- 1HIV Dynamics and Replication Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
- 2Department of Physics, Faculty of Science, Minia University, Minya, Egypt
Editorial on the Research Topic
Reviews in: Radiopharmaceuticals in nuclear medicine
Radiopharmaceuticals are ever evolving
Medical application of radiopharmaceuticals began in the 1920s. The first diagnostic procedure utilizing a radiotracer was performed with 214Bi, then known as radium C, to measure arm-to-arm blood transit time (1, 2). Applications are far more sophisticated now, with nuclear medicine currently using radiopharmaceuticals for both clinical care and research evaluations. For example, 18F-Fluorodeoxyglucose (FDG)-positron emission tomography (PET) is commonly used to detect metabolically active cancer lesions; it is also being evaluated for ability to detect Human Immunodeficiency Virus (HIV) reservoirs (3). In addition to diagnostic uses, radiopharmaceuticals can be employed therapeutically or as theranostics, which combine diagnostic with therapeutic functions that share a specific target (4). This collection of reviews of radiopharmaceuticals in nuclear medicine (https://www.frontiersin.org/research-topics/38645/reviews-in-radiopharmaceuticals-in-nuclear-medicine) highlights ongoing innovation in the field.
Key messages from the collection
Two articles in the collection address PET imaging for lung cancer. Martucci et al. have performed a meta-analysis to quantitatively assess the impact of 18F-FDG PET/Computed tomography (CT) on small cell lung cancer (SCLC) staging. This builds on prior evidence, which relied primarily on 18F-FDG PET without concordant anatomical imaging. Based on 6 publications, the meta-analysis indicates that 18F-FDG PET/CT changes binary stage (limited vs. extensive disease) in approximately 15% of cases when compared to conventional staging. Since clinical management depends on the disease stage, incorporation of 18F-FDG PET/CT into SCLC staging has the potential to improve outcomes. Prospective studies will be helpful to validate this finding and to assess actual impact on outcomes such as morbidity and mortality.
As the armamentarium of radiopharmaceuticals is continuously expanding, Zhu et al. provide a helpful review of PET tracers for lung cancer beyond 18F-FDG. These alternative radioligands target tumor markers and cellular function, including proliferation, amino acid metabolism and transport, tumor hypoxia, angiogenesis, tyrosine kinases and cancer-associated fibroblasts. Many of these tracers are still under evaluation. As the more promising candidates progress to clinical and human research applications, they present the opportunity to target specific features of lung cancer and personalize patient management. Furthermore, the rational approach to ligand discovery, based on understanding of lung cancer pathogenesis, can be applied to diverse other conditions.
Identifying and understanding CNS disease via molecular imaging has long been a goal of the field. As exemplified by the pursuit of radiotracers for Alzheimer's Disease, for which targets include β-amyloid plaques, TAU tangles, and neurodegeneration (5), diverse targets must be explored based on the current understanding of disease features. Tong et al. offer an overview of Poly (ADP-ribose) polymerases (PARPs) as radioligand targets in central nervous system (CNS) diseases. Building on recent studies demonstrating PARP1 activation in neurodegenerative conditions and aging (6), the authors delve into how the PARP1 pathway might be targeted for diagnostic and therapeutic purposes. The development of PARP1 radiotracers, which are being evaluated in clinical trials for CNS conditions as well as malignancies, will hopefully lead to new insights in efforts to mitigate CNS disease. A major question will be how and if they contribute to improved outcomes.
The last article in the collection focuses on radionuclide therapy for metastatic or otherwise inoperable paragangliomas and pheochromocytomas (Prado-Wohlwend et al.). Radionuclide therapy serves as an alternative to chemotherapy and other targeted medications currently under investigation. Using modeling, Prado-Wohlwend et al. found that 177Lu-Lu-DOTA-TATE resulted in better progression free survival when compared to 131I-metaiodobenzylguanidine (MBG). Progression free survival with 177Lu-Lu-DOTA-TATE improved with proportion of adrenal lesions and pheochromocytomas. Understanding the impact of different therapies on disease course, particularly when treatment options are limited, is critical and should be validated with prospective clinical studies.
Building on current knowledge
While these reviews demonstrate incredible progress in nuclear medicine over the past several decades, they also highlight the great potential for further advancement. Identification of novel disease-specific ligands will engender greater clinical relevance and support more personalized use of radiopharmaceuticals. Researchers should strive to optimize the impact on disease course by using these agents to elucidate pathogenesis and identify novel opportunities for intervention. The development of such probes must consider disease attributes, current treatment and management approaches, and target characteristics. For results stemming from use of diagnostic radioligands, potential ethical concerns about whether knowledge it provides regarding a condition is actionable must be considered when seeking full licensure of the agent.
Ensuring that radioligands can access protected sites, particularly the CNS will also be necessary. In addition to optimizing the size and isoelectric point of a candidate, modifications can be made to use carriers or metabolic pathways that actively accumulate the ligand at the desired site. Another burgeoning arm of nuclear medicine is targeting of specific pathogens. For example, progress made in imaging tuberculosis and HIV must be continued (7–9). A particular challenge will be imaging of diffuse, low-concentration targets as is the case with certain infections.
Conclusions
Molecular imaging contributes significantly to research on and clinical management of many conditions. It also has the potential for an even greater impact. Figure 1 shows how functional imaging could impact clinical management, using the example of CNS diseases (10).
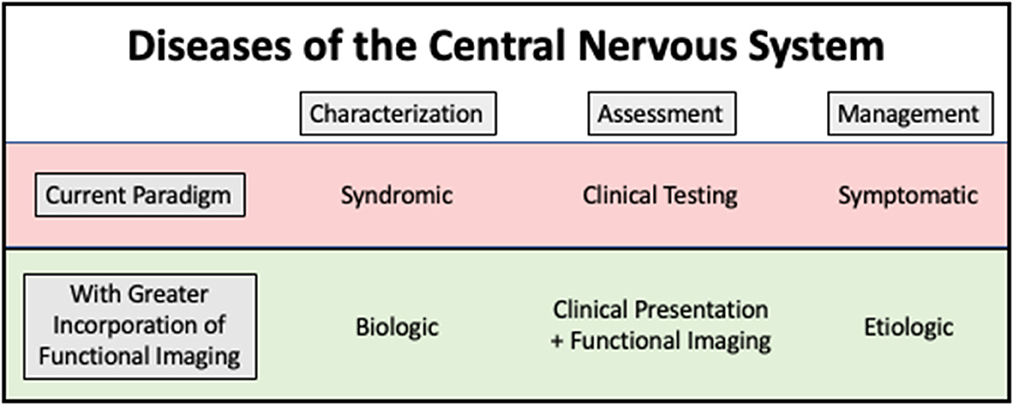
Figure 1. Conceptual model showing how functional imaging has the potential to change our approach to treatment and monitoring of central nervous system diseases. By using functional imaging to understand biology and pathogenesis, underlying causes of disease can be more effectively addressed.
Many conclusions derived from modeling, retrospective, or observational data need prospective high-quality clinical studies to validate results and guide clinical use. For example, we need additional data to conclude that use of 18F-FDG PET/CT for staging improves morbidity and mortality from SCLC. Continued development of additional disease and infection-specific ligands should also be supported, with the goal to personalize management more effectively, improve morbidity and mortality, and optimize quality of life. Radiopharmaceuticals have a very bright future (11). Ongoing assessment and building on current evidence, as has been done by the articles in this collection, will facilitate continued progress.
Author contributions
All authors contributed to conceptualization, writing, and approval of the final version of the manuscript.
Funding
This project was supported in part by the Intramural Research Program, National Cancer Institute, Center for Cancer Research, National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Blumgart HL, Weiss S. Studies on the velocity of blood flow. The physiological and the pathological significance of the velocity of blood flow. J Clin Invest. (1927) 4:199–209. doi: 10.1172/JCI100118
2. Blumgart HL, Yens OC. Studies on the velocity of blood flow. The method utilized. J Clin Invest. (1927) 4:1–13. doi: 10.1172/JCI100106
3. Lau CY, Adan MA, Earhart J, Seamon C, Nguyen T, Savramis A, et al. Imaging and biopsy of HIV-infected individuals undergoing analytic treatment interruption. Front Med (Lausanne). (2022) 9:979756. doi: 10.3389/fmed.2022.979756
4. Gomes Marin JF, Nunes RF, Coutinho AM, Zaniboni EC, Costa LB, Barbosa FG, et al. Theranostics in nuclear medicine: emerging and re-emerging integrated imaging and therapies in the era of precision oncology. Radiographics. (2020) 40:1715–40. doi: 10.1148/rg.2020200021
5. Bao W, Xie F, Zuo C, Guan Y, Huang YH, PET. Neuroimaging of Alzheimer's disease: radiotracers and their utility in clinical research. Front Aging Neurosci. (2021) 13:624330. doi: 10.3389/fnagi.2021.624330
6. Mao K, Zhang G. The role of PARP1 in neurodegenerative diseases and aging. FEBS J. (2022) 289:2013–24. doi: 10.1111/febs.15716
7. Beckford-Vera DR, Flavell RR, Seo Y, Martinez-Ortiz E, Aslam M, Thanh C, et al. First-in-human immunoPET imaging of HIV-1 infection using (89)Zr-labeled VRC01 broadly neutralizing antibody. Nat Commun. (2022) 13:1219. doi: 10.1038/s41467-022-28727-5
8. Ordonez AA, Tucker EW, Anderson CJ, Carter CL, Ganatra S, Kaushal D, et al. Visualizing the dynamics of tuberculosis pathology using molecular imaging. J Clin Invest. (2021) 131:5. doi: 10.1172/JCI145107
9. Lau CY, Maldarelli F, Eckelman WC, Neumann RD. Rational development of radiopharmaceuticals for HIV-1. Nucl Med Biol. (2014) 41:299–308. doi: 10.1016/j.nucmedbio.2014.01.005
10. Weber WA, Czernin J, Anderson CJ, Badawi RD, Barthel H, Bengel F, et al. The future of nuclear medicine, molecular imaging, and theranostics. J Nucl Med. (2020) 61:263S–72S. doi: 10.2967/jnumed.120.254532
Keywords: radiopharmaceutical, nuclear medicine, molecular imaging, radionuclide therapy, tracer
Citation: Lau C-Y and Mostafa MYA (2023) Editorial: Reviews in: Radiopharmaceuticals in nuclear medicine. Front. Med. 10:1178528. doi: 10.3389/fmed.2023.1178528
Received: 02 March 2023; Accepted: 06 March 2023;
Published: 15 March 2023.
Edited and reviewed by: Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), Switzerland
Copyright © 2023 Lau and Mostafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuen-Yen Lau, bGF1Y0BtYWlsLm5paC5nb3Y=
 Chuen-Yen Lau
Chuen-Yen Lau Mostafa Yuness Abdelfatah Mostafa
Mostafa Yuness Abdelfatah Mostafa