- 1Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Department of General Surgery, Huaihua Second People's Hospital, Huaihua, Hunan, China
Rationale: Synovial sarcoma is a subtype of soft tissue sarcoma. Synovial sarcoma in the head and neck region is relatively unusual. Primary synovial sarcoma of the thyroid gland (PSST) is first reported in 2003 by Inako Kikuchi. PSST is extremely rare with only 15 cases documented globally. PSST shows rapid disease progression and a relatively poor prognosis. However, diagnosis and therapy are challenging for clinical surgeons. In this article, we reported the 16th PSST case and reviewed the PSST cases globally for further clinical application.
Patient concerns: The patient was referred to us because of gradually worsened dyspnea and dysphagia for 20 days. Physical examination showed a 5 × 4 cm mass with a clear boundary and good mobility. Contrast-enhanced ultrasonography (CEUS) and computed tomography (CT) showed a mass in the isthmus of the thyroid gland. The imageology diagnosis tends to be a benign thyroid nodule.
Diagnosis: After surgery, histopathology, immunohistochemistry, and fluorescence, in situ hybridization indicated the mass to be primary synovial sarcoma of the thyroid gland with no local and distant metastasis.
Interventions: The patient underwent total thyroidectomy and dissected the lymph nodes in the central compartment. This patient received postoperative chemotherapy (a combination of ifosfamide and epirubicin for five cycles). Patients tolerated chemotherapy well. No recurrence was found during the 9-month follow-up.
Lessons: Although PSST is an extremely rare disease, we should raise our awareness when we encounter a rapidly growing, cystic-solid mixed thyroid mass with neck compression symptoms to avoid misdiagnosis. Intraoperatively, surgeons should refine surgical procedures to avoid capsular rupture and tumor local implantation metastasis. Intraoperative frozen section pathology is necessary sometimes, especially when the diagnosis could not be established before surgery.
Introduction
Synovial sarcoma is a kind of histological subtype of soft tissue sarcoma, making up 8–10% of all soft tissue sarcomas. It is a high-grade malignant neoplasm of mesenchymal pluripotent cells, characterized by a specific t(X;18) (p11.2; q11.2) translocation leading to the generation of SYT-SSX fusion gene transcripts (1, 2). According to different ultra structures, synovial sarcoma was divided into monophasic patterns and classic biphasic patterns (2). Synovial sarcoma in the head and neck region is relatively unusual, and primary synovial sarcoma in the thyroid gland is extremely rare with only 15 cases documented globally (3–13). Due to the rarity of the disease, challenges exist in clinical practice from diagnosis to follow-up. In the study, we report a case of a middle-aged female who was diagnosed with thyroid synovial sarcoma post-surgery. This is the 16th PSST case reported. For deep investigation, the clinical characteristics of 15 previously reported cases were also carefully summarized. Because of a lower survival rate (5-year survival rate of ~60%) than differentiated thyroid cancer (14), we hope our case report and literature review could arise awareness of thyroid synovial sarcoma and improve the diagnosis and treatment of this disease.
Case presentation
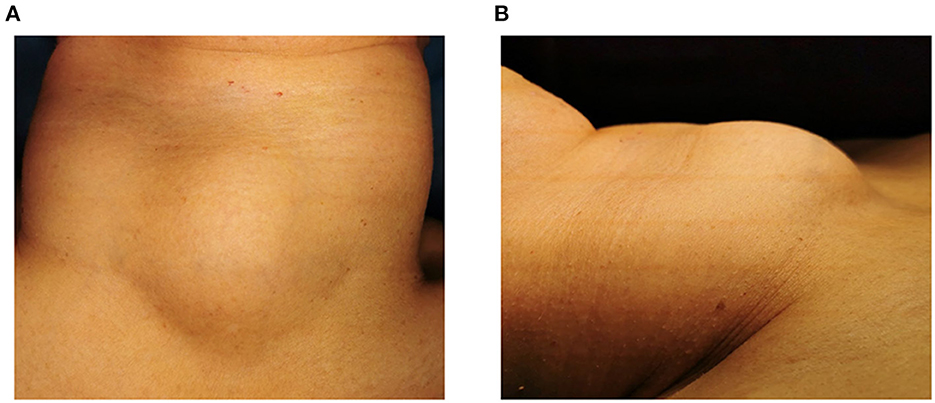
A 59-year-old female patient was admitted to our hospital in June 2022. Her chief complaints were dyspnea and dysphagia for 20 days. She felt dyspnea 20 days before, and the discomfort gradually worsened accompanied by cough and dysphagia. The physical examination showed a palpable hard mass in the center of the neck. The size of the mass was 5 × 4 cm (Figure 1). The boundary of the mass is clear, and no tenderness was found. The mass could move up and down with swallowing. There is no jugular vein distension, and the trachea is centered. No enlarged lymph node was found through palpation. No other signs were found in the physical examination. The patient denied symptoms such as fever, palpitations, shaky hands, and irascibility. There were no abnormalities in defecation and urine. There was no significant change in weight over the past 3 months. The patient underwent a cholecystectomy 10 years ago. She had a history of asthma for a year. Intermittent oral and inhaled drug therapy were used for asthma. The patient has no familial-hereditary disease, and no similar diseases were diagnosed in her family.
Accessory examination before surgery
A Blood test showed no obvious abnormalities. The level of thyroid hormone and calcitonin is in the normal range. No abnormalities were found in the laryngoscopy, chest X-ray, and chest CT. Contrast-enhanced ultrasonography showed a heterogeneous echoic, cystic-solid mixed mass ~48.8 × 37.2 mm in the isthmus of the thyroid gland. The mass is with clear boundary, regular shape. In total, 0.6 ml perflubutane microspheres were injected through the elbow intravenous for CEUS. The isthmus nodule was mostly equally enhanced and partially no enhancement. The peripheral nodule showed annular enhancement. No obvious enlarged lymph nodes were observed in the cervical region. The mass was classified as TI-RADS 3 (Figure 2). CT scan showed a mass lesion in or below the isthmus of the thyroid gland. The mass was heterogeneous density. There are microcalcifications and liquefaction necrosis areas inside the mass. The average CT value is 28 HU. The edge of the mass is mostly clear. The contrast-enhanced CT demonstrated heterogeneous enhancement. The degree of enhancement is lower than that of the normal thyroid gland. The average CT value is 58 HU. There are multiple small lymph nodes in the I-II area of the cervical region. The CT could not establish a confirmed diagnosis but a low-grade tumor could not be ruled out (Figure 3).
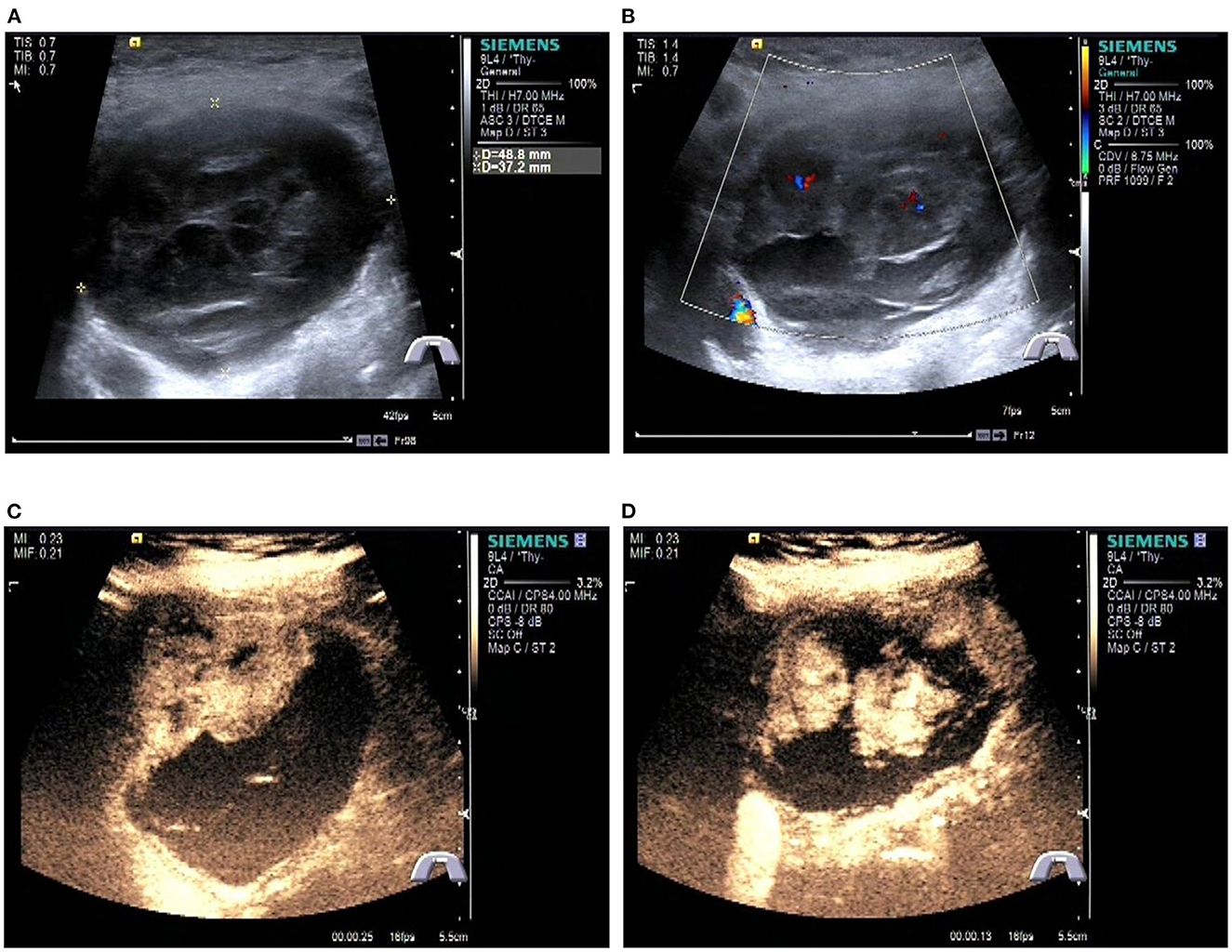
Figure 2. Preoperative contrast-enhanced ultrasonography of the patient. (A) Gray scale ultrasonography of the mass. (B) Color Doppler imaging of the mass. (C, D) Contrast-enhanced ultrasonography of the tumor.
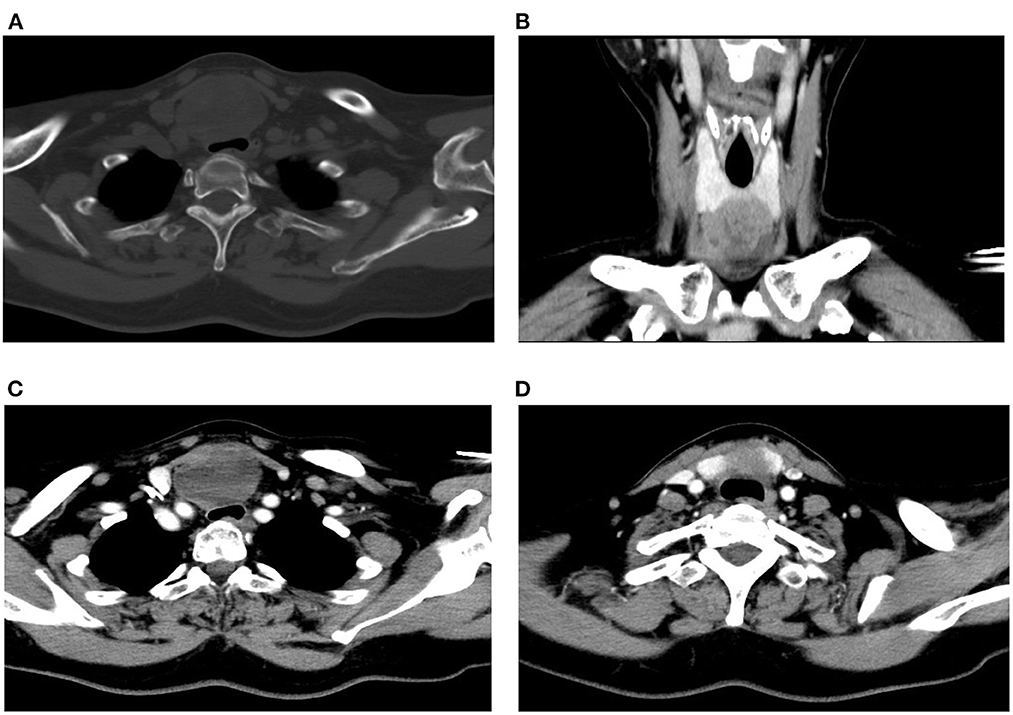
Figure 3. Preoperative CT scan of the patient. (A) Plain CT of the neck (horizontal plane). (B) CT scan of the neck (coronal plane). (C, D) Contrast-enhanced CT of the neck (horizontal plane).
Therapy
The patient was intended for partial thyroidectomy. We made a transverse arc-shaped incision in the suprasternal notch, dissociated the subcutaneous tissue, and opened the interval between the bilateral strap muscles in the neck to expose the thyroid mass. The mass was located in the isthmus of the thyroid gland and extended to the back of the sternum. The mass was cystic-solid mixed. The cyst wall was closely adhered to the trachea. Intraoperatively, the mass was difficult to be mobilized and led to a capsular rupture and an effusion of necrotic, hemorrhagic, and gelatinous liquid. When the mass was cut open, chocolate-like contents could be seen in the capsule. The solid section was fish-like, gray-red, and gray-yellow, and hard calcification was visible in some areas. Frozen section pathology showed soft tissue sarcoma, after which we choose to carry out total thyroidectomy and central cervical lymph nodes dissection.
Histopathology
Histopathology showed that the thyroid isthmus mass was a mesenchymal tissue-derived spindle cell malignant tumor, with epithelioid differentiation in the focal area. The nucleus was atypical. Lots of mitotic figures could be seen. Invasion of surrounding soft tissues was observed. There are no obvious masses in the left and right lobes of the thyroid gland. There was no metastasis in the central cervical lymph nodes (0/6) (Figure 4).
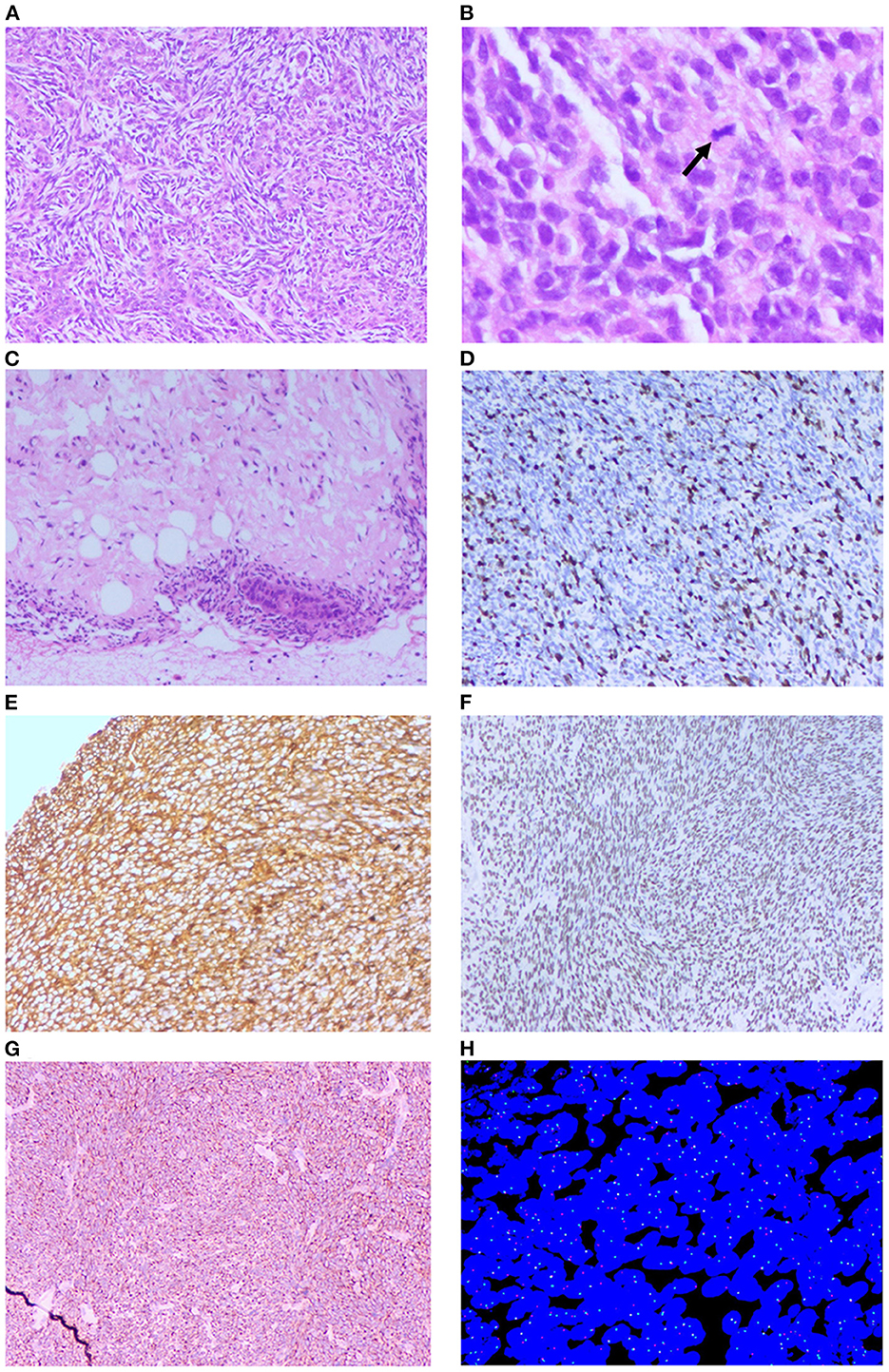
Figure 4. Histopathology examination and FISH of the patient. (A) Microscopic images showing biphasic tumor (H&E × 100). (B) Microscopic images showing pathological mitotic (Black arrow, H&E × 400). (C) Microscopic images showing the tumor invasion to adipose tissue (H&E × 100). (D) Immunohistochemistry positive to Ki-67 (H&E × 100). (E) Immunohistochemistry positive to Vim (H&E × 100). (F) Immunohistochemistry positive to TLE1 (H&E × 100). (G) Immunohistochemistry positive to Bcl-2 (H&E × 100). (H) Fluorescence in situ hybridization (FISH) break-apart probe confirmed SS18 gene rearrangement (split red and green signal).
Immunohistochemistry
CK(-), EMA(-), CK7(-), CK19(-), CD99(-), Bcl-2(+), WT-1(-), TLE1(+), Ki-67(50%), P53(30%+), Vim(+), Syn(focally +), S100(-), SMA(-), MyoD1(-), STAT6(-), and SOX-10(-) (Figure 4).
Fluorescence in situ hybridization (FISH)
Molecular analysis, performed on the formalin-fixed paraffin-embedded tissue by fluorescent in situ hybridization (FISH), showed an SS18 (SYT) gene rearrangement, highly consistent with the diagnosis of a PSST (Figure 4).
Pathology Stage: pT2N0M0. Stage IIA
Postoperative adjuvant therapy and follow-up
After the initial surgery, chest CT, abdominal CT, MRI of the head, and whole-body bone scan were performed for evaluation. No obvious local and distant recurrence or metastases was found. After evaluation, this patient received chemotherapy (a combination of ifosfamide 3,000 mg and epirubicin 120 mg). The patient tolerated chemotherapy well. The patient received five-cycle chemotherapy. Postoperative adjuvant radiation therapy was recommended by the oncologist, but the patient refused for personal reasons. After 9 months of the surgery, no remarkable recurrence and metastases were detected.
Discussion
In this article, we reported the 16th PSST case globally. Although the mass of this patient was mostly composed of spindle cells, a lack of normal thyroid follicular epithelial cells, the boundary between the mass and the surrounding thyroid tissue was unclear, and a part of the normal thyroid tissue was replaced by the synovial sarcoma. In synovial sarcoma of the head and neck regions originating from other structures, the main tumor foci are usually situated around their local regions instead of the thyroid gland. Therefore, according to a definition in a previous study, this mass was identified to be primary thyroid synovial sarcoma (5). When summarizing the clinical features of this case, we could find that the course of the disease was only 20 days, and the mass has some similarities with the benign thyroid nodule (including clear boundary, regular shape, and annular enhancement on ultrasound). These characteristics may lead to a missed diagnosis of malignancy in preoperative evaluation. However, when trying for total excision, we found that the mass closely adhered to the trachea, which is different from a typical benign thyroid tumor. Therefore, we choose to perform frozen section pathology intraoperatively. The intraoperative frozen section pathology assisted the surgeon to make clinical decisions and helped the patient to avoid reoperation. According to the research by Maduekwe et al. synovial sarcoma was thought to have a predilection for metastases to lymph nodes, necessitating the need for possible sentinel node biopsy (15, 16). Therefore, when we encounter rapidly growing thyroid nodules, we should be careful in perioperative assessment and perform intraoperative frozen section pathology when necessary.
For further investigation, we searched the PSST case in PubMed and Web of Science. A total of 15 cases were reported previously. We summarized the clinical characteristics of the 15 cases in Table 1. In most PSST cases, the course of the disease is < 1 year and progressing rapidly. In cases with detailed follow-up, most PSST patients presented recurrence within 2 years. A total of eight cases have detailed survival data. Among them, six patients have fatal events. The longest survival time in these patients is 126 months (in a study by Carina Owen) and four patients died within 3.5 years. The 5-year survival rate is consistent with a previous study (14). Generally, PSST showed rapid disease progression and relatively poor prognosis. Regarding the preoperative evaluation of PSST, the tumor is usually cystic-solid mixed, larger than 5 cm, and presented symptoms of neck compression. In eight cases, fine needle aspiration (FNA) was performed for diagnosis. However, only one case has been suspected to be a malignant spindle cell tumor. Most patients were diagnosed with MTC or undifferentiated thyroid carcinoma by FNA, and some patients could not be diagnosed by FNA. These data indicated that the cytopathological diagnosis of PSST is challenging. Although Aisner et al. proposed that FNA is important for diagnosing soft tissue synovial sarcoma, establishing the diagnosis of PSST is difficult due to its rarity (17). We should be careful in clinical practice in order to reduce misdiagnose rate of this rare disease even when FNA indicated a benign thyroid tumor, especially in a rapidly growing, cystic-solid mixed thyroid mass with neck compression symptoms.
PSST was usually misdiagnosed with MTC, undifferentiated/anaplastic thyroid carcinoma, or SETTLE. In this part, we would discuss the differential diagnosis of PSST. For PSST, the main differential diagnosis is MTC. An elevated calcitonin level is typical in MTC patients. In a microscopic vision, the MTC tissue usually consists of lympho/plasmacytoid cells, atypical cells, or spindle cells. However, a pure spindle cell pattern MTC is uncommon. Giant cells or multinucleated cells could be seen. The MTC tumor cells are generally positive for calcitonin, thyroglobulin, and neuroendocrine markers (18). SETTLE is simple for spindle epithelioid tumors with thymus-like differentiation. SETTLE is common in children and young adults. The mass is slow-growing. The incidence in male is higher than in female. Similar to PSST, the tumor consists of spindle cells with a variable epithelial component. In SETTLE, the mitotic rate is usually low and necrosis is relatively rare. The spindle cells are positive for high-molecular-weight keratin and vimentin (19). Occasionally, PSST would be misdiagnosed as undifferentiated/anaplastic thyroid carcinoma (UTC/ATC). UTC/ATC is usually presented as a rapidly enlarging thyroid mass. It is more common in elderly patients. The tumor is composed of spindle cells with ample cytoplasm, coarse chromatin, frequent mitoses, giant cells, and neutrophils. UTC/ATC is positive for Pax-8 and has p53 mutations (20).
Until now, there is no standard and ideal therapeutic regimen for PSST. Surgical resection remains the mainstay of management for localized synovial sarcoma with or without radiation (11). When the tumor invades important structures and cannot be completely removed, postoperative adjuvant chemoradiotherapy is generally recommended. Some retrospective studies have reported an improvement in local control and disease-free survival conferred by radiotherapy (21–23). A combination of doxorubicin and ifosfamide was considered to be the recommended chemotherapeutic regimen when the primary tumor could not be resected completely in most cases. While PSST did not show an obvious genetic predisposition, the consanguinity of the patient should raise their awareness and perform a regular physical examination.
Limitation
Although we tried our best to document this clinical case report in detail, there are still some limitations. First, because the tumor, in this case, was suspected to be benign in the preoperative examination, we did not perform screening for systemic metastases before surgery, but relevant examinations including chest CT, abdominal CT, and MRI of the head and whole-body bone scan were performed before and during chemotherapy. Second, due to capsular rupture and necrotic effusion during the operation, we are pitiful for failing to take photos of the tumor. The necrotic effusion might lead to tumor local implantation metastasis, making postoperative adjuvant therapy necessary and important. Last but not the least, despite the follow-up of the case being less than 1 year, complete treatment for PSST in this patient has been completed, and no recurrence and metastasis have been detected in 9 months postoperatively. We will continue to focus on the case. We will update this case report and literature review if recurrence or death occurs in the current case.
Established facts
1. Synovial sarcoma is a subtype of soft tissue sarcoma, which is extremely rare in the thyroid gland. PSST showed rapid disease progression and a relatively poor prognosis. However, diagnosis and therapy are challenging.
2. Complete surgical excision and postoperative adjuvant chemotherapy are usually the typical therapeutic regimen for PSST.
Novel insights
1. PSST is difficult to be diagnosed by preoperative evaluation, even FNA. PSST was occasionally misdiagnosed as a benign thyroid tumor. Surgeons should raise their awareness when encountering rapidly growing, cystic-solid mixed thyroid mass with neck compression symptoms.
2. The initial surgery of PSST is of vital importance. Surgeons should avoid capsular rupture and effusion of necrotic during surgery. Intraoperative frozen section pathology should be performed when necessary. Postoperative adjuvant chemotherapy should be scheduled for reduced recurrence and prolonged survival time.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from patient for the publication.
Author contributions
JH, YG, FY, and CR performed the surgery. CR, ZC, QJ, and YL collected the data from the patient. CR, XL, and SL drafted the first manuscript. YG and FY revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Key R&D Program of China (Grant No. 2019YFE0190500), the Hunan Provincial Natural Science Foundation of China (No. 2022JJ30806), and the Scientific research project of Hunan Provincial Health Commission (No. 202204014372).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PSST, primary synovial sarcoma of the thyroid gland; CEUS, Contrast-enhanced ultrasonography; CT, computed tomography; MTC, medullary thyroid carcinoma; SETTLE, spindle epithelioid tumor with thymus-like differentiation; UTC/ATC, undifferentiated/anaplastic thyroid carcinoma; MRI, magnetic resonance imaging.
References
1. Fonseca AS, Azevedo AC, Magalhaes FM, Andrade NA. Synovial sarcoma in head and neck: a case report. Int Arch Otorhinolaryngol. (2014) 18:87–9. doi: 10.1055/s-0033-1361081
2. Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol. (2014) 18:369–80. doi: 10.1016/j.anndiagpath.2014.09.002
3. Kikuchi I, Anbo J, Nakamura S, Sugai T, Sasou S, Yamamoto M, et al. Synovial sarcoma of the thyroid. Report of a case with aspiration cytology findings and gene analysis. Acta Cytol. (2003) 47:495–500. doi: 10.1159/000326558
4. Jang KS, Min KW, Jang SH, Paik SS, Tae K, Jang SJ, et al. Primary synovial sarcoma of the thyroid gland. J Korean Med Sci. (2007) 22(Suppl):S154–8. doi: 10.3346/jkms.2007.22.S.S154
5. Ryu CH, Cho KJ, Choi SH. Synovial sarcoma of the thyroid gland. Clin Exp Otorhinolaryngol. (2011) 4:204–6. doi: 10.3342/ceo.2011.4.4.204
6. Ghafouri A, Anbara T, Mir A, Lashkari M, Nazari M. Thyroid synovial sarcoma: a case report. Acta Med Iran. (2013) 51:69–72.
7. Boudin L, Fakhry N, Chetaille B, Perrot D, Nguyen AT, Daidj N, et al. Primary synovial sarcoma of the thyroid gland: case report and review of the literature. Case Rep Oncol. (2014) 7:6–13. doi: 10.1159/000357913
8. Shi RL, Qu N, Gao LL, Lu ZW, Sun GH Ji QH. Primary synovial sarcoma of the thyroid with locally repeated relapses in short periods: a case report. Biomed Rep. (2016) 5:79–82. doi: 10.3892/br.2016.670
9. Park CS, Kim Y, Jeong EH, Kim NI, Choi YD. Cytologic features of primary monophasic synovial sarcoma of the thyroid gland. Cytojournal. (2017) 14:24. doi: 10.4103/cytojournal.cytojournal_14_17
10. Yadav D, Agarwal S, Sharma A, Malik E, Kandasamy D, Thakar A, et al. Synovial sarcoma masquerading as medullary thyroid carcinoma. Cytopathology. (2018) 29:468–70. doi: 10.1111/cyt.12582
11. Owen C, Constantinidou A, Miah AB, Thway K, Fisher C, Benson C, et al. Synovial sarcoma of the thyroid gland, diagnostic pitfalls and clinical management. Anticancer Res. (2018) 38:5275–82. doi: 10.21873/anticanres.12853
12. Demirel D, Erkul E, Erkilic S, Narli Issin G, Ramzy I. Primary synovial sarcoma of the thyroid: challenges in cytologic diagnosis and review of the literature. Acta Cytol. (2020) 64:498–506. doi: 10.1159/000507312
13. Seyed-Alagheband SA, Sharifian M, Adeli OA, Sohooli M, Shekouhi R. Primary synovial sarcoma of thyroid gland: a case report and review of literature. Int J Surg Case Rep. (2021) 85:106245. doi: 10.1016/j.ijscr.2021.106245
14. Salcedo-Hernandez RA, Lino-Silva LS, Luna-Ortiz K. Synovial sarcomas of the head and neck: comparative analysis with synovial sarcoma of the extremities. Auris Nasus Larynx. (2013) 40:476–80. doi: 10.1016/j.anl.2012.11.015
15. Maduekwe UN, Hornicek FJ, Springfield DS, Raskin KA, Harmon DC, Choy E, et al. Role of sentinel lymph node biopsy in the staging of synovial, epithelioid, and clear cell sarcomas. Ann Surg Oncol. (2009) 16:1356–63. doi: 10.1245/s10434-009-0393-9
16. Gazendam AM, Popovic S, Munir S, Parasu N, Wilson D, Ghert M. Synovial sarcoma: a clinical review. Curr Oncol. (2021) 28:1909–20. doi: 10.3390/curroncol28030177
17. Aisner SC, Seidman JD, Burke KC, Young JW. Aspiration cytology of biphasic and monophasic synovial sarcoma. A report of two cases Acta Cytol. (1993) 37:413–7.
18. Murro D, Slade JM, Syed S, Gattuso P. Fine needle aspiration of secondary synovial sarcoma of the thyroid gland. Diagn Cytopathol. (2015) 43:928–32. doi: 10.1002/dc.23327
19. Tong GX, Hamele-Bena D, Wei XJ, O'Toole K. Fine-needle aspiration biopsy of monophasic variant of spindle epithelial tumor with thymus-like differentiation of the thyroid: report of one case and review of the literature. Diagn Cytopathol. (2007) 35:113–9. doi: 10.1002/dc.20579
20. Pusztaszeri M, Wang H, Cibas ES, Powers CN, Bongiovanni M, Ali S, et al. Fine-needle aspiration biopsy of secondary neoplasms of the thyroid gland: a multi-institutional study of 62 cases. Cancer Cytopathol. (2015) 123:19–29. doi: 10.1002/cncy.21494
21. Guadagnolo BA, Zagars GK, Ballo MT, Patel SR, Lewis VO, Pisters PW, et al. Long-term outcomes for synovial sarcoma treated with conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. (2007) 69:1173–80. doi: 10.1016/j.ijrobp.2007.04.056
22. Shi W, Indelicato DJ, Morris CG, Scarborough MT, Gibbs CP, Zlotecki RA. Long-term treatment outcomes for patients with synovial sarcoma: a 40-year experience at the University of Florida. Am J Clin Oncol. (2013) 36:83–8. doi: 10.1097/COC.0b013e31823fe450
Keywords: primary synovial sarcoma of the thyroid gland, SS18 gene, perioperative evaluation, case report, literature review
Citation: Ren C, Li Y, Huang J, Liu S, Cao Z, Jiang Q, Lin X, Ye F and Gong Y (2023) Primary synovial sarcoma of the thyroid gland: a CARE compliant case report and literature review. Front. Med. 10:1158334. doi: 10.3389/fmed.2023.1158334
Received: 07 February 2023; Accepted: 29 March 2023;
Published: 10 May 2023.
Edited by:
Liam Chen, University of Minnesota, United StatesReviewed by:
Mousumi Chaudhury, Agricultural Research Service (USDA), United StatesSakinah Mohamad, Universiti Sains Malaysia Health Campus, Malaysia
Copyright © 2023 Ren, Li, Huang, Liu, Cao, Jiang, Lin, Ye and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Ye, eWVmZWlAY3N1LmVkdS5jbg==; Yi Gong, Z29uZ3lpQDEyNi5jb20=; Z29uZ3lpNTIyNkBjc3UuZWR1LmNu
†These authors have contributed equally to this work
 Chutong Ren
Chutong Ren Yashan Li
Yashan Li Jiangsheng Huang
Jiangsheng Huang Sushun Liu
Sushun Liu Zhexu Cao
Zhexu Cao Qin Jiang
Qin Jiang Xiang Lin2
Xiang Lin2 Fei Ye
Fei Ye Yi Gong
Yi Gong