- 1School of Rehabilitation Medicine, Weifang Medical University, Weifang, China
- 2Weifang People’s Hospital and Brain Hospital, Weifang, China
- 3School of First Clinical Medical, Weifang Medical University, Weifang, China
- 4Weifang People’s Hospital, Weifang, China
- 5Affiliated Hospital of Weifang Medical University, Weifang, China
Background: Breathing exercises improve oxidative stress in healthy young adults and patients with diabetes, hypertension, and chronic obstructive pulmonary disease. Furthermore, the mechanism of respiratory intervention is controversial. Therefore, in this meta-analysis, we aimed to systematically evaluate the effects of breathing exercises on oxidative stress biomarkers in humans and provide evidence for the clinical application of breathing exercises.
Methods: The Embase, PubMed, Cochrane Library, Web of Science, CNKI, and WANFANG databases were searched for studies about the effects of breathing exercises on human oxidative stress levels, with no restraints regarding time, race, or language. The experimental group included various breathing exercises, and the outcome index included malondialdehyde, superoxide dismutase, and glutathione, nitric oxide, vitamin C, or total antioxidant capacity levels from a randomized controlled trial. Data were extracted by more than two authors and reviewed by one author.
Results: Ten studies were included from five countries. Data from patients with no disease, chronic obstructive pulmonary disease, hypertension, or diabetes were included. Participants who performed breathing exercises had greater changes in the included biomarkers than those who did not, suggesting that these biomarkers can be used to evaluate oxidative stress after respiratory interventions.
Conclusion: Breathing exercises increased SOD and GSH activities and decreased MDA content.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022337119, identifier CRD42022337119.
1. Introduction
Oxidative stress (OS) is the imbalance between the production of the reactive oxygen species (ROS) by oxidation and the clearance of antioxidants in organisms (1, 2), which leads to the accumulation of free radicals, vascular endothelial lipid peroxidation, and inflammatory reactions in the body (3–6). ROS consist of O2 and oxygen-containing molecules that have not been completely reduced (7). ROS are mainly produced in mitochondria and released into the mitochondrial matrix, intermembrane space, and cytoplasm (8–10). A small amount of ROS promotes cell proliferation, although a large quantities accumulated ROS negatively affects cell functions, resulting in lipid peroxidation that produces malondialdehyde (MDA) and, ultimately, cell death (4). Superoxide dismutase (SOD), which is widely distributed in the body, plays an important role in protecting the body from injury by dismutating the superoxide ions in internal and external environments and is an important free radical scavenger in the body (11). Reducing glutathione (GSH) combines with superoxide and free radicals to resist ROS damage to thiol groups, protecting the cell membrane that contains sulfhydryl proteins and sulfhydryl enzymes and preventing free radical damage to important organs (12). Nitric oxide (NO) bioavailability–which is mediated by ROS via endothelial NO synthase (eNOS) activation–leads to chronic inflammatory responses and cardiovascular injury (13).
When the body is exposed to harmful stimuli, the oxidant-antioxidant imbalance leads to the imbalance of musculin breakdown and synthesis, ultimately resulting in varying degrees of skeletal muscle atrophy and thereby leading to decreased muscle strength, which affects exercise endurance and quality of life (14). Via the attenuation of the mitochondrial oxidative respiratory chain and cell death, inflammatory cells activate and accumulate a large number of free oxygen radicals to promote the lipid generation of MDA, aggravating the damage of OS (15–17). Changes in the OS index are related to harmful stimulation, aging, and disease progression (18–20). The diagnosis of a disease or chronic inflammation is associated with changes in OS markers and, often, with an increase in antioxidant biomarker levels and a decrease in oxidative stress biomarker levels as the disease ameliorates (21–28). OS is measured by analyzing the composition of urine, sputum, blood, and exhaled gas (29–31). The lipid peroxidation product MDA, antioxidant enzyme SOD, and non-enzyme GSH are the most commonly used indexes (2, 32).
Moderate-intensity training improves OS while high-intensity training does not reverse OS and muscle damage during aerobic exercises (33–37). Breathing exercises, a moderate-intensity training technique used in pulmonary rehabilitation, have been widely used in clinical practice (38). By prolonging the breathing time, slowing the breathing rate, and reducing the alveolar-arterial oxygen gradient, breathing exercises enhance the strength and endurance of the respiratory muscles to increase pulmonary ventilation and improve the efficiency of gas exchange (39–44). These exercises effectively relieve the body of hypoxia and improve endurance, maintaining the overall health of the body.
Breathing exercises improve OS in healthy young adults and patients with diabetes, hypertension, and COPD (12, 45–53). However, the sample sizes of the previous studies were small, and the impact of respiratory interventions on patients without primary lung diseases is also controversial (54, 55). Therefore, in this meta-analysis, we aimed to systematically evaluate the effects of breathing exercises on OS biomarkers in humans to provide evidence for the clinical application of breathing exercises.
2. Methods
The meta-analysis was registered with PROSPERO (registration number: CRD42022337119).
2.1. Data sources and eligibility criteria
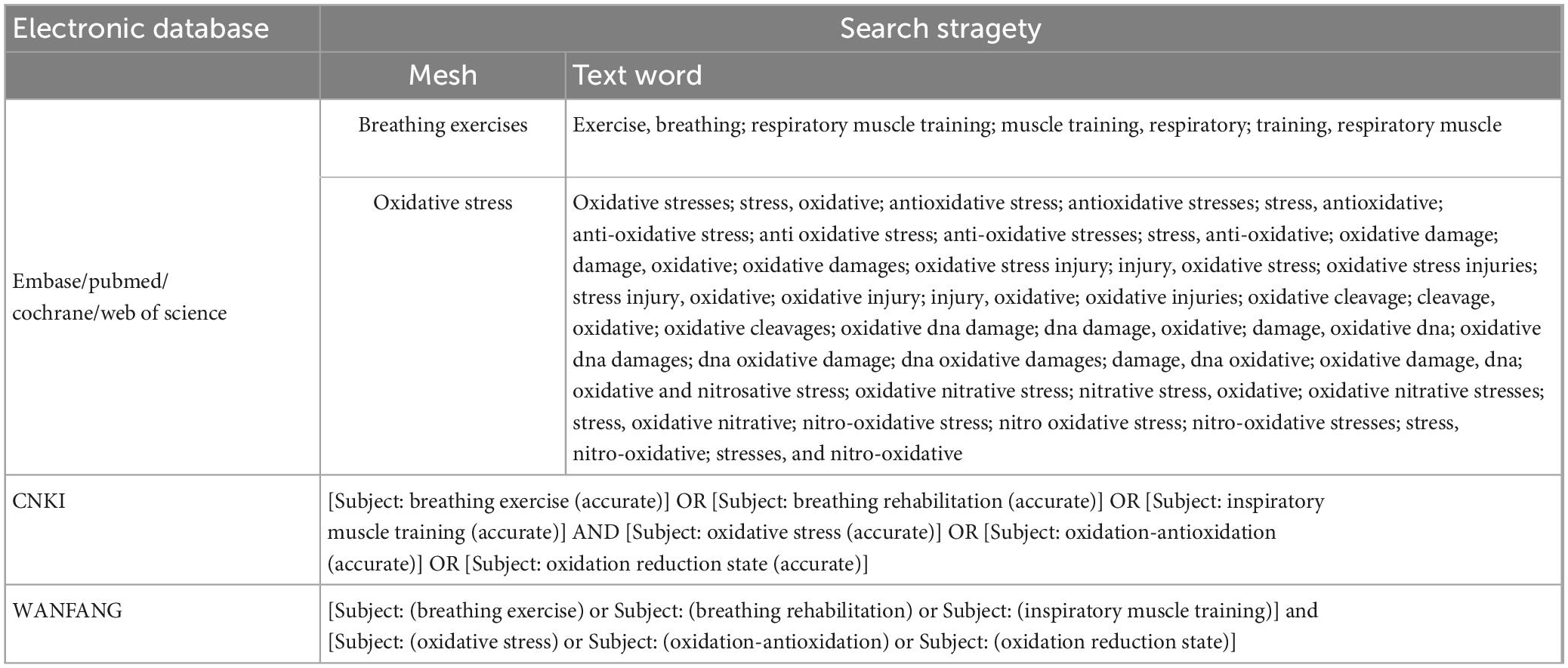
The Embase, PubMed, Cochrane Library, Web of Science, CNKI, and WANFANG databases were searched for randomized controlled trials regarding the effects of breathing exercises. There were no restraints in terms of time, race, or language. The keywords “breathing exercise” and “oxidative stress” were used to link the corresponding free words for the advanced retrieval of studies. In two Chinese databases, Chinese vocabulary retrieval was used to obtain Chinese studies (Table 1).
2.2. Inclusion criteria
Randomized controlled trials in which the intervention was breathing training, such as slow and fast deep breathing, a simple prototype respiratory muscle trainer, diaphragmatic breathing exercises, pranayama of yoga, straight leg raising breathing, inspiratory muscle training, or time-efficient inspiratory muscle strength training and the outcome indexes of OS were MDA, SOD, GSH, NO, vitamin C, or total antioxidant capacity (TAC) levels were included in this meta-analysis. The participants of the included studies were healthy individuals or patients with COPD, hypertension, or diabetes.
Studies that were not randomized controlled trials, including abstracts of conference papers, and those with missing data or data that could not be extracted or obtained from the author were excluded from the meta-analysis. Studies that used non-blood outcome indicators such as urine, sputum, and exhaled gas and those that did not include OS outcome indexes were also excluded. If the clinical randomized controlled trial design was determined as not rigorous, or the Jadad score was <3, the study was not included in the meta-analysis. Moreover, studies in which the breathing exercises were not included in the experimental group were excluded from the meta-analysis.
2.3. Study selection and data collection processes
Data extraction, including the title, author, year, country, the population in each group, intervention methods, intervention time, and outcome index data, was performed by two authors. Mean ± standard deviation values were directly extracted, while the data included in the figures were extracted using error bar graphs with error lines. Disputes were resolved by the examiner, and a consensus was achieved through discussion.
2.4. Quality evaluation
Quality assessment was independently performed by two authors using the Cochrane risk-of-bias tool to assess the literature grade based on the low risk criteria for A, B, and C. In cases of disagreement, the authors reevaluated their judgment and reached an agreement through discussion.
2.5. Statistical analysis
The extracted data were meta-analyzed using RevMan 5.4 and Stata 16.0 software. Continuous variables are presented as weighted mean differences (MD) or e mean differences, and the 95% CI was determined. Bias risk maps and forest maps were created. When I2 was 50% or less, the fixed-effects model was used. When I2 was more than 50%, the heterogeneity was considered to be large, and a sensitivity analysis was performed to reduce the heterogeneity. Statistical significance was set at P < 0.05.
3. Results
3.1. Description of studies
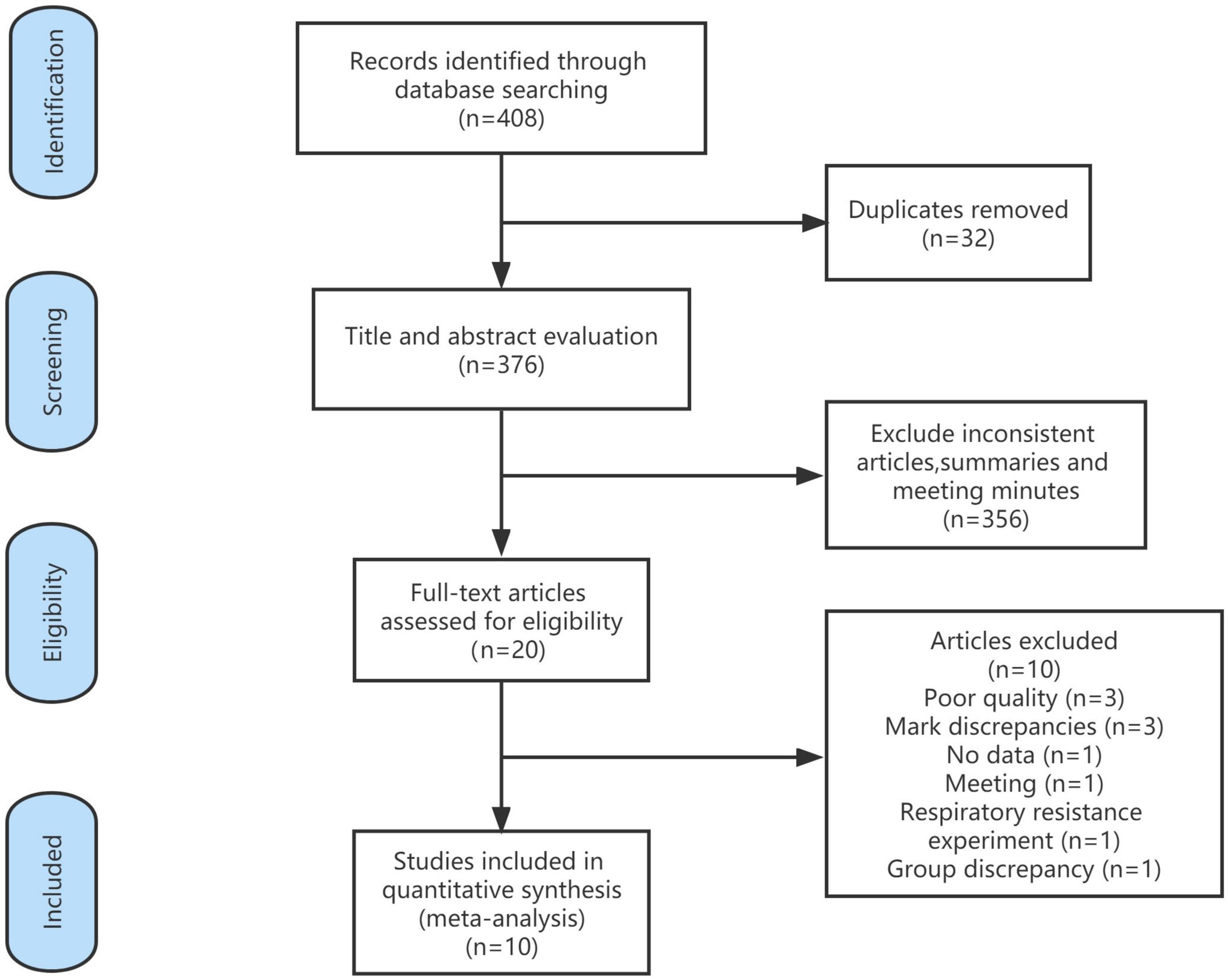
A total of 408 articles were identified in the database search. Of these, 32 duplicate articles were excluded and 356 were excluded based on the title and abstract. A total of 20 articles were read in full, and 10 articles were included in this meta-analysis. The references of the included studies were searched for additional studies, but none met the inclusion criteria (Figure 1).
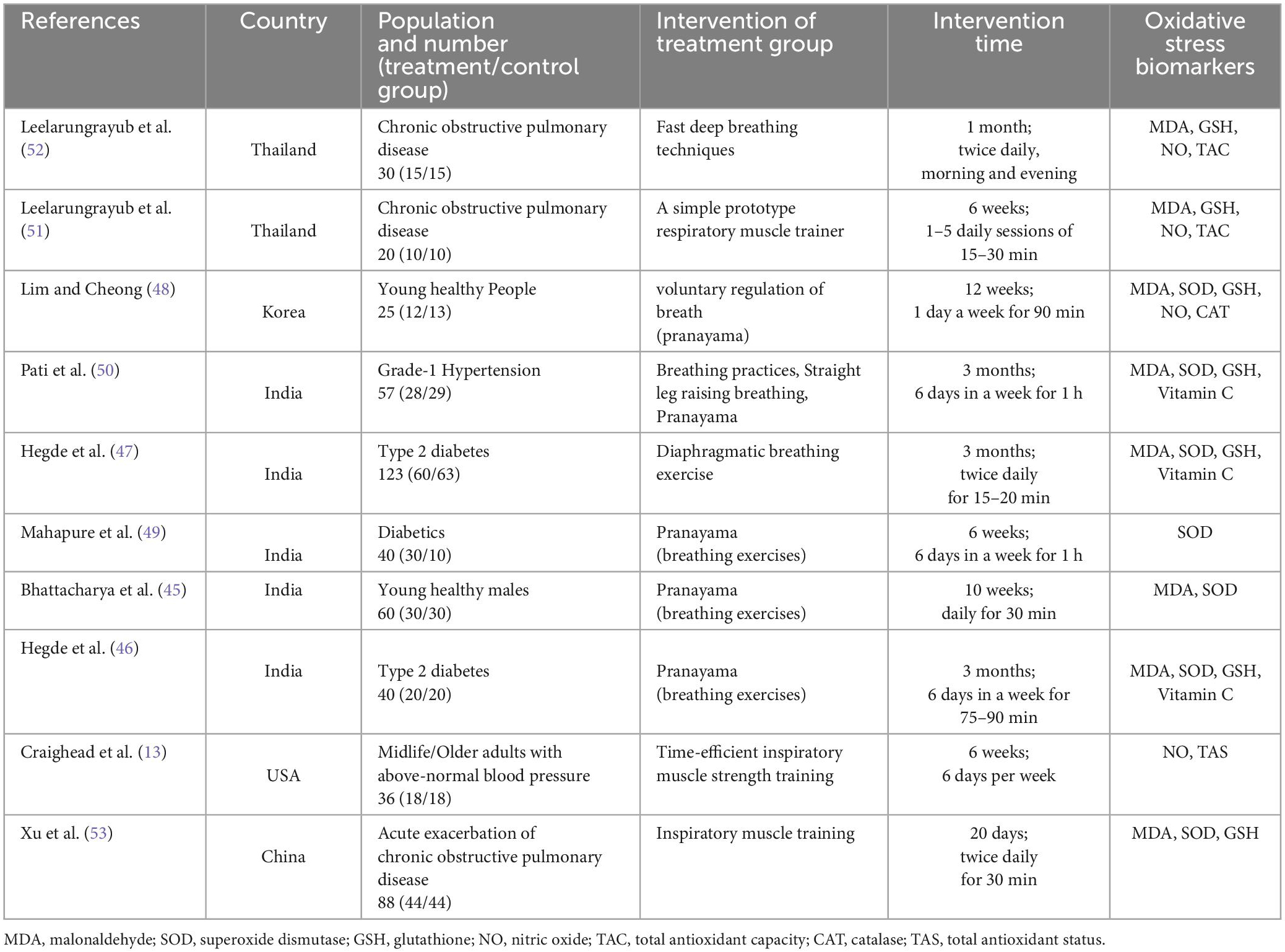
The 10 studies included in this meta-analysis were conducted in five different countries and included 519 participants, including 267 in the treatment group and 252 in the control group (Table 2). Breathing exercises included slow and fast deep breathing (52), a simple prototype respiratory muscle trainer (51), diaphragmatic breathing exercises (47), pranayama of yoga (45, 46, 48, 49), straight leg raising breathing (50), inspiratory muscle training (13), and time-efficient inspiratory muscle strength training (53). The shortest intervention time was 20 days (53), and the longest was 3 months (46, 47, 50). The earliest study was published in 2002 and revealed the changes in MDA and SOD levels in healthy, young individuals after pranayama yoga breathing interventions (45). Two studies were conducted in Thailand (51, 52), one in South Korea (48), five in India (45–47, 49, 50), one in the United States (14), and one in China (53). The most recent studies (13, 53) included inspiratory muscle training exercises. Patients with COPD (51–53), diabetes (46, 47, 49), and hypertension (13, 50) were included in some studies, and two studies included healthy, young adults (45, 48). The main indexes were MDA (45–48, 50–53), SOD (45–50, 53), GSH (46, 47, 49–53), and NO levels (13, 48, 51, 52). TAC (51, 52) and catalase (CAT) (48), vitamin C (46, 47, 50), and F2-isoprostane levels (48) were also measured in some studies (Table 2).
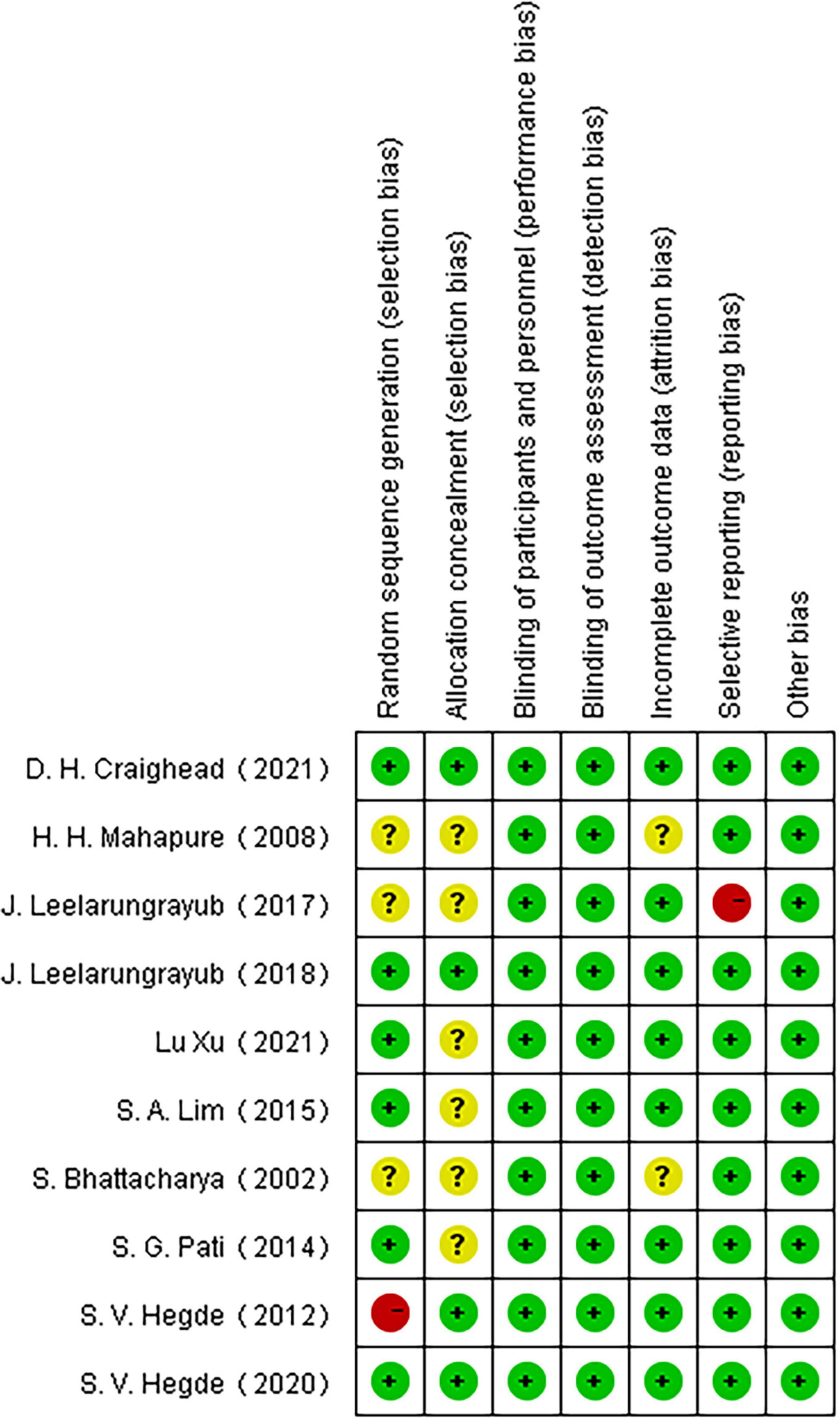
3.2. Risk of bias in included studies
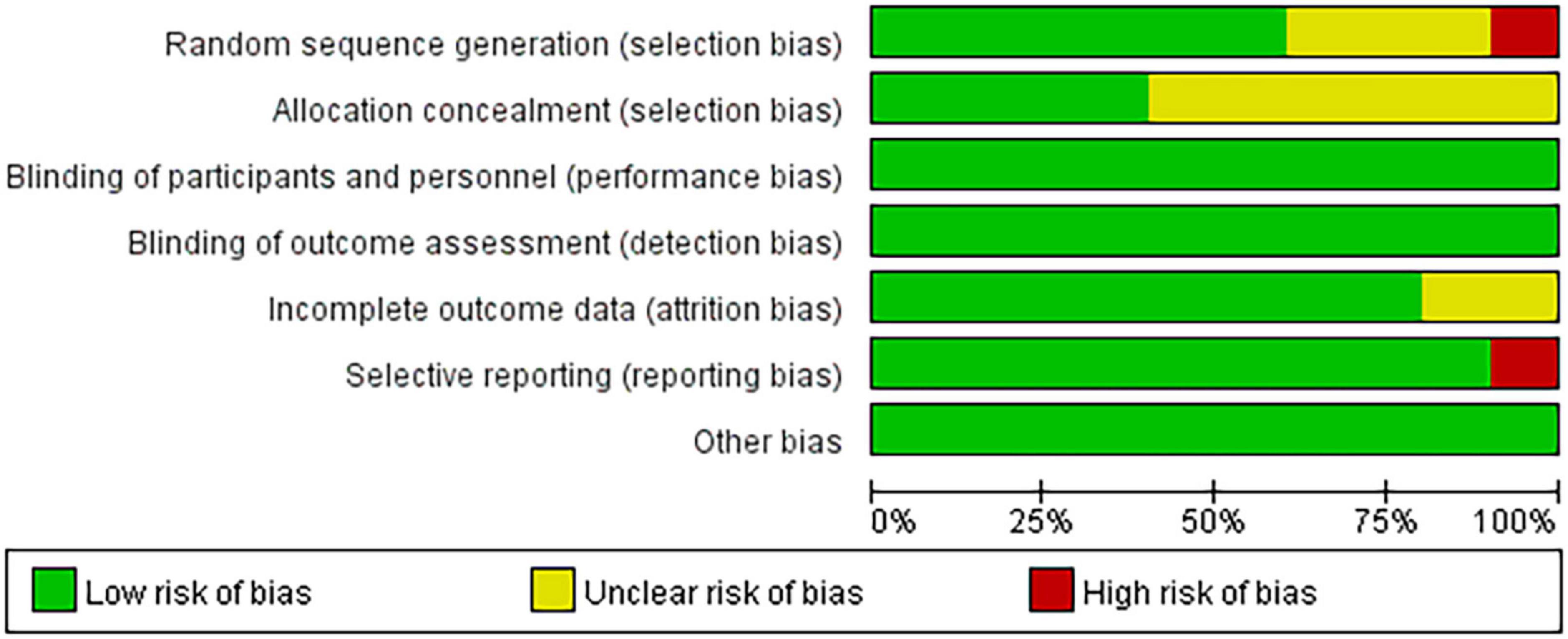
Among the 10 included articles, three were grade A and seven were grade B. Studies that did not explicitly refer to randomized trials and allocation concealment were included in the unclear category. One study (47) did not use randomness and was considered to have a high risk of bias for the generation of random sequences. Another study (51) regarding the application of a simple prototype respiratory muscle trainer for breathing exercises did not include a complete explanation of the experimental design (Figures 2, 3).
3.3. Meta-analysis
3.3.1. MDA
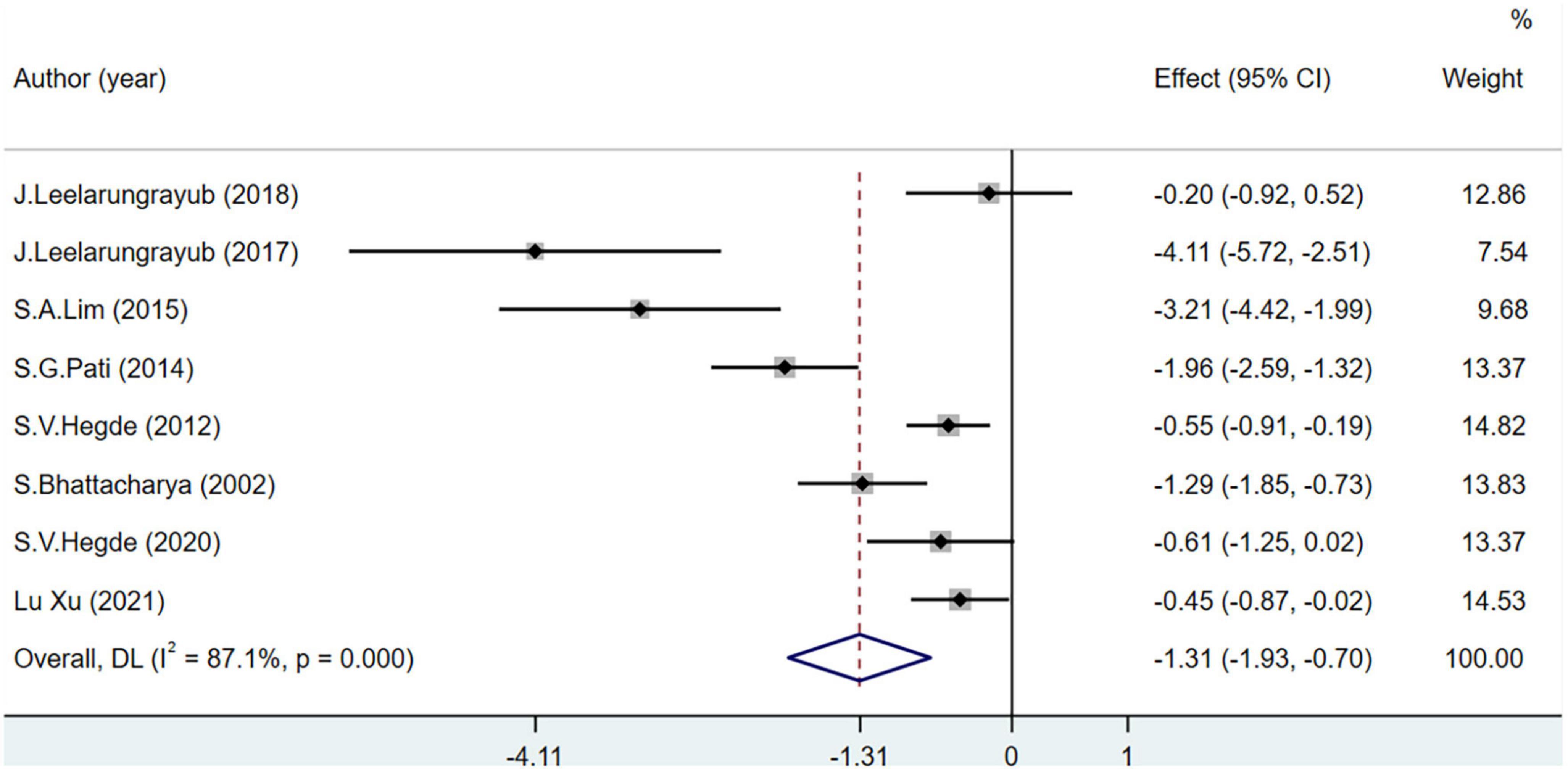
The MDA level was used as the main OS index in eight studies including 443 participants. MDA levels decreased significantly more in the experimental group than in the control group (MD = −1.31; 95% CI = −1.93 to −0.7; P < 0.001) (Figure 4).
3.3.2. SOD
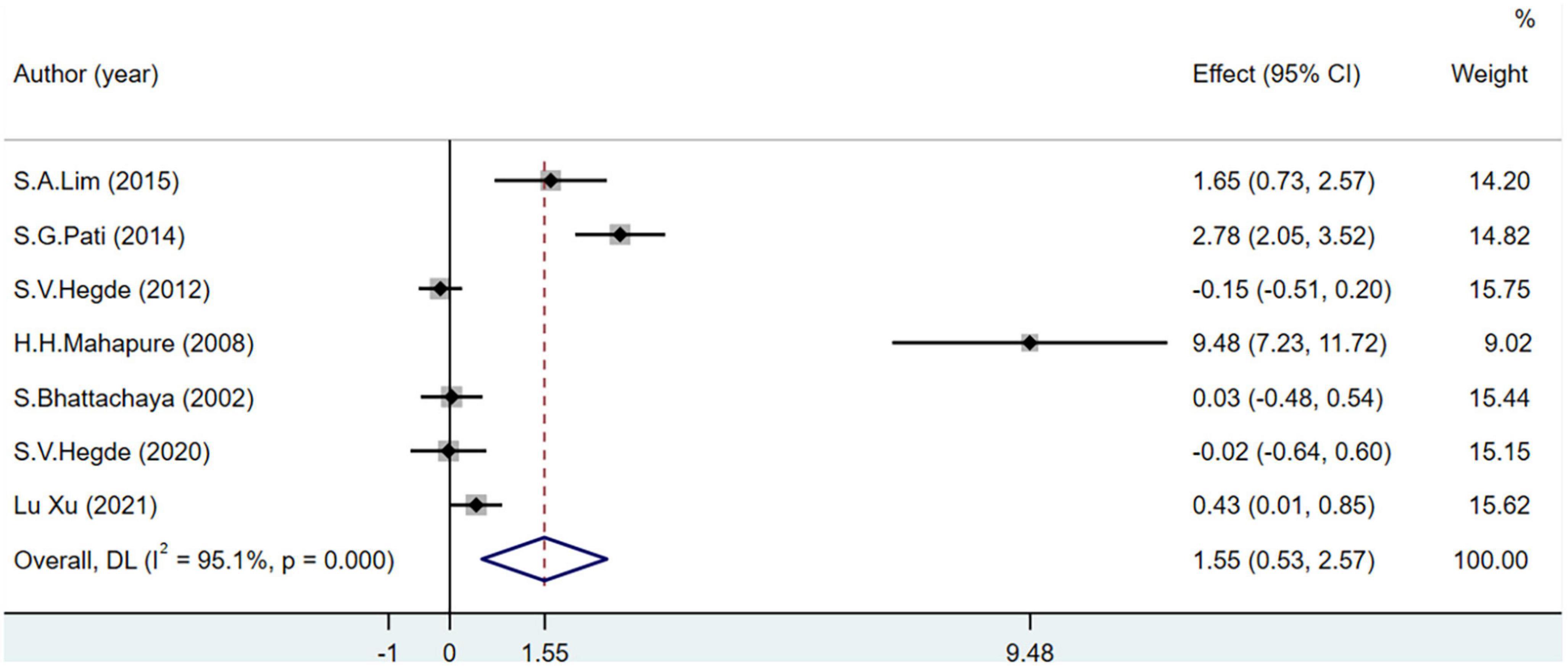
The SOD level was used as an indicator of OS in seven studies including 433 participants. I2 was greater than 50%. The increase in SOD levels was significantly greater in the experimental group than in the control group (MD = 1.55; 95% CI = 0.53–2.57; P < 0.001). However, after removing one study (48) from the sensitivity analysis, there was no significant difference in the levels of SOD between the groups (MD = 0.73; 95% CI = −0.03–1.50; P = 0.001) (Figure 5).
3.3.3. GSH
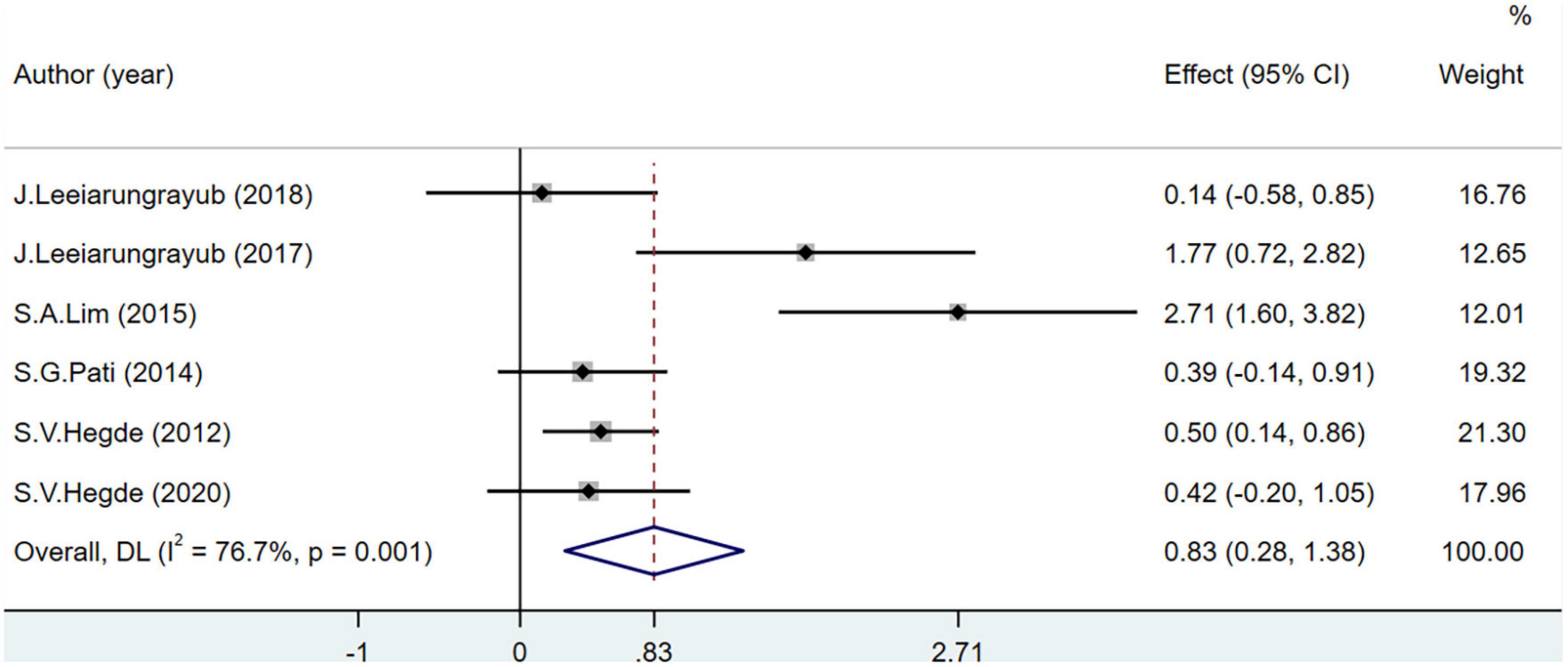
Glutathione levels were recorded in seven studies including 383 participants. One study (53) may have included bias as the heterogeneity reduced after its removal (I2 = 76.7%). The GSH level of the experimental group was significantly higher than that of the control group (MD = 0.83; 95% CI = 0.28–1.38; P < 0.001) (Figure 6).
3.3.4. NO
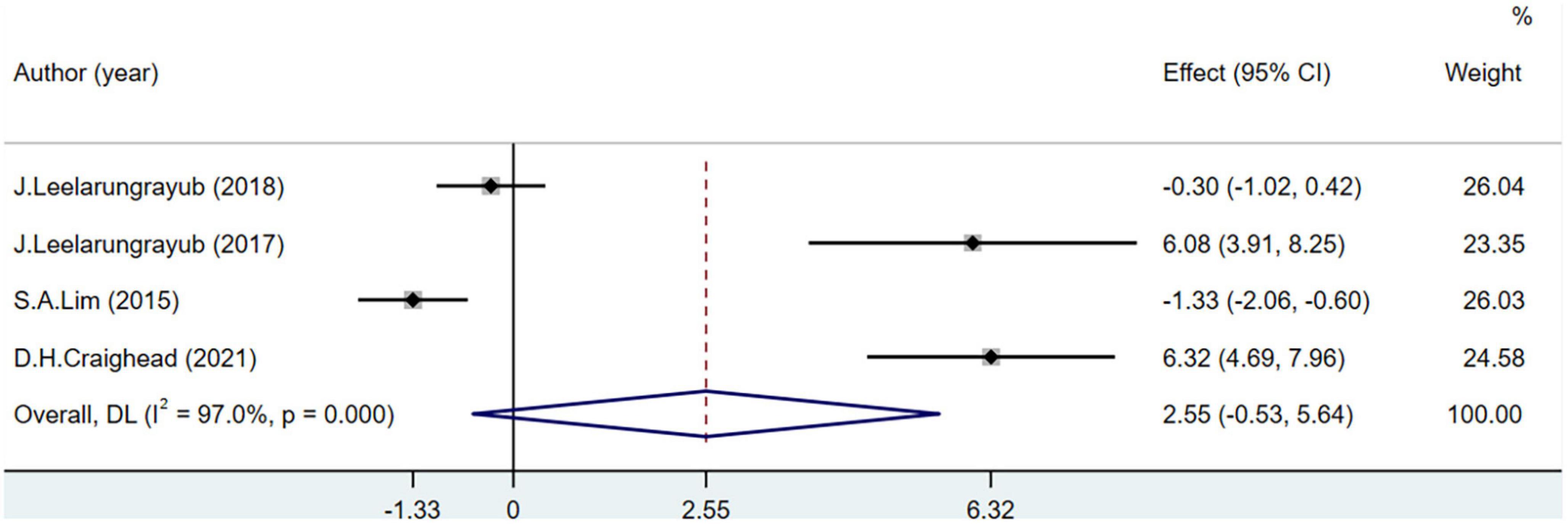
Four studies including 111 participants reported NO level. The NO levels were not significantly different between the groups (MD = 2.55; 95% CI = –0.53 to 5.64; P < 0.001) (Figure 7).
3.3.5. Vitamin C
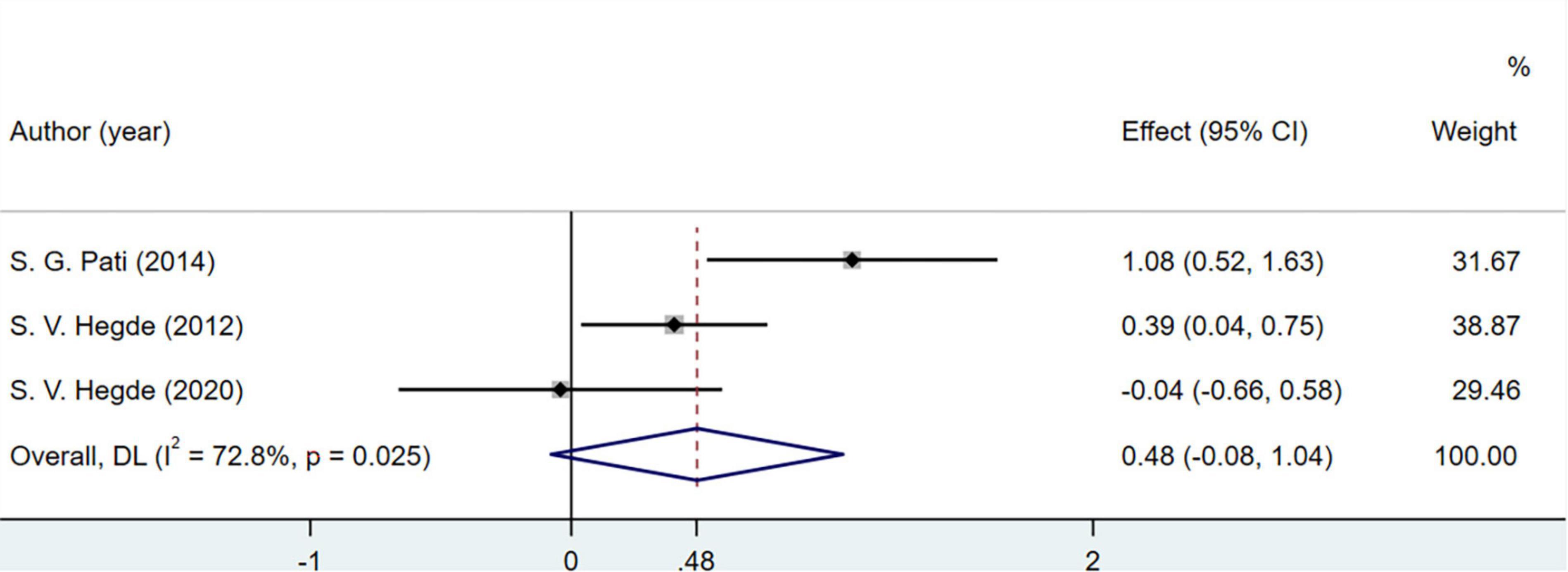
A total of three studies involving 220 patients discussed the changes in vitamin C levels in the human body. Vitamin C concentration did not change significantly (MD = 0.48; 95% CI = −0.08 to 1.04; P = 0.025) (Figure 8).
3.3.6. TAC
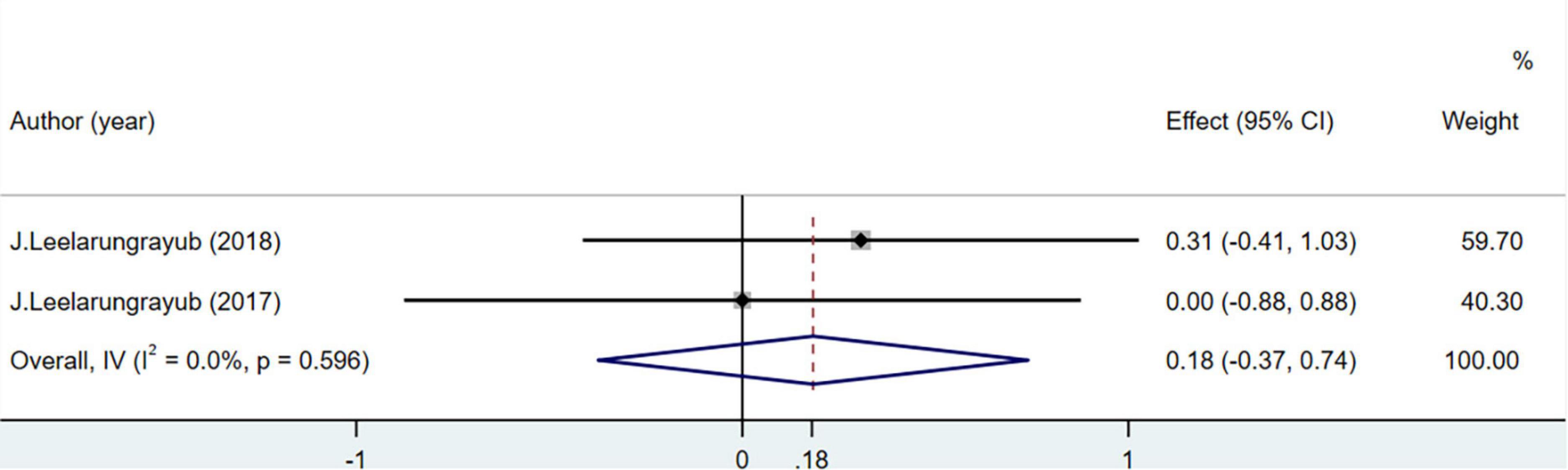
Total antioxidant capacity levels were measured in 50 people in two studies. The difference was not statistically significant (MD = 0.18; 95% CI = –0.37 to 0.74; P = 0.596) (Figure 9).
4. Discussion
Breathing exercises have been a method of rehabilitation of patients with respiratory system diseases for decades (56). Respiratory muscle training has been proven to improve lung function in healthy participants and in patients with COPD, hypertension, diabetes, and chronic kidney disease (57), as it increases exercise endurance and the overall quality of life. OS, as an indicator to measure the survival and mortality of human aging and later disease stages (26, 58–60), is a hotspot of current research, although few studies regarding the impact of breathing exercises on OS have been conducted. The role of breathing exercises in aging or disease progression can be investigated as the effect of respiratory interventions on OS indexes.
In this study, MDA, SOD, and GSH levels, the main outcome indexes of breathing exercises for OS, were investigated. Breathing exercise increased SOD and GSH activities and decreased MDA content. SOD is converted into hydrogen peroxide by catalyzing oxygen reduction, which is then converted to water by GSH. It can effectively remove oxygen free radicals, protect endothelial cells from damage, relax vascular smooth muscle by increasing NO release, thus achieving the purpose of lowering OS (48). Participants who performed breathing exercises had greater changes in these biomarkers than those who did not, suggesting that these biomarkers can be used to evaluate OS after respiratory interventions.
Malondialdehyde is an oxidation product. MDA levels decreased significantly more in participants who performed breathing exercises than in those who did not (45, 47, 48, 50, 51, 53). This may be due to the fact that MDA is the end product of lipid peroxidation (32), a reaction caused by oxygen free radicals by attacking polyunsaturated fatty acids of biofilms. MDA participates in various physiological and pathological processes in the body (4). Leelarungrayub et al. (52) and Hegde et al. (46) reported a significant decrease in MDA levels in the experimental and control groups in their studies but no statistical differences between the groups. In two studies (35, 54), non-aerobic exercises as part of the slow deep breathing method and yoga required a certain intensity of training, and both improved the body’s antioxidant capacity and reduced the MDA content compared with those in the control groups (35), accounting for the lack of significant differences between the groups. These results are similar to those of another study (54) that compared breathing exercises to aerobic exercises in patients undergoing hemodialysis.
In two studies (45, 47), SOD levels decreased in patients with diabetes who performed breathing exercises. This decrease may be due to the adverse effects of long-term, low-intensity training on the body’s antioxidant capacity (61). Chronically increased blood glucose levels increase the content of glycation end products in the body, leading to increased oxidase activity, self-oxidation of glucose, and glycosylation of proteins, resulting in OS reactions (62–64). However, Mahapure et al. (49) reported that SOD levels increased significantly in patients with diabetes performing breathing exercises, which may be related to yoga exercises that are more intense than the breathing exercises. High-intensity exercises resulted in increased SOD levels in patients with diabetes, improving the OS state (65). After a sensitivity analysis and the exclusion of this previous study, the total effect size was not statistically significant in this meta-analysis. In healthy participants and patients with hypertension and COPD (45, 48, 50, 53), SOD dismutates the superoxide anions to generate hydrogen peroxide and oxygen during stable breathing exercises, protecting the body from damage caused by the internal and external environments and maintaining normal physiological activities (11).
The total effect size of the increase in GSH levels was significant in participants performing breathing exercises in this meta-analysis; however, Leelarungrayub et al. (51, 52) concluded that there was no significant change in GSH levels in patients with COPD. This may be due to the training method or the disease period of the population of the included studies. Another study (53) used a Drager Evi-ta-II ventilator to conduct respiratory interventions on patients with acute exacerbations of COPD and reported increased GSH levels, which improved the antioxidant capacity of the body. However, the sensitivity analysis in this study showed that there may be some bias in the previous study. In studies regarding the increase in GSH levels in patients with metabolic diseases and in healthy participants (46–48, 50), GSH improved the antioxidant defense mechanism by reducing the hydrogen peroxide dismutated from SOD into water during breathing exercises (12).
In this analysis, the variation of NO levels was the biggest difference, which may be due to the specific exercise programs in each included study. Moderate exercise increases the secretion of NO by endothelial cells, while long-term and high-intensity exercise can reduce the secretion of NO by endothelial cells (65). In patients with COPD, different respiratory interventions have resulted in conflicting data (13, 48, 51). These differences may also be related to the stimulation degree of endothelial cells in different disease states (66). A high NO level in patients with COPD is related to the severity of the disease and airflow obstruction (67). Breathing exercises stimulate endothelial cells to reduce NO secretion, which helps to prevent deterioration due to the disease (52). In patients with hypertension, oxidation imbalance leads to the production of a large quantity of ROS (68). The bioavailability of NO is increased by the activation of eNOS with ROS, improving OS (13). Under normal conditions, no changes in OS will occur in healthy individuals, and the increase in NO levels will cause damage to the tissues and cells of the body (69). Breathing exercises do not increase NO levels in healthy individuals (48). Therefore, when NO is used as an indicator of OS in different physiological states of the body, its influence on overall OS should be further determined according to the reaction mechanism in the body.
Total antioxidant capacity and CAT, vitamin C, and F2-isoprostane levels were not included in the meta-analysis. TAC and CAT levels were not significantly different between the groups in three studies (48, 51, 52), which may be due to the low sensitivity of these indicators or because respiratory interventions did not result in significant changes. Vitamin C may not be an accurate biomarker of oxidation-antioxidant status, as it can be obtained through food and vitamins (70, 71). Of the 10 studies included in this meta-analysis, one (48) showed the serum F2-isoprostane levels in healthy individuals and reported that the experimental group had lower levels than the control group. The F2-isoprostane content in exhaled air among patients with COPD increased after breathing exercises were performed but decreased after 6 min of walking (72). This may be due to the high variability and small sample size in the previous study. Rapid or gentle breathing regulates the contraction and relaxation of skeletal muscle, which can affect the F2-isoprostane content in exhaled air (73, 74). Aerobic exercise has been reported to reduce F2-isoprostane levels in patients with circulatory system or metabolic diseases (75, 76). However, the effects and action mechanism of breathing exercises on F2-isoprostane remain unknown. Future studies evaluating the role of F2-isoprostane in the OS state are needed to confirm the reference values in patients performing breathing exercises.
In the early stages of aging and diseases, the body reacts to harmful environmental stimuli by increasing the antioxidant levels (77). However, when these levels exceed the antioxidant capacity of the body, exogenous ROS cause inflammation (78), stimulate the release of endogenous superoxide and hydrogen peroxide to produce OS, and lead to a series of pathological reactions such as mitochondrial disorders, lipid peroxidation, and apoptosis. Inflammatory and pathological reactions further promote OS, creating a vicious cycle that worsens the disease (79). Breathing exercises are steady and medium-intensity exercises (54, 74), which effectively inhibit lipid peroxidation reactions, maintain the antioxidant capacity at a high level, enhance the stability of the internal environment, and promote the removal of harmful substances (80, 81).
4.1. Study limitations
Owing to the limited number of published articles, the specific methods of respiratory interventions and disease types were not strictly distinguished in this study. The differences in conclusions regarding changes in OS indicators in the included studies may be related to different breathing exercises and disease states included in the studies.
5. Conclusion
Breathing exercises can improve the main biological indicators of OS toward the direction of antioxidation and improve the OS state by increasing the levels of antioxidants and reducing those of oxidative markers. In addition, the MDA level is the most commonly used and most sensitive indicator to evaluate the impact of breathing exercises on the OS state. However, the effect of SOD on patients with diabetes and the role of GSH in patients with COPD require further research. The reaction mechanism of NO in patients with different diseases should be considered when it is used as an indicator of OS.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
TTL: conceptualization, methodology, formal analysis, investigation, and writing—original draft. HYW and HZ: investigation and editing. PPZ: conceptualization and writing—review and editing. MCZ and HYF: investigation. XYD and WBL: supervision. XWW and ZGS: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Youth Project of Shandong Natural Science Foundation (grant number: ZR2022QH094) and the National Food Safety Risk Assessment Center Scientific Research Joint Research Plan Project (grant number: LH2022GG11).
Acknowledgments
We are grateful to XYD and WBL for providing language support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Betteridge D. What is oxidative stress? Metabolism. (2000) 49 (Suppl 1):3–8. doi: 10.1016/S0026-0495(00)80077-3
2. Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev. (2017) 2017:6501046. doi: 10.1155/2017/6501046
3. Alkadi H. A review on free radicals and antioxidants. Infect Disord Drug Targets. (2020) 20:16–26. doi: 10.2174/1871526518666180628124323
4. Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. (2017) 524:13–30. doi: 10.1016/j.ab.2016.10.021
5. El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. (2013) 65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003
6. Reuter S, Gupta S, Chaturvedi M, Aggarwal B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. (2010) 49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006
7. Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K. Reactive oxygen species - sources, functions, oxidative damage. Pol Merkur Lekarski. (2020) 48:124–7.
8. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. (2004) 55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701
9. Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. (2020) 467:1–12. doi: 10.1007/s11010-019-03667-9
10. Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. (2016) 2016:4350965. doi: 10.1155/2016/4350965
11. Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. (2018) 217:1915–28. doi: 10.1083/jcb.201708007
12. Diaz-Vivancos P, de Simone A, Kiddle G, Foyer C. Glutathione–linking cell proliferation to oxidative stress. Free Radic Biol Med. (2015) 89:1154–64. doi: 10.1016/j.freeradbiomed.2015.09.023
13. Craighead D, Heinbockel T, Freeberg K, Rossman M, Jackman R, Jankowski L, et al. Time-efficient inspiratory muscle strength training lowers blood pressure and improves endothelial function, NO bioavailability, and oxidative stress in midlife/older adults With Above-normal blood pressure. J Am Heart Assoc. (2021) 10:e020980. doi: 10.1161/JAHA.121.020980
14. Rossi P, Marzani B, Giardina S, Negro M, Marzatico F. Human skeletal muscle aging and the oxidative system: cellular events. Curr Aging Sci. (2008) 1:182–91. doi: 10.2174/1874609810801030182
15. Cheresh P, Kim S, Tulasiram S, Kamp D. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. (2013) 1832:1028–40. doi: 10.1016/j.bbadis.2012.11.021
16. Kudryavtseva A, Krasnov G, Dmitriev A, Alekseev B, Kardymon O, Sadritdinova A, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. (2016) 7:44879–905. doi: 10.18632/oncotarget.9821
17. Peoples J, Saraf A, Ghazal N, Pham T, Kwong J. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. (2019) 51:1–13. doi: 10.1038/s12276-019-0355-7
18. Liu C, Lyass A, Larson M, Massaro J, Wang N, D’Agostino R Sr., et al. Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. Age (Dordr). (2016) 38:1. doi: 10.1007/s11357-015-9864-z
19. Saum K, Dieffenbach A, Jansen E, Schöttker B, Holleczek B, Hauer K, et al. Association between oxidative stress and frailty in an elderly German population: results from the Esther cohort study. Gerontology. (2015) 61:407–15. doi: 10.1159/000380881
20. Soysal P, Isik A, Carvalho A, Fernandes B, Solmi M, Schofield P, et al. Oxidative stress and frailty: a systematic review and synthesis of the best evidence. Maturitas. (2017) 99:66–72. doi: 10.1016/j.maturitas.2017.01.006
21. Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis. (2014) 42 (Suppl 3):S125–52. doi: 10.3233/JAD-132738
22. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. (2017) 14:470–85. doi: 10.1038/nrurol.2017.69
23. Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull. (2014) 30:271–81. doi: 10.1007/s12264-013-1423-y
24. Kattoor A, Pothineni N, Palagiri D, Mehta J. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. (2017) 19:42. doi: 10.1007/s11883-017-0678-6
25. Khatri N, Thakur M, Pareek V, Kumar S, Sharma S, Datusalia A. Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets. (2018) 17:689–95. doi: 10.2174/1871527317666180627120501
26. Shaw P, Werstuck G, Chen Y. Oxidative stress and aging diseases. Oxid Med Cell Longev. (2014) 2014:569146. doi: 10.1155/2014/569146
27. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez M, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. (2019) 2019:7092151. doi: 10.1155/2019/7092151
28. Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. (2019) 2019:8267234. doi: 10.1155/2019/8267234
29. Antus B. Oxidative stress markers in sputum. Oxid Med Cell Longev. (2016) 2016:2930434. doi: 10.1155/2016/2930434
30. Tóthová L, Kamodyová N, Èervenka T, Celec P. Salivary markers of oxidative stress in oral diseases. Front Cell Infect Microbiol. (2015) 5:73. doi: 10.3389/fcimb.2015.00073
31. Patsiris S, Stelios G, Papanikolaou I, Exarchos T, Vlamos P. Exhaled breath condensate: a potential tool to evaluate the relationship between inflammation and dyspnea in patients with chronic obstructive pulmonary disease. J Lung Health Dis. (2021) 5:3–7. doi: 10.29245/2689-999X/2021/1.1168
32. Sánchez-Rodríguez M, Mendoza-Núñez V. Oxidative stress indexes for diagnosis of health or disease in humans. Oxid Med Cell Longev. (2019) 2019:4128152. doi: 10.1155/2019/4128152
33. Mendoza-Núñez V, Arista-Ugalde T, Rosado-Pérez J, Ruiz-Ramos M, Santiago-Osorio E. Hypoglycemic and antioxidant effect of Tai chi exercise training in older adults with metabolic syndrome. Clin Interv Aging. (2018) 13:523–31. doi: 10.2147/CIA.S157584
34. Niu A. Effect of “TAI chi” exercise on antioxidant enzymes activities and immunity function in middle-aged participants. Afr J Tradit Complement Altern Med. (2016) 13:87–90.
35. Powers S, Radak Z, Ji L. Exercise-induced oxidative stress: past, present and future. J Physiol. (2016) 594:5081–92. doi: 10.1113/JP270646
36. Fiorenza M, Gunnarsson T, Ehlers T, Bangsbo J. High-intensity exercise training ameliorates aberrant expression of markers of mitochondrial turnover but not oxidative damage in skeletal muscle of men with essential hypertension. Acta Physiol (Oxf). (2019) 225:e13208. doi: 10.1111/apha.13208
37. Cezar M, Oliveira Junior S, Damatto R. Moderate-intensity resistance training improves oxidative stress in heart. Arq Bras Cardiol. (2021) 116:12–3.
38. Cornelison S, Pascual R. Pulmonary rehabilitation in the management of chronic lung disease. Med Clin North Am. (2019) 103:577–84. doi: 10.1016/j.mcna.2018.12.015
39. Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: a systematic review and meta-analysis. Clin Respir J. (2018) 12:2178–88. doi: 10.1111/crj.12905
40. Illi S, Held U, Frank I, Spengler C. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med. (2012) 42:707–24. doi: 10.1007/BF03262290
41. Yan H, Ouyang Y, Wang L, Luo X, Zhan Q. Effect of respiratory rehabilitation training on elderly patients with COVID-19: a protocol for systematic review and meta-analysis. Med (Baltim). (2020) 99:e22109. doi: 10.1097/MD.0000000000022109
42. Khandekar J, Vasavi V, Singh V, Samuel S, Sudhan S, Khandelwal B. Effect of yoga on blood pressure in prehypertension: a systematic review and meta-analysis. Sci World J. (2021) 2021:4039364. doi: 10.1155/2021/4039364
43. de Medeiros A, Fuzari H, Rattesa C, Brandão D, de Melo Marinho P. Inspiratory muscle training improves respiratory muscle strength, functional capacity and quality of life in patients with chronic kidney disease: a systematic review. J Physiother. (2017) 63:76–83. doi: 10.1016/j.jphys.2017.02.016
44. Senaratna C, Perret J, Lodge C, Lowe A, Campbell B, Matheson M, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. (2017) 34:70–81. doi: 10.1016/j.smrv.2016.07.002
45. Bhattacharya S, Pandey U, Verma N. Improvement in oxidative status with yogic breathing in young healthy males. Indian J Physiol Pharmacol. (2002) 46:349-354.
46. Hegde S, Adhikari P, Kotian S, Shastry R. Effects of yoga Versus sham yoga on oxidative stress, glycemic status, and anthropometry in type 2 diabetes mellitus: a single-blinded randomized pilot study. Int J Yoga Therap. (2020) 30:33-39. doi: 10.17761/D-18-2020-00018
47. Hegde S, Adhikari P, Subbalakshmi N, Nandini M, Rao G, D’Souza V. Diaphragmatic breathing exercise as a therapeutic intervention for control of oxidative stress in type 2 diabetes mellitus. Complement Ther Clin Pract. (2012) 18:151-153. doi: 10.1016/j.ctcp.2012.04.002
48. Lim S, Cheong K. Regular yoga practice improves antioxidant status, immune function, and stress hormone releases in young healthy people: a randomized, double-blind, controlled pilot study. J Altern Complement Med. (2015) 21:530-538. doi: 10.1089/acm.2014.0044
49. Mahapure H, Shete S, Bera T. Effect of yogic exercise on super oxide dismutase levels in diabetics. Int J Yoga. (2008) 1:21-26. doi: 10.4103/0973-6131.36792
50. Patil S, Dhanakshirur G, Aithala M, Naregal G, Das K. Effect of yoga on oxidative stress in elderly with grade-I hypertension: a randomized controlled study. J Clin Diagn Res. (2014) 8:BC04-BC07. doi: 10.7860/JCDR/2014/9498.4586
51. Leelarungrayub J, Pinkaew D, Puntumetakul R, Klaphajone J. Effects of a simple prototype respiratory muscle trainer on respiratory muscle strength, quality of life and dyspnea, and oxidative stress in COPD patients: a preliminary study. Int J Chron Obstruct Pulmon Dis. (2017) 12:1415-1425. doi: 10.2147/COPD.S131062
52. Leelarungrayub J, Puntumetakul R, Sriboonreung T, Pothasak Y, Klaphajone J. Preliminary study. Preliminary study: comparative effects of lung volume therapy between slow and fast deep-breathing techniques on pulmonary function, respiratory muscle strength, oxidative stress, cytokines, 6-minute walking distance, and quality of life in persons with COPD. Int J Chron Obstruct Pulmon Dis. (2018) 13:3909-3921. doi: 10.2147/COPD.S181428
53. Xu L, Li G, Gan Y, Zhang J, Li X. Effects of external diaphragmatic pacing combined with inspiratory muscle training on arterial blood gas parameters, respiratory mechanical parameters, and oxidative stress response in patients with AECOPD. Anhui Med Pharm J. (2021) 25:777–81.
54. Figueiredo P, Lima M, Costa H, Martins J, Flecha O, Gonçalves P, et al. Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: a randomized clinical trial. PLoS One. (2018) 13:e0200727. doi: 10.1371/journal.pone.0200727
55. Campos N, Marizeiro D, Florêncio A, Silva Í, Meneses G, Bezerra G, et al. Effects of respiratory muscle training on endothelium and oxidative stress biomarkers in hemodialysis patients: a randomized clinical trial. Respir Med. (2018) 134:103–9. doi: 10.1016/j.rmed.2017.12.005
56. Pardy R, Reid W, Belman M. Respiratory muscle training. Clin Chest Med. (1988) 9:287–96. doi: 10.1016/S0272-5231(21)00505-0
57. Göhl O, Walker D, Walterspacher S, Langer D, Spengler C, Wanke T, et al. [Respiratory muscle training: state of the art]. Pneumologie. (2016) 70:37–48. doi: 10.1055/s-0041-109312
58. Mills D, Johnson M, Barnett Y, Smith W, Sharpe G. The effects of inspiratory muscle training in older adults. Med Sci Sports Exerc. (2015) 47:691–7. doi: 10.1249/MSS.0000000000000474
59. Nakamura T, Naguro I, Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj. (2019) 1863:1398–409. doi: 10.1016/j.bbagen.2019.06.010
60. Brieger K, Schiavone S, Miller F Jr., Krause K. Reactive oxygen species: from health to disease. Swiss Med Wkly. (2012) 142:w13659. doi: 10.4414/smw.2012.13659
61. Woo J, Yeo N, Shin K, Lee H, Yoo J, Kang S. Antioxidant enzyme activities and DNA damage in children with type 1 diabetes mellitus after 12 weeks of exercise. Acta Paediatr. (2010) 99:1263–8. doi: 10.1111/j.1651-2227.2010.01736.x
62. Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Metab Syndr. (2019) 13:1165–72. doi: 10.1016/j.dsx.2019.01.040
63. Rehman K, Akash M. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J Cell Biochem. (2017) 118:3577–85. doi: 10.1002/jcb.26097
64. Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. (2015) 5:194–222. doi: 10.3390/biom5010194
65. Gordon L, Morrison E, McGrowder D, Young R, Fraser Y, Zamora E, et al. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Altern Med. (2008) 8:21. doi: 10.1186/1472-6882-8-21
66. Wong B, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. (2017) 36:2187–203. doi: 10.15252/embj.201696150
67. ben Anes A, Fetoui H, Bchir S, ben Nasr H, Chahdoura H, Chabchoub E, et al. Increased oxidative stress and altered levels of nitric oxide and peroxynitrite in Tunisian patients with chronic obstructive pulmonary disease: correlation with disease severity and airflow obstruction. Biol Trace Elem Res. (2014) 161:20–31. doi: 10.1007/s12011-014-0087-4
68. Guzik T, Touyz R. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. (2017) 70:660–7. doi: 10.1161/HYPERTENSIONAHA.117.07802
69. Pacher P, Beckman J, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. (2007) 87:315–424. doi: 10.1152/physrev.00029.2006
70. Oudemans-van Straaten H, Spoelstra-de Man A, de Waard M. Vitamin C revisited. Crit Care. (2014) 18:460. doi: 10.1186/s13054-014-0460-x
71. Kaźmierczak-Barańska J, Boguszewska K, Adamus-Grabicka A, Karwowski B. Two faces of vitamin C-antioxidative and pro-oxidative agent. Nutrients. (2020) 12:1501. doi: 10.3390/nu12051501
72. Kaminsky D, Guntupalli K, Lippmann J, Burns S, Brock M, Skelly J, et al. Effect of yoga breathing (pranayama) on exercise tolerance in patients with chronic obstructive pulmonary disease: a randomized, controlled trial. J Altern Complement Med. (2017) 23:696–704. doi: 10.1089/acm.2017.0102
73. Nikolaidis M, Kyparos A, Vrabas ISF. 2-isoprostane formation, measurement and interpretation: the role of exercise. Prog Lipid Res. (2011) 50:89–103. doi: 10.1016/j.plipres.2010.10.002
74. Briskey D, Vogel K, Johnson M, Sharpe G, Coombes J, Mills D. Inspiratory flow-resistive breathing, respiratory muscle-induced systemic oxidative stress, and diaphragm fatigue in healthy humans. J Appl Physiol (1985). (2020) 129:185–93. doi: 10.1152/japplphysiol.00091.2020
75. Liu G, Zhao J, Cao Y. Arterial stiffness response to regular aerobic training in hypercholesterolemia patients. Int J Sports Med. (2018) 39:413–8. doi: 10.1055/a-0574-3096
76. Ikizler T, Robinson-Cohen C, Ellis C, Headley S, Tuttle K, Wood R, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. (2018) 29:250–9. doi: 10.1681/ASN.2017010020
77. Zhang P, Xu X, Li X. Cardiovascular diseases: oxidative damage and antioxidant protection. Eur Rev Med Pharmacol Sci. (2014) 18:3091–6.
78. Barnes P. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). (2017) 131:1541–58. doi: 10.1042/CS20160487
79. Barnes P. Oxidative stress-based therapeutics in COPD. Redox Biol. (2020) 33:101544. doi: 10.1016/j.redox.2020.101544
80. Ryrsø C, Thaning P, Siebenmann C, Lundby C, Lange P, Pedersen B, et al. Effect of endurance versus resistance training on local muscle and systemic inflammation and oxidative stress in COPD. Scand J Med Sci Sports. (2018) 28:2339–48. doi: 10.1111/sms.13227
Keywords: breathing exercises, oxidative stress, malondialdehyde, superoxide dismutase, glutathione, nitric oxide
Citation: Li T-t, Wang H-y, Zhang H, Zhang P-p, Zhang M-c, Feng H-y, Duan X-y, Liu W-b, Wang X-w and Sun Z-g (2023) Effect of breathing exercises on oxidative stress biomarkers in humans: A systematic review and meta-analysis. Front. Med. 10:1121036. doi: 10.3389/fmed.2023.1121036
Received: 11 December 2022; Accepted: 20 March 2023;
Published: 05 April 2023.
Edited by:
Shuibang Wang, National Institutes of Health (NIH), United StatesCopyright © 2023 Li, Wang, Zhang, Zhang, Zhang, Feng, Duan, Liu, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong-guang Sun, c3VuemhvbmdndWFuZ0BvdXRsb29rLmNvbQ==; Xiao-wen Wang, V2FuZ3hpYW93ZW4yMDAzQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Ting-ting Li
Ting-ting Li Hong-ying Wang1†
Hong-ying Wang1† Xiao-yong Duan
Xiao-yong Duan Wen-bo Liu
Wen-bo Liu Xiao-wen Wang
Xiao-wen Wang