- Division of Nephrology, Department of Medicine, National Defense Medical Center, Tri-Service General Hospital, Taipei, Taiwan
Background: Rare cases of de novo or relapsed kidney diseases associated with vaccination against coronavirus disease 2019 (COVID-19) have been increasingly reported. The aim of this study was to report the incidence, etiologies, and outcomes of acute kidney disease (AKD) following COVID-19 vaccination.
Methods: This retrospective study extracted cases from renal registry of a single medical center from 1 March 2021 to 30 April 2022, prior to the significant surge in cases of the Omicron variant of COVID-19 infection in Taiwan. Adult patients who developed AKD after COVID-19 vaccination were included. We utilized the Naranjo score as a causality assessment tool for adverse vaccination reactions and charts review by peer nephrologists to exclude other causes. The etiologies, characteristics, and outcomes of AKD were examined.
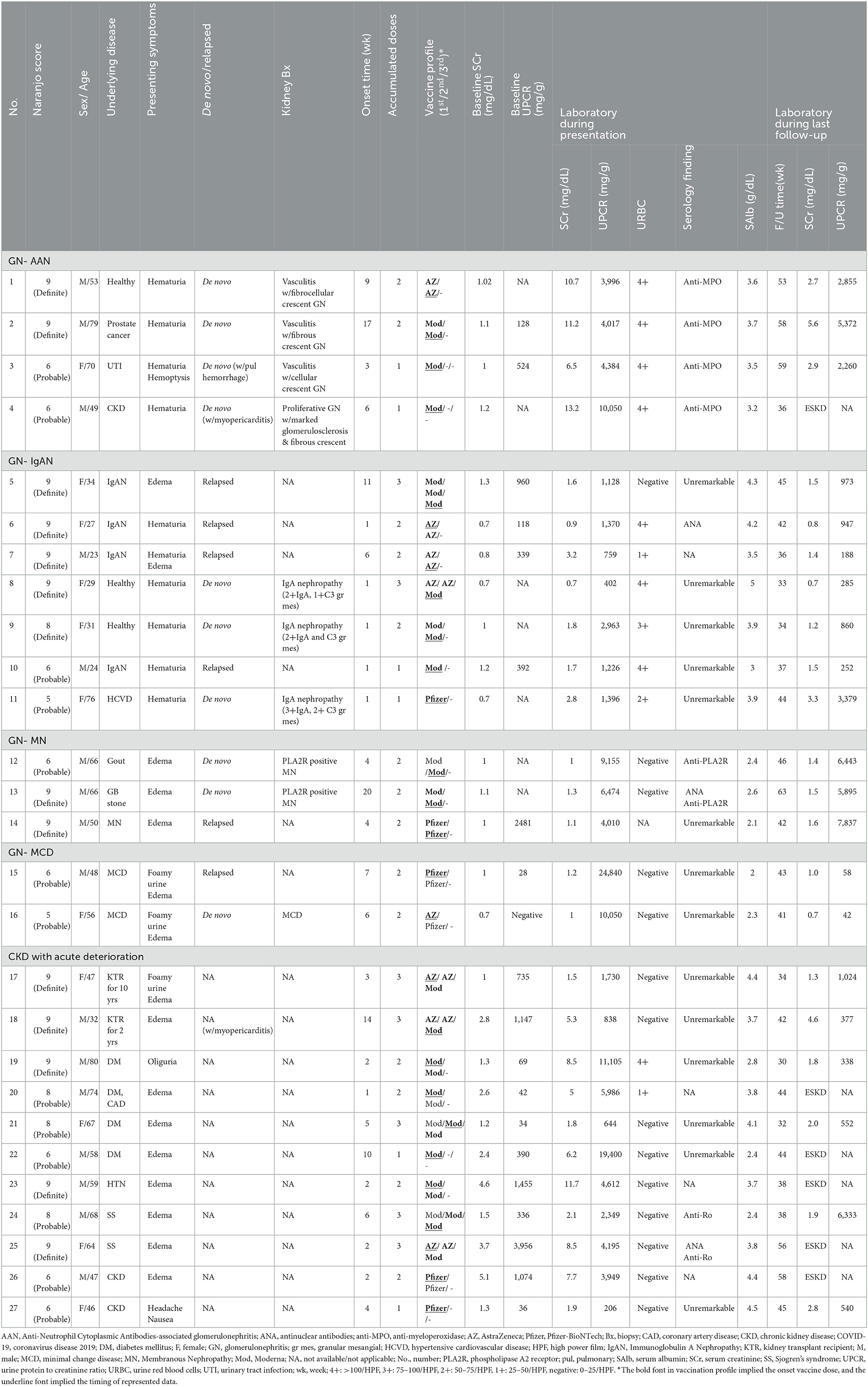
Results: Twenty-seven patients (aged 23 to 80 years) with AKD were identified from 1,897 vaccines (estimated rate of 13.6 per 1000 patient-years within the renal registry). A majority (77.8%) of vaccine received messenger RNA-based regimens. Their median (IQR) Naranjo score was 8 (6-9) points, while 14 of them (51.9%) had a definite probability (Naranjo score ≥ 9). The etiologies of AKD included glomerular disease (n = 16) consisting of seven IgA nephropathy, four anti-neutrophil cytoplasmic antibodies-associated glomerulonephritis (AAN), three membranous glomerulonephritis, two minimal change diseases, and chronic kidney disease (CKD) with acute deterioration (n = 11). Extra-renal manifestations were found in four patients. Over a median (IQR) follow-up period of 42 (36.5–49.5) weeks, six patients progressed to end-stage kidney disease (ESKD).
Conclusion: Besides glomerulonephritis (GN), the occurrence of AKD following COVID-19 vaccination may be more concerning in high-risk CKD patients receiving multiple doses. Patients with the development of de novo AAN, concurrent extra-renal manifestations, or pre-existing moderate to severe CKD may exhibit poorer kidney prognosis.
Introduction
In response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, vaccination against the coronavirus disease of 2019 (COVID-19) has been rapidly deployed under emergency use authorization (EUA) (1). Before the emergence of omicron variants in 2022, two types of vaccination against COVID-19 were approved, based on messenger RNA (mRNA) via lipid nanoparticles such as BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) (2, 3), viral vectors such as AZD1222 (Oxford-AstraZeneca), Ad26.COV2.S (Janssen), and Gam-COVID-Vac (Sputnik V) (4–6). These COVID-19 vaccines can trigger and enhance innate and adaptive immune response with the activation of neutrophils, T cells, and B cells (7, 8). With the strengthened immunity, rare serious side effects have been increasingly reported after COVID-19 vaccination. These included immune-mediated myocarditis, vaccine-induced immune thrombotic thrombocytopenia (VITT), and various neurological complications such as seizures, Guillain–Barré syndrome, transverse myelitis, and Bell's palsy (9–11).
In the kidney, growing literature had reinforced the linkage between COVID-19 vaccination and the rapid development of de novo or relapsed glomerulonephritis (GN) (12, 13). To date, the establishment of COVID-19 vaccination-related kidney diseases mostly depended on temporal association with the exclusion of other clear causes due to the lack of mechanistic proof for confirmation. Hence, there is a discrepancy in the research regarding the link between COVID-19 vaccination and the development of acute kidney disease (AKD) (14). One large retrospective study from the nationwide institute of pathology did not find a higher incidence of GN during the COVID-19 vaccination campaign (15), but a separate study from a nationwide GN registry indicated an increased risk of relapse with a second or third dose of the COVID-19 vaccine, especially the mRNA type (16). Given these diverse results, it is crucial to utilize logical and analytical tools to acquire a comprehensive evaluation of adverse vaccine events. The Naranjo score as a causality assessment tool validated to determine the likelihood of an adverse drug reaction has been used to evaluate the relationship between adverse vaccine events and vaccines (17, 18). Nevertheless, its application has not yet extended to AKD and COVID-19 vaccines. Moreover, the impact of COVID-19 vaccination on accelerating chronic kidney disease (CKD) progression was still less appreciated.
In this study, we first utilized the Naranjo score as a probability measurement tool for adverse vaccination reactions and then chart review by peer nephrologists to exclude other causes at a renal registry of a single medical center in Taiwan prior to the severe COVID-19 pandemic outbreak (19). The etiologies, characteristics, and outcomes of COVID-19 vaccination-related AKD were also examined.
Materials and methods
Patient selection
The study was approved by the Institutional Review Board (IRB) of Tri-service General Hospital, Taipei, Taiwan (IRB No. B202205155). The data of the study were based on an institutional clinical database, collected by Tri-service General Hospital Renal Registry (TSGHRR). The TSGHRR consisted of 2,426 adult patients with either a current or past occurrence of AKI or CKD, but not yet on renal replacement therapy (RRT) from both inpatient and outpatient services. In the TSGHRR, adult patients would regularly receive blood and urine examinations every 3 months. If any uncommon symptoms developed, further additional investigation will be arranged. The algorithm for the selection of AKD related to COVID-19 vaccination is shown in Figure 1. The definition of COVID-19 vaccination is based on the administration of two main approved types of vaccines against COVID-19 in Taiwan, including messenger RNA (mRNA) via lipid nanoparticles such as BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), viral vectors such as AZD1222 (Oxford-AstraZeneca), Ad26.COV2.S (Janssen). The adult patients aged 18 years or more presenting with AKD within 90 days after COVID-19 vaccination from 1 March 2021 to 30 April 2022 (before the significant surge in cases of the Omicron variant of COVID-19 in Taiwan) were preliminarily enrolled. Patients who had a confirmed history of COVID-19 infection through data from Taiwan's National Health Insurance MediCloud System (NHI MediCloud System) were excluded.
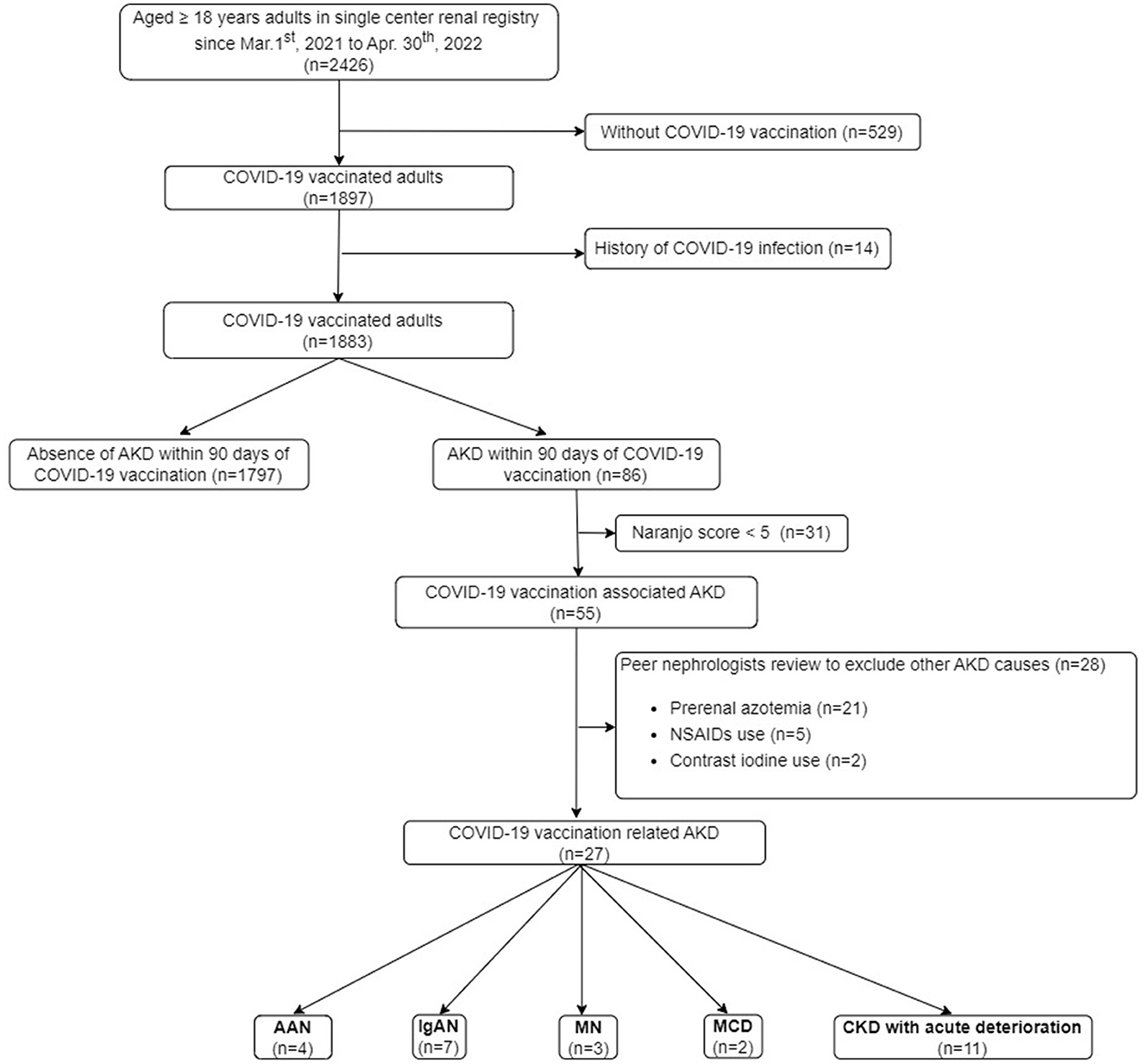
Figure 1. Flowchart of the study. AAN, anti-neutrophil cytoplasmic antibodies-associated glomerulonephritis; AKD, acute kidney disease; KTR, kidney transplant recipient; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; ESKD, end-stage kidney disease; IgAN, immunoglobulin A nephropathy; MCD, minimal change disease; MN, membranous nephropathy; NSAIDs, non-steroidal anti-inflammatory drugs; RRT, renal replacement therapy.
In order to objectively adjudicate the cause of AKD linked to vaccination for each case, Naranjo score, a causality assessment tool to determine the likelihood of an adverse drug reaction was utilized (17). The physicians computed the Naranjo score for each patient who experienced AKD within 90 days of receiving the COVID-19 vaccine. Patients with a Naranjo score of <5 (doubtful or possible probability) were excluded. Furthermore, the manual review of every chart by three peer nephrologists would also be performed to exclude other identifiable causes of AKD.
Acute kidney disease was defined as acute kidney injury (AKI) with structural damage lasting for over 7 days and persisting not yet over 90 days according to Kidney Disease Improving Global Outcomes (KDIGO) criteria (20). AKD should be also more suitable than AKI in a retrospective analysis since patients might have non-specific prodromal symptoms of kidney injury but had not been medically evaluated within 1 week (14). The term “relapsed” kidney disease was defined as recurrent biopsy-proven nephropathy that had never been present prior to vaccination. Based on the definition of “de novo” kidney disease, indeed, an undetected subclinical disease could have been uncovered after the trigger of vaccination. CKD with acute deterioration was defined as the accelerated decline of renal function without any identifiable etiologies of AKD but temporal associated with vaccination in patients with the underlying CKD, stage III (estimated glomerular filtration rate, eGFR< 60 mL/min/1.73m2) or more (21).
Study procedures
The included patients were classified according to the etiologies of AKD. Parameters including demographics, vaccine dose and type, temporality of vaccine to the onset of symptoms, laboratory and kidney biopsy data, medical history, treatments, and clinical follow-up were collected. Data from these subjects were extracted from clinical databases by electronic medical records (EMRs). The values of the estimated eGFR were calculated using the renal disease equation (IDMS-MDRD) (22). Hematuria was defined as the coexistence of ≥1+ heme by dipstick and ≥25 urine red blood cells (URBCs) per high power field. The timing of the baseline serum creatinine (SCr) and urine protein to creatinine ratio (UPCR) was recognized as the latest data before the first dose of COVID-19 vaccination. The onset time among SCr, urine analysis, UPCR, lipid profiles, and serologic exams was determined within 48 h. To describe the relationship between time course, presentation, management, and outcome of AKD after COVID-19 vaccination, some representative cases were illustrated.
Statistical analysis
Descriptive statistics were used to characterize subjects, etiologies, and laboratory data as the sample size is relatively small. We reported continuous data with median and interquartile ranges. Categorical data were found with numbers and percentages. Paired Student's t-test was compared in the patients with acute deteriorated CKD prior to and after COVID-19 vaccination.
Results
Incidence of vaccination-related AKD
As shown in Figure 1, 78.2% (1,897 out of 2,426) of the adult patients in our renal registry had been vaccinated against COVID-19. There were 86 patients developing AKD within 90 days after COVID-19 vaccination. After the exclusion of the patients with Naranjo score < 5 (n = 31) and other causes of AKD recognized by peer nephrologists (n = 28), 27 patients of COVID-19-associated AKD (estimated rate of 13.6 per 1,000 patient-years within the renal registry) were identified.
Naranjo probability score
Their median (IQR) Naranjo score was 8 (6–9). Among the 27 cases of vaccination-related AKD in this study, we found that 14 patients who scored 9 points or more on the Naranjo score (definite probability) and seven patients scoring 5–8 points (probable causality) had received two or more doses of COVID-19 vaccinations. The remaining six patients (four GN and two CKD with acute deterioration) who had received only one dose of COVID-19 vaccination had a Naranjo score of 5–6 points.
Types and doses of COVID-19 vaccination
As shown in Table 1, the most common type of COVID-19 vaccine was the mRNA (23 of 27, 85.2%). Twelve of 16 (75%) patients who developed GN and all patients with CKD with acute deterioration received mRNA vaccination. Regarding the doses of vaccination, AKD occurred after the first dose and both the first and second dose mRNA vaccination in 16 (16 of 23, 69.6%) and seven (7 of 23, 30.4%) patients, respectively. Eight of 27 (29.6%) patients receiving adenovectors vaccination developed AKD in one after the first dose and seven after both the first and second doses. Three developed AKD after the heterologous booster doses of vaccination (Cases 8, 18, and 25), and the other three after the homologous booster doses (Cases 5, 21, and 24).
Etiologies, characteristics, and outcome of vaccination-related AKD
The etiologies of AKD included GN (n = 16, 59.3%) and CKD with acute deterioration (n = 11, 40.7%) with a median (IQR) follow-up period of 42 (36.5–49.5) weeks (Table 1).
GN
They consisted of IgA nephropathy (IgAN) (n = 7, 25.9%), AAN (n = 4, 14.8%), membranous glomerulonephritis (MN) (n = 3, 11.1%), and minimal change disease (MCD) (n = 2, 6.9%). Ten of 16 (62.5%) were de novo with biopsy-proved diagnosis and the others were relapsing. Their median age was 49.5 years (range, 23–79 years) and exhibited relatively normal median (IQR) SCr of 1 (0.78–1.1) mg/dL prior to vaccination.
Four patients with de novo biopsy-proven AAN exhibited severe hematuria, nephrotic proteinuria with median (IQR) UPCR of 4,201 (4,012–5,801) mg/g, and acute renal failure with median (IQR) SCr level from baseline 1.06 (1.0–1.1) to 11.0 (9.7–11.7) mg/dL) after COVID-19 vaccination. Fibrocellular and cellular crescents were predominantly found in kidney biopsies from two AAN patients with shorter onset duration (9 and 3 weeks between the vaccination and the onset in Cases 1 and 3, respectively). However, fibrous crescents were more prominent in another two AAN patients with longer onset duration (17 and 6 weeks between the vaccination and the onset in Cases 2 and 4, respectively). They all received aggressive management including pulse steroid therapy, emergent plasma exchange, intravenous immunoglobulin (IVIG), and anti-CD20 (Rituximab) treatment (Figure 2A). One patient with severe myopericarditis rapidly progressed to ESKD (Table 1, Case 4).
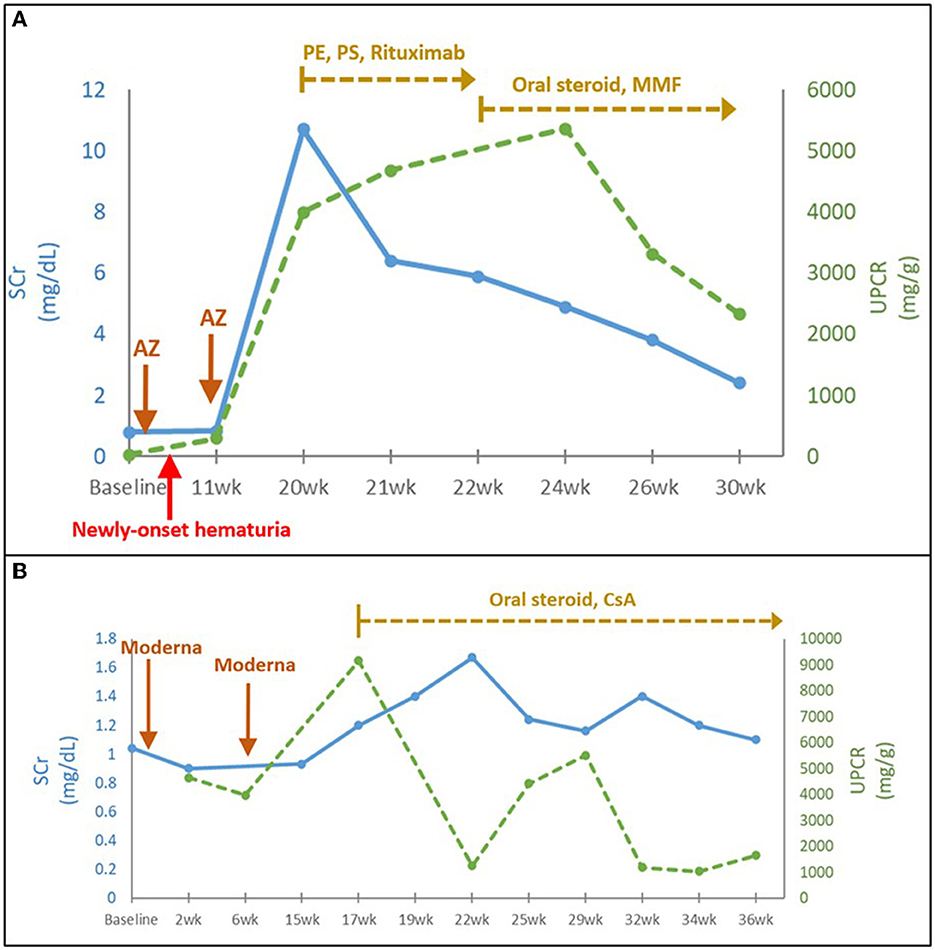
Figure 2. (A) Time course of AAN following COVID-19 vaccination (Case 1). (B) Time course of MN following COVID-19 vaccination (Case 12). AZ, AstraZeneca; CsA, cyclosporine; eGFR, estimated glomerular filtration rate; P.E., plasma exchange; P.S., pulse steroid; SCr, serum creatinine; UPCR, urine protein to creatinine ratio; wk., week.
Seven IgAN patients including three de novo, biopsy-proven and four flared ones were relatively younger median (IQR) age of 29 (25.5–32.5) years. Most patients (six of seven, 85.7%) exhibited a rapid onset of gross hematuria after vaccination, accompanied by AKI with median (IQR) SCr from 0.8 (0.7–1.1) to 1.7 (1.3–2.3) mg/dL and significant proteinuria with median (IQR) UPCR of 1,226 (944–1,383) mg/g. Kidney biopsy performed within 1 week of onset symptoms in all three patients with gross hematuria, significant proteinuria, and various renal function impairments revealed the presence of glomerular immune deposits predominantly stained for IgA and C3, indicative of early de novo IgAN (Table 1, Cases 8, 9, and 11). Steroids and/or cyclosporine were given for IgAN patients with UPCR > 2,000 mg/g and/or impaired renal function. One patient developed from AKI to advanced CKD (Case 11). Of note, the patient in Case 10 had a recurrent episode of IgAN shortly after receiving pneumococcal vaccination, whereas the patient in Case 7 experienced a recurrent episode of IgAN 6 months after contracting COVID-19 infection.
Three middle-aged MN patients consisted of two phospholipase A2 receptor antibody (PLA2R)-positive and one PLA2R-negative cases. They exhibited typical nephrotic syndrome with various degrees of AKI. Despite the immunotherapy, all MN patients had AKI transition to CKD and persistent nephrotic syndrome during the follow-up for almost 1 year (Table 1; Figure 2B). In contrast, the two middle-aged MCD patients with the typical nephrotic syndrome had achieved complete remission with intravenous and/or oral steroid treatment (Table 1).
CKD with acute deterioration
The other 11 patients with pre-existing CKD, stage IIIa to V with median (IQR) SCr of 2.4 (1.3–3.3) mg/dL had acute deteriorated renal function without any identifiable causes such as pre-renal, post-renal, drugs, infection, poor control of hypertension, and malignancy. Their median age was 64 years (range, 46–80 years) with male-to-female ratio 6:3. Among them, four individuals had underlying diabetes mellitus, two had undergone cadaveric kidney transplantation, two had autoimmune disease (Sjogren's syndrome), two had CKD of unknown cause, and one had hypertension.
Eleven patients with CKD and acute deterioration also had a higher median (IQR) Naranjo score of 8 (7–9) despite none of them receiving further histological investigation after patient-centered shared decision-making (SDM) communication. Their median (IQR) eGFR decline rate per 4 months after vaccination was significantly accelerated from 3 (1.2–4.8) mL/min/1.73 m2 [over 4 (3.6–4) months] to 10.9 (9.0–14.3) mL/min/1.73 m2 [follow-up for 4.3 (3.9–5.6) months] (Figure 3). Edema and heavy foamy urine were the most common manifestation (9 of 11, 81.8%). One of the two kidney transplant recipients (KTRs) exhibited extra-renal features with myopericarditis in addition to declined renal function (Case 18). After the strengthened immunosuppressants and pulse steroid therapy, both KTR patients had improved renal function and proteinuria, but still had a transition from AKI to worsened CKD (Cases 17 and 18) (Table 1 and Figure 4A). Of the other nine non-KTR patients, all four patients with CKD stage III had worsened kidney function after the AKD episodes (Cases 19, 21, 24, and 27), while 75% of them (3 of 4) were with repeated COVID-19 vaccination. One diabetic patient with CKD stage III but insignificant proteinuria (SCr 1.3 mg/dL, UPCR 69 mg/g) developed AKI, KDIGO stage 3, and nephrotic syndrome (SCr 8.5 mg/dL, UPCR 11,105 mg/g) after vaccination (Case 19). He achieved improved renal function after two sessions of emergent hemodialysis (HD) and pulse steroid therapy (Table 1). One patient with Sjogren's syndrome had acute deteriorated kidney function with worsened proteinuria accompanied by a marked increase anti-Ro antibody after the repeated AstraZeneca (AZ) COVID-19 vaccination (SCr 3.7 to 8.5 mg/dL, anti-Ro antibody 128 to >100,000 U/mL), she had received emergent HD shortly after the scheduled booster dose of mRNA COVID-19 vaccination despite strengthened immunosuppressants (Case 25, Figure 4C). All five non-KTR patients with pre-existing advanced CKD has worsened to ESKD (Cases 20, 22, 23, 25, and 26), while 80% of them (4 of 5) had received multiple vaccinations (Figures 4B, C).
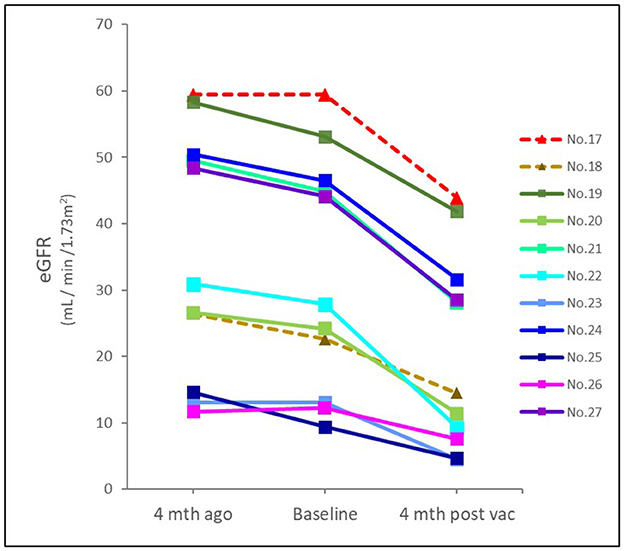
Figure 3. Time and eGFR change prior to and after COVID-19 vaccination in patients with CKD. The median (IQR) eGFR decline rate per 4 months prior and after vaccination was 3 (1.2–4.8) mL/min/1.73m2 and 10.9 (9.0–14.3) mL/min/1.73m2, respectively. Dotted line indicated KTR; solid line indicated non-KTR. eGFR, estimated glomerular filtration rate; mth, month; vac, vaccination.
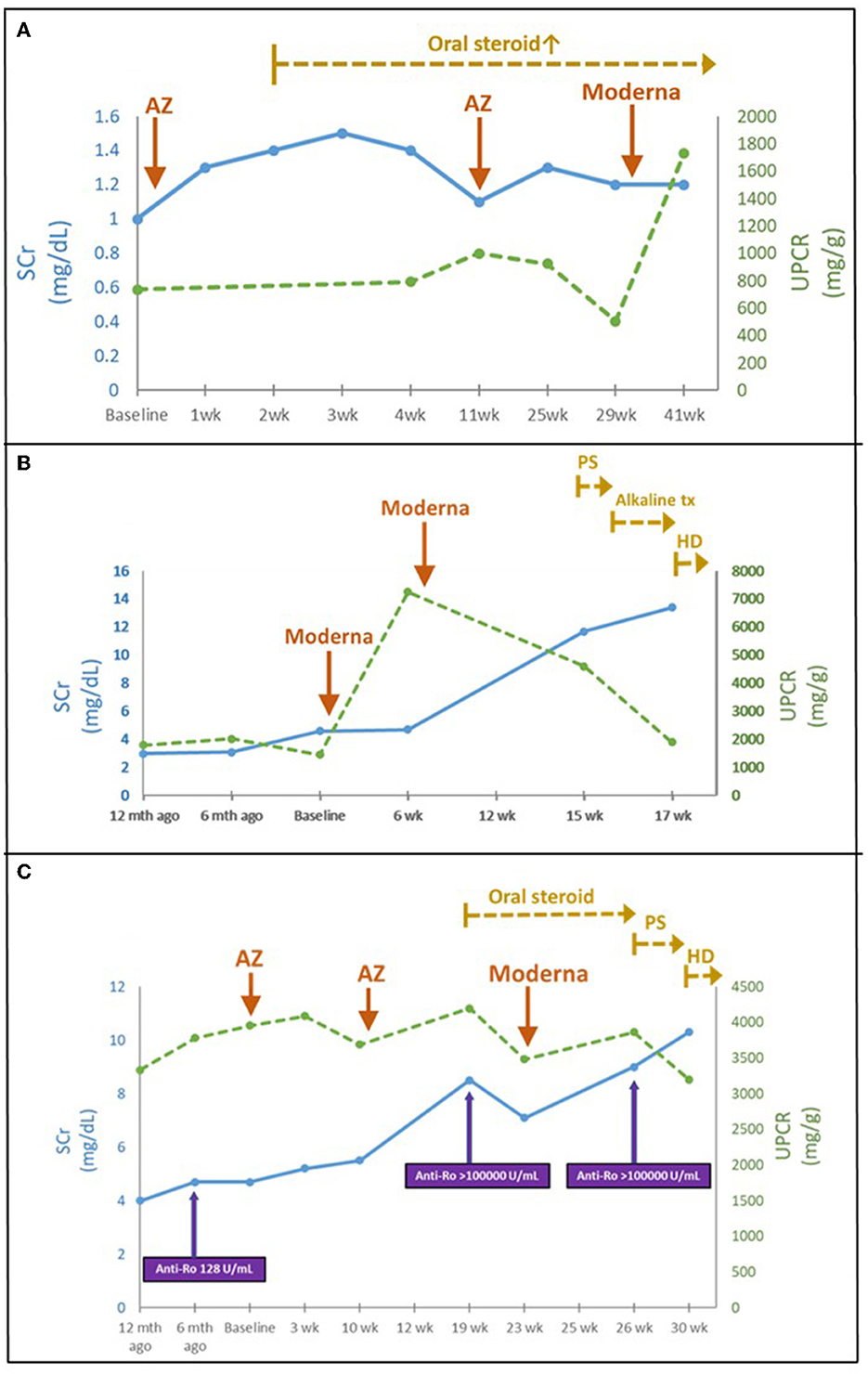
Figure 4. (A) Time course of KTR following COVID-19 vaccination (Case 17). (B) Acute deterioration of CKD following COVID-19 vaccination (Case 23). (C) Acute deterioration of CKD in a Sjogren's syndrome patient following COVID-19 vaccination (Case 25). AZ, AstraZeneca; HD, hemodialysis; Mycophenolate mofetil; P.S., pulse steroid; SCr, serum creatinine; tx, treatment; UPCR, urine protein to creatinine ratio; wk., week.
Discussion
This was the first retrospective study of AKD linked to COVID-19 vaccination using the Naranjo score and peer nephrologists' review from the renal registry of a single medical center in Taiwan. An estimated rate of 13.6 per 1,000 patient-years for AKD following COVID-19 vaccination in this single-center renal registry study may be less rare than expected, as compared with an incidence rate ratio of 0.86 per 1,000,000 person-months for GN after COVID-19 mRNA vaccination from a population-based retrospective pathological cohort study (15). IgAN and de novo AAN were the two leading causes of GN, followed by MN and MCD. Of note, approximately 40% of them had CKD with acute deterioration characterized by worsening proteinuria and renal function without identifiable causes. Four patients exhibited extra-renal manifestations (lung and heart). Even with aggressive management, patients with de novo AAN and pre-existing moderate to severe CKD had poorer renal outcomes.
The COVID-19 vaccine can be viewed as a type of drug. Like any drug, COVID-19 vaccine can cause adverse drug reactions. Due to the clinical complexity involved, it remains challenging to use an empirical approach to distinguish AKD as an adverse drug reaction to the COVID-19 vaccine from other causes. Our study had a merit and novelty to first use the Naranjo score as a standard questionnaire-designed assessment for the causality of adverse reactions to the COVID-19 vaccine. Aside from temporal association, the Naranjo score gave greater weight to the recurrence of adverse events upon the re-administration of the vaccine, making it effective in assessing the impact of repeated COVID-19 vaccinations. Using the Naranjo score, we initially identified 55 of 86 patients with AKD following COVID-19 vaccination had a Naranjo score > 5 as probable or definite causality. Of note, the validity of the obtained Naranjo score should be verified by comprehensive peer reviews since the unaware confounding factors such as volume depletion, concurrent infection, inflammation, diseases progression, and other drug-related nephrotoxicity rather than COVID-19 vaccine could have easily masked intrinsic AKD. With further peer nephrologists' reviews, we excluded 28 patients with AKD caused by other causes rather than the COVID-19 vaccine.
Prior to vast COVID-19 vaccination, glomerular diseases and AKD have been reported to be temporally associated with the immunization of vaccines such as influenza, pneumococcal, and hepatitis B (23). Similarly, AKD following COVID-19 vaccination might share similar immune-mediated mechanisms to the previously reported infection, triggering subclinical disorders and manifesting them into obvious clinical diseases (24, 25). The possible mechanisms include molecular mimicry, cross-reactivity to antigens or adjuvants, and the production of certain autoantibodies (26, 27).
In the etiology of GN associated with COVID-19 vaccination, IgAN was the most common nephritic form in this study. We observed that almost all IgAN exhibited gross hematuria (within 2–3 days) after COVID-19 vaccination, similar to the rapid development of IgAN following upper respiratory tract or gastrointestinal tract infection. The post-vaccination glomerular disease mostly evaluated by the changes in eGFR and proteinuria other than hematuria in cohort studies may make IgAN largely underestimated (16, 28). As the second most nephritic form in this renal registry, all four patients with de novo biopsy-proven AAN also exhibited nephrotic-range proteinuria to indicate podocyte injury. Apart from a high proportion of extra-renal manifestation, their renal function rapidly progressed to either ESKD or advanced CKD yet on dialysis with persistent nephrotic-range proteinuria. Given its ominous prognosis, re-administration of COVID-19 vaccine must be evaluated with more caution in AAN patients.
Like previous reports, MCD and MN were two of the most common nephrotic syndromes after COVID-19 vaccination in our study. Both patients developed MCD after the first dose, coinciding with the possible mechanisms of rapid T cells' response to foreign mRNA by producing soluble factors, leading to podocyte dysfunction (12, 29). They were highly steroid-sensitive and did not have recurrent proteinuria after the second dose, probably related to the legacy effect of steroids. In contrast, all three patients with MN exhibited a poorer response to steroid treatment, cyclosporine, and even anti-CD20 therapy.
In CKD, both T- and B-cell dysregulation can result in reduced immune response to COVID-19 vaccination and affect the efficiency and potency of COVID-19 vaccination (30). Highly potent vaccines such as mRNA vaccines were preferred (31). Nevertheless, we still found 11 CKD patients with acute deterioration who were linked with the stronger mRNA COVID-19 vaccination. They were characterized by markedly worsened CKD and proteinuria after the exclusion of other identifiable causes. It is known that many CKD patients may have co-existing autoimmune disorders, as observed in our patients, such as Sjogren's syndrome and kidney transplant recipients under immunosuppressant therapy. These patients with acute CKD might have the potential immune and autoimmune predisposition or trigger to vaccination for renal injury. It appeared that poor steroid and/or immunotherapy responsiveness with persistent or worsening proteinuria featured in poorer kidney outcomes. Since a single episode of AKI superimposed on patients with pre-existing CKD (low renal function reserve) may cause a rapid decline in renal function, early identification of high-risk CKD patients with undiscovered autoimmune disease flaring and vulnerability to COVID-19 vaccines was needed.
There were some limitations of this study. First, the patient number was still small, while other causes of GN such as lupus nephritis or anti-glomerular basement membrane glomerulonephritis were lacking. Second, given early non-specific prodromal symptoms related to vaccination, the patients with mildly asymptomatic AKD may not be evaluated. Third, due to limited generalizability and a lack of external validity, it is challenging to apply the estimated rate of vaccine-related AKD generated by a single-center renal registry to other centers. Fourth, most COVID-19 vaccines used in Taiwan were limited to BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and AZD1222 (Oxford-AstraZeneca). As the administered vaccine types were affected by the government's policy, the risk of selection bias remains inevitable.
In conclusion, in addition to GN, AKD following COVID-19 vaccination may draw greater concern among CKD patients, particularly in those who receive repeated doses. As the COVID-19 vaccination-related AKD mainly attribute to immune dysregulation, individualized immunological evaluation and patient-centered shared decision-making (SDM) communication in patients with current or past occurrence of kidney diseases are warranted before the scheduled vaccination. Comprehensive evaluation of the adverse vaccine effects via Naranjo score and peer nephrologists' review is also recommended once AKD developed following COVID-19 vaccination. Patients with the development of de novo AAN, concurrent extra-renal manifestations, or pre-existing moderate to severe CKD may exhibit poorer kidney prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Tri-Service General Hospital, Taipei, Taiwan (IRB No. B202205155). The patients/participants provided their written informed consent to participate in this study.
Author contributions
C-CC and S-HL initiated and formulated the study idea and its design. S-SY, Y-JH, C-CS, PC, C-CW, S-NH, and H-EW participated in data analysis and interpretation. D-JL prepared the figures. C-CC authored the original manuscript. S-HL supervised the study. All authors contributed to the article and approved the submitted version.
Acknowledgments
We sincerely appreciate Dr. Chia-Ter Chao for his excellent clinical advice for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Krause PR, Gruber MF. Emergency use authorization of COVID vaccines - safety and efficacy follow-up considerations. N Engl J Med. (2020) 383:e107. doi: 10.1056/NEJMp2031373
2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
3. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the MRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
4. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the Chadox1 NCOV-19 vaccine (Azd1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the Uk. Lancet. (2021) 397:99–111. doi: 10.1016/s0140-6736(20)32661-1
5. Bos R, Rutten L, van der Lubbe JEM, Bakkers MJG, Hardenberg G, Wegmann F, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. (2020) 5:91. doi: 10.1038/s41541-020-00243-x
6. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an RAD26 and RAD5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. (2021) 397:671–81. doi: 10.1016/s0140-6736(21)00234-8
7. Speiser DE, Bachmann MF. COVID-19: mechanisms of vaccination and immunity. Vaccines. (2020) 8:404. doi: 10.3390/vaccines8030404
8. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. (2021) 21:195–7. doi: 10.1038/s41577-021-00526-x
9. Arepally GM, Ortel TL. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. (2021) 138:293–8. doi: 10.1182/blood.2021012152
10. Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acute myocarditis following mrna COVID-19 vaccination. JAMA Cardiol. (2021) 6:1196–201. doi: 10.1001/jamacardio.2021.2828
11. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. (2022) 43:3–40. doi: 10.1007/s10072-021-05662-9
12. Bomback AS, Kudose S, D'Agati VD. De Novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. (2021) 78:477–80. doi: 10.1053/j.ajkd.2021.06.004
13. Izzedine H, Bonilla M, Jhaveri KD. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol Dial Transplant. (2021) 36:1565–9. doi: 10.1093/ndt/gfab215
14. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute Kidney Injury. Nat Rev Dis Primers. (2021) 7:52. doi: 10.1038/s41572-021-00284-z
15. Diebold M, Locher E, Boide P, Enzler-Tschudy A, Faivre A, Fischer I, et al. Incidence of new onset glomerulonephritis after SARS-CoV-2 MRNA vaccination is not increased. Kidney Int. (2022) 102:1409–19. doi: 10.1016/j.kint.2022.08.021
16. Canney M, Atiquzzaman M, Cunningham AM, Zheng Y, Er L, Hawken S, et al. A population-based analysis of the risk of glomerular disease relapse after COVID-19 vaccination. J Am Soc Nephrol. (2022) 33:2247–57. doi: 10.1681/ASN.2022030258
17. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. (1981) 30:239–45. doi: 10.1038/clpt.1981.154
18. Staltari O, Cilurzo F, Caroleo B, Greco A, Corasaniti F, Genovesi MA, et al. Annual report on adverse events related with vaccines use in Calabria (Italy): 2012. J Pharmacol Pharmacother. (2013) 4:S61–5. doi: 10.4103/0976-500X.120951
19. Yen MY, Yen YF, Chen SY, Lee TI, Huang KH, Chan TC, et al. Learning from the Past: Taiwan's responses to COVID-19 versus sars. Int J Infect Dis. (2021) 110:469–78. doi: 10.1016/j.ijid.2021.06.002
20. Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, et al. Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (Kdigo) conference. Kidney Int. (2020) 98:294–309. doi: 10.1016/j.kint.2020.04.020
21. Yan MT, Chao CT, Lin SH. Chronic kidney disease: strategies to retard progression. Int J Mol Sci. (2021) 22:10084. doi: 10.3390/ijms221810084
22. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. (2006) 145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004
23. Patel C, Shah HH. Vaccine-associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl. (2019) 30:1002–9. doi: 10.4103/1319-2442.270254
24. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. (2020) 98:209–18. doi: 10.1016/j.kint.2020.05.006
25. Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. (2020) 31:1959–68. doi: 10.1681/ASN.2020060802
26. Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. (2022) 165:386–401. doi: 10.1111/imm.13443
27. Jara LJ, Vera-Lastra O, Mahroum N, Pineda C, Shoenfeld Y. Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clin Rheumatol. (2022) 41:1603–9. doi: 10.1007/s10067-022-06149-4
28. Waldman M, Sinaii N, Lerma EV, Kurien AA, Jhaveri KD, Uppal NN, et al. COVID-19 vaccination and new onset glomerular disease: results from the irocgn2 international registry. Kidney360. (2023) 4:349–62. doi: 10.34067/KID.0006832022
29. Ritter A, Helmchen B, Gaspert A, Bleisch J, Fritschi B, Buchkremer F, et al. Clinical spectrum of gross haematuria following SARS-CoV-2 vaccination with MRNA vaccines. Clin Kidney J. (2022) 15:961–73. doi: 10.1093/ckj/sfab284
30. Syed-Ahmed M, Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. (2019) 26:8–15. doi: 10.1053/j.ackd.2019.01.004
Keywords: acute kidney disease (AKD), chronic kidney disease, COVID-19, glomerulonephritis (GN), Naranjo score, vaccination
Citation: Chen C-C, Yang S-S, Hsu Y-J, Sung C-C, Chu P, Wu C-C, Hsu S-N, Wang H-E, Lee D-J and Lin S-H (2023) Acute kidney disease following COVID-19 vaccination: a single-center retrospective study. Front. Med. 10:1189243. doi: 10.3389/fmed.2023.1189243
Received: 18 March 2023; Accepted: 03 May 2023;
Published: 22 May 2023.
Edited by:
Michifumi Yamashita, Cedars Sinai Medical Center, United StatesReviewed by:
Yoshihito Nihei, Juntendo University, JapanKenji Okumura, Westchester Medical Center, United States
Masaaki Yamada, The University of Iowa, United States
Copyright © 2023 Chen, Yang, Hsu, Sung, Chu, Wu, Hsu, Wang, Lee and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shih-Hua Lin, bDUyMTExNkBuZG1jdHNnaC50dw==; bDUyMTExNkBnbWFpbC5jb20=
 Chien-Chou Chen
Chien-Chou Chen Sung-Sen Yang
Sung-Sen Yang Yu-Juei Hsu
Yu-Juei Hsu Chih-Chien Sung
Chih-Chien Sung Pauling Chu
Pauling Chu Chia-Chao Wu
Chia-Chao Wu Shun-Neng Hsu
Shun-Neng Hsu Han-En Wang
Han-En Wang Shih-Hua Lin
Shih-Hua Lin