- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, China
Premature ovarian failure (POF) is an insidious cause of female infertility and a devastating condition for women. POF also has a strong familial and heterogeneous genetic background. Management of POF is complicated by the variable etiology and presentation, which are generally characterized by abnormal hormone levels, gene instability and ovarian dysgenesis. To date, abnormal regulation associated with POF has been found in a small number of genes, including autosomal and sex chromosomal genes in folliculogenesis, granulosa cells, and oocytes. Due to the complex genomic contributions, ascertaining the exact causative mechanisms has been challenging in POF, and many pathogenic genomic characteristics have yet to be elucidated. However, emerging research has provided new insights into genomic variation in POF as well as novel etiological factors, pathogenic mechanisms and therapeutic intervention approaches. Meanwhile, scattered studies of transcriptional regulation revealed that ovarian cell function also depends on specific biomarker gene expression, which can influence protein activities, thus causing POF. In this review, we summarized the latest research and issues related to the genomic basis for POF and focused on insights gained from their biological effects and pathogenic mechanisms in POF. The present integrated studies of genomic variants, gene expression and related protein abnormalities were structured to establish the role of etiological genes associated with POF. In addition, we describe the design of some ongoing clinical trials that may suggest safe, feasible and effective approaches to improve the diagnosis and therapy of POF, such as Filgrastim, goserelin, resveratrol, natural plant antitoxin, Kuntai capsule et al. Understanding the candidate genomic characteristics in POF is beneficial for the early diagnosis of POF and provides appropriate methods for prevention and drug treatment. Additional efforts to clarify the POF genetic background are necessary and are beneficial for researchers and clinicians regarding genetic counseling and clinical practice. Taken together, recent genomic explorations have shown great potential to elucidate POF management in women and are stepping from the bench to the bedside.
Introduction
Premature ovarian failure (POF), also known as premature menopause, causes absent menstruation and infertility before expected female menopause (1). It is characterized by the loss of normal ovarian function, the cessation of menstruation or loss of follicles and the cessation of follicle production (2). Approximately 1% of females are affected by sporadic POF in reproductive years under the age of 40, 1‰ before 30 years old and 0.1% before 20 years old, where familial POF presented the highest incidence frequency of nearly 12.7% (3, 4). Females with POF usually exhibit follicular atresia, hypoestrogenism and ovarian function loss and then present with primary or secondary amenorrhea and infertility (5). Clinically, diagnostic criteria are usually based on follicle-stimulating hormone (FSH) levels in the menopausal range (over 40 IU/L) and estradiol levels (less than 50 pmol/L) in females aged before 40 (6, 7). However, ovarian biopsy and ultrasound have minimal effects in the early diagnosis and screening of POF. The diagnosed POF female will suffer a serious psychosocial, economic and health burden (8). Thus, the prediagnosis of the high-risk POF population becomes particularly important for protection and treatment.
The etiology of POF is highly heterogeneous, with a wide spectrum of causes, such as genetics, living habits, the environment and infection, whereas the genetic contribution is generally considered paramount (9). POF, as one component of genetic disorders, has been widely recognized (10). An increasing number of plausible causative genes have been proposed, including genomic mutation, dysregulated gene expression and signaling cascade functions, whereas the gene framework for interpreting the etiology of POF has yet to be elucidated (11). However, the precise identification of causative genes remains a challenge. Causative genes potentially influence various aspects of the ovary, such as gonadal development, oogenesis, folliculogenesis, meiosis, DNA repair, hormone secretion, metabolism, and immune response (9, 11). Defects in multiple genes cause ovarian failure in animal models. It is reasonable to begin by searching causative genes from different angles related to POF development. With contemporary genetic strategy development, genome-wide association studies (GWASs) and genome-wide sequencing of exomes (WES) approaches provide us with more opportunities to locate susceptible loci and more candidate genes related to POF (12–14).
In particularly, for the early diagnosis of a disease, biomarkers are measurable indicators of a particular disease or physiological state of an organism. In terms of this, any epigenetic phenotype can be attributed to DNA, RNA, or protein changes, which in turn are biomarkers for a specific phenotype (15). The ideal biomarker should be easily detected in blood or primary tissues and meet regulatory approval for the test to be used to make clinically valid decisions (16). Understanding specific POF genetic mechanisms will provide guidance for the diagnosis and treatment of high-risk females.
In this review, we summarized POF-related genomic variants, transcriptome abnormalities and protein molecular functions according to recent thought-provoking articles and projects. The etiology and molecular basis of POF are highlighted through different prospective cytogenetic constitutions. In addition, some novel concepts challenging the therapeutic options for POF in clinical reproductive trials have also aroused great interest in the management of this issue. We describe the design of several ongoing clinical trials that might propose safe, feasible and effective approaches to improve the diagnosis and treatment of POF.
Genomic instability involved in POF
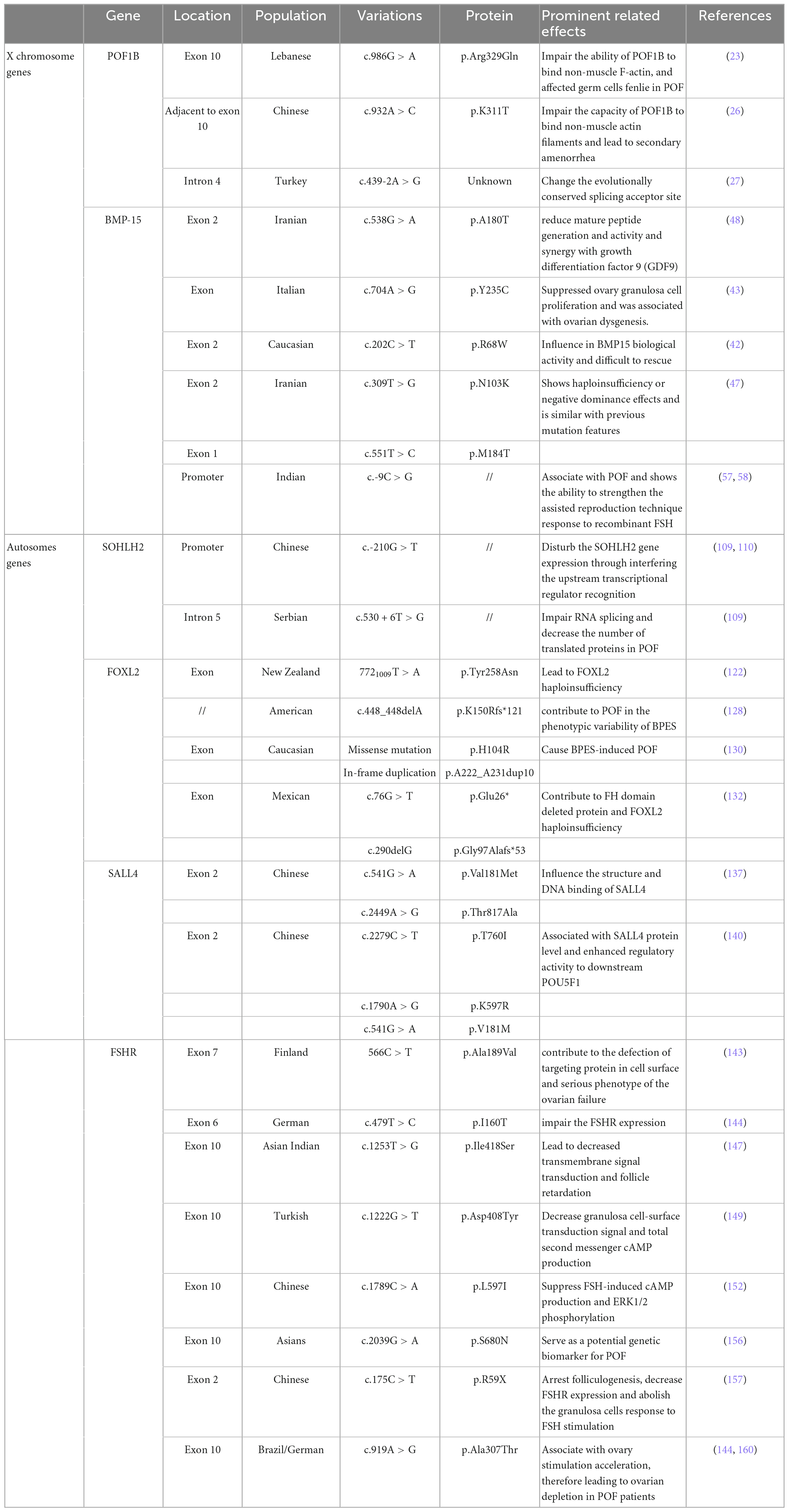
Chromosomal gene abnormalities have long been regarded as a cause of POF, but the percentages vary widely among reported series (17). Genetic heterogeneity and disease development have been widely investigated and observed to have a close relationship (18). With the development of sequencing technology, increasing solid evidence suggests that genomic instability underlies the pathogenesis of POF (13). Therefore, as an obstacle to basic ovarian development, key gene variants are particularly harmful to ovarian cells and promote POF (Table 1). Variants located in conserved regions are more likely to influence the inherent gene functions (19) 31871067. Thus, variation-induced protein and cell signaling perturbations might yield severe functional defects in ovarian cell development, thereby causing a negative effect on POF. In this section, we summarize several pivotal genes (X chromosome genes and autosomal genes) and their latest research advances (Figures 1, 2), whose variants warrant strong consideration as pathogenic candidates for POF.
Abnormal X chromosome genes
The premature ovarian failure 1B (POF1B)
The premature ovarian failure 1B evolutionary novel gene was found only in vertebrates (19) and has been identified by breakpoint mapping of X-autosome translocations (20). Generally, this gene alludes to a specific region following the law of Online Mendelian Inheritance in Man (OMIM) (21). It was previously detected that POF1B (an interrupted gene) in POF patients appeared to be mutated (20). Whole-genome sequencing of a Lebanese POF familial genetic group revealed point mutations localized in POF1B exon 10 characterized by a nucleotide (G > A) at position 1,123 (22). Among these, the protein sequence alteration occurred at 329 amino acid site p. Arg329Gln (c.986 G > A), similar to arginine to glutamine, and the mutant POF1B protein showed a lower ability to bind non-muscle F-actin (Figure 1), thus influencing the POF patients’ germ-cell division (22, 23). Moreover, the POF1B p.Arg329Gln variant influenced the F-actin amount and contributed to the loss of tight junctions in polarized epithelial cells in POF (24). More recently, Zhang et al. demonstrated that there was a novel POF1B missense variant in Chinese POF patients, namely, p.K311T (c. 932A > C), which was adjacent to p.Arg329Gln and was supposed to have similar functions to non-muscle F-actin (25). Recently, in a 21-year-old Chinese POF woman with long-term oligomenorrhea and high sex hormone levels, exome sequencing revealed p.K311T (c.932A > C) variant in POF1B was closely associated with POF (25). The p.K311T was milder than p.Arg329Gln and was more likely to lead to secondary amenorrhea (25). Through comparative analysis with p.Arg329Gln, p. K311T was suspected to damage the capacity of POF1B to bind non-muscle F-actin and lead to secondary amenorrhea (25). Moreover, another variant (c.439-2A > G) in intron 4 of POF1B was observed to be associated with POF, which mainly changes the evolutionally conserved splicing acceptor site (26).
In some keratinocytes, the POF1B expression level was observed to be inversely associated with keratinocyte number and correlated with defects in cell adhesion (27). Keratinocyte-related cytokines might promote the primordial to primary follicle transition (28). As an upstream modulator, CBX2.1 has the ability to stimulate POF1B activation, whereas silencing CBX2.1 significantly downregulated POF1B expression and potentially was associated with POF development (29). CBX2.1 in human sex development has been reported to inhibit the female pathway and is related to female hypoplasia (30). The pathogenic variants of POF1B have been continuously studied (23), and increasing evidence supports the importance of POF1B variants in POF occurrence.
Bone morphogenetic protein 15 (BMP15)
Bone morphogenetic protein 15 is an X-linked coding gene and is related to primary-stage protein generation from oocytes (31). As a member of the TGF-ß superfamily family, BMP-15 also has significant advances in our understanding of early follicle development and oocyte regulation in mammals (32, 33). BMP-15 was demonstrated to be expressed in oocytes and pituitary cells but to a much lesser extent in other organs, such as the kidney and heart (34). The importance of BMP-15 as a fertility marker in females has attracted great attention because it influences all statuses of oocyte development and embryonic quality, especially the regulation of granulosa generation and ovarian functions (35, 36). Expression of BMP15 in oocytes stimulates granulosa cell growth and inhibits FSH action by suppressing follicle-stimulating hormone receptor (FSHR) expression, which is related to ovulation rate and fertility (37). Compared to other TGFß superfamily members, BMP-15 has undergone rapid evolution and subjective positive selection in mammalian clades, and its important and particular functional role eventually leads to its importance in female fertility (38). Abnormal regulation of BMP-15 might be related to female POF and infertility (39, 40). Recently, attention has been focused on the potential biological impact of BMP15 genomic variation on the POF population. BMP15 variation may predispose to POF and stimulate ovarian defects in cooperation with other alteration features (41).
Notably, Di Pasquale et al. (42) reported that the p. Y235C in the X-linked BMP15 gene in 2 sisters, a heterozygous non-conservative substitution in the pro region, suppressed ovary granulosa cell proliferation and was associated with ovarian dysgenesis. Among these, wild-type BMP15 showed the ability to stimulate 3H-thymidine in ovary granulosa cells and was restored at a concentration nearly five-fold higher than that of p. Y235C BMP15 group. Granulosa cells were demonstrated to deliver nutrients and metabolites through gap junctions to oocytes and are involved in oocyte secretion of paracrine signals (43). In addition, BMP15 knockdown female mice have a reduced ovulation rate and therefore show lower fertility (44). BMP15 variation not only influences granulosa cells but also plays an important biological role in the secretion and activation of TGF-β superfamily members in oocytes (45). Moreover, there are two missense alterations (p.R68W and p.A180T) and one insertion (p.262insLeu) were observed in a POF cohort study (33). The p.R68W (c.202C > T) showed a larger and significant influence on BMP15 biological functions, and p.R68W carriers develop POF before the age of 20 years old (41). In vitro cotransfection experiments with wild-type cDNA indicated that p.R68W is a deleterious mutation in POF patients, where BMP15 activity is difficult to rescue (41). Moreover, in Iranian POF familial history patients, the detectable p. A180T (c.538G > A) was accompanied by a long history of oligomenorrhea, high FSH and very low anti-Mullerian hormone (AMH) levels, whereas without sonographic abnormal symptoms (46). The p.A180T nucleotide site alteration in exon 2 of BMP15 (named rs104894767) has been recorded in databases. As previously reported, the p. A180T variation was demonstrated to be associated with non-familial POF patients (33). By Sanger sequencing analysis, the POF family members who carry the p.A180T variant presented premature physiological menopause, while healthy members without p. A180T variant presented regular menses (46). Besides, BMP15 with p. A180T variant presented fourfold lower activity than the wild-type, which changes the potential to reduce mature peptide generation and activity and synergy with growth differentiation factor 9 (GDF9) (47). BMP15 protein binds to its closely related paralog GDF9 to form homodimers or heterodimers that regulate many aspects of development by activating transmembrane serine/threonine kinase receptors (42, 48, 49). The interaction between GDF9 and BMP15 mutation is regarded as an important internal factor for ovarian hypofunction in POF patients (47, 48).
Some novel BMP15 mutations were detected in a number of POF patients. In the BMP15 pro-domain region, p.N103K and p.M184T were observed to prevent the protein from binding to mature dimers, consequently leading to decreased BMP15 activity (46, 50). The pro-domain is usually uncoupled during the maturation process and modulates the folding and dimerization of the protein structure (51). BMP15 with p.N103K and p.M184T variant usually showed haploinsufficiency or negative dominance effects and is similar to previous mutation features (46), which is potentially associated with POF symptoms (52–54). Moreover, BMP15 with the c.-9C > G promoter polymorphism presented a functional association with the POF phenotype (49). Although c.-9C > G in 398 PCOS female cohort studies was not significantly associated with disease pathogenesis, it was related to some specific clinical features, such as anovulation and infertility (55). Indeed, c.-9C > G is a frequent variant in BMP15 and has been reported to be functionally associated with POF (56). Due to the high reactivity of the G allele, c.-9C > G also showed the ability to strengthen the assisted reproduction technique response to recombinant FSH (57). Additionally, in silico analysis in combination with the BMP15 promoter sequence showed that the -14 to -8 bp region of the BMP15 promoter is the primary regulatory target for the pituitary homebox 1 protein (PITX1) (49), in which two of the active binding regions were validated in vitro, namely, prom-G and prom-C constructs. PITX1 was reported to be associated with gonadotroph cell activation and estrogenic signals (58), and the estrus cycle-related estrogen signaling pathway was positively involved in POF traditional Chinese medicine treatment (59). Despite recent findings demonstrating the involvement of BMP15 mutation in POF, further studies elucidating the roles of modulators would lead to a better understanding of the disease pathogenesis.
Fragile X messenger ribonucleoprotein 1 (FMR1)
Fragile X messenger ribonucleoprotein 1 is an X-linked gene encoding RNA binding protein, and its mutation in the 5′UTR dynamic triplet CGG repeat is related to fragile X syndrome (60–62). The CGG trinucleotide repeat is usual but unique in the region of FMR1 exon 1, which conducts mRNA transcription but not translation into protein amino acids (61). The copies of FMR1 premutation CGG can be expanded to varying degrees and associated with different biological function performance (63). Mutations of FMR1 are usually categorized by the number of CGG trinucleotide repeats: the classic normal is 6-45 CGG repeats in the 5′UTR; FMR1 premutation alleles have 50-200 CGG repeats in the 5′UTR; FMR1 full mutation has an expansion of more than 200 CGG repeats in the 5′UTR; and the intermediate range (also called the gray zone) has an expansion of 45–54 repeats in the FMR1 5′UTR (64, 65). The FMR1 gene is primarily associated with neuro/psychiatric risks, while it appeared to control the function of follicle recruitment and ovarian reserve in recent evidences (63, 66, 67). Moreover, the premutation CGG repeat-related increased FMR1 mRNA transcription was potentially involved in POF pathogenesis compared to the general population, such as 35 and 54 repeats (64). Two independent studies indicated that FMR1 premutation carriers have an earlier average menopausal age and are more susceptible to POF than non-carriers (68, 69). Notably, in familial trait ovarian failure, FMR1 premutation CGG was associated with a large number of ascertained idiopathic POF cases (61). FMR1 premutation expansions are not merely high-risk factors for POF occurrence but are also relevant to cytokine levels, such as serum FSH, in ovarian aging pathological conditions (70, 71).
Recently, the FMR1 premutation aroused great attention to female reproductive fertility, and women with monogenic FMR1 premutation have an equivalent risk of POF to the top 1% of genetic susceptibility (72). Approximately 15–20% of females with an FMR1 premutation develop POF (73). In Europe, the frequency of premutation of FMR1 in POF was higher than that in the general population, and FMR1 sequencing has become part of the recommended monitoring indicators for women with POF (74). However, the FMR1 premutation frequency is relatively lower in Chinese women. In Guo et al. (75) indicated that 0.49% (2 to 379) FMR1 premutation was found in limited sporadic Chinese POF samples, while none were observed in 402 controls. Recently, another case-control study reported that only 1.6% (2 to 124) of FMR1 permutations were observed in Chinese POF patients, and a 0.9% (1 to 111) premutation frequency existed in the control group (76). Studies from a Chinese cohort indicated that the distribution of allele 1 (smaller number of CGG repeats) rather than allele 2 in POI was closely associated with POF occurrence, wherein <26 and ≥29 CGG repeats potentially have a higher risk for reproduction and POF (76, 77). The ectopic expression of FMR1 CGG repeats leading to POF was also validated in a mouse model (78). However, the underlying mechanism of FMR1 CGG repeat-relevant ovarian function modulation remains unclear, including the pathological condition of the reduced ovarian reserve and abnormal follicular and oocyte growth. One hypothesis is that various FMR1 CGG expansions might contribute to transcriptional level changes and lead to ratios of distinct RNA isoforms (61, 64, 79). The above changes potentially decrease the fragile X messenger ribonucleoprotein 1 (FMRP) level or intercellular localization, thereby affecting steroidogenic enzymes and hormonal receptors and ultimately affecting the occurrence of POF (79, 80). The increased CGG repeat in FMR1 has the ability to impede the 40S ribosomal subunit in the process of downstream initiation codon recognition, resulting in translational debility and consequently reducing the FMRP level (80). Generally, during normal folliculogenesis, FMRP is primarily expressed in granulosa cells, which is crucial for oocyte maturation and growth. POF patients with FMR1 CGG repeats, such as 19 < n < 90, have impaired ovarian reserve, and abnormal FMR1 transcript levels related to FMRP influence the process of oocyte and follicular maturation (81, 82). Moreover, in these reproduction-related cells, the intracellular signaling cascade is also involved in FMR1 expression and regulation. Rehnitz et al. (67) reported that inhibition of AKT increases FMR1 expression and decreases FMRP levels, whereas recombinant FSH (rFSH) and mTOR inhibition lead to the opposite phenomenon, indicating that a feedback loop between FMR1/FMRP and the mTOR/AKT signaling cascade interacts during GC proliferation and oocyte maturation. In terms of this, FMRP phosphorylation and activation are thought to be potentially associated with S6K, a downstream molecule of mTOR (83). The FMR1 knockdown mouse exhibited larger ovaries in mass and volume compared to age-matched controls, wherein increased protein levels of Tsc2 and mTOR were detected (84). As previously reported, FMR1 expression influenced cholesterol and steroid hormone generation and impaired ovary responses to hormonal stimulation and growth (78, 82), while the mTOR pathway was involved in ovarian development and enlarged ovaries histologically with precocious follicular development (84, 85). On the other hand, an in vivo experiment in mice observed that permutation transcripts were closely associated with mitochondrial and ovarian abnormalities (86). mTOR is closely related to the metabolic process of mitochondria in ovarian cells (87), and inhibition of mTOR significantly improved follicular development and endocrine functions of the ovaries, thereby extending reproductive aging and premature aging of POF mice (88). These observations suggested that abnormal FMR1 transcription potentially causes POF, but it remains unclear which process plays a prior role in disease initiation.
DACH2
The Dachshund (DACH) gene family is known as a transcriptional cofactor on the basis of highly conserved protein interaction domains, while the DACH genomic mutation was observed to be closely associated with poor female reproductive tract development, namely, DACH1 and DACH2 (89). The DACH gene was first described in Drosophila, encoding nuclear proteins involved in different organs, such as eyes, limbs, and genital discs, which are required for the genital development of male and female phenotypes (90, 91). In mammals, the female and male reproductive tracts develop from the Müllerian duct (MD) and Wolffian duct (WD), respectively, wherein the WD regresses, retains the MD and then differentiates into the oviduct, uterus, and cervix (92, 93). Both DACH1 and DACH2 were observed in MD, and DACH1/DACH2 double mutation is associated with female reproductive tract development retrogression (89). In addition, DACH2 is expressed in human ovarian tissue and contributes to some ovarian malignant diseases, and variants in DACH2 may be associated with the POF phenotype (20, 94). Specifically, DACH2 has been implicated in human POF syndrome, where the genomic alteration of DACH2 was demonstrated to hinder the correct process of ovarian follicle differentiation (95).
The DACH2 gene in Xq21 is located 700 KB distal to POF1B (96–98). The genomic mutation analysis of the DACH2 coding region observed that missense mutations of three single nucleotide polymorphisms (SNPs) (P36 L, G59D, and R412K), non-conservative amino acid substitutions, were frequently present in POF patients compared to the control group (20). These three mutations were proven to be specific in the DACH2 subfamily rather than DACH1, and they occur in the N-terminal region of the DACH2 protein close to the DD1 domain (amino acids 66–162). The DD1 domain at the N-terminus was demonstrated to be involved in DNA binding and EYA protein interaction; thus, DACH1 genomic mutation potentially influenced its signaling biological functions (99, 100). In an animal model, the EYA gene plays an important role in mammalian gonad development, whose response to estrogen-related receptors will directly influence reproduction (101). Therefore, the interaction between DACH2 alteration and EYA protein might increase the risk of POF by affecting the biological process of ovarian follicle differentiation (20, 102). Moreover, another study indicated that genomic mutation was specific to one POF patient, which occurred in the third intron of DACH2 with a C to T transition downstream of Exon_3 (103). On the basis of their results, this variant was not within the splice or receptor sequences and was unlikely to influence the DACH2 level, and there is no clear evidence to support its direct function in POF pathology. Strengthening the screening of more candidate disease-causing mutation sites, including exons and introns, is helpful for the early diagnosis and prevention of POF. Generally, the nucleotide translocation might disrupt the transcription of itself or nearby genes by affecting the cis/trans-acting regulatory factors from the transcription units and consequently deleterious changes in the expression and protein level (89, 104). Nevertheless, there is evidence indicating that DACH2 knockout mice are viable and fertile, which is reminiscent of null mutations of DACH2 that might independently affect the reproductive phenotypes of POF patients (105).
Autosomal gene abnormalities
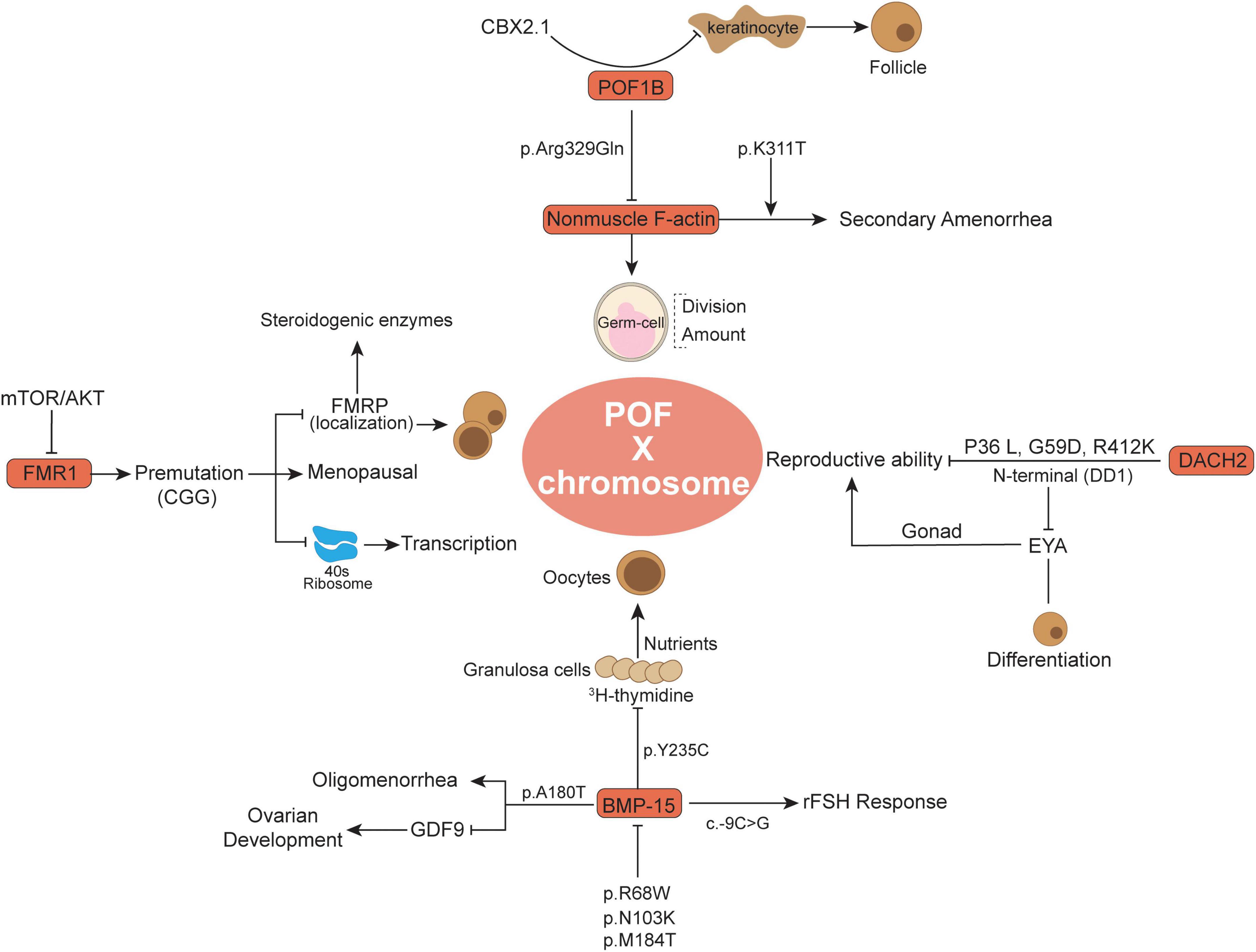
SOHLH2
The transcriptional regulator SOHLH2 was observed to be preferentially expressed in oocytes of immature ovaries and has been deemed a critical regulator for early germ cell development (106). The SOHLH2-silenced mouse model showed infertility and atrophied ovaries devoid of follicles (107). Previously, Qin et al. (108) indicated that SOHLH2 variants were implicated in primary POF, which exhibited several non-synonymous mutations in POF patients compared to normal controls. For example, p.Glu79Lys (c.235G > A) and p.Glu105Gly (c.314A > G) have high conservation among mammalian potential to be pathogenic for Chinese POF patients. In addition, the SOHLH2 promoter variant c.-210G > T was observed in a Chinese POF patient and is located in a region with transcription factor-binding sites and CpG islands (Figure 2) (108, 109). This variation may potentially disturb SOHLH2 gene expression by interfering with upstream transcriptional regulator recognition (109). Notably, the loss of SOHLH2 expression contributes to the rapid loss of oocytes, as well as increased oocyte apoptosis (110). In this respect, SOHLH2 has been regarded as the downstream target of the BMP4/Smad signaling pathway in the survival and apoptosis of oocytes (111). Moreover, SOHLH2 could bind to the C-kit promoter, act as a positive transcriptional regulator for C-kit and modulate the C-kit/PI3K/Akt/Foxo3a cascade in oocytes, which is directly associated with oocyte survival and follicle development in POF (111, 112). Furthermore, there is another variant (c.530 + 6T > G) in introns that is supposed to affect RNA splicing, thereby decreasing the number of translated proteins in POF (108).
FOXL2
Forkhead box L2 (FOXL2) belongs to the winged helix/forkhead transcription factor family, containing a 110 amino acid DNA-binding domain, which is associated with protein mislocation, aggregation, and intranuclear mobility, including ovarian development and postpartum recovery (113). FOXL2, the earliest recognized ovarian differentiation marker in mammals, was observed in the granulosa cells of the ovary as well as the mesenchyme of the developing eyelids (114–117). FOXL2 might be a pivotal modulator of ovarian development and eyelid formation. Previous studies also concluded that the FOXL2 variant results in a relatively mild blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) phenotype (118), which is a rare autosomal dominant genetic developmental disorder in the eyelids and ovary (114). BPES has emerged to have two subtypes: type I is associated with POF, and type 2 has no systemic associations (119), wherein the different amino acid site alterations will lead to the different subtypes (120).
Previously, the FOXL2 variants A221-A230del and p.Tyr258Asn (7721009T > A) were observed in POF patients from the New Zealand and Slovenia cohorts, respectively (121). The non-conservative substitution of 7721009T > A might lead to FOXL2 haploinsufficiency, thus promoting POF development (121). After that, some studies indicated that FOXL2 variants of p.Gly187Asp and c.627delT favor the implication of FOXL2 variants in POF, and more systematic genetic screening of FOXL2 variants is important in POF premature diagnosis and hormonal replacement therapy (122, 123). Recently, several FOXL2 variants were reported to be associated with a severe BPES phenotype, such as p.Arg103Cys (c.307C > T), p.His104Pro (c.311A > C), p.Ser107Asn (c.320G > A), and p.Phe112Tyr (c.335T > A) (124). Of note, these variants potentially damage the function of STAR and OSR2 protein by abolishing transcriptional activity (124), and STAR is important for differentiation of granulosa cells, where the FOXL2 variant will lose suppression for the STAR promoter, thereby accelerating differentiation of granulosa cells and secondary depletion of the primordial follicle pool (125, 126). In addition, the FOXL2 heterozygous deletion variant p.K150Rfs*121 (c.448_448delA) and its coexisting gene BMP15 act synergistically and contribute to POF in the phenotypic variability of BPES, and this variant leads to polyalanine deletion and truncated protein at 269 amino acids (aa) (127, 128). Herein, the digenic inheritance of the FOXL2 variant and its related effector potentially contribute to BPES-related ovarian function impairment and POF 25988799. Moreover, two variants, p.H104R and p.A222_A231dup10 in Caucasian FOXL2 leads to mislocalization and aggregation, thus impairing transactivation, which could cause BPES-induced POF (129). In one case, estrogen treatment successfully improved menarche and secondary sexual characteristics, which showed promising therapeutic potential in the reproductive outcomes of BPES-related POF patients (130). In a Latin American cohort, two FOXL2 variants (c.76G > T and c.290delG) were also deemed pathogenic factors for POF patients, wherein the former contributes to FH domain deleted protein and FOXL2 haploinsufficiency, while the latter mechanism is still unclear (131). Generally, the FOXL2 protein acts as a transcription factor for some important molecules in ovarian maintenance and function, such as binding to TESCO to suppress Sox9 expression in ovaries, ultimately contributing to the development of ovaries (117). The polyalanine tract deletion/expansion-related variant is likely to abolish FOXL2 protein function and thereby abrogate the above process in ovarian maintenance (132), where the polyalanine tract deletion variant is more likely related to BPES type I (with POF) (121, 133). Meanwhile, there are many variants present in unaffected POF family members that have not been confirmed to be pathogenic variants. Identification of FOXL2 variants is critical for suspected BPES and/or POF evaluation as well as prevention in due course.
SALL4
Spalt-like transcription factor 4 (SALL4), a zinc finger transcription factor expressed in murine oocytes, binds to POU5F1 and regulates its expression (134, 135). Previously, genomic sequencing of 100 Han Chinese POF women showed that the SALL4 variants p.Val181Met (c.541G > A) and p.Thr817Ala (c.2449A > G) potentially influences the structure and DNA binding of SALL4 and is associated with POF development (136). The pathogenesis of the SALL4 variants might be achieved by abolishing the recognition of the downstream POU5F1 gene and suppressing its expression (135, 137), ultimately affecting primordial oogonia development and triggering POF (138). Recently, another POF study in 50 Han Chinese individuals through whole-exome sequencing (WES) analysis discovered several novel variants in POF patients, including p.T760I (c.2279C > T) and p.K597R (c.1790A > G), as well as the verified variant p.V181M (c.541G > A) (139). Intriguingly, in vitro functional experiments showed that these variants were positively associated with the SALL4 protein level and enhanced regulatory activity to downstream POU5F1. According to this phenomenon, posttranslational regulation of SALL4 protein levels might be the molecular mechanism underlying POI (139). Taken together, the above observations suggested that SALL4 variants are closely associated with POF development, whereas the different variation types of SALL4 might present different manifestations of SALL4 activity and phenotypic variability.
FSHR
Follicle-stimulating hormone receptor has a pivotal role in recognizing FSH, thereby controlling granulosa cells of the ovary and female reproduction (140). FSHR variants have been identified in women with hypergonadotropic POF symptoms, especially inactivating mutation subtypes (141). Doherty et al. (142) reported that inactivating extracellular FSHR variants existed in the ligand recognition region of the receptor, namely, p.Ala189Val (566C > T), whose occurrence contributed to the defect of targeting protein in the cell surface and a more serious phenotype of ovarian failure. Interestingly, another transmembrane variant, p.Ala419Thr, of FSHR reduced symptoms when it compounded with p.Ala189Val, which was potentially due to the higher residual activity retained by the transmembrane variant and neutralizing p.Ala189Val variant inactivating functions (142). Notably, Ledig et al. (143) observed that p. I160T (c.479T > C) is an inactivation variant in FSHR and is associated with POF, which will impair FSHR expression on the cell surface. They simultaneously indicated that p.I160T variant-induced FSHR inhibition potentially further enhanced the BMP15 variant (p.A180T)-related granular cells decrease in the developing follicle (143). In addition, the interaction between FSHR SNP rs6166 and CYP19A1 SNP rs4646/rs10046 was demonstrated to be involved in POF development by regulating folliculogenesis (144). Thus, synergistic effects of digenic variants might promote POF development to a considerable extent.
Moreover, there are still many FSHR variants that have been demonstrated to have independent pathogenic roles in POF. FSHR is important in human reproduction and was proven to be the first single gene to cause POF (145). In an Asian Indian descent family, an inactivating pathogenic variant p.Ile418Ser (c.1253T > G) in FSHR was detected in POF patients (146). The p.Ile418Ser (c.1253T > G) occurred in exon 10 of FSHR and impaired the transmembrane helix of the FSHR protein, which led to decreased transmembrane signal transduction and follicle retardation (146). Based on characteristics in the FSHR helix transmembrane domain (TMD) and highly conserved across species (147), inactivated p.Ile418Ser variant in FSHR was supposed to cause POF. In the second TMD of FSHR, another inactivating variant, p.Asp408Tyr (c.1222G > T) has been observed in two Turkish POF patients, which was also accompanied by decreased granulosa cell-surface transduction signal and total second messenger cAMP production (148). The lack of sufficient FSHR expression and function in ovarian granulosa cells are unable to promote follicle maturation and ovulation, despite high levels of FSH stimulation (149, 150). Recently, a large cohort study in Han Chinese with more than 190 POF patients and normal controls detected some novel variants. The p. M265 V (c.793A > G) and p.L597I (c.1789C > A) variants exclusively existed in Chinese POF patients, former located in extracellular domain (ECD) and later in TMD, wherein the p.L597I can suppress FSH-induced cAMP production and ERK1/2 phosphorylation, thereby stimulating the POF phenotype (151). Generally, the FSHR variant in ECD impaired trafficking and cell surface expression, while the variant in TMD was characterized by abolished signal transduction (152, 153). However, the p.L597I decreased FSHR expression in the membrane (151), which is similar to another inactivating variant p.A575V (TMD) manifestation in primary amenorrhea patients (154). In addition, another POF-associated FSHR variant p.S680N (c.2039G > A) was specifically proven in Asian people rather than other ethnicities and is supposed to serve as a potential genetic biomarker for POF in Asians (155). Through comparison between a Chinese POF family and 192 control women, Liu et al. (156) demonstrated that a novel FSHR variant p.R59X (c.175C > T) in exon 2 was causative for POF by arresting folliculogenesis. The p.R59X is positively related to decreased FSHR expression and deemed a loss-of-function variant. Additionally, p.R59X variant will abolish the granulosa cell response to FSH stimulation, mainly due to the truncated FSHR protein-related ECD and TMD function region absence (156). It is thus clear that inactivation of FSHR variants will impair FSHR functions and follicular development, thereby leading to POF (157). Further study should focus on pharmacological and assisted reproductive treatments targeting disrupted FSHR.
Patients with specific FSHR variants, such as p.Ala307Thr polymorphism in Brazilian patients, while it was not associated with ovarian endocrine variables or clinical ultrasonographic findings (158). Moreover, an expanded comparative sample identified that p.Ala307Thr is more frequent in POF patients than in controls (159). Among these, increased FSH levels in p.Ala307Thr carrier might be associated with ovary stimulation acceleration, therefore leading to ovarian depletion in POF patients (159). Notably, in another study, the p.Ala307Thr variant has also been identified in 3 German patients with POF (143). In terms of this, menstrual dysfunction precedes the initiation of amenorrhea, such as oligomenorrhea, transient amenorrhea and short cycles, which might be a special signature for ovarian failure and are regarded as “prodromal POF” (160). In terms of this, future investigations are needed to explore the potential roles of other FSHR variants in the development of POF.
Dysregulated gene expression in POF
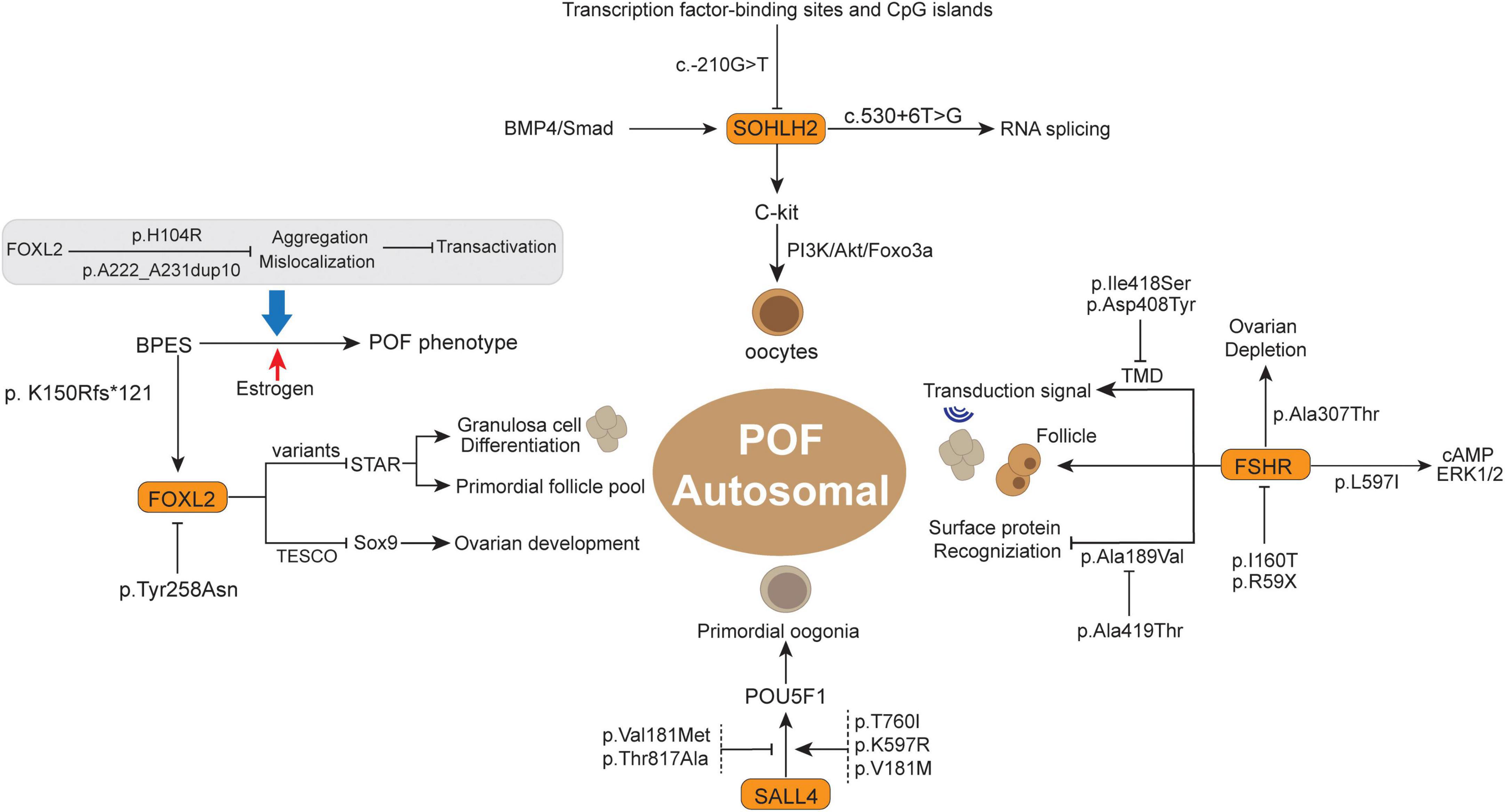
Apart from genomic alterations, abnormal expression of the transcriptome caused by multiple factors also has a great impact on POF. Ovarian insufficiency is a continuum of impaired ovarian function or ovarian aging, which is always accompanied by signature marker gene expression abnormalities (161). In POF, there are three consecutive but progressive stages, occult, biochemical, and overt ovarian failure (162), and gene expression changes with pathogenesis can provide better evidence for clinical diagnosis (163, 164). The monitoring of gene expression has been widely used in the discovery of disease biomarkers or therapeutic targets (165). However, to our knowledge, the molecular mechanism of POF has not yet been clarified. Below, we summarize the dysregulated expression of POF-related genes identified by recent studies, including Ki-67, proliferating cell nuclear antigen (PCNA), chemokine (C-X-C motif) ligand 12 gene (CXCL12), insulin-like peptide 3 (INSL3), and PTX3 (Figure 3).
Ki-67
Ki-67 is expressed in proliferating cells, and its protein is associated with different nuclear domains, whereas the Ki-67 protein was detected in mouse oocytes rather than mature sperm (166, 167). Ki-67 is a DNA-binding protein expressed in all active cell cycle stages and can be used as a marker for cell proliferation (168, 169). Herein, Ki-67 expression has been regarded as the signature for ovarian tissue transplantation activity and was used to evaluate transplantation-associated follicle dynamics, where the higher Ki-67 expression in ovarian granulosa cells indicated an increase in activated growing/primordial follicles (170). Moreover, an in vivo POF model indicated that the ovarian proliferation index can be evaluated by Ki-67, which is expressed in epithelial cells of the endometrium and glands (171). Additionally, lower expression of Ki-67 was detected in the POF group than in the control group. In contrast, ovarian tissue with higher FSH levels showed morphologically normal follicles and significantly decreased Ki-67 expression (172, 173), and gonadotropin inhibited mitosis in granulosa cells early in the periovulatory interval (174). When follicular atresia occurs, the mutual intensive interaction between granulosa cell death and oocytes plays an indispensable role (175). Moreover, the inhibition of NF-κB signaling reversed the loss of Ki-67 expression in granulosa cells (176). A previous study indicated that CYP19A1 expression and estradiol secretion in human ovarian granulosa cells were directly modulated by NF-κB signaling (177), and these two elements and Ki-67 expression have served as indicators in ovarian disease estrogen treatment (178). The intervention of Ki-67 expression will directly promote ribosome synthesis during cell division, which is necessary for ovarian granulosa cell proliferation (179). This evidence supports it as a useful feature to better understand ovarian granulosa cells and follicles, as well as abnormalities in human POF.
PCNA
Proliferating cell nuclear antigen (PCNA) is a key factor for DNA replication and cell cycling, which can be used as an indicator of ovarian cell proliferation and define the extent of departure from Strzalka and Ziemienowicz (180), Thomas et al. (181), Muskhelishvili et al. (182). Through immunohistochemical detection, PCNA has been proven to distinguish follicles with different maturation statuses, where PCNA expression is significant in oocytes, granulosa cells and growing follicles and acts as a primary regulator (183, 184). However, atretic follicles undergoing atresia were negative for PCNA expression (185). In a secondary POF model, the mRNA expression of PCNA was significantly decreased in ovarian tissue compared with healthy controls (186). Moreover, coenzyme Q10 (CoQ10) and/or cryptotanshinone treatment promoted primary follicle and granulosa PCNA expression and improved ovarian injury in POF (186, 187). A previous study indicated that miR-376a influenced primordial follicle assembly and oocyte apoptosis by binding to the mRNA 3’ untranslated region (3’-UTR), thereby inhibiting PCNA expression (188). The microRNA modulatory effect on gene expression has been proven to be associated with POF in a mouse model (189). In POF, increased p53 might disturb oocyte quality and damage ovarian functions (190), mainly through a posttranslational mechanism (191). In this respect, the cyclin-dependent kinase suppressor protein p21 is a key downstream target for p53, where p53 boosts downstream effector expression by elevating nascent RNA amounts (192, 193). Meanwhile, p21 is known as a dual inhibitor for both cyclin-dependent kinases and PCNA, and p21 has the ability to displace chromatin-bound PCNA to interfere with S phase (194, 195). Not surprisingly, the upregulated p53 expression and enhanced p21 transcription simultaneously existed in POF ovarian tissues and were positively associated with disease development (196). The reduction in PCNA will lose the balance between apoptosis and fail to ensure successful follicle development and protect follicular growth tissue homeostasis (197). In addition, another regulator of follicular growth is the Wnt signaling pathway, which is important for pregranulosa cell transition during the period of primordial follicle activation (198). Notably, significant expression of Wnt-2 in all stages of follicles was positively associated with PCNA and induced granulosa cell proliferation (199). A study in human cumulus cells demonstrated that Wnt-2 recognizes its receptor FZD9 to regulate the formation of β-catenin and E-cadherin (200). The knockdown of β-catenin in granulosa cells inhibited PCNA expression but did not affect Wnt-2 expression (199). Herein, the Wnt-2/β-catenin cascade controls diverse ovarian developmental processes and has the potential to modulate PCNA expression in POF (201).
CXCL12
Chemokine (C-X-C motif) ligand 12 gene, also known as stromal cell-derived factor (SDF-1), interacts with its receptor CXCR4 and plays an essential role in primordial germ cell (PGC) migration, proliferation, and survival (202). Moreover, there is evidence indicating that the expression of CXCL12/CXCR4 was increased in a POF mouse model and was negatively associated with primordial-to-primary follicle transition (203). In the Chinese Han population, CXCL12 polymorphism-related CXCL12 expression and high CXCL12 protein levels were supposed to be associated with POF and have the potential to be candidate biomarkers (204). A high level of CXCL12 expression has been deemed a potentiator for primordial follicle densities and smaller follicle sizes due to chemokine and receptor interactions, and increased CXCL12 expression inhibits follicle activation and ovarian functions (205). However, the detailed CXCL12 regulation mechanisms in the pathogenesis of POF are still unclear. In terms of inflammation, POF-related atretic follicles and granulosa cells are usually characterized by an inflammatory response involving leukocytes and their secretory inflammatory factors (206, 207). Among them, the interaction between CXCL12 and its receptor CXCR4 was demonstrated to participate in physiological inflammatory processes, including follicular dysregulation (208, 209). In line with this, the cyclophosphamide- and busulfan-induced POF mouse model presented a positive correlation between CXCL12/CXCR4 protein expression and the inflammatory response compared to the normal control, namely, increased proinflammatory cytokines (IL-6, IL-8, and TNF-α) and decreased anti-inflammatory cytokines (IL-10) (203). On the other hand, the chemotaxis role of the CXCL12/CXCR4 axis in mesenchymal stem cell (MSC) transplantation also attracted great interest in POF therapy. Ling et al. (210) reported that CXCL12 can induce CXCR4-expressing MSC migration and homing in the ovaries of POF mice, and blocking the CXCL12/CXCR4 axis significantly reduced MSC homing to ovaries and reduced their therapeutic efficacy in POF. As in previous studies, the CXCL12/CXCR4 axis might be a prerequisite for MSC homing, where CXCL12 acts as a chemoattractant molecule to guide CXCR4+ MSC directional migration (211–213). In this process, the activated PI3K/Akt signaling pathway was implicated in the CXCL12-CXCR4 interaction as a downstream factor and is thought to be involved in POF (210, 214). The CXCL12/CXCR4 pathway is known as an upstream switch for Akt phosphorylation, which in turn modulates various biological effects, such as cell migration, chemotaxis and adhesion (215). The binding of CXCL12 to CXCR4 was accompanied by PI3K/Akt signaling activation, while CXCL12/CXCR4 inhibition synchronously inhibited PI3K/Akt signaling, which directly decreased the MSC transplant treatment effect in the POF model (210). The higher homing rate and survival of MSCs to the ovary in individuals will reduce depletion of germline stem cells and increase the therapeutic efficacy for POF (216). Moreover, activation of PI3K/Akt signaling has been reported to promote MSC differentiation into endothelial cells (217, 218) and is positively associated with granulosa cells and follicle proliferation in therapy for POF (219–222). In contrast, suppressed PI3K/AKT signaling probably contributed to large-scale oocyte loss and more serious POF (223). Moreover, PI3K/Akt signaling in MSCs post-transplant also showed the ability to increase the Th17/Tc17 and Th17/Treg ratios to improve the inflammatory immune environment, thereby promoting the recovery of ovarian function in POF (222). Together, the balance of CXCL12 expression is important in POF development, while the pros and cons of mechanisms should be further investigated.
INSL3
Insulin-like peptide 3 (INSL3) is a member of the relaxin family of neohormones, which is thought to be specific for mammalian traits with respect to reproduction (224). INSL3 is mainly produced by interna cells of the growing antral follicle and is recognized by the specific receptor RXFP2, which modulates the synthesis of the steroid precursor androstenedione (225). In the follicle, INSL3 expression and activation orchestrate the generation of steroid precursors and rostenedione and promote estradiol release in granulosa cells (225, 226). Of note, INSL3 expression was absent in preantral, atretic follicles, granulosa cells or oocytes (227, 228), while RXFP2 was expressed in mammalian oocytes and was associated with oocyte maturation (229, 230). Moreover, INSL3 can also be secreted into the circulation and detected in serum, with a level of immunofluorescence detection of ∼100 pg/ml in women, by which we can monitor the growth of antral follicles (231). As described above, INSL3 expression is consequently increased in polycystic ovary syndrome (PCOS) and decreased in females with POF, which might be a valuable biomarker for POF patients. Importantly, the effect of high INSL3 expression on follicle development might be attributed to the stimulation of GDF9 in oocytes, and the inhibition of GDF9 specifically blocks the INSL3 growth-stimulating effect (232). Moreover, INSL3 from theca cells induces the generation of the enzyme 17α-hydroxylase (CYP17A1) in the same cell, which can modulate the production of the follicular steroid precursor and androstenedione from pregnenolone or progesterone (225). Additionally, released androstenedione will be absorbed by granulosa cells and act as a precursor for estrogens (estrone, estradiol). In the development of POF, the serum INSL3 level was observed to be continuously decreased and showed a strong negative association with FSH (233). Thus, INSL3 might be a promising new specific biomarker for POF progression.
PTX3
PTX3 is a glycoprotein with two structural domains: one is in the C-terminal region and homology with C-reactive protein (CRP)/serum amyloid P component (SAP), and the other is a unique N-terminal domain without homology (234). PTX3 is specifically expressed in the ovarian cycle and has multifunctional properties under different conditions. Of note, PTX3 was demonstrated to be expressed in cumulus cells, a subtype of granulosa cells surrounding oocytes (235). Cumulus cells are special and have different fates compared to other granulosa cells, such as facilitating oocyte release and fertilization (236). In a mouse model, PTX3 expression is significantly increased in cumulus cells before ovulation and is associated with cumulus matrix formation, and PTX3 blockade in mice results in infertility (237). Due to the specific recognition of heavy chains (HC) to the PTX3 N-terminal domain and its covalent linkage to hyaluronan (HA) polymers (238), PTX3 indirectly influences the HA biological context in cumulus cells, which can be reversed by PTX3 blockade (239). Herein, PTX3 might modulate the HA cascade via the interaction between the HC (240). Notably, HA was reported to be an excellent cell scaffold for MSC transplantation in POF, which not only promotes cell secretory function but also prolongs the retention of MSCs to improve therapeutic efficiency (241, 242). Moreover, HA also protects ovarian function in an immunosuppressive drug-induced POF mouse model, where HA improves granulosa cell damage, estradiol concentration, and the number of follicles (243). In ovarian granulosa cells, HA activation potentially increased progesterone receptor membrane component 1 (PGRMC1) expression, thereby preventing abnormal granulosa cell apoptosis and follicle loss in POF (244). Together, these data indicate that altered expression of PTX3 might influence the POF ovarian microenvironment and cell functions, likely ameliorating proliferation, damage, and hormone levels.
POF relevant clinical trials
Although the POF incidence rate only accounts for 1% of women under the age of 40 (245), monitoring and management show potential to prevent POF from devastating outcomes (246). POF, a term that appropriately describes the end-stage of premature ovarian insufficiency, is typically diagnosed when amenorrhea combined with high gonadotrophins and hypoestrogenemia, wherein some adolescent patients have follicular depletion or insult to the ovary and present with delayed puberty or amenorrhea (247, 248). Current therapeutic management of POF includes psychosocial support, hormone replacement therapy, and fertility management (249). The diverse etiologies of POF, such as genetics, immune disorders, and microenvironmental dysregulation, have also attracted great attention in the treatment of POF (250). Interestingly, providing more information regarding the etiology, diagnosis, and treatment of POF in adolescents or high-risk populations will provide new insights into preventing disease development, along with the development of more sensitive markers. Below, we will present the latest clinical trials on the diagnosis and treatment of POF.
Diagnosis
The POF is serious clinical disease with high FSH, low hormonal and ovarian failure. Women of reproductive age diagnosed with POF or premature ovarian failure according to ESHRE criteria are usually based on a sinus follicle count (AFC), AMH, and early follicular serum FSH levels (251). The premature ovarian insufficiency is usually accompanied with anovulatory cycles leading to abnormal uterine bleeding (AUB), it is important to identify the pathogenies for irregular cycles such as FSH, LH, and estradiol measurements (252). The FSH levels of premature ovarian insufficiency women are typically higher than 25 mIU/ml (253), but precise cutoff levels have not been determined. Some patients with premature ovarian insufficiency symptoms show low FSH levels compared with above standard, while FSH > 40 mIU/ml was deemed as POF (254, 255). In addition, simultaneous measurement of upregulated basal luteinizing hormone (LH) levels is helpful to determine whether a high FSH level is associated with ovulation (254). In clinical practice, more stable and detectable indicators are urgently needed. A clinical trial using a combination of FSH stimulation and transvaginal ultrasound examination developed a method for the precisely detection of POF (NCT00006156). Among these interventions, FSH intervention in normal ovaries showed a significant stimulation in serum inhibin B, and ultrasound examination will further define parameters that could improve the earlier diagnosis of POF (256). Moreover, ovary ultrasound characteristics appear to predict ovarian activity, where ovarian volume/area and follicle count are associated with the age of menopause and primordial follicles in POF patients (257, 258). Some clinical trials have been established for endogenous etiological substances, such as the etiological elements endocan, sFlt-1, PIGF, and niacin, in POF patients (NCT03924648, NCT03932877 and NCT04641624). The above prospective studies included POF patients and normal controls with some potential molecular analysis, and blood was obtained at the early follicular phase of the menstrual cycle. These molecular functions are not well studied in the POF mechanism and diagnosis, whereas they are significant in follicle development and POF population (259–261). These works underscore the idea that additional attempts are needed to understand the impact of POF discrete microenvironments and potential diagnostic markers.
Therapeutic strategies
A randomized clinical trial from South Valley University proposed a hypothesis by using filgrastim to recover ovary functions, with 10 participants from 16 to 40 years (NCT02783937). Filgrastim is a granulocyte-colony stimulating factor (G-CSF) and has been approved to stimulate peripheral blood stem cell numbers (262) and potentially improve ovarian follicle formation (263). In ovarian failure, there are still some residual very small embryonic-like stem cells (VSELs) that serve as a backup pool for mature stem cells and are mobilized under stress conditions, which might be involved in gonadal rescue after exogenous/endogenous stimuli (264, 265). Filgrastim treatment in combination with stem cell studies in a mouse model showed promising results in the recovery of oogenesis and reproductive capacity (266, 267); thus, further clinical studies in humans potentially promote more appropriate treatment for POF. Recently, another clinical trial with 150 participants used the natural plant antitoxin resveratrol to target the NOX/ROS cascade and improve oxidative stress in POF patients (NCT05410093). NOX function in oxidative damage is harmful to ovarian function and structure (268). Moreover, resveratrol exhibited estrogen-like effects and showed great potential in estrogen deficiency-related osteoporosis, as well as increasing ovarian serum estrogen (269). Estrogen upregulation will improve the POF clinical presentation. In this respect, some chemotherapy-induced POF was potentially prevented by the gonadotropin-releasing hormone agonist goserelin (270). The application of goserelin is associated with amelioration of ovarian reserve markers such as AMH, estradiol and FSH, as well as improving ovarian function (271). Nevertheless, further exploration of goserelin function in chemotherapy-induced POF through ovarian function biomarkers are still ongoing (NCT04536467). On the other hand, many trials have focused on systemic balance regulation in POF treatment. Zhang et al. (272) demonstrated that Kuntai capsule, a traditional Chinese medicine, has been widely used for the clinical treatment of menopausal syndrome and showed the ability to improve damaged ovarian function. By improving atretic follicles, AMH expression, the antioxidant pathway, and the Bcl-2/Bax-related apoptotic pathway, Kuntai capsule presented therapeutic potential in the symptoms caused by ovarian failure and ovarian endocrine function recovery. In addition, another POF mouse model demonstrated that Kuntai capsules might inhibit PI3K/AKT/mTOR signaling by decreasing the phosphorylation of pathway protein members, as well as recovering AMH, FSH, and estradiol, ultimately improving ovarian function and protecting reproductive capacity in POF (273). More recently, a random clinical trial with 120 participants was established, which aimed at the efficacy and safety of the Kuntai capsule in POF patients and first used the Kupperman score to evaluate the therapeutic efficacy of the Kuntai capsule (NCT05021094). Another traditional Chinese medicine (HuYang YangKun Formula) was also put forward for POF treatment (NCT02794948), whose systematic regulatory effect significantly improves the ovarian function of POF, such as AMH, FSH, follicle number, TGF-β/TAK1 signaling, and JAK2/STAT3 signaling (274, 275). The ongoing clinical trial is exploring more effective strategies to address these issues so that we can begin to provide etiology-based management for POF-affected women.
Conclusion and prospective
Despite the increase in emerging research, the underlying mechanisms of POF remain poorly understood due to its variable etiology and complex microenvironment. We summarized the recent dynamic changes in POF that are partly important relevant biomarkers in ovarian development by animal and clinical data, which provided essential evidence to confirm the heterogeneity of genomic variants, gene expression and relevant proteins in POF phenotype and etiology. This review evaluated the role of the above important factor status and pathogenic mechanisms in POF, as well as the downstream and downstream consequences. Future genetic studies should involve different ethnic groups and larger sample sizes to promote the understanding of underlying genetic mechanisms in POF. Additionally, effective diagnosis and management necessitate more reliable biomarker targets in the early point POF, wherein the residual ovarian functions may offer an invaluable chance to intervene early. Currently, the therapeutic options for ovarian functional decline, either physiologically or pathologically, are limited. Established clinical trials provide new insights into developing strategies for ovarian resumption and fertility improvement in POF patients. However, the limitations of current genomic studies have restricted the clinical exploration of POF therapy. Therefore, exploring gene-based effective diagnosis and treatment strategies will be beneficial for the physical, mental, and reproductive health of POF patients.
Author contributions
XY: original draft and visualization preparation. LY: supervision and concepts of the manuscript. Both authors read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bilgin EM, Kovanci E. Genetics of premature ovarian failure. Current Opin Obstet Gynecol. (2015) 27:167–74.
3. Conway GS. Clinical manifestations of genetic defects affecting gonadotrophins and their receptors. Clin Endocrinol. (1996) 45:657–63.
4. van Kasteren YM, Hundscheid RD, Smits AP, Cremers FP, van Zonneveld P, Braat DD. Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod (Oxford, England). (1999) 14:2455–9. doi: 10.1093/humrep/14.10.2455
5. Kalantaridou SN, Davis SR, Nelson LM. Premature ovarian failure. Endocrinol Metab Clin North Am. (1998) 27:989–1006.
6. Vujović S, Ivović M, Tancić-Gajić M, Marina L, Barać M, Arizanović Z, et al. Premature ovarian failure. Srpski Arhiv Celokupno Lekarstvo. (2012) 140:806–11.
8. Benetti-Pinto CL, de Almeida DM, Makuch MY. Quality of life in women with premature ovarian failure. Gynecol Endocrinol. (2011) 27:645–9.
9. Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. (2015) 21:787–808.
10. Vegetti W, Marozzi A, Manfredini E, Testa G, Alagna F, Nicolosi A, et al. Premature ovarian failure. Mol Cell Endocrinol. (2000) 161:53–7.
11. Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. Endocr Rev. (2016) 37:609–35. doi: 10.1210/er.2016-1047
12. Qin Y, Sun M, You L, Wei D, Sun J, Liang X, et al. ESR1, HK3 and BRSK1 gene variants are associated with both age at natural menopause and premature ovarian failure. Orphanet J Rare Dis. (2012) 7:5. doi: 10.1186/1750-1172-7-5
13. Jin H, Ahn J, Park Y, Sim J, Park HS, Ryu CS, et al. Identification of potential causal variants for premature ovarian failure by whole exome sequencing. BMC Med Genomics. (2020) 13:159. doi: 10.1186/s12920-020-00813-x
14. Mehrjooy S, Nikbakht R, Mohammadi Asl J, Ghadiri A, Ghandil P. Utilization of whole exome sequencing in non-syndromic premature ovarian failure: ficolin-3 gene mutation in an iranian family. Iran Biomed J. (2021) 25:441–6. doi: 10.52547/ibj.25.6.441
15. Taryma-Leśniak O, Sokolowska KE, Wojdacz TK. Current status of development of methylation biomarkers for in vitro diagnostic IVD applications. Clin. Epigenet. (2020) 12:100.
16. Russell MR, Walker MJ, Williamson AJ, Gentry-Maharaj A, Ryan A, Kalsi J, et al. Protein Z: a putative novel biomarker for early detection of ovarian cancer. Int J Cancer. (2016) 138:2984–92. doi: 10.1002/ijc.30020
17. Wood-Trageser MA, Gurbuz F, Yatsenko SA, Jeffries EP, Kotan LD, Surti U, et al. MCM9 mutations are associated with ovarian failure, short stature, and chromosomal instability. Am J Hum Genet. (2014) 95:754–62. doi: 10.1016/j.ajhg.2014.11.002
18. Quilter CR, Karcanias AC, Bagga MR, Duncan S, Murray A, Conway GS, et al. Analysis of X chromosome genomic DNA sequence copy number variation associated with premature ovarian failure (POF). Hum Reprod (Oxford, England). (2010) 25:2139–50.
19. Rizzolio F, Bione S, Villa A, Berti E, Cassetti A, Bulfone A, et al. Spatial and temporal expression of POF1B, a gene expressed in epithelia. Gene Expr Patterns. (2007) 7:529–34. doi: 10.1016/j.modgep.2006.10.005
20. Bione S, Rizzolio F, Sala C, Ricotti R, Goegan M, Manzini MC, et al. Mutation analysis of two candidate genes for premature ovarian failure, DACH2 and POF1B. Hum Reprod (Oxford, England). (2004) 19:2759–66. doi: 10.1093/humrep/deh502
21. Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: online mendelian inheritance in man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. (2015) 43:D789–98. doi: 10.1093/nar/gku1205
22. Lacombe A, Lee H, Zahed L, Choucair M, Muller JM, Nelson SF, et al. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am J Hum Genet. (2006) 79:113–9. doi: 10.1086/505406
23. Tucker EJ, Bell KM, Robevska G, van den Bergen J, Ayers KL, Listyasari N, et al. Meiotic genes in premature ovarian insufficiency: variants in HROB and REC8 as likely genetic causes. Eur J Hum Genet. (2022) 30:219–28. doi: 10.1038/s41431-021-00977-9
24. Padovano V, Lucibello I, Alari V, Mina P. Della, Crespi A, Ferrari I, et al. The POF1B candidate gene for premature ovarian failure regulates epithelial polarity. J Cell Sci. (2011) 124:3356–68. doi: 10.1242/jcs.088237
25. Yuan ZZ, Wang CY, Jin JY, Sheng Y, Zhao MF, Fan LL, et al. A novel POF1B variant in a Chinese patient is associated with premature ovarian failure. Clin Genet. (2021) 100:773–4. doi: 10.1111/cge.14048
26. Oral E, Toksoy G, Sofiyeva N, Celik HG, Karaman B, Basaran S, et al. Clinical and genetic investigation of premature ovarian insufficiency cases from Turkey. J Gynecol Obstetr Hum Reprod. (2019) 48:817–23. doi: 10.1016/j.jogoh.2019.04.007
27. Crespi A, Bertoni A, Ferrari I, Padovano V, Mina P. Della, Berti E, et al. POF1B localizes to desmosomes and regulates cell adhesion in human intestinal and keratinocyte cell lines. J Invest Dermatol. (2015) 135:192–201. doi: 10.1038/jid.2014.327
28. Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. (2005) 11:461–71.
29. Bouazzi L, Sproll P, Eid W, Biason-Lauber A. The transcriptional regulator CBX2 and ovarian function: a whole genome and whole transcriptome approach. Sci Rep. (2019) 9:17033. doi: 10.1038/s41598-019-53370-4
30. Eid W, Opitz L, Biason-Lauber A. Genome-wide identification of CBX2 targets: insights in the human sex development network. Molec Endocrinol (Baltimore, Md.). (2015) 29:247–57. doi: 10.1210/me.2014-1339
31. Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol (Baltimore, Md.). (1998) 12:1809–17. doi: 10.1210/mend.12.12.0206
32. Sanfins A, Rodrigues P, Albertini DF. G9 and BMP-15 direct the follicle symphony. J Assist Reproduct Genet. (2018) 35:1741–50. doi: 10.1007/s10815-018-1268-4
33. Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. (2006) 91:1976–9. doi: 10.1210/jc.2005-2650
34. Clelland E, Kohli G, Campbell RK, Sharma S, Shimasaki S, Peng C. Bone morphogenetic protein-15 in the zebrafish ovary: complementary deoxyribonucleic acid cloning, genomic organization, tissue distribution, and role in oocyte maturation. Endocrinology. (2006) 147:201–9. doi: 10.1210/en.2005-1017
35. Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update. (2016) 23:1–18. doi: 10.1093/humupd/dmw039
36. Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update. (2014) 20:869–83. doi: 10.1093/humupd/dmu036
37. Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem. (2000) 275:39523–8.
38. Ahmad HI, Liu G, Jiang X, Edallew SG, Wassie T, Tesema B, et al. Maximum-likelihood approaches reveal signatures of positive selection in BMP15 and GDF9 genes modulating ovarian function in mammalian female fertility. Ecol Evol. (2017) 7:8895–902. doi: 10.1002/ece3.3336
39. Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. (2008) 14:159–77.
40. Tiotiu D, Alvaro Mercadal B, Imbert R, Verbist J, Demeestere I, Leener A. De, et al. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod (Oxford, England). (2010) 25:1581–7. doi: 10.1093/humrep/deq073
41. Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, Grammatico P, et al. BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat. (2009) 30:804–10. doi: 10.1002/humu.20961
42. Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. (2004) 75:106–11. doi: 10.1086/422103
43. Alam MH, Miyano T. Interaction between growing oocytes and granulosa cells in vitro. Reproduct Med Biol. (2020) 19:13–23.
44. Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol (Baltimore, Md.). (2001) 15:854–66.
45. Li JJ, Sugimura S, Mueller TD, White MA, Martin GA, Ritter LJ, et al. Modifications of human growth differentiation factor 9 to improve the generation of embryos from low competence oocytes. Mol Endocrinol (Baltimore, Md.). (2015) 29:40–52. doi: 10.1210/me.2014-1173
46. Afkhami F, Shahbazi S, Farzadi L, Danaei S. Novel bone morphogenetic protein 15 (BMP15) gene variants implicated in premature ovarian insufficiency. Reproduct Biol Endocrinol. (2022) 20:42.
47. Patiño LC, Walton KL, Mueller TD, Johnson KE, Stocker W, Richani D, et al. BMP15 mutations associated with primary ovarian insufficiency reduce expression, activity, or synergy with GDF9. J Clin Endocrinol Metab. (2017) 102:1009–19.
48. Kumar R, Alwani M, Kosta S, Kaur R, Agarwal S. BMP15 and GDF9 gene mutations in premature ovarian failure. J Reprod Infertil. (2017) 18:185–9.
49. Fonseca DJ, Ortega-Recalde O, Esteban-Perez C, Moreno-Ortiz H, Patiño LC, Bermúdez OM, et al. BMP15 c.-9C>G promoter sequence variant may contribute to the cause of non-syndromic premature ovarian failure. Reproduct Biomed Online. (2014) 29:627–33. doi: 10.1016/j.rbmo.2014.07.018
50. Inagaki K, Shimasaki S. Impaired production of BMP-15 and GDF-9 mature proteins derived from proproteins WITH mutations in the proregion. Mol Cell Endocrinol. (2010) 328:1–7. doi: 10.1016/j.mce.2010.05.017
51. Belli M, Shimasaki S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam Horm. (2018) 107:317–48. doi: 10.1016/bs.vh.2017.12.003
52. Rossetti R, Ferrari I, Bestetti I, Moleri S, Brancati F, Petrone L, et al. Fundamental role of BMP15 in human ovarian folliculogenesis revealed by null and missense mutations associated with primary ovarian insufficiency. Hum Mutat. (2020) 41:983–97. doi: 10.1002/humu.23988
53. Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction (Cambridge, England). (2006) 132:191–206.
54. Mayer A, Fouquet B, Pugeat M, Misrahi M. BMP15 “knockout-like” effect in familial premature ovarian insufficiency with persistent ovarian reserve. Clin Genet. (2017) 92:208–12. doi: 10.1111/cge.12970
55. Gónzalez A, Ramírez-Lorca R, Calatayud C, Mendoza N, Ruiz A, Sáez ME, et al. Association of genetic markers within the BMP15 gene with anovulation and infertility in women with polycystic ovary syndrome. Fertil Steril. (2008) 90:447–9. doi: 10.1016/j.fertnstert.2007.06.083
56. Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, et al. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. (2006) 119:408–15.
57. Hanevik HI, Hilmarsen HT, Skjelbred CF, Tanbo T, Kahn JA. A single nucleotide polymorphism in BMP15 is associated with high response to ovarian stimulation. Reprod Biomed Online. (2011) 23:97–104.
58. Gajewska A, Herman AP, Wolińska-Witort E, Kochman K, Zwierzchowski L. In vivo oestrogenic modulation of Egr1 and Pitx1 gene expression in female rat pituitary gland. J Mol Endocrinol. (2014) 53:355–66. doi: 10.1530/JME-14-0092
59. Liu H, Yang H, Qin Z, Chen Y, Yu H, Li W, et al. Exploration of the danggui buxue decoction mechanism regulating the balance of ESR and AR in the TP53-AKT signaling pathway in the prevention and treatment of POF. Evid Based Complement Altern Med. (2021) 2021:4862164. doi: 10.1155/2021/4862164
60. Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. (1991) 67:1047–58. doi: 10.1016/0092-8674(91)90283-5
62. Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, et al. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. (1993) 3:36–43. doi: 10.1038/ng0193-36
63. Pastore LM, Johnson J. The FMR1 gene, infertility, and reproductive decision-making: a review. Front Genet. (2014) 5:195. doi: 10.3389/fgene.2014.00195
64. Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. (2005) 117:376–82. doi: 10.1007/s00439-005-1326-8
65. Schufreider A, McQueen DB, Lee SM, Allon R, Uhler ML, Davie J, et al. Diminished ovarian reserve is not observed in infertility patients with high normal CGG repeats on the fragile X mental retardation 1 (FMR1) gene. Hum Reprod (Oxford, England). (2015) 30:2686–92.
66. Gleicher N, Barad DH. The FMR1 gene as regulator of ovarian recruitment and ovarian reserve. Obstet Gynecol Surv. (2010) 65:523–30. doi: 10.1097/OGX.0b013e3181f8bdda
67. Rehnitz J, Alcoba DD, Brum IS, Hinderhofer K, Youness B, Strowitzki T, et al. FMR1 and AKT/mTOR signalling pathways: potential functional interactions controlling folliculogenesis in human granulosa cells. Reprod Biomed Online. (2017) 35:485–93. doi: 10.1016/j.rbmo.2017.07.016
68. Hundscheid RD, Sistermans EA, Thomas CM, Braat DD, Straatman H, Kiemeney LA, et al. Imprinting effect in premature ovarian failure confined to paternally inherited fragile X premutations. Am J Hum Genet. (2000) 66:413–8. doi: 10.1086/302774
69. Murray A, Ennis S, MacSwiney F, Webb J, Morton NE. Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet. (2000) 8:247–52. doi: 10.1038/sj.ejhg.5200451
70. Eslami A, Farahmand K, Totonchi M, Madani T, Asadpour U, Zari Moradi S, et al. FMR1 premutation: not only important in premature ovarian failure but also in diminished ovarian reserve. Hum Fertil (Cambridge, England). (2017) 20:120–5.
71. Chatterjee S, Maitra A, Kadam S, Patel Z, Gokral J, Meherji P. CCG repeat sizing in the FMR1 gene in Indian women with premature ovarian failure. Reprod Biomed Online. (2009) 19:281–6. doi: 10.1016/s1472-6483(10)60086-7
72. Ruth KS, Day FR, Hussain J, Martínez-Marchal A, Aiken CE, Azad A, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. (2021) 596:393–7.
73. Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, et al. The FMR1 premutation and reproduction. Fertil Steril. (2007) 87:456–65.
74. European Society of Human Genetics, European Society of Human Reproduction and Embryology. The need for interaction between assisted reproduction technology and genetics: recommendations of the European Societies of Human Genetics and Human Reproduction and Embryology. Hum Reprod (Oxford, England). (2006) 21:1971–3.
75. Guo T, Qin Y, Jiao X, Li G, Simpson JL, Chen ZJ. FMR1 premutation is an uncommon explanation for premature ovarian failure in Han Chinese. PLoS one (2014) 9:e103316. doi: 10.1371/journal.pone.0103316
76. Tang R, Yu Q. The significance of FMR1 CGG repeats in Chinese women with premature ovarian insufficiency and diminished ovarian reserve. Reprod Biol Endocrinol. (2020) 18:82.
77. Lu CL, Li R, Chen XN, Xu YY, Yan LY, Yan J, et al. The ‘normal’ range of FMR1 triple CGG repeats may be associated with primary ovarian insufficiency in China. Reprod Biomed Online. (2017) 34:175–80.
78. Shelly KE, Candelaria NR, Li Z, Allen EG, Jin P, Nelson DL. Ectopic expression of CGG-repeats alters ovarian response to gonadotropins and leads to infertility in a murine FMR1 premutation model. Hum Mol Genet. (2021) 30:923–38. doi: 10.1093/hmg/ddab083
79. Wang Q, Barad DH, Darmon SK, Kushnir VA, Wu YG, Lazzaroni-Tealdi E, et al. Reduced RNA expression of the FMR1 gene in women with low (CGGn<26) repeats. PLoS One. (2018) 13:e0209309. doi: 10.1371/journal.pone.0209309
80. Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. (2001) 10:1449–54. doi: 10.1093/hmg/10.14.1449
81. Schuettler J, Peng Z, Zimmer J, Sinn P, von Hagens C, Strowitzki T, et al. Variable expression of the Fragile X Mental Retardation 1 (FMR1) gene in patients with premature ovarian failure syndrome is not dependent on number of (CGG)n triplets in exon 1. Hum Reprod (Oxford, England). (2011) 26:1241–51. doi: 10.1093/humrep/der018
82. Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: i. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Müllerian hormone. Fertil Steril. (2009) 91:1700–6. doi: 10.1016/j.fertnstert.2008.01.098
83. Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, et al. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem. (2008) 283:18478–82. doi: 10.1074/jbc.C800055200
84. Ascano M Jr., Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. (2012) 492:382–6.
85. Mok-Lin E, Ascano M Jr., Serganov A, Rosenwaks Z, Tuschl T, Williams Z. Premature recruitment of oocyte pool and increased mTOR activity in Fmr1 knockout mice and reversal of phenotype with rapamycin. Sci. Rep. (2018) 8:588. doi: 10.1038/s41598-017-18598-y
86. Conca Dioguardi C, Uslu B, Haynes M, Kurus M, Gul M, Miao DQ, et al. Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency. Mol Hum Reprod. (2016) 22:384–96. doi: 10.1093/molehr/gaw023
87. Shiratsuki S, Hara T, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Low oxygen level increases proliferation and metabolic changes in bovine granulosa cells. Mol Cell Endocrinol. (2016) 437:75–85.
88. Heng D, Sheng X, Tian C, Li J, Liu L, Gou M, et al. Mtor inhibition by INK128 extends functions of the ovary reconstituted from germline stem cells in aging and premature aging mice. Aging Cell. (2021) 20:e13304. doi: 10.1111/acel.13304
89. Davis RJ, Harding M, Moayedi Y, Mardon G. Mouse Dach1 and Dach2 are redundantly required for Müllerian duct development. Genesis (New York, N.Y.). (2008) 46:205–13. doi: 10.1002/dvg.20385
90. Keisman EL, Baker BS. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development (Cambridge, England). (2001) 128:1643–56. doi: 10.1242/dev.128.9.1643
91. Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development (Cambridge, England). (1994) 120:3473–86.
92. Behr SC, Courtier JL, Qayyum A. Imaging of müllerian duct anomalies. Radiographics. (2012) 32:E233–50.
93. Venkata VD, Jamaluddin MFB, Goad J, Drury HR, Tadros MA, Lim R, et al. Development and characterization of human fetal female reproductive tract organoids to understand Müllerian duct anomalies. Proc Natl Acad Sci U S A. (2022) 119:e2118054119. doi: 10.1073/pnas.2118054119
94. Suzumori N, Pangas SA, Rajkovic A. Candidate genes for premature ovarian failure. Curr Med Chem. (2007) 14:353–7.
95. Nodin B, Fridberg M, Uhlén M, Jirström K. Discovery of dachshund 2 protein as a novel biomarker of poor prognosis in epithelial ovarian cancer. J Ovarian Res. (2012) 5:6. doi: 10.1186/1757-2215-5-6
96. Sala C, Arrigo G, Torri G, Martinazzi F, Riva P, Larizza L, et al. Eleven X chromosome breakpoints associated with premature ovarian failure (POF) map to a 15-Mb YAC contig spanning Xq21. Genomics. (1997) 40:123–31. doi: 10.1006/geno.1996.4542
97. Prueitt RL, Ross JL, Zinn AR. Physical mapping of nine Xq translocation breakpoints and identification of XPNPEP2 as a premature ovarian failure candidate gene. Cytogenet Cell. Genet. (2000) 89:44–50. doi: 10.1159/000015560
98. Mumm S, Herrera L, Waeltz PW, Scardovi A, Nagaraja R, Esposito T, et al. X/autosomal translocations in the Xq critical region associated with premature ovarian failure fall within and outside genes. Genomics. (2001) 76:30–6. doi: 10.1006/geno.2001.6611
99. Kim SS, Zhang RG, Braunstein SE, Joachimiak A, Cvekl A, Hegde RS. Structure of the retinal determination protein Dachshund reveals a DNA binding motif. Structure (London, England). (2002) 10:787–95. doi: 10.1016/s0969-2126(02)00769-4
100. Davis RJ, Shen W, Sandler YI, Heanue TA, Mardon G. Characterization of mouse Dach2, a homologue of Drosophila dachshund. Mech Dev. (2001) 102:169–79. doi: 10.1016/s0925-4773(01)00307-0
101. Misra S, Pandey AK, Gupta S, Kumar A, Khanna P, Shankar J, et al. Estrogen related receptor is required for the testicular development and for the normal sperm axoneme/mitochondrial derivatives in Drosophila males. Sci Rep. (2017) 7:40372. doi: 10.1038/srep40372
102. Xu H, Jiao Y, Yi M, Zhao W, Wu K. EYA2 correlates with clinico-pathological features of breast cancer, promotes tumor proliferation, and predicts poor survival. Front Oncol. (2019) 9:26. doi: 10.3389/fonc.2019.00026
103. Prueitt RL, Chen H, Barnes RI, Zinn AR. Most X;autosome translocations associated with premature ovarian failure do not interrupt X-linked genes. Cytogenet Genome Res. (2002) 97:32–8.
104. Busslinger GA, Stocsits RR, van der Lelij P, Axelsson E, Tedeschi A, Galjart N, et al. Cohesin is positioned in mammalian genomes by transcription, CTCF and Wapl. Nature. (2017) 544:503–7.
105. Davis RJ, Pesah YI, Harding M, Paylor R, Mardon G. Mouse Dach2 mutants do not exhibit gross defects in eye development or brain function. Genesis (New York, N.Y.). (2006) 44:84–92.
106. Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A. Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns. (2006) 6:1014–8.
107. Toyoda S, Miyazaki T, Miyazaki S, Yoshimura T, Yamamoto M, Tashiro F, et al. Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev Biol. (2009) 325:238–48. doi: 10.1016/j.ydbio.2008.10.019
108. Qin Y, Jiao X, Dalgleish R, Vujovic S, Li J, Simpson JL, et al. Novel variants in the SOHLH2 gene are implicated in human premature ovarian failure. Fertil Steril. (2014) 101:1104–9.e6. doi: 10.1016/j.fertnstert.2014.01.001
109. Pan B, Chao H, Chen B, Zhang L, Li L, Sun X, et al. DNA methylation of germ-cell-specific basic helix-loop-helix (HLH) transcription factors, Sohlh2 and Figlα during gametogenesis. Mol Hum Reprod. (2011) 17:550–61.
110. Choi Y, Yuan D, Rajkovic A. Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol Reprod. (2008) 79:1176–82. doi: 10.1095/biolreprod.108.071217
111. Zhang X, Zhang H, Gao Q, Ji S, Bing L, Hao J. Sohlh2 inhibits the apoptosis of mouse primordial follicle oocytes via C-kit/PI3K/Akt/Foxo3a signalling pathway. Reprod Biomed Online. (2015) 30:514–21. doi: 10.1016/j.rbmo.2015.01.015
112. Ding X, Zhang X, Mu Y, Li Y, Hao J. Effects of BMP4/SMAD signaling pathway on mouse primordial follicle growth and survival via up-regulation of Sohlh2 and c-kit. Mol Reprod Dev. (2013) 80:70–8. doi: 10.1002/mrd.22138
115. Castets S, Roucher-Boulez F, Saveanu A, Mallet-Motak D, Chabre O, Amati-Bonneau P, et al. Hypopituitarism in patients with blepharophimosis and FOXL2 mutations. Horm Res Paediatr. (2020) 93:30–9. doi: 10.1159/000507249
116. Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. (2001) 27:159–66.
117. Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. (2009) 139:1130–42.
118. Fan J, Zhou Y, Huang X, Zhang L, Yao Y, Song X, et al. The combination of polyalanine expansion mutation and a novel missense substitution in transcription factor FOXL2 leads to different ovarian phenotypes in blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) patients. Hum Reprod (Oxford, England). (2012) 27:3347–57. doi: 10.1093/humrep/des306
119. Gupta AK, Gupta DC, Khan SA, Razi SM. Blepharophimosis ptosis epicanthus inversus syndrome (BPES) type 1 in an Indian Family. J ASEAN Fed Endocr Soc. (2017) 32:68–71. doi: 10.15605/jafes.032.01.13
120. De Baere E, Beysen D, Oley C, Lorenz B, Cocquet J, De Sutter P, et al. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am J Hum Genet. (2003) 72:478–87. doi: 10.1086/346118
121. Harris SE, Chand AL, Winship IM, Gersak K, Aittomäki K, Shelling AN. Identification of novel mutations in FOXL2 associated with premature ovarian failure. Mol Hum Reprod. (2002) 8:729–33.
122. Laissue P, Lakhal B, Benayoun BA, Dipietromaria A, Braham R, Elghezal H, et al. Functional evidence implicating FOXL2 in non-syndromic premature ovarian failure and in the regulation of the transcription factor OSR2. J Med Genet. (2009) 46:455–7. doi: 10.1136/jmg.2008.065086
123. Corrêa FJ, Tavares AB, Pereira RW, Abrão MS. A new FOXL2 gene mutation in a woman with premature ovarian failure and sporadic blepharophimosis-ptosis-epicanthus inversus syndrome. Fertil Steril. (2010) 93:1006.e3–6. doi: 10.1016/j.fertnstert.2009.08.034
124. Li F, Chen H, Wang Y, Yang J, Zhou Y, Song X, et al. Functional studies of novel FOXL2 variants in chinese families with blepharophimosis-ptosis-epicanthus inversus syndrome. Front Genet. (2021) 12:616112. doi: 10.3389/fgene.2021.616112
125. Méjécase C, Nigam C, Moosajee M, Bladen JC. The genetic and clinical features of FOXL2-related blepharophimosis, ptosis and epicanthus inversus syndrome. Genes. (2021) 12:364. doi: 10.3390/genes12030364
126. França MM, Mendonca BB. Genetics of ovarian insufficiency and defects of folliculogenesis. Best Pract Res Clin Endocrinol Metab. (2022) 36:101594.
127. Settas N, Anapliotou M, Kanavakis E, Fryssira H, Sofocleous C, Dacou-Voutetakis C, et al. A novel FOXL2 gene mutation and BMP15 variants in a woman with primary ovarian insufficiency and blepharophimosis-ptosis-epicanthus inversus syndrome. Menopause (New York, N.Y.). (2015) 22:1264–8. doi: 10.1097/GME.0000000000000473
128. Georges A, Auguste A, Bessière L, Vanet A, Todeschini AL, Veitia RA. FOXL2: a central transcription factor of the ovary. J Mol Endocrinol. (2014) 52:R17–33.
129. Nuovo S, Passeri M, Di Benedetto E, Calanchini M, Meldolesi I, Giacomo M.C. Di, et al. Characterization of endocrine features and genotype-phenotypes correlations in blepharophimosis-ptosis-epicanthus inversus syndrome type 1. J Endocrinol Invest. (2016) 39:227–33. doi: 10.1007/s40618-015-0334-3
130. González-Niño C, Yeste D, Carrascosa A. Tamoxifen treatment in a patient with blepharophimosis-ptosis-epicanthus inversus syndrome and peripheral precocious puberty. J Pediatr Endocrinol Metab. (2007) 20:445–8. doi: 10.1515/jpem.2007.20.3.445
131. Chacón-Camacho OF, Salgado-Medina A, Alcaraz-Lares N, ópez-Moreno DL, Barragán-Arévalo T, Nava-Castañeda A, et al. Clinical characterization and identification of five novel FOXL2 pathogenic variants in a cohort of 12 Mexican subjects with the syndrome of blepharophimosis-ptosis-epicanthus inversus. Gene. (2019) 706:62–8. doi: 10.1016/j.gene.2019.04.073
133. Méduri G, Bachelot A, Duflos C, Bständig B, Poirot C, Genestie C, et al. FOXL2 mutations lead to different ovarian phenotypes in BPES patients: case report. Hum Reprod (Oxford, England). (2010) 25:235–43. doi: 10.1093/humrep/dep355
134. Xu K, Chen X, Yang H, Xu Y, He Y, Wang C, et al. Maternal Sall4 is indispensable for epigenetic maturation of mouse oocytes. J Biol Chem. (2017) 292:1798–807. doi: 10.1074/jbc.M116.767061
135. Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. (2006) 8:1114–23. doi: 10.1038/ncb1481
136. Wang B, Li L, Ni F, Song J, Wang J, Mu Y, et al. Mutational analysis of SAL-Like 4 (SALL4) in Han Chinese women with premature ovarian failure. Mol Hum Reprod. (2009) 15:557–62. doi: 10.1093/molehr/gap046
137. Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod. (2007) 77:312–9. doi: 10.1095/biolreprod.107.060459
138. Wang J, Wang B, Song J, Suo P, Ni F, Chen B, et al. New candidate gene POU5F1 associated with premature ovarian failure in Chinese patients. Reprod Biomed Online. (2011) 22:312–6. doi: 10.1016/j.rbmo.2010.11.008
139. Wang Q, Li D, Cai B, Chen Q, Li C, Wu Y, et al. Whole-exome sequencing reveals SALL4 variants in premature ovarian insufficiency: an update on genotype-phenotype correlations. Hum Genet. (2019) 138:83–92. doi: 10.1007/s00439-018-1962-4
140. Bhartiya D, Patel H. An overview of FSH-FSHR biology and explaining the existing conundrums. J Ovarian Res. (2021) 14:144. doi: 10.1186/s13048-021-00880-3
141. Tapanainen JS, Aittomäki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. (1997) 15:205–6. doi: 10.1038/ng0297-205
142. Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, et al. A Novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab. (2002) 87:1151–5. doi: 10.1210/jcem.87.3.8319
143. Ledig S, Röpke A, Haeusler G, Hinney B, Wieacker P. BMP15 mutations in XX gonadal dysgenesis and premature ovarian failure. Am J Obstetr Gynecol. (2008) 198:84.e1–5.
144. Kim S, Pyun JA, Cha DH, Ko JJ, Kwack K. Epistasis between FSHR and CYP19A1 polymorphisms is associated with premature ovarian failure. Fertil Steril. (2011) 95:2585–8. doi: 10.1016/j.fertnstert.2010.12.042
145. Aittomäki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. (1995) 82:959–68.
146. Katari S, Wood-Trageser MA, Jiang H, Kalynchuk E, Muzumdar R, Yatsenko SA, et al. Novel inactivating mutation of the fsh receptor in two siblings of indian origin with premature ovarian failure. J Clin Endocrinol Metab. (2015) 100:2154–7. doi: 10.1210/jc.2015-1401
147. Banerjee AA, Achrekar SK, Joseph S, Pathak BR, Mahale SD. Functional characterization of two naturally occurring mutations V(221)G and T(449)N in the follicle stimulating hormone receptor. Mol Cell Endocrinol. (2017) 440:69–79. doi: 10.1016/j.mce.2016.11.020
148. Bramble MS, Goldstein EH, Lipson A, Ngun T, Eskin A, Gosschalk JE, et al. A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: a fertility application of whole exome sequencing. Hum Reprod (Oxford, England). (2016) 31:905–14. doi: 10.1093/humrep/dew025
149. Ghezelayagh Z, Totonchi M, Zarei-Moradi S, Asadpour O, Maroufizadeh S, Eftekhari-Yazdi P, et al. The impact of genetic variation and gene expression level of the follicle-stimulating hormone receptor on ovarian reserve. Cell J. (2018) 19:620–6. doi: 10.22074/cellj.2018.4183
150. Oduwole OO, Huhtaniemi IT, Misrahi M. The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int J Mol Sci. (2021) 22:12735. doi: 10.3390/ijms222312735
151. Liu H, Guo T, Gong Z, Yu Y, Zhang Y, Zhao S, et al. Novel FSHR mutations in Han Chinese women with sporadic premature ovarian insufficiency. Mol Cell Endocrinol. (2019) 492:110446. doi: 10.1016/j.mce.2019.05.005
152. Siegel ET, Kim HG, Nishimoto HK, Layman LC. The molecular basis of impaired follicle-stimulating hormone action: evidence from human mutations and mouse models. Reprod Sci (Thousand Oaks, Calif.). (2013) 20:211–33. doi: 10.1177/1933719112461184
153. Desai SS, Roy BS, Mahale SD. Mutations and polymorphisms in FSH receptor: functional implications in human reproduction. Reproduction (Cambridge, England). (2013) 146:R235–48.
154. Desai SS, Achrekar SK, Sahasrabuddhe KA, Meharji PK, Desai SK, Mangoli VS, et al. Functional characterization of two naturally occurring mutations (Val514Ala and Ala575Val) in follicle-stimulating hormone receptor. J Clin Endocrinol Metab. (2015) 100:E638–45. doi: 10.1210/jc.2014-3662
155. Huang W, Cao Y, Shi L. Effects of FSHR polymorphisms on premature ovarian insufficiency in human beings: a meta-analysis. Reprod Biol Endocrinol. (2019) 17:80. doi: 10.1186/s12958-019-0528-1
156. Liu H, Xu X, Han T, Yan L, Cheng L, Qin Y, et al. A novel homozygous mutation in the FSHR gene is causative for primary ovarian insufficiency. Fertil Steril. (2017) 108:1050–55.e2.
157. Polyzos NP, Neves AR, Drakopoulos P, Spits C, Alvaro Mercadal B, Garcia S, et al. The effect of polymorphisms in FSHR and FSHB genes on ovarian response: a prospective multicenter multinational study in Europe and Asia. Hum Reprod (Oxford, England). (2021) 36:1711–21. doi: 10.1093/humrep/deab068
158. Vilodre LC, Kohek MB, Spritzer PM. Screening of follicle-stimulating hormone receptor gene in women with premature ovarian failure in southern Brazil and associations with phenotype. J Endocrinol Invest. (2008) 31:552–7. doi: 10.1007/BF03346407
159. Cordts EB, Santos MC, Bianco B, Barbosa CP, Christofolini DM. Are FSHR polymorphisms risk factors to premature ovarian insufficiency? Gynecol Endocrinol. (2015) 31:663–6.
160. Hu KL, Gan K, Ying Y, Zheng J, Chen R, Xue J, et al. Oligo/Amenorrhea is an independent risk factor associated with low ovarian response. Front Endocrinol. (2021) 12:612042. doi: 10.3389/fendo.2021.612042
161. Li X, Xie J, Wang Q, Cai H, Xie C, Fu X. miR-21 and Pellino-1 expression profiling in autoimmune premature ovarian insufficiency. J Immunol Res. (2020) 2020:3582648. doi: 10.1155/2020/3582648
162. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin. Endocrinol. (2008) 68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x
163. Li J, Fan S, Han D, Xie J, Kuang H, Ge P. Microarray gene expression profiling and bioinformatics analysis of premature ovarian failure in a rat model. Exp Mol Pathol. (2014) 97:535–41. doi: 10.1016/j.yexmp.2014.10.015
165. Kuang H, Han D, Xie J, Yan Y, Li J, Ge P. Profiling of differentially expressed microRNAs in premature ovarian failure in an animal model. Gynecol Endocrinol. (2014) 30:57–61. doi: 10.3109/09513590.2013.850659
166. Winking H, Gerdes J, Traut W. Expression of the proliferation marker Ki-67 during early mouse development. Cytogenet Genome Res. (2004) 105:251–6.
167. Islam MR, Ichii O, Nakamura T, Irie T, Masum MA, Otani Y, et al. Developmental changes of the ovary in neonatal cotton rat (Sigmodon hispidus). Front Physiol. (2020) 11:601927. doi: 10.3389/fphys.2020.601927
168. Sobecki M, Mrouj K, Colinge J, Gerbe F, Jay P, Krasinska L, et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res. (2017) 77:2722–34.
169. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell. Physiol. (2000) 182:311–22.
170. Gavish Z, Spector I, Peer G, Schlatt S, Wistuba J, Roness H, et al. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J. Assist Reprod Genet. (2018) 35:61–9. doi: 10.1007/s10815-017-1079-z
171. Chen XY, Gu C, Ma M, Cong Q, Guo T, Ma D, et al. A mouse model of premature ovarian insufficiency induced by tripterygium glycoside via subcutaneous injection. Int J Clin Exp Pathol. (2014) 7:144–51.
172. Aguiar FLN, Gastal GDA, Ishak GM, Gastal MO, Teixeira DIA, Feugang JM, et al. Effects of FSH addition to an enriched medium containing insulin and EGF after long-term culture on functionality of equine ovarian biopsy tissue. Theriogenology. (2017) 99:124–33. doi: 10.1016/j.theriogenology.2017.04.045
173. Li S, Liu M, Ma H, Jin Q, Ma Y, Wang C, et al. Ameliorative effect of recombinant human lactoferrin on the premature ovarian failure in rats after cyclophosphamide treatments. J Ovarian Res. (2021) 14:17. doi: 10.1186/s13048-020-00763-z
174. Chaffin CL, Schwinof KM, Stouffer RL. Gonadotropin and steroid control of granulosa cell proliferation during the periovulatory interval in rhesus monkeys. Biol Reprod. (2001) 65:755–62. doi: 10.1095/biolreprod65.3.755
175. Alonso-Pozos I, Rosales-Torres AM, valos-Rodríguez AA, Vergara-Onofre M, Rosado-García A. Mechanism of granulosa cell death during follicular atresia depends on follicular size. Theriogenology. (2003) 60:1071–81.
176. Luo X, Xu J, Zhao R, Qin J, Wang X, Yan Y, et al. The role of inactivated NF-κB in premature ovarian failure. Am J Pathol. (2022) 192:468–83.
177. Guan HY, Xia HX, Chen XY, Wang L, Tang ZJ, Zhang W. Toll-like receptor 4 inhibits estradiol secretion via NF-κB signaling in human granulosa cells. Front Endocrinol. (2021) 12:629554. doi: 10.3389/fendo.2021.629554
178. Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, et al. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro. (2014) 28:1215–21. doi: 10.1016/j.tiv.2014.05.015
179. MacCallum DE, Hall PA. The location of pKi67 in the outer dense fibrillary compartment of the nucleolus points to a role in ribosome biogenesis during the cell division cycle. J Pathol. (2000) 190:537–44.
180. Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot. (2011) 107:1127–40.
181. Thomas H, Nasim MM, Sarraf CE, Alison MR, Love S, Lambert HE, et al. Proliferating cell nuclear antigen (PCNA) immunostaining–a prognostic factor in ovarian cancer? Br J Cancer. (1995) 71:357–62. doi: 10.1038/bjc.1995.72
182. Muskhelishvili L, Wingard SK, Latendresse JR. Proliferating cell nuclear antigen–a marker for ovarian follicle counts. Toxicol Pathol. (2005) 33:365–8. doi: 10.1080/01926230590930164
183. Mahran YF, Badr AM, Aldosari A, Bin-Zaid R, Alotaibi HN. Carvacrol and thymol modulate the cross-talk between TNF-α and IGF-1 signaling in radiotherapy-induced ovarian failure. Oxid Med Cell Longev. (2019) 2019:3173745. doi: 10.1155/2019/3173745
184. Tománek M, Chronowska E. Immunohistochemical localization of proliferating cell nuclear antigen (PCNA) in the pig ovary. Folia Histochem Cytobiol. (2006) 44:269–74.
185. Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. (2006) 11:422–34.
186. Delkhosh A, Delashoub M, Tehrani AA, Bahrami AM, Niazi V, Shoorei H, et al. Upregulation of FSHR and PCNA by administration of coenzyme Q10 on cyclophosphamide-induced premature ovarian failure in a mouse model. J Biochem Mol Toxicol. (2019) 33:e22398. doi: 10.1002/jbt.22398
187. Huang J, Zeng F, Xu Q, Ma J. Cryptotanshinone decreases granulosa cell apoptosis and restores ovarian function in mice with premature ovarian failure. Gen Physiol Biophys. (2020) 39:277–83. doi: 10.4149/gpb_2019059
188. Zhang H, Jiang X, Zhang Y, Xu B, Hua J, Ma T, et al. microRNA 376a regulates follicle assembly by targeting Pcna in fetal and neonatal mouse ovaries. Reproduction (Cambridge, England). (2014) 148:43–54. doi: 10.1530/REP-13-0508
189. Fu X, He Y, Wang X, Peng D, Chen X, Li X, et al. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. (2017) 8:187. doi: 10.1186/s13287-017-0641-z
190. Haraguchi H, Hirota Y, Saito-Fujita T, Tanaka T, Shimizu-Hirota R, Harada M, et al. Mdm2-p53-SF1 pathway in ovarian granulosa cells directs ovulation and fertilization by conditioning oocyte quality. FASEB J. (2019) 33:2610–20. doi: 10.1096/fj.201801401R
191. Del Rosario BC, Kriz AJ, Del Rosario AM, Anselmo A, Fry CJ, White FM, et al. Exploration of CTCF post-translation modifications uncovers Serine-224 phosphorylation by PLK1 at pericentric regions during the G2/M transition. eLife. (2019) 8:e42341. doi: 10.7554/eLife.42341
192. Alexander KA, Coté A, Nguyen SC, Zhang L, Gholamalamdari O, Agudelo-Garcia P, et al. p53 mediates target gene association with nuclear speckles for amplified RNA expression. Mol Cell. (2021) 81:1666–81.e6. doi: 10.1016/j.molcel.2021.03.006
193. Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. (1992) 71:587–97. doi: 10.1016/0092-8674(92)90593-2
194. Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. (2016) 42:63–71.
195. Mansilla SF, de la Vega MB, Calzetta NL, Siri SO, Gottifredi V. CDK-independent and PCNA-dependent functions of p21 in DNA replication. Genes. (2020) 11:593. doi: 10.3390/genes11060593
196. Liu TE, Zhang L, Wang S, Chen C, Zheng J. Tripterygium glycosides induce premature ovarian failure in rats by promoting p53 phosphorylation and activating the serine/threonine kinase 11-p53-p21 signaling pathway. Exp Ther Med. (2015) 10:12–8. doi: 10.3892/etm.2015.2498
197. El-Derany MO, Said RS, El-Demerdash E. Bone marrow-derived mesenchymal stem cells reverse radiotherapy-induced premature ovarian failure: emphasis on signal integration of TGF-β, Wnt/β-catenin and hippo pathways. Stem Cell Rev Rep. (2021) 17:1429–45.
198. Habara O, Logan CY, Kanai-Azuma M, Nusse R, Takase HM. WNT signaling in pre-granulosa cells is required for ovarian folliculogenesis and female fertility. Development (Cambridge, England). (2021) 148:dev198846. doi: 10.1242/dev.198846
199. Wang HX, Li TY, Kidder GM. WNT2 regulates DNA synthesis in mouse granulosa cells through beta-catenin. Biol. Reprod. (2010) 82:865–75.
200. Wang HX, Tekpetey FR, Kidder GM. Identification of WNT/beta-CATENIN signaling pathway components in human cumulus cells. Mol Hum Reprod. (2009) 15:11–7. doi: 10.1093/molehr/gan070
201. Hernandez Gifford JA. The role of WNT signaling in adult ovarian folliculogenesis. Reproduction (Cambridge, England). (2015) 150:R137–48.
202. Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med (Berlin, Germany). (2014) 92:433–9.
203. Luo Q, Yin N, Zhang L, Yuan W, Zhao W, Luan X, et al. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci. (2017) 179:103–9. doi: 10.1016/j.lfs.2017.05.001
204. Wang B, Suo P, Chen B, Wei Z, Yang L, Zhou S, et al. Haplotype analysis of chemokine CXCL12 polymorphisms and susceptibility to premature ovarian failure in Chinese women. Hum Reprod (Oxford, England). (2011) 26:950–4. doi: 10.1093/humrep/der001
205. Holt JE, Jackson A, Roman SD, Aitken RJ, Koopman P, McLaughlin EA. CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev Biol. (2006) 293:449–60. doi: 10.1016/j.ydbio.2006.02.012
206. Han Y, Yao R, Yang Z, Li S, Meng W, Zhang Y, et al. Interleukin-4 activates the PI3K/AKT signaling to promote apoptosis and inhibit the proliferation of granulosa cells. Exp Cell Res. (2022) 412:113002. doi: 10.1016/j.yexcr.2021.113002
207. Sanverdi I, Kilicci C, Cogendez E, Abide Yayla C, Ozkaya E. Utility of complete blood count parameters to detect premature ovarian insufficiency in cases with oligomenorrhea/amenorrhea. J Clin Lab Anal. (2018) 32:e22372. doi: 10.1002/jcla.22372
208. Townson DH, Liptak AR. Chemokines in the corpus luteum: implications of leukocyte chemotaxis. Reprod Biol Endocrinol. (2003) 1:94. doi: 10.1186/1477-7827-1-94
209. Chatterjee M, Rath D, Gawaz M. Role of chemokine receptors CXCR4 and CXCR7 for platelet function. Biochem Soc Trans. (2015) 43:720–6.
210. Ling L, Hou J, Liu D, Tang D, Zhang Y, Zeng Q, et al. Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI). Stem Cell Res. Ther. (2022) 13:79. doi: 10.1186/s13287-022-02759-6
211. Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. (2014) 35:5627–35. doi: 10.1016/j.biomaterials.2014.03.070
212. Wei FY, Leung KS, Li G, Qin J, Chow SK, Huang S, et al. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS One. (2014) 9:e106722. doi: 10.1371/journal.pone.0106722
214. Xiu G, Li X, Yin Y, Li J, Li B, Chen X, et al. SDF1/CXCR4 augments the therapeutic effect of bone marrow mesenchymal stem cells in the treatment of lipopolysaccharide-induced liver injury by promoting their migration through PI3K/Akt signaling pathway. Cell Trans. (2020) 29:963689720929992. doi: 10.1177/0963689720929992
215. Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. (2010) 16:2927–31.
216. Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J. Transl. Med. (2015) 13:155. doi: 10.1186/s12967-015-0516-y
217. Sheng L, Mao X, Yu Q, Yu D. Effect of the PI3K/AKT signaling pathway on hypoxia-induced proliferation and differentiation of bone marrow-derived mesenchymal stem cells. Exp Ther Med. (2017) 13:55–62.
218. Jozkowiak M, Hutchings G, Jankowski M, Kulcenty K, Mozdziak P, Kempisty B, et al. The stemness of human ovarian granulosa cells and the role of resveratrol in the differentiation of MSCs-A review based on cellular and molecular knowledge. Cells. (2020) 9:1418. doi: 10.3390/cells9061418
219. Basini G, Falasconi I, Bussolati S, Grolli S, Lecce R. Di, Grasselli F. Swine granulosa cells show typical endothelial cell characteristics. Reprod Sci (Thousand Oaks, Calif.). (2016) 23:630–7. doi: 10.1177/1933719115612130
220. Liu M, Qiu Y, Xue Z, Wu R, Li J, Niu X, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res. Ther. (2020) 11:3. doi: 10.1186/s13287-019-1508-2
221. Cho J, Kim TH, Seok J, Jun JH, Park H, Kweon M, et al. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab Invest A J Techn Methods Pathol. (2021) 101:304–17.
222. Yin N, Wang Y, Lu X, Liu R, Zhang L, Zhao W, et al. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res. Ther. (2018) 9:37.
223. Liang QX, Wang ZB, Lin F, Zhang CH, Sun HM, Zhou L, et al. Ablation of beta subunit of protein kinase CK2 in mouse oocytes causes follicle atresia and premature ovarian failure. Cell Death Dis. (2018) 9:508. doi: 10.1038/s41419-018-0505-1
224. Ivell R, Anand-Ivell R. Biology of insulin-like factor 3 in human reproduction. Hum Reprod Update. (2009) 15:463–76.
225. Dai Y, Ivell R, Anand-Ivell R. Theca cell INSL3 and steroids together orchestrate the growing bovine antral follicle. Front Physiol. (2017) 8:1033. doi: 10.3389/fphys.2017.01033
226. Bonser J, Walker J, Purohit A, Reed MJ, Potter BV, Willis DS, et al. Human granulosa cells are a site of sulphatase activity and are able to utilize dehydroepiandrosterone sulphate as a precursor for oestradiol production. J Endocrinol. (2000) 167:465–71. doi: 10.1677/joe.0.1670465
227. Irving-Rodgers HF, Bathgate RA, Ivell R, Domagalski R, Rodgers RJ. Dynamic changes in the expression of relaxin-like factor (INSL3), cholesterol side-chain cleavage cytochrome p450, and 3beta-hydroxysteroid dehydrogenase in bovine ovarian follicles during growth and atresia. Biol Reprod. (2002) 66:934–43. doi: 10.1095/biolreprod66.4.934
228. Ivell R, Anand-Ivell R. Insulin-like peptide 3 (INSL3) is a major regulator of female reproductive physiology. Hum Reprod Update. (2018) 24:639–51. doi: 10.1093/humupd/dmy029
229. Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl. Acad Sci U S A. (2004) 101:7323–8. doi: 10.1073/pnas.0307061101
230. Dai Y, Ivell R, Liu X, Janowski D, Anand-Ivell R. Relaxin-family peptide receptors 1 and 2 are fully functional in the bovine. Front. Physiol. (2017) 8:359. doi: 10.3389/fphys.2017.00359
231. Anand-Ivell R, Tremellen K, Dai Y, Heng K, Yoshida M, Knight PG, et al. Circulating insulin-like factor 3 (INSL3) in healthy and infertile women. Hum Reprod (Oxford, England). (2013) 28:3093–102.
232. Xue K, Kim JY, Liu JY, Tsang BK. Insulin-like 3-induced rat preantral follicular growth is mediated by growth differentiation factor 9. Endocrinology. (2014) 155:156–67. doi: 10.1210/en.2013-1491
233. Zhu C, Luo W, Li Z, Zhang X, Hu J, Zhao S, et al. New theca-cell marker insulin-like factor 3 is associated with premature ovarian insufficiency. Fertil Steril. (2021) 115:455–62. doi: 10.1016/j.fertnstert.2020.08.005
234. Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. (1996) 87:1862–72.
235. Camaioni A, Klinger FG, Campagnolo L, Salustri A. The influence of pentraxin 3 on the ovarian function and its impact on fertility. Front Immunol. (2018) 9:2808. doi: 10.3389/fimmu.2018.02808
236. Di Giacomo M, Camaioni A, Klinger FG, Bonfiglio R, Salustri A. Cyclic AMP-elevating agents promote cumulus cell survival and hyaluronan matrix stability, thereby prolonging the time of mouse oocyte fertilizability. J Biol Chem. (2016) 291:3821–36. doi: 10.1074/jbc.M115.680983
237. Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol (Baltimore, Md.). (2002) 16:1154–67. doi: 10.1210/mend.16.6.0859
238. Nagyova E, Camaioni A, Prochazka R, Salustri A. Covalent transfer of heavy chains of inter-alpha-trypsin inhibitor family proteins to hyaluronan in in vivo and in vitro expanded porcine oocyte-cumulus complexes. Biol Reprod. (2004) 71:1838–43. doi: 10.1095/biolreprod.104.029595
239. Scarchilli L, Camaioni A, Bottazzi B, Negri V, Doni A, Deban L, et al. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem. (2007) 282:30161–70. doi: 10.1074/jbc.M703738200
240. Ievoli E, Lindstedt R, Inforzato A, Camaioni A, Palone F, Day AJ, et al. Implication of the oligomeric state of the N-terminal PTX3 domain in cumulus matrix assembly. Matrix Biol. (2011) 30:330–7. doi: 10.1016/j.matbio.2011.05.002
241. Jiao W, Mi X, Yang Y, Liu R, Liu Q, Yan T, et al. Mesenchymal stem cells combined with autocrosslinked hyaluronic acid improve mouse ovarian function by activating the PI3K-AKT pathway in a paracrine manner. Stem Cell Res Ther. (2022) 13:49. doi: 10.1186/s13287-022-02724-3
242. Shin EY, Kim DS, Lee MJ, Lee AR, Shim SH, Baek SW, et al. Prevention of chemotherapy-induced premature ovarian insufficiency in mice by scaffold-based local delivery of human embryonic stem cell-derived mesenchymal progenitor cells. Stem Cell Res Ther. (2021) 12:431. doi: 10.1186/s13287-021-02479-3
243. Zhao G, Yan G, Cheng J, Zhou X, Fang T, Sun H, et al. Hyaluronic acid prevents immunosuppressive drug-induced ovarian damage via up-regulating PGRMC1 expression. Sci Rep. (2015) 5:7647. doi: 10.1038/srep07647
244. Zhao G, Zhou X, Fang T, Hou Y, Hu Y. Hyaluronic acid promotes the expression of progesterone receptor membrane component 1 via epigenetic silencing of miR-139-5p in human and rat granulosa cells. Biol Reprod. (2014) 91:116. doi: 10.1095/biolreprod.114.120295
245. Hewlett M, Mahalingaiah S. Update on primary ovarian insufficiency. Curr Opin Endocrinol Diabetes Obes. (2015) 22:483–9.
246. Kovanci E, Schutt AK. Premature ovarian failure: clinical presentation and treatment. Obstetr Gynecol Clin North Am. (2015) 42:153–61.
247. Arrigo T, Bertelloni S, Carcione L, De Luca F, De Sanctis C, Einaudi S, et al. Characterization of early presentation idiopathic ovarian failure in girls and adolescents. J Pediatr Endocrinol Metab. (2003) 16:835–42. doi: 10.1515/jpem.2003.16.6.835
248. Bachelot A, Rouxel A, Massin N, Dulon J, Courtillot C, Matuchansky C, et al. Phenotyping and genetic studies of 357 consecutive patients presenting with premature ovarian failure. Eur J Endocrinol (2009) 161:179–87. doi: 10.1530/EJE-09-0231
250. Ayesha, Jha V, Goswami D. Premature ovarian failure: an association with autoimmune diseases. J Clin Diagnost Res (2016) 10:Qc10–2.
251. Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod (Oxford, England). (2016) 31:926–37.
252. Michala L, Stefanaki K, Loutradis D. Premature ovarian insufficiency in adolescence: a chance for early diagnosis? Hormones (Athens, Greece). (2020) 19:277–83.
253. Baker VL. Primary ovarian insufficiency in the adolescent. Curr Opin Obstetr Gynecol. (2013) 25:375–81.
254. Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI). Front Endocrinol. (2021) 12:626924. doi: 10.3389/fendo.2021.626924
255. Wang MY, Wang YX, Li-Ling J, Xie HQ. Adult stem cell therapy for premature ovarian failure: from bench to bedside. Tissue Eng Part B Rev. (2022) 28:63–78. doi: 10.1089/ten.TEB.2020.0205
256. Bidet M, Bachelot A, Bissauge E, Golmard JL, Gricourt S, Dulon J, et al. Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab. (2011) 96:3864–72. doi: 10.1210/jc.2011-1038
257. Wallace WH, Kelsey TW. Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Hum Reprod (Oxford, England). (2004) 19:1612–7.
258. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. (2009) 30:465–93.
259. Wang S, Sun M, Yu L, Wang Y, Yao Y, Wang D. Niacin Inhibits Apoptosis and Rescues Premature Ovarian Failure. Cell Physiol Biochem. (2018) 50:2060–70. doi: 10.1159/000495051
260. Shimizu T, Jayawardana BC, Tetsuka M, Miyamoto A. Differential effect of follicle-stimulating hormone and estradiol on expressions of vascular endothelial growth factor (VEGF) 120, VEGF164 and their receptors in bovine granulosa cells. J Reprod Dev. (2007) 53:105–12. doi: 10.1262/jrd.18088
261. Ovayolu A, Karaman E, Turgut A, Cekici Y, Ortabag T, Rapisarda A.M, et al. Endothelial cell-specific-molecule-1 (endocan) levels in women with premature ovarian insufficiency: a prospective comparative study. J Obstetr Gynaecol. (2021) 41:637–41. doi: 10.1080/01443615.2020.1789952
262. Donato ML, Gershenson D, Ippoliti C, Wharton JT, Bast RC Jr., Aleman A, et al. High-dose ifosfamide and etoposide with filgrastim for stem cell mobilization in patients with advanced ovarian cancer. Bone Marrow Transpl. (2000) 25:1137–40.
263. Noory P, Navid S, Zanganeh BM, Talebi A, Borhani-Haghighi M, Gholami K, et al. Human menstrual blood stem cell-derived granulosa cells participate in ovarian follicle formation in a rat model of premature ovarian failure in vivo. Cell Reprogr. (2019) 21:249–59. doi: 10.1089/cell.2019.0020
264. Bhartiya D, Shaikh A, Anand S, Patel H, Kapoor S, Sriraman K, et al. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod Update. (2016) 23:41–76. doi: 10.1093/humupd/dmw030
265. Bhartiya D, Sharma D. Ovary does harbor stem cells - size of the cells matter! J Ovarian Res. (2020) 13:39. doi: 10.1186/s13048-020-00647-2
266. Höglund M. Glycosylated and non-glycosylated recombinant human granulocyte colony-stimulating factor (rhG-CSF)–what is the difference? Med Oncol (Northwood, London, England). (1998) 15:229–33. doi: 10.1007/BF02787205
267. Skaznik-Wikiel ME, McGuire MM, Sukhwani M, Donohue J, Chu T, Krivak TC, et al. Granulocyte colony-stimulating factor with or without stem cell factor extends time to premature ovarian insufficiency in female mice treated with alkylating chemotherapy. Fertil Steril. (2013) 99:2045–54.e3.
268. Mantawy EM, Said RS, Kassem DH, Abdel-Aziz AK, Badr AM. Novel molecular mechanisms underlying the ameliorative effect of N-acetyl-L-cysteine against γ-radiation-induced premature ovarian failure in rats. Ecotoxicol Environ Saf. (2020) 206:111190. doi: 10.1016/j.ecoenv.2020.111190
269. Zhang Y, Liu MW, He Y, Deng N, Chen Y, Huang J, et al. Protective effect of resveratrol on estrogen deficiency-induced osteoporosis though attenuating NADPH oxidase 4/nuclear factor kappa B pathway by increasing miR-92b-3p expression. Int J Immunopathol Pharmacol. (2020) 34:2058738420941762. doi: 10.1177/2058738420941762
270. Recchia F, Necozione S, Bratta M, Rosselli M, Guerriero G, Rea S, et al. analogues in the treatment of young women with early breast cancer: long-term follow-up of a phase II study. Int J Oncol. (2015) 46:1354–60. doi: 10.3892/ijo.2014.2811
271. Wang S, Pei L, Hu T, Jia M, Wang S. Protective effect of goserelin on ovarian reserve during (neo)adjuvant chemotherapy in young breast cancer patients: a prospective cohort study in China. Hum Reprod (Oxford, England). (2021) 36:976–86. doi: 10.1093/humrep/deaa349
272. Zhang J, Fang L, Shi L, Lai Z, Lu Z, Xiong J, et al. Protective effects and mechanisms investigation of Kuntai capsule on the ovarian function of a novel model with accelerated aging ovaries. J Ethnopharmacol. (2017) 195:173–81.
273. Zhang H, Qin F, Liu A, Sun Q, Wang Q, Li Q, et al. Kuntai capsule attenuates premature ovarian failure through the PI3K/AKT/mTOR pathway. J Ethnopharmacol. (2019) 239:111885.
274. Li M, Xie L, Li Y, Liu J, Nie G, Yang H. Synergistic effect of Huyang Yangkun Formula and embryonic stem cells on 4-vinylcyclohexene diepoxide induced premature ovarian insufficiency in mice. Chin Med. (2020) 15:83. doi: 10.1186/s13020-020-00362-6
275. Xie L, Wu S, Cao D, Li M, Liu J, Nie G, et al. Huyang yangkun formula protects against 4-Vinylcyclohexene diepoxide-induced premature ovarian insufficiency in rats via the Hippo-JAK2/STAT3 signaling pathway. Biomed Pharmacother Biomed Pharmacother. (2019) 116:109008. doi: 10.1016/j.biopha.2019.109008
Keywords: premature ovarian failure (POF), infertility, genomic variants, treatment, clinical trials
Citation: Yang X and Yang L (2023) Current understanding of the genomic abnormities in premature ovarian failure: chance for early diagnosis and management. Front. Med. 10:1194865. doi: 10.3389/fmed.2023.1194865
Received: 27 March 2023; Accepted: 17 May 2023;
Published: 02 June 2023.
Edited by:
Ali Çetin, University of Health Sciences, TürkiyeReviewed by:
Numan Cim, Demiroğlu Bilim University, TürkiyeHarun Egemen Tolunay, Liv Hospital, Türkiye
Copyright © 2023 Yang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Yang, aHh5eXlsQHllYWgubmV0
 Xu Yang1
Xu Yang1 Lin Yang
Lin Yang