- 1Hematology Department, Hamad Medical Corporation, Doha, Qatar
- 2Internal Medicine Department, Hamad Medical Corporation, Doha, Qatar
Sickle cell leg ulcers (SCLUs) are usually chronic, painful, and devastating complications of sickle cell disease. Skin vaso-occlusion with compromised blood flow, chronic inflammation, and endothelial dysfunction is thought to be the underlying mechanism. It is usually slow to heal, and it may become chronic and superinfected. The management of SCLUs is usually challenging and requires a multidisciplinary team. Multiple systemic and local therapies have been tried in SCLU treatment. However, the outcome is variable: currently, there are no official recommendations for the best effective treatment. Herein, we report a 34-year-old male patient with non-transfusion-dependent sickle cell disease who was suffering from a chronic left ankle ulcer and was successfully managed with hyperbaric oxygen therapy, resulting in a complete resolution of this devastating complication.
Introduction
Sickle cell disease (SCD) is an inherited disorder that results from a point mutation in the beta-globin gene leading to the production of sickle hemoglobin. When the red blood cells become deoxygenated, it leads to abnormal hemoglobin S polymers that destroy the normal shape of the red cell. These rigid sickled-shaped red blood cells can subsequently occlude blood flow in many parts, leading to both acute and chronic tissue damage (1–3).
Sickle cell leg ulcers (SCLUs) are relatively common and can be disabling complications of sickle cell disease. The pathogenesis is complex and may include mechanical obstruction, bacterial infection, local thrombi, and deficiency of functional nitric oxide leading to endothelial dysfunction. The geographic origin is an important factor in the occurrence of SCLUs, with a reported prevalence of 40% in tropical areas like Jamaica compared to approximately 18% in the USA (4, 5).
Multiple systemic and local therapies have been tried in SCLU treatment. However, the outcome is variable: currently, there are no official recommendations for the best effective treatment (6, 7). Hyperbaric oxygen is a treatment modality for different medical conditions. Accelerated wound healing may be mediated by the combined effects of reactive oxygen species and the increased production of nitric oxide, an essential cellular signal for tissue repair (8, 9).
Herein, we report a 34-year-old male patient with sickle cell disease who was suffering from chronic recurrent left ankle ulcers and received four sessions of hyperbaric oxygen therapy, which resulted in the complete resolution of his chronic ulcers.
Case presentation
A 33-year-old gentleman presented with a known case of sickle cell disease and was on folic acid and analgesics as needed. With regular follow-ups in the hematology clinic, the hematological laboratory values range for hemoglobin was between 8 and 9 gm/dl without transfusions, platelets were between 200 and 300 × 109/L, hemoglobin S was 91.4%, hemoglobin F was 3.9%, total bilirubin was 32 μmol/L, indirect bilirubin was 21 μmol/L, LDH was 503 u/l, and retic percentage was 7.9%, and his disease was under control without hydroxyurea. Throughout the course, he did not show any clinical manifestations of SCD, such as stroke, retinopathy, acute chest syndrome, and priapism. Moreover, he was physically active without any limitations, and the latest painful crisis was 4 years ago. For the last 6 months, he was suffering from a left ankle ulcer on the left medial and lateral malleoli for which he was following up in the podiatry clinic for regular dressings.
Despite that, after 2 months, the ulcers continued to expand and became more painful, so cultures were sent and the results were negative. An MRI of the ankle was performed, which showed an increased signal with irregular outline synovium of the ankle joint due to synovitis and the posterior lateral part of the calcaneum adjacent skin thickening and subcutaneous edema extending distally to the level of the calcaneocuboid articulation and to a lesser extent adjacent to its medial part.
After that, in the podiatry clinic, they tried different types of dressing, and then he was referred to orthopedic and pain clinics with minimal resolution of his ankle ulcers, which were disabling and devastating for him (Figure 1A).
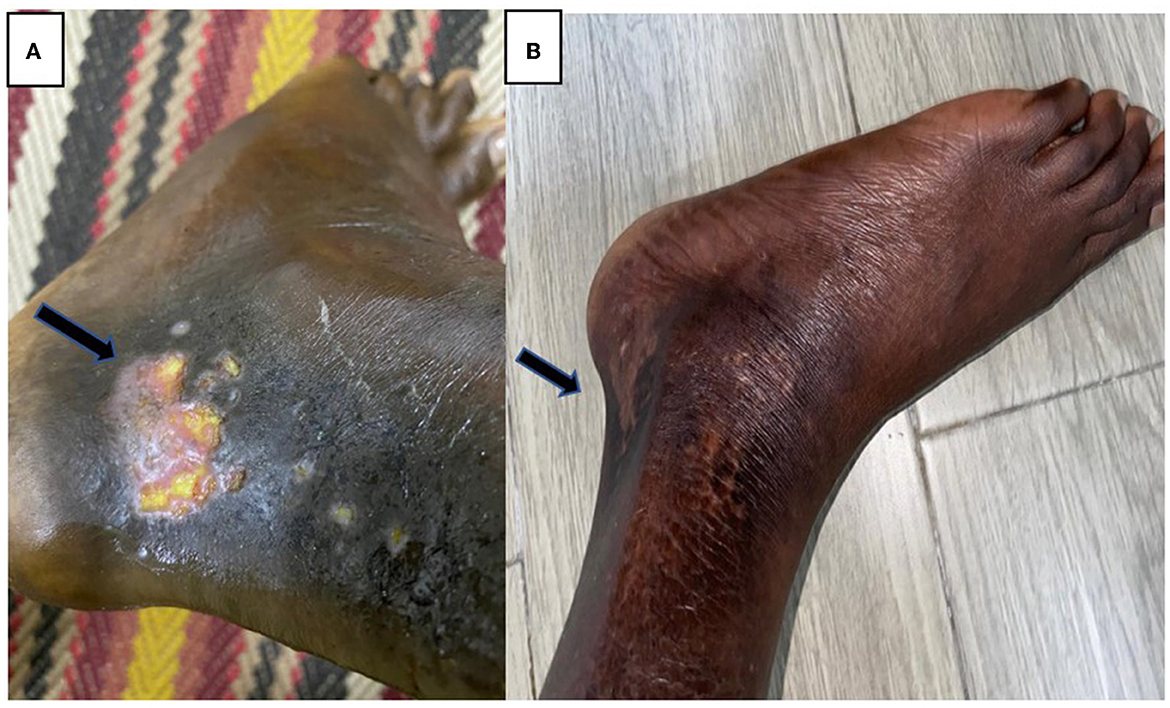
Figure 1. (A) Left ankle ulcer before using HBOT. (B) Almost complete resolution of his ulcers after using HBOT.
After 4 months, the ulcers increased in size with a length of 1.9 cm and width of 1.5 cm and became extremely painful, so cultures were sent from the wound, which did not grow any organisms, and subsequently, conservative measures with regular dressings, leg elevation, offloading, physiotherapy, and pain medications were tried but without significant clinical improvement.
Consequently, we decided to change our management strategy. Therefore, we counseled our patient about hyperbaric oxygen therapy to treat the ankle ulcer and reduce pain, and he agreed.
He received four sessions of hyperbaric oxygen therapy in 4 weeks, with one session per week that lasted for 2 h with a pressure of 2.5 pounds per square inch. Afterward, he achieved dramatic clinical improvement (without blood transfusion), and the examination showed well-granulated wounds. He had less pain and was healing properly without signs of active infection. He tolerated HOBT sessions very well without notable complications. Subsequent follow-up showed almost complete resolution of his ulcers (Figure 1B), and he was able to practice his daily life activities without limitations.
Discussion
Sickle cell leg ulcersare associated with significant medical and psychological morbidity and are an independent risk factor for death. They may occur spontaneously or after trauma with medial and lateral malleoli being the most common sites. They are usually slow to heal and may become chronic and superinfected. Risk factors for chronicity include venous incompetence, lower socioeconomic status, older age, a high degree of hemolysis, geographic distribution, and low steady-state hemoglobin (5, 10, 11).
Prevention is the best approach to sickle cell leg ulcer management, including avoiding trauma, wearing properly adapted shoes, preventing insect bites, and early counseling in case of skin injury. One study showed that a hemoglobin F of more than 25% is needed in order to reduce leg ulcer incidence by one-third (12). However, when a sickle cell disease patient develops a leg ulcer, the mainstays of treatment are non-specific measures with local wound care, pain control, bedrest, treatment of infection, venous compression therapy, and general sickle cell disease-based therapy chronic blood transfusion and hydroxyurea. Moreover, there are multiple treatment options that may be of benefit but remain unproven due to the lack of sufficient evidence. Options include Apligraf (a skin equivalent), hyperbaric oxygen, RGD peptide matrix, topical timolol, topical sodium nitrite cream, topical triple anti-biotherapy, solcoseryl, and collagen/glycosaminoglycan matrix (7, 13). The role of novel sickle cell disease drugs in the management of leg ulcers is not yet clear (14–16).
Hyperbaric oxygen (HBO) is a treatment modality for various conditions. It works by supplying oxygen at a pressure greater than the sea level leading to an increase in oxygen levels in the blood and body tissues. HBO has been used in chronic wounds that have not improved with conventional measures. This includes surgical and traumatic wounds, diabetic foot ulcers, skin grafts, and reconstructive flaps with vascular compromise. The use of HBO has not been specifically studied in sickle cell leg ulcer management. A recent meta-analysis evaluating the role of hyperbaric oxygen therapy in chronic wound healing showed that diabetic foot ulcers significantly improved in the short-term follow-up; however, this benefit was not demonstrated in the long term. However, it did not show any benefits of hyperbaric oxygen therapy in other chronic wounds. Sickle cell leg ulcer was not specifically studied. In our reported case, the patient was suffering from a painful chronic ulcer that did not improve with usual conservative measures, including regular dressing with different creams, leg elevations, pain management, and antibiotics. Using hyperbaric oxygen relieved his pain and resulted in almost complete healing. This finding supports the need for further large studies to confirm this therapeutic benefit (17, 18).
Hyperbaric oxygen improves wound healing by different mechanisms. It (1) reduces ischemia-reperfusion-induced inflammatory changes, (2) augments angiogenesis and fibroblast proliferation, (3) promotes neutrophil bacterial killing activity, suppresses clostridial spore and exotoxin generation, and kills anaerobes such as Clostridium perfringens, (4) induces vasoconstriction, which in turn reduces vasogenic edema, and (5) may work with growth factors such as plateletderived growth factor (PDGF), which require the presence of oxygen for successful function. As with other medical conditions, it may take up to 30 or more sessions to have good results (9, 19).
In conclusion, considering the limited data, this case report indicates that hyperbaric oxygen therapy is an effective and safe treatment option for sickle cell leg ulcers; however, further studies are needed to confirm this finding.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Research Council, Hamad Medical Corporation. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Academic Health System, Hamad Medical Corporation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ata F, Rahhal A, Malkawi L, Iqbal P, Khamees I, Alhiyari M, et al. Genotypic and phenotypic composition of sickle cell disease in the arab population - a systematic review. Pharmacogenomics Pers Med. (2023). 16:133–44. doi: 10.2147/PGPM.S391394
2. Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. (2009) 84:618–25. doi: 10.1002/ajh.21475
3. Wali Y, Kini V, Yassin MA. Distribution of sickle cell disease and assessment of risk factors based on transcranial Doppler values in the Gulf region. Hematol Amst Neth. (2020) 25:55–62. doi: 10.1080/16078454.2020.1714113
4. Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell disease. Am J Hematol. (2010) 85:831–3. doi: 10.1002/ajh.21838
5. Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore). (2005) 84:363–76. doi: 10.1097/01.md.0000189089.45003.52
6. Delaney K-MH, Axelrod KC, Buscetta A, Hassell KL, Adams-Graves PE, Seamon C, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. (2013) 37:789968. doi: 10.3109/03630269.2013.789968
7. Monfort J-B, Senet P. Leg ulcers in sickle-cell disease: treatment update. Adv Wound Care. (2020) 9:348–56. doi: 10.1089/wound.2018.0918
8. Boykin JV, Baylis C. Hyperbaric oxygen therapy mediates increased nitric oxide production associated with wound healing: a preliminary study. Adv Skin Wound Care. (2007) 20:382–8. doi: 10.1097/01.ASW.0000280198.81130.d5
9. Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM Mon J Assoc Physic. (2004) 97:385–95. doi: 10.1093/qjmed/hch074
10. Umeh NI, Ajegba B, Buscetta AJ, Abdallah KE, Minniti CP, Bonham VL. The psychosocial impact of leg ulcers in patients with sickle cell disease: I don't want them to know my little secret. PLoS ONE. (2017) 12:e0186270. doi: 10.1371/journal.pone.0186270
11. Antwi-Boasiako C, Andemariam B, Colombatti R, Vicky Asare E, Strunk C, Piccone CM, et al. A study of the geographic distribution and associated risk factors of leg ulcers within an international cohort of sickle cell disease patients: the CASiRe group analysis. Ann Hematol. (2020) 99:2073–9. doi: 10.1007/s00277-020-04057-8
12. Tolu SS, Crouch A, Choi J, Gao Q, Reyes-Gil M, Ogu UO, et al. Hydroxyurea and fetal hemoglobin effect on leg ulcers in patients with sickle cell disease. Ann Hematol. (2022) 101:541–8. doi: 10.1007/s00277-021-04635-4
13. Rozi W, Rahhal A, Ali EA, Al-Mashdali A, Hilan Y, Khamees I, et al. Direct oral anticoagulants in sickle cell disease: a systematic review and meta-analysis. Blood Adv. (2022) 6:5061–6. doi: 10.1182/bloodadvances.2022007308
14. Adel AA, Abushanab D, Hamad A, Al-Badriyeh D, Yassin MA. L-Glutamine and crizanlizumab for adults with sickle cell disease (SCD) in Qatar: a cost effectiveness analysis. Blood. (2021) 138:4945. doi: 10.1182/blood-2021-144636
15. Alshurafa A, Yassin MA. Short- and long-term follow-up and additional benefits in a sickle cell disease patient experienced severe crizanlizumab infusion-related vaso-occlusive crisis: A case report. Front Med. (2022) 9:1048571. doi: 10.3389/fmed.2022.1048571
16. Minniti CP, Knight-Madden J, Tonda M, Gray S, Lehrer-Graiwer J, Biemond BJ. The impact of voxelotor treatment on leg ulcers in patients with sickle cell disease. Am J Hematol. (2021) 96:E126–8. doi: 10.1002/ajh.26101
17. Alshurafa A, Elhissi M, Yassin MA. Complete resolution of stage II avascular necrosis affecting three joints by hyperbaric oxygen in a patient with sickle cell disease: A case report. Front Med. (2023) 9:1063255. doi: 10.3389/fmed.2022.1063255
18. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. (2009) 17:1–18. doi: 10.1111/j.1524-475X.2008.00436.x
Keywords: sickle cell disease, leg ulcer, hyperbaric oxygen, wound care, wound healing
Citation: Alshurafa A, Alkhatib M, Abu-Tineh M and Yassin MA (2023) Sickle cell leg ulcer successfully managed by hyperbaric oxygen: a case report. Front. Med. 10:1171971. doi: 10.3389/fmed.2023.1171971
Received: 22 February 2023; Accepted: 18 May 2023;
Published: 15 June 2023.
Edited by:
Ahmet Emre Eskazan, Istanbul University-Cerrahpasa, TürkiyeReviewed by:
Raffaella Colombatti, University Hospital of Padua, ItalyAnazoeze Jude Madu, University of Nigeria, Nigeria
Copyright © 2023 Alshurafa, Alkhatib, Abu-Tineh and Yassin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Alkhatib, ZHJta2hhdGVlYjkxQGdtYWlsLmNvbQ==
 Awni Alshurafa
Awni Alshurafa Mohammed Alkhatib2*
Mohammed Alkhatib2* Mohammad Abu-Tineh
Mohammad Abu-Tineh Mohamed A. Yassin
Mohamed A. Yassin