- 1Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 2Center for Infection and Immunity, Mailman School of Public Health, Vagelos College of Physicians and Surgeons of Columbia University, New York, NY, United States
Some patients remain unwell for months after “recovering” from acute COVID-19. They develop persistent fatigue, cognitive problems, headaches, disrupted sleep, myalgias and arthralgias, post-exertional malaise, orthostatic intolerance and other symptoms that greatly interfere with their ability to function and that can leave some people housebound and disabled. The illness (Long COVID) is similar to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) as well as to persisting illnesses that can follow a wide variety of other infectious agents and following major traumatic injury. Together, these illnesses are projected to cost the U.S. trillions of dollars. In this review, we first compare the symptoms of ME/CFS and Long COVID, noting the considerable similarities and the few differences. We then compare in extensive detail the underlying pathophysiology of these two conditions, focusing on abnormalities of the central and autonomic nervous system, lungs, heart, vasculature, immune system, gut microbiome, energy metabolism and redox balance. This comparison highlights how strong the evidence is for each abnormality, in each illness, and helps to set priorities for future investigation. The review provides a current road map to the extensive literature on the underlying biology of both illnesses.
Introduction
Shortly after the onset of the COVID-19 pandemic, it became clear that some patients remained unwell for months to years after “recovering” from the acute infection. They suffered from fatigue, cognitive problems, headaches, disrupted sleep, myalgias and arthralgias, post-exertional malaise, orthostatic intolerance, tachyarrhythmias and gastrointestinal complaints, all of which greatly interfered with their ability to function at home and at work. In this post-COVID period, the most severely affected people were housebound and disabled. This was true even of people who had not been severely ill with acute COVID-19. The illness has been given several names, including post-acute SARS-CoV-2 sequelae (PASC), post-COVID-19 condition (PCC), post-acute COVID-19 syndrome (PACS), and “Long COVID”—the name we use in this review. We distinguish it from other post-COVID conditions in Table 1, below.
In this review, we compare the symptoms of Long COVID and ME/CFS, noting considerable similarities and some differences. Early in the study of both illnesses, the lack of objective biomarkers led some to question whether the illnesses were “real”—whether people might be imagining or even fabricating their symptoms.
In this review we summarize the emerging evidence that, in fact, there are many underlying biological abnormalities reported in both illnesses, documented by multiple laboratories. Moreover, we show that the two illnesses share many of these underlying abnormalities, just as they share many symptoms. That is, both illnesses are “real,” and both share similar biological abnormalities.
Understanding the underlying biology of these illnesses is critically important, given the burden they are placing on all societies. The National Academy of Medicine and Centers for Disease Control and Prevention (CDC) estimate that, in the U.S., ME/CFS affects up to 2.5 million people and generates direct and indirect expenses of approximately $17–24 billion annually (1). It also has been reported in many countries around the world.
Post-COVID-19 conditions may affect 65 million people, globally (2). In the U.S., nearly 2% of the total civilian labor force is unable to work because of these illnesses, with foregone wages of $170–230 billion annually (3). Senior economists have estimated that the aggregate cost of medical care, lost productivity and disability may be $3.7 trillion over the next 5 years (4, 5). The most important, and unanswered, question regarding the economic impact of Long COVID is how long it will last. If, as with most people with ME/CFS, it lasts decades, the burden will be even greater than this projection. Because of the exceptional anticipated burden, the National Institutes of Health has dedicated over $1 billion in support of studies of the underlying biology and natural history of various post-COVID conditions.
In Tables 4–7 we try to efficiently summarize the findings from the large body of ME/CFS and post-COVID-19 literature. Our intention is to provide both an overview to general readers and a road map to a very large literature for investigators interested in pursuing the role of specific abnormalities in either or both illnesses. In summarizing what is known about biological abnormalities in both illnesses, we have cited both the “positive” studies that find an abnormality and the “negative” ones that do not. This allows readers to assess the strength (or weakness) of a reported finding.
As in any review, the studies we include are of varying size and quality. It is our hope that the road map we provide will help all interested readers judge for themselves how solid the evidence is for each of the underlying biological abnormalities, in each of the two illnesses, and where holes exist in our knowledge that need to be filled.
Brief summary of the illnesses
Overview of different conditions following acute COVID-19
Most people recover completely from acute COVID-19. However, others develop a variety of different post-acute sequelae of SARS-CoV-2 infection (PASC). The terminology for these different endotypes has not been standardized, in part because the detailed longitudinal studies necessary to generate the empirical data by which to define and distinguish different PASC endotypes have not yet been completed.
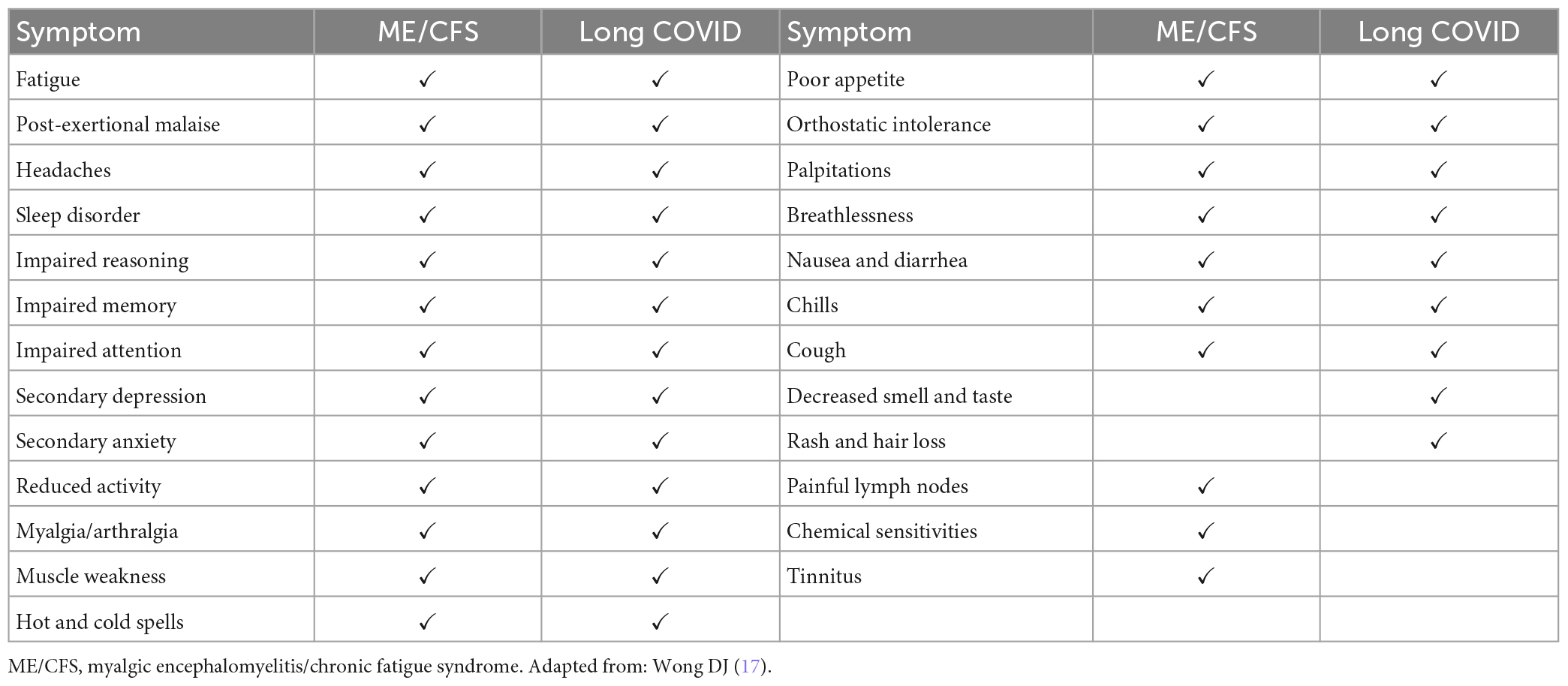
In Table 1, we propose three broad categories of PASC. Although we mention the first two categories for completeness, this review discusses only the third category—the condition called Long COVID. Some authorities refer to Long COVID as “PASC.” We think that is imprecise, since the tissue injury to multiple organs and the new onset of major diseases listed in Table 1 surely also are “sequelae” of COVID-19. In any event, the data summarized in Tables 3–7 of this review refer only to Long COVID.
The symptoms of Long COVID limit an individual’s ability to function at home or at work. The CDC states that in the “post-COVID condition” (which we take as equivalent to “Long COVID”) symptoms can be present four or more weeks after infection with SARS-CoV-2. The World Health Organization defines the condition as the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection and lasting for at least 2 months with no other explanation.
Some evidence indicates that Long COVID is more likely to develop in: people who are sickest and have the greatest evidence of inflammation with acute COVID-19; were PCR positive; are female; and have a premorbid history of asthma, chronic obstructive pulmonary disease and depression. However, many people with Long COVID have none of these risk factors (6–8).
Long COVID
How frequently does Long COVID follow acute COVID-19? A wide range of values for the incidence of Long COVID following infection with SARS-CoV-2 has been reported, depending on how the investigators have defined Long COVID and how rigorously they have pursued alternative explanations for the persisting symptoms.
A meta-analysis of 57 studies involving over 250,000 people found that ongoing symptoms impairing functional mobility persisted in 43% of people with acute COVID-19 for at least 6 months after acute infection (511). A study of nearly 100,000 cases and matched never-infected controls found that over 40% of cases remained with persistent symptoms and impairment across all daily activities at 18 months (8). A study of six thousand hospitalized patients compared to uninfected matched controls found persistent symptoms in approximately 30% of cases—much more often than in uninfected controls—at least 2 years after acute infection (512). Among people with persistent, debilitating symptoms following acute COVID-19, an estimated 13–45% meet the National Academy of Medicine case definition for ME/CFS (447, 513–515).
ME/CFS
Interest in ME/CFS (originally called “chronic fatigue syndrome”) surged in the mid-1980s, although a very similar illness has been described in the medical literature for several centuries. In the 19th and early 20th centuries the name “neurasthenia” was used to refer to a similar illness (516, 517). Since the 1980s, the names “chronic fatigue syndrome” and “systemic exertional intolerance disease” have been used. The illness also has several case definitions. We prefer the one proposed by the U.S. National Academy of Medicine (1), that is summarized in Table 2.
Myalgic encephalomyelitis/CFS often, but not always, follows in the wake of an apparent “infectious-like” illness characterized by respiratory and gastrointestinal symptoms, fatigue, myalgias and other symptoms as well as fever and lymphadenopathy (518). This “infectious-like” illness often is little different, initially, from the common, transient infectious illnesses that most people experience throughout life. It is not standard medical practice to test for the responsible infectious agent in people with common and transient infectious illnesses. Thus, typically no testing has been done to determine the cause of the initial “infectious-like” illness that then becomes a chronic illness in subsequent months and years.
Although in this review we are describing cases of ME/CFS that occur sporadically, there also have been apparent epidemics of a similar illness described in communities (141, 519–523) and in large hospitals over the past century (524). In one report, 6% of a community of 2,500 people—most young or middle-aged adults—became suddenly ill with an infectious-like illness followed by at least 5 months of a cyclic, debilitating illness characterized by fatigue, cognitive impairment (confirmed on objective testing) and pain (520). Details on symptoms, physical examination findings and laboratory test results in these studies are not sufficient to determine how similar these illnesses were to ME/CFS.
The severity of the symptoms, and the functional impairment, can range widely from one person to another. The functional impairment in people with ME/CFS may be even greater than in those with congestive heart failure and major depression (525, 526), and greater than in those with Long COVID (527). Some people remain able to fulfill their main responsibilities at work and at home, although hobbled. Others are bed-ridden or housebound, and unable to work. For most individuals with ME/CFS the symptoms are cyclic, with some relatively “good” days and frequent “bad” days. Several stressors—exercise, prolonged upright position, cognitive and emotional upset—typically produce a worsening of all of the symptoms of the illness. This condition, called post-exertional malaise, is a cardinal feature of the illness (1).
Attempts to identify a single and possibly novel infectious agent as the cause of most cases of ME/CFS have been unsuccessful. For example, claims that murine leukemia viruses cause ME/CFS have been refuted (528, 529), as have similar claims for Borna disease virus (530).
Several other conditions often are present in some people with ME/CFS: autonomic dysfunction (particularly postural orthostatic tachycardia syndrome), various rheumatologic conditions (particularly fibromyalgia), neurologic deficits (such as sensory hypersensitivity and small fiber neuropathy), secondary depression and anxiety, new or worsened allergic disorders, and endometriosis (531, 532).
Comparison of the symptoms
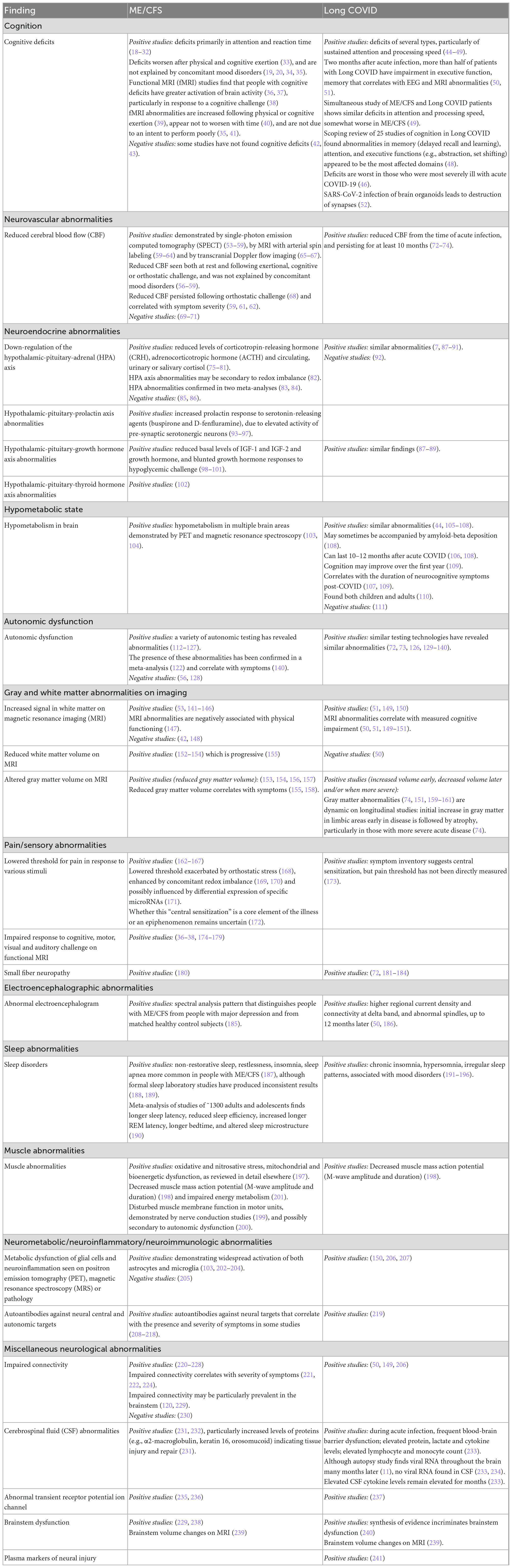
The symptoms reported by people with both ME/CFS and Long COVID have been integrated in a recent meta-analysis of 21 studies (17). In Table 3, we summarize the long list of symptoms shared by these two illnesses (and shared with other illnesses). The cardinal symptom of ME/CFS, post-exertional malaise, also is reported by the vast majority of people with Long COVID (533, 534). As seen in Table 3, most symptoms in the two illnesses are similar, although decreased smell and taste, rash and hair loss are frequent in Long COVID but only rarely reported by people with ME/CFS, and painful lymph nodes, chemical sensitivities and tinnitus are frequent in ME/CFS but rarely reported in Long COVID.
Comparison of the underlying objective biological abnormalities
A wide range of objective abnormalities have been reported in both Long COVID and ME/CFS. Multi-organ magnetic resonance imaging (MRI) imaging of many Long COVID patients and non-symptomatic post-COVID control subjects has revealed abnormalities involving many organs up to 1 year following acute COVID-19, although it is unclear if the anatomic MRI abnormalities reflect defective organ function: correlation of MRI abnormalities with persistent symptoms was poor (535).
Probably with both illnesses, and certainly with ME/CFS, underlying biological abnormalities appear to change over time. Initially, there is activation of neuroendocrine axes and cytokine production, followed after several years by an apparent “exhaustion” of neuroendocrine and immunologic activity (260, 536).
The most compelling findings thus far have been described in the central and autonomic nervous system, immune system, infectious agents, metabolism, and the cardiopulmonary system. We will focus on those abnormalities in this review.
Central and autonomic nervous system
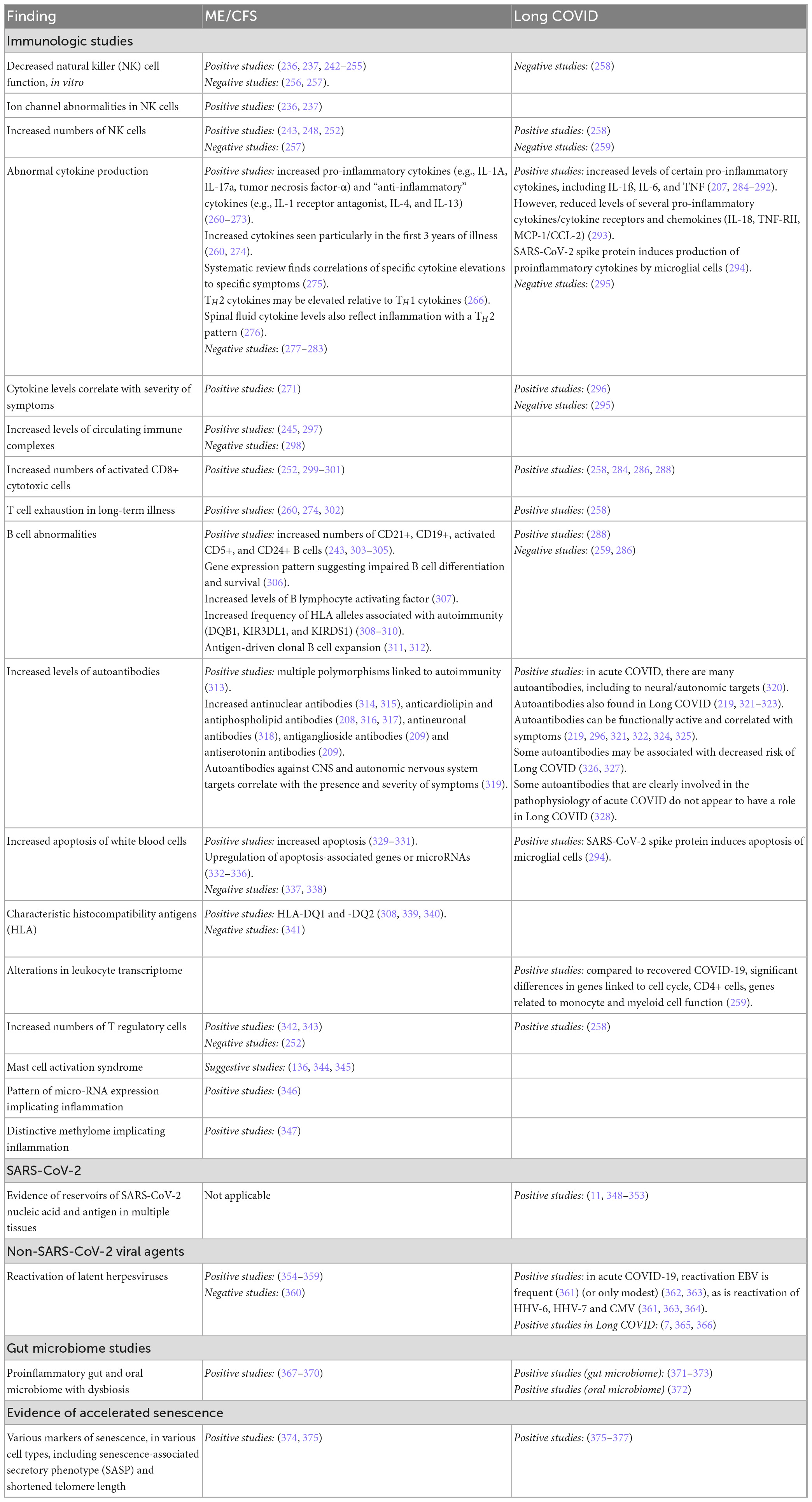
Table 4 summarizes the different abnormalities that have been identified in the central nervous system (CNS) and autonomic nervous system (ANS) in ME/CFS and in Long COVID. Most of them have been reported by multiple laboratories and are common to both illnesses. Often, the measured abnormality has been shown to be correlated with the presence and severity of symptoms. We comment in the text only on those abnormalities where the evidence is most extensive.
Cognition
Psychometric testing has revealed cognitive deficits in both illnesses. In people with ME/CFS, testing typically has been performed years after the illness began: there have been few longitudinal studies of the trajectory of cognitive deficits. In contrast, because the inciting infectious agent is clear in Long COVID, and because longitudinal studies were launched shortly after the onset of the pandemic, it is clear that the initial cognitive deficits can persist for many months. One study found there may be some improvement by the end of the first year (537).
With both illnesses, the most consistently observed abnormalities have been impaired attention and information processing speed. Long-lasting objective cognitive impairment has been demonstrated even in people with mild acute COVID-19 (538, 539). One study estimated that the measured cognitive deficit was equivalent to 10 years of aging (540). Measured cognitive impairment correlated significantly with abnormalities seen with EEG and MRI studies (50, 51).
The most persuasive evidence that the persistent cognitive deficits in Long COVID reflect damage to the CNS comes from a large population-based longitudinal study in which MRI scans and cognitive testing were performed both before the pandemic and after. Compared to a matched group that did not develop COVID-19, those who developed COVID-19 (even those not requiring hospitalization) had greater reduction in gray matter thickness and in global brain size, and greater cognitive decline (541).
Neurovascular abnormalities
In both illnesses, there is a reduction in cerebral blood flow. This likely results from autonomic dysfunction (Table 4) and possibly from reduced blood volume (Table 7). It may contribute to some of the symptoms of both illnesses, including fatigue and impaired cognition; it may also reflect reduced physical activity and reduced oxygen consumption (hypometabolism).
Neuroendocrine abnormalities
The first neurological abnormalities to be documented in ME/CFS were a group of different neuroendocrine abnormalities. In particular, there appears to be down-regulation of activity in several hypothalamic-pituitary axes (Table 4) in ME/CFS, with some similar reports in Long COVID (542). These neuroendocrine changes could have bidirectional interactions with various immunologic and vascular abnormalities that also are seen in both illnesses (542).
Autonomic nervous system
Many of the common symptoms seen in people with both illnesses could reflect autonomic dysfunction. In addition, autonomic dysfunction could explain other underlying biological disturbances, such as the reported neurovascular abnormalities. The autonomic dysfunction, in turn, could be caused by other biological abnormalities seen in both illnesses, particularly antibodies directed at autonomic receptors (129) and biopsy-documented small-fiber neuropathy (72, 180–182).
As summarized in Table 4, autonomic abnormalities have been well documented in both illnesses. Abnormalities of both the sympathetic and parasympathetic arms of the autonomic nervous system reflect “dyshomeostasis” (130): poor modulation of the balance between the two systems, with the imbalance favoring expression of the sympathetic system (72, 73, 131–134). Objective autonomic dysfunction is common in the first 6–12 months following acute COVID-19, becoming less common by 24 months following infection, in comparison to matched comparison groups without COVID-19 (73).
Magnetic resonance imaging (MRI)
Magnetic resonance abnormalities involving both gray matter and white matter have been found in both illnesses and been shown to correlate positively with measured objective cognitive impairment (50, 51, 149, 150). Impaired responses to a variety of challenges have been revealed by functional MRI.
Immune system and infectious agents
Myalgic encephalomyelitis/CFS often follows in the wake of an “infectious-like” illness. Long COVID (by definition) follows in the wake of acute infection with SARS-CoV-2. As summarized in Table 5, a variety of immunological parameters distinguish people with ME/CFS from healthy control subjects of the same age and gender. The same is true of Long COVID: immune parameters distinguish patients from post-COVID patients who no longer have symptoms, and from non-infected controls subjects. Moreover, many of the same immune parameters distinguish patients with ME/CFS and with Long COVID from comparison/control groups.
In addition, as summarized in Table 5, differences in the frequency of several reactivated herpesviruses, and differences in the gut microbiome, distinguish patients with these two illnesses from comparison/control groups.
Metabolic abnormalities
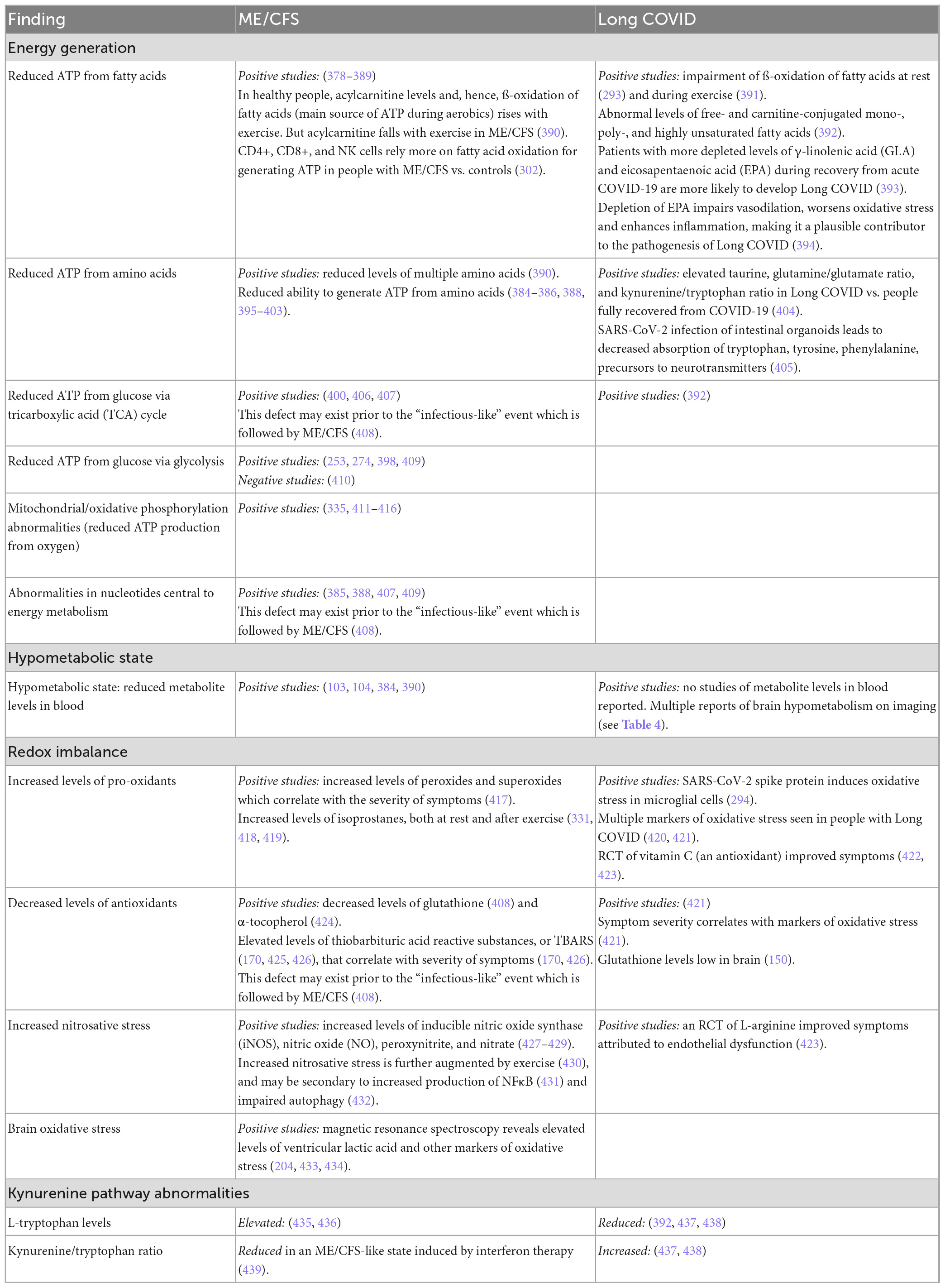
Beginning in the 1990s, evidence began to accumulate indicating that in people with ME/CFS who experienced a lack of “energy,” a contributing factor might be a cellular failure to generate and utilize adenosine triphosphate (ATP). As summarized in Table 6, considerable evidence has since emerged in support of that hypothesis. The ability to generate energy from multiple sources is impaired: from fatty acids, amino acids, glucose and oxygen. Moreover, similar evidence is emerging in people with Long COVID.
In addition to energy metabolic deficits, evidence of a systemic hypometabolic state (also manifest in the brain, Table 4), abnormalities in redox balance, and abnormalities in the kynurenine pathway have emerged in both illnesses, as summarized in Table 6. Later, we discuss briefly how abnormalities in redox balance may have bidirectional connections to abnormalities in the immune response and energy generation.
Cardiopulmonary and vascular abnormalities
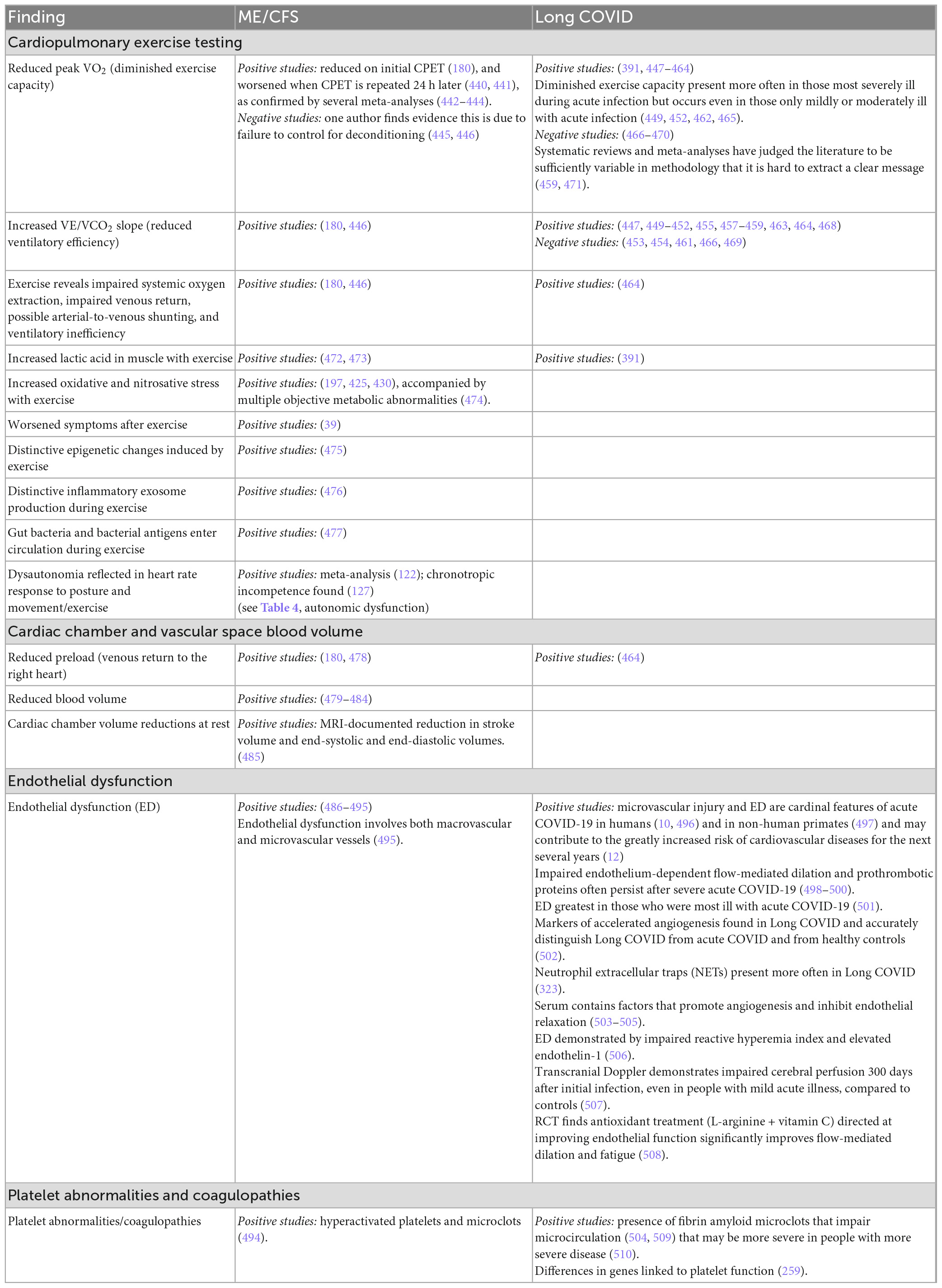
Although less extensively studied than neurological, immunological, infectious or metabolic abnormalities, a growing number of cardiopulmonary abnormalities have been identified, as summarized in Table 7. The most well documented abnormalities are diminished exercise capacity on exercise testing, particularly when a second exercise test is performed 24 h after the first; reduced ventilatory efficiency; and endothelial dysfunction (particularly in Long COVID but also in ME/CFS). Some of the metabolic abnormalities noted in Table 6 are provoked by exercise, and thus there is some overlap between Tables 6, 7.
Discussion
Similarities and differences in symptoms
As summarized in Table 3, most of the symptoms reported with ME/CFS and Long COVID are similar. Decreased smell and taste, rash and hair loss are more likely in Long COVID than ME/CFS; this may reflect pathology induced specifically by SARS-CoV-2 (543).
Not only is a common core of symptoms shared by ME/CFS and Long COVID: these same symptoms also are also reported following multiple infectious illnesses (544) and major non-infectious injury such as post-critical illness syndrome or post-intensive care unit syndrome (545, 546), including heat stroke (547).
Similarities and differences in underlying biology
In this review we have compared what is known about the underlying biology of ME/CFS and Long COVID. Both clearly are systemic illnesses involving multiple organs and physiological systems. Long COVID is triggered by infection with SARS-CoV-2. ME/CFS often is triggered by an “infectious-like” illness. We think it is unlikely that ME/CFS is triggered by a single, novel infectious agent: more likely, it represents a dysfunctional response to infection with any of multiple agents, as recently described (544).
As summarized in this review, both illnesses share abnormalities involving the central and autonomic nervous systems, the immune system, reactivation of latent infectious agents (primarily herpesviruses), the gut microbiome, energy metabolism, a hypometabolic state, redox imbalance, and various cardiac, pulmonary and vascular abnormalities.
Many of these abnormalities bidirectionally influence each other. This creates the potential for multiple, self-reinforcing “vicious” pathophysiological cycles that could lead to persisting illness (548, 549). It also means that the precipitating event, which sets in motion those vicious cycles, may be different in one person with the illness from the precipitating event in another person.
In summarizing what is known about biological abnormalities in both illnesses, we have cited both the “positive” studies that find an abnormality and the “negative” ones that do not. These citations allow readers to assess the strength (or weakness) of a reported finding.
As shown by the tables, some reported findings are supported by robust evidence—confirmatory reports from multiple laboratories: even though there may be some dissenting reports, the preponderance of the evidence supports the finding. The tables also identify abnormalities that are not supported at this time by robust evidence and require future investigation.
Post-infection/post-injury syndrome
The similar symptoms and pathology of ME/CFS and Long COVID raise the question of whether these disorders represent just two examples of a broader illness in which symptoms occur because they are generated by a carefully orchestrated, stereotyped, multi-system response to infection and injury.
Why might such a carefully orchestrated group of symptoms be generated following infection or injury? We speculate, as have others (550–552), that these symptoms are generated because they lead to metabolic reprogramming (553) as well as to behavioral changes that reduce non-essential, energy-consuming activities—thereby maximizing the amount of energy available to facilitate recovery. Fatigue, myalgia and orthostatic intolerance, for example, lead to reduced physical activity, redirecting energy stores to eradicate infection and heal tissue injury.
Such a carefully orchestrated response to vital threats exists throughout the animal kingdom. The best studied examples are hibernation and torpor in “higher” animals (554–556), and the larval state of dauer in the worm C. elegans (557–559).
Dauer, hibernation and torpor all involve “abnormal” innate immune responses, redox imbalance, increased glycolysis, decreased aerobic respiration, and possibly even alteration in the organisms’ microbiome. They also involve orchestration by the autonomic nervous system (and its counterparts in the nervous systems of more primitive organisms). Thus, these well-recognized “hunkering down” mechanisms all exhibit similarities to the emerging pathophysiology of ME/CFS and Long COVID, as summarized in this review.
Conclusion
The goal of this report is to provide a road map to the state of knowledge about the underlying biology of ME/CFS and Long COVID. The often-similar findings suggest that insights into each disorder will have implications for the other. They may also enhance our understanding of evolutionarily preserved biological responses that fight infection and heal injury. We urge that investigators studying the underlying biology of Long COVID take note of the robust findings in ME/CFS that have not yet been investigated in Long COVID: given the many similarities in the underlying biology of the two illnesses, it is likely that pursuing such abnormalities in Long COVID will prove instructive.
Research into the pathophysiology of these responses has the potential to lead to new strategies for reducing the morbidity of ME/CFS and Long COVID, and of similar illnesses that can follow a variety of infections and non-infectious traumatic injury.
Author contributions
AK and WL conceptualized and wrote the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
The authors’ time in writing this review was supported by the US Public Health Service Grant (AI138370) to the Center for Solutions for ME/CFS at Columbia University.
Acknowledgments
We thank Jill Mazzetta for help in retrieving and cataloguing the literature.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington, DC: The National Academies Press (2015).
2. Davis H, McCorkell L, Vogel J, Topol E. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46.
3. Bach K. Is Long COVID Worsening the Labor Shortage?. Washington, D. C: Brookings Institution (2022).
6. Sudre C, Murray B, Varsavsky T, Graham M, Penfold R, Bowyer R, et al. Attributes and predictors of long COVID. Nat Med. (2021) 27:626–31.
7. Su Y, Yuan D, Chen D, Ng R, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. (2022) 185:881–95.e20.
8. Hastie C, Lowe D, McAuley A, Winter A, Mills N, Black C, et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. (2022) 13:5663.
9. You J, Zhang L, Ni-Jia-Ti M, Zhang J, Hu F, Chen L, et al. Abnormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect. (2020) 81:e150–2. doi: 10.1016/j.jinf.2020.06.003
10. Lee M, Perl D, Nair G, Li W, Maric D, Murray H, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. (2021) 384:481–3.
11. Stein S, Ramelli S, Grazioli A, Chung J, Singh M, Yinda C, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. (2022) 612:758–63. doi: 10.1038/s41586-022-05542-y
12. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. (2022) 28:583–90.
13. Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. (2021) 32:151–60.
14. Xu E, Xie Y, Al-Aly Z. Long-term gastrointestinal outcomes of COVID-19. Nat Commun. (2023) 14:983.
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. (2021) 594:259–64.
16. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. (2022) 10:311–21.
17. Wong T, Weitzer D. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—A systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). (2021) 57:418. doi: 10.3390/medicina57050418
18. Sandman C, Barron J, Nackoul K, Goldstein J, Fidler F. Memory deficits associated with chronic fatigue immune dysfunction syndrome. Biol Psychiatry. (1993) 33:618–23.
19. DeLuca J, Johnson S, Beldowicz D, Natelson B. Neuropsychological impairments in chronic fatigue syndrome, multiple sclerosis, and depression. J Neurol Neurosurg Psychiatry. (1995) 58:38–43.
20. Marcel B, Komaroff A, Fagioli L, Kornish R, Albert M. Cognitive deficits in patients with chronic fatigue syndrome. Biol Psychiatry. (1996) 40:535–41.
21. DeLuca J, Johnson S, Ellis S, Natelson B. Cognitive functioning is impaired in patients with chronic fatigue syndrome devoid of psychiatric disease. J Neurol Neurosurg Psychiatry. (1997) 62:151–5.
22. Tiersky L, Johnson S, Lange G, Natelson B, DeLuca J. Neuropsychology of chronic fatigue syndrome: a critical review. J Clin Exp Neuropsychol. (1997) 19:560–86.
23. Daly E, Komaroff A, Bloomingdale K, Wilson S, Albert M. Neuropsychological functioning in patients with chronic fatigue syndrome, multiple sclerosis and depression. App Neuropsychol. (2001) 8:12–22.
24. Michiels V, Cluydts R. Neuropsychological functioning in chronic fatigue syndrome: a review. Acta Psychiatr Scand. (2001) 103:84–93.
25. DeLuca J, Christodoulou C, Diamond B, Rosenstein, Kramer N, Natelson B. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. (2004) 10:101–9. doi: 10.1017/S1355617704101124
26. Dickson A, Toft A, O’Carroll R. Neuropsychological functioning, illness perception, mood and quality of life in chronic fatigue syndrome, autoimmune thyroid disease and healthy participants. Psychol Med. (2009) 39:1567–76. doi: 10.1017/S0033291708004960
27. Cockshell S, Mathias J. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. (2010) 40:1253–67.
28. Den Eede F, Moorkens G, Hulstijn W, Maas Y, Schrijvers D, Stevens S, et al. Psychomotor function and response inhibition in chronic fatigue syndrome. Pscyhiatry Res. (2011) 186:367–72. doi: 10.1016/j.psychres.2010.07.022
29. Rasouli O, Gotaas M, Stensdotter A, Skovlund E, Landrø N, Dåstøl P, et al. Neuropsychological dysfunction in chronic fatigue syndrome and the relation between objective and subjective findings. Neuropsychology. (2019) 33:658–69.
30. Murga I, Aranburu L, Gargiulo P, Gomez-Esteban J, Lafuente J. The maintained attention assessment in patients affected by myalgic encephalomyelitis/chronic fatigue syndrome: a reliable biomarker? J Transl Med. (2021) 19:494. doi: 10.1186/s12967-021-03153-1
31. Aoun Sebaiti M, Hainselin M, Gounden Y, Sirbu C, Sekulic S, Lorusso L, et al. Systematic review and meta-analysis of cognitive impairment in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Sci Rep. (2022) 12:2157.
32. Fernandez-Quiros J, Lacasa-Cazcarra M, Alegre-Martin J, Sanmartin-Sentanes R, Almirall M, Launois-Obregon P, et al. The conners continuous performance test CPT3: is it a reliable marker to predict neurocognitive dysfunction in Myalgic encephalomyelitis/chronic fatigue syndrome? Front Psychol. (2023) 14:1127193. doi: 10.3389/fpsyg.2023.1127193
33. Cook D, Nagelkirk P, Peckerman A, Poluri A, Mores J, Natelson B. Exercise and cognitive performance in chronic fatigue syndrome. Med Sci Sports Exerc. (2005) 37:1460–7.
34. Schmaling K, DiClementi J, Cullum C, Jones J. Cognitive functioning in chronic fatigue syndrome and depression: a preliminary comparison. Psychosom Med. (1994) 56:383–8.
35. Constant E, Adam S, Gillain B, Lambert M, Masquelier E, Seron X. Cognitive deficits in patients with chronic fatigue syndrope compared to those with major depressive disorder and healthy controls. Clin Neurol Neurosurg. (2011) 113:295–302.
36. Lange G, Steffener J, Cook D, Bly B, Christodoulou C, Liu W, et al. Objective evidence of cognitive complaints in chronic fatigue syndrome: a BOLD fMRI study of verbal working memory. NeuroImage. (2005) 26:513–24. doi: 10.1016/j.neuroimage.2005.02.011
37. Cook D, O’Connor P, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. NeuroImage. (2007) 36:108–22.
38. Shan Z, Finegan K, Bhuta S, Ireland T, Staines D, Marshall-Gradisnik S, et al. Brain function characteristics of chronic fatigue syndrome: a task fMRI study. Neuroimage Clin. (2018) 19:279–86.
39. Cook D, Light A, Light K, Broderick G, Shields M, Dougherty R, et al. Neural consequences of post-exertion malaise in myalgic encephalomyelitis/chronic fatigue syndrome. Brain Behav Immun. (2017) 62:87–99. doi: 10.1016/j.bbi.2017.02.009
40. Santamarina-Perez P, Eiroa-Orosa F, Freniche V, Moreno-Mayos A, Alegre J, Saez N, et al. Length of illness does not predict cognitive dysfunction in chronic fatigue syndrome. Appl Neuropsychol. (2011) 18:216–22.
41. Cockshell S, Mathias J. Test effort in persons with chronic fatigue syndrome when assessed using the validity indicator profile. J Clin Exp Neuropsychol. (2012) 34:679–87. doi: 10.1080/13803395.2012.668176
42. Cope H, Pernet A, Kendall B, David A. Cognitive functioning and magnetic resonance imaging in chronic fatigue. Br J Psychiatry. (1995) 167:86–94.
43. Mahurin R, Goldberg J, Claypoole K, Arguelles L, Ashton S, Buchwald D. Cognitive processing in monozygotic twins discordant for chronic fatigue syndrome. Neuropsychology. (2004) 18:232–9.
44. Hosp J, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. (2021) 144:1263–76.
45. Hampshire A, Chatfield D, Am M, Jolly A, Trender W, Hellyer P, et al. Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. EClinicalMedicine. (2022) 47:101417.
46. Ollila H, Pihlaja R, Koskinen S, Tuulio-Henriksson A, Salmela V, Tiainen M, et al. Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: a comprehensive controlled neuropsychological study. Crit Care. (2022) 26:223.
47. Jennings G, Monaghan A, Xue F, Duggan E, Romero-Ortuno R. Comprehensive clinical characterisation of brain fog in adults reporting long COVID symptoms. J Clin Med. (2022) 11:3440.
48. Bertuccelli M, Ciringione L, Rubega M, Bisiacchi P, Masiero S, Del Felice A. Cognitive impairment in people with previous COVID-19 infection: a scoping review. Cortex. (2022) 154:212–30. doi: 10.1016/j.cortex.2022.06.002
49. Azcue N, Gomez-Esteban J, Acera M, Tijero B, Fernandez T, Ayo-Mentxakatorre N, et al. Brain fog of post-COVID-19 condition and chronic fatigue syndrome, same medical disorder? J Transl Med. (2022) 20:569. doi: 10.1186/s12967-022-03764-2
50. Cecchetti G, Agosta F, Canu E, Basaia S, Barbieri A, Cardamone R, et al. Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J Neurol. (2022) 269:3400–12. doi: 10.1007/s00415-022-11047-5
51. Andriuta D, Si-Ahmed C, Roussel M, Constans J, Makki M, Aarabi A, et al. Clinical and imaging determinants of neurocognitive disorders in post-acute COVID-19 patients with cognitive complaints. J Alzheimers Dis. (2022) 87:1239–50. doi: 10.3233/JAD-215506
52. Samudyata, Oliveira AO, Malwade S, Rufino de Sousa N, Goparaju SK, Gracias J, et al. SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol Psychiatry. (2022) 27:3939–50. doi: 10.1038/s41380-022-01786-2
53. Schwartz R, Komaroff A, Garada B, Gleit M, Doolittle T, Bates D, et al. SPECT imaging of the brain: comparison of findings in patients with chronic fatigue syndrome, AIDS dementia complex, and major unipolar depression. AJR Am J Roentgenol. (1994) 162:943–51. doi: 10.2214/ajr.162.4.8141022
54. Tanaka H, Matsushima R, Tamai H, Kajimoto Y. Impaired postural cerebral hemodynamics in young patients with chronic fatigue with and without orthostatic intolerance. J Pediatr. (2002) 140:412–7.
55. Schmaling K, Lewis D, Fiedelak J, Mahurin R, Buchwald D. Single-photon emission computerized tomography and neurocognitive function in patients with chronic fatigue syndrome. Psychosom Med. (2003) 65:129–36. doi: 10.1097/01.psy.0000038942.33335.9b
56. Razumovsky A, DeBusk K, Calkins H, Snader S, Lucas K, Vyas P, et al. Cerebral and systemic hemodynamics changes during upright tilt in chronic fatigue syndrome. J Neuroimag. (2003) 13:57–67.
57. Yoshiuchi K, Farkas J, Natelson B. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging. (2006) 26:83–6.
58. Stewart J, Medow M, Messer Z, Baugham I, Terilli C, Ocon A. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. (2012) 302:1185–94.
59. Natelson B, Mao X, Stegner A, Lange G, Vu D, Blate M, et al. Multimodal and simultaneous assessments of brain and spinal fluid abnormalities in chronic fatigue syndrome and the effects of psychiatric comorbidity. J Neurol Sci. (2017) 375:411–6. doi: 10.1016/j.jns.2017.02.046
60. Biswal B, Kunwar P, Natelson B. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. (2011) 301:9–11.
61. Finkelmeyer A, He J, Maclachlan L, Blamire A, Newton J. Intracranial compliance is associated with symptoms of orthostatic intolerance in chronic fatigue syndrome. PLos One. (2018) 13:e0200068. doi: 10.1371/journal.pone.0200068
62. Boissoneault J, Letzen J, Robinson M, Staud R. Cerebral blood flow and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain Imaging Behav. (2018) 13:789–97. doi: 10.1007/s11682-018-9897-x
63. Staud R, Boissoneault J, Craggs J, Lai S, Robinson M. Task related cerebral blood flow changes of patients with chronic fatigue syndrome: an arterial spin labeling study. Fatigue. (2018) 6:63–79.
64. Li X, Julin P, Li T. Limbic perfusion is reduced in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Tomography. (2021) 7:675–87. doi: 10.3390/tomography7040056
65. van Campen C, Verheugt F, Rowe P, Visser F. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: a quantitative, controlled study using Doppler echography. Clin Neurophysiol Pract. (2020) 5:50–8. doi: 10.1016/j.cnp.2020.01.003
66. van Campen C, Rowe P, Visser F. Cerebral blood flow is reduced in severe myalgic encephalomyelitis/chronic fatigue syndrome patients during mild orthostatic stress testing: an exploratory study at 20 degrees of head-up tilt testing. Healthcare (Basel). (2020) 8:169. doi: 10.3390/healthcare8020169
67. van Campen C, Visser F. Psychogenic pseudosyncope: real or imaginary? Results from a case-control study in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients. Medicina (Kaunas). (2022) 58:98.
68. van Campen C, Rowe P, Visser F. Cerebral blood flow remains reduced after tilt testing in myalgic encephalomyelitis/chronic fatigue syndrome patients. Clin Neurophysiol Pract. (2021) 6:245–55. doi: 10.1016/j.cnp.2021.09.001
69. Fischler B, D’Haenen H, Cluydts R, Michiels V, Demets K, Bossuyt A, et al. Comparison of 99mTc HMPAO SPECT scan between chronic fatigue syndrome, major depression and healthy controls: an exploratory study of clinical correlates of regional cerebral blood flow. Neuropsychobiology. (1996) 34:175–83. doi: 10.1159/000119307
70. Malfliet A, Pas R, Brouns R, De Win J, Hatem S, Meeus M, et al. Cerebral blood flow and heart rate variability in chronic fatigue syndrome: a randomized cross-over study. Pain Physician. (2018) 21:E13–24.
71. Lewis D, Mayberg H, Fischer M, Goldberg J, Ashton S, Graham M, et al. Monozygotic twins discordant for chronic fatigue syndrome: regional cerebral blood flow SPECT. Radiology. (2001) 219:766–73.
72. Novak P, Giannetti M, Weller E, Hamilton M, Mukerji S, Alabsi H, et al. Network autonomic analysis of post-acute sequelae of COVID-19 and postural tachycardia syndrome. Neurol Sci. (2022) 43:6627–38. doi: 10.1007/s10072-022-06423-y
73. van Campen C, Visser F. Long-haul COVID patients: prevalence of POTS are reduced but cerebral blood flow abnormalities remain abnormal with longer disease duration. Healthcare (Basel). (2022) 10:2105. doi: 10.3390/healthcare10102105
74. Tian T, Wu J, Chen T, Li J, Yan S, Zhou Y, et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight. (2022) 7:e155827. doi: 10.1172/jci.insight.155827
75. Demitrack M, Dale J, Straus S, Laue L, Listwak S, Kruesi M, et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. (1991) 73:1224–34.
76. Dinan T. Neuroendocrinology of chronic fatigue syndrome. J Chronic Fatigue Syndrome. (1996) 2:69–70.
77. Cleare A. The HPA axis and the genesis of chronic fatigue syndrome. TRENDS Endocrin Metabol. (2004) 15:55–9. doi: 10.1016/j.tem.2003.12.002
78. Roberts A, Wessely S, Chalder T, Papadopoulos A, Cleare A. Salivary cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry. (2004) 184:136–41.
79. Jerjes W, Taylor N, Wood P, Cleare A. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. (2007) 32:192–8. doi: 10.1016/j.psyneuen.2006.12.005
80. Van Den Eede F, Moorkens G, Hulstijn W, van Houdenhove B, Cosyns P, Sabbe B, et al. Combined dexamethasone/corticotropin-releasing factor test in chronic fatigue syndrome. Psychol Med. (2008) 38:963–73.
81. Wyller V, Vitelli V, Sulheim D, Fagermoen E, Winger A, Godang K, et al. Altered neuroendocrine control and association to clinical symptoms in adolescent chronic fatigue syndrome: a cross-sectional study. J Transl Med. (2016) 14:121.
82. Morris G, Anderson G, Maes M. Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol Neurobiol. (2017) 54:6806–19.
83. Tak L, Cleare A, Ormel J, Manoharan A, Kok I, Wessely S, et al. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. (2011) 87:183–94.
84. Powell D, Liossi C, Moss-Morris R, Schlotz W. Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: a systematic review and subset meta-analysis. Psychoneuroendocrinology. (2013) 38:2405–22. doi: 10.1016/j.psyneuen.2013.07.004
85. Meeus M, Nijs J, Van de Wauwer N, Toeback L, Truijen S. Diffuse noxious inhibitory control is delayed in chronic fatigue syndrome: an experimental study. Pain. (2008) 139:439–48. doi: 10.1016/j.pain.2008.05.018
86. Inder W, Prickett T, Mulder R. Normal opioid tone and hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome despite marked functional impairment. Clin Endocrinol. (2005) 62:343–8. doi: 10.1111/j.1365-2265.2005.02220.x
87. Urhan E, Karaca Z, Unuvar G, Gundogan K, Unluhizarci K. Investigation of pituitary functions after acute coronavirus disease 2019. Endocrine J. (2022) 69:649–58.
88. Sunada N, Nakano Y, Otsuka Y, Tokumasu K, Honda H, Sakurada Y, et al. Characteristics of sleep disturbance in patients with long COVID: a retrospective observational study in Japan. J Clin Med. (2022) 11:7332. doi: 10.3390/jcm11247332
89. Sunada N, Honda H, Nakano Y, Yamamoto K, Tokumasu K, Sakurada Y, et al. Hormonal trends in patients suffering from long COVID symptoms. Endocrine J. (2022) 69:1173–81. doi: 10.1507/endocrj.EJ22-0093
90. Hamazaki K, Nishigaki T, Kuramoto N, Oh K, Konishi H. Secondary adrenal insufficiency after COVID-19 diagnosed by insulin tolerance test and corticotropin-releasing hormone test. Cureus. (2022) 14:e23021.
91. Yavropoulou M, Tsokos G, Chrousos G, Sfikakis P. Protracted stress-induced hypocortisolemia may account for the clinical and immune manifestations of Long COVID. Clin Immunol. (2022) 245:109133. doi: 10.1016/j.clim.2022.109133
92. Clarke S, Phylactou M, Patel B, Mills E, Muzi B, Izzi-Engbeaya C, et al. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J Clin Endocrinol Metab. (2021) 106:2208–20. doi: 10.1210/clinem/dgab349
93. Bakheit A, Behan P, Dinan T, Gray C, O’Keane V. Possible upregulation of hypothalamic 5-hydroxytryptamine receptors in patients with postviral fatigue syndrome. BMJ. (1992) 304:1010–2. doi: 10.1136/bmj.304.6833.1010
94. Cleare A, Bearn J, Allain T, McGregor A, Wessely S, Murray R, et al. Contrasting neuroendocrine responses in depression and chronic fatigue syndrome. J Affect Disord. (1995) 35:283–9. doi: 10.1016/0165-0327(95)00026-j
95. Bearn J, Allain T, Coskeran P, Munro N, Butler J, McGregor A, et al. Neuroendocrine responses to d-fenfluramine and insulin-induced hypoglycemia in chronic fatigue syndrome. Biol Psychiatry. (1995) 37:245–52. doi: 10.1016/0006-3223(94)00121-I
96. Yatham L, Morehouse R, Chisholm B, Haase D, MacDonald D, Marrie T. Neuroendocrine assessment of serotonin (5-HT) function in chronic fatigue syndrome. Can J Psychiatry. (1995) 40:93–6.
97. Vassallo C, Feldman E, Peto T, Castell L, Sharpley A, Cowen P. Decreased tryptophan availability but normal post-synaptic 5-HT 2c receptor sensitivity in chronic fatigue syndrome. Psychol Med. (2001) 31:585–91. doi: 10.1017/s0033291701003580
98. Buchwald D, Umali J, Stene M. Insulin-like growth factor-I (somatomedin C) levels in chronic fatigue syndrome and fibromyalgia. J Rheumatol. (1996) 23:739–42.
99. Allain T, Bearn J, Coskerman P, Jones J, Checkley A, Butler J, et al. Changes in growth hormone, insulin, insulinlike growth factors (IGFs), and IGF-binding protein-1 in chronic fatigue syndrome. Biol Psychiatry. (1997) 41:567–73. doi: 10.1016/s0006-3223(96)00074-1
100. Berwaerts J, Moorkens G, Abs R. Secretion of growth hormone in patients with chronic fatigue syndrome. Growth Horm IGF Res. (1998) 8:127–9.
101. Moorkens G, Berwaerts J, Wynants H, Abs R. Characterization of pituitary function with emphasis on GH secretion in the chronic fatigue syndrome. Clin Endocrinol. (2000) 53:99–106. doi: 10.1046/j.1365-2265.2000.01049.x
102. Ruiz-Nunez B, Tarasse R, Vogelaar E, Janneke Dijck-Brouwer DA, Muskiet FAJ. Higher prevalence of “low T3 syndrome” in patients with chronic fatigue syndrome: a case-control study. Front Endocrinol. (2018) 9:97. doi: 10.3389/fendo.2018.00097
103. Mueller C, Lin J, Sheriff S, Maudsley A, Younger J. Evidence of widespread metabolite abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. (2019) 14:562–72.
104. Mairal E, Barberon B, Laine N, Coulange M, Guedj E. Reversible widespread brain 18F-FDG PET hypometabolism in chronic fatigue syndrome treated by hyperbaric oxygen therapy. Eur J Nucl Med Mol Imaging. (2021) 48:1680–1.
105. Guedj E, Campion J, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. (2021) 48:2823–33.
106. Verger A, Kas A, Dudouet P, Goehringer F, Salmon-Ceron D, Guedj E. Visual interpretation of brain hypometabolism related to neurological long COVID: a French multicentric experience. Eur J Nucl Med Mol Imaging. (2022) 49:3197–202. doi: 10.1007/s00259-022-05753-5
107. Goehringer F, Bruyere A, Doyen M, Bevilacqua S, Charmillon A, Heyer S, et al. Brain (18)F-FDG PET imaging in outpatients with post-COVID-19 conditions: findings and associations with clinical characteristics. Eur J Nucl Med Mol Imaging. (2022) 50:1084–9.
108. Ferrucci R, Cuffaro L, Capozza A, Rosci C, Maiorana N, Groppo E, et al. Brain positron emission tomography (PET) and cognitive abnormalities one year after COVID-19. J Neurol. (2023) 270:1823–34.
109. Martini A, Carli G, Kiferle L, Piersanti P, Palumbo P, Morbelli S, et al. Time-dependent recovery of brain hypometabolism in neuro-COVID-19 patients. Eur J Nucl Med Mol Imaging. (2022) 50:90–102. doi: 10.1007/s00259-022-05942-2
110. Morand A, Campion J, Lepine A, Bosdure E, Luciani L, Cammilleri S, et al. Similar patterns of [(18)F]-FDG brain PET hypometabolism in paediatric and adult patients with long COVID: a paediatric case series. Eur J Nucl Med Mol Imaging. (2022) 49:913–20. doi: 10.1007/s00259-021-05528-4
111. Miskowiak K, Bech J, Henriksen A, Johnsen S, Podlekareva D, Marner L. Cerebral metabolic rate of glucose and cognitive tests in long COVID patients. Brain Sci. (2022) 13:23. doi: 10.3390/brainsci13010023
112. Rowe P, Bou-Holaigah I, Kan J, Calkins H. Is neurally mediated hypotension an unrecognised cause of chronic fatigue? Lancet. (1995) 345:623–4.
113. Freeman R, Komaroff A. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med. (1997) 102:357–62.
114. Stewart J. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res. (2000) 48:218–26. doi: 10.1203/00006450-200008000-00016
115. Wyller V, Saul J, Amlie J, Thaulow E. Sympathetic predominance of cardiovascular regulation during mild orthostatic stress in adolescents with chronic fatigue. Clin Physiol Funct Imaging. (2007) 27:231–8.
116. Wyller V, Barbieri R, Thaulow E, Saul J. Enhanced vagal withdrawal during mild orthostatic stress in adolescents with chronic fatigue. Ann Noninvasive Electrocardiol. (2008) 13:67–73. doi: 10.1111/j.1542-474X.2007.00202.x
117. Hoad A, Spickett G, Elliott J, Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. QJM. (2008) 101:961–5. doi: 10.1093/qjmed/hcn123
118. Wyller V, Barbieri R, Saul J. Blood pressure variability and closed-loop baroreflex assessment in adolescent chronic fatigue syndrome during supine rest and orthostatic stress. Eur J Appl Physiol. (2011) 497:507. doi: 10.1007/s00421-010-1670-9
119. Van Cauwenbergh D, Nijs J, Kos D, Van Weijnen L, Struyf F, Meeus M. Malfunctioning of the autonomic nervous system in patients with chronic fatigue syndrome: a systematic literature review. Eur J Clin Invest. (2014) 44:516–26. doi: 10.1111/eci.12256
120. Barnden L, Kwiatek R, Crouch B, Burnet R, Del Fante P. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in chronic fatigue syndrome. Neuroimage Clin. (2016) 11:530–7. doi: 10.1016/j.nicl.2016.03.017
121. Oosterwijck J, Marusic U, De Wandele I, Paul L, Meeus M, Moorkens G, et al. The role of autonomic function in exercise-induced endogenous analgesia: a case-control study in myalgic encephalomyelitis/chronic fatigue syndrome and healthy people. Pain Physician. (2017) 20:E389–99.
122. Nelson M, Bahl J, Buckley J, Thomson R, Davison K. Evidence of altered cardiac autonomic regulation in myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review and meta-analysis. Medicine (Baltimore). (2019) 98:e17600. doi: 10.1097/MD.0000000000017600
123. Vuong Q, Allison J, Finkelmeyer A, Newton J, Durham J. Brain responses in CFS and TMD to autonomic challenges: an exploratory fMRI study. JDR Clin Trans Res. (2020) 5:224–32. doi: 10.1177/2380084419872135
124. Van Oosterwijck J, Marusic U, De Wandele I, Meeus M, Paul L, Lambrecht L, et al. Reduced parasympathetic reactivation during recovery from exercise in myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Med. (2021) 10:4527.
125. Natelson B, Lin J, Blate M, Khan S, Chen Y, Unger E. Physiological assessment of orthostatic intolerance in chronic fatigue syndrome. J Transl Med. (2022) 20:95.
126. Vernon S, Funk S, Bateman L, Stoddard G, Hammer S, Sullivan K, et al. Orthostatic challenge causes distinctive symptomatic, hemodynamic and cognitive responses in long COVID and myalgic encephalomyelitis/chronic fatigue syndrome. Front Med. (2022) 9:917019. doi: 10.3389/fmed.2022.917019
127. Miwa K. Orthostatic intolerance and chronotropic incompetence in patients with myalgic encephalomyelitis or chronic fatigue syndrome. Circ Rep. (2023) 5:55–61.
128. Jones J, Nicholson A, Nisenbaum R, Papanicolaou D, Solomon L, Boneva R, et al. Orthostatic instability in a population-based study of chronic fatigue syndrome. Am J Med. (2005) 118:1415.e19–e28.
129. Dotan A, David P, Arnheim D, Shoenfeld Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun Rev. (2022) 21:103071.
130. Goldstein D. The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res. (2020) 30:299–315. doi: 10.1007/s10286-020-00714-0
131. Barizien N, Le Guen M, Russel S, Touche P, Huang F, Vallee A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. (2021) 11:14042.
132. Aranyo J, Bazan V, Llados G, Dominguez M, Bisbal F, Massanella M, et al. Inappropriate sinus tachycardia in post-COVID-19 syndrome. Sci Rep. (2022) 12:298.
133. Marques K, Silva C, Trindade S, Santos M, Rocha R, Vasconcelos P, et al. Reduction of cardiac autonomic modulation and increased sympathetic activity by heart rate variability in patients with long COVID. Front Cardiovasc Med. (2022) 9:862001. doi: 10.3389/fcvm.2022.862001
134. van Campen C, Visser F. Orthostatic intolerance in long-haul COVID after SARS-CoV-2: a case-control comparison with post-EBV and insidious-onset myalgic encephalomyelitis/chronic fatigue syndrome patients. Healthcare (Basel). (2022) 10:2058. doi: 10.3390/healthcare10102058
135. Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci. (2020) 21:100276. doi: 10.1016/j.ensci.2020.100276
136. Petracek L, Suskauer S, Vickers R, Patel N, Violand R, Swope R, et al. Adolescent and young adult ME/CFS after confirmed or probable COVID-19. Front Med. (2021) 8:668944. doi: 10.3389/fmed.2021.668944
137. Becker R. Autonomic dysfunction in SARS-COV-2 infection acute and long-term implications COVID-19 editor’s page series. J Thromb Thrombolysis. (2021) 52:692–707. doi: 10.1007/s11239-021-02549-6
138. Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan T, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. (2021) 184:5902–15.e17.
139. Buoite Stella A, Furlanis G, Frezza N, Valentinotti R, Ajcevic M, Manganotti P. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: a prospective multidomain observational study. J Neurol. (2022) 269:587–96. doi: 10.1007/s00415-021-10735-y
140. Steinsvik E, Hausken T, Fluge O, Mella O, Gilja O. Gastric dysmotility and gastrointestinal symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. Scand J Gastroenterol. (2023). Online ahead of print. doi: 10.1080/00365521.2023.2173533
141. Buchwald D, Cheney P, Peterson D, Henry B, Wormsley S, Geiger A, et al. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann Intern Med. (1992) 116:103–13. doi: 10.7326/0003-4819-116-2-103
142. Natelson B, Cohen J, Brassloff I, Lee HJ. A controlled study of brain magnetic resonance imaging in patients with the chronic fatigue syndrome. J Neurosurg Sci. (1993) 120:213–7.
143. Schwartz R, Garada B, Komaroff A, Tice H, Gleit M, Jolesz F, et al. Detection of intracranial abnormalities in patients with chronic fatigue syndrome: comparison of MR imaging and SPECT. AJR Am J Roentgenol. (1994) 162:935–41. doi: 10.2214/ajr.162.4.8141020
144. Lange G, DeLuca J, Maldjian J, Lee H, Tiersky L, Natelson B. Brain MRI abnormalities exist in a subset of patients with chronic fatigue syndrome. J Neurosci. (1999) 171:3–7. doi: 10.1016/s0022-510x(99)00243-9
145. Barnden L, Crouch B, Kwiatek R, Burnet R, Mernone A, Chryssidis S, et al. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR Biomed. (2011) 24:1302–12. doi: 10.1002/nbm.1692
146. Barnden L, Shan Z, Staines D, Marshall-Gradisnik S, Finegan K, Ireland T, et al. Hyperintense sensorimotor T1 spin echo MRI is associated with brainstem abnormality in chronic fatigue syndrome. Neuroimage Clin. (2018) 20:102–9. doi: 10.1016/j.nicl.2018.07.011
147. Cook D, Lange G, DeLuca J, Natelson B. Relationship of brain MRI abnormalities and physical functional status in chronic fatigue syndrome. Int J Neurosci. (2001) 107:1–6. doi: 10.3109/00207450109149754
148. Perrin R, Embleton K, Pentreath V, Jackson A. Longitudinal MRI shows no cerebral abnormality in chronic fatigue syndrome. Br J Radiol. (2010) 83:419–23.
149. Benedetti F, Palladini M, Paolini M, Melloni E, Vai B, De Lorenzo R, et al. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: a multimodal magnetic resonance imaging study. Brain Behav Immun Health. (2021) 18:100387. doi: 10.1016/j.bbih.2021.100387
150. Poletti S, Paolini M, Mazza M, Palladini M, Furlan R, Querini P, et al. Lower levels of glutathione in the anterior cingulate cortex associate with depressive symptoms and white matter hyperintensities in COVID-19 survivors. Eur Neuropsychopharmacol. (2022) 61:71–7. doi: 10.1016/j.euroneuro.2022.06.008
151. Heine J, Schwichtenberg K, Hartung T, Rekers S, Chien C, Boesl F, et al. Structural brain changes in patients with post-COVID fatigue: a prospective observational study. eClinicalMedicine. (2023) 58:101874. doi: 10.1016/j.eclinm.2023.101874
152. Zeineh M, Kang J, Atlas S, Raman M, Reiss A, Norris J, et al. Right arcuate fasciculus abnormality in chronic fatigue syndrome. Radiology. (2014) 274:517–26.
153. Finkelmeyer A, He J, Maclachlan L, Watson S, Gallagher P, Newton J, et al. Grey and white matter differences in chronic fatigue syndrome - a voxel-based morphometry study. Neuroimage Clin. (2018) 17:24–30.
154. Thapaliya K, Marshall-Gradisnik S, Staines D, Su J, Barnden L. Alteration of cortical volume and thickness in myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurosci. (2022) 16:848730. doi: 10.3389/fnins.2022.848730
155. Shan Z, Kwiatek R, Burnet R, Del Fante P, Staines D, Marshall-Gradisnik S, et al. Progressive brain changes in patients with chronic fatigue syndrome: a longitudinal MRI study. J Magn Reson Imaging. (2016) 44:1301–11.
156. Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. (2004) 4:14. doi: 10.1186/1471-2377-4-14
157. de Lange F, Kalkman J, Bleijenberg G, Hagoort P, van der Meer J, Toni I. Gray matter volume reduction in the chronic fatigue syndrome. NeuroImage. (2005) 26:777–81.
158. Putra H, Park K, Yamashita F, Nakagawa Y, Murai T. Cerebral gray matter volume correlates with fatigue and varies between desk workers and non-desk workers. Front Behav Neurosci. (2022) 16:951754. doi: 10.3389/fnbeh.2022.951754
159. Besteher B, Machnik M, Troll M, Toepffer A, Zerekidze A, Rocktaschel T, et al. Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatry Res. (2022) 317:114836. doi: 10.1016/j.psychres.2022.114836
160. Hafiz R, Gandhi T, Mishra S, Prasad A, Mahajan V, Di X, et al. Higher limbic and basal ganglia volumes in surviving COVID-negative patients and the relations to fatigue. Neuroimage Rep. (2022) 2:100095.
161. Rothstein T. Cortical grey matter volume depletion links to neurological sequelae in post COVID-19 “long haulers”. BMC Neurol. (2023) 23:22. doi: 10.1186/s12883-023-03049-1
162. Van Oosterwijck J, Nijs J, Meeus M, Lefever I, Huybrechts L, Lambrecht L, et al. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: an experimental study. J Intern Med. (2010) 268:265–78.
163. Meeus M, Roussel N, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med. (2010) 42:884–90. doi: 10.2340/16501977-0595
164. Meeus M, Nijs J, Huybrechts S, Truijen S. Evidence for generalized hyperalgesia in chronic fatigue syndrome. Clin Rheumatol. (2010) 29:393–8.
165. Nijs J, Meeus M, Van Oosterwijck J, Ickmans K, Moorkens G, Hans G, et al. In the mind or in the brain? Scientific evidence for central sensitisation in chronic fatigue syndrome. Eur J Clin Invest. (2012) 42:203–12.
166. Collin S, Nijs J, Meeus M, Polli A, Willekens B, Ickmans K. Endogenous pain facilitation rather than inhibition differs between people with chronic fatigue syndrome, multiple sclerosis, and controls: an observational study. Pain Physician. (2017) 20:E489–97.
167. Bourke J, Wodehouse T, Clark L, Constantinou E, Kidd B, Langford R, et al. Central sensitisation in chronic fatigue syndrome and fibromyalgia; a case control study. J Psychosom Res. (2021) 150:110624.
168. van Campen C, Rowe P, Verheugt F, Visser F. Orthostatic stress testing in myalgic encephalomyelitis/chronic fatigue syndrome patients with or without concomitant fibromyalgia: effects on pressure pain thresholds and temporal summation. Clin Exp Rheumatol. (2021) 39 Suppl:39–47.
169. Polli A, Van Oosterwijck J, Nijs J, Marusic U, De Wandele I, Paul L, et al. Relationship between exercise-induced oxidative stress changes and parasympathetic activity in chronic fatigue syndrome: an observational study in patients and healthy subjects. Clin Ther. (2019) 41:641–55.
170. Vecchiet J, Cipollone F, Falasca K, Mezzetti A, Pizzigallo E, Bucciarelli T, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. (2003) 335:151–4.
171. Al-Rawaf H, Alghadir A, Gabr S. MicroRNAs as biomarkers of pain intensity in patients with chronic fatigue syndrome. Pain Pract. (2019) 19:848–60. doi: 10.1111/papr.12817
172. Walitt B, Ceko M, Gracely J, Gracely R. Neuroimaging of central sensitivity syndromes: key insights from the scientific literature. Curr Rheumatol Rev. (2016) 12:55–87. doi: 10.2174/1573397112666151231111104
173. Pacho-Hernandez J, Fernandez-de-Las-Penas C, Fuensalida-Novo S, Jimenez-Antona C, Ortega-Santiago R, Cigaran-Mendez M. Sleep quality mediates the effect of sensitization-associated symptoms, anxiety, and depression on quality of life in individuals with post-COVID-19 pain. Brain Sci. (2022) 12:1363. doi: 10.3390/brainsci12101363
174. de Lange F, Kalkman J, Bleijenberg G, Hagoort P, van der Werf S, van der Meer J, et al. Neural correlates of the chronic fatigue syndrome–an fMRI study. Brain. (2004) 127:1948–57.
175. Tanaka M, Sadato N, Okada T, Mizuno K, Sasabe T, Tanabe H, et al. Reduced responsiveness is an essential feature of chronic fatigue syndrome: a fMRI study. BMC Neurol. (2006) 6:9. doi: 10.1186/1471-2377-6-9
176. Caseras X, Mataix-Cols D, Giampietro V, Rimes K, Brammer M, Zelaya F, et al. Probing the working memory system in chronic fatigue syndrome: a functional magnetic resonance imaging study using the n-back task. Psychosom Med. (2006) 68:947–55. doi: 10.1097/01.psy.0000242770.50979.5f
177. Mizuno K, Kawatani J, Tajima K, Sasaki A, Yoneda T, Komi M, et al. Low putamen activity associated with poor reward sensitivity in childhood chronic fatigue syndrome. Neuroimage Clin. (2016) 12:600–6. doi: 10.1016/j.nicl.2016.09.016
178. Wortinger L, Endestad T, Melinder A, Oie M, Sulheim D, Fagermoen E, et al. Emotional conflict processing in adolescent chronic fatigue syndrome: a pilot study using functional magnetic resonance imaging. J Clin Exp Neuropsychol. (2017) 39:355–68. doi: 10.1080/13803395.2016.1230180
179. Almutairi B, Langley C, Crawley E, Thai N. Using structural and functional MRI as a neuroimaging technique to investigate chronic fatigue syndrome/myalgic encephalopathy: a systematic review. BMJ Open. (2020) 10:e031672.
180. Joseph P, Arevalo C, Oliveira R, Faria-Urbina M, Felsenstein D, Oaklander A, et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. (2021) 160:642–51.
181. Oaklander A, Mills A, Kelley M, Toran L, Smith B, Dalakas M, et al. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1146.
182. Novak P, Mukerji S, Alabsi H, Systrom D, Marciano S, Felsenstein D, et al. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann Neurol. (2022) 91:367–79.
183. Abrams R, Simpson D, Navis A, Jette N, Zhou L, Shin S. Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve. (2022) 65:440–3.
184. Bitirgen G, Korkmaz C, Zamani A, Ozkagnici A, Zengin N, Ponirakis G, et al. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. (2022) 106:1635–41. doi: 10.1136/bjophthalmol-2021-319450
185. Duffy F, McAnulty G, McCreary M, Cuchural G, Komaroff ALEEG. spectral coherence data distinguish chronic fatigue syndrome patients from healthy controls and depressed patients - A case control study. BMC Neurol. (2011) 11:82. doi: 10.1186/1471-2377-11-82
186. Rubega M, Ciringione L, Bertuccelli M, Paramento M, Sparacino G, Vianello A, et al. High-density EEG sleep correlates of cognitive and affective impairment at 12-month follow-up after COVID-19. Clin Neurophysiol. (2022) 140:126–35. doi: 10.1016/j.clinph.2022.05.017
187. Unger E, Nisenbaum R, Moldofsky H, Cesta A, Sammut C, Reyes M, et al. Sleep assessment in a population-based study of chronic fatigue syndrome. BMC Neurol. (2004) 4:6. doi: 10.1186/1471-2377-4-6
188. Maksoud R, Eaton-Fitch N, Matula M, Cabanas H, Staines D, Marshall-Gradisnik S. Systematic review of sleep characteristics in myalgic encephalomyelitis/chronic fatigue syndrome. Healthcare (Basel). (2021) 9:568.
189. McCarthy M. Circadian rhythm disruption in myalgic encephalomyelitis/chronic fatigue syndrome: implications for the post-acute sequelae of COVID-19. Brain Behav Immun Health. (2022) 20:100412. doi: 10.1016/j.bbih.2022.100412
190. Mohamed A, Andersen T, Radovic S, Del Fante P, Kwiatek R, Calhoun V, et al. Objective sleep measures in chronic fatigue syndrome patients: a systematic review and meta-analysis. Sleep Med Rev. (2023) 69:101771.
191. Moura A, Oliveira D, Torres D, Tavares-Junior J, Nobrega P, Braga-Neto P, et al. Central hypersomnia and chronic insomnia: expanding the spectrum of sleep disorders in long COVID syndrome - a prospective cohort study. BMC Neurol. (2022) 22:417. doi: 10.1186/s12883-022-02940-7
192. Mahmoudi H, Saffari M, Movahedi M, Sanaeinasab H, Rashidi-Jahan H, Pourgholami M, et al. A mediating role for mental health in associations between COVID-19-related self-stigma, PTSD, quality of life, and insomnia among patients recovered from COVID-19. Brain Behav. (2021) 11:e02138. doi: 10.1002/brb3.2138
193. Alzueta E, Perrin P, Yuksel D, Ramos-Usuga D, Kiss O, Iacovides S, et al. An international study of post-COVID sleep health. Sleep Health. (2022) 8:684–90. doi: 10.1016/j.sleh.2022.06.011
194. Merikanto I, Dauvilliers Y, Chung F, Wing Y, De Gennaro L, Holzinger B, et al. Sleep symptoms are essential features of long-COVID - comparing healthy controls with COVID-19 cases of different severity in the international COVID sleep study (ICOSS-II). J Sleep Res. (2022):e13754. doi: 10.1111/jsr.13754
195. Goldstein C, Kagan D, Rizvydeen M, Warshaw S, Troost J, Burgess H. The possibility of circadian rhythm disruption in long COVID. Brain Behav Immun Health. (2022) 23:100476. doi: 10.1016/j.bbih.2022.100476
196. Badenoch J, Rengasamy E, Watson C, Jansen K, Chakraborty S, Sundaram R, et al. Persistent neuropsychiatric symptoms after COVID-19: a systematic review and meta-analysis. Brain Commun. (2022) 4:fcab297.
197. Rutherford G, Manning P, Newton J. Understanding muscle dysfunction in chronic fatigue syndrome. J Aging Res. (2016) 2016:2497348.
198. Retornaz F, Rebaudet S, Stavris C, Jammes Y. Long-term neuromuscular consequences of SARS-Cov-2 and their similarities with myalgic encephalomyelitis/chronic fatigue syndrome: results of the retrospective CoLGEM study. J Transl Med. (2022) 20:429.
199. Klaver-Krol E, Hermens H, Vermeulen R, Klaver M, Luyten H, Henriquez N, et al. Chronic fatigue syndrome: abnormally fast muscle fiber conduction in the membranes of motor units at low static force load. Clin Neurophysiol. (2021) 132:967–74.
200. Wirth K, Scheibenbogen C. Pathophysiology of skeletal muscle disturbances in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. (2021) 19:162. doi: 10.1186/s12967-021-02833-2
201. Petter E, Scheibenbogen C, Linz P, Stehning C, Wirth K, Kuehne T, et al. Muscle sodium content in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. (2022) 20:580.
202. Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an11C-(R)-PK11195 PET study. J Nucl Med. (2014) 55:945–50. doi: 10.2967/jnumed.113.131045
203. Renz-Polster H, Tremblay M, Bienzle D, Fischer J. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: the case for neuroglial failure. Front Cell Neurosci. (2022) 16:888232. doi: 10.3389/fncel.2022.888232
204. Godlewska B, Williams S, Emir U, Chen C, Sharpley A, Goncalves A, et al. Neurochemical abnormalities in chronic fatigue syndrome: a pilot magnetic resonance spectroscopy study at 7 Tesla. Psychopharmacology (Berl). (2022) 239:163–71. doi: 10.1007/s00213-021-05986-6
205. Raijmakers R, Roerink M, Keijmel S, Joosten L, Netea M, van der Meer J, et al. No signs of neuroinflammation in women with chronic fatigue syndrome or Q fever fatigue syndrome using the TSPO ligand [(11)C]-PK11195. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1113.
206. Kiatkittikul P, Promteangtrong C, Kunawudhi A, Siripongsatian D, Siripongboonsitti T, Ruckpanich P, et al. Abnormality pattern of f-18 FDG PET whole body with functional MRI brain in post-acute COVID-19. Nucl Med Mol Imaging. (2022) 56:29–41. doi: 10.1007/s13139-021-00730-6
207. Fernandez-Castaneda A, Lu P, Geraghty A, Song E, Lee M, Wood J, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. (2022) 185:2452–68.e16. doi: 10.1016/j.cell.2022.06.008
208. Klein R, Berg P. High incidence of antibodies to 5-hydroxytryptamine, gangliosides and phospholipids in patients with chronic fatigue and fibromyalgia syndrome and their relatives: evidence for a clinical entity of both disorders. Eur J Med Res. (1995) 1:21–6.
209. Tanaka S, Kuratsune H, Hidaka Y, Hakariya Y, Tatsumi K, Takano T, et al. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. Int J Mol Med. (2003) 12:225–30.
210. Yamamoto S, Ouchi Y, Nakatsuka D, Tahara T, Mizuno K, Tajima S, et al. Reduction of [11C](+)3-MPB binding in brain of chronic fatigue syndrome with serum autoantibody against muscarinic cholinergic receptor. PLos One. (2012) 7:e51515. doi: 10.1371/journal.pone.0051515
211. Loebel M, Grabowski P, Heidecke H, Bauer S, Hanitsch L, Wittke K, et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with chronic fatigue syndrome. Brain Behav Immun. (2016) 52:32–9.
212. Sotzny F, Blanco J, Capelli E, Castro-Marrero J, Steiner S, Murovska M, et al. Myalgic encephalomyelitis/chronic fatigue syndrome – evidence for an autoimmune disease. Autoimmun Rev. (2018) 17:601–9.
213. Shoenfeld Y, Ryabkova V, Scheibenbogen C, Brinth L, Martinez-Lavin M, Ikeda S, et al. Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clin Immunol. (2020) 214:108384. doi: 10.1016/j.clim.2020.108384
214. Fujii H, Sato W, Kimura Y, Matsuda H, Ota M, Maikusa N, et al. Altered structural brain networks related to adrenergic/muscarinic receptor autoantibodies in chronic fatigue syndrome. J Neuroimaging. (2020) 30:822–7. doi: 10.1111/jon.12751
215. Wirth K, Scheibenbogen C. A unifying hypothesis of the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): recognitions from the finding of autoantibodies against ss2-adrenergic receptors. Autoimmun Rev. (2020) 19:102527. doi: 10.1016/j.autrev.2020.102527
216. Szklarski M, Freitag H, Lorenz S, Becker S, Sotzny F, Bauer S, et al. Delineating the association between soluble CD26 and autoantibodies against G-protein coupled receptors, immunological and cardiovascular parameters identifies distinct patterns in post-infectious vs. non-infection-triggered myalgic encephalomyelitis/chronic fatigue syndrome. Front Immunol. (2021) 12:644548. doi: 10.3389/fimmu.2021.644548
217. De Bellis A, Bellastella G, Pernice V, Cirillo P, Longo M, Maio A, et al. Hypothalamic-pituitary autoimmunity and related impairment of hormone secretions in chronic fatigue syndrome. J Clin Endocrinol Metab. (2021) 106:e5147–55. doi: 10.1210/clinem/dgab429
218. Freitag H, Szklarski M, Lorenz S, Sotzny F, Bauer S, Philippe A, et al. Autoantibodies to vasoregulative G-protein-coupled receptors correlate with symptom severity, autonomic dysfunction and disability in myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Med. (2021) 10:3675. doi: 10.3390/jcm10163675
219. Szewczykowski C, Mardin C, Lucio M, Wallukat G, Hoffmanns J, Schroder T, et al. Long COVID: association of functional autoantibodies against G-protein-coupled receptors with an impaired retinal microcirculation. Int J Mol Sci. (2022) 23:7209. doi: 10.3390/ijms23137209
220. Kim B, Namkoong K, Kim J, Lee S, Yoon K, Choi M, et al. Altered resting-state functional connectivity in women with chronic fatigue syndrome. Psychiatry Res. (2015) 234:292–7.
221. Wortinger L, Endestad T, Melinder A, Oie M, Sevenius A, Bruun Wyller V. Aberrant resting-state functional connectivity in the salience network of adolescent chronic fatigue syndrome. PLos One. (2016) 11:e0159351. doi: 10.1371/journal.pone.0159351
222. Gay C, Robinson M, Lai S, O’Shea A, Craggs J, Price D, et al. Abnormal resting-state functional connectivity in patients with chronic fatigue syndrome: results of seed and data-driven analyses. Brain Connect. (2016) 6:48–56. doi: 10.1089/brain.2015.0366
223. Zinn M, Zinn M, Jason L. Intrinsic functional hypoconnectivity in core neurocognitive networks suggests central nervous system pathology in patients with myalgic encephalomyelitis: a pilot study. Appl Psychophysiol Biofeedback. (2016) 41:283–300. doi: 10.1007/s10484-016-9331-3
224. Boissoneault J, Letzen J, Lai S, O’Shea A, Craggs J, Robinson M, et al. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: an arterial spin-labeling fMRI study. Magn Reson Imaging. (2016) 34:603–8.
225. Wortinger L, Glenne Oie M, Endestad T, Bruun Wyller V. Altered right anterior insular connectivity and loss of associated functions in adolescent chronic fatigue syndrome. PLos One. (2017) 12:e0184325. doi: 10.1371/journal.pone.0184325
226. Shan Z, Finegan K, Bhuta S, Ireland T, Staines D, Marshall-Gradisnik S, et al. Decreased connectivity and increased blood oxygenation level dependent complexity in the default mode network in individuals with chronic fatigue syndrome. Brain Connect. (2018) 8:33–9.
227. Barnden L, Shan Z, Staines D, Marshall-Gradisnik S, Finegan K, Ireland T, et al. Intra brainstem connectivity is impaired in chronic fatigue syndrome. Neuroimage Clin. (2019) 24:102045. doi: 10.1016/j.nicl.2019.102045
228. Zinn M, Jason L. Cortical autonomic network connectivity predicts symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Int J Psychophysiol. (2021) 170:89–101.
229. Nelson T, Zhang L, Guo H, Nacul L, Song X. Brainstem abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: a scoping review and evaluation of magnetic resonance imaging findings. Front Neurol. (2021) 12:769511. doi: 10.3389/fneur.2021.769511
230. Josev E, Malpas C, Seal M, Scheinberg A, Lubitz L, Rowe K, et al. Resting-state functional connectivity, cognition, and fatigue in response to cognitive exertion: a novel study in adolescents with chronic fatigue syndrome. Brain Imaging Behav. (2019) 14:1815–30. doi: 10.1007/s11682-019-00119-2
231. Baraniuk J, Casado B, Maibach H, Clauw D, Pannell L, Hess S. A chronic fatigue syndrome-related proteome in human cerebrospinal fluid. BMC Neurol. (2005) 5:22. doi: 10.1186/1471-2377-5-22
232. Schutzer S, Angel T, Liu T, Schepmoes A, Clauss T, Adkins J, et al. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. PLos One. (2011) 6:e17287. doi: 10.1371/journal.pone.0017287
233. Jarius S, Pache F, Kortvelyessy P, Jelcic I, Stettner M, Franciotta D, et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflamm. (2022) 19:19. doi: 10.1186/s12974-021-02339-0
234. Schweitzer F, Goereci Y, Franke C, Silling S, Bosl F, Maier F, et al. Cerebrospinal fluid analysis post-COVID-19 is not suggestive of persistent central nervous system infection. Ann Neurol. (2022) 91:150–7.
235. Du Preez S, Eaton-Fitch N, Cabanas H, Staines D, Marshall-Gradisnik S. Characterization of IL-2 stimulation and TRPM7 pharmacomodulation in NK cell cytotoxicity and channel co-localization with PIP2 in myalgic encephalomyelitis/chronic fatigue syndrome patients. Int J Environ Res Public Health. (2021) 18:11879. doi: 10.3390/ijerph182211879
236. Du Preez S, Cabanas H, Staines D, Marshall-Gradisnik S. Potential implications of mammalian transient receptor potential melastatin 7 in the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome: a review. Int J Environ Res Public Health. (2021) 18:10708. doi: 10.3390/ijerph182010708
237. Sasso E, Muraki K, Eaton-Fitch N, Smith P, Lesslar O, Deed G, et al. Transient receptor potential melastatin 3 dysfunction in post COVID-19 condition and myalgic encephalomyelitis/chronic fatigue syndrome patients. Mol Med. (2022) 28:98. doi: 10.1186/s10020-022-00528-y
238. Baraniuk J. Review of the midbrain ascending arousal network nuclei and implications for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Gulf War Illness (GWI) and postexertional malaise (PEM). Brain Sci. (2022) 12:132. doi: 10.3390/brainsci12020132
239. Thapaliya K, Marshall-Gradisnik S, Barth M, Eaton-Fitch N, Barnden L. Brainstem volume changes in myalgic encephalomyelitis/chronic fatigue syndrome and long COVID patients. Front Neurosci. (2023) 17:1125208. doi: 10.3389/fnins.2023.1125208
240. Yong S. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. (2021) 12:573–80.
241. Peluso M, Sans H, Forman C, Nylander A, Ho H, Lu S, et al. Plasma markers of neurologic injury and inflammation in people with self-reported neurologic postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e200003. doi: 10.1212/NXI.0000000000200003
242. Caligiuri M, Murray C, Buchwald D, Levine H, Cheney P, Peterson D, et al. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J Immunol. (1987) 139:3306–13.
243. Klimas N, Salvato F, Morgan R, Fletcher M. Immunologic abnormalities in chronic fatigue syndrome. J Clin Microbiol. (1990) 28:1403–10.
244. Aoki T, Miyakoshi H, Usuda Y, Herberman R. Low NK syndrome and its relationship to chronic fatigue syndrome. Clin Immunol Immunopathol. (1993) 69:253–65.
245. Vojdani A, Thrasher J. Cellular and humoral immune abnormalities in Gulf War veterans. Environ Health Perspect. (2004) 112:840–6.
246. Brenu E, van Driel M, Straines D, Ashton K, Hardcastle S, Keane J, et al. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. (2012) 10:88. doi: 10.1186/1479-5876-10-88
247. Brenu E, Huth T, Hardcastle S, Fuller K, Kaur M, Johnston S, et al. Role of adaptive and innate immune cells in chronic fatigue syndrome/myalgic encephalomyelitis. Int Immunol. (2014) 26:233–42.
248. Hardcastle S, Brenu E, Johnston S, Nguyen T, Huth T, Kaur M, et al. Analysis of the relationship between immune dysfunction and symptom severity in patients with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Clin Cell Immunol. (2014) 5:1000190.
249. Chacko A, Staines D, Johnston S, Marshall-Gradisnik S. Dysregulation of protein kinase gene expression in NK cells from chronic fatigue syndrome/myalgic encephalomyelitis patients. Gene Regul Syst Bio. (2016) 10:85–93.
250. Huth T, Brenu E, Ramos S, Nguyen T, Broadley S, Staines D, et al. Pilot study of natural killer cells in chronic fatigue syndrome/myalgic encephalomyelitis and multiple sclerosis. Scand J Immunol. (2016) 83:44–51.
251. Nguyen T, Johnston S, Clarke L, Smith P, Staines D, Marshall-Gradisnik S. Impaired calcium mobilization in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients is associated with transient receptor potential melastatin 3 ion channels. Clin Exp Immunol. (2017) 187:284–93.
252. Rivas J, Palencia T, Fernandez G, Garcia M. Association of T and NK cell phenotype with the diagnosis of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front Immunol. (2018) 9:1028. doi: 10.3389/fimmu.2018.01028
253. Nguyen T, Staines D, Johnston S, Marshall-Gradisnik S. Reduced glycolytic reserve in isolated natural killer cells from myalgic encephalomyelitis/chronic fatigue syndrome patients: a preliminary investigation. Asian Pac J Allergy Immunol. (2019) 37:102–8.
254. Eaton-Fitch N, du Preez S, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of natural killer cells profile and cytotoxic function in myalgic encephalomyelitis/chronic fatigue syndrome. Syst Rev. (2019) 8:279.
255. Sung A, Tang J, Guglielmo M, Smith-Gagen J, Bateman L, Navarrete-Galvan L, et al. Antibody-dependent cell-mediated cytotoxicity (ADCC) in familial myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Fatigue. (2021) 8:226–44.
256. Theorell J, Bileviciute-Ljungar I, Tesi B, Schlums H, Johnsgaard M, Asadi-Azarbaijani B, et al. Unperturbed cytotoxic lymphocyte phenotype and function in myalgic encephalomyelitis/chronic fatigue syndrome patients. Front Immunol. (2017) 8:723. doi: 10.3389/fimmu.2017.00723
257. Cliff J, King E, Lee J, Sepulveda N, Wolf A, Kingdon C, et al. Cellular immune function in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front Immunol. (2019) 10:796. doi: 10.3389/fimmu.2019.00796
258. Galan M, Vigon L, Fuertes D, Murciano-Anton M, Casado-Fernandez G, Dominguez-Mateos S, et al. Persistent overactive cytotoxic immune response in a Spanish cohort of individuals with long-COVID: identification of diagnostic biomarkers. Front Immunol. (2022) 13:848886. doi: 10.3389/fimmu.2022.848886
259. Ryan F, Hope C, Masavuli M, Lynn M, Mekonnen Z, Yeow A, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. (2022) 20:26. doi: 10.1186/s12916-021-02228-6
260. Hornig M, Montoya J, Klimas N, Levine S, Felsenstein D, Bateman L, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. (2015) 1:e1400121. doi: 10.1126/sciadv.1400121
261. Bennett A, Chao C, Hu S, Buchwald D, Fagioli L, Schur P, et al. Elevation of bioactive transforming growth factor-B in serum from patients with chronic fatigue syndrome. J Clin Immunol. (1997) 17:160–6.
262. Moss R, Mercandetti A, Vojdani AT. NF-α and chronic fatigue syndrome. J Clin Immunol. (1999) 19:314–6.
263. Kerr J, Barah F, Mattey D, Laing I, Hopkins S, Hutchinson I, et al. Circulating tumour necrosis factor-α and interferon- g are detectable during acute and convalescent parvovirus B19 infection and are associated with prolonged and chronic fatigue. J Gen Virol. (2001) 82:3011–9.
265. Fletcher M, Zeng X, Barnes Z, Levis S, Klimas N. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. (2009) 7:96.
266. Broderick G, Fuite J, Kreitz A, Vernon S, Klimas N, Fletcher MA. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. (2010) 24:1209–17.
267. Maes M, Twisk F, Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. (2012) 81:286–95. doi: 10.1159/000336803
268. Stringer E, Baker K, Carroll I, Montoya J, Chu L, Maecker H, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J Transl Med. (2013) 11:93. doi: 10.1186/1479-5876-11-93
269. Khaiboullina S, DeMeirleir K, Rawat S, Berk G, Gaynor-Berk R, Mijatovic T, et al. Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine. (2015) 72:1–8. doi: 10.1016/j.cyto.2014.11.019
270. Roerink M, Knoop H, Bronkhorst E, Mouthaan H, Hawinkels L, Joosten L, et al. Cytokine signatures in chronic fatigue syndrome patients: a case control study and the effect of anakinra treatment. J Transl Med. (2017) 15:267. doi: 10.1186/s12967-017-1371-9
271. Montoya J, Holmes T, Anderson J, Maecker H, Rosenberg-Hasson Y, Valencia I, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci USA. (2017) 114:E7150–8.
272. Strawbridge R, Sartor M, Scott F, Cleare A. Inflammatory proteins are altered in chronic fatigue syndrome-A systematic review and meta-analysis. Neurosci Biobehav Rev. (2019) 107:69–83. doi: 10.1016/j.neubiorev.2019.08.011
273. Jonsjö M, Olsson G, Wicksell R, Alving K, Holmström L, Andreasson A. The role of low-grade inflammation in ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) - associations with symptoms. Psychoneuroendocrinology. (2020) 113:104578.
274. Mandarano A, Maya J, Giloteaux L, Peterson D, Maynard M, Gottschalk C, et al. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J Clin Invest. (2020) 130:1491–505. doi: 10.1172/JCI132185
275. Raanes E, Stiles T. Associations between psychological and immunological variables in chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. Front Psychiatry. (2021) 12:716320. doi: 10.3389/fpsyt.2021.716320
276. Hornig M, Gottschalk G, Peterson D, Knox K, Schultz A, Eddy M, et al. Cytokine network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome. Mol Psychiatry. (2016) 21:261–9.
277. Nakamura T, Schwander S, Donnelly R, Ortega F, Togo F, Broderick G, et al. Cytokines across the night in chronic fatigue syndrome with and without fibromyalgia. Clin Vaccine Immunol. (2010) 17:582–7. doi: 10.1128/CVI.00379-09
278. Clark L, Buckland M, Murphy G, Taylor N, Vleck V, Mein C, et al. Cytokine responses to exercise and activity in patients with chronic fatigue syndrome: case-control study. Clin Exp Immunol. (2017) 190:360–71.
279. Blundell S, Ray K, Buckland M, White P. Chronic fatigue syndrome and circulating cytokines: a systematic review. Brain Behav Immun. (2015) 50:186–95.
280. Wyller V, Sorensen O, Sulheim D, Fagermoen E, Ueland T, Mollnes T. Plasma cytokine expression in adolescent chronic fatigue syndrome. Brain Behav Immun. (2015) 46:80–6.
281. Kashipaz M, Swinden D, Todd I, Powell R. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clin Exp Immunol. (2003) 132:360–5. doi: 10.1046/j.1365-2249.2003.02149.x
282. VanElzakker M, Brumfield S, Lara Mejia P. Neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a critical review of research methods. Front Neurol. (2019) 9:1033. doi: 10.3389/fneur.2018.01033
283. Corbitt M, Eaton-Fitch N, Staines D, Cabanas H, Marshall-Gradisnik S. A systematic review of cytokines in chronic fatigue syndrome/myalgic encephalomyelitis/systemic exertion intolerance disease (CFS/ME/SEID). BMC Neurol. (2019) 19:207. doi: 10.1186/s12883-019-1433-0
284. Patterson B, Guevara-Coto J, Yogendra R, Francisco E, Long E, Pise A, et al. Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front Immunol. (2021) 12:700782. doi: 10.3389/fimmu.2021.700782
285. Schultheiss C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes S, et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. (2022) 3:100663.
286. Shuwa H, Shaw T, Knight S, Wemyss K, McClure F, Pearmain L, et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med (N Y). (2021) 2:720–35.e4. doi: 10.1016/j.medj.2021.03.013
287. Ruenjaiman V, Sodsai P, Kueanjinda P, Bunrasmee W, Klinchanhom S, Reantragoon R, et al. Impact of SARS-CoV-2 infection on the profiles and responses of innate immune cells after recovery. J Microbiol Immunol Infect. (2022) 55(6 Pt 1):993–1004.
288. Acosta-Ampudia Y, Monsalve D, Rojas M, Rodriguez Y, Zapata E, Ramirez-Santana C, et al. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J Infect Dis. (2022) 225:2155–62. doi: 10.1093/infdis/jiac017
289. Bellan M, Apostolo D, Albe A, Crevola M, Errica N, Ratano G, et al. Determinants of long COVID among adults hospitalized for SARS-CoV-2 infection: a prospective cohort study. Front Immunol. (2022) 13:1038227. doi: 10.3389/fimmu.2022.1038227
290. Frere J, Serafini R, Pryce K, Zazhytska M, Oishi K, Golynker I, et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations post recovery. Sci Trans Med. (2022) 14:eabq3059. doi: 10.1126/scitranslmed.abq3059
291. Phetsouphanh C, Darley D, Wilson D, Howe A, Munier C, Patel S, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. (2022) 23:210–6. doi: 10.1038/s41590-021-01113-x
292. Low R, Low R, Akrami A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front Med. (2023) 10:1011936. doi: 10.3389/fmed.2023.1011936
293. Kovarik J, Bileck A, Hagn G, Meier-Menches S, Frey T, Kaempf A, et al. A multi-omics based anti-inflammatory immune signature characterizes long COVID-19 syndrome. iScience. (2023) 26:105717. doi: 10.1016/j.isci.2022.105717
294. Clough E, Inigo J, Chandra D, Chaves L, Reynolds J, Aalinkeel R, et al. Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: implications for neuro-COVID. J Neuroimmune Pharmacol. (2021) 16:770–84.
295. Sommen S, Havdal L, Selvakumar J, Einvik G, Leegaard T, Lund-Johansen F, et al. Inflammatory markers and pulmonary function in adolescents and young adults 6 months after mild COVID-19. Front Immunol. (2023) 13:1081718. doi: 10.3389/fimmu.2022.1081718
296. Son K, Jamil R, Chowdhury A, Mukherjee M, Venegas C, Miyasaki K, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J. (2023) 61:2200970. doi: 10.1183/13993003.00970-2022
297. Bates D, Buchwald D, Lee J, Doolittle T, Kornish J, Rutherford C, et al. Laboratory abnormalities in patients with chronic fatigue syndrome. Clin Infect Dis. (1994) 18(Suppl 1):S86–S.
298. Clark M, Katon W, Russo J, Kith P, Sintay M, Buchwald D. Chronic fatigue: risk factors for symptom persistence in a 2 1/2-year follow-up study. Am J Med. (1995) 88:187–95. doi: 10.1016/S0002-9343(99)80403-3
299. Landay A, Jessop C, Lennette E, Levy J. Chronic fatigue syndrome: clinical condition associated with immune activation. Lancet. (1991) 338:707–12.
300. Barker E, Fujimura S, Fadem M, Landay A, Levy J. Immunologic abnormalities associated with chronic fatigue syndrome. Clin Infect Dis. (1994) 18(Suppl. 1):S136–41.
301. Nijs J, de Becker P, de Meirleir K, Demanet C, Vincken W, Schuermans D, et al. Associations between bronchial hyperresponsiveness and immune cell parameters in patients with chronic fatigue syndrome. Chest. (2003) 123:998–1007. doi: 10.1378/chest.123.4.998
302. Maya J, Leddy S, Gottschalk C, Peterson D, Hanson M. Altered fatty acid oxidation in lymphocyte populations of myalgic encephalomyelitis/chronic fatigue syndrome. Int J Mol Sci. (2023) 24:2010.
303. Tirelli U, Marotta G, Improta S, Pinto A. Immmunological abnormalities in patients with chronic fatigue syndrome. Scand J Immunol. (1994) 40:601–8.
304. Mensah F, Bansal A, Berkovitz S, Sharma A, Reddy V, Leandro M, et al. Extended B cell phenotype in patients with myalgic encephalomyelitis/chronic fatigue syndrome: a cross-sectional study. Clin Exp Immunol. (2016) 184:237–47.
305. Mensah F, Armstrong C, Reddy V, Bansal A, Berkovitz S, Leandro M, et al. CD24 expression and B cell maturation shows a novel link with energy metabolism: potential implications for patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Immunol. (2018) 9:2421. doi: 10.3389/fimmu.2018.02421
306. Nguyen C, Alsoe L, Lindvall J, Sulheim D, Fagermoen E, Winger A, et al. Whole blood gene expression in adolescent chronic fatigue syndrome: an exploratory cross-sectional study suggesting altered B cell differentiation and survival. J Transl Med. (2017) 15:102. doi: 10.1186/s12967-017-1201-0
307. Lunde S, Kristoffersen E, Sapkota D, Risa K, Dahl O, Bruland O, et al. Serum BAFF and APRIL Levels, T-lymphocyte subsets, and immunoglobulins after B-cell depletion using the monoclonal anti-CD20 antibody rituximab in myalgic encephalopathy/chronic fatigue syndrome. PLos One. (2016) 11:e0161226. doi: 10.1371/journal.pone.0161226
308. Smith J, Fritz E, Kerr J, Cleare A, Wessely S, Mattey D. Association of chronic fatigue syndrome with human leucocyte antigen class II alleles. J Clin Pathol. (2005) 58:860–3.
309. Carlo-Stella N, Bozzini S, De Silvestri A, Sbarsi I, Pizzochero C, Lorusso L, et al. Molecular study of receptor for advanced glycation endproduct gene promoter and identification of specific HLA haplotypes possibly involved in chronic fatigue syndrome. Int J Immunopathol Pharmacol. (2009) 22:745–54. doi: 10.1177/039463200902200320
310. Pasi A, Bozzini S, Carlo-Stella N, Martinetti M, Bombardieri S, De Silvestri A, et al. Excess of activating killer cell immunoglobulin-like receptors and lack of HLA-Bw4 ligands: a two-edged weapon in chronic fatigue syndrome. Mol Med Rep. (2011) 4:535–40. doi: 10.3892/mmr.2011.447
311. Milivojevic M, Che X, Bateman L, Cheng A, Garcia B, Hornig M, et al. Plasma proteomic profiling suggests an association between antigen driven clonal B cell expansion and ME/CFS. PLos One. (2020) 15:e0236148. doi: 10.1371/journal.pone.0236148
312. Sato W, Ono H, Matsutani T, Nakamura M, Shin I, Amano K, et al. Skewing of the B cell receptor repertoire in myalgic encephalomyelitis/chronic fatigue syndrome. Brain Behav Immun. (2021) 95:245–55. doi: 10.1016/j.bbi.2021.03.023
313. Steiner S, Becker S, Hartwig J, Sotzny F, Lorenz S, Bauer S, et al. Autoimmunity-related risk variants in PTPN22 and CTLA4 are associated with ME/CFS with infectious onset. Front Immunol. (2020) 11:578. doi: 10.3389/fimmu.2020.00578
314. Konstantinov K, von Mikecz A, Buchwald D, Jones J, Gerace L, Tan E. Autoantibodies to nuclear envelope antigens in chronic fatigue syndrome. J Clin Invest. (1996) 98:1888–96.
315. Nishikai M. Antinuclear antibodies in patients with chronic fatigue syndrome. Nippon Rinsho. (2007) 65:1067–70.
316. Hokama Y, Empey-Campora C, Hara C, Higa N, Siu N, Lau R, et al. Acute phase phospholipids related to the cardiolipin of mitochondria in the sera of patients with chronic fatigue syndrome (CFS), chronic ciguatera fish poisoning (CCFP), and other diseases attributed to chemicals, Gulf War and marine toxins. J Clin Lab Anal. (2008) 22:99–105. doi: 10.1002/jcla.20217
317. Hokama Y, Campora C, Hara C, Kuribayashi T, Huynh D, Yabusaki K. Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. J Clin Lab Anal. (2009) 23:210–2.
318. Buchwald D, Wener M, Komaroff A. Anti-neuronal antibody levels in chronic fatigue syndrome patients with neurologic abnormalities. Arthritis Rheum. (1991) 34:1485–6.
319. Ryabkova V, Gavrilova N, Poletaeva A, Pukhalenko A, Koshkina I, Churilov L, et al. Autoantibody correlation signatures in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome: association with symptom severity. Biomedicines. (2023) 11:257.
320. Wang E, Mao T, Klein J, Dai Y, Huck J, Jaycox J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. (2021) 595:283–8.
321. Wallukat G, Hohberger B, Wenzel K, Furst J, Schulze-Rothe S, Wallukat A, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. (2021) 4:100100. doi: 10.1016/j.jtauto.2021.100100
322. L’Huillier A, Pagano S, Baggio S, Meyer B, Andrey D, Nehme M, et al. Autoantibodies against apolipoprotein A-1 after COVID-19 predict symptoms persistence. Eur J Clin Invest. (2022) 52:e13818. doi: 10.1111/eci.13818
323. Pisareva E, Badiou S, Mihalovicova L, Mirandola A, Pastor B, Kudriavtsev A, et al. Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients. J Med Virol. (2023) 95:e28209. doi: 10.1002/jmv.28209
324. Sotzny F, Filgueiras I, Kedor C, Freitag H, Wittke K, Bauer S, et al. Dysregulated autoantibodies targeting vaso- and immunoregulatory receptors in Post COVID Syndrome correlate with symptom severity. Front Immunol. (2022) 13:981532. doi: 10.3389/fimmu.2022.981532
325. Seessle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis. (2022) 74:1191–8.
326. Lui D, Lee C, Chow W, Lee A, Tam A, Pang P, et al. Long COVID in patients with mild to moderate disease: do thyroid function and autoimmunity play a role? Endocr Pract. (2021) 27:894–902. doi: 10.1016/j.eprac.2021.06.016
327. Moody R, Sonda S, Johnston F, Smith K, Stephens N, McPherson M, et al. Antibodies against Spike protein correlate with broad autoantigen recognition 8 months post SARS-CoV-2 exposure, and anti-calprotectin autoantibodies associated with better clinical outcomes. Front Immunol. (2022) 13:945021. doi: 10.3389/fimmu.2022.945021
328. Peluso M, Mitchell A, Wang C, Takahashi S, Hoh R, Tai V, et al. Low prevalence of interferon-α autoantibodies in people experiencing long COVID symptoms. J Infect Dis. (2023) 227:246–50. doi: 10.1093/infdis/jiac372
329. Vojdani A, Ghoneum M, Choppa P, Magtoto L, Lapp C. Elevated apoptotic cell population in patients with chronic fatigue syndrome: the pivotal role of protein kinase RNA. J Intern Med. (1997) 242:465–78. doi: 10.1111/j.1365-2796.1997.tb00019.x
330. Kennedy G, Spence V, Underwood C, Belch J. Increased neutrophil apoptosis in chronic fatigue syndrome. J Clin Pathol. (2004) 57:891–3.
331. Kennedy G, Khan F, Hill A, Underwood C, Belch J. Biochemical and vascular aspects of pediatric chronic fatigue syndrome. Arch Pediatr Adolesc Med. (2010) 164:817–23. doi: 10.1001/archpediatrics.2010.157
332. Fang H, Xie Q, Boneva R, Fostel J, Perkins R, Tong W. Gene expression profile exploration of a large dataset on chronic fatigue syndrome. Pharmacogenomics. (2006) 7:429–40. doi: 10.2217/14622416.7.3.429
333. Gow J, Hagan S, Herzyk P, Cannon C, Behan P, Chaudhuri A. A gene signature for post-infectious chronic fatigue syndrome. BMC Med Genomics. (2009) 2:38. doi: 10.1186/1755-8794-2-38
334. Brenu E, Ashton K, van Driel M, Staines D, Peterson D, Atkinson G, et al. Cytotoxic lymphocyte microRNAs as prospective biomarkers for chronic fatigue syndrome/myalgic encephalomyelitis. J Affect Disord. (2012) 141:261–9. doi: 10.1016/j.jad.2012.03.037
335. Sweetman E, Kleffmann T, Edgar C, de Lange M, Vallings R, Tate W. A SWATH-MS analysis of myalgic encephalomyelitis/chronic fatigue syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J Transl Med. (2020) 18:365. doi: 10.1186/s12967-020-02533-3
336. Jahanbani F, Maynard R, Sing J, Jahanbani S, Perrino J, Spacek D, et al. Phenotypic characteristics of peripheral immune cells of myalgic encephalomyelitis/chronic fatigue syndrome via transmission electron microscopy: a pilot study. PLos One. (2022) 17:e0272703. doi: 10.1371/journal.pone.0272703
337. Swanink C, Vercoulen J, Galama J, Roos M, Meyaard L, van der Ven-Jongekrijg J, et al. Lymphocyte subsets, apoptosis, and cytokines in patients with chronic fatigue syndrome. J Infect Dis. (1996) 173:460–3.
338. Hassan I, Bannister B, Akbar A, Weir W, Bofill M. A study of the immunology of the chronic fatigue syndrome: correlation of immunologic parameters to health dysfunction. Clin Immunol Immunopathol. (1998) 87:60–7.
339. Schacterle R, Milford E, Komaroff A. The frequency of HLA class II antigens in chronic fatigue syndrome. J Chron Fatigue Synd. (2003) 11:33–42.
340. Hajdarevic R, Lande A, Rekeland I, Rydland A, Strand E, Sosa D, et al. Fine mapping of the major histocompatibility complex (MHC) in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) suggests involvement of both HLA class I and class II loci. Brain Behav Immun. (2021) 98:101–9. doi: 10.1016/j.bbi.2021.08.219
341. Underhill J, Mahalingam M, Peakman M, Wessely S. Lack of association between HLA genotype and chronic fatigue syndrome. Eur J Immunogenet. (2001) 28:425–8. doi: 10.1046/j.1365-2370.2001.00235.x
342. Ramos S, Brenu E, Broadley S, Kwiatek R, Ng J, Nguyen T, et al. Regulatory T, natural killer T and gammadelta T cells in multiple sclerosis and chronic fatigue syndrome/myalgic encephalomyelitis: a comparison. Asian Pac J Allergy Immunol. (2016) 34:300–5. doi: 10.12932/AP0733
343. Curriu M, Carillo J, Massanella M, Rigau J, Alegre J, Puig J, et al. Screening NK-, B- and T-cell phenotype and function in patients suffering from chronic fatigue syndrome. J Transl Med. (2013) 11:68. doi: 10.1186/1479-5876-11-68
344. Nguyen T, Johnston S, Chacko A, Gibson D, Cepon J, Smith P, et al. Novel characterisation of mast cell phenotypes from peripheral blood mononuclear cells in chronic fatigue syndrome/myalgic encephalomyelitis patients. Asian Pac J Allergy Immunol. (2017) 35:75–81. doi: 10.12932/ap0771
345. Balinas C, Nguyen T, Johnston S, Smith P, Staines D, Marshall-Gradisnik S. Investigation of mast cell toll-like receptor 3 in chronic fatigue syndrome/myalgic encephalomyelitis and systemic mastocytosis using the novel application of autoMACS magnetic separation and flow cytometry. Asian Pac J Allergy Immunol. (2018) 36:257–64. doi: 10.12932/AP-200517-0086
346. Nepotchatykh E, Caraus I, Elremaly W, Leveau C, Elbakry M, Godbout C, et al. Circulating microRNA expression signatures accurately discriminate myalgic encephalomyelitis from fibromyalgia and comorbid conditions. Sci Rep. (2023) 13:1896.
347. Helliwell A, Stockwell P, Edgar C, Chatterjee A, Tate W. Dynamic epigenetic changes during a relapse and recovery cycle in myalgic encephalomyelitis/chronic fatigue syndrome. Int J Mol Sci. (2022) 23:11852.
348. Zuo T, Liu Q, Zhang F, Lui G, Tso E, Yeoh Y, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. (2021) 70:276–84. doi: 10.1136/gutjnl-2020-322294
349. Gaebler C, Wang Z, Lorenzi J, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. (2021) 591:639–44.
350. Swank Z, Senussi Y, Manickas-Hill Z, Yu X, Li J, Alter G, et al. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis. (2022) 76:e487–e490.
351. Cheung C, Goh D, Lim X, Tien T, Lim J, Lee J, et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. (2022) 71:226–9.
352. Natarajan A, Zlitni S, Brooks E, Vance S, Dahlen A, Hedlin H, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med (N Y). (2022) 3:371–87.e9.
353. Tejerina F, Catalan P, Rodriguez-Grande C, Adan J, Rodriguez-Gonzalez C, Munoz P, et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect Dis. (2022) 22:211. doi: 10.1186/s12879-022-07153-4
354. Straus S, Tosato G, Armstrong G, Lawley T, Preble O, Henle W, et al. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med. (1985) 102:7–16.
355. Jones J, Ray C, Minnich L, Hicks M, Kibler R, Lucas D. Evidence for active Epstein-Barr virus infection in patients with persistent, unexplained illnesses: elevated anti-early antigen antibodies. Ann Intern Med. (1985) 102:1–7. doi: 10.7326/0003-4819-102-1-
356. Wray B, Gaughf C, Chandler F Jr., Berry S, Latham J, Wood L, et al. Detection of Epstein-Barr virus and cytomegalovirus in patients with chronic fatigue. Ann Allergy. (1993) 71:223–6.
357. Di Luca D, Zorzenon M, Mirandola P, Colle R, Botta G, Cassai E. Human herpesvirus 6 and human herpesvirus 7 in chronic fatigue syndrome. J Clin Microbiol. (1995) 33:1660–1.
358. Patnaik M, Komaroff A, Conley E, Ojo-Amaize E, Peter J. Prevalence of IgM antibodies to human herpesvirus 6 early antigen (p41/38) in patients with chronic fatigue syndrome. J Infect Dis. (1995) 172:1364–7. doi: 10.1093/infdis/172.5.1364
359. Komaroff A. Is human herpesvirus-6 a trigger for chronic fatigue syndrome? J Clin Virol. (2006) 37(Suppl. 1):S39–46. doi: 10.1016/S1386-6532(06)70010-5
360. Koelle D, Barcy S, Huang M, Ashley R, Corey L, Zeh J, et al. Markers of viral infection in monozygotic twins discordant for chronic fatigue syndrome. Clin Infect Dis. (2002) 35:518–25. doi: 10.1086/341774
361. Simonnet A, Engelmann I, Moreau A, Garcia B, Six S, El Kalioubie A, et al. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. (2021) 51:296–9. doi: 10.1016/j.idnow.2021.01.005
362. Brooks B, Tancredi C, Song Y, Mogus A, Huang M, Zhu H, et al. Epstein-Barr virus and human herpesvirus-6 reactivation in acute COVID-19 patients. Viruses. (2022) 14:1872.
363. Shafiee A, Teymouri Athar M, Amini M, Hajishah H, Siahvoshi S, Jalali M, et al. Reactivation of herpesviruses during COVID-19: a systematic review and meta-analysis. Rev Med Virol. (2023) 33:e2437.
364. Carneiro V, Alves-Leon S, Sarmento D, Coelho W, Moreira O, Salvio A, et al. Herpesvirus and neurological manifestations in patients with severe coronavirus disease. Virol J. (2022) 19:101.
365. Gold J, Okyay R, Licht W, Hurley D. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. (2021) 10:763.
366. Peluso M, Deveau T, Munter S, Ryder D, Buck A, Beck-Engeser G, et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest. (2023) 133:e163669.
367. Giloteaux L, Goodrich J, Walters W, Levine S, Ley R, Hanson M. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. (2016) 4:30. doi: 10.1186/s40168-016-0171-4
368. Nagy-Szakal D, Williams B, Mishra N, Che X, Lee B, Bateman L, et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. (2017) 5:44.
369. Guo C, Che X, Briese T, Ranjan A, Allicock O, Yates R, et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe. (2023) 31:288–304.e8. doi: 10.1016/j.chom.2023.01.004
370. Xiong R, Gunter C, Fleming E, Vernon S, Bateman L, Unutmaz D, et al. Multi-’omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients. Cell Host Microbe. (2023) 31:273–87.e5.
371. Yeoh Y, Zuo T, Lui G, Zhang F, Liu Q, Li A, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. (2021) 70:698–706. doi: 10.1136/gutjnl-2020-323020
372. Haran J, Bradley E, Zeamer A, Cincotta L, Salive M, Dutta P, et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight. (2021) 6:e152346.
373. Liu Q, Mak J, Su Q, Yeoh Y, Lui G, Ng S, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. (2022) 71:544–52.
374. Rajeevan M, Murray J, Oakley L, Lin J, Unger E. Association of chronic fatigue syndrome with premature telomere attrition. J Transl Med. (2018) 16:44. doi: 10.1186/s12967-018-1414-x
375. Sfera A, Osorio C, Zapata Martin Del Campo CM, Pereida S, Maurer S, Maldonado JC, et al. Endothelial senescence and chronic fatigue syndrome, a COVID-19 based hypothesis. Front Cell Neurosci. (2021) 15:673217. doi: 10.3389/fncel.2021.673217
376. Lynch S, Guo G, Gibson D, Bjourson A, Rai T. Role of senescence and aging in SARS-CoV-2 infection and COVID-19 Disease. Cells. (2021) 10:3367.
377. Kuo C, Pilling L, Atkins J, Masoli J, Delgado J, Tignanelli C, et al. Biological aging predicts vulnerability to COVID-19 severity in UK Biobank participants. J Gerontol A Biol Sci Med Sci. (2021) 76:e133–41. doi: 10.1093/gerona/glab060
378. Kuratsune H, Yamaguti K, Takahashi M, Misaki H, Tagawa S, Kitani T. Acylcarnitine deficiency in chronic fatigue syndrome. Clin Infect Dis. (1994) 18(Suppl. 1):S62–7.
379. Majeed T, de Simone C, Famularo G, Marcellini S, Behan P. Abnormalities of carnitine metabolism in chronic fatigue syndrome. Eur J Neurol. (1995) 2:425–8.
380. Kuratsune H, Yamaguti K, Lindh G, Evengard B, Takahashi H, Machii T, et al. Low levels of serum acylcarnitine in chronic fatigue syndrome and chronic hepatitis type C, but not seen in other diseases. Int J Mol Med. (1998) 2:51–6. doi: 10.3892/ijmm.2.1.51
381. Jones M, Goodwin C, Amjad S, Chalmers R. Plasma and urinary carnitine and acylcarnitines in chronic fatigue syndrome. Clin Chim Acta. (2005) 360:173–7.
382. Reuter S, Evans A. Long-chain acylcarnitine deficiency in patients with chronic fatigue syndrome. Potential involvement of altered carnitine palmitoyltransferase-l activity. J Intern Med. (2011) 270:76–84.
383. Armstrong C, McGregor N, Lewis D, Butt H, Gooley P. The association of fecal microbiota and fecal, blood serum and urine metabolites in myalgic encephalomyelitis/chronic fatigue syndrome. Metabolomics. (2017) 13:1–13.
384. Naviaux R, Naviaux J, Li K, Bright A, Alaynick W, Wang L, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. (2016) 113:E5472–80.
385. Germain A, Ruppert D, Levine S, Hanson M. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol Biosyst. (2017) 13:371–9.
386. Nagy-Szakal D, Barupal D, Lee B, Che X, Williams B, Kahn E, et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci Rep. (2018) 8:10056. doi: 10.1038/s41598-018-28477-9
387. Germain A, Barupal D, Levine S, Hanson M. Comprehensive circulatory metabolomics in ME/CFS reveals disrupted metabolism of acyl lipids and steroids. Metabolites. (2020) 10:34. doi: 10.3390/metabo10010034
388. Hoel F, Hoel A, Pettersen I, Rekeland I, Risa K, Alme K, et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight. (2021) 6:e149217.
389. Nkiliza A, Parks M, Cseresznye A, Oberlin S, Evans J, Darcey T, et al. Sex-specific plasma lipid profiles of ME/CFS patients and their association with pain, fatigue, and cognitive symptoms. J Transl Med. (2021) 19:370. doi: 10.1186/s12967-021-03035-6
390. Glass K, Germain A, Huang Y, Hanson M. Urine metabolomics exposes anomalous recovery after maximal exertion in female ME/CFS patients. Int J Mol Sci. (2023) 24:3685. doi: 10.3390/ijms24043685
391. de Boer E, Petrache I, Goldstein N, Olin J, Keith R, Modena B, et al. Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am J Respir Crit Care Med. (2022) 205:126–9.
392. Guntur V, Nemkov T, de Boer E, Mohning M, Baraghoshi D, Cendali F, et al. Signatures of mitochondrial dysfunction and impaired fatty acid metabolism in plasma of patients with post-acute sequelae of COVID-19 (PASC). Metabolites. (2022) 12:1026. doi: 10.3390/metabo12111026
393. Stromberg S, Baxter B, Dooley G, LaVergne S, Gallichotte E, Dutt T, et al. Relationships between plasma fatty acids in adults with mild, moderate, or severe COVID-19 and the development of post-acute sequelae. Front Nutr. (2022) 9:960409. doi: 10.3389/fnut.2022.960409
394. Yang C, Chang C, Yang C, Pariante C, Su K. Long COVID and long chain fatty acids (LCFAs): Psychoneuroimmunity implication of omega-3 LCFAs in delayed consequences of COVID-19. Brain Behav Immun. (2022) 103:19–27.
395. Jones M, Cooper E, Amjad S, Goodwin C, Barron J, Chalmers R. Urinary and plasma organic acids and amino acids in chronic fatigue syndrome. Clin Chim Acta. (2005) 361:150–8.
396. Armstrong C, McGregor N, Sheedy J, Buttfield I, Butt H, Gooley PR. NMR metabolic profiling of serum identifies amino acid disturbances in chronic fatigue syndrome. Clin Chim Acta. (2012) 413:1525–31. doi: 10.1016/j.cca.2012.06.022
397. Armstrong C, McGregor N, Butt H, Gooley P. Metabolism in chronic fatigue syndrome. In: Makowski G editor. Advances in Clinical Chemistry. Waltham, MA: Elsevier (2014). p. 121–72.
398. Armstrong C, McGregor N, Lewis D, Butt H, Gooley P. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. (2015) 11:1626–39.
399. Fluge O, Mella O, Bruland O, Risa K, Dyrstad S, Alme K, et al. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight. (2016) 1:e89376.
400. Yamano E, Sugimoto M, Hirayama A, Kume S, Yamato M, Jin G, et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci Rep. (2016) 6:34990. doi: 10.1038/srep34990
401. Huth T, Eaton-Fitch N, Staines D, Marshall-Gradisnik S. A systematic review of metabolomic dysregulation in chronic fatigue syndrome/myalgic encephalomyelitis/systemic exertion intolerance disease (CFS/ME/SEID). J Transl Med. (2020) 18:198.
402. Groven N, Reitan S, Fors E, Guzey I. Kynurenine metabolites and ratios differ between chronic fatigue syndrome, fibromyalgia, and healthy controls. Psychoneuroendocrinology. (2021) 131:105287. doi: 10.1016/j.psyneuen.2021.105287
403. Simonato M, Dall’Acqua S, Zilli C, Sut S, Tenconi R, Gallo N, et al. Tryptophan metabolites, cytokines, and fatty acid binding protein 2 in myalgic encephalomyelitis/chronic fatigue syndrome. Biomedicines. (2021) 9:1724. doi: 10.3390/biomedicines9111724
404. Holmes E, Wist J, Masuda R, Lodge S, Nitschke P, Kimhofer T, et al. Incomplete systemic recovery and metabolic phenoreversion in post-acute-phase nonhospitalized COVID-19 patients: implications for assessment of post-acute COVID-19 syndrome. J Proteome Res. (2021) 20:3315–29. doi: 10.1021/acs.jproteome.1c00224
405. Nataf S, Pays L. Molecular insights into SARS-CoV2-induced alterations of the gut/brain axis. Int J Mol Sci. (2021) 22:10440. doi: 10.3390/ijms221910440
406. Ciregia F, Kollipara L, Giusti L, Zahedi R, Giacomelli C, Mazzoni M, et al. Bottom-up proteomics suggests an association between differential expression of mitochondrial proteins and chronic fatigue syndrome. Transl Psychiatry. (2016) 6:e904. doi: 10.1038/tp.2016.184
407. Germain A, Ruppert D, Levine S, Hanson M. Prospective biomarkers from plasma metabolomics of myalgic encephalomyelitis/chronic fatigue syndrome implicate redox imbalance in disease symptomatology. Metabolites. (2018) 8:90. doi: 10.3390/metabo8040090
408. Jason L, Conroy K, Furst J, Vasan K, Katz B. Pre-illness data reveals differences in multiple metabolites and metabolic pathways in those who do and do not recover from infectious mononucleosis. Mol Omics. (2022) 18:662–5. doi: 10.1039/d2mo00124a
409. McGregor N, Armstrong C, Lewis D, Gooley P. Post-exertional malaise is associated with hypermetabolism, hypoacetylation and purine metabolism deregulation in ME/CFS cases. Diagnostics (Basel). (2019) 9:E70. doi: 10.3390/diagnostics9030070
410. Lawson N, Hsieh C, March D, Wang X. Elevated energy production in chronic fatigue syndrome patients. J Nat Sci. (2016) 2:e221.
411. Tomas C, Brown A, Strassheim V, Elson J, Newton J, Manning P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLos One. (2017) 12:e0186802. doi: 10.1371/journal.pone.0186802
412. Tomas C, Brown A, Newton J, Elson J. Mitochondrial complex activity in permeabilised cells of chronic fatigue syndrome patients using two cell types. PeerJ. (2019) 7:e6500. doi: 10.7717/peerj.6500
413. Tomas C, Elson J, Newton J, Walker M. Substrate utilisation of cultured skeletal muscle cells in patients with CFS. Sci Rep. (2020) 10:18232.
414. Tomas C, Elson J, Strassheim V, Newton J, Walker M. The effect of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) severity on cellular bioenergetic function. PLos One. (2020) 15:e0231136. doi: 10.1371/journal.pone.0231136
415. Missailidis D, Annesley S, Allan C, Sanislav O, Lidbury B, Lewis D, et al. An isolated Complex V inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int J Mol Sci. (2020) 21:1074. doi: 10.3390/ijms21031074
416. Missailidis D, Sanislav O, Allan C, Smith P, Annesley S, Fisher P. Dysregulated provision of oxidisable substrates to the mitochondria in ME/CFS lymphoblasts. Int J Mol Sci. (2021) 22:2046.
417. Maes M, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med Sci Monit. (2011) 17:SC11–5.
418. Robinson M, Gray S, Watson M, Kennedy G, Hill A, Belch J, et al. Plasma IL-6, its soluble receptors and F2-isoprostanes at rest and during exercise in chronic fatigue syndrome. Scand J Med Sci Sports. (2010) 20:282–90. doi: 10.1111/j.1600-0838.2009.00895.x
419. Kennedy G, Spence V, McLaren M, Hill A, Underwood C, Belch J. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. (2005) 39:584–9.
420. Block T, Kuo J. Rationale for nicotinamide adenine dinucleotide (n.d.) metabolome disruption as a pathogenic mechanism of post-acute COVID-19 syndrome. Clin Pathol (2022) 15:2632010X221106986.
421. Al-Hakeim H, Al-Rubaye H, Al-Hadrawi D, Almulla A, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry. (2023) 28:564–78.
422. Vollbracht C, Kraft K. Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue. Nutrients. (2021) 13:1154.
423. Izzo R, Trimarco V, Mone P, Aloe T, Capra Marzani M, Diana A, et al. Combining L-arginine with vitamin C improves long-COVID symptoms: the LINCOLN Survey. Pharmacol Res. (2022) 183:106360. doi: 10.1016/j.phrs.2022.106360
424. Miwa K, Fujita M. Increased oxidative stress suggested by low serum vitamin E concentrations in patients with chronic fatigue syndrome. Int J Cardiol. (2009) 136:238–9.
425. Jammes Y, Guillaume Steinberg J, Guieu R, Delliaux S. Chronic fatigue syndrome with history of severe infection combined altered blood oxidant status, and reduced potassium efflux and muscle excitability at exercise. Open J Int Med. (2013) 3:98–105.
426. Fenouillet E, Vigouroux A, Steinberg J, Chagvardieff A, Retornaz F, Guieu R, et al. Association of biomarkers with health-related quality of life and history of stressors in myalgic encephalomyelitis/chronic fatigue syndrome patients. J Transl Med. (2016) 14:251. doi: 10.1186/s12967-016-1010-x
427. Maes M, Mihaylova I, Leunis J. Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neoepitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett. (2006) 27:615–21.
428. Morris G, Berk M, Klein H, Walder K, Galecki P, Maes M. Nitrosative stress, hypernitrosylation, and autoimmune responses to nitrosylated proteins: new pathways in neuroprogressive disorders including depression and chronic fatigue syndrome. Mol Neurobiol. (2017) 54:4271–91. doi: 10.1007/s12035-016-9975-2
429. Monro J, Puri BK. A molecular neurobiological approach to understanding the aetiology of chronic fatigue syndrome (myalgic encephalomyelitis or systemic exertion intolerance disease) with treatment implications. Mol Neurobiol. (2018) 55:7377–88. doi: 10.1007/s12035-018-0928-9
430. Suárez A, Guillamó E, Roig T, Blázquez A, Alegre J, Bermúdez J, et al. Nitric oxide metabolite production during exercise in chronic fatigue syndrome: a case-control study. J Womens Health. (2010) 19:1073–7. doi: 10.1089/jwh.2008.1255
431. Morris G, Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol. (2014) 12:168–85.
432. Gottschalk G, Peterson D, Knox K, Maynard M, Whelan R, Roy A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol Cell Neurosci. (2022) 120:103731. doi: 10.1016/j.mcn.2022.103731
433. Shungu D, Weiduschat N, Murrough J, Mao X, Pillemer S, Dyke J, et al. Increased ventricular lactate in chronic fatigue syndrome. III. relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. (2012) 25:1073–87. doi: 10.1002/nbm.2772
434. Natelson B, Vu D, Coplan J, Mao X, Blate M, Kang G, et al. Elevations of ventricular lactate levels occur in both chronic fatigue syndrome and fibromyalgia. Fatigue. (2017) 5:15–20.
435. Georgiades E, Behan W, Kilduff L, Hadjicharalambous M, Mackie E, Wilson J, et al. Chronic fatigue syndrome: new evidence for a central fatigue disorder. Clin Sci. (2003) 105:213–8.
436. Badawy A, Morgan C, Llewelyn M, Albuquerque S, Farmer A. Heterogeneity of serum tryptophan concentration and availability to the brain in patients with chronic fatigue syndrome. J Psychopharm. (2005) 19:385–91.
437. Thomas T, Stefanoni D, Reisz J, Nemkov T, Bertolone L, Francis R, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. (2020) 5:e140327.
438. Ruffieux H, Hanson A, Lodge S, Lawler N, Whiley L, Gray N, et al. A patient-centric modeling framework captures recovery from SARS-CoV-2 infection. Nat Immunol. (2023) 24:349–58. doi: 10.1038/s41590-022-01380-2
439. Russell A, Hepgul N, Nikkheslat N, Borsini A, Zajkowska Z, Moll N, et al. Persistent fatigue induced by interferon-alpha: a novel, inflammation-based, proxy model of chronic fatigue syndrome. Psychoneuroendocrinology. (2019) 100:276–85. doi: 10.1016/j.psyneuen.2018.11.032
440. van Campen C, Visser F. Comparing idiopathic chronic fatigue and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in males: response to two-day cardiopulmonary exercise testing protocol. Healthcare (Basel). (2021) 9:683.
441. van Campen C, Visser F. Female patients with myalgic encephalomyelitis/chronic fatigue syndrome or idiopathic chronic fatigue: comparison of responses to a two-day cardiopulmonary exercise testing protocol. Healthcare (Basel). (2021) 9:682.
442. Franklin J, Atkinson G, Atkinson J, Batterham A. Peak oxygen uptake in chronic fatigue syndrome/myalgic encephalomyelitis: a meta-analysis. Int J Sports Med. (2018) 40:77–87.
443. Lim E, Kang E, Jang E, Son C. The prospects of the two-day cardiopulmonary exercise test (CPET) in ME/CFS patients: a meta-analysis. J Clin Med. (2020) 9:4040.
444. Davenport T, Stevens S, Stevens J, Snell C, Van Ness J, Mooney A. Properties of measurements obtained during cardiopulmonary exercise testing in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Work. (2020) 66:247–56.
445. Cook D, Nagelkirk P, Poluri A, Mores J, Natelson B. The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis Rheum. (2006) 54:3351–62. doi: 10.1002/art.22124
446. Cook D, VanRiper S, Dougherty R, Lindheimer J, Falvo M, Chen Y, et al. Cardiopulmonary, metabolic, and perceptual responses during exercise in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a multi-site clinical assessment of ME/CFS (MCAM) sub-study. PLos One. (2022) 17:e0265315. doi: 10.1371/journal.pone.0265315
447. Mancini D, Brunjes D, Lala A, Trivieri M, Contreras J, Natelson B. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. (2021) 9:927–37. doi: 10.1016/j.jchf.2021.10.002
448. Clavario P, De Marzo V, Lotti R, Barbara C, Porcile A, Russo C, et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol. (2021) 340:113–8.
449. Vonbank K, Lehmann A, Bernitzky D, Gysan M, Simon S, Schrott A, et al. Predictors of prolonged cardiopulmonary exercise impairment after COVID-19 infection: a prospective observational study. Front Med. (2021) 8:773788. doi: 10.3389/fmed.2021.773788
450. Vannini L, Quijada-Fumero A, Martin M, Pina N, Afonso J. Cardiopulmonary exercise test with stress echocardiography in COVID-19 survivors at 6 months follow-up. Eur J Intern Med. (2021) 94:101–4. doi: 10.1016/j.ejim.2021.10.004
451. Szekely Y, Lichter Y, Sadon S, Lupu L, Taieb P, Banai A, et al. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr. (2021) 34:1273–84.e9.
452. Skjorten I, Ankerstjerne O, Trebinjac D, Bronstad E, Rasch-Halvorsen O, Einvik G, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. (2021) 58:2100996.
453. Rinaldo R, Mondoni M, Parazzini E, Baccelli A, Pitari F, Brambilla E, et al. Severity does not impact on exercise capacity in COVID-19 survivors. Respir Med. (2021) 187:106577.
454. Rinaldo R, Mondoni M, Parazzini E, Pitari F, Brambilla E, Luraschi S, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. (2021) 58:2100870.
455. Motiejunaite J, Balagny P, Arnoult F, Mangin L, Bancal C, Vidal-Petiot E, et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur Respir J. (2021) 58:2101578.
456. Mohr A, Dannerbeck L, Lange T, Pfeifer M, Blaas S, Salzberger B, et al. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidisc Resp Med. (2021) 16:732. doi: 10.4081/mrm.2021.732
457. Debeaumont D, Boujibar F, Ferrand-Devouge E, Artaud-Macari E, Tamion F, Gravier F, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther. (2021) 101:1–9. doi: 10.1093/ptj/pzab099
458. Cassar M, Tunnicliffe E, Petousi N, Lewandowski A, Xie C, Mahmod M, et al. Symptom persistence despite improvement in cardiopulmonary health - Insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine. (2021) 41:101159. doi: 10.1016/j.eclinm.2021.101159
459. Aparisi A, Ladron R, Ybarra-Falcon C, Tobar J, San Roman J. Exercise intolerance in post-acute sequelae of COVID-19 and the value of cardiopulmonary exercise testing- a mini-review. Front Med. (2022) 9:924819. doi: 10.3389/fmed.2022.924819
460. Margalit I, Yelin D, Sagi M, Rahat M, Sheena L, Mizrahi N, et al. Risk factors and multidimensional assessment of long coronavirus disease fatigue: a nested case-control study. Clin Infect Dis. (2022) 75:1688–97. doi: 10.1093/cid/ciac283
461. Longobardi I, Prado D, Goessler K, Meletti M, de Oliveira Junior G, de Andrade D, et al. Oxygen uptake kinetics and chronotropic responses to exercise are impaired in survivors of severe COVID-19. Am J Physiol Heart Circ Physiol. (2022) 323:H569–76. doi: 10.1152/ajpheart.00291.2022
462. Ladlow P, O’Sullivan O, Bennett A, Barker-Davies R, Houston A, Chamley R, et al. The effect of medium-term recovery status after COVID-19 illness on cardiopulmonary exercise capacity in a physically active adult population. J Appl Physiol. (2022) 132:1525–35. doi: 10.1152/japplphysiol.00138.2022
463. Ladlow P, O’Sullivan O, Houston A, Barker-Davies R, May S, Mills D, et al. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms but is associated with objective functional limitations. Heart Rhythm. (2022) 19:613–20.
464. Singh I, Joseph P, Heerdt P, Cullinan M, Lutchmansingh D, Gulati M, et al. Persistent exertional intolerance after COVID-19. insights from invasive cardiopulmonary exercise testing. Chest. (2022) 161:54–63.
465. Kimmig L, Rako Z, Ziegler S, Richter M, Ashkan Tolou GS, Roller F, et al. Long-term comprehensive cardiopulmonary phenotyping of COVID-19. Respir Res. (2022) 23:263.
466. Alba G, Ziehr D, Rouvina J, Hariri L, Knipe R, Medoff B, et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. EClinicalMedicine. (2021) 39:101066.
467. Wood G, Kirkevang T, Agergaard J, Leth S, Hansen E, Laustsen C, et al. Cardiac performance and cardiopulmonary fitness after infection with SARS-CoV-2. Front Cardiovasc Med. (2022) 9:871603. doi: 10.3389/fcvm.2022.871603
468. van Voorthuizen E, van Helvoort H, Peters J, van den Heuvel M, van den Borst B. Persistent exertional dyspnea and perceived exercise intolerance after mild COVID-19: a critical role for breathing dysregulation? Phys Ther. (2022) 102:zac105.
469. Kersten J, Hoyo L, Wolf A, Hull E, Nunn S, Tadic M, et al. Cardiopulmonary exercise testing distinguishes between post-COVID-19 as a dysfunctional syndrome and organ pathologies. Int J Environ Res Public Health. (2022) 19:11421.
470. Beaudry R, Brotto A, Varughese R, de Waal S, Fuhr D, Damant R, et al. Persistent dyspnea after COVID-19 is not related to cardiopulmonary impairment; a cross-sectional study of persistently dyspneic COVID-19, non-dyspneic COVID-19 and controls. Front Physiol. (2022) 13:917886. doi: 10.3389/fphys.2022.917886
471. Durstenfeld M, Sun K, Tahir P, Peluso M, Deeks S, Aras M, et al. Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: a systematic review and meta-analysis. JAMA Netw Open. (2022) 5:e2236057.
472. Jones D, Hollingsworth K, Jakovljevic D, Fattakhova G, Pairman J, Blamire A, et al. Loss of capacity to recover from acidosis on repeat exercise in chronic fatigue syndrome: a case-control study. Eur J Clin Invest. (2012) 42:186–94. doi: 10.1111/j.1365-2362.2011.02567.x
473. Lien K, Johansen B, Veierod M, Haslestad A, Bohn S, Melsom M, et al. Abnormal blood lactate accumulation during repeated exercise testing in myalgic encephalomyelitis/chronic fatigue syndrome. Physiol Rep. (2019) 7:e14138.
474. Germain A, Giloteaux L, Moore G, Levine S, Chia J, Keller B, et al. Plasma metabolomics reveals disrupted response and recovery following maximal exercise in myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight. (2022) 7:e157621.
475. Van Booven D, Gamer J, Joseph A, Perez M, Zarnowski O, Pandya M, et al. Stress-induced transcriptomic changes in females with myalgic encephalomyelitis/chronic fatigue syndrome reveal disrupted immune signatures. Int J Mol Sci. (2023) 24:2698.
476. Tsilioni I, Natelson B, Theoharides T. Exosome-associated mitochondrial DNA from patients with myalgic encephalomyelitis/chronic fatigue syndrome stimulates human microglia to release IL-1beta. Eur J Neurosci. (2022) 56:5784–94. doi: 10.1111/ejn.15828
477. Shukla S, Cook D, Meyer J, Vernon S, Le T, Clevidence D, et al. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLos One. (2015) 10:e0145453. doi: 10.1371/journal.pone.0145453
478. Joseph P, Pari R, Miller S, Warren A, Stovall M, Squires J, et al. Neurovascular dysregulation and acute exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome: a randomized, placebo-controlled trial of pyridostigmine. Chest. (2022) 162:1116–26. doi: 10.1016/j.chest.2022.04.146
479. Streeten D, Thomas D, Bell D. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. (2000) 320:1–8. doi: 10.1097/00000441-200007000-00001
480. Farquhar W, Hunt B, Taylor J, Darling S, Freeman R. Blood volume and its relation to peak O2 consumption and physical activity in patients with chronic fatigue. Am J Physiol Heart Circ Physiol. (2002) 282:H66–71. doi: 10.1152/ajpheart.2002.282.1.H66
481. Stewart J. Chronic fatigue syndrome: comments on deconditioning, blood volume and resulting cardiac function. Clin Sci. (2009) 118:121–3.
482. Hurwitz B, Coryell V, Parker M, Martin P, Laperriere A, Klimas N, et al. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin Sci (Lond). (2010) 118:125–35.
483. Newton J, Finkelmeyer A, Petrides G, Frith J, Hodgson T, Maclachlan L, et al. Reduced cardiac volumes in chronic fatigue syndrome associate with plasma volume but not length of disease: a cohort study. Open Heart. (2016) 3:e000381.
484. van Campen C, Rowe P, Visser F. Blood volume status in ME/CFS correlates with the presence or absence of orthostatic symptoms: preliminary results. Front Pediatr. (2018) 6:352. doi: 10.3389/fped.2018.00352
485. Moore Y, Serafimova T, Anderson N, King H, Richards A, Brigden A, et al. Recovery from chronic fatigue syndrome: a systematic review-heterogeneity of definition limits study comparison. Arch Dis Child. (2021) 106:1087–94. doi: 10.1136/archdischild-2020-320196
486. Newton D, Kennedy G, Chan K, Lang C, Belch J, Khan F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol. (2012) 154:335–6.
487. Scherbakov N, Szklarski M, Hartwig J, Sotzny F, Lorenz S, Meyer A, et al. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. (2020) 7:1064–71.
488. Fluge Ø, Tronstad KJ, Mella O. Pathomechanisms and possible interventions in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Clin Invest (2021) 131:e150377.
489. Blauensteiner J, Bertinat R, León L, Riederer M, Sepúlveda N, Westermeier F. Altered endothelial dysfunction-related miRs in plasma from ME/CFS patients. Sci Rep. (2021) 11:10604.
490. Bond J, Nielsen T, Hodges L. Effects of post-exertional malaise on markers of arterial stiffness in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Int J Environ Res Public Health. (2021) 18:2366. doi: 10.3390/ijerph18052366
491. Sørland K, Sandvik M, Rekeland I, Ribu L, Småstuen M, Mella O, et al. Reduced endothelial function in myalgic encephalomyelitis/chronic fatigue syndrome–results from open-label cyclophosphamide intervention study. Front Med. (2021) 8:642710. doi: 10.3389/fmed.2021.642710
492. Bertinat R, Villalobos-Labra R, Hofmann L, Blauensteiner J, Sepulveda N, Westermeier F. Decreased NO production in endothelial cells exposed to plasma from ME/CFS patients. Vascul Pharmacol. (2022) 143:106953. doi: 10.1016/j.vph.2022.106953
493. Lubell J. Letter: could endothelial dysfunction and vascular damage contribute to pain, inflammation and post-exertional malaise in individuals with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)? J Transl Med. (2022) 20:40. doi: 10.1186/s12967-022-03244-7
494. Nunes J, Kruger A, Proal A, Kell D, Pretorius E. The occurrence of hyperactivated platelets and fibrinaloid microclots in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Pharmaceuticals (Basel). (2022) 15:931. doi: 10.3390/ph15080931
495. Sandvik M, Sorland K, Leirgul E, Rekeland I, Stavland C, Mella O, et al. Endothelial dysfunction in ME/CFS patients. PLos One. (2023) 18:e0280942. doi: 10.1371/journal.pone.0280942
496. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. (2020) 41:3038–44.
497. Rutkai I, Mayer M, Hellmers L, Ning B, Huang Z, Monjure C, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. (2022) 13:1745.
498. Oikonomou E, Souvaliotis N, Lampsas S, Siasos G, Poulakou G, Theofilis P, et al. Endothelial dysfunction in acute and long standing COVID-19: a prospective cohort study. Vascul Pharmacol. (2022) 144:106975.
499. Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. (2021) 19:2546–53.
500. Ambrosino P, Calcaterra I, Molino A, Moretta P, Lupoli R, Spedicato G, et al. Persistent endothelial dysfunction in post-acute COVID-19 syndrome: a case-control study. Biomedicines. (2021) 9:957. doi: 10.3390/biomedicines9080957
501. Santoro L, Falsetti L, Zaccone V, Nesci A, Tosato M, Giupponi B, et al. Impaired endothelial function in convalescent phase of COVID-19: a 3 month follow up observational prospective study. J Clin Med. (2022) 11:1774. doi: 10.3390/jcm11071774
502. Patel M, Knauer M, Nicholson M, Daley M, Van Nynatten L, Martin C, et al. Elevated vascular transformation blood biomarkers in long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol Med. (2022) 28:122. doi: 10.1186/s10020-022-00548-8
503. Flaskamp L, Roubal C, Uddin S, Sotzny F, Kedor C, Bauer S, et al. Serum of post-COVID-19 syndrome patients with or without ME/CFS differentially affects endothelial cell function in vitro. Cells. (2022) 11:2376.
504. Kruger A, Vlok M, Turner S, Venter C, Laubscher G, Kell D, et al. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. (2022) 21:190.
505. Beltran-Camacho L, Eslava-Alcon S, Rojas-Torres M, Sanchez-Morillo D, Martinez-Nicolas M, Martin-Bermejo V, et al. The serum of COVID-19 asymptomatic patients up-regulates proteins related to endothelial dysfunction and viral response in circulating angiogenic cells ex-vivo. Mol Med. (2022) 28:40.
506. Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N, et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med. (2022) 20:138. doi: 10.1186/s12967-022-03346-2
507. Marcic M, Marcic L, Lovric Kojundzic S, Marinovic Guic M, Marcic B, Caljkusic K. Chronic endothelial dysfunction after COVID-19 infection shown by transcranial color-coded Doppler: a cross-sectional study. Biomedicines. (2022) 10:2550. doi: 10.3390/biomedicines10102550
508. Tosato M, Calvani R, Picca A, Ciciarello F, Galluzzo V, Coelho-Junior H, et al. Effects of L-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: a single-blind randomized controlled trial. Nutrients. (2022) 14:4984. doi: 10.3390/nu14234984
509. Kell D, Laubscher G, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. (2022) 479:537–59. doi: 10.1042/BCJ20220016
510. Grobbelaar L, Kruger A, Venter C, Burger E, Laubscher G, Maponga T, et al. Relative hypercoagulopathy of the SARS-CoV-2 beta and delta variants when compared to the less severe omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Hemost. (2022) 48:858–68. doi: 10.1055/s-0042-1756306
511. Groff D, Sun A, Ssentongo A, Ba D, Parsons N, Poudel G, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. (2021) 4:e2128568.
512. Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respiratory Med. (2022) 10:863–76.
513. González-Hermosillo J, Martínez-López J, Carrillo-Lampón S, Ruiz-Ojeda D, Herrera-Ramírez S, Amezcua-Guerra L, et al. Post-acute COVID-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: a 6-month survey in a Mexican cohort. Brain Sci. (2021) 11:760. doi: 10.3390/brainsci11060760
514. Mirfazeli F, Sarabi-Jamab A, Pereira-Sanchez V, Kordi A, Shariati B, Shariat S, et al. Chronic fatigue syndrome and cognitive deficit are associated with acute-phase neuropsychiatric manifestations of COVID-19: a 9-month follow-up study. Neurol Sci. (2022) 43:2231–9.
515. Bonilla H, Quach T, Tiwari A, Bonilla A, Miglis M, Yang P, et al. Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic. Front Neurol. (2023) 14:1090747. doi: 10.3389/fneur.2023.1090747
518. Komaroff A, Buchwald D. Symptoms and signs of chronic fatigue syndrome. Rev Infect Dis. (1991) 13:S8–11.
519. Shelokov A, Habel K, Verder E, Welsh W. Epidemic neuromyasthenia: an outbreak of poliomyelitislike illness in student nurses. N Engl J Med. (1957) 257:345–55. doi: 10.1056/NEJM195708222570801
520. Poskanzer D, Henderson D, Kunkle E, Kalter S, Clement W, Bond J. Epidemic neuromyasthenia: an outbreak in Punta Gorda. Florida. N Engl J Med. (1957) 257: 356–64.
521. Acheson ED. The clinical syndrome variously called benign myalgic encephalomyelitis, Iceland disease and epidemic neuromyasthenia. Am J Med. (1959) 4:569–95. doi: 10.1016/0002-9343(59)90280-3
522. Henderson D, Shelokov A. Epidemic neuromyasthenia – clinical syndrome. N Engl J Med. (1959) 260:757–64.
523. Levine P, Jacobson S, Pocinki A, Cheney P, Peterson D, Connelly R, et al. Clinical, epidemiologic, and virologic studies in four clusters of the chronic fatigue syndrome. Arch Intern Med. (1992) 152:1611–6.
524. Medical staff of the Royal Free Hospital. An outbreak of encephalomyelitis in the Royal Free Hospital Group, London, in l955. BMJ. (1957) 2:895–904.
525. Komaroff A, Fagioli L, Doolittle T, Gandek B, Gleit M, Guerriero R, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med. (1996) 101:281–90.
526. Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med. (1996) 171:364–70.
527. Raman B, Cassar M, Tunnicliffe E, Filippini N, Griffanti L, Alfaro-Almagro F, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. (2021) 31:100683.
528. Simmons G, Glynn S, Komaroff A, Mikovits J, Tobler L, Hackett J Jr., et al. Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Science. (2011) 334:814–7.
529. Alter H, Mikovits J, Switzer W, Ruscetti F, Lo S, Klimas N, et al. A multicenter blinded analysis indicates no association between chronic fatigue syndrome/myalgic encephalomyelitis and either xenotropic murine leukemia virus-related virus or polytropic murine leukemia virus. mBio. (2012) 3:e266–212.
530. Evengård B, Briese T, Lindh G, Lee S, Lipkin W. Absence of evidence of Borna disease virus infection in Swedish patients with chronic fatigue syndrome. J Neurovirol. (1999) 5:495–9.
531. Dansie E, Furberg H, Afari N, Buchwald D, Edwards K, Goldberg J, et al. Conditions comorbid with chronic fatigue in a population-based sample. Psychosomatics. (2012) 53:44–50. doi: 10.1016/j.psym.2011.04.001
532. Bateman L, Bested A, Bonilla H, Chheda B, Chu L, Curtin J, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. (2021) 96:2861–78.
533. Davis H, Assaf G, McCorkell L, Wei H, Low R, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
534. Vernon S, Hartle M, Sullivan K, Bell J, Abbaszadeh S, Unutmaz D, et al. Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work. (2023) 74:1179–86. doi: 10.3233/WOR-220581
535. Dennis A, Cuthbertson D, Wootton D, Crooks M, Gabbay M, Eichert N, et al. Multi-organ impairment and long COVID: a 1-year prospective, longitudinal cohort study. J R Soc Med. (2023) 116:97–112. doi: 10.1177/01410768231154703
536. Nacul L, O’Boyle S, Palla L, Nacul F, Mudie K, Kingdon C, et al. How myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) progresses: the natural history of ME/CFS. Front Neurol. (2020) 11:826. doi: 10.3389/fneur.2020.00826
537. Ferrucci R, Dini M, Rosci C, Capozza A, Groppo E, Reitano M, et al. One-year cognitive follow-up of COVID-19 hospitalized patients. Eur J Neurol. (2022) 29:2006–14.
538. Wild C, Norton L, Menon D, Ripsman D, Swartz R, Owen A. Disentangling the cognitive, physical, and mental health sequelae of COVID-19. Cell Rep Med. (2022) 3:100750. doi: 10.1016/j.xcrm.2022.100750
539. Ortelli P, Benso F, Ferrazzoli D, Scarano I, Saltuari L, Sebastianelli L, et al. Global slowness and increased intra-individual variability are key features of attentional deficits and cognitive fluctuations in post COVID-19 patients. Sci Rep. (2022) 12:13123. doi: 10.1038/s41598-022-17463-x
540. Holdsworth D, Chamley R, Barker-Davies R, O’Sullivan O, Ladlow P, Mitchell J, et al. Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLos One. (2022) 17:e0267392. doi: 10.1371/journal.pone.0267392
541. Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. (2022) 604:697–707.
542. Stanculescu D, Bergquist J. Perspective: drawing on findings from critical illness to explain myalgic encephalomyelitis/chronic fatigue syndrome. Front Med. (2022) 9:818728. doi: 10.3389/fmed.2022.818728
543. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. (2020) 24:168–75.
544. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. (2022) 28:911–23.
545. Preiser J, Ichai C, Orban J, Groeneveld A. Metabolic response to the stress of critical illness. Br J Anaesth. (2014) 113:945–54.
546. Stanculescu D, Larsson L, Bergquist J. Hypothesis: mechanisms that prevent recovery in prolonged ICU patients also underlie myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front Med. (2021) 8:628029. doi: 10.3389/fmed.2021.628029
547. Stanculescu D, Sepulveda N, Lim C, Bergquist J. Lessons from heat stroke for understanding myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. (2021) 12:789784. doi: 10.3389/fneur.2021.789784
548. Komaroff A, Lipkin W. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. (2021) 27:895–906.
549. Paul B, Lemle M, Komaroff A, Snyder S. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci USA. (2021) 118:e2024358118.
550. Dantzer R, Heijnen C, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. (2014) 37:39–46.
551. Miller A, Haroon E, Felger J. The immunology of behavior-Exploring the role of the immune system in brain health and illness. Neuropsychopharmacology. (2017) 42:1–4.
552. Raizen D, Mullington J, Anaclet C, Clarke G, Critchley H, Dantzer R, et al. Beyond the symptom: the biology of fatigue. Sleep (2023) (in press).
553. Wang T, Cao Y, Zhang H, Wang Z, Man C, Yang Y, et al. COVID-19 metabolism: mechanisms and therapeutic targets. MedComm (2020). (2022) 3:e157.
554. Storey K, Storey J. Metabolic rate depression: the biochemistry of mammalian hibernation. Adv Clin Chem. (2010) 52:77–108.
555. Tessier S, Storey K. Transitioning between entry and exit from mammalian torpor: the involvement of signal transduction pathways. Temperature (Austin). (2014) 1:92–3. doi: 10.4161/temp.29972
556. Drew K, Buck C, Barnes B, Christian S, Rasley B, Harris M. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. (2007) 102:1713–26.
557. Bargmann C, Horvitz H. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. (1991) 251:1243–6. doi: 10.1126/science.2006412
558. Wang Y, Hou X. Dauer in nematodes as a way to persist or obviate. Nematropica. (2015) 45:128–37.
Keywords: chronic fatigue syndrome (CFS), myalgic encephalomyelitis (ME), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Long COVID, post-acute sequelae of SARS-CoV-2 infection, post-infectious fatigue syndrome, post-intensive care unit syndrome
Citation: Komaroff AL and Lipkin WI (2023) ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front. Med. 10:1187163. doi: 10.3389/fmed.2023.1187163
Received: 15 March 2023; Accepted: 09 May 2023;
Published: 02 June 2023.
Edited by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoReviewed by:
Jeremy S. Rossman, Research-Aid Networks, United StatesJohannes Fessler, Medical University of Graz, Austria
Copyright © 2023 Komaroff and Lipkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony L. Komaroff, a29tYXJvZmZAaG1zLmhhcnZhcmQuZWR1
 Anthony L. Komaroff
Anthony L. Komaroff W. Ian Lipkin2
W. Ian Lipkin2