti-anaerobic drugs and anti-pathogenic drugs are commonly used in combination. The CDC currently recommends considering the addition of metronidazole in all outpatient treatments of PID patients and in patients who have BV, trichomoniasis or who were exposed to recent uterine instrumentation (26, 27).
The clinical resolution rate of the phase IV clinical trial was close to that achieved in the relevant treatment regimens reported in the previous literature (28–30). There were some differences in the combination of drugs used. In addition, the actual infection rate of anaerobes was relatively unclear, so a stratified analysis and other methods should be considered in future studies. Individualized medication should be given to patients who are highly suspected of having an anaerobic infection to improve the clinical resolution rate.
The trial showed that morinidazole was safe and well-tolerated. The drug-related AEs mainly occurred in the digestive system and nervous system, which were also common drug-related AEs observed in previous clinical practice (31, 32), and there were no unexpected drug-related AEs. The bacteriological evaluation showed that morinidazole had a similar antibacterial spectrum for targeting the tested anaerobic bacterial strains to those observed for ornidazole and metronidazole. Morinidazole had obvious antibacterial activity against the tested anaerobic bacterias, and the antibacterial effect against most of the tested strains was sensitive.
The phase IV trial had a larger sample size than did the phase III trial. Moreover, this trial involved 30 hospitals, covering not only most geographical areas in China but also people of different ages and ethnic groups. Our results represent the basic situation of PID in secondary and tertiary hospitals in China. The main purpose of phase IV clinical trials is to verify the effectiveness and safety of morinidazole, so we chose levofloxacin, which has a weak effect on anaerobic bacteria, as the combination drug. The trial protocol used in the phase IV trial was more consistent with the clinical situation and the requirements of the guidelines than the protocol used in the phase III trial, so we consider this study to have higher external validity. In addition, we used the same data collection format in all hospitals to improve consistency and reliability. Finally, all pathogenic bacteria were uniformly transported to the central laboratory for reverification and MIC determination. The MICs of the anaerobes were evaluated. The strains were kept in the central laboratory for inspection by a supervisory department, and the information collected from this study is important for implementing more relevant and effective intervention strategies for each area. Furthermore, because it is inconvenient to perform a gynecological examination during menstruation, we conducted an effectiveness evaluation 7 and 30 days after the end of treatment to rule out this situation to provide more objective data.
Some limitations of the study deserve consideration. First, 84 subjects dropped out (17.54%) from the trial. The main reasons for the loss of participants were early withdrawal because of adverse events and self-withdrawal or loss of patients to follow-up. However, the results still fall into the category of statistical acceptability. When possible, various measures should be taken to reduce the occurrence of these situations. Additionally, we noticed that the clinical resolution rate was slightly lower than that observed in the phase III trial. Whether the presence of anaerobic bacteria is universal in the pathogenesis of PID is unknown. There may be other confounding factors. Beyond these speculations, it is unknown whether the results were related to the different combinations of drugs used. More studies should be performed to explore the results. Additionally, the phase III and phase IV trials were conducted at different periods and in different settings and were not head-to-head studies; thus, it may not be easy to compare them directly. Finally, although the cervical secretions of each patient were collected and examined, the culture rate of anaerobes was generally low due to the high difficulty of culturing anaerobes.
This study was a multicenter, prospective, open-label phase IV trial evaluating the effectiveness, safety and population pharmacokinetics of morinidazole in the treatment of adult females with PID. The results showed that treatment with a 14-day course of intravenous morinidazole, 500 mg twice daily, plus levofloxacin 500 mg daily, was effective. At the same time, the bacteriological success rate was satisfactory, and there were few side effects. The results of this study were basically consistent with the results of a phase III clinical trial, which verified the effectiveness and safety of morinidazole. Therefore, we believe that morinidazole is a new drug option for patients with PID.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology approved the study. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TZ, MY, SW, and RL contributed to the conceptualization of the study. MY, JL, and FJ assisted in data collection. TZ and RL are responsible for the supervision of the clinical trial. RL has access to the final trial dataset. All authors have read and approved the manuscript for publication.
Funding
This study received funding from Jiangsu Hansoh Pharmaceuticals Limited. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
Acknowledgments
We would like to thank the following hospitals: LiuZhou Worker's Hospital; LiuZhou Maternity and Child Healthcare Hospital; Panjin Central Hospital; Yue Bei People's Hospital; Hebei General Hospital; Guangzhou Panyu Central Hospital; The Second Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine; Renmin Hospital of Wuhan University; Fifth People's Hospital of Shanghai; Affiliated Hospital of Guangdong Medical University; The First Affiliated Hospital of Hainan Medical College; Xiangyang Central Hospital; Chifeng Municipal Hospital; The Second Nanning People's Hospital; The Second Affiliated Hospital of Shantou University Medical College; Qinghai University Affiliated Hospital; Traditional Chinese Medicine Hospital of Xinjiang Uygur Autonomous Region; Anhui Provincial Cancer Hospital; The First People's Hospital of Foshan; The Second People's Hospital of Yibin; The Second Affiliated Hospital of Wenzhou Medical University; The Second Affiliated Hospital of Chongqing Medical University; The First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of Science and Technology; The Second Hospital of Shanxi Medical University; Huangshi Central Hospital; The First Affiliated Hospital of Xinjiang University; Yanbian University Hospital; The Affiliated Hospital of Shaanxi University of Traditional Chinese Medicine, and Jilin Province People's Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. (2015) 64:1–137. doi: 10.15585/mmwr.rr7004a1
2. Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. (2015) 372:2039–48. doi: 10.1056/NEJMra1411426
3. Greydanus DE, Cabral MD, Patel DR. Pelvic inflammatory disease in the adolescent and young adult: an update. Dis Mon. (2022) 68:101287. doi: 10.1016/j.disamonth.2021.101287
4. Ravel J, Moreno I, Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol. (2021) 224:251–7. doi: 10.1016/j.ajog.2020.10.019
5. Anyalechi GE, Hong J, Kreisel K, Torrone E, Boulet S, Gorwitz R, et al. Self-reported infertility and associated pelvic inflammatory disease among women of reproductive age-national health and nutrition examination survey, United States, 2013-2016. Sex Transm Dis. (2019) 46:446–51. doi: 10.1097/OLQ.0000000000000996
6. Soper DE. Pelvic inflammatory disease. Obstet Gynecol. (2020) 116:419–28. doi: 10.1097/AOG.0b013e3181e92c54
7. Jinlan Piao, Lee EJ, Lee M. Association between pelvic inflammatory disease and risk of ovarian cancer: an updated meta-analysis. Gynecol Oncol. (2020) 157:542–8. doi: 10.1016/j.ygyno.2020.02.002
8. Snydman DR, Jacobus NV, McDermott LA, Ruthazer R, Golan Y, Goldstein EJC, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. (2007) 51:1649–55. doi: 10.1128/AAC.01435-06
9. Mikamo H, Matsumizu M, Nakazuru Y, Nagashima M. Efficacy and safety of metronidazole injection for the treatment of infectious peritonitis, abdominal Abscess and pelvic inflammatory diseases in Japan. J Infect Chemother. (2015) 21:96–104. doi: 10.1016/j.jiac.2014.10.005
10. Wiesenfeld HC, Meyn LA, Darville T, Macio IS, Hillier SL. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. (2021) 72:1181–9. doi: 10.1093/cid/ciaa101
11. Bugg CW, Taira T. Pelvic inflammatory disease: diagnosis and treatment in the emergency department. Emerg Med Pract. (2016) 18:1–24.
12. Jamal W, Shahin M, Rotimi VO. Surveillance and trends of antimicrobial resistance among clinical isolates of anaerobes in Kuwait hospitals from 2002 to (2007). Anaerobe. (2010) 16:1–5. doi: 10.1016/j.anaerobe.2009.04.004
13. Petrina MAB, Cosentino LA, Wiesenfeld HC, Darville T, Hillier SL. Susceptibility of endometrial isolates recovered from women with clinical pelvic inflammatory disease or histological endometritis to antimicrobial agents. Anaerobe. (2019) 56:61–5. doi: 10.1016/j.anaerobe.2019.02.005
14. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. (2012) 56:1247–52. doi: 10.1128/AAC.05823-11
15. Fernández-Canigia L, Litterio M, Legaria MC, Castello L, Predari SC, Martino AD, et al. First national survey of antibiotic susceptibility of the Bacteroides fragilis group: emerging resistance to carbapenems in Argentina. Antimicrob Agents Chemother. (2012) 56:1309–14. doi: 10.1128/AAC.05622-11
16. Cereija TB, Castro J, Alves P, Cerca N. Influence of anaerobic conditions on vaginal microbiota recovery from bacterial vaginosis patients. Sex Transm Infect. (2013) 89:307. doi: 10.1136/sextrans-2013-051100
17. Duarte R, Fuhrich D, Ross JD. A review of antibiotic therapy for pelvic inflammatory disease. Int J Antimicrob Agents. (2015) 46:272–7. doi: 10.1016/j.ijantimicag.2015.05.004
18. Savaris RF, Fuhrich DG, Maissiat J, Duarte RV, Ross J. Antibiotic therapy for pelvic inflammatory disease. Cochrane Database Syst Rev. (2020) 20:CD010285. doi: 10.1002/14651858.CD010285.pub3
19. Cao C, Luo AY, Wu P, Weng DH, Zheng H, Wang SX. Efficacy and safety of morinidazole in pelvic inflammatory disease: results of a multicenter, double-blind, randomized trial. Eur J Clin Microbiol Infect Dis. (2017) 36:1225–30. doi: 10.1007/s10096-017-2913-z
20. Infectious Diseases Cooperation Group Chinese Chinese Society of Obstetrics and Gynecology Chinese Medical Association. Chin J Obstetr Gynecol. (2014) 49:401–3. doi: 10.3760/cma.j.issn.0529-567x.2014.06.001
21. Ross J, Guaschino S, Cusini M, Jensen J. 2017European guideline for the management of pelvic inflammatory disease. Int J STD AIDS. (2018) 29:108–14. doi: 10.1177/0956462417744099
22. Ross J. United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease. London: British Association for Sexual Health and HIV (2019).
23. Brun JL, Graessllin O, Fauconnier A, Verdon R, Agostini A, Bourret A, et al. Updated French guidelines for diagnosis and management of pelvic inflammatory disease. Int J Gynaecol Obstet. (2016) 134:121–5. doi: 10.1016/j.ijgo.2015.11.028
24. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. (2013) 40:117–22. doi: 10.1097/OLQ.0b013e31827c5a5b
25. Haggerty CL, Totten PA, Tang G, Astete SG, Ferris MJ, Norori J, et al. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect. (2016) 92:441–6. doi: 10.1136/sextrans-2015-052285
26. Dalby J, Stoner BP. Sexually transmitted infections: updates from the 2021 CDC guidelines. Am Fam Phys. (2022) 105:514–20.
27. Hazra A, Collison MW, Davis AM. CDC sexually transmitted infections treatment guidelines, 2021. JAMA. (2022) 327:870–1. doi: 10.1001/jama.2022.1246
28. Dean G, Soni S, Pitt R, Ross J, Sabin C, Whetham J. Treatment of mild-to-moderate pelvic inflammatory disease with a short-course azithromycin-based regimen versus ofloxacin plus metronidazole: results of a multicentre, randomised controlled trial. Sex Transm Infect. (2021) 97:177–82. doi: 10.1136/sextrans-2020-054468
29. Heystek M, Ross JD; PID Study Group. A randomized double-blind comparison of moxifloxacin and doxycycline/metronidazole/ciprofloxacin in the treatment of acute, uncomplicated pelvic inflammatory disease. Int J STD AIDS. (2009) 20:690–5. doi: 10.1258/ijsa.2008.008495
30. Aşicioglu O, Gungorduk K, Ozdemir A, Ertas IE, Yildirim G, Sanci M, et al. Single daily dose of moxifloxacin versus ofloxacin plus metronidazole as a new treatment approach to uncomplicated pelvic inflammatory disease: a multicentre prospective randomized trial. Eur J Obstet Gynecol Reprod Biol. (2013) 171:116–21. doi: 10.1016/j.ejogrb.2013.08.012
31. Judlin P, Liao Q, Liu Z, Reimnitz P, Hampel B, Arvis P. Efficacy and safety of moxifloxacin in uncomplicated pelvic inflammatory disease: the MONALISA study. BJOG. (2010) 117:1475–84. doi: 10.1111/j.1471-0528.2010.02687.x
Keywords: morinidazole, pelvic inflammatory disease, anaerobes, levofloxacin, safety
Citation: Zhou T, Yuan M, Cui P, Li J, Jia F, Wang S and Liu R (2022) Effectiveness and safety of morinidazole in the treatment of pelvic inflammatory disease: A multicenter, prospective, open-label phase IV trial. Front. Med. 9:888186. doi: 10.3389/fmed.2022.888186
Received: 02 March 2022; Accepted: 13 July 2022;
Published: 03 August 2022.
Edited by:
Aleksandra Barac, University of Belgrade, SerbiaReviewed by:
David Leitsch, Medical University of Vienna, AustriaTimothy Abiola Olusesan Oluwasola, University of Ibadan, Nigeria
Copyright © 2022 Zhou, Yuan, Cui, Li, Jia, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronghua Liu, TGl1emh1MzhAMTI2LmNvbQ==
 Ting Zhou
Ting Zhou Ming Yuan1
Ming Yuan1 Pengfei Cui
Pengfei Cui Shixuan Wang
Shixuan Wang Ronghua Liu
Ronghua Liu