- 1Department of Allergy, Immunology, and Rheumatology, Tungs’ Taichung MetroHarbor Hospital, Taichung, Taiwan
- 2Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
- 3Department of Nursing, Jen Teh Junior College of Medicine, Nursing and Management, Miaoli, Taiwan
- 4Rheumatology and Immunology Center, China Medical University Hospital, Taichung, Taiwan
- 5Department of Medicine, China Medical University, Taichung, Taiwan
- 6Department of Psychiatry, National Taiwan University Hospital Yunlin Branch, Douliu, Taiwan
- 7Department of Psychiatry, College of Medicine, National Taiwan University, Taipei, Taiwan
- 8National Center for Geriatrics and Welfare Research, National Health Research Institutes, Miaoli, Taiwan
Purpose: This study aimed to estimate the lifetime healthcare costs and loss of life expectancy (loss-of-LE) among patients with incident rheumatoid arthritis (RA) with and without depression.
Methods: This 18 years longitudinal cohort study used data from Taiwan’s National Health Insurance Research Database. In total, 43,311 patients with RA were included. Among them, 1,663 patients had depressive disorders in the year preceding the RA diagnosis. The survival function for patients with RA with or without depression was estimated and extrapolated over a lifetime using the rolling extrapolation algorithm. The loss-of-LE was calculated by comparing the sex, age, and calendar year-matched referents from vital statistics. The average monthly cost was calculated as the sum of the monthly costs for all patients divided by the number of surviving patients. Lifetime healthcare costs were estimated by multiplying the monthly average cost by the monthly survival probability.
Results: The loss-of-LE for RA patients with and without depression was 5.60 years and 4.76 years, respectively. The lifetime costs of RA patients with and without depression were USD$ 90,346 and USD$ 92,239, respectively. However, the annual healthcare costs were USD$ 4,123 for RA patients with depression and USD$ 3,812 for RA patients without depression. Regardless of sex or age, RA patients with depression had higher annual healthcare costs than those without depression.
Conclusion: Patients with RA and depression have a high loss-of-LE and high annual healthcare costs. Whether treating depression prolongs life expectancy and reduces healthcare costs warrants further investigation.
1. Introduction
Rheumatoid arthritis (RA) is a chronic medical condition characterized by systemic joint inflammation. It has numerous negative health indicators, including pain, disability, low quality of life, and increased mortality (1, 2). In addition, RA is associated with multiple comorbid conditions (3), with depressive disorder being one of the most common. A meta-analysis study showed a depression prevalence of approximately 16.8% among patients with RA (4). Notably, depressive disorders lead to a poor prognosis for many chronic diseases, including diabetes mellitus and cardiovascular diseases (5, 6). Depressive disorder is also an independent risk for mortality (7).
Literature reviews have shown that patients with RA and depression experience more pain, fatigue, and disability than those with RA alone (8, 9). However, few studies have demonstrated that depression is associated with high mortality in RA. One hospital-based cohort study, including 1,290 RA patients with an 18 years observation period, demonstrated that clinical depression was associated with a 2.2-fold increased risk of mortality (10). Another longitudinal study of 882 RA patients with a 14 years follow-up found that depression was associated with 1.35-fold hazard ratios of mortality (11). Moreover, a Finnish case study identified that 10 women and 9 men with RA died by suicide. The authors found that patients who died by suicide had more depressive disorders than the control group, whereas such associations were not found among male patients with RA (12).
The economic burden of RA is substantial (1) and may be increased by comorbid depression. Two studies using the U.S. insurance claim database revealed that RA patients with depression had higher health service utilization during the 12 months post-diagnosis period. This included hospitalization, physician visits, and emergency room visits, thereby having higher all-cause direct healthcare costs than patients with RA alone (13, 14). Another study conducted in Japan also showed that comorbid depression was associated with increased total healthcare utilization and costs (15). However, these studies only estimated healthcare costs in the first 12 months after diagnosis. The increase in lifetime healthcare expenditures due to depression has not yet been explored.
Using a nationwide longitudinal cohort of incident RA patients with an 18 years follow-up period, this study aimed to estimate the lifetime healthcare costs and loss of life expectancy (loss-of-LE) of RA patients with and without depression.
2. Materials and methods
2.1. Study approval
The study was approved by the Research Ethics Committee of China Medical University and Hospital (IRB number: CMUH108-REC2-119).
2.2. Study population and datasets
Taiwan’s National Health Insurance (NHI) was implemented in 1995. Since 2004, more than 99% of Taiwanese citizens have been covered (16). In the NHI Registry for Catastrophic Illness Patients, two physicians confirmed the catastrophic illness diagnosis. First, we identified a retrospective cohort of RA patients from the Registry for Catastrophic Illness Patients between 1999 and 2017 (International Classification of Diseases, Ninth Revision, ICD-9-CM codes 714.0, 714.1, 714.2, and 714.81, and ICD-10-CM codes M05, M06.0, M06.2, M06.3, M06.8, and M06.9). Patients younger than 16 years old at the time of diagnosis were excluded. To ensure data accuracy, an examination was carried out involving patients’ birth year and sex in both the NHI Registry for Catastrophic Illness and the Registry of Beneficiaries. Subsequently, individuals displaying discrepancies or missing information were excluded from the analysis. The survival status of patients with RA was verified by linkage to the National Mortality Registry. All patients with RA were followed until death or 31 December 2017. RA patients with depression were defined as having a diagnosis of depression in the year before the RA diagnosis. According to the ICD diagnosis codes, depression was categorized into major depression (ICD-9-CM codes 296.2, 296.3; ICD-10-CM codes: F32.1–F32.9; F33.1–F33.9) and minor depression (ICD-9-CM code: 300.4, 311; ICD-10-CM code: F34.1). In cases where a patient presented with both major and minor depression, the classification would prioritize major depression. In the analyses of life expectancy (LE) and lifetime costs, the RA cohort was further stratified by depression subtype, sex, and age at RA diagnosis (<50, between 50 and 64, and ≥65 years).
2.3. Estimation of LE and loss-of-LE
To estimate the LE of RA patients, we applied the semiparametric survival extrapolation method by Hwang and Wang (17, 18) and mathematically validated it by Fang et al. (19). This method has been used in RA by Chiu et al. (1) and is widely applied in other medical or psychiatric disorders (20, 21). Survival functions were generated by the Kaplan–Meier method until the end of the follow-up. First, a reference cohort was matched to the age, sex, and calendar year at diagnosis of the RA cohort. It was established using the Monte Carlo method based on Taiwan’s National Vital Statistics life table. Second, the survival rates of the RA cohort and the reference cohort were logit-transformed at each time point “t.” By fitting a restricted cubic spline model, we could predict the survival rate for the next month. Third, the predicted survival rate for the next month was regarded as the actual observation, and the data used in the previous extrapolation for the first month were ignored. Furthermore, the restricted cubic spline model was refitted again by repeating this step until the survival rate was close to zero. This procedure is called the rolling extrapolation algorithm.
The area under the estimated survival curve of the RA cohort was the LE after RA diagnosis. Moreover, loss-of-LE refers to the area difference between the survival curves of the RA and reference cohorts. We used the open-source R package iSQoL2 to infer the survival curves. The standard error and 95% confidence intervals were estimated through a permutation test. Group differences were assessed using a z-test to compute p-values.
To facilitate comparisons with previous studies, we conducted a Cox regression model to estimate the hazard ratios of mortality while adjusting for age, sex, and calendar year at diagnosis.
2.4. Estimation of lifetime healthcare expenditures for patients
We collected reimbursement data from the NHI database to estimate lifetime healthcare costs for patients with RA. First, monthly patient costs were summed and divided by each month’s surviving cases to calculate the average monthly cost. Assuming that healthcare costs would increase in the months near the end of life, the average cost function was estimated by weighing the patient’s average cost over the previous months. The lifetime healthcare costs were obtained by multiplying the monthly average cost to the survival probability (18). We adjusted annual NHI costs based on the Consumer Price Index (CPI) to account for inflation. For consistency, the extrapolated costs were adjusted at a discount rate of 3% per year. Currency values were expressed in 2017 US dollars (1 USD = 30.44 TWD).
2.5. Sensitivity analysis
Given that biological therapies were introduced in 2003 in Taiwan, healthcare costs increased dramatically. The sensitivity analysis included only patients diagnosed between 2003 and 2017.
3. Results
3.1. Patient characteristics
There were 43,311 new cases of RA from 1999 to 2017, of which 1,662 (3.8%) had a diagnosis of depression prior to the RA diagnosis. Among the 33,225 female patients with RA, 1,383 (4.2%) had depressive disorders. Among the 10,086 male patients with RA, 279 (2.8%) had depressive disorders.
3.2. Estimation of LE and loss-of-LE
The average age at diagnosis of patients with RA without depression was 52.68 years old, the mean LE after diagnosis was 27.05 years, and the loss-of-LE was 4.76 years (95% CI: 3.63–5.72). In contrast, the average age at diagnosis of RA patients with depression was 55.41 years old, the mean LE after diagnosis was 24.00 years, and the loss-of-LE was 5.60 years (1.99–8.92). While the 95% confidence intervals for loss-of-LE overlapped between RA patients with and without depression (p-value = 0.667), the hazard ratio for mortality associated with depression among RA patients was 1.38 (1.24–1.54; p-value <0.001). Regarding subtypes of depression, the average age at diagnosis of RA patients with major and minor depression was 53.43 and 56.26, respectively. The mean LE for major and minor depression was 26.70 and 23.33 years, respectively. The loss-of-LE was 4.68 years (1.56–10.64) for major depression and 5.46 years (1.64–9.19) for minor depression (refer to Table 1 and Figure 1). However, no statistically significant difference was observed (p-value = 0.822).
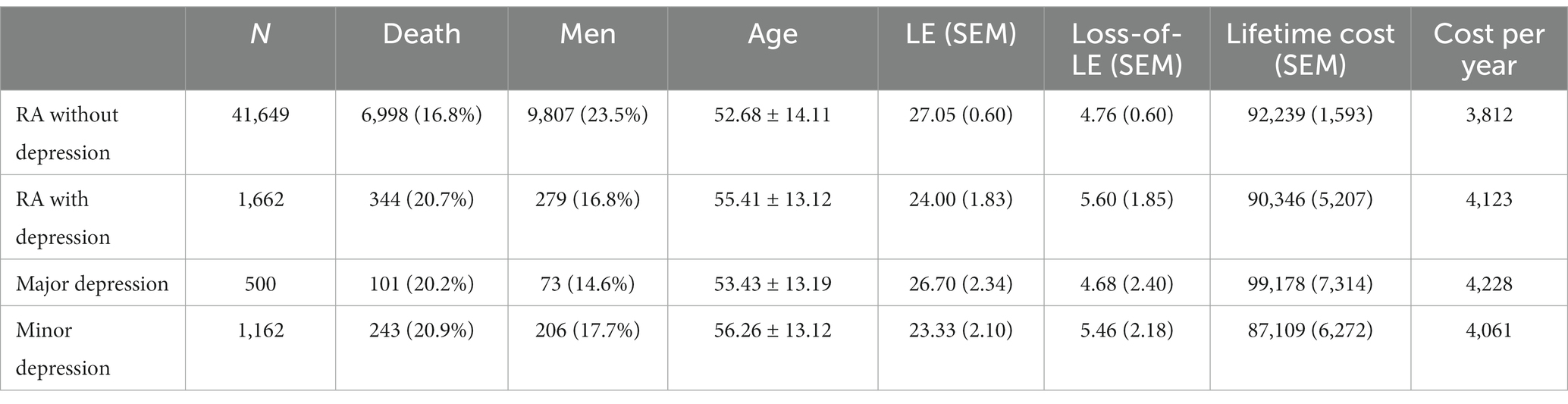
Table 1. Life expectancy, loss of life expectancy, lifetime cost, and cost per life-year of rheumatoid arthritis patients, stratified by depression.
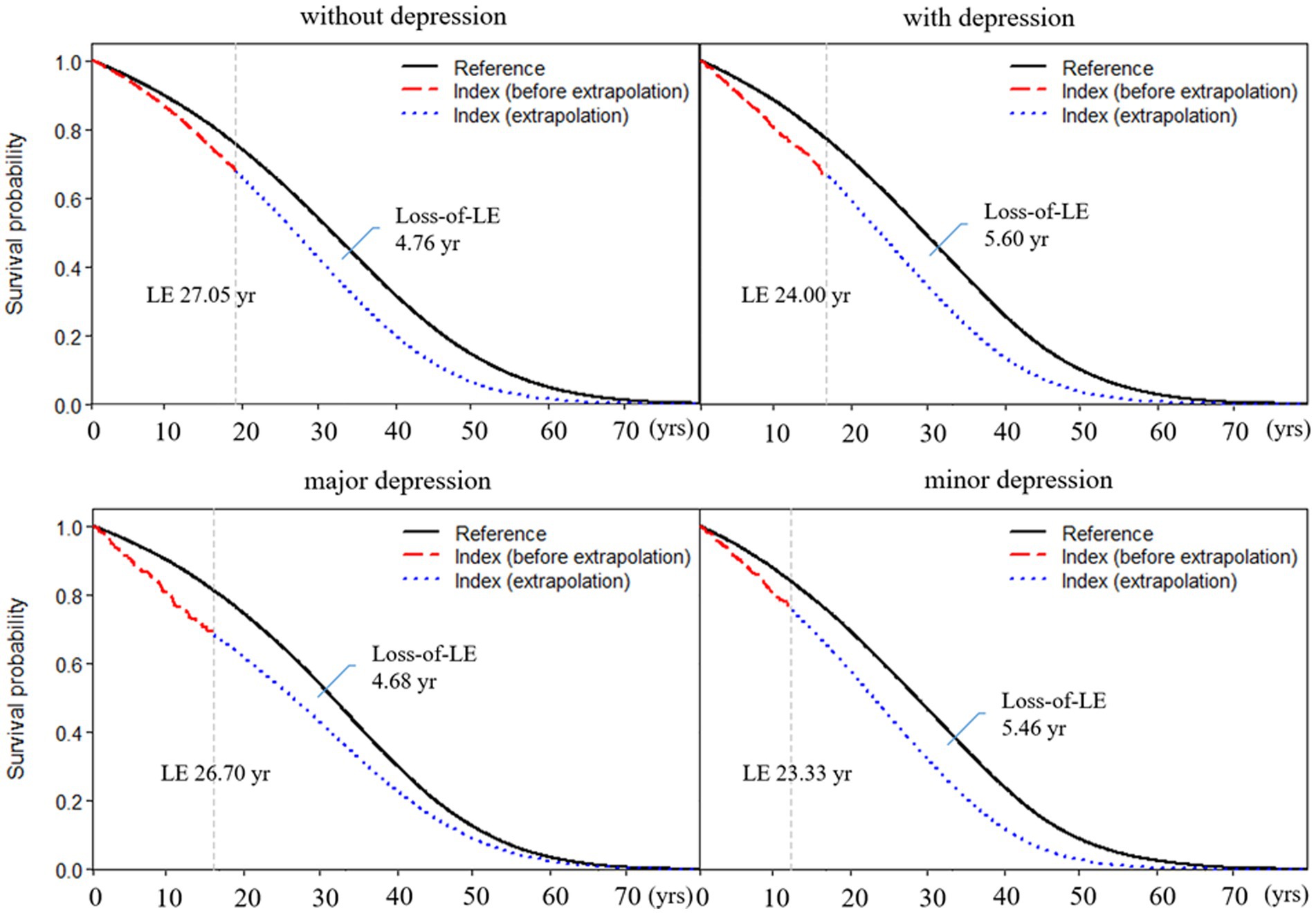
Figure 1. Life expectancy and loss of life expectancy after diagnosis of rheumatoid arthritis stratified by depression. The red line indicates the survival curve of true data from rheumatoid arthritis patients, and the blue line indicates the survival curve of extrapolation from rheumatoid arthritis. The black line indicates the survival curve of the sex-, age-, and calendar year-matched reference cohort.
Table 2 shows subgroup analysis stratified by sex and age for RA patients with and without depression. We found that the loss-of-LE for male RA patients with and without depression was 7.40 years (2.14–11.4) and 5.57 years (4.70–11.0), respectively. Similarly, the loss-of-LE for female RA patients with and without depression was 5.91 years (1.53–9.93) and 3.64 years (2.31–5.65), respectively. These results indicate that, in our study, male patients had lower LE when compared to female patients. It is worth noting that the difference in loss-of-LE due to depression was not significant for both sexes. The loss-of-LE for RA patients without depression was 8.66 (2.88–12.16), 5.75 (4.77–6.34), and 2.36 (2.12–2.66) years for those aged <50, between 50 and 64, and ≥65, respectively. Among RA patients with depression, the loss-of-LE was 15.69 (2.86–21.83), 8.17 (4.42–9.89), and 4.12 (2.50–5.52) years for those aged <50, between 50 and 64, and ≥65, respectively. These findings indicate that study patients with RA onset at younger and middle-aged ages have significantly greater loss-of-LE than older-aged patients, particularly those with depression. In addition, when comparing the loss-of-LE between RA patients with and without depression, it was only statistically significant among those aged ≥65 years (p-value = 0.036). Figures 2, 3 indicate the correlation between LE and loss-of-LE in these stratifications.
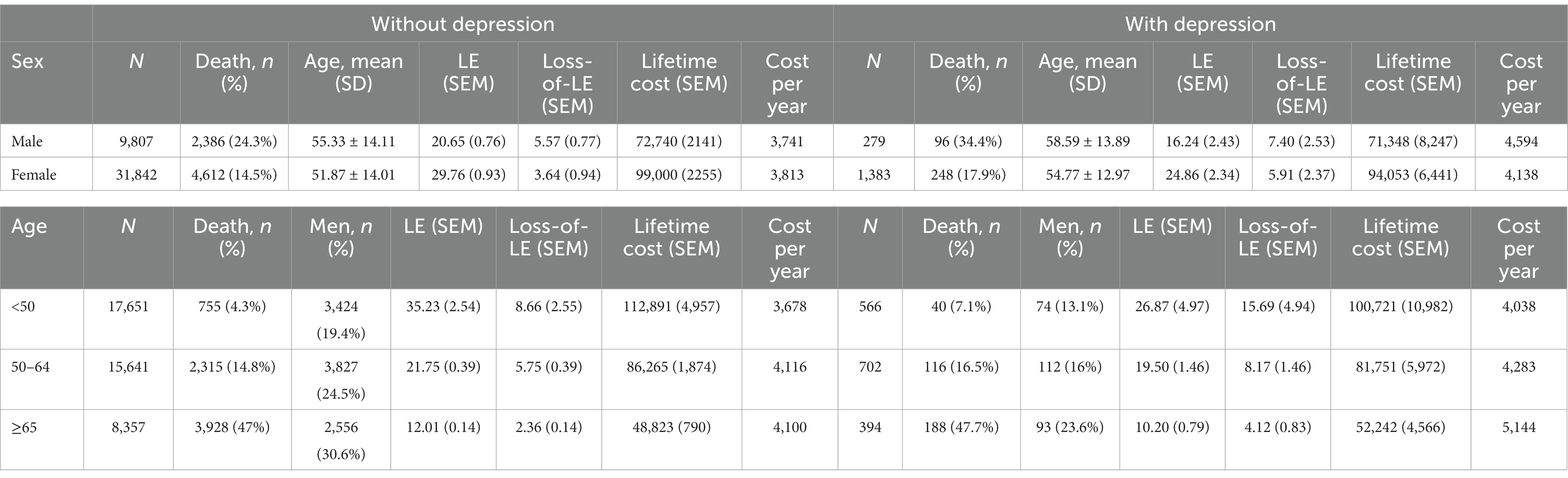
Table 2. Life expectancy, loss of life expectancy, lifetime cost, and cost per life-year of rheumatoid arthritis patients, stratified by depression, sex, and age at diagnosis.
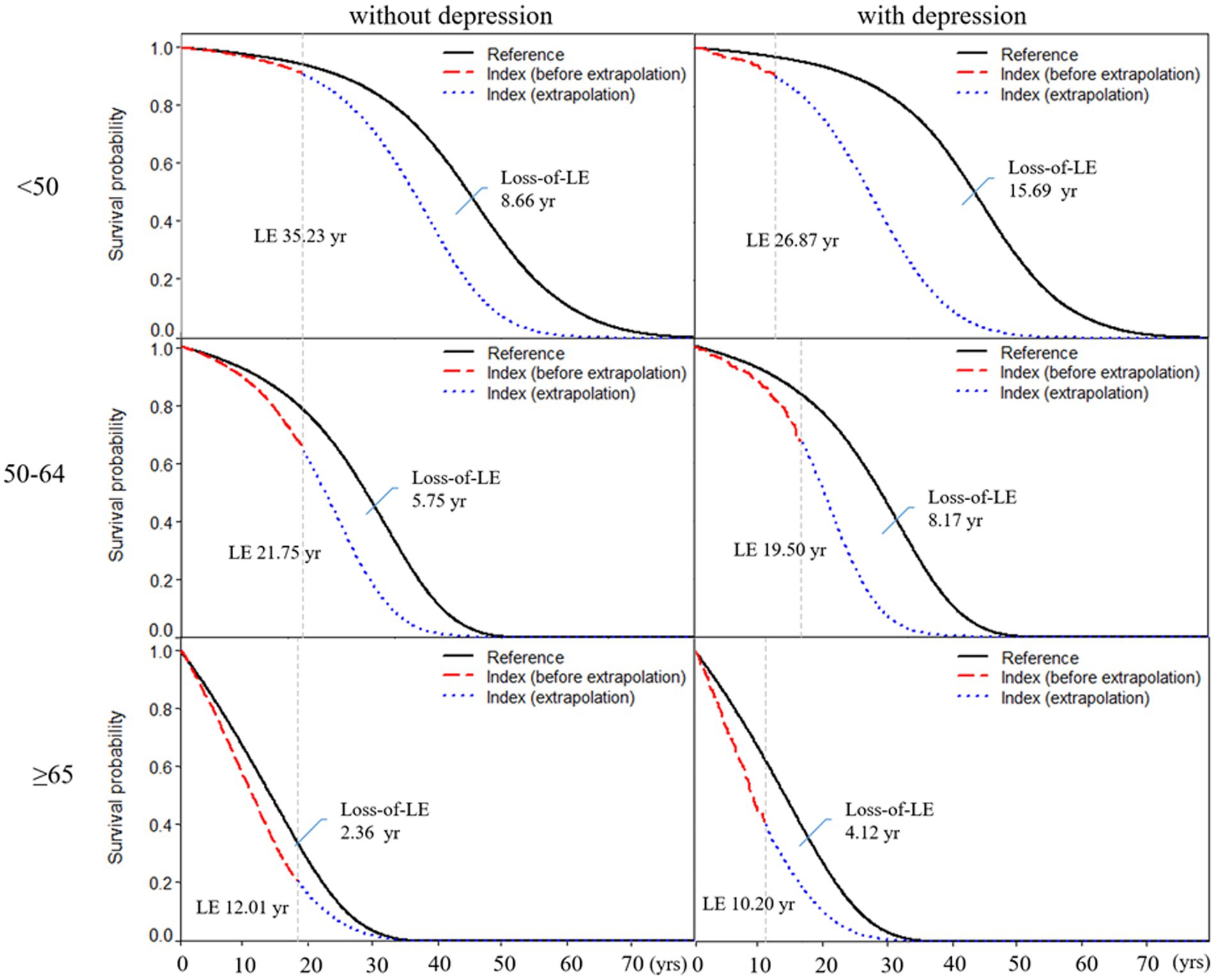
Figure 2. Life expectancy and loss of life expectancy after diagnosis of rheumatoid arthritis stratified by depression and age at diagnosis. The red line indicates the survival curve of true data from rheumatoid arthritis patients, and the blue line indicates the survival curve of extrapolation from rheumatoid arthritis. The black line indicates the survival curve of the sex-, age-, and calendar year-matched reference cohort.
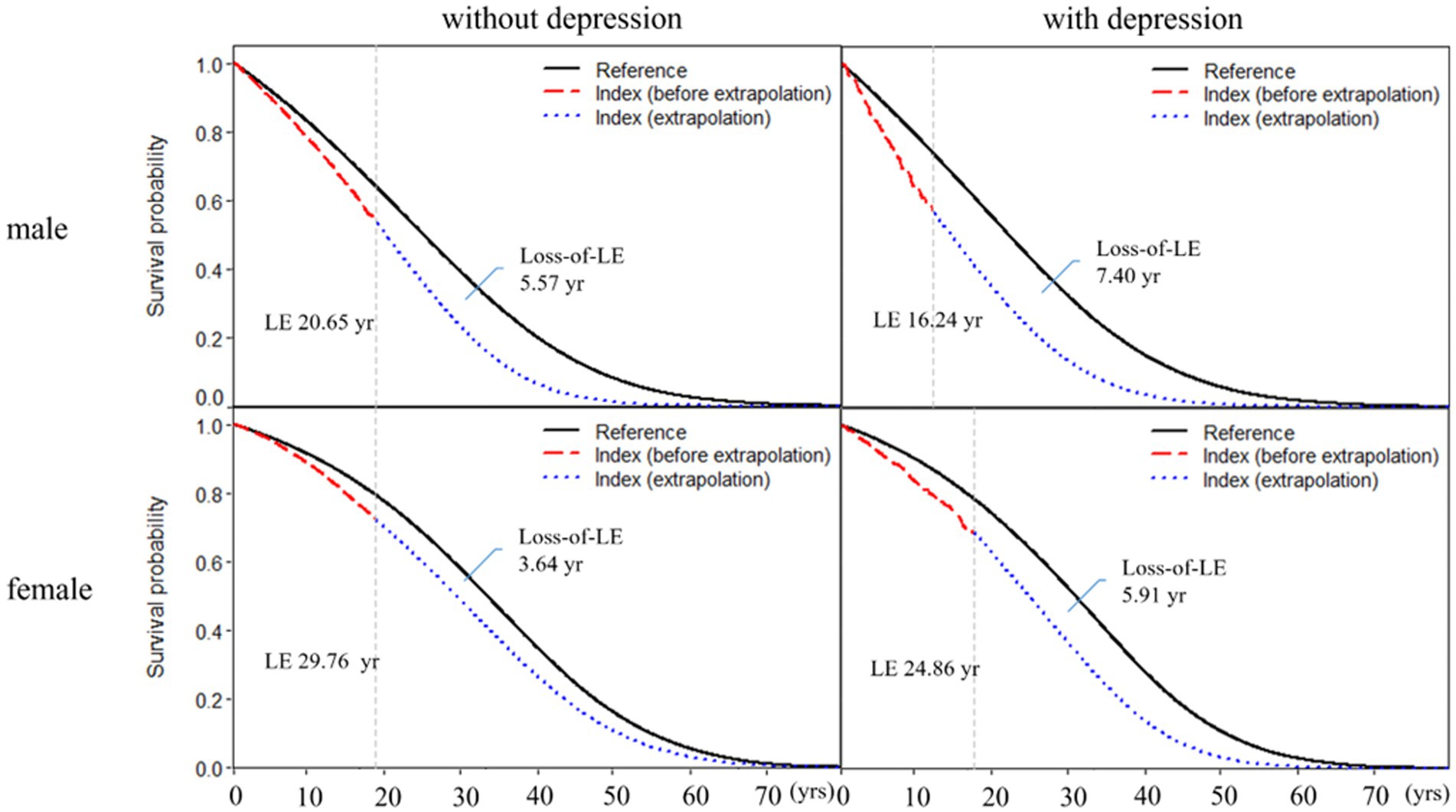
Figure 3. Life expectancy and loss of life expectancy after diagnosis of rheumatoid arthritis stratified by depression and sex. The red line indicates the survival curve of true data from rheumatoid arthritis patients, and the blue line indicates the survival curve of extrapolation from rheumatoid arthritis. The black line indicates the survival curve of the sex-, age-, and calendar year-matched reference cohort.
3.3. Estimation of lifetime healthcare expenditures for patients
The lifetime costs of RA patients without depression and with depression were USD $92,239 and USD $90,346, respectively. Patients with RA without depression had higher lifetime costs than those with depression due to longer LE. However, to account for LE, we divided lifetime costs by LE to obtain the cost per year. The annual healthcare cost, derived from the division of lifetime healthcare costs by life expectancy, amounted to USD $4,123 for patients with both RA and depression and USD $3,812 for patients with RA alone (p-value = 0.043).
Figure 4 illustrates the annual healthcare costs categorized by the sex and age groups. When comparing the annual healthcare costs between patients with and without depression, a disparity of USD $853 (p-value = 0.024) was observed among male patients and USD $325 (p-value = 0.046) among female patients. Regarding age groups, the difference amounted to USD $360 (p-value = 0.206) for individuals aged <50 years, USD $167 (p-value = 0.473) for those aged between 50 and 64 years, and USD $1,044 (p-value = 0.001) for those aged ≥65 years.

Figure 4. Annual healthcare cost after diagnosis of rheumatoid arthritis stratified by the sex and age groups.
3.4. Sensitivity analysis
The findings from the sensitivity analysis, encompassing RA patients diagnosed from 2003 to 2017, remained consistent (see Table 3). A modest elevation in LE and a slight reduction in loss-of-LE were observed alongside a marginal increase in annual healthcare costs. It was noted that most of the initial study cohort was incorporated after introducing biological therapy. Consequently, the outcomes exhibited no significant alteration.
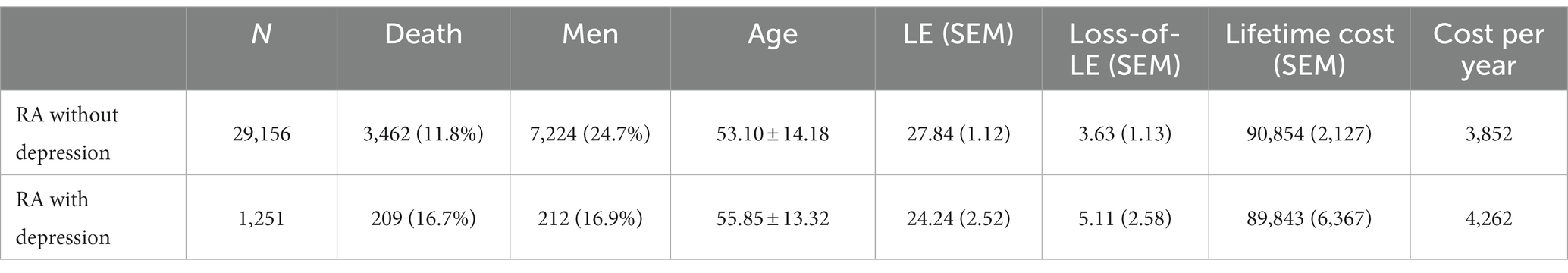
Table 3. Sensitivity analysis for life expectancy, loss of life expectancy, lifetime cost, and cost per life-year of rheumatoid arthritis patients diagnosed between 2003 and 2017.
4. Discussion
To the best of our knowledge, this is the first study to estimate both LE and lifetime healthcare costs in a cohort of patients with incident RA with and without depression. We found that the loss-of-LE was 5.60 years for patients with RA with depression and 4.76 years for those with only RA. The annual healthcare costs were USD$ 4,123 for RA patients with depression and USD$ 3,812 for those without depression. In the subgroup analysis, the loss-of-LE for male patients with RA was larger than that for female patients, but the sex difference in loss-of-LE was similar across patients with and without depression. On the other hand, the annual medical costs were similar for both sexes among patients with RA alone but much higher for male RA patients with depression than for female RA patients with depression. Regarding group, the loss-of-LE was much higher among RA patients with depression aged <50 years; however, the annual medical costs were much higher among RA patients with depression aged ≥65 years.
In this study, the prevalence of depression among patients with RA was 3.8%, which was higher than that of the general population in Taiwan (22). However, the prevalence is much lower than the pooled prevalence (16.8%) estimated in one meta-analysis (4). It should be noted that the prevalence of depression among patients with RA varied across different countries and study designs. The prevalence of depression in the general population is approximately 1%–2% in Taiwan (23), which is much lower than that in other countries (24). Moreover, the prevalence of depression was relatively higher using self-reported instruments than that diagnosed by psychiatric interviews (4). A study using US claim records found that the prevalence was 7.5% among incident RA patients (14). Another study conducted in Japan found that the prevalence was 5% (15). Notably, the treatment rate also affects the estimated prevalence using the claim database. Overall, the treatment rate for depression varied, ranging from 16.8% in low-income countries to 48.3% in high-income countries (25). In Taiwan, the treatment rate was approximately 27% of patients (26).
Furthermore, we only identified pre-existing depression cases in the year preceding the RA diagnosis. Patients who developed depressive disorders after RA onset were not identified in our study but would be in a cross-sectional design. Thus, the prevalence of depression in the present study was relatively low. In our post-hoc analysis, we identified 5,316 individuals (12.8%) who developed depressive disorders among the comparison groups during the follow-up period. The incidence rate was calculated at 25.7 per 1,000 person-years.
We observed a greater loss-of-LE in patients with both RA and depression compared to those with RA alone, despite the overlapping 95% confidence intervals for the loss-of-LE in these two groups. Nevertheless, employing the Cox regression model, we identified a statistically significant hazard ratio for mortality associated with depression. This finding was consistent with previous studies that showed that RA patients with depression had high mortality (10, 11). The underlying mechanism is multifactorial. Although RA patients with depression might be more likely to die by suicide (12), increased mortality was not restricted to suicide. Patients with depressive disorder have unhealthy lifestyles, including a lack of exercise and an unhealthy diet (27), which could be attributed to non-suicide mortality. One study demonstrated that depression increased the risk of myocardial infarction among patients with RA (28). Furthermore, depression is associated with the inflammation process, which might exacerbate RA activity (29). Moreover, depression might be associated with a poor treatment response for RA, which further deteriorates the prognosis of RA (30, 31). All of these factors are associated with the increasing loss-of-LE among RA patients with depression.
Unsurprisingly, patients with RA and depression had higher annual healthcare costs than those with RA alone. In this study, we did not assess the disease-specific costs. Several studies found that patients with RA and depression had higher all-cause healthcare utilization and costs (13–15). One study demonstrated annual healthcare costs of USD$ 12,225 for RA patients with depression and USD$ 11,404 for those with RA only, with a 7% incremental cost (14). However, the percentage of incremental healthcare costs for depression was 46% in a Japanese study (15). In our study, the annual medical cost of depression increased by 8.2%, which was much closer to the findings in the US. The increased healthcare cost might be due to the cost of depression treatment; however, previous studies showed that RA-related healthcare utilization also increased (13–15). Comorbid depression might also be associated with poor RA treatment responses (30, 31). In addition, we only calculated the costs reimbursed in the NHI program, and this study did not include out-of-pocket expenses. Therefore, medical costs were underestimated. The components of increased healthcare costs and the underlying mechanisms should be explored in detail.
In terms of depression subtypes, the annual healthcare cost for major depression was higher than that for dysthymia or minor depression. However, we found that there was no difference in the loss-of-LE between major and minor depression. A previous study showed that the mortality risk for dysthymia was similar to that for major depression (32). Patients with dysthymia had fewer depression symptoms but lower rates of remission (33). Further research is needed to determine which symptom severity or illness duration determinants had a higher effect on LE among RA patients.
We found that older patients with RA had higher annual healthcare costs but less loss-of-LE. Older patients with depression might have more comorbidities and healthcare costs (34). Additionally, older patients have more risk factors for mortality; therefore, the effect of depression on mortality was less prominent than other risk factors. In contrast, depression may be among the few major risk factors among younger adults. Hence, depression had an important effect on mortality in our study.
The sex difference in loss-of-LE between patients with and without depression was similar; however, the annual medical costs of male patients with depression were higher than those of female patients. Whether there is a sex-related difference in the outcome of RA remains inconclusive (35). Possible factors associated with the outcome include hormonal factors, help-seeking behaviors, and medication compliance. The treatment rate for depression was lower in men than in women, and undertreatment might deteriorate treatment response and increase costs (26). No previous study has explored the role of sex on the association between depression and mortality or medical costs among patients with RA. Further investigations are necessary to explore these differences.
This study has several limitations. First, we identified patients with depressive disorders based on claim records. The prevalence of depressive disorders was found to be lower than in a previous study (4). According to our previous investigation, only 27% of patients sought treatment for depression (26). It is possible that some patients with depression who were neither diagnosed nor treated could have been misclassified into the comparison group. Conversely, while the accuracy of ICD codes for depressive disorders is substantial, some patients without depressive disorders might have been misclassified into the depression groups. These misclassifications could, overall, reduce the observed differences in LE and lifetime healthcare costs between RA patients with depression and those without depression. Therefore, our results may have been underestimated. Second, in order to avoid immortal time bias (36)—wherein RA patients must survive until depression diagnosis—we refrained from categorizing patients who developed depressive disorders after RA diagnosis into the depression groups. It is important to acknowledge that such misclassifications could potentially lead to underestimations in our findings. Third, we only measured direct healthcare costs. Quality of life, functional disability, loss of productivity, and caregiver load were not included. The economic burden of depression with RA is greater than the direct healthcare costs. Fourth, given the limited sample size, we did not explore the causes of death, and these findings should be explored in future. Fifth, we matched for age, sex, and calendar year. Nevertheless, certain variables, including socioeconomic status, disease severity, comorbid medical conditions, and the introduction of biological therapies throughout the study duration, could have significantly affected costs and outcomes, potentially confounding our findings. These variables may also act as mediators in the relationship between depression and lifetime healthcare costs in individuals with RA. Consequently, we refrained from making adjustments for these factors. Finally, we did not investigate the treatment effect of depression on the loss-of-LE and direct medical costs. Previous studies have shown that antidepressants might be associated with a reduced risk of diabetic complications among patients with diabetes (37). It should also be investigated whether depression treatment could alleviate the adverse effects of depression.
Despite the abovementioned limitations, this study’s strengths include the use of a nationally representative cohort with an 18 years follow-up period, a relatively large cohort size, and a novel method for estimating LE and lifetime healthcare expenditure.
5. Conclusion
We found that patients with RA had a higher loss-of-LE and higher direct medical costs when they had comorbid depressive disorders. Depression is a treatable illness but is commonly unrecognized and undertreated. Because the effect of depression is noticeable, clinicians should focus on screening for and detecting depression among patients with RA. Whether treatment can prolong LE and reduce healthcare costs remains unclear. Further research should focus on evaluating the effect of depression treatment on advanced complications and mortality in patients with RA.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: data used in this study are from the Health and Welfare Data Science Center at Ministry of Health and Welfare, which are not publicly available. Requests to access these datasets should be directed to the Health and Welfare Data Science Center (HWDC), https://www.apre.mohw.gov.tw/.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of China Medical University and Hospital (IRB number: CMUH108-REC2-119). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because all personal information is de-identified.
Author contributions
Y-MC, W-LH, and C-SW contributed to conceptualization, methodology, data curation, and original draft preparation. J-LL and W-LH supervised, reviewed, and edited the draft. All authors contributed to the article and approved the submitted version.
Funding
This study was partly supported by the National Health Research Institutes of Taiwan (CG-110-GP-01, PI: C-SW). The funding agency had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Acknowledgments
The authors are grateful to the Health Data Science Centre, China Medical University Hospital, for providing administrative, technical, and funding support and to Ya-Chu Yang for data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chiu, YM, Lu, YP, Lan, JL, Chen, DY, and Wang, JD. Lifetime risks, life expectancy, and health care expenditures for rheumatoid arthritis: a nationwide cohort followed up from 2003 to 2016. Arthritis Rheumatol. (2021) 73:750–8. doi: 10.1002/art.41597
2. Matcham, F, Scott, IC, Rayner, L, Hotopf, M, Kingsley, GH, Norton, S, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. (2014) 44:123–30. doi: 10.1016/j.semarthrit.2014.05.001
3. Dougados, M. Comorbidities in rheumatoid arthritis. Curr Opin Rheumatol. (2016) 28:282–8. doi: 10.1097/BOR.0000000000000267
4. Matcham, F, Rayner, L, Steer, S, and Hotopf, M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology. (2013) 52:2136–48. doi: 10.1093/rheumatology/ket169
5. Wu, C-S, Hsu, L-Y, and Wang, S-H. Association of depression and diabetes complications and mortality: a population-based cohort study. Epidemiol Psychiatr Sci. (2020) 29:29. doi: 10.1017/S2045796020000049
6. Hare, DL, Toukhsati, SR, Johansson, P, and Jaarsma, T. Depression and cardiovascular disease: a clinical review. Eur Heart J. (2013) 35:1365–72. doi: 10.1093/eurheartj/eht462
7. Machado, MO, Veronese, N, Sanches, M, Stubbs, B, Koyanagi, A, Thompson, T, et al. The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Med. (2018) 16:1–13. doi: 10.1186/s12916-018-1101-z
8. Bruce, TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep. (2008) 10:258–64. doi: 10.1007/s11920-008-0042-1
9. Dickens, C, McGowan, L, Clark-Carter, D, and Creed, F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. (2002) 64:52–60. doi: 10.1097/00006842-200201000-00008
10. Ang, DC, Choi, H, Kroenke, K, and Wolfe, F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol. (2005) 32:1013–9.
11. van den Hoek, J, Boshuizen, H, Roorda, L, Tijhuis, G, Nurmohamed, M, Dekker, J, et al. Association of somatic comorbidities and comorbid depression with mortality in patients with rheumatoid arthritis: a 14-year prospective cohort study. Arthritis Care Res. (2016) 68:1055–60. doi: 10.1002/acr.22812
12. Timonen, M, Viilo, K, Hakko, H, Särkioja, T, Ylikulju, M, Meyer-Rochow, VB, et al. Suicides in persons suffering from rheumatoid arthritis. Rheumatology. (2003) 42:287–91. doi: 10.1093/rheumatology/keg082
13. Li, N, Chan, E, and Peterson, S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J Med Econ. (2019) 22:372–8. doi: 10.1080/13696998.2019.1572015
14. Joyce, AT, Smith, P, Khandker, R, Melin, JM, and Singh, A. Hidden cost of rheumatoid arthritis (RA): estimating cost of comorbid cardiovascular disease and depression among patients with RA. J Rheumatol. (2009) 36:743–52. doi: 10.3899/jrheum.080670
15. Guelfucci, F, Kaneko, Y, Mahlich, J, and Sruamsiri, R. Cost of depression in Japanese patients with rheumatoid arthritis: evidence from administrative data. Rheumatol Ther. (2018) 5:171–83. doi: 10.1007/s40744-018-0096-4
16. Lin, L-y, Warren-Gash, C, Smeeth, L, and Chen, P-C. Data resource profile: the national health insurance research database (NHIRD). Epidemiol Health. (2018) 40:40. doi: 10.4178/epih.e2018062
17. Hwang, JS, and Wang, JD. Monte Carlo estimation of extrapolation of quality-adjusted survival for follow-up studies. Stat Med. (1999) 18:1627–40. doi: 10.1002/(SICI)1097-0258(19990715)18:13<1627::AID-SIM159>3.0.CO;2-D
18. Hwang, J-S, Hu, T-H, Lee, LJ-H, and Wang, J-D. Estimating lifetime medical costs from censored claims data. Health Econ. (2017) 26:e332–44. doi: 10.1002/hec.3512
19. Fang, CT, Chang, YY, Hsu, HM, Twu, SJ, Chen, KT, Lin, CC, et al. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM. (2007) 100:97–105. doi: 10.1093/qjmed/hcl141
20. Kuo, S-C, Lin, C-N, Lin, Y-J, Chen, W-Y, Hwang, J-S, and Wang, J-D. Optimal intervals of ultrasonography screening for early diagnosis of hepatocellular carcinoma in Taiwan. JAMA Netw Open. (2021) 4:e2114680. doi: 10.1001/jamanetworkopen.2021.14680
21. Lêng, CH, Chou, MH, Lin, S-H, Yang, YK, and Wang, J-D. Estimation of life expectancy, loss-of-life expectancy, and lifetime healthcare expenditures for schizophrenia in Taiwan. Schizophr Res. (2016) 171:97–102. doi: 10.1016/j.schres.2016.01.033
22. Wang, H-H, Chang, C-M, Chang, S-S, Yang, AC, Liu, Y-H, Liao, S-C, et al. Ten-year trends in depression care in Taiwan. J Formos Med Assoc. (2022) 121:2001–11. doi: 10.1016/j.jfma.2022.02.011
23. Liao, S-C, Chen, W, Lee, M-B, Lung, F-W, Lai, T-J, Liu, C-Y, et al. Low prevalence of major depressive disorder in Taiwanese adults: possible explanations and implications. Psychol Med. (2012) 42:1227–37. doi: 10.1017/S0033291711002364
24. Lim, GY, Tam, WW, Lu, Y, Ho, CS, Zhang, MW, and Ho, RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. (2018) 8:1–10. doi: 10.1038/s41598-018-21243-x
25. Mekonen, T, Chan, GC, Connor, JP, Hides, L, and Leung, J. Estimating the global treatment rates for depression: a systematic review and meta-analysis. J Affect Disord. (2021) 295:1234–42. doi: 10.1016/j.jad.2021.09.038
26. Chang, T-Y, Liao, S-C, Chang, C-M, Wu, C-S, Huang, W-L, Hwang, J-J, et al. Barriers to depression care among middle-aged and older adults in Taiwan’s universal healthcare system. Lancet Reg Health. (2022) 26:100501. doi: 10.1016/j.lanwpc.2022.100501
27. Jacka, FN, and Berk, M. Depression, diet and exercise. Med J Aust. (2013) 199:S21–3. doi: 10.5694/mja12.10508
28. Scherrer, JF, Chrusciel, T, Zeringue, A, Garfield, LD, Hauptman, PJ, Lustman, PJ, et al. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed veterans administration patients. Am Heart J. (2010) 159:772–9. doi: 10.1016/j.ahj.2010.02.033
29. Nerurkar, L, Siebert, S, McInnes, IB, and Cavanagh, J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry. (2019) 6:164–73. doi: 10.1016/S2215-0366(18)30255-4
30. Covic, T, Tyson, G, Spencer, D, and Howe, G. Depression in rheumatoid arthritis patients: demographic, clinical, and psychological predictors. J Psychosom Res. (2006) 60:469–76. doi: 10.1016/j.jpsychores.2005.09.011
31. Matcham, F, Davies, R, Hotopf, M, Hyrich, KL, Norton, S, Steer, S, et al. The relationship between depression and biologic treatment response in rheumatoid arthritis: an analysis of the British Society for Rheumatology biologics register. Rheumatology. (2018) 57:835–43. doi: 10.1093/rheumatology/kex528
32. Byers, AL, Covinsky, KE, Barnes, DE, and Yaffe, K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. (2012) 20:664–72. doi: 10.1097/JGP.0b013e31822001c1
33. Rhebergen, D, Beekman, AT, de Graaf, R, Nolen, WA, Spijker, J, Hoogendijk, WJ, et al. The three-year naturalistic course of major depressive disorder, dysthymic disorder and double depression. J Affect Disord. (2009) 115:450–9. doi: 10.1016/j.jad.2008.10.018
34. Ho, CS, Feng, L, Fam, J, Mahendran, R, Kua, EH, and Ng, TP. Coexisting medical comorbidity and depression: multiplicative effects on health outcomes in older adults. Int Psychogeriatr. (2014) 26:1221–9. doi: 10.1017/S1041610214000611
35. van Vollenhoven, RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med. (2009) 7:1–4. doi: 10.1186/1741-7015-7-12
36. Yadav, K, and Lewis, RJ. Immortal time bias in observational studies. JAMA. (2021) 325:686–7. doi: 10.1001/jama.2020.9151
Keywords: rheumatoid arthritis, depression, healthcare cost, life expectancy, disease burden
Citation: Chiu Y-M, Lan J-L, Huang W-L and Wu C-S (2023) Estimation of life expectancy and healthcare cost in rheumatoid arthritis patients with and without depression: a population-based retrospective cohort study. Front. Med. 10:1221393. doi: 10.3389/fmed.2023.1221393
Edited by:
Hubert Marotte, INSERM U1059 SAnté INgéniérie BIOlogie, FranceReviewed by:
Caleb Cornaby, Children’s Hospital of Los Angeles, United StatesArnulfo Hernan Nava-Zavala, Mexican Social Security Institute (IMSS), Mexico
Copyright © 2023 Chiu, Lan, Huang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Shin Wu, Y2hpc2hpbnd1QG5ocmkuZWR1LnR3
 Ying-Ming Chiu1,2,3
Ying-Ming Chiu1,2,3 Wei-Lieh Huang
Wei-Lieh Huang Chi-Shin Wu
Chi-Shin Wu