- Department of Ophthalmology, The First Affiliated Hospital of Chongqing Medical University, Chongqing Key Laboratory of Ophthalmology and Chongqing Eye Institute, Chongqing, China
Purpose: This research aims to study the corneal morphological changes in adult patients with myopic anisometropia after small incision lenticule extraction (SMILE) and the safety, efficacy, and predictability of clinical outcomes.
Methods: This was a prospective cohort study. Patients with myopic anisometropia [refractive difference >2.0 diopters (D)] were included in this study who underwent SMILE at our hospital from September 2019 to March 2021. For the two eyes of each patient, the one with higher myopia was defined as group A, and the fellow eye was group B. The follow-up time points were set as 1 week, 1 month, 3 months, and 6 months after the surgery. The data collected were uncorrected and best-corrected distance visual acuity (UDVA and CDVA), spherical equivalent (SE), efficacy and safety indexes, posterior corneal elevation (PCE), anterior and posterior corneal radius of curvature in the 3 mm area at the center of the thinnest point of the cornea (ARC and PRC), and higher-order aberrations (HOAs).
Results: The study included 36 patients (72 eyes), and the mean age was 25.2 ± 6.4 years. The preoperative SEs were −6.45 ± 1.25 D in group A and −3.76 ± 1.29 D in group B. Six months after surgery, the SEs in groups A and B were −0.09 ± 0.50 D and 0.07 ± 0.47 (P = 0.059), respectively. The efficacy indexes were 1.06 ± 0.16 in group A and 1.07 ± 0.14 in group B (P = 0.750). The safety indexes were 1.08 ± 0.14 in group A and 1.12 ± 0.15 in group B (P = 0.173). The PCE was significantly reduced at 6 months after surgery in pagebreak both groups (P < 0.05). The ARC was significantly higher than before the surgery (P < 0.05) in the two groups. The two groups showed significant increases in total HOAs, coma 90°, and spherical aberrations (P < 0.05).
Conclusion: SMILE is predictable, effective, and safe in correcting myopic anisometropia. The postoperative changes in HOAs are characteristic.
Introduction
Anisometropia refers to the unequal refraction of two eyes. The mild differences in refraction do not cause clinical symptoms due to binocular fusion. However, obvious anisometropia may impair central fusion function, resulting in stereoscopic and contrast sensitivity dysfunction, asthenopia, and even strabismus or amblyopia (1). Anisometropia can be classified into physiological anisometropia and pathological anisometropia. Refractive differences of up to 1 diopter (D) are considered physiological (2), and more than 2.0 D are defined as pathological anisometropia (3).
Spectacles and contact lenses are the traditional treatment for anisometropia. With the advancement of refractive surgery, more and more people choose refractive surgery to correct anisometropia. Many studies have demonstrated that corneal refractive surgeries treat anisometropia safely and effectively. Common refractive surgeries include photorefractive keratectomy (PRK), laser in situ keratomileusis (LASIK), laser-assisted subepithelial keratectomy (LASEK), and small incision lenticule extraction (SMILE) (4–11). SMILE surgery is preferred for patients with anisometropia due to its safety, efficacy, predictability, and fewer valve-related complications (12–16).
Changed corneal morphology and higher-order aberrations (HOAs) after SMILE have been reported (17–19). However, it was rarely reported whether the corneal morphological changes after SMILE are characteristic in the two eyes of a patient with anisometropia. Therefore, this study investigated the corneal morphological changes in adult patients with myopic anisometropia after SMILE and the safety, efficacy, and predictability.
Patients and methods
This was a prospective cohort study. Patients with myopic anisometropia were included in this study who underwent SMILE at the Ophthalmology Department of the First Affiliated Hospital of Chongqing Medical University from September 2019 to March 2021. The study was conducted in compliance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Written informed consent for surgery was obtained from patients and their immediate family members.
Inclusion criteria
(1) Age ≥18 years; (2) binocular myopic anisometropia [spherical equivalent (SE) >2.0 D]; (3) corrected cylinder power ranging from −9 D to −0.25 D; (4) the best-corrected distance visual acuities (CDVA) in both eyes ≥20/25; (5) stable refractive status ≥2 years; and (6) normal corneal topography.
Exclusion criteria
(1) Corrected distance visual acuity in one or both eyes <20/25; (2) patients diagnosed with other ocular and systemic diseases or more likely to scar; (3) history of ocular surgery; (4) patients with severe dry eye or other cornea diseases; and (5) pregnant or lactating women.
Surgical methods
All the patients were administered levofloxacin eye drops (5 ml: 24.4 mg, Cravit®, Santen, Osaka, Japan) and sodium hyaluronate eye drops (10 ml: 0.1%, Hylo-comod®, RSAPHARM Arzneimittel GmbH, Industriestraße, Saarbrücken, Germany) four times a day, 3 days before the surgery. Intraoperatively, oxybuprocaine hydrochloride eye drops (20 ml: 80 mg, Benoxil®, Santen, Osaka, Japan) were administered twice for topical anesthesia. All SMILE surgeries were conducted using a VisuMax femtosecond laser system (Carl Zeiss Meditec AG, Jena, Germany). The femtosecond laser parameters were set as follows: pulse frequency, 500 kHz; pulse energy, 135 nJ; spot size, 4.3 μm; optical zone diameter, 6.0–6.7 mm; corneal cap diameter, 6.8–7.5 mm; cap thickness, 120 μm; incision length, 2.8 mm; position of incision, 90°; and the edge cutting angle, 90°. After the canning, a micro-separator was used to separate and lift the corneal cap’s edge and separate the lens’s front and back surfaces in turn. Then, micro tweezers were employed to remove the stromal lens from the small incision under the corneal cap, and the wholeness of the stromal lens was carefully checked. Extra water was absorbed by a sterile sponge, and the operation was completed.
Postoperative treatment
Tobramycin and dexamethasone eye drops (TobraDex®, Novartis, Rijksweg, Puurs) were administered immediately after the operation four times at 10 min intervals. Subsequently, the following eye drops were required to be applied concomitantly: 0.5% levofloxacin eye drops, four times a day for 21 days; tobramycin dexamethasone eye drops, four times a day for 1 week, and then replaced with loteprednol eye drops (5 ml: 0.5%, Lotemax®, Bausch & Lomb Incorporated, Tampa, FL, United States), three times a day for 1 week, then twice a day for 1 week, and finally once a day for 1 week; sodium hyaluronate eye drops, four times a day for 1 month.
Follow-up examinations
Before the operation, patients underwent a slip lamp examination for the anterior segment and a fundus examination using an indirect ophthalmoscope after dilatation of pupils using compound tropicamide eye drops (1 ml, Zhuobi’an®, Sinqi Pharmaceutical, Shenyang, China). Snellen chart was used to check the distance vision before and after the surgery; corneal topography was obtained by performing Pentacam® AXL panoramic biometer (Oculus GmbH, Wetzlar, Germany) by an experienced clinician. Image acquisition was performed on an automatic mode. A total of three images were obtained for each eye to collect the total corneal aberration data with a 6 mm diameter. Images with data over 8 mm diameter and quality marked as “OK” were selected for the data processing. Images with data equal to or less than 8 mm diameter or failed quality tests were excluded. Higher order aberration (HOA), coma aberration (Coma), trefoil aberration (Trefoil), and spherical aberration (SA) were analyzed by the root mean square (RMS, μm). Furthermore, the dilated and small pupil tests were conducted by the same experienced optometrist. SE was obtained by adding the sum of the sphere power with half of the cylinder power. Follow-up examinations were conducted at 1 week, 1 month, 3 months, and 6 months after the surgery, which included uncorrected distance visual acuity (UDVA), CDVA, diopters, effectiveness index (defined as preoperative CDVA/postoperative UDCA), safety index (defined as postoperative CDVA/preoperative CDVA), posterior corneal elevation (PCE), anterior and posterior corneal radius of curvature of the 3 mm area at the center of the thinnest point of the cornea (ARC and PRC), and the wavefront aberrations. A standardized pupil diameter of 6.0 mm was used to analyze aberration.
Statistical analysis
SPSS 17.0 (IBM Corp., Armonk, NY, United States) software was used to analyze data. Data were presented as “mean ± standard deviation.” Paired t-test was applied to compare the two groups of continuous normally distributed variables. Pearson Chi-squared test was used for intra-group comparison. P-value < 0.05 was considered statistically significant.
Results
Baseline information
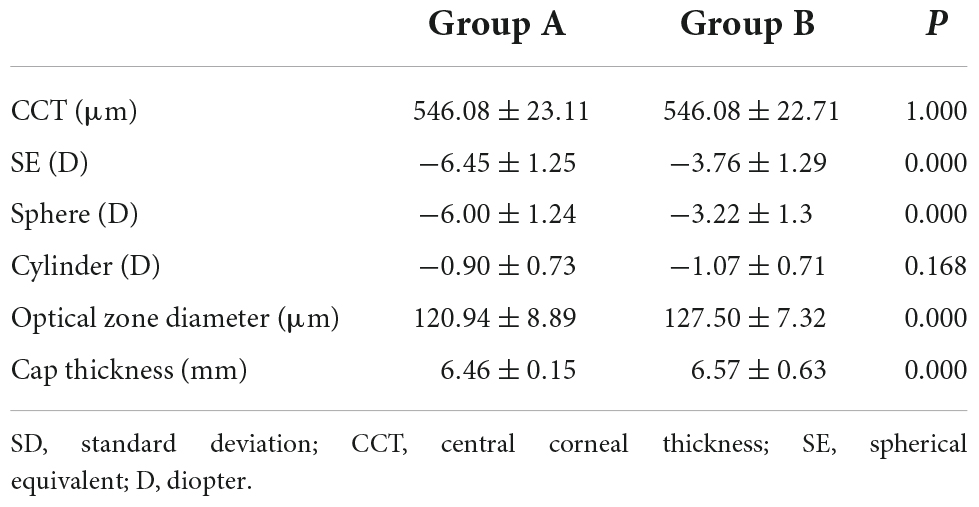
The study included 36 consecutive patients (mean age: 25.2 ± 6.4 years). Among them, 14 (38.9%) were males and 22 (61.1%) were females. For the two eyes of each patient, the one with higher myopia was defined as group A, and the fellow eye was group B. The preoperative refractive status and corneal thickness of both groups are presented in Table 1.
Efficacy and safety
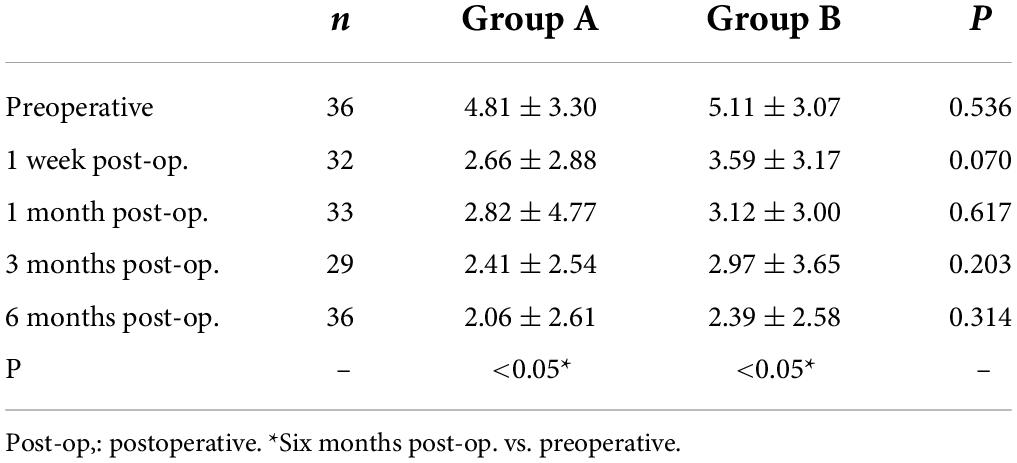
Preoperative CDVAs in groups A and B had no significant difference and were equal to or higher than 20/20. Six months after the surgery, the postoperative UDVAs were 20/32 or better in all the eyes from group A or group B. Among them, 34 eyes (94.4%) in group A and 34 eyes (94.4%) in group B had UDVAs of 20/20 or better, 26 eyes (72.2%) in group A and 27 eyes (75.0%) in group B had UDVAs of 20/16 or better (Figures 1A, 2A). The effectiveness indexes 6 months after surgery were 1.06 ± 0.16 in group A and 1.07 ± 0.14 in group B (P = 0.750).
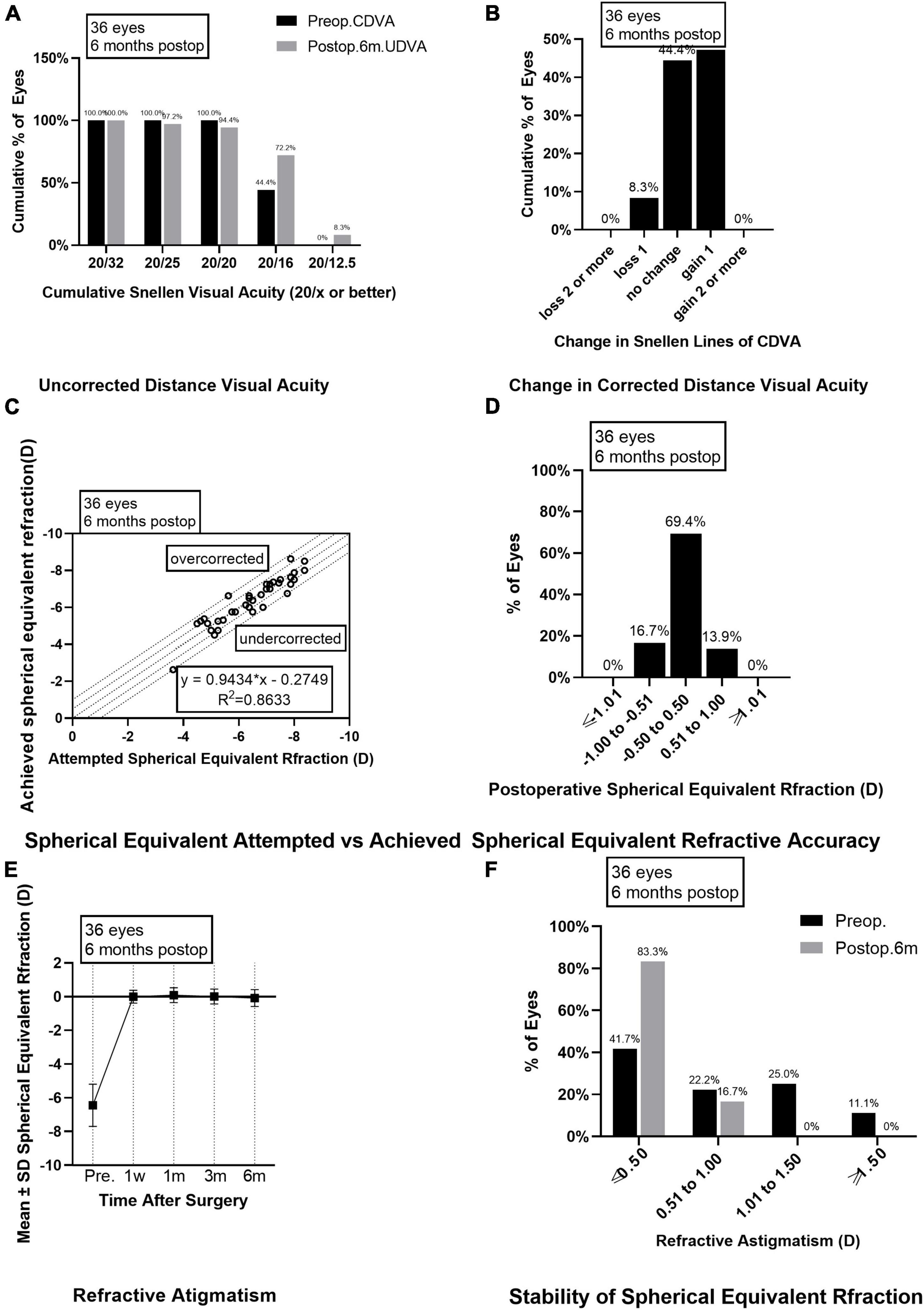
Figure 1. Outcomes of group A after SMILE surgery. (A) Cumulative percentage of preoperative CDVA and postoperative UDVA in group A at 6 months follow-up; (B) Change in CDVA in group A at 6 months follow-up; (C) Achieved versus attempted change in SE at 6 months follow-up in group A; (D) Accuracy of SE refraction in group A at 6 months follow-up; (E) Stability of SE in group A at 1 week, 1 month, 3 months, and 6 months after surgery; (F) The percentage of preoperative postoperative refractive astigmatism in group A at 6 months follow-up. CDVA, corrected distance visual acuity; UDVA, uncorrected distance visual acuity; SE, spherical equivalent.
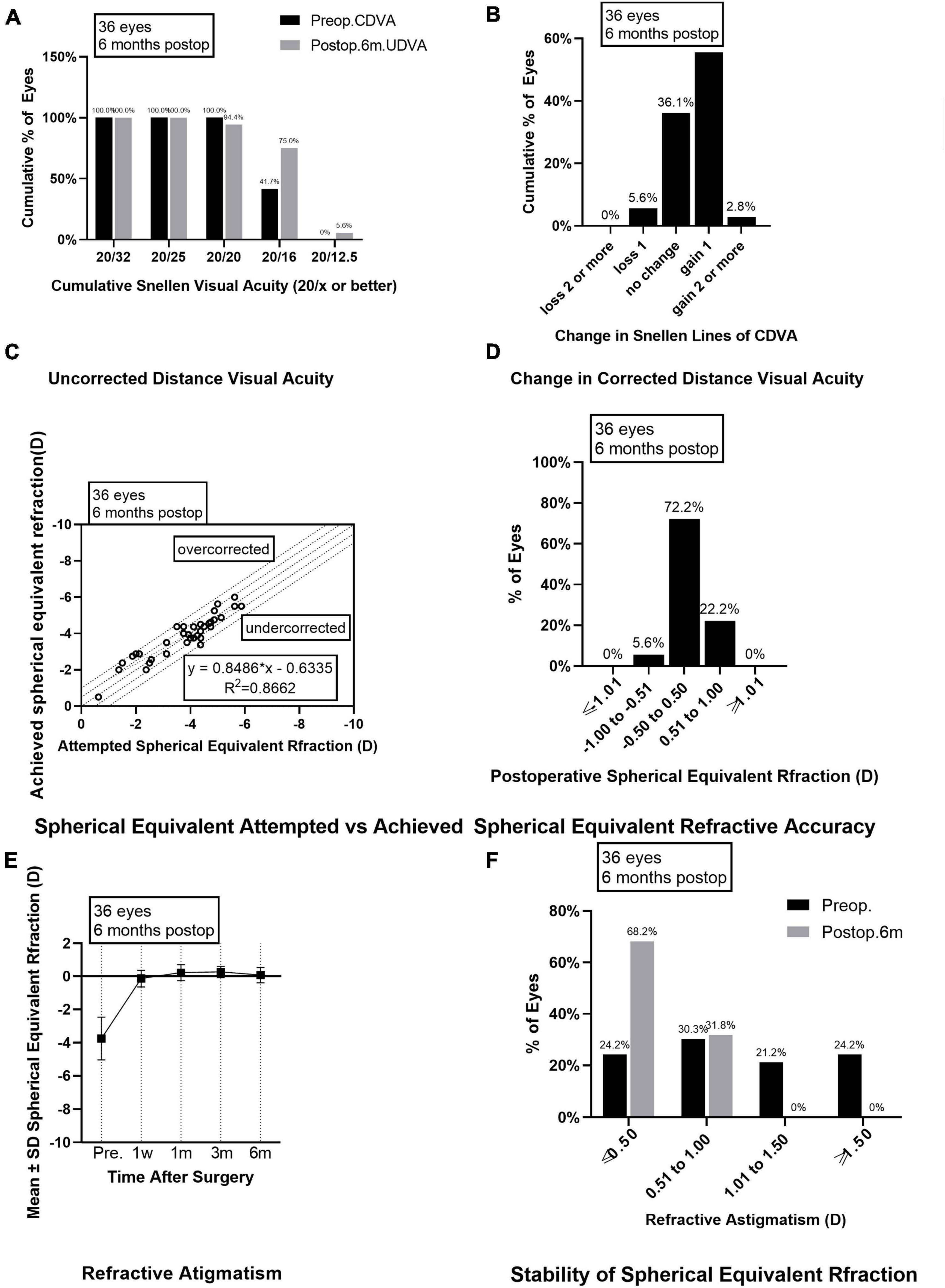
Figure 2. Outcomes of group B after SMILE surgery. (A) Cumulative percentage of preoperative CDVA and postoperative UDVA in group B at 6 months follow-up; (B) Change in CDVA in group B at 6 months follow-up; (C) Achieved versus attempted change in SE at 6 months follow-up in group B; (D) Accuracy of SE refraction in group B at 6 months follow-up; (E) Stability of SE in group B at 1 week, 1 month, 3 months and 6 months after surgery; (F) The percentage of preoperative postoperative refractive astigmatism in group B at 6 months follow-up. CDVA, corrected distance visual acuity; UDVA, uncorrected distance visual acuity; SE, spherical equivalent.
As for the safety evaluation, the number of eyes with no change in CDVA was 16 (44.4%) in group A and 13 (36.1%) in group B; with one line improved in 17 eyes (47.2%) from group A and 20 eyes (55.6%) in group B; with two lines improved in none eyes from group A and one eye (2.8%) in group B (Figures 1B, 2B); with one line decreased in three eyes (8.3%) from group A and two eyes (5.6%) from group B; none of the eyes in groups A and B lost two or more lines when compared the postoperative CDVAs to the preoperative CDVAs. The safety indexes were 1.08 ± 0.14 in group A and 1.12 ± 0.15 in group B (P = 0.173).
Predictability and stability
Six months after the surgery, the linear regression equation of achieved SE after SMILE and attempted SE before SMILE was y = 0.9434 × x-0.2749 (R2 = 0.8633, P < 0.0001) in group A and y = 0.8486 × x-0.6355 (R2 = 0.8662, P < 0.0001) in group B, without difference between the two groups (P > 0.0001, Figures 1C, 2C). The SE correction errors were less than ± 0.5 D in 25 eyes (69.4%) in group A and 26 eyes (72.2%) in group B (Figures 1D, 2D). The mean SE refraction changes over time after SMILE in the two groups are presented in Figures 1E, 2E. There was a significant reduction in the mean SEs at 1 week, 1 month, 3 months, and 6 months after the surgery compared with the preoperative SEs (P < 0.05). However, there was no significant change in SEs from 1 week to 6 months after surgery in both groups A and B. Three months after surgery, SE in group B was significantly higher than in group A (P = 0.003). The mean SEs at 6 months after surgery were −0.09 ± 0.50 in group A and 0.07 ± 0.47 in group B, without a significant difference between them (P = 0.059).
Astigmatism
The preoperative astigmatism was less than 0.5 D in 15 eyes (41.7%) in group A and 10 eyes (27.8%) in group B. The numbers and percentages of eyes with different levels of postoperative astigmatism at 6 months after surgery were as follows: (1) 0.00–0.50 D, 30 eyes (83.3%) in group A and 28 eyes (77.8%) in group B; (2) 0.50–1.00 D, 6 eyes (16.7%) in group A and 8 eyes (22.2%) in group B; (3) 1.00 D–1.50 D and >1.50 D, none eyes in groups A and B (Figures 1F, 2F).
Corneal morphology
Posterior corneal elevation: There was no significant difference in PCE between group A and group B before SMILE or at 1 week, 1 month, 3 months, and 6 months after SMILE. In groups A and B, compared to the preoperative PCE, there was no significant change in PCE at 1 week, 1 month, and 3 months after surgery (P > 0.05). However, it significantly decreased 6 months after surgery (P < 0.05). There was no significant change in PCE from 1 week to 6 months after surgery in groups A and B (P > 0.05, Table 2). ARC: There was no significant difference in ARC between group A (7.76 ± 0.22) and group B (7.77 ± 0.22) preoperatively. The ARC was significantly increased postoperatively than before the surgery in the two groups (P < 0.000). The ARCs were higher in group A than in group B at 1 week, 1 month, 3 months, and 6 months after surgery (P = 0.000, Table 3). PRC: There was no significant difference in PRC between group A and group B preoperatively and postoperatively at 1 week, 1 month, 3 months, and 6 months. PRCs did not change significantly after the surgery compared to the preoperative values in the two groups (P > 0.05). HOAs: As is seen in Table 4, before surgery, the root means square (RMS) value was 0.34 ± 0.08 in group A and 0.36 ± 0.10 in group B (P = 0.063). RMS values increased after SMILE in both groups compared to those before surgery (P < 0.05). As is seen in Figure 3, the postoperative RMS value in group A was significantly higher than in group B (P = 0.000). Compared to the values before surgery, there were significant increases in postoperative SA in groups A and B (P < 0.05). In both groups, coma 90° increased after surgery. The coma 90° value in group A was significantly higher than that in group B at 1 week, 1 month, 3 months and 6 months after surgery (P = 0.000). In both groups, there was no significant change in trefoil 0°, trefoil 30°, and coma 0° during the 6 months follow-up. For RMS values of total HOAs, SA, and coma, no significant changes were found between 1 week and 6 months postoperatively (P > 0.05). At 6 months postoperatively, there was no significant correlation between CDVA and HOAs in group A (r = 0.002, P = 0.990). Moreover, similar results were found in group B (r = −0.313, P = 0.076).
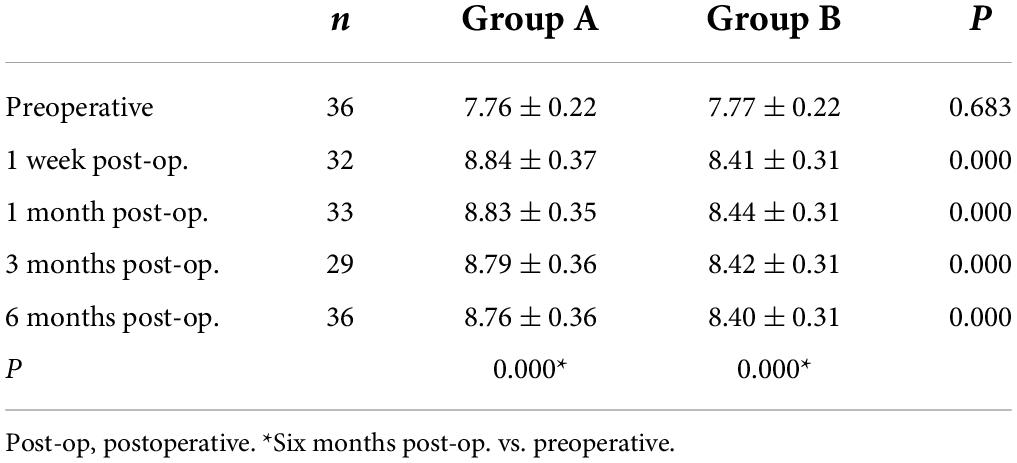
Table 3. Anterior corneal radius of curvature of the 3 mm area at the center of the thinnest point of the cornea (ARC).
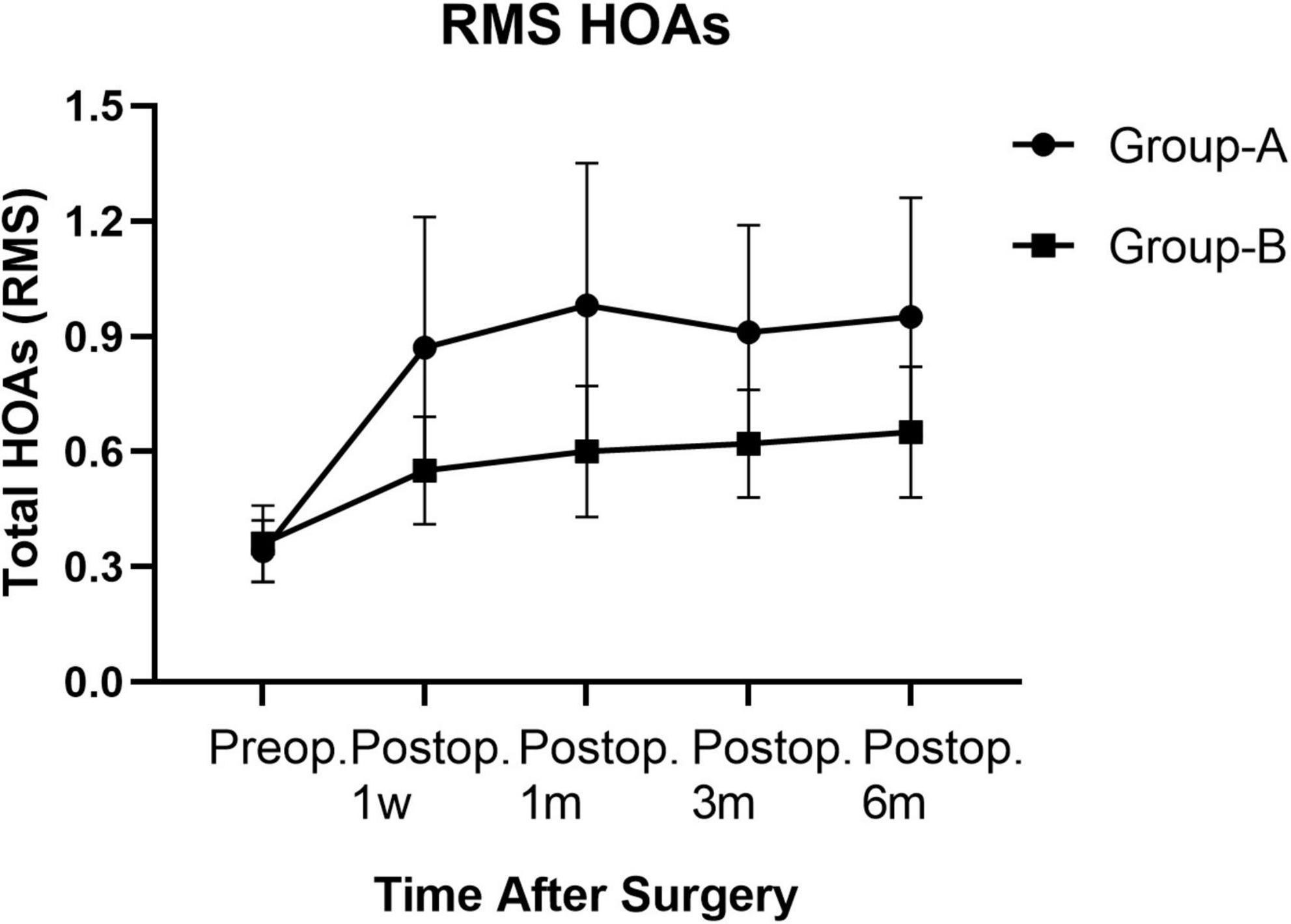
Figure 3. Root mean square HOAs of groups A and B from preoperative baseline to the 6 months after surgery.
Discussion
The results from the present study demonstrated that SMILE is an effective and safe procedure for correcting the refraction of myopic anisometropia. The predictability and stability of the achieved SE are also good and in agreement with the previous reports. Our data supported why SMILE has been selected as one of the mainstream procedures among the cornea stromal refractive surgeries correcting anisometropia. Even though characteristic changes of corneal morphology after SMILE have been investigated in many studies (20, 21), few revealed the characteristics and differences of these changes between the two eyes in a myopic anisometropia patient. In the present study, we provided information that should be useful to guide our practice in the future.
Small incision lenticule extraction procedure seems to have similar predictability, independent of the diopters of myopic correction (20, 22). No significant difference after SMILE was found in the efficacy index and safety index between high myopia and mild or moderate myopia from the previous studies (22–24). In the present study, there was no difference in the efficacy and safety indexes between the two groups, which is consistent with the results of the previous studies. However, it was previously shown in studies (25, 26) that the inflammatory response and refractive regression after SMILE were more severe in the high myopia eyes, which was related to the recovery of postoperative vision and might adversely affect the efficacy index.
Changes in PCE after SMILE have been reported that PCE tended to decrease as compared with that preoperatively, and this decline remained stable from 1 week to a 5 years follow-up period (27, 28). In the present study, changes in PCE in the higher myopia eyes were consistent with the previous studies in the higher and lower myopia eyes. This may be explained by the corneal reaction induced by laser surgery. However, a study on LASIK showed that eyes with higher preoperative refraction require more laser ablation, thus making them more prone to anterior corneal shift (29). It should be noted that although there was a tendency for posterior corneal surface elevation in the high myopia group, no iatrogenic keratectasia was observed in the present study.
Higher-order aberration changes after SMILE and is related to the visual quality. Induction of spherical aberration is a typical side effect in the refractive surgery of myopia (30). The increase of coma may be related to laser energy density and the laser inclination angle during peripheral corneal ablation, the number of corrected diopters, the position of cap rim cut, and the decentration ablation (20). In the present study, RMS values of total HOAs changed after SMILE. SA and coma 90° increased significantly after surgery; while coma 0°, trefoil 0°, and trefoil 30° did not change significantly. HOAs also presented stable without changes during follow-up from 1 week to 6 months. The eyes with higher myopia had significantly higher postoperative HOA, SA, and coma 90° than those of the fellow eyes. Our results were consistent with some previous studies (31), but not all. For example, Jin et al. (20) reported that RMS values and SA in the high myopia group were higher than in mild to moderate myopia groups, but this difference did not appear in coma 90°. Zhao et al. (32) found that RMS, coma 90°, and SA of the high and mild myopia groups increased significantly after surgery. However, there was no significant difference between the two groups in the RMS values of total HOAs and specific HOAs. It has been reported that RMS values and SA significantly increased after surgery, decreasing from 3 months to 3 years postoperatively. However, Coma 90° increased and remained stable over 3 years postoperatively (18). Future studies are warranted to address the different results between those studies and the present study.
There are differences in postoperative corneal aberration changes caused by different surgical methods. Wu et al. (33) suggested that the SMILE procedure induced similar optical changes with FS-LASIK. Chen et al. (34) also found no significant difference in trefoil, horizontal coma, SA, and total HOA postoperatively between SMILE and wavefront-guided (WFG) FS-LASIK. However, higher vertical coma was shown in SMILE than in WFG FS-LASIK, which may be related to the difference in the location of the two surgical incisions. However, in other studies (35), at 3 months postoperatively, the average values for SA and horizontal coma were lower in the SMILE group compared with the FS-LASIK group. Yu et al. (36) reported that the changes in a vertical coma, SA, and HOA were significantly lower in SMILE than in LASEK 3 years after surgery. In conclusion, the magnitudes of the changes of HOAs after different surgical methods are still inconclusive, which may be related to the size of the optical zone (33), follow-up period, ethnic difference, corneal thickness, degree of myopia, and tear film stability. (18) Studies are warranted to answer the question.
In general, the present study had some limitations. The sample size of this study was not large enough, and the follow-up period could be longer. A larger sample size and more extended observation after SMILE are warranted in the future. The correlation between the HOAs after SMILE and the objective and subjective visual quality should also be evaluated.
Conclusion
From the present study’s results, SMILE was safe, effective, predictable and stable in treating adult myopic anisometropia. The postoperative corneal morphological changes in HOAs were characteristic, i.e., RMS values, SA, and coma 90° increased significantly after SMILE and remained stable during the 6 months follow-up. The eyes with higher myopia had significantly higher postoperative HOA, SA, and coma 90° than those of the fellow eyes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 81970832 and 81870650), the Key Project of the Chongqing Health Commission (grant numbers: 2018MSXM003 and 2018GDRC008), and the Natural Science Foundation of Chongqing Science and Technology Bureau (grant numbers: CSTC2021jscx-gksb-N0017 and cstc2021jcyj-msxmX0967). The funder had no role in the study design, data collection and analysis, publication decision, and manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smith E, Hung L, Arumugam B, Wensveen J, Chino Y, Harwerth R. Observations on the relationship between anisometropia, amblyopia and strabismus. Vision Res. (2017) 134:26–42. doi: 10.1016/j.visres.2017.03.004
2. Weale R. On the age-related prevalence of anisometropia. Ophthalmic Res. (2002) 34:389–92. doi: 10.1159/000067040
3. Zhang F, Sugar A, Jacobsen G, Collins M. Visual function and patient satisfaction: comparison between bilateral diffractive multifocal intraocular lenses and monovision pseudophakia. J Cataract Refract Surg. (2011) 37:446–53.
4. Autrata R, Rehurek J. Laser-assisted subepithelial keratectomy and photorefractive keratectomy versus conventional treatment of myopic anisometropic amblyopia in children. J Cataract Refract Surg. (2004) 30:74–84.
5. Autrata R, Krejčíøová I, Griščíková L, Doležel Z. [Refractive Surgery in Children with Myopic Anisometropia and Amblyopia in Comparison with Conventional Treatment by Contact Lenses]. Cesk Slov Oftalmol. (2016) 72:12–9.
6. Paysse E, Coats D, Hussein M, Hamill M, Koch D. Long-term outcomes of photorefractive keratectomy for anisometropic amblyopia in children. Ophthalmology. (2006) 113:169–76. doi: 10.1016/j.ophtha.2005.06.010
7. Lin X, Yan X, Wang Z, Yang B, Chen Q, Su J, et al. Long-term efficacy of excimer laser in situ keratomileusis in the management of children with high anisometropic amblyopia. Chin Med J. (2009) 122:813–7.
8. Nucci P, Drack A. Refractive surgery for unilateral high myopia in children. J AAPOS. (2001) 5:348–51. doi: 10.1067/mpa.2001.119787
9. Roszkowska A, Biondi S, Chisari G, Messina A, Ferreri F, Meduri A. Visual outcome after excimer laser refractive surgery in adult patients with amblyopia. Eur J Ophthalmol. (2006) 16:214–8. doi: 10.1177/112067210601600204
10. Meduri A, Scalinci S, Morara M, Ceruti P, Grenga P, Zigiotti G, et al. Effect of basic fibroblast growth factor in transgenic mice: corneal epithelial healing process after excimer laser photoablation. Ophthalmologica. (2009) 223:139–44. doi: 10.1159/000187686
11. Meduri A, Scorolli L, Scalinci S, Grenga P, Lupo S, Rechichi M, et al. Effect of the combination of basic fibroblast growth factor and cysteine on corneal epithelial healing after photorefractive keratectomy in patients affected by myopia. Indian J Ophthalmol. (2014) 62:424–8. doi: 10.4103/0301-4738.119420
12. Kim T, Alió Del Barrio J, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. (2019) 393:2085–98. doi: 10.1016/S0140-6736(18)33209-4
13. Liu M, Chen Y, Wang D, Zhou Y, Zhang X, He J, et al. Clinical outcomes after SMILE and femtosecond laser-assisted LASIK for myopia and myopic astigmatism: a prospective randomized comparative study. Cornea. (2016) 35:210–6. doi: 10.1097/ICO.0000000000000707
14. He S, Luo Y, Chen P, Ye Y, Zheng H, Lan M, et al. Prospective, randomized, contralateral eye comparison of functional optical zone, and visual quality after SMILE and FS-LASIK for high myopia. Transl Vis Sci Technol. (2022) 11:13. doi: 10.1167/tvst.11.2.13
15. Liu T, Lu G, Chen K, Kan Q, Bai J. Visual and optical quality outcomes of SMILE and FS-LASIK for myopia in the very early phase after surgery. BMC Ophthalmol. (2019) 19:88. doi: 10.1186/s12886-019-1096-z
16. Samir A, Lotfy A, Heikal M, Abdelrahman Elsayed A. Small incision lenticule extraction for correction of pediatric unilateral anisometropic myopia. J Refract Surg. (2021) 37:510–5. doi: 10.3928/1081597X-20210506-02
17. Damgaard I, Sejersen H, Ivarsen A, Hjortdal J. 7-year results of SMILE for high myopia: visual and refractive outcomes and aberrations. J Refract Surg. (2021) 37:654–61. doi: 10.3928/1081597X-20210712-02
18. Bohac M, Koncarevic M, Dukic A, Biscevic A, Cerovic V, Merlak M, et al. Unwanted astigmatism and high-order aberrations one year after excimer and femtosecond corneal surgery. Optom Vis Sci. (2018) 95:1064–76. doi: 10.1097/OPX.0000000000001298
19. Pedersen I, Ivarsen A, Hjortdal J. Three-year results of small incision lenticule extraction for high myopia: refractive outcomes and aberrations. J Refract Surg. (2015) 31:719–24. doi: 10.3928/1081597X-20150923-11
20. Jin H, Wan T, Wu F, Yao K. Comparison of visual results and higher-order aberrations after small incision lenticule extraction (SMILE): high myopia vs. mild to moderate myopia. BMC Ophthalmol. (2017) 17:118. doi: 10.1186/s12886-017-0507-2
21. Cao K, Liu L, Yu T, Chen F, Bai J, Liu T. Changes in corneal biomechanics during small-incision lenticule extraction (SMILE) and femtosecond-assisted laser in situ keratomileusis (FS-LASIK). Lasers Med Sci. (2020) 35:599–609. doi: 10.1007/s10103-019-02854-w
22. Kim J, Kim B, Mun S, Chung Y, Kim H. One-year outcomes of small-incision lenticule extraction (SMILE): mild to moderate myopia vs. high myopia. BMC Ophthalmol. (2015) 15:59. doi: 10.1186/s12886-015-0051-x
23. Torky M, Alzafiri Y. Visual and refractive outcomes of small-incision lenticule extraction in mild, moderate, and high myopia: six-month results. J Cataract Refract Surg. (2017) 43:459–65. doi: 10.1016/j.jcrs.2017.01.015
24. Qin B, Zhao J, Li M, Yao P, Zhou X. The comparison of visual outcomes, aberrations, and Bowman’s layer micro-distortions after femtosecond laser small-incision lenticule extraction (SMILE) for the correction of high and moderate myopia and myopic astigmatism. BMC Ophthalmol. (2019) 19:138. doi: 10.1186/s12886-019-1135-9
25. Dong Z, Zhou X, Wu J, Zhang Z, Li T, Zhou Z, et al. Small incision lenticule extraction (SMILE) and femtosecond laser LASIK: comparison of corneal wound healing and inflammation. Br J Ophthalmol. (2014) 98:263–9. doi: 10.1136/bjophthalmol-2013-303415
26. Liu Y, Teo E, Lwin N, Yam G, Mehta J. Early corneal wound healing and inflammatory responses after SMILE: comparison of the effects of different refractive corrections and surgical experiences. J Refract Surg. (2016) 32:346–53. doi: 10.3928/1081597X-20160217-05
27. Li M, Yang D, Zhao Y, Yang W, Shang J, Zhou X, et al. Impact of ablation ratio on 5-year postoperative posterior corneal stability after refractive surgery: SMILE and FS-LASIK. Eye Vis. (2020) 7:53. doi: 10.1186/s40662-020-00218-y
28. Yu M, Chen M, Dai J. Comparison of the posterior corneal elevation and biomechanics after SMILE and LASEK for myopia: a short- and long-term observation. Graefes Arch Clin Exp Ophthalmol. (2019) 257:601–6. doi: 10.1007/s00417-018-04227-5
29. Baek T, Lee K, Kagaya F, Tomidokoro A, Amano S, Oshika T. Factors affecting the forward shift of posterior corneal surface after laser in situ keratomileusis. Ophthalmology. (2001) 108:317–20. doi: 10.1016/S0161-6420(00)00502-9
30. Kohnen T, Bühren J, Kühne C, Mirshahi A. Wavefront-guided LASIK with the Zyoptix 3.1 system for the correction of myopia and compound myopic astigmatism with 1-year follow-up: clinical outcome and change in higher order aberrations. Ophthalmology. (2004) 111:2175–85. doi: 10.1016/j.ophtha.2004.06.027
31. Tülü Aygün B, Çankaya K, Aðca A, Yıldırım Y, Yıldız B, Sucu M, et al. Five-year outcomes of small-incision lenticule extraction vs femtosecond laser-assisted laser in situ keratomileusis: a contralateral eye study. J Cataract Refract Surg. (2020) 46:403–9. doi: 10.1097/j.jcrs.0000000000000067
32. Zhao P, Hu Y, Wang Y, Fu C, Zhang J, Zhai C. Comparison of correcting myopia and astigmatism with SMILE or FS-LASIK and postoperative higher-order aberrations. Int J Ophthalmol. (2021) 14:523–8.
33. Wu W, Wang Y. Corneal higher-order aberrations of the anterior surface, posterior surface, and total cornea after SMILE, FS-LASIK, and FLEx surgeries. Eye Contact Lens. (2016) 42:358–65. doi: 10.1097/ICL.0000000000000225
34. Chen X, Wang Y, Zhang J, Yang S, Li X, Zhang L. Comparison of ocular higher-order aberrations after SMILE and Wavefront-guided Femtosecond LASIK for myopia. BMC Ophthalmol. (2017) 17:42. doi: 10.1186/s12886-017-0431-5
35. Wu W, Wang Y. The correlation analysis between corneal biomechanical properties and the surgically induced corneal high-order aberrations after small incision lenticule extraction and femtosecond laser in situ keratomileusis. J Ophthalmol. (2015) 2015:758196. doi: 10.1155/2015/758196
Keywords: refractive surgery, small incision lenticule extraction (SMILE), myopic anisometropia, higher-order aberrations (HOA), posterior corneal elevation (PCE)
Citation: Zhu L, Ji Y, Yang X, Lu X, Wu Q, Wang Q, Xia J, Li M, Hu K and Wan W (2022) Corneal morphological changes after small incision lenticule extraction for myopic anisometropia. Front. Med. 9:977586. doi: 10.3389/fmed.2022.977586
Received: 24 June 2022; Accepted: 08 August 2022;
Published: 24 August 2022.
Edited by:
Alessandro Meduri, University of Messina, ItalyReviewed by:
Laura De Luca, University of Messina, ItalyFrancesco Gazia, University of Messina, Italy
Copyright © 2022 Zhu, Ji, Yang, Lu, Wu, Wang, Xia, Li, Hu and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjuan Wan, d2Fud2VuanVhbmNxdW1zQDE2My5jb20=; Ke Hu, NDIyMjJAcXEuY29t
 Lu Zhu
Lu Zhu Ke Hu
Ke Hu Wenjuan Wan
Wenjuan Wan