- 1Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Mondino Foundation, Pavia, Italy
- 2Unit of Human and Clinical Nutrition, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
- 3Endocrinology and Nutrition Unit, Azienda di Servizi alla Persona “Istituto Santa Margherita”, University of Pavia, Pavia, Italy
- 4R&D Department, Indena SpA, Milan, Italy
- 5Department of Biology, College of Science, University of Bahrain, Zallaq, Bahrain
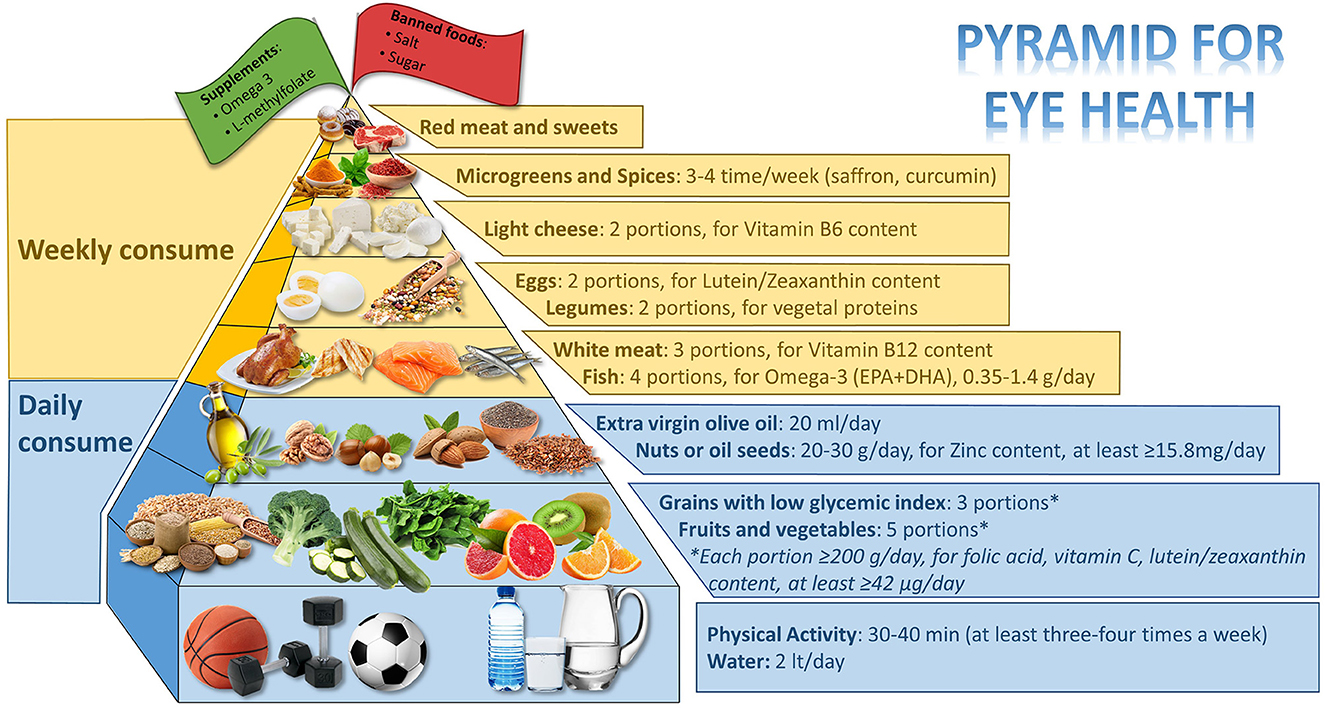
Many eye diseases, such as diabetic retinopathy (DR), age-related macular degeneration (AMD), and cataracts are preventable and treatable with lifestyle. The objective of this review is to assess the most recent research on the ideal dietary approach to prevent or support the treatment of DR, AMD, and cataracts, as well as to construct a food pyramid that makes it simple for people who are at risk of developing these pathologies to decide what to eat. The food pyramid presented here proposes what should be consumed every day: 3 portions of low glycemic index (GI) grains (for fiber and zinc content), 5 portions (each portion: ≥200 g/day) of fruits and vegetables (spinach, broccoli, zucchini cooked, green leafy vegetables, orange, kiwi, grapefruit for folic acid, vitamin C, and lutein/zeaxanthin content, at least ≥42 μg/day, are to be preferred), extra virgin olive (EVO) oil (almost 20 mg/day for vitamin E and polyphenols content), nuts or oil seeds (20–30 g/day, for zinc content, at least ≥15.8 mg/day); weekly: fish (4 portions, for omega-3 content and eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) 0.35–1.4 g/day), white meat (3 portions for vitamin B12 content), legumes (2 portions for vegetal proteins), eggs (2 portions for lutein/zeaxanthin content), light cheeses (2 portions for vitamin B6 content), and almost 3–4 times/week microgreen and spices (saffron and curcumin). At the top of the pyramid, there are two pennants: one green, which indicates the need for personalized supplementation (if daily requirements cannot be met through diet, omega-3, and L-methylfolate supplementation), and one red, which indicates that certain foods are prohibited (salt and sugar). Finally, 3–4 times per week, 30–40 min of aerobic and resistance exercises are required.
1. Introduction
The 2019 WHO World Report on Vision confirms that more than a billion people worldwide suffer from vision disorders, which can be prevented or treated to avoid blindness. Furthermore, the number of people suffering from partial or severe blindness is increasing alarmingly. Cataracts and refractive defects make up half of the cases of blindness or severe vision impairment; diabetic retinopathy (DR) is the major cause among persons of working age, whereas age-related macular degeneration (AMD) is the major cause in the elderly (1). While there is a surgical treatment for cataracts, there is still no cure for many eye conditions that cause blindness. Among these is AMD. This is why it is very important to study mechanisms that lead to disease and to slow down the progression through prevention. Diet and lifestyle are two of the most important thoroughly studied factors, but are still little known by patients. Both seem to significantly influence the onset of the disease and rate of progression. Many eye diseases are treatable and preventable, especially in the first phase in which they occur and lifestyle, understood as nutrition and physical activity (PA), plays an essential role. The growth of studies in the literature on the subject suggests that various eye diseases, including glaucoma, AMD, and DR are associated with lower levels of physical activity. Similarly, physical activity levels are lower in people with lower vision (2). The relationship between PA and three of the most common vision diseases has abundant evidence supporting a possible protective role of PA against vision loss. A very recent narrative review (3) analyzed evidence in the literature between dietary styles and common eye diseases: specifically, the authors conclude that there is enough evidence in the literature to suggest that the Mediterranean diet and the “Asian diet” are linked with a reduced incidence of AMD, whereas the Western diet is associated with a greater incidence. Moreover, there seems to be evidence of a positive correlation between the Western diet and the development of cataracts, while there are not enough data to identify a correct dietary style that prevents this pathology. The data currently available come mainly from observational studies and some randomized clinical studies related to nutritional epidemiology. Among these, the most important to-date remain the Age-Related Eye Disease Study (AREDS) and AREDS2 studies (4, 5).
1.1. Antioxidants
1.1.1. Carotenoids
Only zeaxanthin and meso-zeaxanthin (a lutein metabolite formed in the macula through metabolic transformation) are present in significant amounts in the macula of human plasma (6). Together, these two carotenoids form the pigment of the macula, an essential component for maintaining vision at optimal levels, and this pigment can be used as a marker to assess the risk of AMD. Diet and supplements can alter the concentration of lutein and zeaxanthin, and thus their potential biological function. In 1997, the study by Hammon showed that the modification of one's diet can modify retinal receptor density: the addition of 60 mg of spinach (10.8 mg of lutein, 0.3 mg of zeaxanthin, and 5 mg of b-carotene) and/or 150 g of corn (0.4 mg of lutein and 0.3 of zeaxanthin) for 15 weeks to one's daily diet affected retinal receptor density (+19%) in 8 out of 12 subjects studied (7). In 2007, Schalch administered lutein (10 mg), zeaxanthin (10 mg), or a combination of the two (10+10 mg) to 126 male subjects for 1 year to assess their ability to influence macular pigment optical density (MPOD), by measuring monthly retinal parameters. It was found that the administration of one of the two components alone or the combined administration of both can improve MPOD up to 15% of the initial value. Furthermore, it was found that lutein tends to act mainly on the fovea, while zeaxanthin acts on the entire surface of the retina (8). Johnson (2008) investigated the effect of lutein (12 mg) and docosahexaenoic acid (DHA) (800 mg) supplementation for 4 months in preventing AMD in a sample of 49 women (aged 60–80 years): the subjects were randomized into four treatment groups (placebo, DHA, lutein, and DHA+lutein) and were evaluated before and after treatment for blood parameters and MPOD. The study showed that both lutein and DHA can significantly increase MPOD individually and in combination (p < 0.01) at 2 and 4 months after administration (9). Another feature of lutein is that it can be retained in the human retina for an extended period. In the study carried out by Landrum, two healthy subjects were supplemented with lutein esters equivalent to 30 mg of free lutein for 140 days, and during the intake period, it was possible to highlight a significant increase (+20–40% depending on the eye and the subject analyzed) of the MPOD, and this parameter continued to increase up to 50 days after the suspension of the supplementation and then progressively decreased (10). The articles by Eisenhauer and Perry report the content of lutein and zeaxanthin in foods; foods rich in lutein (lutein>900 mg/100 g) are in descending order of content: cooked spinach, cooked kale, cilantro, raw spinach, parsley, green leafy vegetables (lettuce and romaine), pistachios, zucchini cooked with skin, cooked asparagus. Foods rich in zeaxanthin (zeaxanthin>500 mg/100 g) are in descending order of content: scallions cooked in oil, oranges, raw egg yolk, and cooked egg yolk (11, 12).
The behavior of carotenoids in cooking has been investigated in the literature, but mainly for lutein, while studies on zeaxanthin are lacking. From a review by Palermo (13) regarding the effects of cooking on phytochemicals, several studies have analyzed lutein content in various vegetables before and after different types of cooking. Lutein tends to increase with steam cooking, probably due to the degradation of cellulose which allows for greater release and tends to be reduced with frying in proportion to the temperatures and surfaces exposed to cooking (cutting into smaller pieces tends to increase the surface in contact with the oil and therefore the loss of lutein). Evidence is conflicting for microwave cooking. A new frontier in the food sector is the use of “microgreens” or young seedlings (harvested 7–21 days after sowing) of various species of vegetables, wild plants, and aromatic herbs. These foods are richer in vitamins, micronutrients, and antioxidant compounds more than matured vegetables and plants. Xiao analyzed the content of lutein, zeaxanthin, tocopherol, beta carotene, and violaxanthin of these “young” vegetables (14): the microgreens richest in beta carotene (beta carotene:>10 mg/100 g fresh weight) are: cilantro, peppercress, red cabbage, and red sorrel, while the microgreens richest in lutein/zeaxanthin (lutein/zeaxanthin>8 mg/100 g fresh weight) include cilantro, garnet amaranth, and red cabbage. In consideration of these high contents of compounds useful for eye health, microgreens can be an excellent addition to a diet aimed at the prevention of eye diseases. Egg yolk is the finest non-vegetarian food source of lutein and zeaxanthin because eggs' high-fat content boosts the absorption of carotenoids (15), even though their level mostly depends on the hen's diet, which includes lutein and zeaxanthin in its esterified forms along with trace amounts of lycopene and β-carotene (16). In-depth knowledge of release into the circulation and before that of the absorption, transport, and accumulation of carotenoids in the eye is essential to evaluate their beneficial aspects. Carotenoids are generally lipophilic, however, lutein and zeaxanthin are more polar substances than hydrocarbon carotenoids like beta-carotene and lycopene because of the presence of the hydroxyl group. Lutein and zeaxanthin absorption from meals determines their bioavailability in ocular tissue (17), and intestinal absorption is in turn influenced by several factors: the type of the food matrix (natural food or supplement), the amount and type of fats consumed, which let carotenoids circulate, the potential existence of phospholipids, and the availability of dietary fiber. The characteristics of the food matrices have a significant impact on the bioavailability of carotenoids (18). Lutein, zeaxanthin, and beta-cryptoxanthin have been found to release almost completely from fruits (orange, kiwi, grapefruit, and sweet potato), but only 19–38% from green vegetables (spinach and broccoli) (19). Human tissues do not all contain the same amounts of lutein, with the macula having the highest concentration (20).
1.1.2. Vitamin A
Unsaturated isoprenoid chain structure distinguishes the group of fat-soluble, vegetal, and animal-derived chemicals known as vitamin A and, in general, they are defined “retinoids.” All vitamin A types have the same physiological effects on an organism and a comparable structural makeup and they could be either from a natural or synthetic source. Unlike water-soluble vitamins, all of these substances are liposoluble and can easily accumulate in the body, particularly in the liver and adipose tissue (21). In this instance, 11-cis-retinol is the active vitamin A derivate; it is connected to the G-coupled protein receptor in the retina known as opsin. The complex is referred to as rhodopsin, and it is the essential pigment for seeing in the dark (22). Vitamin A deficiency, common in the presence of generalized malnutrition, is associated with night blindness, conjunctival xerosis, and corneal ulceration, particularly with concomitant measles infection (23, 24). Two recent reviews have shown significant effects of vitamin A in preventing ocular diseases such as cataracts: data from the meta-analysis presented by Wang A et al. showed that ingesting enough vitamin A decreased the risk of cataracts by 17% (95% CI, 0.757–0.913) (25) and the review of Jiang H et al. showed a significant reduced risk of cataract by the consumption of carotenoids [relative risk (RR), 0.81; 95% CI, 0.71–0.92] (26). Although data from the National Health and Nutrition Examination Survey (NHANES I) initially showed a protective effect of a diet based on the high amount of fruit and vegetables rich in vitamin A on developing AMD (27), the following epidemiological studies did not found any significative evidence on the association between dietary intake of vitamin A and reduced risk of AMD (28), so further investigations are needed.
In light of this background, the objective of this review is to assess the most recent information regarding the ideal dietary approach to prevent or support the treatment of DR, AMD, and cataracts, and to construct a food pyramid that enables subjects who are at risk of developing these pathologies or subjects who have these pathologies to easily figure out what to eat.
Figure 1 summarizes the main risk factors common to the three eye diseases discussed in the review.
2. Methods
The procedures used to carry out this narrative review are as follows (29): (1) Three clinical nutrition-trained operators compose the working group (one acting as a methodological operator and two participating as clinical operators); (2) Formulation of the revision question based on the abstract's points: “the most recent information on the optimal dietary approach to prevent or support the treatment of DR, AMD, and cataracts”; (3) Identification of pertinent studies: The following research method was planned on PubMed [Public MEDLINE, operated by the National Center for Biotechnology Information (NCBI) of the National Library of Medicine of Bethesda (Bethesda, MD, USA)]: (a) the definition of the keywords (DR, foods, nutrients, and diet), which can be used singly or in combination, (b) the use of the Boolean operator, which enables the establishment of logical relationships between concepts, (c) advanced search as a research modality, (d) Limitations: human subjects; English; articles published within the last 30 years; and (e) manual search by senior researchers skilled in clinical nutrition through the revision of reviews and particular patient dietary therapy publications published in journals qualified in the Index Medicus; (4) analysis and presentation of outcomes: the data extrapolated from the “revised studies” were allocated in tables; in particular, for each study, the authors, year of publication, and study characteristics were reported; (5) A narrative review of the reports was used to carry out the analysis. Each section's introduction includes a list of the studies that were considered as well as the type of study and keywords. We reviewed studies of any design that took account of the importance of diet, foods, nutrients, and dietary patterns (DPs) to prevent or support the treatment of DR, AMD, and cataracts.
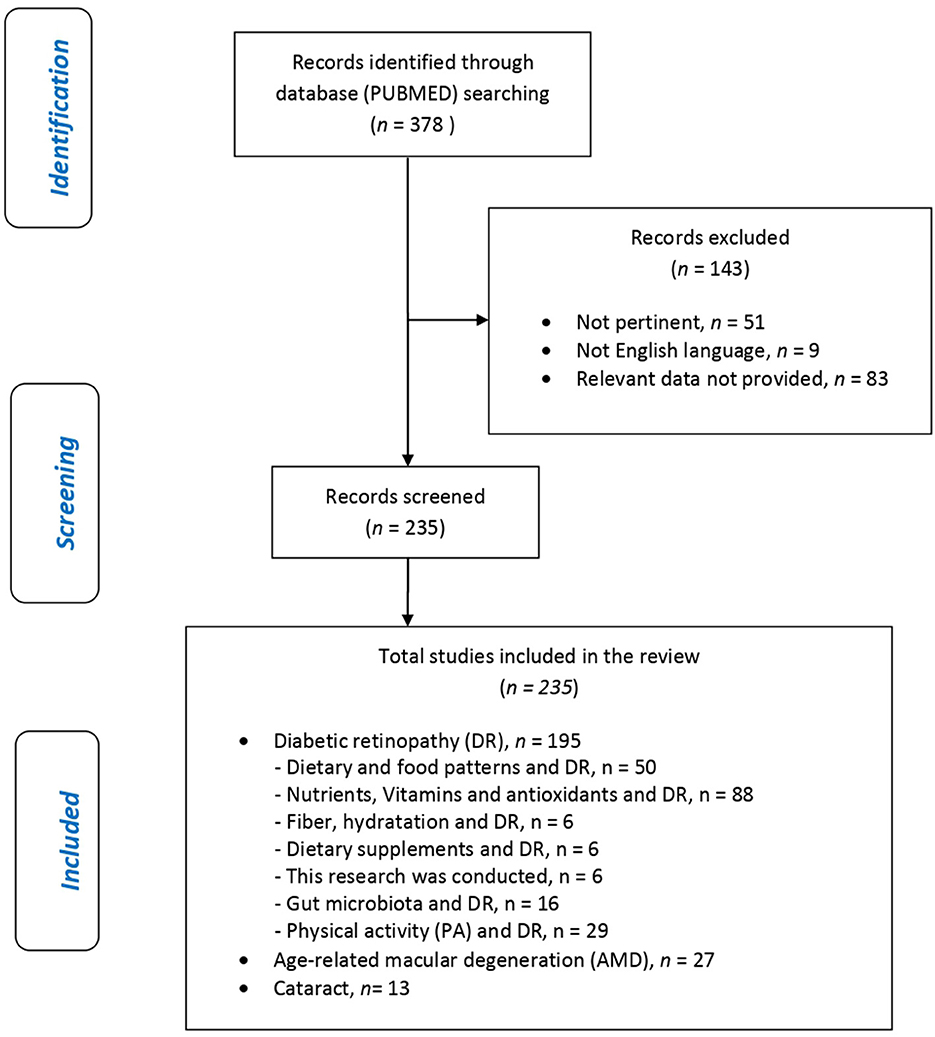
Figure 2 shows the eligible studies and Figure 3 represents proper nutrition and lifestyle to prevent or support the treatment of DR, AMD, and cataracts, specifying the quality and amount of food needed to provide ideal dietary management and to construct a food pyramid.
3. Results
3.1. DR
3.1.1. Dietary and food patterns
The following keywords served as the basis for this research: “diet” OR “Mediterranean diet” OR “natural food “OR “Fruits and vegetables” OR “nuts” OR “saffron” OR “curcumin” OR “Tea and coffee” AND “diabetic retinopathy” OR “eye diseases” OR “diabetes.” Thirty-one articles were consulted, including five narrative reviews, four systematic reviews, one systematic review & meta-analysis, a review of in vitro studies, 11 clinical trials (two post hoc analyses of randomized trials, one cross-sectional study, one retrospective study, two cohort studies, one randomized controlled trial, two case–control studies, and one prospective trial), six in vitro studies, and three studies on animal models.
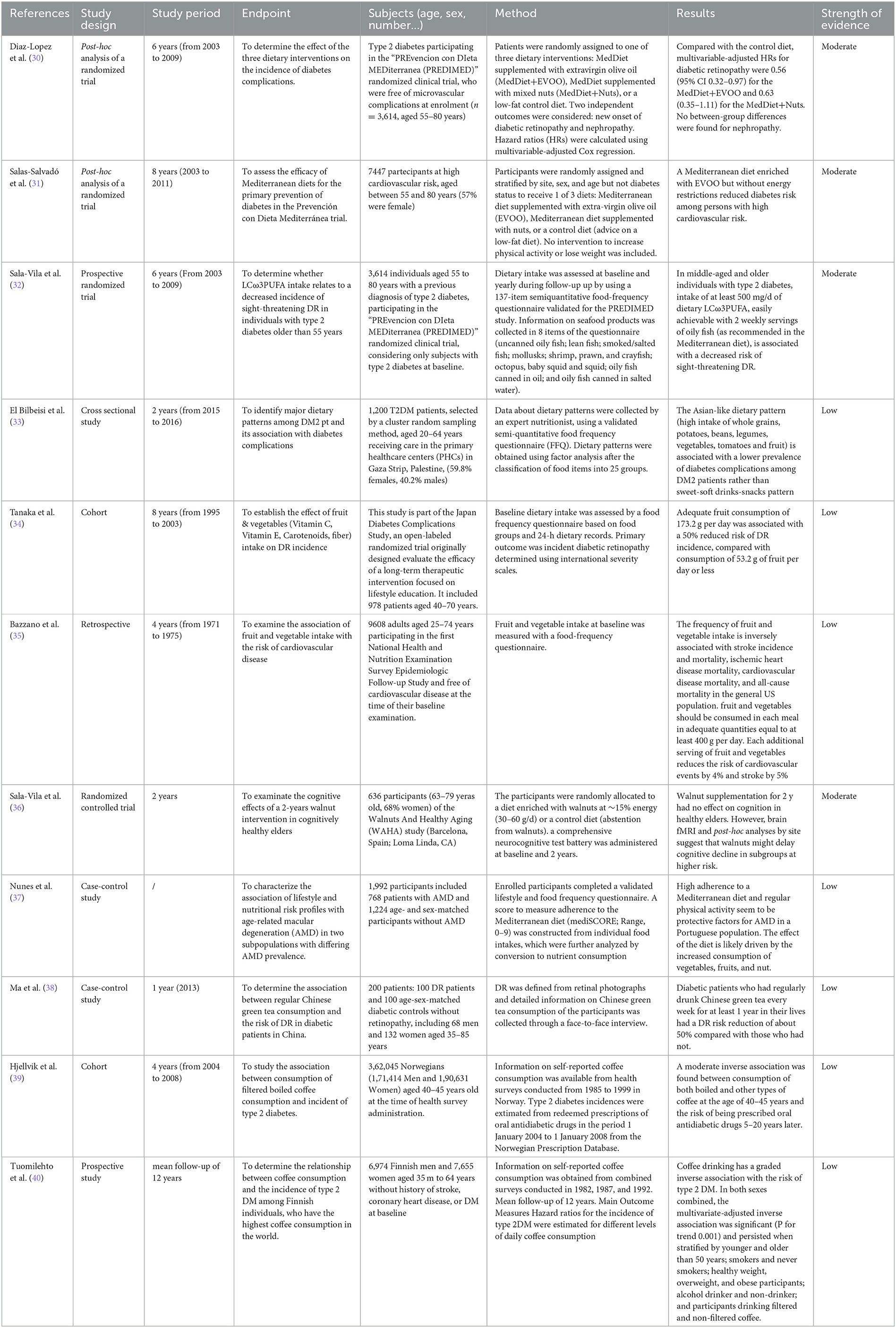
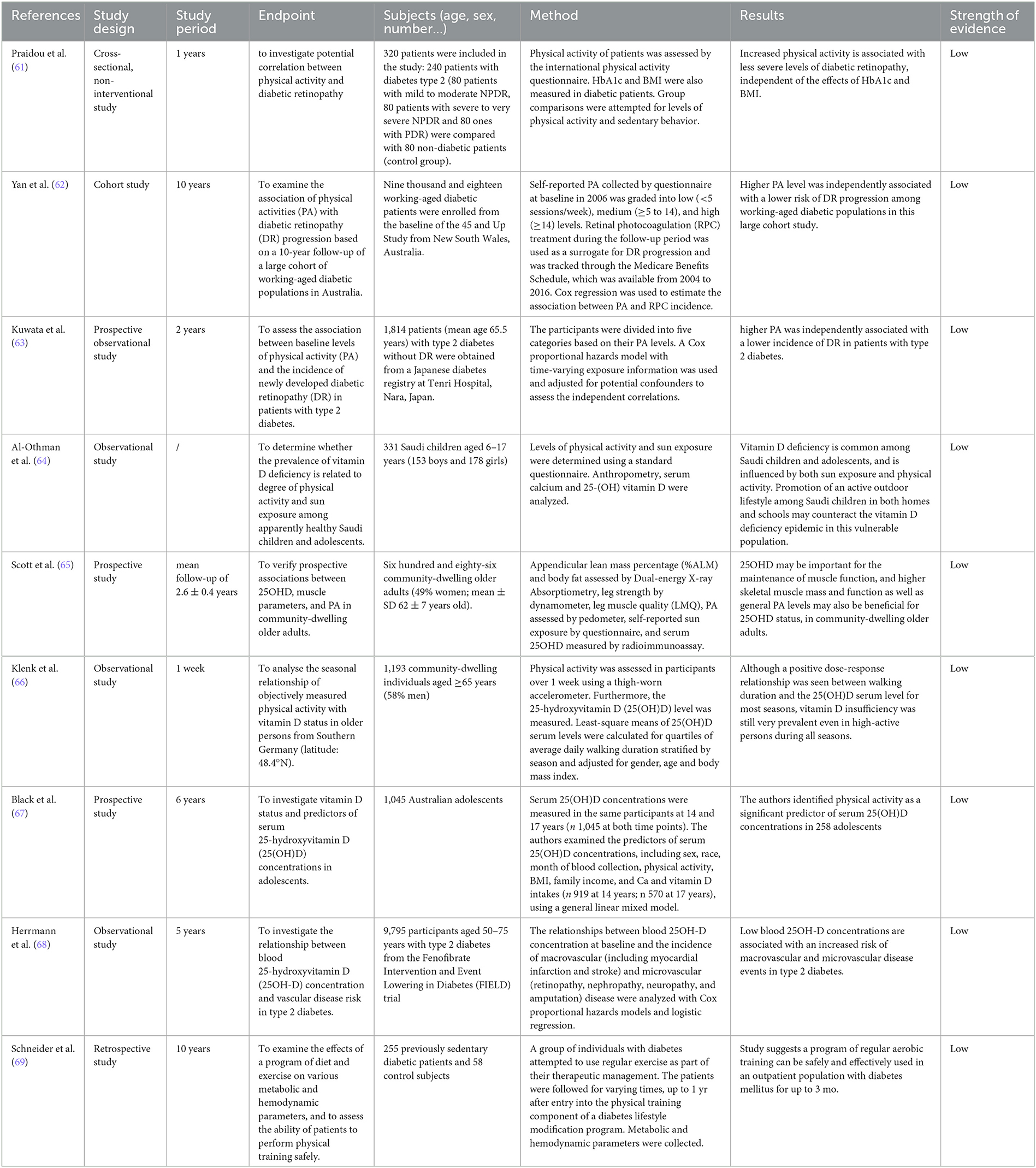
Table 1 shows the studies that evaluated the relationship between DPs and food and diabetic retinopathy with their strength of evidence.
3.1.2. Nutrients, vitamins, and antioxidants and DR
This research was conducted based on the keywords: “nutrients” OR “Vitamins” OR “antioxidants” OR “Vitamin A and carotenoids “OR “vitamin E” OR “vitamin D and 25-hydroxyvitamin D” OR “Polyphenols” OR “vitamin C” OR “B vitamins” OR “Fatty acids” OR “zinc” AND “diabetic retinopathy” OR “eye diseases” OR “diabetes.” Forty-one studies have been referenced, including nine narrative reviews, one systematic review, 19 clinical trials (two cross-sectional studies, five retrospective studies, three cohort studies, five randomized controlled trials, two case–control studies, one prospective study, and one longitudinal study), seven in vitro studies, three animal model studies, one book, and one Health Professional Fact Sheet.
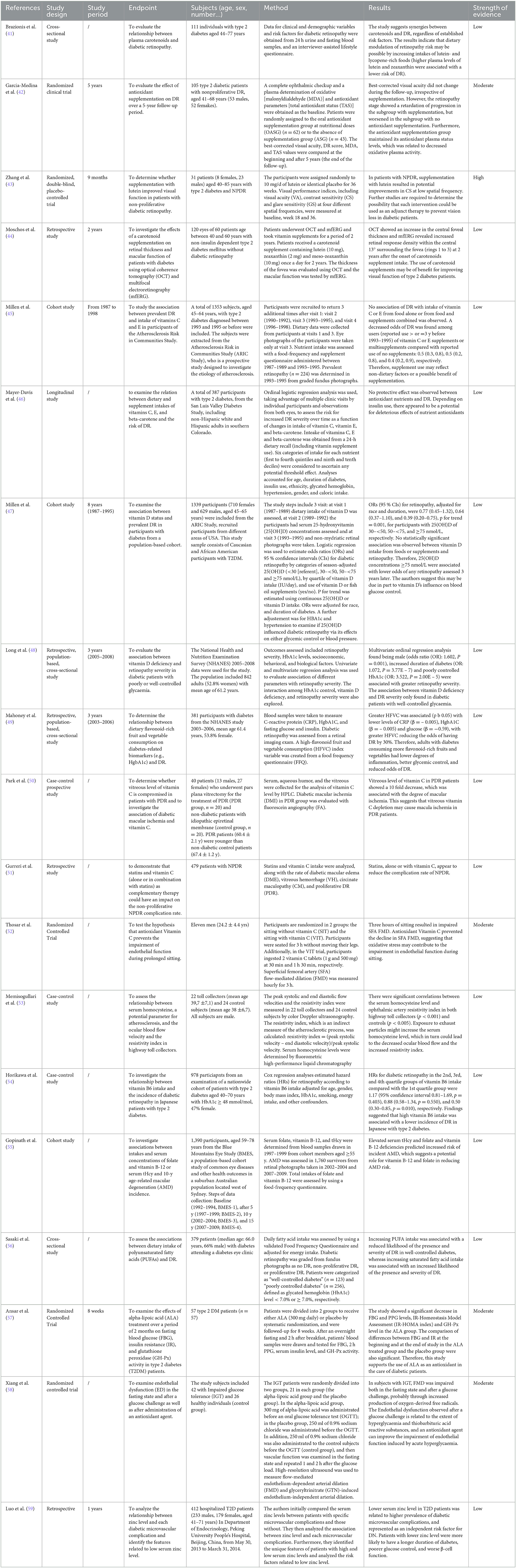
Table 2 shows the studies that evaluated the relationship between Nutrients, Vitamins, and antioxidants and diabetic retinopathy with their strength of evidence.
3.1.3. Fiber and hydratation, and DR
The keywords used in this research were: “fiber” OR “hydration status” OR “water intake” AND “DR” OR “eye diseases” OR “diabetes.” Six articles were sourced: one narrative review, one systematic review, two cross-sectional studies, one randomized controlled trial, and one post-hoc analysis of a randomized trial.
Table 3 includes studies that assessed the connection between fiber and hydration, and DR alongside the strength of evidence.
3.1.4. Gut microbiota and DR
These keywords were used as the basis for the research: “Gut microbiota” OR “dysbiosis;” AND “diabetic retinopathy” OR “eye diseases” OR “diabetes.” Four articles were sourced: one narrative review, two studies on animal models, and one comment on a study based on animal models.
3.1.5. PA and DR
This study was done based on the following keywords: “physical activity” OR “sedentary behavior” OR “lifestyle” OR “resistance training” OR “aerobic exercise” AND “diabetic retinopathy” OR “eye diseases” OR “diabetes.” Twenty-four articles were sourced: four narrative reviews, three systematic reviews and meta-analysis, one review of in vitro studies, one mini review, nine clinical trials (one cross-sectional study, one retrospective study, one cohort study, three prospective trials, and three observational studies), five studies on animal models, and one Clinician's Guide.
The studies that assessed the connection between PA and DR are listed in Table 4 along with their strength of evidence.
Table 5 shows the reviews about DR and DPs.
3.2. AMD
This research was conducted based on the keywords: “AMD” OR “AMD” AND “diet” OR “nutrients” OR “nutrition” OR “food” OR “supplements” OR “supplementation.” Nineteen articles were sourced: one randomized controlled trial, one cross-sectional trial, seven cohort studies, two multi-center studies, four population-based prospective studies, three case–control studies, and one clinical trial.
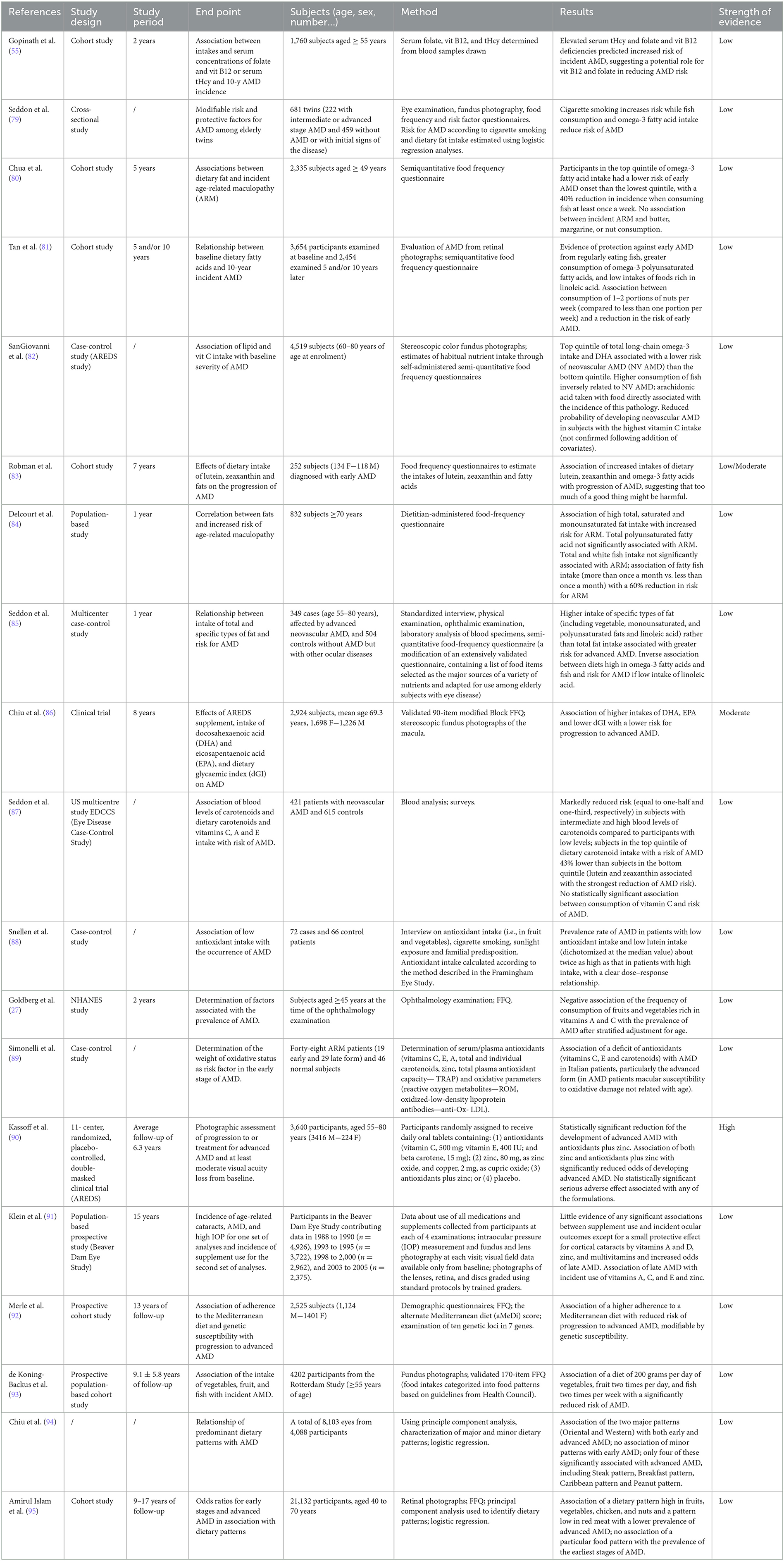
Table 6 includes the research that assessed the connection between AMD nutrition, including supplement use, and their level of evidence.
3.3. Cataracts
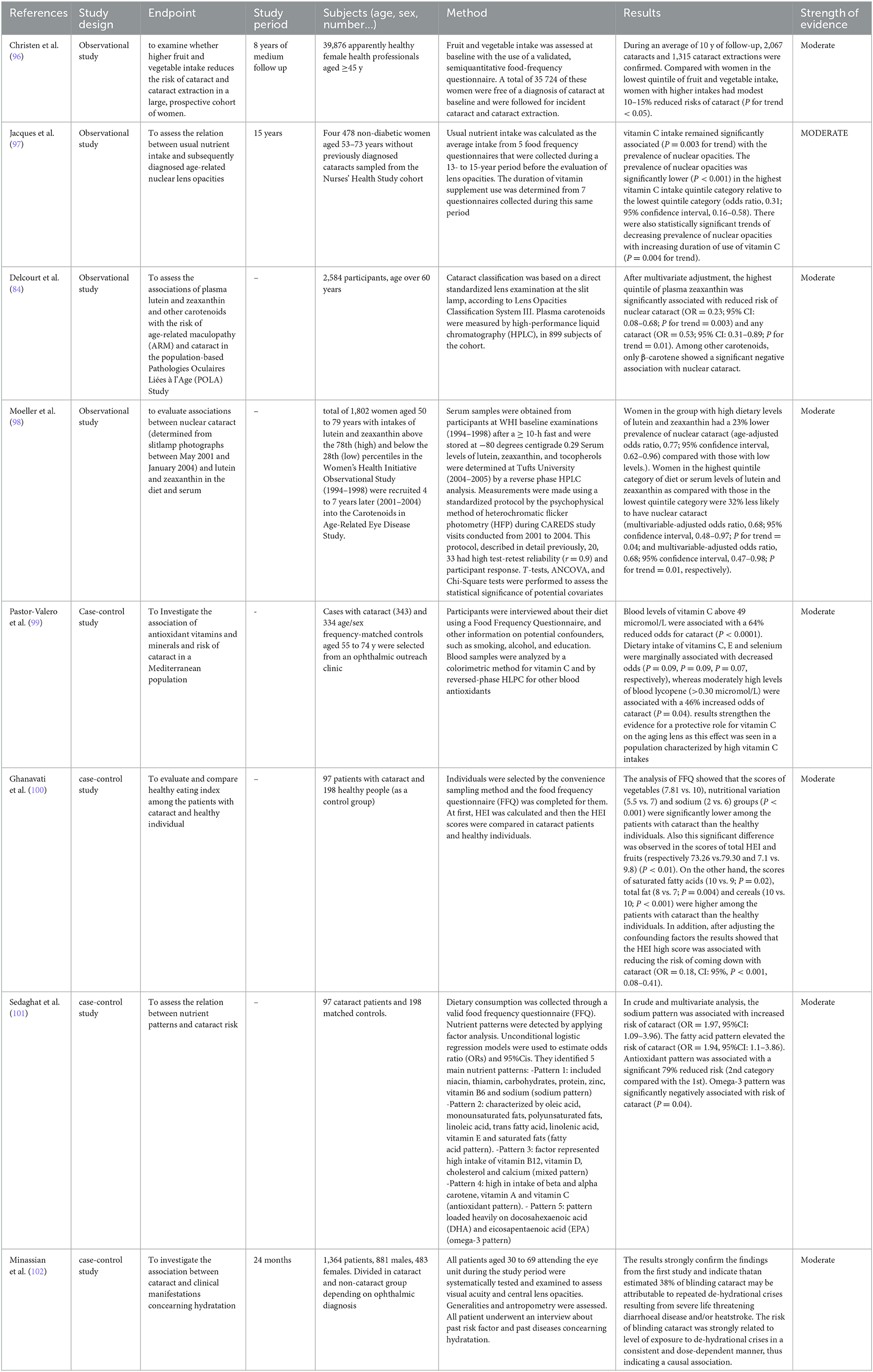
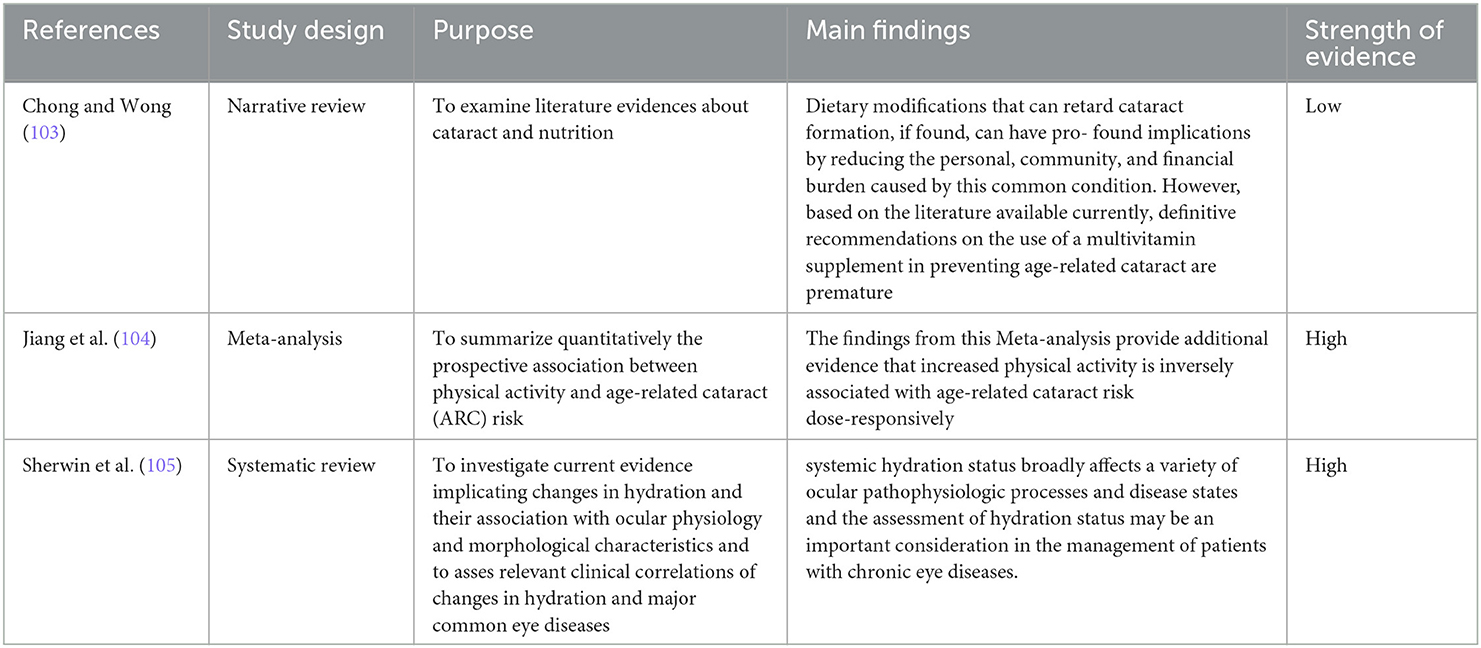
These keywords served as the basis for the research: “cataract” OR “lens opacities” AND “nutrition” OR “supplementation” OR “supplementation” OR “physical activity” OR “hydration.” Eleven articles were sourced: Four observational studies, four case–control studies, one narrative review, one systematic review, and one meta-analysis.
Tables 7, 8 include the research that evaluated the relationship between PA and bone along with the strength of the evidence.
4. Discussion
4.1. DR
DR is a major microvascular complication of diabetic disease and is a major cause of vision loss in working-age populations globally (106–108). In a meta-analysis of 35 studies conducted worldwide between 1980 and 2008, an overall prevalence of DR of 34.6% (95% CI 34.5–34.8), proliferative DR (PDR) of 6.96% (6.87–7.04), and diabetic macular edema (DME) of 6.81% (6.74–6.89) was recorded; it has also been estimated that 10.2% (10.1–10.3) of diabetic patients are at risk of visual impairment from retinopathy (107). Complex microvascular, neurodegenerative, immunological, genetic/epigenetic, and inflammatory interactions contribute to the development of DR (109). Among the various factors involved, there are both modifiable and non-modifiable risk factors. Modifiable risk factors include hyperglycemia, arterial hypertension, dyslipidemia, obesity and inadequate nutritional status, hyperhomocysteinemia, chronic kidney disease, alcohol consumption, and smoking. Those that cannot be modified are represented by gender, age, myopia, duration of the diabetic disease, type of diabetes, and family history of DR (110, 111).
This broad range of pathogenic pathways explains how hyperglycemia is etiologically related to aging and other pathologies, including DR and AMD. Therefore, in this context, these pathologies can be considered metabolic diseases of the retina in all aspects (112).
Obesity is frequently linked to DM and cardiovascular disease as a risk factor. It can be defined by waist-to-hip ratio, waist circumference, and body mass index (BMI). Both higher waist-to-hip ratio and waist circumference are risk factors for DR (113–115). The OR of DR is 1.28 per 5 cm increase in waist circumference (OR = 1.28; 95% CI, 1.05–1.56; P = 0.014) (115). Also, malnutrition is a potential risk factor for the development of DR (116).
4.1.1. Hyperhomocysteinemia
The enzyme methylenetetrahydrofolate reductase (MTHFR) is essential for adding the methyl group to folates. Polymorphisms in the MTHFR gene that reduce its activity, impairing the enzyme's ability to generate L-methylfolate, are common (117). These mutations are associated with hyperhomocysteinemia and other diseases, including DR (118, 119). At the cellular level, it has been demonstrated that a high level of homocysteine is harmful to the hemo-retinal barrier and has a pro-inflammatory effect on the epithelial cells of the retinal pigment, with the risk of increasing apoptosis phenomena (120). Elevated homocysteine levels increase the risk of hypertension, hypertensive retinopathy, diabetes, and DR (121) and are also associated with increased incidence and progression of DR (118, 119).
Supplementation with L-methylfolate [the bioactive form of folic acid (118)] can lead to the conversion of homocysteine into methionine, restoring its stocks, regardless of dietary deficiencies or genetic polymorphisms (122, 123). Optimal combinations of vitamins B1, B2, B6, L-methylfolate, methylcobalamin (B12), C, D, natural vitamin E complex, lutein, zeaxanthin, and alpha-lipoic acid are identified for protecting the retina and choroid. Nutritional interventions can support conventional therapies for DR to reduce the disease risk and severity of DR (122).
As far as alcohol is concerned, two important publications in literature have dealt with the relationship between DR and the consumption of alcoholic beverages (124, 125). Both concluded that there was no statistically significant association between alcohol consumption and DR risk. A first meta-analysis was conducted by Zhu in 2017 and included a total of 15 studies. Interestingly, in the statistical analysis analyzing different types of alcoholic beverages, wine or sherry intake was associated with a reduced risk of DR. In the publication, however, it was not possible to establish the dose responsible for this reduction, since there were no statistically significant differences between the various quantities taken (124, 125). The authors attributed this result to the potential protective effects of consuming low-to-moderate alcohol levels on the risk of diabetes mellitus (DM) and cardiovascular disease (126). However, the inflammatory response and oxidative stress could be influenced by alcohol, and are significantly associated with the risk of DR (127, 128). The stratified analyses of this meta-analysis were mixed due to the presence of various types of included studies; therefore, the results of these analyses are unreliable. A second meta-analysis in 2020 by Chen undertaken to correct the previous one and to implement the analysis with the new works that had been published in the meantime, confirmed the results of the previous one, not finding any significant association between alcohol intake and risk of DR of alcoholic beverages (125). However, even in this case, most of the studies considered reported inconsistent results. The Casteldaccia study showed that the duration of alcohol intake between 1 and 19 years was not associated with a risk of DR, but conversely, there was a reduction in the risk of DR with alcohol intake for a greater or equal number of years at 20 (129). According to Beulens' study, people with type 1 diabetes who drink moderate amounts of alcohol had a lower risk of microvascular problems (130). Fenwick showed that people with type 2 diabetes who occasionally consumed white wine had a lower risk of developing diabetic complications (131). This cross-sectional study was conducted in 2015 in patients with type 2DM, who were given a questionnaire that evaluated alcohol consumption and lifestyle. Patients included in the study then underwent retinography, and DR was staged as absent, present without, and at risk of vision loss. The relationship of DR intensity to alcohol consumption was adjusted for clinical-demographic, socioeconomic, and lifestyle factors. After adjusting for traditional risk factors and those for which they varied in univariate analysis, it was discovered that moderate drinkers (1–14 units/week) had a decreased risk of developing DR than non-drinkers. Therefore, the study concludes that in type 2 diabetics, the moderate consumption of alcoholic beverages is independently associated with a reduced risk of DR.
4.1.2. Dietary and food patterns
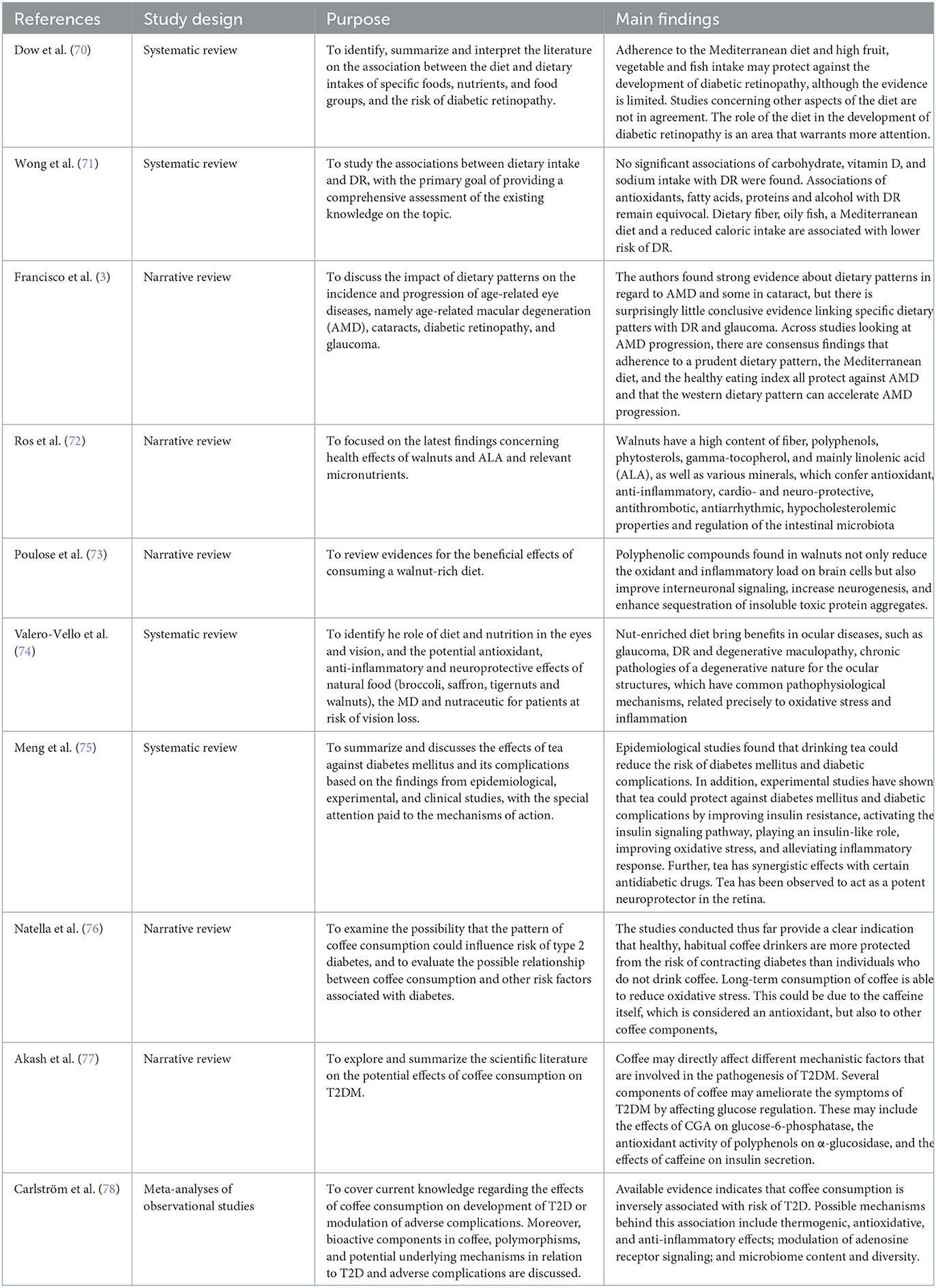
Regarding DPs, there is evidence for the protective effect of the Mediterranean diet on the onset of DR. Diaz-López conducted a nutritional intervention study in type 2 DM patients who did not have microvascular complications at baseline. Three different dietary models were analyzed: the Mediterranean diet supplemented with extra virgin olive (EVO) oil, the Mediterranean diet supplemented with nuts, and a low-fat control diet. After a 6-year follow-up, it was observed that the Mediterranean diet supplemented with EVO oil had a protective effect on the development of DR (30). A 2018 review by Dow examined the association among individual foods, macro- or micronutrients, dietary supplements, DPs, and DR or DME. In particular, the following were taken into consideration: fruit, vegetables, fish, milk, carbohydrates, fibers, fats, proteins, salt, potassium, vitamins C, D, and E, carotenoids, food supplements, green tea, and alcohol. Studies suggest that adherence to the Mediterranean diet and a high intake of fruit, vegetables, and fish may protect against the development of DR, although evidence is limited (70). Another review, also published in 2018, systematically searched the literature for studies on diet and DR published between 1967 and 2017 using standardized criteria for diet and DR. The review concluded that higher dietary intake of fiber and fish and higher adherence to the Mediterranean diet were protective against DR. Conversely, high total caloric intake was associated with increased risk of DR. No significant association was found among carbohydrates, vitamin D, sodium, and DR; however, the association between DR and antioxidants, fatty acid (FA), proteins, and alcohol remained in doubt (71). In a more recent review, released in 2020, the effect of DPs on the occurrence and development of age-related eye illnesses such as DR, degenerative maculopathy, cataracts, and glaucoma was reviewed. Treatments for diabetes should slow the growth of DR. However, only a small number of research have confirmed if following particular DPs or eating a more or less healthy diet affects the prevalence of DR (3). For example, the randomized clinical trial PREDIMED (Prevention with Mediterranean Diet) (132) demonstrated how adherence to the Mediterranean diet could prevent diabetes. To see if DR might be avoided similarly, it seemed sense to do so. According to Dáz-post-hoc López's post-hoc analysis of the PREDIMED research, consuming 500 mg/day of omega-3 fatty acids (a readily feasible intake with strong adherence to the Mediterranean DP) considerably lowers the chance of getting DR (30).
Eating fish (an omega-3-rich dietary source) does, in fact, assist to delay the onset of DR. Eating oily fish at least two times weekly (rather than less frequently) has been related to a roughly 60% lower incidence of retinopathy (32). According to a 2017 cross-sectional study carried out in Palestine, a healthy eating pattern known as “Asian,” which is characterized by a high intake of whole grains, potatoes, legumes, vegetables, and fruit, can be linked to a lower prevalence of diabetes problems. This was contrasted with the “sweet-soft drinks-snacks pattern,” which was described as a harmful eating behavior characterized by high consumption of refined cereals, sugar, sweets, desserts, snacks, and soft drinks (33).
4.1.3. Fruits and vegetables
In a Japanese cohort study that considered type 2 DM patients, a high fruit intake was linked to a decreased risk of DR. It was discovered that daily fruit eating of at least 173.0 g was related to a 50% lower risk of retinopathy incidence than daily fruit consumption of 53.2 g or less (34). Fruits and vegetables are generally good sources of flavonoids, fiber, minerals, and vitamins. They should be consumed in sufficient amounts, or at least 400 g per day, at each meal. The risk of cardiovascular events is decreased by 4% and the risk of stroke is decreased by 5% with each additional serving of fruit and vegetables (35).
4.1.4. Nuts
Gamma-tocopherol, phytosterols, polyphenols, fiber, and linolenic acid (ALA) are all abundant in walnuts, as well as various minerals, which confer antioxidant, anti-inflammatory, cardio- and neuro-protective, antithrombotic, antiarrhythmic, hypocholesterolemic properties, and regulation of the intestinal microbiota (72). Nut consumption has been linked in human clinical trials to enhance cognitive function, with favorable effects on memory, learning, motor coordination, anxiety, and locomotor activity (36, 73). These researches also concluded that a diet high in nuts is beneficial for treating brain disorders and other chronic conditions linked to inflammation and oxidative stress (36, 37, 73). These health benefits also occur at the ocular level in various diseases, such as glaucoma, DR and degenerative maculopathy, and chronic pathologies of a degenerative nature for the ocular structures, which have common pathophysiological mechanisms related precisely to oxidative stress and inflammation (74).
4.1.5. Saffron
In DR, saffron may reduce insulin resistance in patients with “prediabetes” (74). It has been shown in vitro that saffron can control the activation of microglia. Moreover, crocin (the carotenoid that gives saffron its distinctive color) supplementation reduces retinal thickness and enhances visual acuity in patients with diabetic macular edema, perhaps as a result of its anti-inflammatory effects (120).
This was observed in a double-blind, placebo-controlled, randomized phase 2 clinical trial. Sixty patients with diabetic maculopathy refractory to conventional therapy [including macular photocoagulation and intravitreal injection of an endothelial growth factor inhibitor (bevacizumab) with or without steroids (triamcinolone)] were considered. Patients were divided into three groups: patients in the crocin group were given 5 mg or 15 mg crocin tablets daily for 3 months, while patients in the placebo group received one placebo tablet daily during the study. Best corrected visual acuity (BCVA) and central macular thickness (CMT) were measured at baseline, and then monthly for a total of 3 months post-operatively. Blood chemistry tests were also evaluated at baseline and at the end of the study. BCVA and CMT were evaluated as primary outcomes, while glycated hemoglobin (HbA1c) and fasting blood glucose (FBS) were investigated as secondary outcomes in this study. The results showed that administering crocin tablets 15 mg daily could significantly reduce HbA1cg and CMT and improve BCVA compared to the placebo group. Although administering crocin tablets 5 mg daily can improve HbA1c, FBS, CMT, and BCVA, the difference was not significant compared with the placebo group. Thus, this study highlighted how crocin may act as a potent antioxidant and neuroprotective in short-term refractory DME; however, the clinical significance has yet to be demonstrated in a longer study with a larger sample size that includes treatment-naïve patients (120).
4.1.6. Curcumin
Literature has shown that turmeric has an interesting activity on the retina; in vitro, treatment of high-glucose-induced human retinal endothelial cells (HRECs) with curcumin significantly reduced the intracellular production of reactive oxygen species (ROS), as well as the release of tumor necrosis factor-α (TNFα) (133).
Similar results were also obtained when curcumin was added to particular cell lines of the retinal pigment epithelium, called ARPE-19 (a spontaneous human retinal pigment epithelium cell line with normal karyotype that forms polarized epithelial monolayers on porous filter media) (134, 135).
Curcumin reduced the production of ROS and increased the expression of heme oxygenase-1 (HO-1), a type of redox-sensitive protein, whose activation protects cells from various types of stress. These findings imply that curcumin exhibits indirect antioxidant activity in addition to direct antioxidant activity by enhancing the activity of HO-1 and other antioxidant enzymes (134, 136).
Similarly, Maugeri argued that curcumin treatment can largely prevent the changes of DNA methyltransferase activity in high glucose-related stress ARPE-19 cells by downregulation of ROS production (137).
Given this background, although the studies are currently conducted exclusively in vitro and in the animal model, the results of the same encourage the routine intake of curcumin at least weekly.
4.1.7. Tea and coffee
Tea has been found to act as a strong neuroprotector in the retina (75), inhibiting neovascularization and protecting pericytes preventing neovascularization (138). By lowering the production of ROS, boosting the expression of the glutamate transporter, reestablishing intercellular connections, and promoting glutamine/glutamate recycling, green tea can preserve retinal neurons in diabetes patients and control the retinal microenvironment (139). Furthermore, a low dose of green tea improves antioxidant defenses, reduces markers of inflammation, and prevents retinal basement membrane thickening (140). In a Chinese case–control study, including diabetic patients without DR, regular consumption of Chinese green tea every week for at least 1 year was associated with a reduced likelihood of DR in female subjects, but not in male subjects (38). In an animal model study, black tea was shown to lower blood sugar and slow the development of cataracts (141). Green and black tea (in 1.25% water) were administered to streptozotocin-induced diabetic rats for 3 months. Normal and diabetic control groups were also studied. As expected, the tested diabetic animals showed a significant increase in lens and plasma glucose. Red blood cell lens and sorbitol were significantly increased due to activation of the aldose reductase pathway. The thiobarbituric acid reactive substances of plasma, lens lipids, and protein glycation were also significantly elevated. Both teas significantly inhibited diabetic cataracts and caused significant reductions in the biochemical pathway implicated in the development of the disease. After corrections for glucose, teas have been found to delay the development of diabetic cataracts due to a hypoglycemic effect, which in turn inhibits biomarkers of the disease. Significant correlations were found among glucose level, cataract severity, and these indicators. Green tea, but not black tea, caused a significant drop in triglycerides in diabetic animals. The study concludes that tea may be a simple and cost-effective means of preventing or delaying diabetes in humans and resulting complications. Therefore, tea should also be studied as a therapeutic adjuvant in the treatment of diabetes. As for coffee, its long-term consumption can reduce oxidative stress (76). This could be due to the caffeine itself, which is considered an antioxidant, but also due to other coffee components, such as some trace elements (zinc, copper, and iron) and other substances, including chlorogenic acid (CGA), cafestol, trigonelline, and caffeic and ferulic acids (77). By modifying adenosine signaling, inhibiting glucose-6-phosphatase, inhibiting glucose-6-phosphate translocase 1, inhibiting intestinal glucose absorption, decreasing glucose production in the liver, increasing insulin secretion from pancreatic islets, and improving peripheral insulin sensitivity and glucose uptake, caffeine and CGA affect insulin and glucose homeostasis (by stimulation of the glucose transporter type 4 (GLUT4) and modulation of the activation of the intracellular signaling pathway that includes Akt, AMPK, and MAPK) (78). In healthy, obese, and 2DM adults, regular coffee consumption can reduce levels of pro-inflammatory biomarkers. The anti-inflammatory adiponectin, interleukin 4, and interleukin 10 can all be increased by it (76, 77). A Norwegian study found that high compared with low coffee consumption reduced the risk of type 2 DM by about 35% (39). This study looked at more than 360,000 subjects between 40 and 45 years of age, over 20 years, who were divided according to coffee consumption into four groups: <1 cup of coffee per day, 1 to 4 cups/day, 5 to 8, or more than 9 cups of coffee per day. The group that drank less than one cup of coffee per day was used as a reference. Compared to this, the other groups had relative risks of developing type 2 DM (0.87 for the 1–4 cups/day group, and 0.65 for both the 5–8 cups/day and > 9 cups/day groups). This regards the consumption of “boiled” coffee, while for other types of coffee, mainly filtered, the relative risks were as follows: 0.84 (1–4 cups/day), 0.67 (5–8 cups/day), and 0.62 (>9 cups/day). Similar results were obtained in a Finnish cohort study, in which coffee consumption was inversely correlated with type 2 DM (40).
4.1.8. Nutrients
4.1.8.1. Vitamins and antioxidants
It has been demonstrated that vitamins and antioxidants (such as vitamins C, E, and carotenoids) may play a role in the pathogenesis of DR as they lead to a reduction of retinal neovascularization, with the restoration of blood flow and have a protective role against free radicals (142). Furthermore, vitamins C and E appear to suppress vascular endothelial growth factor (VEGF) production in animal models and decrease advanced glycation end-products (AGEs) accumulation. Vitamin C can decrease protein kinase C activation (143), prevent glucose-induced pericyte apoptosis (144), and reduce oxidative stress in human retinal pigment epithelium (145). Given these premises, several studies have attempted to establish whether there was a relationship between DR and dietary antioxidant intake.
4.1.8.2. Vitamin A and carotenoids
According to Brazionis, greater plasma levels of lutein and zeaxanthin were linked to a decreased risk of DR, just like they were for AMD (41). Taking lutein supplements at a level of 6 mg per day for 20 days per month (considered a “nutritional” intake, meaning typically ingested with a healthy and diverse diet) can stop the progression of DR within 5 years, according to a randomized trial on certain antioxidants (42). Patients with non-proliferative DR (NPDR) who take 10 mg of lutein daily report improved contrast sensitivity, glare, and visual acuity (43). In a 2-year study, diabetic individuals without DR who received 10 mg of lutein and 12 mg of zeaxanthin/day showed improved retinal density on multifocal electroretinography and a modest increase in non-edematous foveal thickness (44).
4.1.8.3. Vitamin D
It was observed that plasma concentrations of 25-hydroxyvitamin D ≥75 nmol/L were associated with a reduced probability of developing retinopathy at 3 years (47). Subsequently, in a 2017 retrospective cross-sectional study on over 800 adults emerged that an optimal level of vitamin D is fundamental for reducing the risk and severity of DR (48).
4.1.8.4. Polyphenols
A cross-sectional study by Mayoney examined the effect of flavonoids in diabetic patients who were divided into groups based on the frequency of consumption of fruits and vegetables with high flavonoid contents. It was observed that there was a significant association between a high intake of these foods and lower levels of c-reactive protein (CRP), HgbA1C, and glucose. In addition to lower levels of inflammation and better glycemic control, these patients also had a 30% reduction in the likelihood of DR (49).
4.1.8.5. Vitamin C
It was observed that patients with PDR had a 10-fold lower level of ascorbate in the vitreous humor and a greater tendency to DME (50), and that vitamin C taken with statins decreased NPDR, in a dose-dependent manner, more than statins alone (51). However, regarding the vitamin C–DR relationship, not all studies agree: a Japanese cohort study found that high vitamin C intake (4th quartile) was associated with a 40% reduction in retinopathy risk (34), while two cross-sectional studies showed no association between vitamin C consumption and retinopathy (45, 46), except for an increased likelihood of retinopathy in the 9th decile of vitamin C intake in the study by Mayer-Davis (46). In diabetic subjects, oral supplementation with 1,500 mg of vitamin C reduces capillary endothelial dysfunction (52) and therefore can be a useful support in microvascular pathologies such as RD.
4.1.8.6. B vitamins
Vitamin B1 (thiamine). In addition to controlling intracellular glucose and preventing the activation of the polyol pathway, which is brought on by increased intracellular glucose levels, thiamine is a powerful free radical scavenger (146). This pathway represents one of the mechanisms in the pathogenesis of DR (147). Furthermore, elevated serum thiamine levels protect the vascular endothelium from injury by advanced glycation end products (146, 148, 149). As reported in a 2020 review published in “Eye and Vision” by Shi, for the treatment and prevention of vascular end-organ damage, such as that seen in DR and diabetic nephropathy, high-dose thiamin supplementation (50–100 mg/day) is safe and effective for neuroprotection. Because of the low toxicity, no upper limits (UL) have been recorded (150).
Vitamin B2 (riboflavin). Riboflavin supplementation in humans likely guards against damage caused by oxidative stress, hyperglycemia, and homocysteine (53, 151, 152). Supplementing with vitamins B6 and B12 may also be advantageous since these nutrients lower homocysteine levels (150).
Vitamin B6. A cohort of Japanese 2DM patients was followed for 8 years, monitoring vitamin B6 intake and DR onset. It was noted that low vitamin B6 intake (particularly the lowest quartile of vitamin B6 intake) was correlated with a higher incidence of DR (54). There are various forms of B6, and the naturally occurring active form pyridoxal-5-phosphate (P5P) is the safest and most efficient form for lowering homocysteine levels (153). P5P supplementation may lower the chance of developing DR and diabetes. Vitamin B6 therapy alone, at a dosage of 50–200 mg per day, was associated with a decreased long-term incidence of DR in a small cohort trial of a few participants (150).
Vitamin B9 (folate). The use of supplements with L-methylfolate, B2, B6 (in the form of P5P), and B12 can reduce homocysteine levels, the incidence of DR, and other diabetes-related diseases (150).
Vitamin B12 (Cobalamin). Increased homocysteine levels, as already stated, are linked to decreased cerebral and retinal blood flow, as well as decreased central retinal artery caliber, VEGF expression, and DR (53, 55, 154). Supplementation with vitamin B12 increases the release of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) (155) and allows for the reduction of DR-associated long-term complications (140). The active transport necessary for gastrointestinal absorption of vitamin B12 from food requires the presence of an intrinsic factor, an acidic environment, and an intact intestinal mucosa (156).
4.1.8.7. FA
FA can influence retinopathy through several pathways. First, the accumulation of long-chain FA can lead to activation of the protein kinase C pathway, just as occurs with excess glucose (112, 157). Second, since the retina is an extremely oxidizing and polyunsaturated fatty acids (PUFA)-rich environment, an accumulation of lipids can more easily undergo peroxidation and accumulation of advanced lipoxidation end products (ALEs) (158). Both ALEs and AGEs activate a pro-inflammatory response via the AGE receptor, which activates the proinflammatory transcription factor NF-kB and decreases the antioxidant response (159). Concerning the PUFA and DR relationship in Sazaki's study, an increase in PUFA intake was linked to a lower likelihood of DR occurrence and severity in individuals with well-controlled diabetes, whereas an increase in short-chain fatty acid intake (SFA) was linked to a higher probability of DR occurrence and severity (56). A 500 mg/day consumption of omega-3 FA can greatly lower the possibility of developing DR, as was highlighted in a post-hoc analysis of the PREDIMED study by Diáz-López (32). Alpha lipoic acid, an important cofactor of mitochondrial metabolism, has an antioxidant action by counteracting ROS and enhancing the effects of endogenous antioxidants such as glutathione and vitamins C and E (160). The administration of alpha lipoic acid shields the retina's ganglion cells and pigment epithelial cells, in particular, from ischemic damage and apoptosis (161). Furthermore, alpha lipoic acid reduces hyperglycemia and hyperglycemia-induced endothelial dysfunction in type 2 DM patients (57, 58). Daily supplementation with 600 mg of alpha lipoic acid is safe and well-tolerated (150).
4.1.8.8. Zinc
Several chronic disorders, including metabolic syndrome, diabetes-related complications, such as DR, and metabolic syndrome, are known to advance more quickly when there is zinc deficiency. Low serum zinc levels correlate with DM duration, elevated HbA1c levels, hypertension, and microvascular complications. Blood zinc levels gradually decline with DR duration and severity (59).
4.1.8.9. Fiber
A higher risk of getting DR is linked to lower dietary fiber consumption (162). Furthermore, once this complication occurs, intensive glycemic control can slow the rate of development (163). The Mediterranean diet is rich in food sources of fiber, such as fruits, vegetables, and unrefined carbohydrates, and has been associated with a lower incidence of DR (30, 31).
4.1.8.10. Hydratation
Results emerging from the review by Sherwin et al. showed that chronic activation of the renin-angiotensin-aldosterone system (RAAS) may be implicated in the pathogenesis of DR and glaucoma, thus suggesting a possible new therapeutic target on which to base new studies' intervention (105). The cross-sectional population analysis of the 2005–2008 NHANES study (5220 US adults 40 years of age and older) also found that low levels of hydration, as assessed by measured (or calculated) formulas based on blood levels of glucose, sodium, potassium, and urea (limited to subjects aged ≥65 years), were associated with an increased risk of DR (60). So, the intake of adequate quantities of water, equal to 1.5–2 liters per day, is a fundamental objective to achieve.
4.1.8.11. Gut microbiota
Beli (164) first described the link between the gut microbiome and DR in differently fed rodents. The intermittently fasting mice exhibited retinal histology that was comparable to that of non-diabetic controls, while the ad libitum diet animals displayed ocular symptoms of DR. Intermittently fasted rodents showed increased Firmicutes to Bacteroidetes ratio and changes in bacterial metabolites, with increased levels of taurochenodeoxycholate (TUDCA) derived from bile acids and known to have anti-inflammatory effects. TUDCA enters the bloodstream and activates GPBAR1, even referred to as TGR5, the TUDCA receptor in the retina. The results imply that intermittent fasting may protect against DR by increasing TUDCA levels and, in addition, TGR5 could represent a novel therapeutic target for the diabetic retina (165). Regarding the role of prebiotics, oligofructose, used alone or in combination with metformin, is effective in preventing the development of DM and its microvascular complications, opening the door for new treatment approaches and research ideas (166). These results suggest that the growth of beneficial bacteria in patients with healthy diets, either through pre- and probiotics, or even through intermittent fasting, could protect against the development of T2DM (167).
4.1.8.12. Physical activity
Increased PA reduced the risk of its onset (61, 62). Higher levels of PA were shown to be independently linked to a decreased incidence of DR in type 2 DM patients (63). A minimum of 30 min of PA, 5 days a week, might minimize the risk of DR advancement by 40% (168). Conversely, it has been revealed that diabetic subjects who lead a sedentary lifestyle have a higher risk of developing DR than those who live actively (169). The results of a meta-analysis also revealed a possible mechanism of impact of PA on DR due to improved glycemic control (170). An alteration in 25-hydroxyvitamin D levels could be another probable mechanism. Supporting evidence is the finding in subjects of all ages that 25-hydroxyvitamin D levels improve with increased PA (64–67, 171). Low levels of 25-hydroxyvitamin D in the blood have been linked to an increased risk of macrovascular and microvascular events, including DR (68). Furthermore, exercise has been shown to modulate oxidative stress (172). Research on animal models has shown that exercise reduces oxidative stress in the retinas of DR mice (173–176). It should be remembered, however, that high-intensity resistance and aerobic exercise should be avoided in diabetic patients with DR to lower the risk of vitreous hemorrhage or retinal detachment (69, 177). Moreover, the risk of vitreous hemorrhage is increased by any exercise which can lead to a rise in systolic blood pressure (178, 179). In animal model studies of diabetic disease, resistance exercise has been shown to lead to increased muscle mass (180). Skeletal muscle is an essential reservoir of glucose in the body, and exercise is a powerful stimulator of glucose uptake, which in part is stored within skeletal muscles (181). Resistance exercise has a direct impact on skeletal muscle and may be used to manage individuals with DM2 (182).
4.2. AMD
In the transversal AREDA study conducted on 4,088 participants (whose eyes were divided into three groups: controls, early AMD, and advanced AMD), two major DPs were identified: the Western pattern and the Oriental pattern by using a food frequency questionnaire (FFQ) with subsequent factorial analysis. The first pattern had a higher prevalence of progressive AMD, while an “oriental” dietary style appears to be protective against this pathology (183). Furthermore, according to a recent review, for both early and late AMD, abdominal obesity would be a risk factor (184). In a 2013 cohort study that enrolled 1,760 subjects aged ≥55 years, the authors tried to provide epidemiological evidence for the possible relationship among serum levels of homocysteine, vitamin B12, and folate, and the risk of AMD, finding that high homocysteine levels, as well as a vitamin B12 or folate deficiency, were linked to a higher incidence of AMD at age 10 years; this risk was decreased by 47% with vitamin B12 supplementation (55). Moreover, the effects of several dietary sources, including omega-3 and omega-6 fatty acids, mono-, polyunsaturated, and saturated fats, total fats, trans fats, and cholesterol, on the risk of AMD have been investigated. In particular, omega-3 has anti-inflammatory properties and, when transformed into neuroprotectin, can help prevent oxidation-induced apoptosis in retinal cells and support the fluidity of the photoreceptor membrane (185). The polyunsaturated fatty acids EPA and DHA are linked to a lower incidence of AMD and play a preventive role in the course of the illness, according to a 2018 review of epidemiological, clinical, and experimental data. Indeed, in humans, the retina has a lipid profile that is especially high in long and very long-chain polyunsaturated FA, which is crucial for maintaining retinal structure and function (184). In the US Twin Study of AMD, a cross-sectional study performed on 681 twins, of which 222 subjects had intermediate or advanced stage AMD and 459 did not or just exhibited initial signs of the disease, it was demonstrated that a higher omega-3 FA level (upper quartile, corresponding to a mean daily intake of 0.35 g of omega-3, vs. lower quartile, corresponding to 0.06 g/day) was inversely related to AMD, with a significant risk reduction observed primarily in subjects with a lower than average intake of linoleic acid (an omega-6 FA) (1.8 g/day) (79). In the Blue Mountains Eye Study, 2,335 participants aged 49 years and older underwent reevaluation at 5 years for the development of AMD. Results showed that those in the highest quintile of omega-3 fatty acid intake (0.52–2.11%, expressed as a percentage of total energy intake) had a lower risk of early AMD onset than those in the lowest quintile (0.05–0.26%), with a 40% reduction in incidence when consuming fish at least once a week (80). Consumption of 1–2 portions of nuts per week (compared to less than one portion per week) was also related to a lower risk of early AMD onset, with a protective impact in comparisons of retinal pigment abnormalities reported in non-smokers, subjects with a lower-than-average total cholesterol-to-HDL-cholesterol blood ratio, and those with higher-than-average beta-carotene intake (6836 g/day) (81). Within the Age-Related Eye Disease Study (AREDS), 4,519 subjects (60–80 years) provided an estimate of habitual nutrient intake through self-administered, semi-quantitative FFQ, from which the study showed that those in the top quintile of total long-chain-omega-3 intake (0.110% of total energy intake) and DHA (0.061%) had a lower risk of neovascular (NV) AMD (NV AMD) than bottom quintile (0.013% for total omega-3 and 0.010% for DHA). In general, higher consumption of fish was inversely related to NV AMD, while arachidonic acid taken with food was directly associated with the incidence of this pathology (82). Several other studies have looked into the connection between lipid intake and the risk of AMD, including a cohort study with 6,734 people (aged 58 to 69) who completed the FFQ and also reported using supplements (ascorbic acid, vitamin E, cod liver oil, and fish oil). A greater trans-fat intake was linked to a higher prevalence of late AMD, whereas higher omega-3 FA and olive oil intake would lessen the incidence of both early and late AMD, respectively (upper quartile, 1.4 g/d vs. lower quartile, 1.0 g/d; OR, 0.85; 95% confidence range, 0.71–1.02; P = 0.03). However, neither monounsaturated FA nor oleic acid, of which olive oil is particularly rich, were associated with late AMD; presumably, therefore, other non-FA contained in this oil could be responsible for its protective effect. Conversely, conflicting results emerged from an Australian study on 254 subjects diagnosed with early AMD, in which the possible progression of the disease at 7 years was evaluated: these findings contribute to a relationship between omega-3 intake (as measured by FFQ) and the development of AMD, potentially demonstrating how excessive consumption of a drug having therapeutic effects can be hazardous (83). Furthermore, high consumption of total, saturated, and monounsaturated fats was linked to an elevated risk of age-related maculopathy in the POLANUT trial, which involved a sample of 832 people from southern France. While no significant correlation emerged with polyunsaturated FA intake, a 60% decrease in the risk of maculopathy was associated with fatty fish consumption frequency (more than once per month vs. less than once per month) (84). The multi-center, case–control study by Seddo examined 504 controls without AMD but with other ocular pathologies, as well as 349 patients (55–80 years) with advanced neovascular AMD. It concluded that higher consumption of certain types of fats, especially mono- and polyunsaturated-FA of vegetable origin, may be linked to an increased risk of advanced AMD, while diets high in omega-3s and fish (two or more servings/week vs. less than one serving/week) seemed to be inversely associated with this risk, but limited to subjects with low linoleic acid intake ( ≤ 5.5 g vs. ≥5.6 g) (85). Given that the GI of foods appears to play a role in the pathogenesis of AMD, a significant group of studies have looked into the potential involvement of carbs in AMD. Low dietary GI values (dGI75.2 vs. 81.5, computed as the average of GI of specific items weighed by the presence of carbs) were linked to a lower chance of developing advanced forms of AMD, according to an analysis of the data from the AREDS study: More specifically, it was discovered that a dGI reduction of 6 units (roughly equivalent to substituting 5 slices of white bread with 5 slices of whole grain bread in a subject's daily diet who consumes 250 g/day of total available carbohydrates) could prevent 8% of advanced AMD cases for 5 years. The production of advanced glycosylation products, the aggregation and precipitation of glycosylated protein aggregates, and the ensuing inflammatory and angiogenic responses have all been linked to higher post-prandial glycoxidative stress caused by high GI foods. Furthermore, the compensatory hyperlipidemia that occurs in the late post-prandial phase following the intake of high GI foods could also play a role in the pathogenesis of AMD (86). A higher mean dietary GI (lower quartile vs. upper quartile) is associated with a higher 10-year risk of developing early AMD, according to the Australian Blue Mountain Eye Study (3,654 participants, 49 years and older, examined at baseline in 1992–1994, of whom 2,335 were re-examined after 5 years, and 1952 after 10 years). This is after adjusting data for potential confounders and diet constituents. On the contrary, a greater consumption of whole-meal bread and cereals (in particular, those with a lower GI) was related to a reduction in this risk. In 1993, the Eye Disease Case-Control Study Group found that participants with intermediate and high blood levels of carotenoids had a much lower chance of developing neovascular AMD than those with low levels—equivalent to half and one-third, respectively. Within the same study, surveys performed on a sample of 356 patients with advanced-stage AMD (55–80 years) and 520 controls showed that subjects in the top quintile of dietary carotenoid intake had a risk of AMD 43% lower than subjects in the bottom quintile, and how, among the specific carotenoids, lutein and zeaxanthin (mainly found in green leafy vegetables) had the strongest association with a reduced risk of AMD (consuming spinach and collard greens more frequently was linked to a significantly decreased incidence of AMD) (87). A case–control study on the intake of antioxidants (72 patients and 66 controls) revealed that AMD was almost two times as common in patients who consumed fewer antioxidants and lutein than the typical person compared to those who consumed more, indicating a clear dose–response relationship (88). Vitamin A, which the body stores as retinol, is the source of several carotenoids. Even after adjusting for variables, demographics, and specialists, the National Health and Nutrition Examination Survey (NHANES) study found a negative correlation between the frequency of consumption of vitamin A-rich fruits and vegetables and the prevalence of macular degeneration in subjects under the age of 45 years (27). These observational studies collectively imply that lutein and zeaxanthin are the carotenoids that benefit the retina the most out of all those under investigation. These effects seem to be exclusive to certain types or stages of macular degeneration, with advanced disease benefiting most from a lower risk of damage. It is reasonable to speculate that vitamin C's potent antioxidant activities may be crucial in the onset and progression of the illness given the significance of oxidative stress on the etiopathogenesis of AMD. Most of the early studies were case–control studies. In 2002, Simonelli et al. analyzed the oxidative status of the serum/plasma in 48 Italian patients with macular degeneration (19 with the early form and 29 with the late form) and 46 healthy subjects, showing that subjects with late pathology had plasma levels of vitamin C, vitamin E, total carotenoids, and beta-cryptoxanthin compared to patients with early AMD, but with no differences in plasma levels of vitamin C between patients with ocular disease and healthy controls (89). Other observational studies have confirmed a small effect of vitamin C on the risk of macular degeneration. Data obtained from 4,519 participants in the AREDS study, which suggested a reduced probability of developing neovascular AMD in subjects with the highest vitamin C intake, were then not confirmed following the addition of covariates (82). Even the multicenter Eye Disease Case–Control Study (EDCCS), which included 520 controls with other eye diseases and 356 patients with advanced-stage AMD (55–80 years), failed to detect any statistically significant link between vitamin C consumption and risk of AMD, even though the data appeared to point to a lower risk among those with the highest intake of vitamin C (particularly that contained in food) (87). Following multivariate adjustment, the examination of NHANES data from 1971 to 1972 revealed that there was no correlation between vitamin C intake and the prevalence of AMD at any stage (27). In addition to serving as a catalyst for more than 50 different enzymes, zinc also controls the expression of genes and contributes to the structure of proteins, making it a vital component of many physiological processes (186). Furthermore, zinc, together with copper, is an essential microelement for the retina, particularly concentrated in photoreceptors and pigmented epithelium of the human eye. Zinc and copper also act as cofactors for numerous ocular enzymes, including superoxide dismutase, a component of the main antioxidant system that modulates oxidative stress in the body. Oxidative stress and a reduced antioxidant capacity have been included among the possible pathogenetic factors implicated in the genesis of AMD, as the retina, and in particular the RPE, are particularly susceptible to oxidative stress due to high oxygen tension, high content of polyunsaturated fats, and intense exposure to light. These factors have led some researchers to hypothesize that taking zinc supplements may benefit retinal health (187). Zinc was a component of the antioxidant mixture given to the intervention group in the AREDS study. Participants were first randomized into four groups at random and given one of the following treatments per day: (1) antioxidants (vitamin C, 500 mg; vitamin E, 400 IU; and beta-carotene, 15 mg); (2) zinc, 80 mg; and copper, 2 mg, as cupric oxide; (3) antioxidants plus zinc; and (4) placebos. Data on subjects who took zinc (thus including both those who took zinc alone and those who took zinc plus antioxidants) proved to be suggestive of a reduction in the risk of developing advanced forms of AMD, while no significant effect emerged in subjects taking antioxidants (including both the antioxidants-only group and the antioxidants-plus-zinc group). A statistically significant risk reduction was seen for antioxidants + zinc and suggestive for zinc alone, but not for antioxidants alone, when individual intervention groups were compared with placebo. Additionally, considering only individuals with the most severe forms of AMD, the size of the risk decrease increased (90). The Beaver Dam Eye Study, a prospective population-based study that initially enrolled 4,926 participants in 1990 and then reexamined 3,722, 2,962, and 2,375 participants in 1993–1995, 1998–2000, and 2003–2005, respectively, has revealed a higher risk of late AMD in users of supplements based on vitamins A, C, E, and zinc (91). In more recent times, attempts have been made to analyze the associations between illness and diet not so much understood as a single nutrient or food, but as a food style, comparing healthy styles and not starting from the large studies done in the past. The first and most important investigation of the Mediterranean diet and AMD was the French prospective cohort study by Merle et al. from 2015, conducted on 2,525 participants of the AREDS study (in which 1,028 eyes were found to have progressed to an advanced form of AMD for 13 years). The alternate Mediterranean Diet score (aMeDi, range: 0–9, from non-adherent to fully adherent) was calculated for each subject using a validated, self-administered, semi-quantitative FFQ. This score is widely used to assess adherence to the Mediterranean Diet in the US population based on the individual intake of nine components: vegetables, fruit, legumes, whole grains, nuts, fish, red and processed meats, alcohol, and the ratio of monounsaturated and saturated fats. In addition, 10 genetic loci associated with AMD located in seven different genes were determined and analyzed as covariates (for inherited predisposition). A high aMeDi score (6–9) was significantly associated with a 26% reduced risk of progression to advanced disease after adjusting for demographic, behavioral, ocular, and genetic covariates (HR: 0.74; 95% CI: 0.61–0.91; P-trend = 0.007). Furthermore, the aMeDi score appeared to be associated with a lower risk of incidence of advanced disease among subjects carrying non-at-risk alleles, while no association with AMD emerged among subjects homozygous for the risk allele. Greater adherence to the Mediterranean diet, therefore, appears to be associated with a reduced risk of progression to advanced disease, a risk that can be modified by genetic susceptibility. Finally, the data collected demonstrated that two components of the aMeDi score, in particular, the consumption of fish and that of vegetables, were associated with a lower risk of progression (92). Surveys conducted on 4,202 participants in the Rotterdam Study, through the administration of a validated FFQ comprising 170 items and classifying the data obtained on dietary intakes in nine food patterns according to the Health Councils guidelines, showed an association of fish with 24% reduced risk of AMD occurrence (mean follow-up of 9.1 ± 5.8 years), while no other association with single food categories reached statistical significance. Furthermore, the authors highlighted that only one DP, the one characterized by the intake of ≥200 g/day of vegetables, ≥200 g/day (two servings a day) of fruit, and ≥32 g/day (equivalent to two servings per week) of fish, was significantly associated with a lower risk of developing AMD (hazard ratio 0.58 [95% confidence interval 0.36–0.93]) (93). An additional survey conducted on 4,088 subjects participating in the AREDS study identified, based on the data obtained through FFQ, two major DPs (Oriental and Western) and eight minor DPs (subgroups or extensions of one of the two main patterns, generally including a smaller number of characterizing foods). The two major patterns were significantly associated with both early (OR Oriental pattern: 0.74; OR Western pattern:1.56) and advanced AMD (OR Oriental pattern:0.38; OR Western pattern:3.70), while no minor pattern showed a correlation with early AMD, and only four of these were found to be significantly associated with advanced AMD, including Steak pattern [similar to the Western DP; OR comparing the highest to the lowest quintile of the pattern score = 1.73 (95% confidence interval: 1.24–2.41; P trend = 0.02)], Breakfast pattern [cereals, fruit, and fruit juices; 0.60 (0.44–0.82); P trend = 0.004], Caribbean pattern [white meat, fish, rice, low-fat dairy, and offal; 0.64 (0.47–0.89; P trend = 0.009)], and Peanut pattern [peanuts, snacks, high-fat dairy, and sweets; 0.64 (0.46–0.89; P trend = 0.03)]. The data collected suggested that specific foods may harbor potentially beneficial effects (peanuts, pizza, coffee, and tea) or harmful effects (salad dressing) against the development of AMD (94). Amirul Islam discovered six food patterns (or factors) that are characterized by a preponderance of consumption of fruit (Factor 1), vegetables (Factor 2), grains, fish, steamed or boiled chicken, vegetables, nuts (Factor 3), red meat (Factor 4), processed foods, such as cakes, cookies, pastries, and desserts (Factor 5), and salads (Factor 6). Patterns from factors 1–3 were associated with a lower prevalence of AMD, while factors 4 and 6 were associated with a higher prevalence of advanced AMD. Notably, factor 4, which also included processed fish, eggs, and a low intake of whole grain foods (wheat or rye bread) was associated with an increased risk of late AMD, but not early AMD (OR = 1.46; 95% CI:1.0–2.17). The typical Western DP containing mostly processed foods (Factor 5) was found to have no significant association with AMD. In contrast, the latter pattern also included foods such as dairy, tea, and peanuts, which are known to protect against AMD, demonstrating that the impacts of potentially harmful foods featured in the DP may be mitigated by the consumption of beneficial foods (95).
4.2.1. Physical activity
An active lifestyle, defined by at least 3 h of daily low-to-moderate intensity physical activity, is related to a decreased risk of AMD, according to a recent meta-analysis of nine cross-sectional studies that assessed the effects of PA on AMD in 15 research, with a protective association against both early AMD [8 studies, n = 38,112, odds ratio (OR) 0.92, 95% confidence interval (CI) 0.86–0.98] and late AMD [7 studies, N=28,854, OR 0.59, 95% CI 0.49–0.72] (188).
4.3. Cataracts
The Women's Health Study (WHS) is the largest prospective cataract study that also correlates total fruit and vegetable consumption (96). The study boasts an average of 10 years of follow-up, in which there were 2,067 cases of cataract onset and 1,315 cases of lens replacement due to cataracts. Compared with women in the lowest fruit and vegetable consumption quintile, women in quintiles 2–5 (≥3.4 servings/day) had a moderate (10–15%) reduction in the risk of cataracts (P = 0.05). In the 2013 study by M. Pator-Valero, an inverse association between increasing quartiles of fruit and vegetable intake and the prevalence of cataracts was demonstrated. The study's stated consumption was much higher than what other studies had described. The WHO recommendation of five or more servings of fruit and/or vegetables per day (>400 g/day), with a median of 440 g/day, was actually met by 50% of the Spanish study population (IQR 226). The Alicante diet (study population) is a Mediterranean diet abundant in fruits and vegetables, particularly citrus fruits, and offers high levels of antioxidant vitamins (99) compared to the best American diets of other studies examined. Among the antioxidants examined in the Spanish study, dietary vitamin C has a more consistent effect on cataract prevalence. The results show that daily intake of vitamin C in the diet >107 mg/day are inversely linked with a decreased risk of developing cataracts (P trend between the four quartiles = 0.047). Compared with the lowest quartiles, with vitamin C intakes between 13 mg/day and 83 mg/day, vitamin C intakes between 83 and 107 mg/day were discovered to be 38 times less likely to be related to cataract prevalence and intakes between 107 and 143 mg/day were associated with a 51% lower probability of cataract development. Arrives at 54% with intakes between 143 and 408 mg/day. These data are consistent with previous studies that demonstrated that human eye tissues become saturated with vitamin C with dietary intakes between 200 and 300 mg/day (189). An analysis of the Nutrition and Vision Project (97) also obtained similar results observing a significant 48% reduction in the likelihood of nuclear opacity for vitamin C intakes between 140 and 180 mg/day, a reduction of 53% for intakes between 180 and 240 mg/day, and of 66% for intakes between 240 and 360 mg/day compared to the intakes of the highest quintiles (<140 mg/day). The French study POLA (84) instead found an inverse association between nuclear cataracts and plasmatic zeaxanthin [OR = 0.23 (0.08–0.68)], thus concluding that xanthophylls are important for the prevention of ocular compared to individuals who had low plasma zeaxanthin levels (0.04 mol/L). Nuclear cataract risk was reduced by 75% in people with high plasma zeaxanthin levels (>0.08 mol/L), but not for other types of cataracts. The authors found no association between lutein and cataracts of any type. The CAREDES study (98), composed of women previously enrolled in an observational study and who were above and below the 78th and 28th percentiles, respectively, for consumption of lutein and zeaxanthin, demonstrated that women whose overall scores for HEI-95 (Healthy Eating Index-95) were in the highest vs. lowest quintiles had diets that were less rich in fat, saturated fat, in particular, and contained less sodium. The prevalence of cataracts was related to low values for most of the subscale scores (vegetables, fruit, milk, cereals, total saturated fat, and food variety in general). Furthermore, this study shows that meat consumption is directly related to cataracts (p = 0.07). The analysis of sodium and cholesterol consumption did not lead to any specific results. Two studies on the same population in Iran (100, 101) highlighted how DPs rich in sodium and trans-fats were linked to a higher prevalence of cataracts. Ghanavati used a case–control study evaluating the association of cataracts with a healthy eating style, the Healthy Eating Index (HEI). The analysis of FFQ led to dividing the population into three sub-groups with respect to the diet followed. The two categories of HEI were found to be protective against cataracts, while the population in the lowest quartile [OR = 0.19 (95% CI: 0.09–0.4); P < 0.01] had the greatest prevalence. Factor analysis was used on dietary data (101) to extract nutritional patterns and identified two particularly inadequate nutritional patterns, defined as sodium regimen and fatty acid regimen. Sedaghat has redivided the nutritional models into five models based on nutrients. The regimens are as follows: (1) sodium regimen: included niacin, thiamine, high amounts of carbohydrates and proteins, zinc, vitamin B6, and sodium; (2) fatty acid regimen featuring oleic acid, monounsaturated fatty acids (MUFA), PUFA, linoleic acid, trans FA, vitamin E, and saturated fat; (3) mixed regimen represented a high intake of vitamin B12, vitamin D, cholesterol, and calcium; (4) the antioxidant regimen had high intakes of beta and alpha carotene, vitamin A, and vitamin C; and (5) omega-3 regimen contained a high intake of DHA and EPA. In the crude, multivariate analysis, the sodium model was associated with an increased risk of cataracts (OR = 1.97, 95% CI: 1.09–3.96). The FA pattern (this model represents a surrogate for meats and processed foods) was associated with high risk (OR = 1.94, 95%CI: 1.1–3.86), while the antioxidant regimen was associated with 79% reduced risk compared to the sodium regimen. Finally, the omega-3 model was negatively associated with cataract risk (P = 0.04). The narrative review by Chong in 2008 suggests that the risk of cataracts can be reduced by adhering to diets high in vitamin C, xanthophylls (lutein and zeaxanthin, present not only in the macula but also in the lens), omega-3 FA, and avoiding frequent and abundant intakes of simple carbohydrates with a high GI (103).
4.3.1. Hydration
The high-water content in the eye, as well as the peculiar fluid regulation system in its context, suggest that the state of hydration may also play an important role in determining the health or disease state of the eye itself (105). A 2015 review suggested that dehydration correlates with the onset of some eye diseases, such as dry eye syndrome, cataracts, retinal vascular diseases, and refractive defects (105). In particular, the cornea, the main refractive medium of the eye, is made up of ~80% water, and its transparency mainly depends on its state of hydration. Indeed, changes in the state of hydration of the cornea can result in a change in its central thickness and the ability to recover from such changes decreases with age (190, 191). This could also affect the outcome of cataract surgery (105). Indeed, diabetes has also been observed to increase the risk of developing cataracts, as well as in diabetic patients suffering from cataracts, the total water content of the eye's lens system is reduced (192, 193). In a case–control study conducted in India in 1989 on 434 cases and 930 controls (30–69 years), 38% of the cases suggested that the cause could be attributed to episodes of severe dehydration, in a dose-dependent manner (102). Given this background, water must therefore be taken in a quantity of 1.5–2 liters per day, as per the indications of all international guidelines for a healthy diet.
4.3.2. Physical activity
A recent review (104) evaluated the outcome of PA on cataracts, finding that regular activity decreases the rate of progression and risk of incidence. Results from prospective cohort studies accessible and examined in this review revealed that greater PA was inversely related to cataract risk and that the association was significant in studies that measured the metabolic equivalent of task (MET) PA as opposed to studies that measured it as a weekly activity. According to a dose–response analysis, each increase of 6 METs/day resulted in a 2% reduction in the chance of developing cataracts. The ocular lens is highly susceptible to oxidative damage as it is rich in polyunsaturated FA and the presence of greater quantities of ROS has great toxicity on the components of the lens itself, such as the crystalline proteins, whose damage leads to the development of opacities (194, 195). From this point of view, PA could reduce the levels of oxidative stress by increasing the activity of antioxidant enzymes and thus favoring the prevention of cataracts.
5. Conclusion
Many common eye diseases, in particular DR, AMD, and cataracts, are treatable and preventable, especially in the first phase in which they occur. Lifestyle, especially nutrition and physical activity, plays an essential role. To create a food pyramid that makes it simple for people who are at risk of developing DR, AMD, and cataracts to decide what to eat, this narrative review analyzed the most recent research on the best dietary strategy needed to avoid the development of these pathologies. In preventive terms, the subjects who can benefit most from following the indications given in the pyramid are the following: diabetic and hypertensive subjects as they are at greater risk of diabetic retinopathy since both pathologies tend to damage the retina; subjects who are hypertensive and smokers as they are at increased risk of age-related macular degeneration; subjects suffering from other eye diseases such as glaucoma or uveitis, diabetics, and who have undergone prolonged therapies with cortisone as they are at greater risk of diabetic retinopathy. The pyramid illustrates the recommended daily diet: three portions of grains with low GI (for high fiber and zinc content), five portions (≥200 g/die) of fruits and vegetables, especially spinach and broccoli and cooked zucchini and green leafy vegetables, orange, kiwi, grapefruit (for luteina/zeaxantina at least ≥942 μg/die content, are to be preferred), light yogurt (125 ml), skim milk (200 ml), EVO oil (almost 20 mg/day for high vitamin E and polyphenols content), and nuts or oilseeds (20–30 g/day, for zinc content, at least ≥15.8 mg/die); and weekly: fish (4 portions, for omega-3 content, EPA+DHA at least 0.35 as far as 1.4 g/day), white meat (3 portions for vitamin B12 and folic acid content), legumes (2 portions for vegetal proteins), eggs (2 portions for lutein/zeaxanthin content), fresh and low-fat cheeses for the content of vitamins of group B), and red or processed meats (once/week) and microgreen (at least once a week). There are two pennants at the top of the pyramid: one green indicates the need for individualized supplementation (if daily requirements cannot be met through diet, omega-3 supplementation and L-metilfolate may be a useful strategy with a great benefit-to-cost ratio) and one red indicates the presence of certain foods that are prohibited) (salt and sugar). Finally, 30 to 40 min of aerobic and resistance workouts must be done three to four times per week, and the intake of adequate quantities of water, equal to 1.5–2 liters/day, is a fundamental objective to achieve. Another important topic on which most of the literature agrees is the importance of maintaining a BMI between 19 and 25 kg/m2. Finally, in these conclusions, it is necessary to remember a topic that will be the subject of many studies in the near future: the relationship between intestinal microbiota and eye diseases because the microbiota can influence several metabolic pathways involved in the regulation of ocular health. Inflammation and hyperglycemia can lead to intestinal permeability of microbial products, which can in turn bind to ocular receptors and transmit inflammatory signals. The gut microbiota influences bacterial and host-derived metabolites, which signal distally to the brain and eye and influences systemic lipid metabolism, and has been shown to influence the lipid composition of the retina.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MR, SP, and AR contributed to the conception and design of the study. AC, CR, and AT wrote the first draft of the manuscript. CG, GB, and GP wrote sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of interest
AR and GP are employed by Indena Spa.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GA declared a shared affiliation with the authors MR, CG, GB, AC, CR, and AT to the handling editor at time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Ong SR, Crowston JG, Loprinzi PD, Ramulu PY. Physical activity, visual impairment, and eye disease. Eye. (2018) 32:1296–303. doi: 10.1038/s41433-018-0081-8
3. Francisco SG, Smith KM, Aragonès G, Whitcomb EA, Weinberg J, Wang X, et al. Dietary patterns, carbohydrates, and age-related eye diseases. Nutrients. (2020) 12:2862. doi: 10.3390/nu12092862
4. The age-related eye disease study (AREDS). Control Clin Trials. (1999) 20:573–600. doi: 10.1016/S0197-2456(99)00031-8
5. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration. JAMA. (2013) 309:2005. doi: 10.1001/jama.2013.4997
6. Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. (2016) 50:34–66. doi: 10.1016/j.preteyeres.2015.10.003
7. Hammond BR, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. (1997) 38:1795–801.
8. Schalch W, Cohn W, Barker FM, Köpcke W, Mellerio J, Bird AC, et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin – the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. (2007) 458:128–35. doi: 10.1016/j.abb.2006.09.032
9. Johnson EJ, Chung H-Y, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. (2008) 87:1521–9. doi: 10.1093/ajcn/87.5.1521
10. Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. (1997) 65:57–62. doi: 10.1006/exer.1997.0309
11. Eisenhauer B, Natoli S, Liew G, Flood V. Lutein and zeaxanthin—food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients. (2017) 9:120. doi: 10.3390/nu9020120
12. Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J Food Compos Anal. (2009) 22:9–15. doi: 10.1016/j.jfca.2008.07.006
13. Palermo M, Pellegrini N, Fogliano V. The effect of cooking on the phytochemical content of vegetables. J Sci Food Agric. (2014) 94:1057–70. doi: 10.1002/jsfa.6478
14. Xiao Z, Lester GE, Luo Y, Wang Q. Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. J Agric Food Chem. (2012) 60:7644–51. doi: 10.1021/jf300459b
15. Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J Am Diet Assoc. (1993) 93:284–96. doi: 10.1016/0002-8223(93)91553-3
16. Schaeffer JL, Tyczkowski JK, Parkhurst CR, Hamilton PB. Carotenoid composition of serum and egg yolks of hens fed diets varying in carotenoid composition. Poult Sci. (1988) 67:608–14. doi: 10.3382/ps.0670608
17. Bohn T. Bioavailability of non-provitamin A carotenoids. Curr Nutr Food Sci. (2008) 4:240–58. doi: 10.2174/157340108786263685
18. van het Hof KH, West CE, Weststrate JA, Hautvast JGAJ. dietary factors that affect the bioavailability of carotenoids. J Nutr. (2000) 130:503–6. doi: 10.1093/jn/130.3.503
19. O'Connell OF, Ryan L, O'Brien NM. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr Res. (2007) 27:258–64. doi: 10.1016/j.nutres.2007.04.002
20. Castenmiller JJM, West CE, Linssen JPH, van het Hof KH, Voragen AGJ. The food matrix of spinach is a limiting factor in determining the bioavailability of β-carotene and to a lesser extent of lutein in humans. J Nutr. (1999) 129:349–55. doi: 10.1093/jn/129.2.349
21. Carazo A, Macáková K, Matoušová K, Krčmová LK, Protti M, Mladěnka P. Vitamin a update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. (2021) 13:1703. doi: 10.3390/nu13051703
22. Zhong M, Kawaguchi R, Kassai M, Sun H. Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients. (2012) 4:2069–96. doi: 10.3390/nu4122069
23. Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. (2011) 343:d5094. doi: 10.1136/bmj.d5094
24. Wolf G. The discovery of the visual function of vitamin A. J Nutr. (2001) 131:1647–50. doi: 10.1093/jn/131.6.1647
25. Wang A, Han J, Jiang Y, Zhang D. Association of vitamin A and β-carotene with risk for age-related cataract: a meta-analysis. Nutrition. (2014) 30:1113–21. doi: 10.1016/j.nut.2014.02.025
26. Jiang H, Yin Y, Wu CR, Liu Y, Guo F, Li M, et al. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am J Clin Nutr. (2019) 109:43–54. doi: 10.1093/ajcn/nqy270
27. Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MOM. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol. (1988) 128:700–10. doi: 10.1093/oxfordjournals.aje.a115023
28. Delcourt C, Cristol JP, Tessier F, Léger CL, Descomps B, Papoz L. Age-related macular degeneration and antioxidant status in the POLA study. POLA Study Group. Pathologies Oculaires Liées à l'Age. Arch Ophthalmol. (1999) 117:1384–90. doi: 10.1001/archopht.117.10.1384
29. Egger Matthias, Smith GDavey, Altman DG. Systematic Reviews in Health Care : Meta-Analysis in Context. BMJ Books (2001). p. 487. doi: 10.1002/9780470693926
30. Diaz-Lopez A, Babio N, Martinez-González MA, Corella D, Amor AJ, Fitó M, et al. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: a post hoc analysis of a randomized trial. Diabetes Care. (2015) 38:2134–41. doi: 10.2337/dc15-1117
31. Salas-Salvadó J, Bulló M, Babio N, Martínez-González MÁ, Ibarrola-Jurado N, Basora J, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean DietResults of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. (2011) 34:14–9. doi: 10.2337/dc10-1288
32. Sala-Vila A, Díaz-López A, Valls-Pedret C, Cofán M, García-Layana A, Lamuela-Raventós RM, et al. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol. (2016) 134:1142–49. doi: 10.1001/jamaophthalmol.2016.2906
33. el Bilbeisi AH, Hosseini S, Djafarian K. Association of dietary patterns with diabetes complications among type 2 diabetes patients in Gaza Strip, Palestine: a cross sectional study. J Health Popul Nutr. (2017) 36:1–11. doi: 10.1186/s41043-017-0115-z
34. Tanaka S, Yoshimura Y, Kawasaki R. Fruit intake incident diabetic retinopathy with type 2 diabetes. JSTOR (2013). Available online at: https://www.jstor.org/stable/23487820?casa_token=ED906ID4cTAAAAAA:6ZWHvgjkJQi77Nzc8DKO5HAv4cMoCEQqWLuNbS7yIfk7-4YdQT8XX6ADyKBvqGnaTIJ_ozfiU9edjnPLIuxTPqFdT0b3dt2DsQoV0daS_4XOJ8ybs5w0 (accessed June 30, 2022).
35. Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. (2002) 76:93–9. doi: 10.1093/ajcn/76.1.93
36. Sala-Vila A, Valls-Pedret C, Rajaram S, Coll-Padrós N, Cofán M, Serra-Mir M, et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: a randomized controlled trial. Am J Clin Nutr. (2020) 111:590–600. doi: 10.1093/ajcn/nqz328
37. Nunes S, Alves D, Barreto P, Raimundo M, da Luz Cachulo M, Farinha C, et al. Adherence to a Mediterranean diet and its association with age-related macular degeneration. The Coimbra Eye Study–Report 4. Nutrition. (2018) 51–2:6–12. doi: 10.1016/j.nut.2017.12.010
38. Ma Q, Chen D, Sun HP, Yan N, Xu Y, Pan CW. Regular Chinese green tea consumption is protective for diabetic retinopathy: a clinic-based case-control study. J Diabetes Res. (2015) 2015:231570. doi: 10.1155/2015/231570
39. Hjellvik V, Tverdal A, Strøm H. Boiled coffee intake and subsequent risk for type 2 diabetes. Epidemiology. (2011) 22:418–21. doi: 10.1097/EDE.0b013e31821083e3
40. Tuomilehto J, Hu G, Bidel S, Lindström J. Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. jamanetwork.com. Available online at: https://jamanetwork.com/journals/jama/article-abstract/198353 (accessed July 18, 2022). doi: 10.1001/jama.291.10.1213
41. Brazionis L, Rowley K, Itsiopoulos C, O'dea K. Plasma carotenoids and diabetic retinopathy. Br J Nutr. (2008) 101:270–7. doi: 10.1017/S0007114508006545
42. Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol. (2011) 21:637–43. doi: 10.5301/EJO.2010.6212
43. Zhang PC, Wu CR, Wang ZL, Wang LY, Han Y, Sun SL, et al. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac J Clin Nutr. (2017) 26:406–11. doi: 10.6133/APJCN.032016.13
44. Moschos MM, Dettoraki M, Tsatsos M, Kitsos G, Kalogeropoulos C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. (2017) 4:23. doi: 10.1186/s40662-017-0088-4
45. Millen AE, Klein R, Folsom AR, Stevens J, Palta M, Mares JA. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. (2004) 79:865–73. doi: 10.1093/ajcn/79.5.865
46. Mayer-Davis E, Bell R, Reboussin B. Antioxidant nutrient intake and diabetic retinopathy: the San Luis Valley Diabetes Study. Ophthalmology. 105:2264–70. doi: 10.1016/S0161-6420(98)91227-1
47. Millen AE, Sahli MW, Nie J, LaMonte MJ, Lutsey PL, Klein BEK, et al. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc Diabetol. (2016) 15:1–11. doi: 10.1186/s12933-016-0434-1
48. Long M, Wang C, Liu D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr Diab. (2017) 7:e281. doi: 10.1038/nutd.2017.30
49. Mahoney SE, Loprinzi PD. Influence of flavonoid-rich fruit and vegetable intake on diabetic retinopathy and diabetes-related biomarkers. J Diabetes Complic. (2014) 28:767–71. doi: 10.1016/j.jdiacomp.2014.06.011
50. Park SW, Ghim W, Oh S, Kim Y, Park UC, Kang J, et al. Association of vitreous vitamin C depletion with diabetic macular ischemia in proliferative diabetic retinopathy. PLoS ONE. (2019) 14:e0218433. doi: 10.1371/journal.pone.0218433
51. Gurreri A, Pazzaglia A, Schiavi C. Role of statins and ascorbic acid in the natural history of diabetic retinopathy: a new, affordable therapy? Ophthalmic Surg Lasers Imaging Retina. (2019) 50:S23–7. doi: 10.3928/23258160-20190108-06
52. Thosar SS, Bielko SL, Wiggins CS, Klaunig JE, Mather KJ, Wallace JP. Antioxidant vitamin C prevents decline in endothelial function during sitting. Med Sci Monitor. (2015) 21:1015–21. doi: 10.12659/MSM.893192
53. Memisogullari R, Yüksel H, Coskun A, Yüksel HK, Yazgan Ö, Bilgin C. High serum homocysteine levels correlate with a decrease in the blood flow velocity of the ophthalmic artery in highway toll collectors. Tohoku J Exp Med. (2007) 212:247–52. doi: 10.1620/tjem.212.247
54. Horikawa C, Aida R, Kamada C, Fujihara K, Tanaka S, Tanaka S, et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: analysis of data from the Japan Diabetes Complications Study (JDCS). Eur J Nutr. (2020) 59:1585–94. doi: 10.1007/s00394-019-02014-4
55. Gopinath B, Flood VM, Rochtchina E, Wang JJ, Mitchell P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr. (2013) 98:129–35. doi: 10.3945/ajcn.112.057091
56. Sasaki M, Kawasaki R, Rogers S, Man REK, Itakura K, Xie J, et al. The associations of dietary intake of polyunsaturated fatty acids with diabetic retinopathy in well-controlled diabetes. Invest Ophthalmol Vis Sci. (2015) 56:7473–9. doi: 10.1167/iovs.15-17485
57. Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. (2011) 32:584–8.
58. Xiang G-D, Sun H-L, Zhao L-S, Hou J, Yue L, Xu L. The antioxidant alpha-lipoic acid improves endothelial dysfunction induced by acute hyperglycaemia during OGTT in impaired glucose tolerance. Wiley Online Library. (2008) 68:716–23. doi: 10.1111/j.1365-2265.2007.03099.x
59. Luo YY, Zhao J, Han XY, Zhou XH, Wu J, Ji LN. Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin Med J. (2015) 128:3276–82. doi: 10.4103/0366-6999.171357
60. Zhang J, Ren Z, Zhang Q, Zhang R, Zhang C, Liu J. Lower hydration status increased diabetic retinopathy among middle-aged adults and older adults: results from NHANES 2005-2008. Front Public Health. (2022) 10:1023747. doi: 10.3389/fpubh.2022.1023747
61. Praidou A, Harris M, Niakas D, Labiris G. Physical activity and its correlation to diabetic retinopathy. J Diabetes Complic. (2017) 31:456–61. doi: 10.1016/j.jdiacomp.2016.06.027
62. Yan X, Han X, Wu C, Shang X, Zhang L, He M. Effect of physical activity on reducing the risk of diabetic retinopathy progression: 10-year prospective findings from the 45 and Up Study. PLoS ONE. (2021) 16:e0239214. doi: 10.1371/journal.pone.0239214
63. Kuwata H, Okamura S, Hayashino Y, Tsujii S, Ishii H. Higher levels of physical activity are independently associated with a lower incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: a prospective cohort study, Diabetes Distress and Care Registry at Tenri (DDCRT15). PLoS ONE. (2017) 12:e0172890. doi: 10.1371/journal.pone.0172890
64. Al-Othman A, Al-Musharaf S, Al-Daghri NM, Krishnaswamy S, Yusuf DS, Alkharfy KM, et al. Effect of physical activity and sun exposure on vitamin D status of Saudi children and adolescents. BMC Pediatr. (2012) 12:92. doi: 10.1186/1471-2431-12-92
65. Scott D, Blizzard L, Fell J, Ding C, Winzenberg T, Jones G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol. (2010) 73:581–7. doi: 10.1111/j.1365-2265.2010.03858.x
66. Klenk J, Rapp K, Denkinger M, Nagel G, Nikolaus T, Peter R, et al. Objectively measured physical activity and vitamin D status in older people from Germany. J Epidemiol Community Health. (2015) 69:388–92. doi: 10.1136/jech-2014-204632
67. Black LJ, Burrows SA, Jacoby P, Oddy WH, Beilin LJ, Ping-Delfos WCS, et al. Vitamin D status and predictors of serum 25-hydroxyvitamin D concentrations in Western Australian adolescents. Br J Nutr. (2014) 112:1154–62. doi: 10.1017/S000711451400186X
68. Herrmann M, Sullivan DR, Veillard A-S, McCorquodale T, Straub IR, Scott R, et al. Serum 25-hydroxyvitamin d: a predictor of macrovascular and microvascular complications in patients with type 2 diabetes. Diabetes Care. (2015) 38:521–8. doi: 10.2337/dc14-0180
69. Schneider SH, Khachadurian AK, Amorosa LF, Clemow L, Ruderman NB. Ten-year experience with an exercise-based outpatient life-style modification program in the treatment of diabetes mellitus. Diabetes Care. (1992) 15:1800–10. doi: 10.2337/diacare.15.11.1800
70. Dow C, Mancini F, Rajaobelina K, Boutron-Ruault MC, Balkau B, Bonnet F, et al. Diet and risk of diabetic retinopathy: a systematic review. Eur J Epidemiol. (2018) 33:141–56. doi: 10.1007/s10654-017-0338-8
71. Wong MYZ, Man REK, Fenwick EK, Gupta P, Li LJ, van Dam RM, et al. Dietary intake and diabetic retinopathy: a systematic review. PLoS ONE. (2018) 13:e0186582. doi: 10.1371/journal.pone.0186582
72. Ros E, Izquierdo-Pulido M, Sala-Vila A. Beneficial effects of walnut consumption on human health: role of micronutrients. Curr Opin Clin Nutr Metab Care. (2018) 21:498–504. doi: 10.1097/MCO.0000000000000508
73. Poulose SM, Miller MG, Shukitt-Hale B. Role of walnuts in maintaining brain health with age. J Nutr. (2014) 144:561S−66. doi: 10.3945/jn.113.184838
74. Valero-Vello M, Peris-Martínez C, García-Medina JJ, Sanz-González SM, Ramírez AI, Fernández-Albarral JA, et al. Searching for the antioxidant, anti-inflammatory, and neuroprotective potential of natural food and nutritional supplements for ocular health in the mediterranean. Foods. (2021) 10:1231. doi: 10.3390/foods10061231
75. Meng J-M, Cao S-Y, Wei X-L, Gan R-Y, Wang Y-F, Cai S-X, et al. Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: an updated review. Antioxidants. (2019) 8:170. doi: 10.3390/antiox8060170
76. Natella F, Scaccini C. Role of coffee in modulation of diabetes risk. Nutr Rev. (2012) 70:207–17. doi: 10.1111/j.1753-4887.2012.00470.x
77. Akash MSH, Rehman K, Chen S. Effects of coffee on type 2 diabetes mellitus. Nutrition. (2014) 30:755–63. doi: 10.1016/j.nut.2013.11.020
78. Carlström M, Larsson SC. Coffee consumption and reduced risk of developing type 2 diabetes: a systematic review with meta-analysis. Nutr Rev. (2018) 76:395–417. doi: 10.1093/nutrit/nuy014
79. Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. (2006) 124:995–1001. doi: 10.1001/archopht.124.7.995
80. Chua B, Flood V, Rochtchina E, Wang JJ, Smith W, Mitchell P. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch Ophthalmol. (2006) 124:981–6. doi: 10.1001/archopht.124.7.981
81. Tan JSL, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. (2009) 127:656–65. doi: 10.1001/archophthalmol.2009.76
82. SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, Gensler G, Lindblad AS, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. (2007) 125:1225–32. doi: 10.1001/archopht.125.9.1225
83. Robman L, Vu H, Hodge A, Tikellis G, Dimitrov P, McCarty C, et al. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol. (2007) 42:720–6. doi: 10.3129/i07-116
84. Delcourt C, Carrière I, Cristol JP, Lacroux A, Gerber M. Dietary fat and the risk of age-related maculopathy: the POLANUT study. Eur J Clin Nutr. (2007) 61:1341–4. doi: 10.1038/sj.ejcn.1602685
85. Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. (2001) 119:1191–9. doi: 10.1001/archopht.119.8.1191
86. Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A. Does eating particular diets alter the risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. (2009) 93:1241. doi: 10.1136/bjo.2008.143412
87. Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. (1994) 272:1413–20. doi: 10.1001/jama.272.18.1413
88. Snellen ELM, Verbeek ALM, Van Den Hoogen GWP, Cruysberg JRM, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand. (2002) 80:368–71. doi: 10.1034/j.1600-0420.2002.800404.x
89. Simonelli F, Zarrilli F, Mazzeo S, Verde V, Romano N, Savoia M, et al. Serum oxidative and antioxidant parameters in a group of Italian patients with age-related maculopathy. Clinica Chimica Acta. (2002) 320:111–5. doi: 10.1016/S0009-8981(02)00056-6
90. Kassoff A, Kassoff J, Buehler J, Eglow M, Kaufman F, Mehu M, et al. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. (2001) 119:1417–36. doi: 10.1001/archopht.119.10.1417
91. Klein BEK, Knudtson MD, Lee KE, Reinke JO, Danforth LG, Wealti AM, et al. Supplements and age-related eye conditions the beaver dam eye study. Ophthalmology. (2008) 115:1203–8. doi: 10.1016/j.ophtha.2007.09.011
92. Merle BM, Silver RE, Rosner B, Seddon JM. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study 1-3. Am J Clin Nutr. (2015) 102:1196–206. doi: 10.3945/ajcn.115.111047
93. de Koning-Backus APM, Buitendijk GHS, Kiefte-de Jong JC, Colijn JM, Hofman A, Vingerling JR, et al. Intake of vegetables, fruit, and fish is beneficial for age-related macular degeneration. Am J Ophthalmol. (2019) 198:70–9. doi: 10.1016/j.ajo.2018.09.036
94. Chiu CJ, Chang ML, Li T, Gensler G, Taylor A. Visualization of dietary patterns and their associations with age-related macular degeneration. Invest Ophthalmol Vis Sci. (2017) 58:1404. doi: 10.1167/iovs.16-20454
95. Amirul Islam FM, Chong EW, Hodge AM, Guymer RH, Aung KZ, Makeyeva GA, et al. Dietary patterns and their associations with age-related macular degeneration: the Melbourne collaborative cohort study. Ophthalmology. (2014) 121:1428–34.e2. doi: 10.1016/j.ophtha.2014.01.002
96. Christen WG, Liu S, Schaumberg DA, Buring JE. Fruit and vegetable intake and the risk of cataract in women. Am J Clin Nutr. (2005) 81:1417–22. doi: 10.1093/ajcn/81.6.1417
97. Jacques PF, Chylack LT, Hankinson SE, Khu PM, Rogers G, Friend J, et al. Long-term nutrient intake and early age-related nuclear lens opacities. Arch Ophthalmol. (2001) 119:1009–19. doi: 10.1001/archopht.119.7.1009
98. Moeller SM, Taylor A, Tucker KL, McCullough ML, Chylack LT, Hankinson SE, et al. Overall adherence to the dietary guidelines for Americans is associated with reduced prevalence of early age-related nuclear lens opacities in women. J Nutr. (2004) 134:1812–9. doi: 10.1093/jn/134.7.1812
99. Pastor-Valero M, Fletcher AE, De Stavola BL, Vioque J, Alepuz VC. Vitamin C is associated with reduced risk of cataract in a Mediterranean population. J Nutr. (2002) 132:1299–306. doi: 10.1093/jn/132.6.1299
100. Ghanavati M, Behrooz M, Rashidkhani B, Ashtray-Larky D, Zameni SD, Alipour M. Healthy eating index in patients with cataract: a case-control study. Iran Red Crescent Med J. (2015) 17:e22490. doi: 10.5812/ircmj.22490
101. Sedaghat F, Ghanavati M, Hajian PN, Hajishirazi S, Ehteshami M, Rashidkhani B. Nutrient patterns and risk of cataract: a case-control study. Int J Ophthalmol. (2017) 10:586–92. doi: 10.18240/ijo.2017.04.14
102. Minassian DC, Mehra V, Verrey JD. Dehydrational crises: a major risk factor in blinding cataract. Br J Ophthalmol. (1989) 73:100–5. doi: 10.1136/bjo.73.2.100
103. Chong EW, Wong TY. Multivitamin Supplements and cataract prevention. Ophthalmology. (2008) 115:597–8. doi: 10.1016/j.ophtha.2008.01.033
104. Jiang H, Wang LN, Liu Y, Li M, Wu M, Yin Y, et al. Physical activity and risk of age-related cataract. Int J Ophthalmol. (2020) 13:643–9. doi: 10.18240/ijo.2020.04.18
105. Sherwin JC, Kokavec J, Thornton SN. Hydration, fluid regulation and the eye: in health and disease. Clin Exp Ophthalmol. (2015) 43:749–64. doi: 10.1111/ceo.12546
106. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. (2010) 376:124–36. doi: 10.1016/S0140-6736(09)62124-3
107. Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
108. Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. (2012) 12:346–54. doi: 10.1007/s11892-012-0283-6
109. Grzybowski A, Brona P, Kim SJ. Microbial flora and resistance in ophthalmology: a review. Graefe's Arch Clin Exp Ophthalmol. (2017) 255:851–62. doi: 10.1007/s00417-017-3608-y
110. Raman R, Rani PK, Gnanamoorthy P, Sudhir RR, Kumaramanikavel G, Sharma T. Association of obesity with diabetic retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS Report no. 8). Acta Diabetol. (2010) 47:209–15. doi: 10.1007/s00592-009-0113-8
111. Scanlon PH, Aldington SJ, Stratton IM. Epidemiological issues in diabetic retinopathy. Middle East Afr J Ophthalmol. (2013) 20:293–300. doi: 10.4103/0974-9233.120007
112. Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. (2011) 30:18–53. doi: 10.1016/j.preteyeres.2010.09.001
113. Wong TY, Klein R, Islam FMA, Cotch MF, Folsom AR, Klein BEK, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. (2006) 141:446–55. doi: 10.1016/j.ajo.2005.08.063
114. Van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. (2003) 121:245–51. doi: 10.1001/archopht.121.2.245
115. Rajalakshmi R, Amutha A, Ranjani H, Ali MK, Unnikrishnan R, Anjana RM, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complic. (2014) 28:291–7. doi: 10.1016/j.jdiacomp.2013.12.008
116. Sharma Y, Saxena S, Mishra A, Saxena A, Natu SM. Nutrition for diabetic retinopathy: plummeting the inevitable threat of diabetic vision loss. Eur J Nutr. (2017) 56:2013–27. doi: 10.1007/s00394-017-1406-2
117. Van der Put N Gabreëls Gabreëls FES-TAJ of 1998 undefined. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Elsevier. Available online at: https://www.sciencedirect.com/science/article/pii/S0002929707615249 (accessed July 18, 2022).
118. McNulty H, Strain JJ, Hughes CF, Ward M. Riboflavin, MTHFR genotype and blood pressure: a personalized approach to prevention and treatment of hypertension. Mol Aspects Med. (2017) 53:2–9. doi: 10.1016/j.mam.2016.10.002
119. Yigit S, Karakus N, vision AI-M. Association of MTHFR gene C677T mutation with diabetic peripheral neuropathy diabetic retinopathy. (2013). Available online at: ncbi.nlm.nih.gov; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3724957/ (accessed July 18, 2022).
120. Yaribeygi H, Atkin SL, Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J Cell Physiol. (2019) 234:1300–12. doi: 10.1002/jcp.27164
121. Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, et al. Impaired tissue perfusion. Circulation. (2008) 118:968–76. doi: 10.1161/CIRCULATIONAHA.107.763730
122. Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Am Diabetes Assoc. 24:1403–10. doi: 10.2337/diacare.24.8.1403
123. Scaglione F, Xenobiotica GP. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. (2014) 44:480–8. doi: 10.3109/00498254.2013.845705
124. Zhu W, Meng YF, Wu Y, Xu M, Lu J. Association of alcohol intake with risk of diabetic retinopathy: a meta-analysis of observational studies. Sci Rep. (2017) 7:1–9. doi: 10.1038/s41598-017-00034-w
125. Chen C, Sun Z, Xu W, Tan J, Li D, Wu Y, Zheng T, Peng D. Associations between alcohol intake and diabetic retinopathy risk: a systematic review and meta-analysis. BMC Endocr Disord. (2020) 20:106. doi: 10.1186/s12902-020-00588-3
126. Raum P, Lamparter J, Ponto KA, Peto T, Hoehn R, Schulz A, et al. Prevalence and cardiovascular associations of diabetic retinopathy and maculopathy: results from the Gutenberg health study. PLoS ONE. (2015) 10:e0139527. doi: 10.1371/journal.pone.0139527
127. Yu W, Fu YC, Wang W. Cellular and molecular effects of resveratrol in health and disease. J Cell Biochem. (2012) 113:752–9. doi: 10.1002/jcb.23431
128. Srikanta AH, Kumar A, Sukhdeo SV, Peddha MS, Govindaswamy V. The antioxidant effect of mulberry and jamun fruit wines by ameliorating oxidative stress in streptozotocin-induced diabetic Wistar rats. Food Funct. (2016) 7:4422–31. doi: 10.1039/C6FO00372A
129. Giuffrè G, Lodato G, Dardanoni G. Prevalence and risk factors of diabetic retinopathy in adult and elderly subjects: the Casteldaccia Eye Study. Graefe's Arch Clin Exp Ophthalmol. (2004) 242:535–40. doi: 10.1007/s00417-004-0880-4
130. Beulens JWJ, Kruidhof JS, Grobbee DE, Chaturvedi N, Fuller JH, Soedamah-Muthu SS. Alcohol consumption and risk of microvascular complications in type 1 diabetes patients: the EURODIAB Prospective Complications Study. Diabetologia. (2008) 51:1631–8. doi: 10.1007/s00125-008-1091-z
131. Fenwick EK, Xie J, Man REK, Lim LL, Flood VM, Finger RP, et al. Moderate consumption of white and fortified wine is associated with reduced odds of diabetic retinopathy. J Diabetes Complic. (2015) 29:1009–14. doi: 10.1016/j.jdiacomp.2015.09.001
132. Salas-Salvadó J, Bulló M, Estruch R, Ros E, Covas M-I, Ibarrola-Jurado N, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. (2014) 160:1–10. doi: 10.7326/M13-1725
133. Premanand C, Rema M, Sameer MZ, Sujatha M, Balasubramanyam M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest Opthalmol Visual Sci. (2006) 47:2179. doi: 10.1167/iovs.05-0580
134. Bucolo C, Drago F, Maisto R, Romano GL, D'Agata V, Maugeri G, Giunta S. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. J Cell Physiol. (2019) 234:17295–304. doi: 10.1002/jcp.28347
135. Platania CBM, Fidilio A, Lazzara F, Piazza C, Geraci F, Giurdanella G, et al. Retinal protection and distribution of curcumin in vitro and in vivo. Front Pharmacol. (2018) 9:670. doi: 10.3389/fphar.2018.00670
136. Woo JM, Shin D-Y, Lee SJ, Joe Y, Zheng M, Yim JH, et al. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol Vis. (2012) 18:901–8.
137. Maugeri A, Mazzone MG, Giuliano F, Vinciguerra M, Basile G, Barchitta M, et al. Curcumin modulates dna methyltransferase functions in a cellular model of diabetic retinopathy. Oxid Med Cell Longev. (2018) 2018:1–12. doi: 10.1155/2018/5407482
138. Mustata GT, Rosca M, Biemel KM, Reihl O, Smith MA, Viswanathan A, et al. Paradoxical effects of green tea (Camellia sinensis) and antioxidant vitamins in diabetic rats improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes. (2005) 54:517–26. doi: 10.2337/diabetes.54.2.517
139. Silva KC, Rosales MAB, Hamassaki DE, Saito KC, Faria AM, Ribeiro PAO, et al. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. (2013) 54:1325–36. doi: 10.1167/iovs.12-10647
140. Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. (2012) 47:103–8. doi: 10.1159/000330051
141. Vinson JA, Zhang J. Black and green teas equally inhibit diabetic cataracts in a streptozotocin-induced rat model of diabetes. J Agric Food Chem. (2005) 53:3710–3. doi: 10.1021/jf048052l
142. Silva S Da, Costa J, Pintado M, Ferreira D. Antioxidants in the prevention and treatment of diabetic retinopathy a review. J Diabet Metabol. (2010) 1:111. doi: 10.4172/2155-6156.1000111
143. Lee Chong T, Ahearn EL, Cimmino L. Reprogramming the epigenome with vitamin C. Front Cell Dev Biol. (2019) 7:128. doi: 10.3389/fcell.2019.00128
144. May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. (2013) 19:2068–83. doi: 10.1089/ars.2013.5205
145. Yin J, Thomas F, Lang JC, Chaum E. Modulation of oxidative stress responses in the human retinal pigment epithelium following treatment with vitamin C. Wiley Online Library. (2011) 226:2025–32. doi: 10.1002/jcp.22532
146. Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem. (2006) 281:9307–13. doi: 10.1074/jbc.M600418200
147. Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Am Diabetes Assoc. 53:2404–11. doi: 10.2337/diabetes.53.9.2404
148. Pácal L, Kuricová K, diabetes KK. Evidence for altered thiamine metabolism in diabetes: is there a potential to oppose gluco-and lipotoxicity by rational supplementation? World J Diabetes. 5:288–95. doi: 10.4239/wjd.v5.i3.288
149. Booth AA, Khalifah RG, Hudson BG. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: comparison with aminoguanidine 1. Biochem Biophys Res Commun. (1996) 220:113–9. doi: 10.1006/bbrc.1996.0366
150. Shi C, Wang P, Airen S, Brown C, Liu Z, Townsend JH, et al. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis. (2020) 7:1–16. doi: 10.1186/s40662-020-00199-y
151. Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol. (2014) 9:167. doi: 10.1186/s13000-014-0167-y
152. Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov S, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. (2006) 25:397–424. doi: 10.1016/j.preteyeres.2006.05.003
153. Vrolijk MF, Opperhuizen A, Jansen EHJM, Hageman GJ, Bast A, Haenen GRMM. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol in Vitro. (2017) 44:206–12. doi: 10.1016/j.tiv.2017.07.009
154. Braun DJ, Abner E, Bakshi V, Goulding DS, Grau EM, Lin AL, et al. Blood flow deficits and cerebrovascular changes in a dietary model of hyperhomocysteinemia. ASN Neuro. (2019) 11:1759091419865788. doi: 10.1177/1759091419865788
155. Rathod RS, Khaire AA, Kale AA, Joshi SR. Effect of vitamin B12 and omega-3 fatty acid supplementation on brain neurotrophins and cognition in rats: a multigeneration study. Biochimie. (2016) 128–9:201–8. doi: 10.1016/j.biochi.2016.08.009
156. Vitamin B12-Health Professional Fact Sheet. National Institutes of Health. (2020). Available online at: https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/. - Cerca con Google. https://www.google.com/search?q=Vitamin+B12-Health+Professional+Fact+Sheet.+National+Institutes+of+Health.+2020.+https%3A%2F%2Fods.od.nih.gov%2Ffactsheets%2FVitaminB12-HealthProfessional%2F.&rlz=1C1AVFC_enIT851IT852&oq=Vitamin+B12-Health+Professional+Fact+Sheet.+National+Institutes+of+Health.+2020.+https%3A%2F%2Fods.od.nih.gov%2Ffactsheets%2FVitaminB12-HealthProfessional%2F.&aqs=chrome.0.69i57.404j0j7&sourceid=chrome&ie=UTF-8 (accessed July 14, 2022).
157. Tarr JM, Kaul K, Wolanska K, Kohner EM, Chibber R. Retinopathy in diabetes. Adv Exp Med Biol. (2013) 771:88–106. doi: 10.1007/978-1-4614-5441-0_10
158. Augustine J, Troendle EP, Barabas P, McAleese CA, Friedel T, Stitt AW, et al. The role of lipoxidation in the pathogenesis of diabetic retinopathy. Front Endocrinol. (2021) 11:1146. doi: 10.3389/fendo.2020.621938
159. Bengmark S. Advanced glycation and lipoxidation end products–amplifiers of inflammation: the role of food. J Parenteral Enteral Nutr. (2007) 31:430–40. doi: 10.1177/0148607107031005430
160. Park S, Karunakaran U, Jeoung N, Jeon J-H, Lee I-K. Physiological effect and therapeutic application of alpha lipoic acid. Curr Med Chem. (2014) 21:3636–45. doi: 10.2174/0929867321666140706141806
161. Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R)-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. (2005) 46:4302–10. doi: 10.1167/iovs.04-1098
162. Diabetes Control and Complications Trial Research Group DMNathan Genuth S Lachin J Cleary P Crofford O. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. (1993) 329:977–986. doi: 10.1056/NEJM199309303291401
163. Linee guida per la pratica clinica : gestione delle complicanze oculari del diabete. Retinopatia diabetica ed edema maculare. Rodríguez, F Cabrera Available online at: https://scielo.isciii.es/scielo.php?pid=S0365-66912009000900003&script=sci_arttext&tlng=en (accessed July 20, 2022).
164. Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, et al. Restructuring of the Gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. (2018) 67:1867–79. doi: 10.2337/db18-0158
165. Haluzík M, Mráz M. Intermittent fasting and prevention of diabetic retinopathy: where do we go from here? Diabetes. (2018) 67:1745–7. doi: 10.2337/dbi18-0022
166. Li Q, He R, Zhang F, Zhang J, Lian S, Liu H. Combination of oligofructose and metformin alters the gut microbiota and improves metabolic profiles, contributing to the potentiated therapeutic effects on diet-induced obese animals. Front Endocrinol. (2020) 10:939. doi: 10.3389/fendo.2019.00939
167. Maria Tanase D, Maria Gosav E, Neculae E, Florida Costea C, Ciocoiu M, Liliana Hurjui L, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients. (2020) 12:3719. doi: 10.3390/nu12123719
168. Dirani M, Crowston JG, van Wijngaarden P. Physical inactivity as a risk factor for diabetic retinopathy? A review. Clin Exp Ophthalmol. (2014) 42:574–81. doi: 10.1111/ceo.12306
169. Ren C, Liu W, Li J, Cao Y, Xu J, Lu P. Physical activity and risk of diabetic retinopathy: a systematic review and meta-analysis. Acta Diabetol. (2019) 56:823–37. doi: 10.1007/s00592-019-01319-4
170. Boniol M, Dragomir M, Autier P, Boyle P. Physical activity and change in fasting glucose and HbA1c: a quantitative meta-analysis of randomized trials. Acta Diabetol. (2017) 54:983–91. doi: 10.1007/s00592-017-1037-3
171. Makanae Y, Ogasawara R, Sato K, Takamura Y, Matsutani K, Kido K, et al. Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp Physiol. (2015) 100:1168–76. doi: 10.1113/EP085207
172. Sallam N, Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid Med Cell Longev. (2016) 2016:7239639. doi: 10.1155/2016/7239639
173. Kim CS, Park S, Chun Y, Song W, Kim HJ, Kim J. Treadmill exercise attenuates retinal oxidative stress in naturally-aged mice: an immunohistochemical study. Int J Mol Sci. (2015) 16:21008–20. doi: 10.3390/ijms160921008
174. Kruk J, Kubasik-Kladna K, Aboul-Enein YH. The role oxidative stress in the pathogenesis of eye diseases: currnt status and a dual role of physical activity. Mini Rev Med Chem. (2016) 16:241–57. doi: 10.2174/1389557516666151120114605
175. Allen RS, Hanif AM, Gogniat MA, Prall BC, Haider R, Aung MH, et al. TrkB signalling pathway mediates the protective effects of exercise in the diabetic rat retina. Eur J Neurosci. (2018) 47:1254–65. doi: 10.1111/ejn.13909
176. Cui JZ, Wong M, Wang A, Laher I, Matsubara JA. Exercise inhibits progression of diabetic retinopathy by reducing inflammatory, oxidative stress, and ER stress gene expression in the retina of db/db mice. Invest Ophthalmol Vis Sci. (2016) 57:5434–4.
177. Colberg SR. Exercise and diabetes: a clinician's guide to prescribing physical activity. Am Diabetes Assoc. (2013). doi: 10.2337/9781580404853
178. Graham C, Lasko-m P. Exercise options for persons with diabetic complications. Diabetes Educ. (1990) 16:212–20. doi: 10.1177/014572179001600312
179. Hamdy O, Goodyear LJ, Horton ES. Diet and exercise in type 2 diabetes mellitus. Endocrinol Metab Clin North Am. (2001) 30:883–907. doi: 10.1016/S0889-8529(05)70220-6
180. Farrell PA, Fedele MJ, Hernandez J, Fluckey JD, Miller JL, Lang CH, et al. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. J Appl Physiol. (1999) 87:1075–82. doi: 10.1152/jappl.1999.87.3.1075
181. Liew G, Klein R, Wong TY. The role of genetics in susceptibility to diabetic retinopathy. Int Ophthalmol Clin. (2009) 49:35–52. doi: 10.1097/IIO.0b013e31819fd5d7
182. Irvine C, Taylor NF. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: a systematic review. Austral J Physiother. (2009) 55:237–46. doi: 10.1016/S0004-9514(09)70003-0
183. Chiu CJ, Chang ML, Zhang FF, Li T, Gensler G, Schleicher M, et al. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol. (2014) 158:118–27.e1. doi: 10.1016/j.ajo.2014.04.016
184. Rinninella E, Mele MC, Merendino N, Cintoni M, Anselmi G, Caporossi A, et al. The role of diet, micronutrients and the gut microbiota in age-related macular degeneration: new perspectives from the gut−retina axis. Nutrients. (2018) 10. doi: 10.20944/preprints201810.0369.v1
185. Schweigert FJ, Reimann J. Mikronährstoffe und ihre Bedeutung für das Auge Wirkungsweise von Lutein/Zeaxanthin und Omega-3-Fettsäuren. Klin Monbl Augenheilkd. (2011) 228:537–43. doi: 10.1055/s-0029-1245527
186. King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. (2011) 94: 679S−84. doi: 10.3945/ajcn.110.005744
187. Erie JC, Good JA, Butz JA, Pulido JS. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am J Ophthalmol. (2009) 147:276–82.e1. doi: 10.1016/j.ajo.2008.08.014
188. McGuinness MB, Le J, Mitchell P, Gopinath B, Cerin E, Saksens NTM, et al. Physical activity and age-related macular degeneration: a systematic literature review and meta-analysis. Am J Ophthalmol. (2017) 180:29–38. doi: 10.1016/j.ajo.2017.05.016
189. Taylor A, Jacques PF, Nowell T, Perrone G, Blumberg J, Handelman G, et al. Vitamin C in human and guinea pig aqueous, lens and plasma in relation to intake. Curr Eye Res. (1997) 16:857–64. doi: 10.1076/ceyr.16.9.857.5039
190. Polse KA, Brand R, Mandell R, Vastine D, Demartini D, Flom R. Age differences in corneal hydration control. Invest Ophthalmol Vis Sci. (1989) 30:392–9.
191. Sabetti L, Renzetti A, D'Alessandri L, Balestrazzi E. Eventual error caused by dehydration with pachometry. Ophthalmologica. (2001) 215:97–101. doi: 10.1159/000050837
192. Heys KR, Friedrich MG, Truscott RJW. Free and bound water in normal and cataractous human lenses. Invest Ophthalmol Vis Sci. (2008) 49:1991–7. doi: 10.1167/iovs.07-1151
193. Obrosova IG, Chung SSM, Kador PF. Diabetic cataracts: mechanisms and management. Diabetes Metab Res Rev. (2010) 26:172–80. doi: 10.1002/dmrr.1075
194. Kisic B, Miric D, Zoric L, Ilic A, Dragojevic I. Antioxidant capacity of lenses with age-related cataract. Oxid Med Cell Longev. (2012) 2012:467130. doi: 10.1155/2012/467130
Keywords: diet, food pyramid, diabetic retinopathy, age-related macular degeneration, cataract, phytoextracts
Citation: Rondanelli M, Gasparri C, Riva A, Petrangolini G, Barrile GC, Cavioni A, Razza C, Tartara A and Perna S (2023) Diet and ideal food pyramid to prevent or support the treatment of diabetic retinopathy, age-related macular degeneration, and cataracts. Front. Med. 10:1168560. doi: 10.3389/fmed.2023.1168560
Received: 17 February 2023; Accepted: 09 May 2023;
Published: 30 May 2023.
Edited by:
Roberta Zupo, University of Bari Aldo Moro, ItalyReviewed by:
Giacosa Attilio, University of Pavia, ItalyEmanuele Cereda, San Matteo Hospital Foundation (IRCCS), Italy
Alfredo Caturano, University of Campania Luigi Vanvitelli, Italy
Copyright © 2023 Rondanelli, Gasparri, Riva, Petrangolini, Barrile, Cavioni, Razza, Tartara and Perna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clara Gasparri, Y2xhcmEuZ2FzcGFycmkwMUB1bml2ZXJzaXRhZGlwYXZpYS5pdA==
 Mariangela Rondanelli
Mariangela Rondanelli Clara Gasparri
Clara Gasparri Antonella Riva
Antonella Riva Giovanna Petrangolini
Giovanna Petrangolini Gaetan Claude Barrile3
Gaetan Claude Barrile3